Glaucoma is a leading cause of irreversible blindness, expected to affect 140 million people worldwide by the year 2040. The pathophysiology of glaucoma involves retinal ganglion cell loss, optic nerve atrophy and cupping of the optic disk, culminating in visual field loss (Boia et al., 2020). Glaucoma is a multifactorial disease in which increased intraocular pressure is the main risk factor. Therefore, the majority of experimental models are based on ocular hypertension that mimics in some extend the features of clinical glaucoma (Aires et al., 2017). Elevated hydrostatic pressure is also suitable to disentangle the cellular mechanisms involved in disease progression, using cultured cells or tissues (Aires et al., 2017).
Microglial cells are the immune competent cells of the retina. They surveil the retinal parenchyma by extending and retracting their highly motile processes to detect alterations in homeostasis. Inflammation has long been demonstrated to take part in glaucoma onset and progression (Boia et al., 2020). In the early stages of the disease, the response of microglia may be protective; however chronic sustained exacerbated response is associated with glaucoma progression by initiating a cascade of events that lead to an uncontrolled inflammatory milieu (Madeira et al., 2015a). Microglial cell reactivity occurs early in the disease prior to the detection of retinal ganglion cell death (Madeira et al., 2015a), placing microglial cells as crucial players of glaucomatous degeneration. Indeed, we demonstrated that challenging microglial cells with elevated hydrostatic pressure causes microglia reactivity and cell death (Aires et al., 2019). The exposure of retinal organotypic cultures to elevated hydrostatic pressure increases the levels of pro-inflammatory cytokines tumor necrosis factor and interleukin 1β that mediate the loss of retinal ganglion cells, since the neutralization of these cytokines is able to halt retinal ganglion cell loss (Madeira et al., 2015b). Strong evidence supporting the role of microglia in the death of retinal neurons during conditions of elevated pressure was definitely demonstrated recently using a pharmacologic strategy to deplete microglia (Aires et al., 2019). In such conditions, exposing retinal neural cells to elevated hydrostatic pressure did not increase cell death, pointing to a role of microglia in elevated pressure-mediated degeneration (Aires et al., 2019). All these findings corroborate the hypothesis that controlling microglia-mediated neuroinflammation may have beneficial effects in glaucoma. Therefore, it is of the utmost importance to clarify the mechanisms by which microglia initiate and propagate the inflammatory milieu in the retina. One intriguing fact is the observation that reactive microglia are found along the damaged optic nerve fibers, initiating at the unmyelinated region of the optic nerve, further progressing along the retinal parenchyma (Boia et al., 2020). Taking into account this synchronous response of microglial cells it is envisaged the existence of an intercellular communication system that allows the propagation of the inflammatory signal.
Exosomes have arisen as an important mean for efficient cell to cell communication. These small nanoscale extracellular vesicles are derived from the endocytic pathway and are released into the extracellular space through the fusion of multivesicular bodies with the plasma membrane (Aires et al., 2020). Exosomes may carry lipids, proteins and microRNAs across cell boundaries and directly target desired recipient cells through membrane receptors, fusion or endocytosis (Aires et al., 2020). In the brain, upon a deleterious stimulus microglia were shown to release exosomes that interact with other microglial cells, contributing to the amplification of the inflammatory environment (Paolicelli et al., 2019). Very recently, we reported a role for retinal microglial exosomes in neurodegeneration (Figure 1). Microglia release exosomes constitutively, but elevated hydrostatic pressure approximately doubles the release of exosomes by microglia. An increase in exosome release was already reported and associated with brain inflammatory conditions and microglial cell response (Paolicelli et al., 2019). Also, the release of extracellular vesicles by microglia is promoted by adenosine triphosphate, which might explain the effect of elevated pressure on the increased release of exosomes, since we reported an increase in extracellular adenosine triphosphate when microglial cells are challenged with elevated hydrostatic pressure (Rodrigues-Neves et al., 2018). The exosomes isolated from microglia exposed to elevated hydrostatic pressure promoted a shift in naïve microglia cell response in vitro, with an increase in the production of pro-inflammatory mediators and reactivity markers (Figure 1). They also changed microglia function, promoting motility, proliferation and phagocytosis (Figure 1). These are all features associated with reactive microglial cells, showing that the exosomes derived from reactive microglia (cells exposed to elevated hydrostatic pressure) code for a proinflammatory condition. In this study, it was nicely shown that the effects observed were clearly mediated by exosomes, since impairing their release by the inhibition of neutral sphingomyelinase with GW4869 abolished the effects of exosomes. The exosomes from reactive microglia should be different in some extent from the constitutively released exosomes (control pressure) since the incubation of microglial cells even with the double of exosomes isolated from control cells does not trigger an inflammatory response. In line with this observation, it was recently demonstrated that control microglial exosomes may exert protection against the loss of photoreceptors and neuroinflammation in a model of retinopathy of prematurity (Xu et al., 2019). In this report, microglial exosomes reduce avascular regions in the retina, vascular endothelial growth factor expression, and photoreceptor apoptosis, suggesting that the phenotype of recipient cells may also determine the effects of microglial exosomes.
Figure 1.
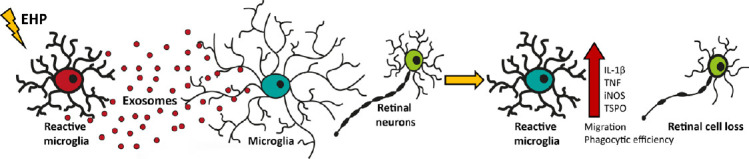
Schematic representation of the effects of exosomes derived from microglia exposed to elevated pressure on naïve microglia and neuronal cells.
Exosomes released by microglia exposed to elevated pressure (EHP) amplify the inflammatory response, enhancing microglia reactivity and leading to retinal degeneration. IL-1β: Interleukin-1β; iNOS: inducible nitric oxide synthase; TNF: tumor necrosis factor; TSPO: translocator protein.
Although we have demonstrated that exosomes derived from microglia interact with recipient microglial cells, suggesting an autocrine function, exosomes from reactive microglia have been also reported to induce oxidative stress and cell death in primary retinal neural cell cultures. Whether exosomes are interfering on microglial cells that then mediate neuronal cell death or act directly on retinal neurons is yet to be explored. It has been reported that microglia preferentially uptake exosomes from different cell populations (Bian et al., 2020). The subretinal administration of exosomes derived from neural progenitor cells hinders photoreceptor loss through the control of microglia reactivity in a model of retinal degeneration (Bian et al., 2020). In this study, the authors demonstrated that neural progenitor cells exosomes were selectively incorporated by retinal microglial cells (Bian et al., 2020). Therefore, it is conceivable that microglia may act as a hub for exosomes, reacting in accordance to the stimulus transmitted. Several reports have shown that exosomes from different cell types such as neurons, oligodendrocytes and astrocytes, and even circulating exosomes, specifically target microglia and change their phenotype that ultimately contribute to disease (Paolicelli et al., 2019). On the other hand, microglia per se also release exosomes that may target other microglia, further propagating the inflammatory signal. All these evidences confirm microglia as sensors for tissue homeostasis and point exosomes as new players in microglia communication.
Exosomes derived from microglial cells exposed to elevated hydrostatic pressure and injected into the vitreous of mice trigger a response in microglia, with increased expression of reactivity markers (major histocompatibility complex class II and translocator protein), and retinal cell death, including retinal ganglion cells. The exosomes from control microglia fail to induce retinal inflammation and cell death, further suggesting that exosome composition can be different. Exosomes from elevated pressure-induced reactive microglia can be foreseen as a new route of communication in pathological conditions, being able to propagate and amplify inflammation in the retina during deleterious conditions. It will be crucial to explore the differential cargo of these extracellular vesicles. It will certainly open new possibilities to address how intercellular communication occurs in retinal degenerative diseases with an inflammatory component, as it may also reveal new targets for treatment of retinal diseases. In the brain hindering exosome formation or depleting microglia has been reported to decrease tau propagation in a model of Alzheimer’s disease (Asai et al., 2015), reinforcing the hypothesis that the modulation of reactive microglia-derived exosomes may be on the genesis of neurodegenerative diseases. These findings pave the way for new approaches for retinal diseases where microglia-mediated neuroinflammation plays a role, opening the possibility that halting microglia communication through exosomes may hinder disease progression. Taking into consideration the fact that exosomes containing retinal proteins are detected in the human vitreous (Zhao et al., 2018) and in the near future there will be the possibility of isolating cell-specific exosomes from the vitreous, the identification of specific microglial exosomes within the humor vitreous may help in disease diagnostics. There is still much to explore regarding retinal microglial exosomes. Intraocular pressure fluctuates along the day, in glaucoma this occurs with peaks of elevated intraocular pressure. Would this be accompanied by microglial cell response with subsequent release of exosomes and worsening of the disease is still unknown. The better understanding of the mechanisms that induce exosome release by microglia and the shift in their content and effects on recipient cells could foresee new advances in the field and in the development of new therapeutic approaches. Since microglia response in the glaucomatous retina occurs in an orchestrated manner with sequential onset, approaches that modulate the release or the content of exosomes by microglial cells may be envisaged to confer protection to the retina.
Given the data obtained so far, it is conceivable that microglial exosomes also play an important role in the progression of other retinal pathologies. Microglial cells are central players in several diseases and as previously demonstrated the modulation of their function could hinder retinal degeneration. The work discussed here paves the way for new studies exploring the involvement of exosomes in retinal disease progression and the discovery of innovative therapeutic options.
In conclusion, exosomes are important entities involved in disease progression, but they have also potential to serve as biomarkers of disease and can be viewed as therapeutic vehicles. Increasing the knowledge about exosomes in retinal diseases may open new possibilities to halt degeneration. However, the field faces many challenges, such as the difficulty to isolate exosomes from ocular structures, to clearly identify their origin and also due to their changeable nature exosomes may present different cargos encoding for distinct downstream effects in accordance to the stage of the disease. In addition, the methodology currently available to ensure reliable and systematic isolation and purification from ocular tissues is not yet available. The perspective of using exosomes as therapeutic agents is really promising, since they cross the blood-retinal barrier and may be loaded and targeted to different cell populations. These mechanisms are yet to be explored in most retinal pathologies and may encompass a step forward in the treatment of ocular diseases.
This work was supported by the Foundation for Science and Technology (FCT), Portugal (Strategic Projects UID/NEU/04539/2013, UID/NEU/04539/2019, UIDB/04539/2020 and UIDP/01/2020); PhD fellowships (PD/BD/52294/2013, PD/BD/114115/2015 and PD/BD/127821/2016); COMPETE-FEDER (FCOMP-01-0124-FEDER-028417 and POCI-01-0145-FEDER-007440); Centro 2020 Regional Operational Programme (CENTRO-01-0145-FEDER-000008: BRAINHEALTH 2020); Banco Santander Totta (grant FMUC-BST-2016-224).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Wang L; T-Editor: Jia Y
References
- 1.Aires ID, Ambrósio AF, Santiago AR. Modeling human glaucoma: lessons from the in vitro models. Ophthalmic Res. 2017;57:77–86. doi: 10.1159/000448480. [DOI] [PubMed] [Google Scholar]
- 2.Aires ID, Boia R, Rodrigues-Neves AC, Madeira MH, Marques C, Ambrósio AF, Santiago AR. Blockade of microglial adenosine A2A receptor suppresses elevated pressure-induced inflammation, oxidative stress, and cell death in retinal cells. Glia. 2019;67:896–914. doi: 10.1002/glia.23579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aires ID, Ribeiro-Rodrigues T, Boia R, Catarino S, Girão H, Ambrósio AF, Santiago AR. Exosomes derived from microglia exposed to elevated pressure amplify the neuroinflammatory response in retinal cells. Glia. 2020;68:2705–2724. doi: 10.1002/glia.23880. [DOI] [PubMed] [Google Scholar]
- 4.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, Wolozin B, Butovsky O, Kügler S, Ikezu T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18:1584–1593. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian B, Zhao C, He X, Gong Y, Ren C, Ge L, Zeng Y, Li Q, Chen M, Weng C, He J, Fang Y, Xu H, Yin ZQ. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J Extracell Vesicles. 2020;9:1748931. doi: 10.1080/20013078.2020.1748931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, Santiago AR. Neuroprotective strategies for retinal ganglion cell degeneration: current status and challenges ahead. Int J Mol Sci. 2020;21:2262. doi: 10.3390/ijms21072262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madeira MH, Boia R, Santos PF, Ambrósio AF, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015a;2015:673090. doi: 10.1155/2015/673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madeira MH, Elvas F, Boia R, Gonçalves FQ, Cunha RA, Ambrósio AF, Santiago AR. Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure. J Neuroinflammation. 2015b;12:115. doi: 10.1186/s12974-015-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues-Neves AC, Aires ID, Vindeirinho J, Boia R, Madeira MH, Gonçalves FQ, Cunha RA, Santos PF, Ambrósio AF, Santiago AR. Elevated pressure changes the purinergic system of microglial cells. Front Pharmacol. 2018;9:16. doi: 10.3389/fphar.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu W, Wu Y, Hu Z, Sun L, Dou G, Zhang Z, Wang H, Guo C, Wang Y. Exosomes from microglia attenuate photoreceptor injury and neovascularization in an animal model of retinopathy of prematurity. Mol Ther Nucleic Acids. 2019;16:778–790. doi: 10.1016/j.omtn.2019.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Weber SR, Lease J, Russo M, Siedlecki CA, Xu LC, Chen H, Wang W, Ford M, Simó R, Sundstrom JM. Liquid biopsy of vitreous reveals an abundant vesicle population consistent with the size and morphology of exosomes. Transl Vis Sci Technol. 2018;7:6. doi: 10.1167/tvst.7.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


