Abstract
The contribution of chronic peripheral inflammation to the pathogenesis of neurodegenerative diseases is an outstanding question. Sustained activation of the peripheral innate and adaptive immune systems occurs in the context of a broad array of disorders ranging from chronic infectious diseases to autoimmune and metabolic diseases. In addition, progressive systemic inflammation is increasingly recognized during aging. Peripheral immune cells could potentially modulate the cellular brain environment via the secretion of soluble molecules. There is an ongoing debate whether peripheral immune cells have the potential to migrate into the brain under certain permissive circumstances. In this perspective, we discuss the possible contribution of chronic peripheral inflammation to the pathogenesis of age-related neurodegenerative diseases with a focus on microglia, the resident immune cells of the brain parenchyma.
Keywords: aging, Alzheimer's disease, inflammation, innate immune system, microglia, neurodegeneration, Parkinson's disease
Introduction
Microglia, the resident innate immune cells of the brain, are involved in the pathogenesis of neurodegenerative diseases like Alzheimer’s disease (AD) and Parkinson’s disease (PD), but the contribution of systemic inflammation to disease risk and progression of these diseases is ambiguous.
The innate immune system is shaped and modulated by the interplay between an individual’s genetic background and environmental factors. Genetic variants linked to AD and PD have been shown to influence gene regulation in monocytes, the peripheral blood mononuclear cells of the innate immune system (Raj et al., 2014). Conditions such as diabetes, hypertension and infectious disease potentially cause a sustained, low-level inflammatory activation state. Aging, the number one risk factor for neurodegenerative diseases, has been shown to alter the state of the innate immune system. These changes include alterations in the molecular signature of monocytes and a reduction in monocytes’ ability to phagocytose and respond properly to inflammatory stimuli (Hearps et al., 2012; Bliederhaeuser et al., 2016).
Search Strategy and Selection Criteria
We searched PubMed to find articles on peripheral inflammation and neurodegeneration that were published between 2000 and 2020.
Crosstalk between Chronic Peripheral Inflammation and Microglia
Little is known about how chronic peripheral inflammation influences the microglia cell compartment. At steady-state, microglia perform functions including immune surveillance and phagocytosis. Interestingly, microglia express numerous genes implicated in AD (Gosselin et al., 2017). It is thought that microglia may be involved in mediating the activation of astrocytes, neuronal dysfunction, aberrant removal of synapses, and eventually cell death via increased production of cytokines, chemokines and reactive oxygen species when responding to pathophysiological conditions (Figure 1; Salter and Stevens, 2017). The precise mechanisms that reprogram microglia into a dysregulated state are unclear. While studies aiming at deciphering the pathogenesis of AD and PD have been primarily conducted in a very brain centric lens, recent findings suggest an involvement of the peripheral immune system in neurodegenerative diseases (Figure 2).
Figure 1.
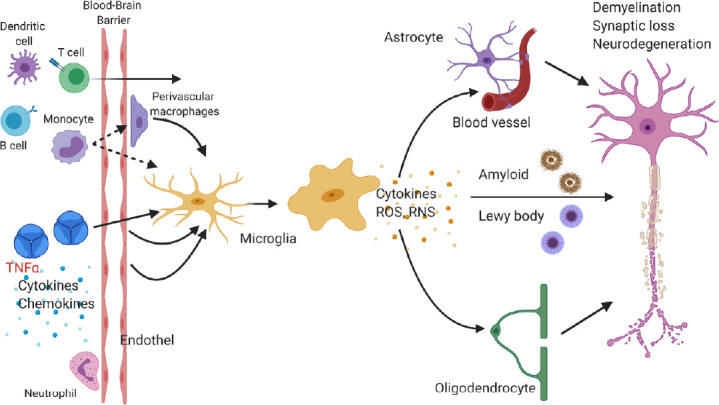
Microglia response to chronic peripheral inflammation may alter the state of neighboring cells.
Cells of the innate and adaptive immune system (e.g., monocytes, dendritic cells, T and B cells) may directly migrate into the central nervous system and inflammatory mediators like cytokines (e.g., tumor necrosis factor alpha (TNFα), interleukin-1β, and interleukin-6) may also indirectly activate endothelial cells or perivascular macrophages. Changes in the environment are sensed by microglia. Microglia may secrete cytokines or reactive oxygen species (ROS) or reactive nitrogen species (RNS), which affect nearby astrocytes, oligodendrocytes, endothelial cells of blood vessels, and neurons. These events can lead to impaired phagocytosis of protein aggregates like amyloid plaques and eventually to neuronal dysfunction, synaptic degeneration, and neuronal loss. Figure 1 was created using BioRender (https://biorender.com).
Figure 2.
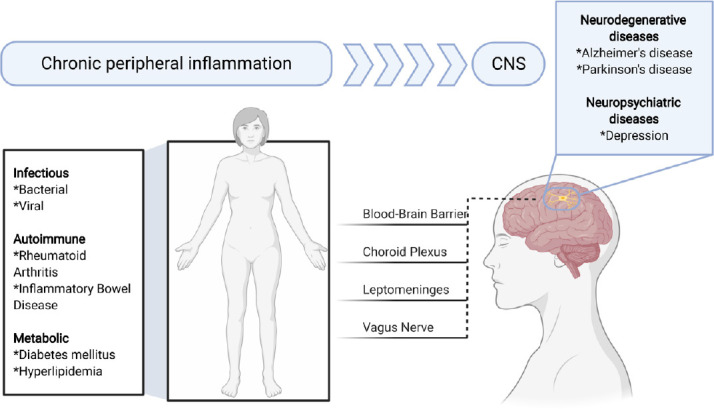
Chronic peripheral inflammation and its possible contribution to neurodegenerative and neuropsychiatric diseases.
Chronic peripheral inflammation may be caused by infectious, autoimmune, and metabolic diseases among many others. Inflammatory mediators may reach the central nervous system (CNS) via the blood-brain barrier, choroid plexus, leptomeninges or vagus nerve. This in turn may cause a response by microglia and thereby contributes to the pathogenesis of Alzheimer’s disease or Parkinson’s disease. Figure 2 was created using BioRender (https://biorender.com).
Microglia show an immediate yet transient change in gene expression and secreted factors after peripheral administration of the bacterial endotoxin lipopolysaccharide and polyinosinic:polycytidyclic (Poly I:C), a synthetic stimulator of viral infections. Lipopolysaccharide and Poly I:C both have been shown to exacerbate neuropathology in mouse models of neurodegenerative diseases like AD and PD (Kitazawa et al., 2005; Krstic et al., 2012). Severe sepsis in humans possibly increases the risk for profound and sustained cognitive impairment (Iwashyna et al., 2010). Likewise, acute infections such as urinary tract infections often lead to the worsening of motor symptoms in patients with PD (Zheng et al., 2012). Microglia maintain long-lasting epigenetic modifications and a conditioned responsiveness to immune stimuli in mice (Wendeln et al., 2018). The effect of acute inflammatory stimuli on long-lasting risk for neurodegenerative disorders has been indicated in humans. For example, individuals who survive an episode of sepsis during their midlife are at an increased risk of accelerated cognitive decline later in life (Walker et al., 2019). There are conflicting data, however, whether in these contexts peripheral immune cells are able to migrate and integrate into the brain parenchyma in neurodegenerative diseases and modulate disease progression or even onset. Under homeostatic conditions, it seems unlikely that peripheral immune cells enter the brain (Ajami et al., 2007; Mildner et al., 2007). Whether circulating monocytes can significantly infiltrate the brain and can be used as potential vehicles for treatment of neurodegenerative diseases is an exciting, but controversial line of research (Koronyo-Hamaoui et al., 2020; Reed-Geaghan et al., 2020). Recent findings suggest that T cells, although at low numbers, are present in the brain of patients with AD or PD and may participate in and modulate disease pathogenesis (Sommer et al., 2019; Gate et al., 2020). Other studies suggest that also accumulation of neutrophils and platelets occurs in the vicinity of plaques (Gowert et al., 2014; Zenaro et al., 2015).
Contribution of Chronic Peripheral Inflammation to Aging and Neurodegenerative Diseases
There are presently limited data studying the effect of chronic peripheral inflammation on the brain and its contribution to aging and neurodegenerative disease. Causes for persistent peripheral inflammation include conditions such as diabetes, hypertension, and hyperlipidemia. Other chronic inflammatory conditions are autoimmune disorders such as inflammatory bowel disease (IBD) and rheumatoid arthritis. For example, midlife diabetes and hypertension have been associated with a higher prevalence of AD (Barnes and Yaffe, 2011) and individuals with IBD show an increased risk to develop PD (Peter et al., 2018). Individuals with rheumatoid arthritis appear to be at an increased risk to develop AD, however, the risk seems to be reduced when patients are treated with a tumor necrosis factor α (TNFα) inhibitor (Chou et al., 2016).
These findings prompted us to ask whether chronic peripheral inflammation alters the immune compartment of the brain. If so, does chronic peripheral inflammation cause microglia heterogeneity and altered gene expression profiles? To address these questions, we used a rodent model (Tg197), which overexpresses human TNFα in the periphery. Human TNFα is not expressed in the brain and cannot enter the brain via the intact rodent blood-brain barrier. We observed that increased chronic peripheral immune activation resulted in an expansion of microglia cells within distinct anatomical brain regions (Süβ et al., 2020). We identified a microglia subpopulation showing a gene expression profile enriched for genes involved in cytokine production, complement, lysosomal function, adenosine triphosphate metabolic process, and response to interferon gamma. Specifically, genes involved in complement (C3 and C1pc) and cytokines/chemokines like Cxcl13, Cxcl16, Ccl2, and Ccl12 were upregulated, whereas homeostatic markers like Fcrls, P2ry12 and Cx3cr1 were decreased. Furthermore, gene expression of Tyrobp, Ctsb, Fth1, Axl, Apoe, Itgax, Nfkbia, Cd9, and Cd63 were increased. This inflammatory microglia population thus shows modulated phago-lysosomal activity and oxidative phosphorylation, resembling a recently identified microglia population in the context of aging and neurodegeneration (Keren-Shaul et al., 2017). However, to what extent these inflammatory phenotypes shown by microglia translate into human microglia in these disease contexts is unclear. First data derived from human postmortem brain tissue and chimeric microglia indicate that human microglia response to neurodegenerative diseases like AD differ substantially from murine microglia (Hasselmann et al., 2019; Srinivasan et al., 2020).
The microglial transcriptomic fingerprints associated with peripheral inflammation are present in distinct brain regions, primarily in the cortex, striatum, and thalamus. In contrast, we did not observe an inflammatory microglia phenotype within the cerebellum and hippocampus. This finding corroborates our earlier findings with this mouse model, which showed that chronic peripheral inflammation does not lead to depressive behavior and does not affect either inflammation or neurogenesis in the hippocampus (Süβ et al., 2015). The causes and mechanisms behind this differential brain region response to chronic peripheral inflammation are unclear. Perhaps different external cues and differences in ontology and homeostatic microglia phenotype may result in the observed altered reactivity. In addition, other factors such as the composition of the blood-brain barrier, vasculature, meninges or choroid plexus may direct signals selectively to certain brain regions (Figure 2). Furthermore, soluble molecules like cytokines such as interleukins (e.g., interleukin-17a) and interferons as well as peripherally derived molecules (e.g., enzymes like GPLD1 derived from the liver) have the potential to modulate region-specific immune cell activation in the brain (Alves de Lima et al., 2020; Horowitz et al., 2020).
To see whether patients with known chronic peripheral inflammation showed brain region specific alterations in the microglia compartment, we analyzed postmortem brain tissue samples from individuals with rheumatoid arthritis. To study microglia in the brain tissue, we stained the frontal cortex and the cerebellum for the microglial markers IBA-1 and P2RY12. Through these studies, we observed an altered microglia phenotype in the frontal cortex but not in the cerebellum, which indicates the presence of a differential microglial response within the human brain to chronic peripheral inflammation. Further work will need to be done in order to identify changes in gene and protein expression and to see whether this finding extends to other diseases with a component of chronic peripheral inflammation.
To examine whether microglial inflammation induced by chronic peripheral inflammation is reversible, we administered Infliximab, an antibody directed against human TNFα in the periphery of Tg197 mice. We observed a loss of the inflammatory microglia transcriptional signature, indicating that the microglia phenotype can be restored by inhibiting TNFα in the periphery. Interestingly, a positive association between serum levels of TNFα and accelerated cognitive decline has been reported in patients with AD (Holmes et al., 2009). This has led also to the exploration of TNFα as a possible target for AD. In mouse models showing increased deposition of amyloid plaques, reduction of TNFα levels led to improved cognition accompanied by a reduction in neuroinflammation and amyloid deposition (MacPherson et al., 2017; Paouri et al., 2017). In individuals with IBD, TNFα targeted treatment appears to lower the incidence of PD (Peter et al., 2018).
Prior studies have suggested that chronic intake of non-steroidal inflammatory drugs may be beneficial for the prevention of neurodegenerative diseases, but there is presently no conclusive data supporting this notion. It is plausible that an efficient treatment of neurodegenerative diseases may require a combinatorial treatment strategy aiming at multiple mechanisms, including the clearance of accumulated toxic substrates, inhibition of inflammation and oxidative stress, and improvement of neuronal homeostasis.
Additional file: Open peer review report 1 (84.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Aysegul Yildiz-Unal, Mugla Sitki Kocman University, Turkey; Cláudio Roque, Universidade da Beira Interior, Portugal.
P-Reviewers: Yildiz-Unal A, Roque C; C-Editors: Zhao M, Qiu Y; T-Editor: Jia Y
References
- 1.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 2.Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, Martelossi Cebinelli G, Mamuladze T, Baker W, Papadopoulos Z, Lopes MB, Cao WS, Xie XS, Herz J, Kipnis J. Meningeal gammadelta T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21:1421–1429. doi: 10.1038/s41590-020-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bliederhaeuser C, Grozdanov V, Speidel A, Zondler L, Ruf WP, Bayer H, Kiechle M, Feiler MS, Freischmidt A, Brenner D, Witting A, Hengerer B, Fandrich M, Ludolph AC, Weishaupt JH, Gillardon F, Danzer KM. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 2016;131:379–391. doi: 10.1007/s00401-015-1504-2. [DOI] [PubMed] [Google Scholar]
- 5.Chou RC, Kane M, Ghimire S, Gautam S, Gui J. Treatment for rheumatoid arthritis and risk of Alzheimer’s disease: a nested case-control analysis. CNS Drugs. 2016;30:1111–1120. doi: 10.1007/s40263-016-0374-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gate D, Saligrama N, Leventhal O, Yang AC, Unger MS, Middeldorp J, Chen K, Lehallier B, Channappa D, De Los Santos MB, McBride A, Pluvinage J, Elahi F, Tam GK, Kim Y, Greicius M, Wagner AD, Aigner L, Galasko DR, Davis MM, et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature. 2020;577:399–404. doi: 10.1038/s41586-019-1895-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356:eaal3222. doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gowert NS, Donner L, Chatterjee M, Eisele YS, Towhid ST, Munzer P, Walker B, Ogorek I, Borst O, Grandoch M, Schaller M, Fischer JW, Gawaz M, Weggen S, Lang F, Jucker M, Elvers M. Blood platelets in the progression of Alzheimer’s disease. PLoS One. 2014;9:e90523. doi: 10.1371/journal.pone.0090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasselmann J, Coburn MA, England W, Figueroa Velez DX, Kiani Shabestari S, Tu CH, McQuade A, Kolahdouzan M, Echeverria K, Claes C, Nakayama T, Azevedo R, Coufal NG, Han CZ, Cummings BJ, Davtyan H, Glass CK, Healy LM, Gandhi SP, Spitale RC, et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron. 2019;103:1016–1033. doi: 10.1016/j.neuron.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hearps AC, Martin GE, Angelovich TA, Cheng WJ, Maisa A, Landay AL, Jaworowski A, Crowe SM. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11:867–875. doi: 10.1111/j.1474-9726.2012.00851.x. [DOI] [PubMed] [Google Scholar]
- 11.Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz AM, Fan X, Bieri G, Smith LK, Sanchez-Diaz CI, Schroer AB, Gontier G, Casaletto KB, Kramer JH, Williams KE, Villeda SA. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science. 2020;369:167–173. doi: 10.1126/science.aaw2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 15.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koronyo-Hamaoui M, Sheyn J, Hayden EY, Li S, Fuchs DT, Regis GC, Lopes DHJ, Black KL, Bernstein KE, Teplow DB, Fuchs S, Koronyo Y, Rentsendorj A. Peripherally derived angiotensin converting enzyme-enhanced macrophages alleviate Alzheimer-related disease. Brain. 2020;143:336–358. doi: 10.1093/brain/awz364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, Riether C, Meyer U, Knuesel I. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacPherson KP, Sompol P, Kannarkat GT, Chang J, Sniffen L, Wildner ME, Norris CM, Tansey MG. Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol Dis. 2017;102:81–95. doi: 10.1016/j.nbd.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 20.Paouri E, Tzara O, Kartalou GI, Zenelak S, Georgopoulos S. Peripheral tumor necrosis factor-alpha (TNF-alpha) modulates amyloid pathology by regulating blood-derived immune cells and glial response in the brain of AD/TNF transgenic mice. J Neurosci. 2017;37:5155–5171. doi: 10.1523/JNEUROSCI.2484-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peter I, Dubinsky M, Bressman S, Park A, Lu C, Chen N, Wang A. Anti-tumor necrosis factor therapy and incidence of parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 2018;75:939–946. doi: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, Feng T, Lee M, Asinovski N, Frohlich I, Imboywa S, Von Korff A, Okada Y, Patsopoulos NA, Davis S, McCabe C, Paik HI, Srivastava GP, Raychaudhuri S, Hafler DA, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344:519–523. doi: 10.1126/science.1249547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed-Geaghan EG, Croxford AL, Becher B, Landreth GE. Plaque-associated myeloid cells derive from resident microglia in an Alzheimer’s disease model. J Exp Med. 2020;217:e20191374. doi: 10.1084/jem.20191374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 25.Sommer A, Marxreiter F, Krach F, Fadler T, Grosch J, Maroni M, Graef D, Eberhardt E, Riemenschneider MJ, Yeo GW, Kohl Z, Xiang W, Gage FH, Winkler J, Prots I, Winner B. Th17 lymphocytes induce neuronal cell death in a human iPSC-based model of Parkinson’s disease. Cell Stem Cell. 2019;24:1006. doi: 10.1016/j.stem.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan K, Friedman BA, Etxeberria A, Huntley MA, van der Brug MP, Foreman O, Paw JS, Modrusan Z, Beach TG, Serrano GE, Hansen DV. Alzheimer’s patient microglia exhibit enhanced aging and unique transcriptional activation. Cell Rep. 2020;31:107843. doi: 10.1016/j.celrep.2020.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Süβ P, Kalinichenko L, Baum W, Reichel M, Kornhuber J, Loskarn S, Ettle B, Distler JH, Schett G, Winkler J, Muller CP, Schlachetzki JC. Hippocampal structure and function are maintained despite severe innate peripheral inflammation. Brain Behav Immun. 2015;49:156–170. doi: 10.1016/j.bbi.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Süβ P, Hoffmann A, Rothe T, Ouyang Z, Baum W, Staszewski O, Schett G, Prinz M, Kroenke G, Glass CK, Winkler J, Schlachetzki J. Chronic peripheral inflammation causes a brain region-specific myeloid response. Cell Rep. 2020;30:4082–4095. doi: 10.1016/j.celrep.2020.02.109. [DOI] [PubMed] [Google Scholar]
- 29.Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH, Jr, Selvin E, Windham BG. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC study. Neurology. 2019;92:e1256–1267. doi: 10.1212/WNL.0000000000007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Häsler LM, Wild K, Skodras A, Blank T, Staszewski O, Datta M, Centeno TP, Capece V, Islam MR, Kerimoglu C, Staufenbiel M, Schultze JL, Beyer M, et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature. 2018;556:332–338. doi: 10.1038/s41586-018-0023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, Turano E, Rossi B, Angiari S, Dusi S, Montresor A, Carlucci T, Nani S, Tosadori G, Calciano L, Catalucci D, Berton G, Bonetti B, Constantin G. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 32.Zheng KS, Dorfman BJ, Christos PJ, Khadem NR, Henchcliffe C, Piboolnurak P, Nirenberg MJ. Clinical characteristics of exacerbations in Parkinson disease. Neurologist. 2012;18:120–124. doi: 10.1097/NRL.0b013e318251e6f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


