Keywords: immune cell subsets, immune function, inflammasomes, leukocyte infiltration, macrophages, microglia, pathways, spinal cord injury
Abstract
Inflammation is a major cause of neuronal injury after spinal cord injury. We hypothesized that inhibiting caspase-1 activation may reduce neuroinflammation after spinal cord injury, thus producing a protective effect in the injured spinal cord. A mouse model of T9 contusive spinal cord injury was established using an Infinite Horizon Impactor, and VX-765, a selective inhibitor of caspase-1, was administered for 7 successive days after spinal cord injury. The results showed that: (1) VX-765 inhibited spinal cord injury-induced caspase-1 activation and interleukin-1β and interleukin-18 secretion. (2) After spinal cord injury, an increase in M1 cells mainly came from local microglia rather than infiltrating macrophages. (3) Pro-inflammatory Th1Th17 cells were predominant in the Th subsets. VX-765 suppressed total macrophage infiltration, M1 macrophages/microglia, Th1 and Th1Th17 subset differentiation, and cytotoxic T cells activation; increased M2 microglia; and promoted Th2 and Treg differentiation. (4) VX-765 reduced the fibrotic area, promoted white matter myelination, alleviated motor neuron injury, and improved functional recovery. These findings suggest that VX-765 can reduce neuroinflammation and improve nerve function recovery after spinal cord injury by inhibiting caspase-1/interleukin-1β/interleukin-18. This may be a potential strategy for treating spinal cord injury. This study was approved by the Animal Care Ethics Committee of Bengbu Medical College (approval No. 2017-037) on February 23, 2017.
Chinese Library Classification No. R453; R392.3; R744
Introduction
Spinal cord injury (SCI) can be devastating for patients and lacks effective drug treatments (Singh et al., 2014; Rubiano et al., 2015). SCI includes two main pathological processes: primary injury and secondary injury. The former is the direct injury caused by mechanical force, and cannot be predicted or intervened with (Hayta and Elden, 2018). The latter includes local ischemia, edema, electrolyte disorder, lipid peroxidation, and inflammation (Popovich, 2014; Ahuja et al., 2017; Rouanet et al., 2017; Hayta and Elden, 2018; Liu et al., 2019a; Chai et al., 2021). Of these, inflammation caused by immune cells is one of the most damaging factors (Rouanet et al., 2017; Ahmed et al., 2018). The inflammatory reaction to SCI includes the activation of local immune cells, the infiltration of peripheral immune cells, and the secretion of proinflammatory cytokines, finally leading to spinal cord dysfunction (Sun et al., 2016). Early and effective anti-inflammatory treatment is therefore important to improve the local immune microenvironment, protect residual neurons, and promote functional recovery. Recently, many basic and clinical studies have been published investigating anti-inflammatory drugs for SCI, such as methylprednisolone (Fehlings et al., 2017), ibuprofen, minocycline (Hayta and Elden, 2018; Aceves et al., 2019), and interleukin (IL)-10 (Thompson et al., 2013; Kitamura et al., 2019). However, only methylprednisolone is currently approved for clinical application in SCI, and it has many unwanted side effects, including wound infection, hyperglycemia, and gastrointestinal bleeding (Karsy and Hawryluk, 2019; Liu et al., 2019b). Therefore, the search for more effective drugs with fewer side effects for the treatment of SCI remains a research hot spot in this field.
Inflammasomes are a kind of high-molecular-weight multi-protein complex, and are mainly composed of intracellular pattern recognition receptors (PRRs), adaptor proteins (namely, apoptosis-associated speck-like protein containing a card [ASC]), and pro-caspase-1 (Martinon et al., 2002; Latz et al., 2013; Jamilloux and Martinon, 2016; Sharma and Kanneganti, 2016; Adornetto et al., 2019; Christgen et al., 2020). When PRRs recognize damage-associated molecular patterns (DAMPs), inflammasomes assemble, which recruit ASC and cause caspase-1 self-cleavage and activation (Martinon et al., 2002; Latz et al., 2013; Jamilloux and Martinon, 2016; Sharma and Kanneganti, 2016; Christgen et al., 2020). Caspase-1, also known as IL-1-converting enzyme, is the key regulator of pro-IL-1β and pro-IL-18 to the active IL-1β and IL-18. This further induces the initial immune cells to differentiate into pro-inflammatory cells, secrete pro-inflammatory cytokines, and induce inflammatory reactions (Martinon et al., 2002; Latz et al., 2013; Jamilloux and Martinon, 2016; Sharma and Kanneganti, 2016; Christgen et al., 2020).
As a key component of the inflammasome, caspase-1 is considered an important target for inhibiting inflammasome activation. Several caspase-1 inhibitors (e.g., VX-740, IDN-6556, and VX-765) have been studied in inflammatory-related diseases (Maroso et al., 2011; Noe et al., 2013; Chen et al., 2018; Flores et al., 2020; Kawahara et al., 2020). Among them, VX-765 (belnacasan), a selective inhibitor of caspase-1 (Wannamaker et al., 2007; Yang et al., 2017), has been demonstrated to be effective in central nervous system diseases (e.g., epilepsy, Alzheimer’s disease, and multiple sclerosis) (Maroso et al., 2011; McKenzie et al., 2018; Flores et al., 2020). Notably, VX-765 has reached phase II clinical trials for the treatment of epilepsy (Marchesan et al., 2020). Using RNA sequencing, we recently found that VX 765 application after SCI can inhibit signaling pathways associated with inflammatory responses (Chen et al., 2020a). This suggests that the immune microenvironment of SCI can be improved by inhibiting caspase-1 activation. We therefore hypothesized that VX-765 might ameliorate neuroinflammation and improve functional recovery following SCI. Here, we studied the role of VX-765 on local immune cell subsets and investigated its neuroprotective effects following SCI.
Materials and Methods
Animals
Eight-week-old female C57BL/6 mice (specific-pathogen-free level, weight 18–20 g, n = 84) were purchased from Changzhou Cavens Laboratory Animal Ltd. (Changzhou, China; license No. SCXK (Su) 2016-0010). The complete experimental protocol is shown in a flow chart (Figure 1). All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines. The animal surgery protocol and postoperative care were approved by the Animal Care Ethics Committee of Bengbu Medical College (approval No. 2017-037) on February 23, 2017.
Figure 1.
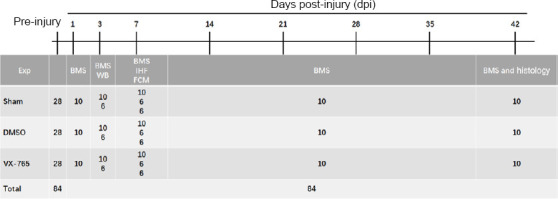
The complete experimental protocol schedule.
BMS: Basso Mouse Scale; DMSO: dimethyl sulfoxide; Exp: experiment; IHF: immunofluorescence; SCI: spinal cord injury; VX-765: caspase-1 selective inhibitor; WB: western blot.
Contusive SCI model establishment and drug administration
The animals were randomly divided into the SCI (n = 56) and sham (n = 28) groups. Establishment of the contusive SCI model was performed using an Infinite Horizon Impactor (Precision Systems & Instrumentation, Lexington, KY, USA) (Horiuchi et al., 2015; Wu et al., 2016). Briefly, all mice (n = 84) were first anesthetized by intraperitoneal injection with 80 mg/kg ketamine and 10 mg/kg xylazine (Sigma-Aldrich, St. Louis, MO, USA). The T7 and T11 spinous processes were then clamped, fixing the spine, and the T9 lamina was excised. A rod (1.3 mm in diameter) was used to produce a moderate contusive SCI model with a force of 50 kdynes. Mice in the sham group received a laminectomy only, without contusion. After the operation, mice were placed into a chamber at 20–22°C and 30–70% humidity. To prevent infections, the mice were administered 50 mg/kg chloramphenicol (Sangon Biotech, Shanghai, China) daily. Artificial bladder emptying was performed three times per day until spontaneous bladder emptying was re-established. The SCI mice were randomly divided into the dimethyl sulfoxide (DMSO; SCI + DMSO, n = 28) and VX-765 (SCI + VX-765, n = 28) groups. DMSO (100 μL) or VX-765 (MedKoo Biosciences, Inc., Morrisville, NC, USA, 100 mg/kg prepared in DMSO) was intraperitoneally injected immediately after injury and continued once daily for 7 days. The selection of this dose was based on a previous report (Wannamaker et al., 2007).
Western blot assay
At 3 days post-injury (dpi), mice (n = 6 per group) were euthanized with 80 mg/kg ketamine (Sigma-Aldrich) and 10 mg/kg xylazine (Sigma-Aldrich), and then perfused with 10 mL phosphate-buffered saline (PBS; 0.01 M, pH 7.4, 4°C). After perfusion, 5 mm segments of spinal cord containing the injury epicenter (or the same spinal cord segments for the sham group) were removed. For western blot assays, the total protein was extracted and analyzed as previously described (Lin et al., 2018). Briefly, the protein extract was obtained using radioimmunoprecipitation assay lysis buffer (Cat# P0013B; Biosharp, Guangzhou, China). Quantitative analysis of the protein concentration was determined using a Bicinchoninic Acid Protein Assay Kit (Cat# P0012; Beyotime, Shanghai, China). For western blot assays, protein supernatants were diluted in sodium dodecyl sulphate-polyacrylamide gel electrophoresis sample loading buffer (Cat# P0015; Beyotime) and boiled for 5 minutes. Next, 40 mg protein was added to each well and electrophoresed in 10% sodium dodecyl sulphate-polyacrylamide gels before being transferred to polyvinylidene difluoride membranes (Cat# SEQ15150; Millipore, Bedford, MA, USA). To block the membranes, 5% (w/v) non-fat dry milk (Cat# P0016; Beyotime) was used at room temperature for 1 hour. The membranes were then incubated with primary antibodies at 4°C overnight. The primary antibodies were as follows: rabbit polyclonal anti-mouse ASC (1:1000; Cat# abx013852; Abbexa, Cambridge, UK), rabbit monoclonal anti-mouse caspase-1 (1:1000; Cat# ab179515; Abcam, Cambridge, MA, USA), rabbit polyclonal anti-mouse β-actin (1:2000; Cat# BL005B; Biosharp), rabbit polyclonal anti-mouse IL-18 (1:2000; Cat# PA5-79481; Invitrogen, Carlsbad, CA, USA), rabbit polyclonal anti-mouse IL-1β (1:2000; Cat# ab9722; Abcam). Next, the membranes were incubated for 1 hour at room temperature with goat anti-rabbit IgG/horseradish peroxidase secondary antibody (1:10,000; Cat# BL003A; Biosharp). Finally, the immunoreactive target proteins were detected using an enhanced chemiluminescence kit (Cat# 35055; Pierce™, Thermo Fisher Scientific, Waltham, MA, USA) and observed using a Gel-Pro Analyzer (Media Cybernetics, Silver Spring, MD, USA). The optical density values of specific bands were analyzed.
Immunofluorescence double-staining
At 7 dpi, mice (n = 6 per group) were euthanized with 80 mg/kg ketamine and 10 mg/kg xylazine and perfused with PBS, as described in the western blot protocol. The mice were then perfused with 20 mL paraformaldehyde (4%, prepared in PBS) at 4°C. After perfusion, 5 mm segments of spinal cord containing the injury epicenter (or the same spinal cord segments for the sham group) were removed. The spinal cords were then postfixed overnight in 4% paraformaldehyde before being transferred to 30% sucrose (in PBS) at 4°C overnight. Next, the spinal cords were embedded in optimal cutting temperature medium (Tissue-Tek, Sakura Finetek USA Inc., Torrance, CA, USA) and cut into 6 μm transverse sections using a cryostat (CM1900; Leica Microsystems, Bannockburn, IL, USA). For the immunofluorescence assay, slides were incubated with primary antibodies overnight at 4°C. The following day, after being rinsed three times with PBS, the sections were incubated with secondary antibodies at 37°C for 1 hour. Additional Table 1 shows the details of the primary and secondary antibodies. Finally, the slides were washed three times, coverslipped with medium containing blue nuclear dye (Hoechst 33342; Cat# B2261; Sigma-Aldrich), and examined under a ZEISS Axio observation microscope (Carl Zeiss AG; Jena, Germany). For cell quantification, six mice were used per group. For each animal, cells were counted from five complete cross-sections containing the injury epicenter (0 mm), from rostral (1 and 0.5 mm) to caudal (–1 and –0.5 mm), as previously described (Wu et al., 2018).
Additional Table 1.
Information of antibodies used in immunohistofluorescence
| Antigen | Host species and clone | Cat# or Lot# | RRID | Source | Used concentration |
|---|---|---|---|---|---|
| CD11b | Rat monoclonal | 14-0112-82 | AB_467108 | Invitrogen | 1:200 |
| CD45 | 14-0451-82 | AB_467251 | |||
| CD68 | MA5-16674 | AB_2538168 | |||
| FOXP3 | 14-4776-82 | AB_467554 | |||
| CD4 | 14-9766-82 | AB_2573008 | |||
| CD4 | Rabbit polyclonal | PA5-87425 | AB_2804136 | ||
| GATA3 | PA5-20892 | AB_11154392 | |||
| T-bet | PA5-40573 | AB_2576589 | |||
| RORγ (t) | PA5-23148 | AB_2540675 | |||
| Arg1 | PA5-29645 | AB_2547120 | |||
| CCR7 | ab191575 | Abcam | |||
| Rat IgG (H+L) | Fluorescein-conjugated goat polyclonal | 112-095-143 | AB_2338199 | Jackson ImmunoResearch | |
| Rabbit | Rhodamine-conjugated goat polyclonal | 111-025-144 | AB_2337932 | ||
| IgG (H+L) |
Arg1: Arginase-1; CCR7: chemokine (C-C motif) receptor 7; FOXP3: forkhead box P3; GATA3: GATA binding protein 3; RORγ (t): retinoid-related orphan nuclear receptor γ-t; T-bet: Th1-specific T box transcription factor.
Flow cytometry
At 7 dpi, mice (n = 6 per group) were euthanized with 80 mg/kg ketamine and 10 mg/kg xylazine and perfused with PBS. After perfusion, 5 mm segments of spinal cord containing the injury epicenter (or the same spinal cord segments for the sham group) were obtained. The spinal cords were then dissociated into a single-cell suspension. Percoll gradient centrifugation was used to isolate the mononuclear cells as previously described (Chen et al., 2020b). The immune cell subtypes were stained with antibodies from Invitrogen. Additional Table 2 shows the details of all antibodies. After incubation with the antibodies for 30 minutes at 4°C, the cells were washed with PBS, fixed with 1% paraformaldehyde, and detected using a BD Accuri flow cytometer (BD Bioscience, San Jose, CA, USA). Non-specific staining was controlled using isotype-matched antibodies. The data were analyzed using FlowJo 7.6.1 software (FlowJo, LLC, Ashland, OR, USA).
Additional Table 2.
Information of antibodies used in flow cytometry
| Antigen | Host species and clone | Cat#or Lot# | RRID | Conjugation | Source | Used concentration |
|---|---|---|---|---|---|---|
| CCR7 | Rat monoclonal | 47-1971-82 | AB_2573974 | AF780 | Invitrogen | 0.25μg/test |
| IgG2bκ isotype control | Rat | 47-4321-82 | AB_1271997 | 0.25μg/test | ||
| CD11b | Rat monoclonal | 12-0112-81 | AB_465546 | PE | 0.125 μg/test | |
| CD3 | Rat monoclonal | 12-0032-82 | AB_2811741 | 0.25μg/test | ||
| IgG2bκ isotype control | Rat | 12-4031-82 | AB_470042 | 0.25μg/test | ||
| CD127 | Rat monoclonal | 48-1273-82 | AB_2574039 | eFlour 450 | 0.5 μg/test | |
| IgG2b κ isotype control | Rat | 48-4031-82 | AB_1272017 | 0.5 μg/test | ||
| CD183 | Armenian hamster monoclonal | 62-1831-82 | AB_2762747 | Super Bright 436 | 0.5 μg/test | |
| IgG isotype control | Armenian hamster | 62-4888-82 | AB_2717007 | 0.5 μg/test | ||
| CD196 | Rat monoclonal | 50-7196-82 | AB_11219682 | eFlour 660 | 0.5 μg/test | |
| IgG2a κ isotype control | Rat | 50-4321-82 | AB_10598503 | 0.5 μg/test | ||
| CD25 | Rat monoclonal | 47-0251-82 | AB_1272179 | AF780 | 0.5 μg/test | |
| IgG1 κ Isotype Control | Rat | 47-4301-80 | AB_1271986 | 0.5 μg/test | ||
| CD28 | Syrian hamster Monoclonal | 45-0281-80 | AB_925744 | PerCP-Cyanine5.5 | 0.5 μg/test | |
| IgG isotype control | Armenian hamster | 45-4888-80 | AB_906260 | 0.5 μg/test | ||
| CD3 | Armenian hamster monoclonal | 47-0031-82 | AB_11149861 | AF780 | 0.5 μg/test | |
| IgG Isotype Control | Armenian hamster | 47-4888-80 | AB_1271978 | 0.5 μg/test | ||
| CD4 | Rat monoclonal | 11-0041-82 | AB_464892 | FITC | 0.25μg/test | |
| CD68 | Rat monoclonal | MA5-16676 | AB_2538170 | 0.25μg/test | ||
| IgG2bκ isotype control | Rat | 11-4031-82 | AB_470004 | 0.25μg/test | ||
| CD45 | Rat monoclonal | 17-0451-82 | AB_469392 | APC | 0.125 μg/test | |
| IgG2b κ Isotype Control | Rat | 17-4031-82 | AB_470176 | 0.125 μg/test |
AF780: APC-eFluor 780; APC: allophycocyanin; CCR7: chemokine (C-C motif) receptor 7; FITC: fluoresceine isothiocyanate; PE: phycoerythrin.
Histological analyses
At 42 dpi, the remaining animals were euthanized with 80 mg/kg ketamine and 10 mg/kg xylazine and the spinal cords were harvested. Next, 5 μm transverse sections (n = 6 per group) were cut as described in the immunofluorescence protocol. The fibrotic area, residual ventral horn motoneurons, and myelinated white matter were then identified using hematoxylin-eosin (Cat# C0105M; Beyotime), Nissl staining (Cat# C0117; Beyotime), and Luxol fast blue (Cat# L0294; Sigma-Aldrich), respectively, as previously described (Chen et al., 2020b). Images were taken using a ZEISS Axio observation microscope. The fibrotic area measurements and cell quantifications were performed in an unbiased stereological manner using ImageJ software (National Institutes of Health, Bethesda, MD, USA) (Karimi-Abdolrezaee et al., 2006). In the hematoxylin-eosin-stained sections, the fibrotic area was notably darker than the other areas. Fibrosis was quantified by the ratio of the fibrotic area to the intact spinal cord area. For Nissl staining, the existence of Nissl substance and euchromatic nuclei were used to identify surviving ventral horn neurons (Teng et al., 1998). The surviving ventral horn neurons were quantified by counting all such cells from the ventral horn. For Luxol fast blue staining, Image-Pro Plus 5.1 (Media Cybernetics, Inc., Rockville, MD, USA) was used to quantify the myelinated white matter (Chen et al., 2020b). The myelinated white matter was quantified by the ratio of the Luxol fast blue-positive area to the intact spinal cord area. For the three histological analyses, nine complete cross-sections containing the injury epicenter (0 mm), from rostral (1.5, 1, 0.5, and 0.25 mm) to caudal (–1.5, –1, –0.5, and –0.25 mm), were analyzed per animal as previously described (Chen et al., 2020b).
Basso Mouse Scale
The Basso Mouse Scale, a 10-point scale (0–9) system (Basso et al., 2006), was used to assess locomotion after SCI. The scores were evaluated at 1, 3, 7, 14, 21, 28, 35, and 42 dpi. The evaluation was performed by two blinded scorers, while the mice (n = 10 per group) walked freely on an open-field surface for 4 minutes. A score of 9 indicates normal motor function, while 0 indicates complete paralysis. Thus, a higher score indicates better motor function.
Statistical analysis
The repeated measures two-way analysis of variance followed by Bonferroni’s post hoc analysis was used for all histological and behavioral data. The non-parametric Kruskal-Wallis analysis of variance followed by individual Mann-Whitney U tests was used to analyze all other data. P < 0.05 was considered statistically significant. The data were analyzed using SPSS software v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
VX-765 inhibits SCI-induced expression and activation of caspase-1 and its related molecules
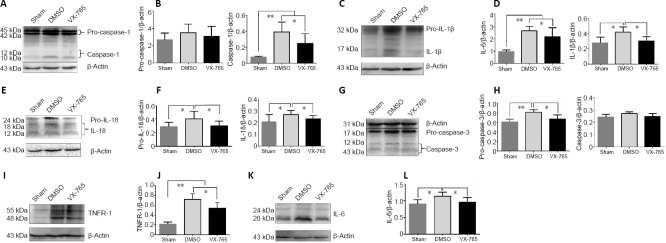
The effects of VX-765 on the SCI-induced expression and activation of caspase-1 and its related molecules were analyzed by western blot. As shown in Figure 2A and B, the 45 and 42 kDa pro-caspase-1 levels were not significantly different among all groups. However, compared with the sham group, the activated 12 and 10 kDa caspase-1 levels were significantly higher in both the DMSO and VX-765 groups (both P < 0.01, n = 6), and the 12 and 10 kDa caspase-1 levels were significantly lower in the VX-765 group than in the DMSO group (P < 0.05, n = 6). The 32 kDa pro-IL-1β and 17 kDa IL-1β levels were significantly higher in the DMSO group compared with the other two groups (P < 0.05 or 0.01, n = 6); in contrast, IL-1β levels were not significantly different between the sham and VX-765 groups (P > 0.05, n = 6; Figure 2C and D). Compared with the other two groups, the pro-IL-18 (24 kDa) and IL-18 (18 kDa and 12 kDa) levels were significantly higher in the DMSO group (P < 0.05, n = 6), while there were no differences between the sham and VX-765 groups (P > 0.05, n = 6; Figure 2E and F). Figure 2G and H show that the levels of 17 and 12 kDa caspase-3, which are related to apoptosis (Porter and Jänicke, 1999), were not significantly different among the groups (P > 0.05, n = 6). However, the 31 kDa pro-caspase-3 was significantly higher in the DMSO group compared with the other two groups (P < 0.1 or 0.05, n = 6), while there was no difference between the sham and VX-765 groups (P > 0.05, n = 6). Compared with the sham group, the 55 and 48 kDa tumor necrosis factor receptor type 1 levels were significantly higher in the DMSO and VX-765 groups (both P < 0.01, n = 6), and they were significantly lower in the VX-765 group compared with the DMSO group (P < 0.05, n = 6; Figure 2I and J). There was no significant difference between the VX-765 and sham groups in 20 kDa IL-6 levels (P > 0.05, n = 6), but levels were significantly higher in the DMSO group than in the other two groups (both P < 0.05, n = 6; Figure 2K and L).
Figure 2.
VX-765 inhibits SCI-induced expression and activities of caspase-1 and related molecules at 3 days post-injury.
(A, C, E, G, I, K) The bands of pro-caspase-1 (45/42 kDa) and cleaved-caspase-1 (10 and 12 kDa) (A), pro-IL-1β (32 kDa) and IL-1β (17 kDa) (C), pro-IL-18 (24 kDa) and IL-18 (18 and 12 kDa) (E), pro-caspase-3 (31 kDa) and caspase-3 (17 and 12 kDa; related to apoptosis) (G), TNFR-1 (55 and 48 kDa) (I), IL-6 (24 and 20 kDa) (K), and β-actin (43 kDa). (B, D, F, H, J, L) Quantitative results of the expression of caspase-1 and related molecules. The original data for Figure 2B, D, F, H, J, and L are shown in Additional file 2 (118.2KB, pdf) . Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). IL: Interleukin; SCI: spinal cord injury; TNFR-1: tumor necrosis factor receptor type 1; VX-765: caspase-1 selective inhibitor.
VX-765 inhibits SCI-induced differentiation of macrophages and microglia into M1 cells and increases the differentiation of microglia into M2 cells
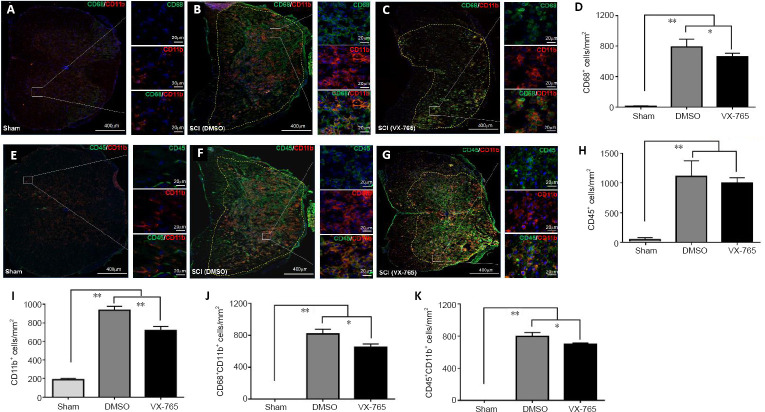
CD68, CD45, and CD11b were used to investigate microglia and infiltrated macrophages using immunofluorescence. CD45+ cells are peripheral leukocytes (Thomas, 1989; Hermiston et al., 2003), CD68+ cells are activated macrophages and microglia (Greaves and Gordon, 2002; Chen et al., 2015), and CD11b+ cells are macrophages and microglia (Martin et al., 2017). Therefore, CD45+CD11b+ cells are peripheral macrophages, while CD68+CD11b+ cells are activated macrophages and microglia. Our immunofluorescence results revealed that, in the sham-operated spinal cord, CD68+ cells (Figure 3A) were very rare. After SCI, there were significantly more CD68+ cells compared with the sham group (P < 0.01, n = 6; Figure 3B–D). The number of CD68+ cells in the VX-765 group (Figure 3C) was significantly lower compared with the DMSO (Figure 3B) group (P < 0.05, n = 6; Figure 3D). In the sham-operated spinal cord, CD45+ cells (Figure 3E) were also very rare. After SCI, there were significantly more CD45+ cells compared with the sham group (P < 0.01, n = 6; Figure 3F, G, and H). The numbers of CD45+ peripheral leukocytes were not significantly different between the DMSO (Figure 3F) and VX-765 (Figure 3G) groups (P > 0.05, n = 6; Figure 3H). In the sham-operated spinal cord, typical resting microglia, characterized by many tiny processes, were observed (Figure 3A and E). After SCI, these cells were activated, with a round or oval morphology (Figure 3B, C, F, and G). The numbers of CD11b+ cells were also significantly higher in the DMSO and VX-765 groups compared with the sham group (P < 0.01, n = 6; Figure 3I). Furthermore, the numbers of CD11b+ cells were significantly lower in the VX-765 group (Figure 3C and G) compared with the DMSO (Figure 3B and F) group (P < 0.05, n = 6; Figure 3I). Although CD68+CD11b+ activated macrophages and microglia (Figure 3A) and CD45+CD11b+ peripheral macrophages (Figure 3E) were not detected in the sham group, both cell types increased significantly in the DMSO and VX-765 groups (both P < 0.01, n = 6; Figure 3B, F, J, and K). Compared with the DMSO group (Figure 3B and F), the numbers of CD68+CD11b+ and CD45+CD11b+ cells were significantly lower in the VX-765 (Figure 3C and G) group (both P < 0.05, n = 6; Figure 3J and K).
Figure 3.
Effects of VX-765 on microglia and infiltrated macrophages in the injured spinal cord at 7 days post-injury: immunofluorescence detection.
(A–C) CD11b (red, stained by rhodamine) for macrophages and/or microglia, CD68 (green, stained by FITC) for activated macrophages and/or microglia. Nuclei are blue, stained by Hoechst 33342. (D) Quantitative results of CD68+ activated macrophages and/or microglia. (E–G) CD11b (red, stained by rhodamine) for macrophages and/or microglia, CD45 (green, stained by FITC) for peripheral leukocytes. Nuclei are blue, stained by Hoechst 33342. (H–K) Quantitative results of CD45+ peripheral leukocytes (H), CD11b+ macrophages and/or microglia (I), CD68+CD11b+ activated macrophages and/or microglia (J), and CD45+CD11b+ macrophages from peripheral blood (K). The original data for Figure 3D, H–K are shown in Additional file 3. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). DMSO: Dimethyl sulfoxide; FITC: fluoresceine isothiocyanate; SCI: spinal cord injury; VX-765: caspase-1 selective inhibitor.
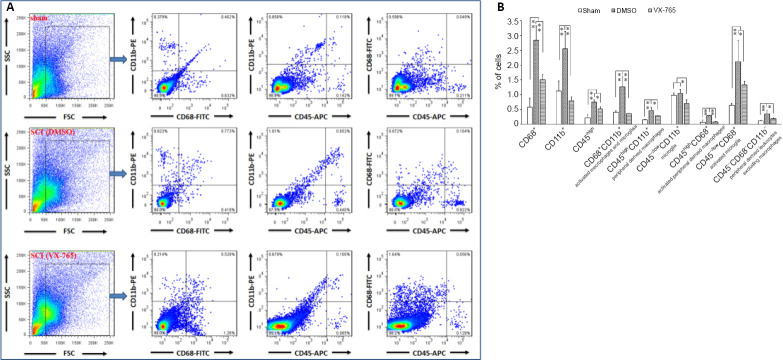
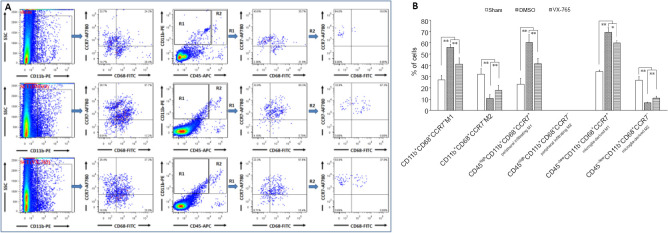
Microglia and infiltrated macrophages were further detected by flow cytometry using CD45, CD11b, and CD68 antibodies (Figure 4). In this experiment, CD45high cells were defined as peripheral infiltrated leukocytes, because activated microglia might have low CD45 expression (Sedgwick et al., 1998; Fu et al., 2009). Therefore, CD68+CD11b+, CD45high CD11b+, CD45–/low CD11b+, CD45high CD68+, CD45–/low CD68+, and CD45high CD68– CD11b– cells were defined as activated macrophages and/or microglia, peripheral-derived macrophages, microglia, activated peripheral-derived macrophages, activated microglia, and peripheral-derived leukocytes excluding macrophages, respectively (Figure 4A). The statistical results (Figure 4B) revealed that, compared with the sham group, the levels of cell subsets (except CD45–/low CD11b+ microglia) in the DMSO group were significantly higher (P < 0.01, n = 6). In the VX-765 group, the levels of all cell subsets were significantly lower than in the DMSO group (P < 0.01 or 0.05, n = 6).
Figure 4.
Effects of VX-765 on microglia and infiltrated macrophages in the injured spinal cord at 7 days post-injury: flow cytometry assay.
CD68+, CD11b+, CD45high, CD68+CD11b+, CD45high CD11b+, CD45–/low CD11b+, CD45high CD68+, CD45–/low CD68+, and CD45high CD68– CD11b– cells were defined as activated cells, macrophages/microglia, peripheral infiltrated leukocytes, activated macrophages and/or microglia, peripheral derived macrophages, microglia, activated peripheral derived macrophages, activated microglia, and peripheral-derived leukocytes excluding macrophages, respectively. (A) Flow cytometry images of cells derived from spinal cord homogenate. (B) Proportional analysis of the indicated cells in the three groups. The original data for Figure 4B are shown in Additional file 4. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). DMSO: Dimethyl sulfoxide; SCI: spinal cord injury; VX-765: caspase-1 selective inhibitor.
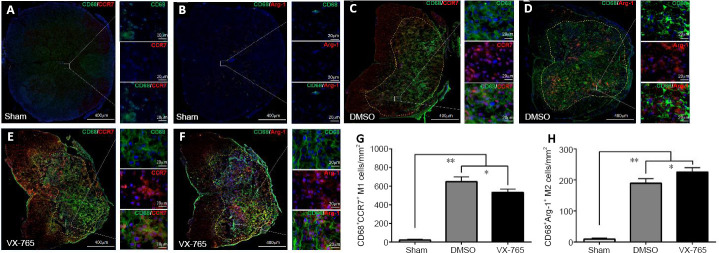
To determine the effects of VX-765 on M1 and M2, which are markers of activated microglia and/or macrophages (CD68), specific markers for M1 (C-C chemokine receptor type 7; CCR7) and M2 (arginase-1; ARG1) (Chen et al., 2015) were detected by immunofluorescence. Figure 5 shows that both CD68+CCR7+M1 cells (Figure 5A) and CD68+Arg1+M2 cells (Figure 5B) were very rare in the sham group. In contrast, after SCI (Figure 5C–F), both CD68+CCR7+M1 cells (Figure 5C and E) and CD68+Arg1+M2 cells (Figure 5D and F) were significantly increased (both P < 0.01, n = 6; Figure 5G and H). The CD68+CCR7+M1 cell numbers were significantly lower (P < 0.01, n = 6; Figure 5C, E, and G), while the CD68+Arg1+M2 cell numbers were significantly higher, in the VX-765 group compared with the DMSO group (P < 0.01, n = 6; Figure 5D, F, and H).
Figure 5.
Effects of VX-765 on M1 and M2 cells in the injured spinal cord at 7 days post-injury: immunofluorescence detection.
(A–F) CD68 (green, stained by FITC) and CCR7 (red, stained by rhodamine) (A, C, and E) for M1 cells, or Arg1 (B, D, and F) for M2 cells, in the different groups. Nuclei are blue, stained by Hoechst 33342. Scale bars: 400 µm, 20 µm (enlarged parts). (G, H) CD68+CCR7+M1 (G) and CD68+Arg1+M2 (H) cell counts in the sham, DMSO, and VX-765 groups. The original data for Figure 5G and H are shown in Additional file 5. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). Arg1: Arginase-1; DMSO: dimethyl sulfoxide; FITC: fluoresceine isothiocyanate; SCI: spinal cord injury; VX-765: caspase-1 selective inhibitor.
Flow cytometry was also used to analyze M1 and M2 in the injured spinal cords. Here, CD11b+CD68+CCR7+ and CD11b+CD68+CCR7– cells were defined as M1 and M2 cells, respectively. In addition, CD45–/low CD11b+CD68+CCR7+ and CD45–/low CD11b+CD68+CCR7– cells were defined as microglia-derived M1 and M2 cells, respectively. Moreover, CD45high CD11b+CD68+CCR7+ and CD45high CD11b+CD68+CCR7– cells were defined as peripheral infiltrating M1 and M2 cells, respectively (Thomas, 1989; Chen et al., 2015; Martin et al., 2017) (Figure 6A). Figure 6B shows that the proportions of total M1, peripheral infiltrating M1, and microglia-derived M1 cells were significantly higher after SCI compared with the sham group (all P < 0.01, n = 6). After VX-765 treatment, their proportions were significantly lower compared with the DMSO group (P < 0.01 or 0.05, n = 6). In contrast, the total proportions of M2 and microglia-derived M2 cells were significantly lower after SCI compared with the sham group (both P < 0.01, n = 6). After VX-765 treatment, their proportions were significantly higher compared with the DMSO group (both P < 0.01, n = 6).
Figure 6.
Effects of VX-765 on M1 and M2 in the injured spinal cord at 7 days post-injury: flow cytometry assay.
CD11b+CD68+CCR7+ and CD11b+CD68+CCR7– cells were defined as M1 and M2, respectively. CD45–/low CD11b+CD68+CCR7+ and CD45–/low CD11b+CD68+CCR7– cells were defined as microglia-derived M1 and M2, respectively. CD45high CD11b+CD68+CCR7+ and CD45high CD11b+CD68+CCR7– cells were defined as peripheral infiltrating M1 and M2, respectively. (A) Flow cytometry images of cells derived from spinal cord homogenate. (B) Proportional analysis of the indicated cells in the sham, DMSO, and VX-765 groups. The original data for Figure 6B are shown in Additional file 6. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). DMSO: Dimethyl sulfoxide; SCI: spinal cord injury; VX-765: caspase-1 selective inhibitor.
VX-765 inhibits SCI-induced differentiation of T helper (Th)1Th17 cells and promotes Th2 cell differentiation
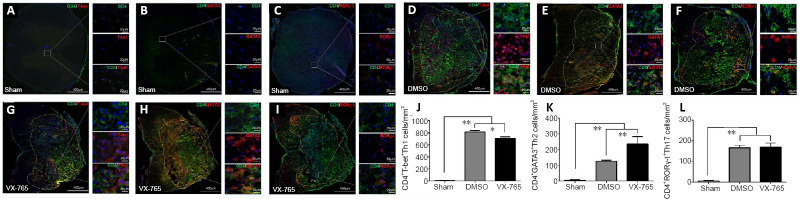
To determine the effects of VX-765 on Th1, Th2, and Th17 cell subsets, immunofluorescence was used to detect a general marker (CD4) of Th cells, as well as Th1-specific T box transcription factor, GATA-binding protein 3, and retinoid-related orphan nuclear receptor γ-t as markers of Th1, Th2, and Th17 cells (Zhang et al., 2014; Hu et al., 2016b), respectively. As shown in Figure 7, all of the Th subsets were very rare in sham-operated spinal cords (Figure 7A–C). After injury, they were significantly higher compared with the sham group (all P < 0.01, n = 6; Figure 7D–L). After VX-765 treatment, Th1 cell numbers were significantly lower (P < 0.05, n = 6; Figure 7G and J) and Th2 cell numbers were significantly higher (P < 0.01, n = 6; Figure 7H and K) compared with the DMSO group (Figure 7D and E). There were no differences in Th17 cell numbers between these two SCI groups (P > 0.05, n = 6; Figure 7F, I, and L).
Figure 7.
Effects of VX-765 on Th1, Th2, and Th17 in the injured spinal cord at 7 days post-injury: immunofluorescence detection.
(A–I) CD4 (green, stained by FITC) and T-bet (red, stained by rhodamine) (A, D, and G) for Th1 cells, or GATA3 (red, stained by rhodamine) (B, E, and H) for Th2 cells, or RORγ-t (red, stained by rhodamine) (C, F, and I) for Th17 cells, in the different groups. Nuclei are blue, stained by Hoechst 33342. Scale bars: 400 µm, 20 µm (enlarged parts). (J–L) CD4+ T-bet+ Th1 (J), CD4+GATA3+ Th2 (K), and CD4+RORγ-t+ Th17 (L) cell counts in the sham, DMSO, and VX-765 groups. The original data for Figure J–L are shown in Additional file 7. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal–Wallis analysis of variance). DMSO: Dimethyl sulfoxide; FITC: fluoresceine isothiocyanate; GATA3: GATA-binding protein 3; SCI: spinal cord injury; RORγ-t: retinoid-related orphan nuclear receptor γ-t; T-bet: Th1-specific T box transcription factor; Th: T helper; VX-765: caspase-1 selective inhibitor.
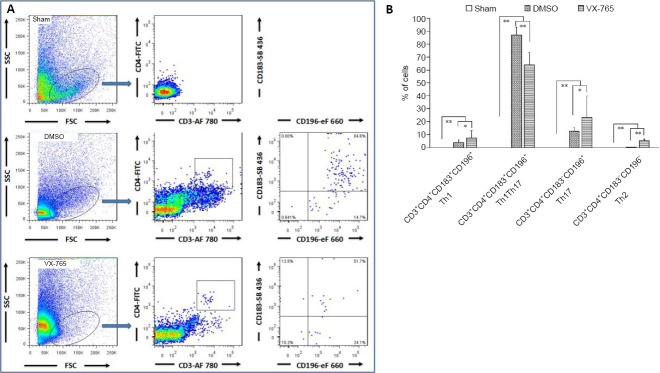
When the Th cell subsets were analyzed using flow cytometry, CD3+CD4+CD183+CD196– cells, CD3+CD4+CD183+CD196+ cells, CD3+CD4+CD183– CD196+ cells, and CD3+CD4+CD183– CD196– cells were defined as Th1, Th1Th17, Th17, and Th2 cells, respectively (Chen et al., 2020b) (Figure 8A). All Th subsets were significantly higher after SCI compared with the sham group (all P < 0.01, n = 6; Figure 8B). However, the Th1Th17 subset was predominant, and its proportion was significantly lower in the VX-765 group compared with the DMSO group (P < 0.01, n = 6). In contrast, compared with the DMSO group, the other Th subsets were significantly higher in the VX-765 group (P < 0.01 or 0.05, n = 6).
Figure 8.
Effects of VX-765 on Th1, Th2, and Th17 in the injured spinal cord at 7 days post-injury (flow cytometry assay).
CD3+CD4+CD183+CD196– cells, CD3+CD4+CD183+CD196+ cells, CD3+CD4+CD183– CD196+ cells, and CD3+CD4+CD183– CD196– cells were defined as Th1, Th1Th17, Th17, and Th2, respectively. (A) Flow cytometry images of cells derived from spinal cord homogenate. (B) Proportional analysis of the indicated cells in the sham, DMSO, and VX-765 groups. The original data for Figure 8B are shown in Additional file 8. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). DMSO: Dimethyl sulfoxide; SCI: spinal cord injury; Th: T helper; VX-765: caspase-1 selective inhibitor.
VX-765 increases the proportion of regulatory T cells (Tregs) in the injured spinal cord
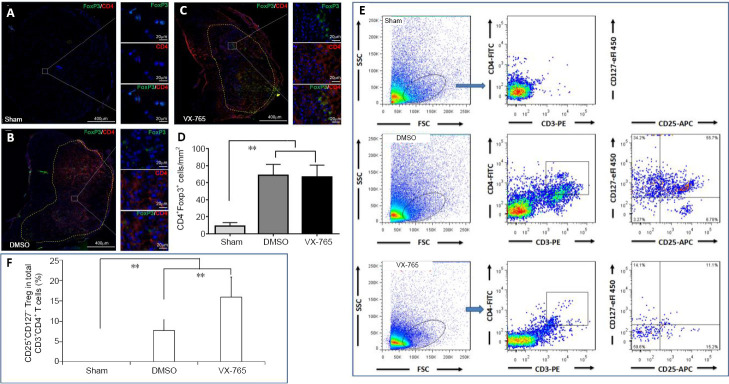
To further clarify the effects of VX-765 on Tregs, CD4+FoxP3+ cells in immunofluorescence were defined as Tregs (Miyara et al., 2009). There were very few Tregs in the sham group (Figure 9A), and there were also few Tregs following SCI (Figure 9B and C). The statistical results (Figure 9D) revealed that, although the number of Tregs was significantly higher after SCI (all P < 0.01, n = 6) compared with the sham group, there was no significant difference between the two SCI groups (P > 0.05, n = 6). For flow cytometry, the CD3+CD4+CD25+CD127– cell subset was defined as Tregs (Yu et al., 2012) (Figure 9E). Figure 9F shows that, compared with the sham group, the proportions of Tregs in the DMSO and VX-765 groups were significantly higher (both P < 0.01, n = 6). After VX-765 treatment, the proportion of Tregs was also significantly higher compared with the DMSO group (P < 0.01, n = 6).
Figure 9.
Effects of VX-765 on Treg in the injured spinal cord at 7 days post-injury: immunofluorescence and flow cytometry assay.
(A–C) CD4 (red, stained by rhodamine) and FoxP3 (green, stained by FITC) for Treg. Nuclei are blue, stained by Hoechst 33342. Scale bars: 400 µm, 20 µm (enlarged parts). (D) CD4+FoxP3+ Treg cell counts in the sham, DMSO, and VX-765 groups. (E) Flow cytometry images of cells derived from spinal cord homogenate in the different groups. (F) Proportional analysis of CD3+CD4+CD25+CD127– Treg. The original data for Figure 9D and F are shown in Additional file 9. Data are represented as the mean ± SD (n = 6). **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). DMSO: Dimethyl sulfoxide; FITC: fluoresceine isothiocyanate; FoxP3: forkhead box P3; SCI: spinal cord injury; Treg: regulatory T cells; VX-765: caspase-1 selective inhibitor.
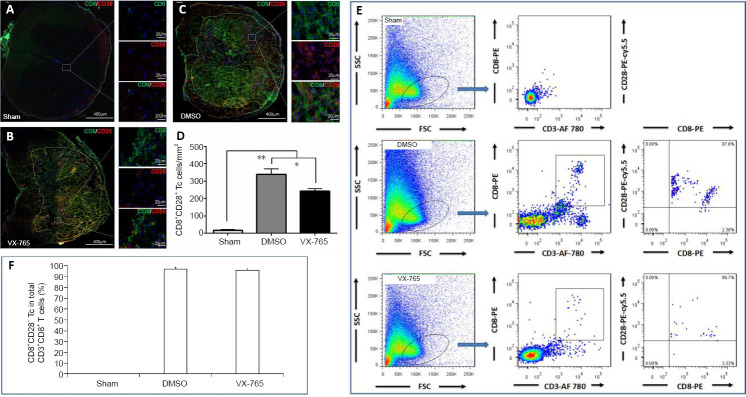
VX-765 decreases the number of cytotoxic T (Tc) cells in the injured spinal cord
To further clarify the effects of VX-765 on Tc cells, CD8+CD28+ cells in immunofluorescence were defined as Tc cells (Wu et al., 2017). There were very few Tc cells in the sham group (Figure 10A), and a larger number of Tc cells following SCI (Figure 10B and C). The statistical results (Figure 10D) revealed that, compared with the sham group, the numbers of Tc cells in the DMSO and VX-765 groups were significantly higher (both P < 0.01, n = 6). Compared with the DMSO group, the numbers of Tc cells were significantly lower in the VX-765 group (P < 0.05, n = 6). CD3+CD8+CD28+ cells were also detected using flow cytometry, as previously described (Wu et al., 2017) (Figure 10E). Figure 10F shows that the proportions of Tc cells in the DMSO and VX-765 groups were significantly higher compared with the sham group (both P < 0.01, n = 6); however, there was no significant difference between the DMSO and VX-765 groups (P > 0.05, n = 6).
Figure 10.
Effects of VX-765 on Tc cells in the injured spinal cord at 7 days post-injury: immunofluorescence and flow cytometry assay.
(A–C) CD8 (green, stained by FITC) and CD28 (red, stained by rhodamine) for Tc cells in the different groups. Nuclei are blue, stained by Hoechst 33342. (D) CD8+CD28+ Tc cell counts in the sham, DMSO, and VX-765 groups. (E) Flow cytometry images of cells derived from spinal cord homogenate. (F) Proportional analysis of CD8+CD28+Tc cells in the three groups. The original data for Figure 10D and F are shown in Additional file 10. Data are represented as the mean ± SD (n = 6). *P < 0.05, **P < 0.01 (non-parametric Kruskal-Wallis analysis of variance). DMSO: Dimethyl sulfoxide; FITC: fluoresceine isothiocyanate; SCI: spinal cord injury; Tc: cytotoxic T; VX-765: caspase-1 selective inhibitor.
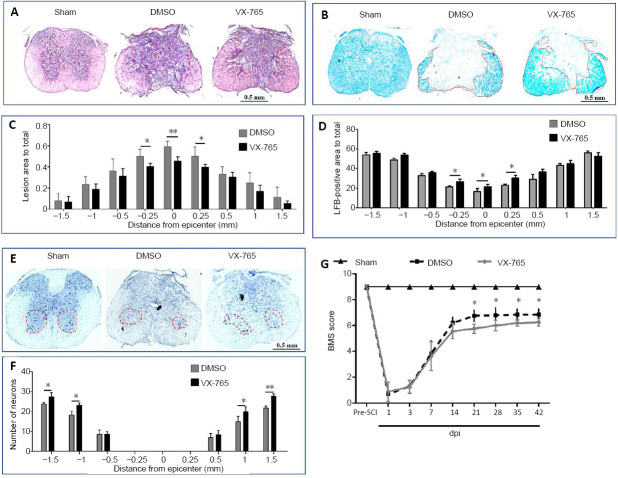
VX-765 reduces spinal cord tissue damage and promotes functional recovery after SCI
The effects of VX-765 on histology and behavior after SCI were detected at 42 dpi. Representative hematoxylin-eosin and Luxol fast blue images of injured centers are shown in Figure 11A and B, respectively. In hematoxylin-eosin-stained sections (Figure 11A), the butterfly-shaped gray matter was intact and clearly demarcated from the white matter in the sham group. After SCI, the boundary between the gray and white matter was unclear, and the color of the fibrotic area was notably darker than other areas. For Luxol fast blue staining, blue represents the myelinated areas (Figure 11B). The statistical results revealed that the effects of VX-765 in fibrotic areas (Figure 11C) and spared white matter tissue (Figure 11D) could be observed within the range of 0.5 mm (compared with the DMSO group, P < 0.01 or 0.05, n = 10). The fibrotic area of the VX-765 group was smaller than that of the DMSO group, whereas the myelinated area of the VX-765 group was larger than that of the DMSO group. (Figure 11E) shows images of Nissl staining. The existence of Nissl substance and euchromatic nuclei were used to identify the surviving ventral horn neurons. Significant differences were detected in an area within 1 mm away from the epicenter at the rostral and caudal spinal cords (Figure 11F). The VX-765 group had more residual ventral horn motoneurons than the DMSO group (P < 0.01 or 0.05, n = 10).
Figure 11.
Effects of VX-765 on histopathology and behavior after SCI at 42 days post-injury.
(A, B) Hematoxylin-eosin (A) and Luxol fast blue (B) staining in the injury epicenter. In hematoxylin-eosin-stained sections (A), the color of the fibrotic area was notably darker than in other areas. There was no fibrotic area in the sham-injured spinal cord. There were marked fibrotic areas in the injured spinal cords, and the fibrotic area of the VX-765 group was smaller than that of the DMSO group. For Luxol fast blue staining (B), blue represents myelinated areas. Although the myelinated areas decreased markedly after SCI, the myelinated area of the VX-765 group was larger than that of the DMSO group. (C, D) Quantitative analysis of the fibrotic area (C) and residual myelination (D). (E) Nissl-stained cross-section, 0.5 mm rostral to the epicenter. Although Nissl-stained neurons were observed in all groups, the number of neurons decreased markedly after SCI, and the neurons were increased in the VX-765 group compared with the DMSO group. Scale bars: 0.5 mm. (F) Quantitative analysis of the residual ventral horn motoneurons. (G) BMS scores. All data are represented as the mean ± SD (n = 10). The original data for C, D, F, and G are shown in Additional file 11. *P < 0.05, **P < 0.01 (repeated measures two-way analysis of variance followed by Bonferroni’s post hocanalysis). BMS: Basso Mouse Scale; DMSO: dimethyl sulfoxide; dpi: day(s) post-injury; SCI: spinal cord injury; VX-765: caspase-1 selective inhibitor.
To investigate behavioral recovery, the Basso Mouse Scale was used. Figure 11G shows that all animals scored 9 points before the operation. After the operation, the sham group still scored 9 points. In contrast, at 1–3 dpi, the scores of the DMSO and VX-765 groups were less than 2 points. From 1–14 dpi, there were no significant differences between the DMSO and VX-765 groups. However, compared with the DMSO group, the Basso Mouse Scale scores of the VX-765 group were significantly higher after 21 dpi (P < 0.05, n = 10).
Discussion
Previous studies have shown that caspase-1 activation occurs in the injured spinal cord (Mortezaee et al., 2018). VX-765, a compound with a molecular weight of 509 Da and formula of C24H33ClN4O6, reportedly inhibits caspase-1 by inhibiting pro-caspase-1 self-cleavage (Stack et al., 2005; Wannamaker et al., 2007; Zhang and Zheng, 2016; Flores et al., 2018). It has been demonstrated to be effective in central nervous system diseases (Maroso et al., 2011; McKenzie et al., 2018; Flores et al., 2020), collagen-induced arthritis (Zhang and Zheng, 2016), infantile spasms (Galanopoulou et al., 2017), asthma (Chen et al., 2019), atherosclerosis (Li et al., 2020), and some other inflammatory diseases in murine models (Stack et al., 2005). Recently, using RNA sequencing, we also found that VX 765 application following SCI can inhibit signaling pathways associated with inflammatory responses (Chen et al., 2020a). We therefore hypothesized that VX-765 may be an effective anti-inflammatory and neuroprotective drug for treating SCI.
Our western blot results revealed no significant differences in pro-caspase-1 levels between all of the groups. However, the 42–45 kDa bands were at the saturation level. This is because the amount of proteins that were needed per well to detect the activated 12 and 10 kDa cleaved-caspase-1 exceeded the linear phase of detection. However, although the 45 and 42 kDa pro-caspase-1 levels were not found to differ significantly between the groups, western blot analysis confirmed that SCI can induce the activation of caspase-1, IL-1β, and IL-18. These are all involved in the inflammasome-related signaling pathway, and the expression of apoptosis-related molecules (such as pro-caspase-3 and tumor necrosis factor receptor type 1) (Porter and Jänicke, 1999; Idriss and Naismith, 2000) and necrosis-related molecules (such as IL-6) is then induced (Rose-John, 2018). In addition, our findings were consistent with those of previous reports (Wannamaker et al., 2007; Yang et al., 2017). However, we noted that VX-765 decreased pro-caspase-3 levels without affecting the level of activation of caspase-3. This may be because the drug works at the level of gene transcription or translation, although the detailed mechanisms need to be further explored. Nevertheless, our results indicate that VX-765 can indeed inhibit apoptosis- and necrosis-related molecular events, suggesting that VX-765 may be a potential therapeutic drug for SCI. Immediate administration of VX-765 may inhibit inflammation and apoptosis- and necrosis-related molecular events in the injured spinal cord. Thus, we next explored the effects of VX-765 on the immune microenvironment and neuroprotection following SCI.
After SCI, there is destruction of the blood-spinal cord barrier and infiltration of peripheral inflammatory cells, resulting in many immune cell subsets with different functions in the injured spinal cord (Ahmed et al., 2018). Previous studies have reported that infiltrated monocytes and T lymphocytes, and locally activated microglia, play important roles in the pathophysiology of SCI (Hu et al., 2012; Popovich, 2014; Chen et al., 2015; Ma et al., 2015; Hu et al., 2016a; Wu et al., 2017). It has also been confirmed that inflammation is most serious in the subacute stage (1–2 weeks) of SCI (Chen et al., 2015; Wu et al., 2017). We therefore investigated changes in the numbers and proportions of local immune cell subsets at 7 dpi to explore the effects of VX-765 on the local immune microenvironment.
To determine the effects of VX-765 on immune cell subsets, immunofluorescence and flow cytometry were used. The combination of these two methods can not only count the numbers and proportions of immune cell subsets, but can also effectively distinguish peripheral-infiltrated and locally activated cell subsets. They thus provide reliable evidence to understand the effects of VX-765 on the local immune microenvironment in SCI. Our results demonstrated that increased M1 cells were mainly the result of transformation of local microglia, rather than infiltrated macrophages, following SCI in mice. This result suggests that inhibition of local microglia activation after SCI is an important strategy to control the inflammatory response. We also found that an interesting proinflammatory Th1Th17 cell subset (a special Th subset producing both Th1 and Th17 cytokines) was predominant in the Th subsets of the injured spinal cord. Th1Th17 has a stronger proinflammatory effect than Th1 or Th17 cells (Gosselin et al., 2010; Nikitina et al., 2018). We therefore speculate that Th1Th17 may be another important target for improving the local immune microenvironment in SCI.
In addition, we found the relevance of a 1–3% change in the percentages of immune cells in the pathophysiology generated after SCI. This is because flow cytometry was used to analyze the proportions of immune cells out of all spinal cord cells. Although the proportions were limited, there was a significant imbalance between immune cell subsets, and destructive cells (such as M1, Th1, Th1Th17, and Tc) were predominant after SCI. Our results also showed that VX-765 was able to effectively change this immune imbalance at the injury site. This finding is consistent with a recent report using a stroke model (Li et al., 2019).
The present study also produced some interesting and even seemingly contradictory results, which need further discussion. For example, M2 expression markers were upregulated after SCI in immunofluorescence, but using flow cytometry, the expression of CD11b+CD68+CCR7– indicated a reduction in M2 cells after SCI. Another way of considering this result is that the level of M2 cells in immunofluorescence was extremely low, while the level of M2 using flow cytometry was high. This is likely a technical issue, because immunofluorescence detects the number of cells, whereas flow cytometry measures the proportion of cells. Similarly, using immunofluorescence, Th1 and Th17 levels were lower or remained the same in terms of positive cell numbers, respectively, with VX-765 treatment (relative to injured controls). However, the proportions of markers for Th1 or Th17 were higher with the drug treatment (relative to injured controls) when flow cytometry technology was used. Moreover, there was no significant difference in Treg numbers between the injured groups using immunofluorescence, but with flow cytometry we found a significant increase in Tregs proportion in the VX-765-treated group relative to injured controls. Furthermore, the results related to Tc cells showed a similar situation. Nevertheless, our results indicated that VX-765 was able to suppress the infiltration of total macrophages, the differentiation of M1 macrophages/microglia, the differentiation of Th1 and Th1Th17, and the activation of cytotoxic T cells. Accordingly, this drug may promote the differentiation of M2 microglia, Th2, and Tregs. Our results therefore demonstrate that VX-765 is an immunomodulatory drug for SCI. Next, the effects of VX-765 on histology and behavior following SCI were further explored.
We demonstrated that treatment with VX-765 for 7 consecutive days reduced the fibrotic area, increased white matter myelination and residual motoneurons, and improved functional recovery. Here, the effects of the drug on lesion volume and spared white matter was only observed within a range of approximately 0.5 mm. One reason for this finding may be that VX-765 mainly inhibits the inflammatory response, and the 0.5 mm range contains the area of the most serious immune response. Another reason may be that VX-765 lacks a target for the injured spinal cord. Therefore, understanding the mechanisms of VX-765 in the injured area and exploring a targeted drug delivery system may improve the efficacy of VX-765. Another puzzling finding was that, although the drug was administered for 7 consecutive days, the first signs of behavioral improvement were observed at 21 dpi, which remained for 6 weeks. The likely reason for this phenomenon is that behavioral improvement is based on improvements in histology, and histological improvement is based on improvements in the immune microenvironment at 7 dpi. The observed lag in behavioral improvement can therefore be explained as such.
At present, many drugs or cytokine inhibitors to treat the inflammatory reaction in SCI are in the experimental phase; however, the mechanisms and extent of such treatments are unclear. Moreover, some drugs are effective in experimental SCI models, but not in humans. Currently, steroids are the best clinical drugs for use in early clinical therapy. Therefore, although VX-765 has been demonstrated here to be beneficial in the treatment of SCI, this drug remains in the primary research stage using a mouse model. There are still many underlying mechanisms that need to be explored. For example, we do not know about the drug’s turn-over in rodents, or its half-life. It therefore remains to be explored whether continuous administration of VX-765 over 7 days is the best option. In addition, it is unclear how the drug delivery scheme might be optimized and made suitable for humans. One limitation of the present study is that we explored an anti-inflammatory strategy for SCI from the perspective of improving the local immune microenvironment only, even though the pathological mechanisms of SCI are complex. In addition to the inflammatory/immune mechanisms, other factors (such as vascular mechanisms, excitatory amino acid toxicity, peroxide pressure, and astrocyte reactions) are involved in the pathological process of SCI. It may therefore be impossible to rely on a single drug alone, and the development of a comprehensive and feasible treatment plan in this field is urgently needed.
In summary, our results confirm that DAMPs produced by SCI can bind to intracellular PRRs in cells of the injured spinal cord, leading to the assembly of intracellular PRRs, ASC, and pro-caspase-1. This forms the activated inflammasome complex, which causes pro-caspase-1 cleavage and activation. The activated caspase-1 can further cleave pro-IL-1β and pro-IL-18 to form mature IL-1β and IL-18, and results in a local immune microenvironment imbalance, producing neuroinflammation. The use of VX-765 can selectively inhibit the activation of caspase-1, thus inhibiting the production of IL-1β and IL-18, which can improve the local immune microenvironment and produce a neuroprotective effect. The early administration of VX-765 is therefore a promising strategy for the treatment of SCI. However, some limitations remain for the clinical application of VX-765. Our future studies will focus on further clarifying the detailed mechanisms and characteristics of the drug (such as its half-life and side effects), and optimizing the administration scheme.
Additional files:
Additional Table 1: Information of antibodies used in immunohistofluorescence.
Additional Table 2: Information of antibodies used in flow cytometry.
Additional file 1: Open peer review report 1 (87.9KB, pdf) .
Additional file 2 (118.2KB, pdf) : Original data of Figure 2.
Additional file 3: Original data of Figure 3.
Quantitative results of microglia and infiltrated macrophages in injured spinal cord
| CD45+ cells/mm2 | |||
|---|---|---|---|
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 20 | 627 | 906 | |
| 25 | 1186 | 1004 | |
| 35 | 1517 | 1087 | |
| 11 | 1243 | 865 | |
| 12 | 1114 | 1074 | |
| 17 | 1135 | 1032 | |
| mean | 20 | 1137 | 994.6666667 |
| SD | 8.988882022 | 289.3682775 | 90.52660751 |
| CD68+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 5 | 658 | 593 | |
| 7 | 872 | 596 | |
| 8 | 779 | 702 | |
| 11 | 676 | 619 | |
| 15 | 879 | 648 | |
| 16 | 814 | 701 | |
| mean | 10.33333333 | 779.6666667 | 643.1666667 |
| SD | 4.457203907 | 94.99614027 | 49.30483411 |
| CD11b+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 188 | 517 | 902 | |
| 185 | 1186 | 970 | |
| 175 | 1627 | 981 | |
| 171 | 1243 | 875 | |
| 172 | 1114 | 964 | |
| 211 | 1135 | 932 | |
| mean | 183.6666667 | 1137 | 937.3333333 |
| SD | 15.09525312 | 357.3989368 | 42.06502902 |
| CD68+CD11b+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 0 | 708 | 615 | |
| 0 | 810 | 682 | |
| 0 | 802 | 664 | |
| 0 | 885 | 689 | |
| 0 | 776 | 674 | |
| 0 | 836 | 623 | |
| mean | 0 | 802.8333333 | 657.8333333 |
| SD | 0 | 59.35795369 | 31.31400113 |
| CD45+CD11b+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 0 | 808 | 695 | |
| 0 | 764 | 672 | |
| 0 | 738 | 720 | |
| 0 | 848 | 695 | |
| 0 | 788 | 712 | |
| 0 | 807 | 703 | |
| mean | 0 | 792.1666667 | 699.5 |
| SD | 0 | 38.29577871 | 16.64632091 |
Additional file 4: Original data of Figure 4.
Microglia and infiltrated macrophages: a flow cytometry assay
| cell subsets (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD68+ | CD11b+ | CD45+ | CD68+CD11b+ | CD45+CD11b+ | CD45-/lowCD11b+ | CD45+CD68+ | CD45-/lowCD68+ | CD45+CD68-CD11b- | ||
| 0.628 | 0.726 | 0.239 | 0.426 | 0.112 | 0.907 | 0.055 | 0.698 | 0.12 | ||
| 0.313 | 1.08 | 0.353 | 0.35 | 0.152 | 0.84 | 0.182 | 0.645 | 0.13 | ||
| 0.41 | 1.254 | 0.226 | 0.435 | 0.143 | 0.81 | 0.01 | 0.689 | 0.1 | ||
| sham | 0.51 | 1.387 | 0.212 | 0.51 | 0.149 | 1.03 | 0.02 | 0.663 | 0.12 | |
| 0.921 | 0.527 | 0.093 | 0.342 | 0.084 | 0.789 | 0.039 | 0.794 | 0.12 | ||
| 0.645 | 1.58 | 0.12 | 0.44 | 0.148 | 0.92 | 0.025 | 0.746 | 0.11 | ||
| mean | 0.571166667 | 1.092333333 | 0.2071667 | 0.417166667 | 0.131333333 | 0.882666667 | 0.055166667 | 0.705833333 | 0.116666667 | |
| SD | 0.213274862 | 0.401182585 | 0.0930493 | 0.062764374 | 0.027449347 | 0.088957668 | 0.06408562 | 0.055257277 | 0.010327956 | |
| CD68+ | CD11b+ | CD45+ | CD68+CD11b+ | CD45+CD11b+ | CD45-/lowCD11b+ | CD45+CD68+ | CD45-/lowCD68+ | CD45+CD68-CD11b- | ||
| 3.07 | 2.15 | 0.885 | 1.18 | 0.414 | 1.12 | 0.276 | 2.74 | 0.219 | ||
| 2.092 | 1.96 | 0.834 | 0.572 | 0.318 | 0.787 | 0.282 | 1.9 | 0.274 | ||
| 2.918 | 2.55 | 0.75 | 1.26 | 0.54 | 1.15 | 0.318 | 3.02 | 0.522 | ||
| SCI(DMSO) | 3.11 | 2.99 | 0.85 | 1.586 | 0.642 | 1.146 | 0.284 | 1.12 | 0.498 | |
| 3.31 | 3.17 | 0.72 | 1.476 | 0.416 | 0.9535 | 0.29 | 2.12 | 0.296 | ||
| 2.412 | 2.56 | 0.701 | 1.512 | 0.418 | 1.157 | 0.302 | 1.7 | 0.374 | ||
| mean | 2.818666667 | 2.563333333 | 0.79 | 1.264333333 | 0.458 | 1.05225 | 0.292 | 2.1 | 0.363833333 | |
| SD | 0.467491462 | 0.46586121 | 0.0761341 | 0.373113209 | 0.114437756 | 0.151007202 | 0.015491933 | 0.695240965 | 0.123910317 | |
| CD68+ | CD11b+ | CD45+ | CD68+CD11b+ | CD45+CD11b+ | CD45-/lowCD11b+ | CD45+CD68+ | CD45-/lowCD68+ | CD45+CD68-CD11b- | ||
| 1.37 | 0.694 | 0.354 | 0.361 | 0.277 | 0.62 | 0.084 | 1.24 | 0.163 | ||
| 1.86 | 0.85 | 0.652 | 0.331 | 0.383 | 0.893 | 0.091 | 1.35 | 0.21 | ||
| 1.42 | 0.76 | 0.552 | 0.365 | 0.271 | 0.571 | 0.065 | 1.14 | 0.182 | ||
| SCI(VX-765) | 1.5 | 0.642 | 0.518 | 0.367 | 0.215 | 0.713 | 0.071 | 0.98 | 0.17 | |
| 1.62 | 1.02 | 0.491 | 0.358 | 0.325 | 0.792 | 0.04 | 1.11 | 0.189 | ||
| 1.495 | 0.7925 | 0.525 | 0.355 | 0.221 | 0.706 | 0.072 | 1.05 | 0.176 | ||
| mean | 1.544166667 | 0.793083333 | 0.5153333 | 0.356166667 | 0.282 | 0.715833333 | 0.0705 | 1.145 | 0.181666667 | |
| SD | 0.176420426 | 0.133018201 | 0.0966885 | 0.013090709 | 0.06388427 | 0.116150621 | 0.017694632 | 0.133078924 | 0.016573071 | |
Additional file 5: Original data of Figure 5.
Quantitative results of M1 and M2 cells in injured spinal cord in injured spinal cord
| CD68+CCR7+ cells/mm2 | |||
|---|---|---|---|
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 22 | 625 | 564 | |
| 15 | 602 | 519 | |
| 23 | 673 | 497 | |
| 21 | 589 | 476 | |
| 13 | 803 | 569 | |
| 18 | 659 | 545 | |
| mean | 18.66666667 | 658.5 | 528.3333333 |
| SD | 4.03319559 | 77.76310179 | 37.44685122 |
| CD68+Arg-1+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 36 | 165 | 245 | |
| 33 | 190 | 224 | |
| 28 | 212 | 240 | |
| 22 | 188 | 215 | |
| 32 | 172 | 218 | |
| 38 | 198 | 250 | |
| mean | 31.5 | 187.5 | 232 |
| SD | 5.787918451 | 17.10847743 | 14.87279395 |
Additional file 6: Original data of Figure 6.
M1 and M2 Microglia/macrophages: a flow cytometry assay
| cell subsets (%) | |||||||
|---|---|---|---|---|---|---|---|
| CD11b+CD68+CCR7+ | CD11b+CD68+CCR7- | CD45highCD11b+CD68+CCR7+ | CD45highCD11b+CD68+CCR7- | CD45-/lowCD11b+CD68+CCR7+ | CD45-/lowCD11b+CD68+CCR7- | ||
| 24.212 | 28.45 | 16.25 | 0 | 35.71 | 21.92 | ||
| 31.75 | 32.12 | 23.28 | 0 | 34.35 | 26.12 | ||
| 29.78 | 25.16 | 29.35 | 0 | 35.08 | 32.15 | ||
| sham | 28.97 | 31.412 | 24.11 | 0 | 32.01 | 25.55 | |
| 21.36 | 36.97 | 18.12 | 0 | 33.17 | 26.13 | ||
| 30.13 | 38.88 | 28.22 | 0 | 35.02 | 28.68 | ||
| mean | 27.70033333 | 32.16533333 | 23.22166667 | 0 | 34.22333333 | 26.75833333 | |
| SD | 4.015182271 | 5.130682866 | 5.252875086 | 0 | 1.385866756 | 3.418616192 | |
| CD11b+CD68+CCR7+ | CD11b+CD68+CCR7- | CD45highCD11b+CD68+CCR7+ | CD45highCD11b+CD68+CCR7- | CD45-/lowCD11b+CD68+CCR7+ | CD45-/lowCD11b+CD68+CCR7- | ||
| 57.18 | 13.23 | 67.23 | 0 | 65.26 | 5.79 | ||
| 55.75 | 10.38 | 60.05 | 0 | 69.95 | 6.73 | ||
| 59.38 | 13.59 | 52.16 | 0 | 73.26 | 7.54 | ||
| SCI(DMSO) | 51.71 | 8.06 | 53.47 | 0 | 72.39 | 6.21 | |
| 53.64 | 5.94 | 69.46 | 0 | 63.11 | 8.13 | ||
| 56.98 | 14.36 | 58.11 | 0 | 71.32 | 6.12 | ||
| mean | 55.77333333 | 10.92666667 | 60.08 | 0 | 69.215 | 6.753333333 | |
| SD | 2.738800224 | 3.393226586 | 7.062679378 | 0 | 4.106749323 | 0.909827823 | |
| CD11b+CD68+CCR7+ | CD11b+CD68+CCR7- | CD45highCD11b+CD68+CCR7+ | CD45highCD11b+CD68+CCR7- | CD45-/lowCD11b+CD68+CCR7+ | CD45-/lowCD11b+CD68+CCR7- | ||
| 37.32 | 20.88 | 37.02 | 0 | 57.58 | 10.41 | ||
| 41.15 | 18.42 | 42.55 | 0 | 59.95 | 11.15 | ||
| 45.36 | 22.36 | 49.42 | 0 | 63.81 | 13.79 | ||
| SCI(VX-765) | 49.51 | 10.86 | 39.98 | 0 | 57.11 | 10.22 | |
| 34.74 | 20.15 | 43.26 | 0 | 59.36 | 11.88 | ||
| 39.22 | 14.22 | 37.54 | 0 | 58.97 | 9.37 | ||
| mean | 41.21666667 | 17.815 | 41.62833333 | 0 | 59.46333333 | 11.13666667 | |
| SD | 5.423447858 | 4.408300126 | 4.580176489 | 0 | 2.386190828 | 1.554498847 | |
Additional file 7: Original data of Figure 7.
Quantitative results of Th1, Th2 and Th17 cells in injured spinal cord in injured spinal cord
| CD4+T-bet+ cells/mm2 | |||
|---|---|---|---|
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 8 | 811 | 719 | |
| 2 | 797 | 705 | |
| 12 | 805 | 715 | |
| 10 | 754 | 685 | |
| 4 | 774 | 635 | |
| 15 | 816 | 702 | |
| mean | 8.5 | 792.8333333 | 693.5 |
| SD | 4.888762625 | 24.06172618 | 31.02096066 |
| CD4+GATA3+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 5 | 133 | 285 | |
| 13 | 126 | 232 | |
| 5 | 123 | 175 | |
| 10 | 125 | 213 | |
| 12 | 138 | 263 | |
| 5 | 144 | 275 | |
| mean | 8.333333333 | 131.5 | 240.5 |
| SD | 3.777124126 | 8.312640976 | 41.9416261 |
| CD4+RORγt+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 2 | 154 | 147 | |
| 6 | 167 | 175 | |
| 5 | 178 | 206 | |
| 3 | 166 | 173 | |
| 5 | 134 | 144 | |
| 4 | 189 | 172 | |
| mean | 4.166666667 | 164.6666667 | 169.5 |
| SD | 1.471960144 | 19.13809465 | 22.52776065 |
Additional file 8: Original data of Figure 8.
Th1, Th2 and Th17 subsets: a flow cytometry assay
| cell subsets (%) | |||||
|---|---|---|---|---|---|
| CD3+CD4+CD183+CD196- | CD3+CD4+CD183+CD196+ | CD3+CD4+CD183-CD196+ | CD3+CD4+CD183-CD196- | ||
| 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | ||
| sham | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | ||
| 0 | 0 | 0 | 0 | ||
| mean | 0 | 0 | 0 | 0 | |
| SD | 0 | 0 | 0 | 0 | |
| CD3+CD4+CD183+CD196- | CD3+CD4+CD183+CD196+ | CD3+CD4+CD183-CD196+ | CD3+CD4+CD183-CD196- | ||
| 0 | 84.62 | 14.74 | 0.64 | ||
| 4.6 | 84.05 | 11.03 | 0.32 | ||
| 3.98 | 85.69 | 10.21 | 0.12 | ||
| SCI(DMSO) | 1 | 94.22 | 4.63 | 0.15 | |
| 4.56 | 83.24 | 11.84 | 0.36 | ||
| 5.18 | 81.01 | 13.58 | 0.23 | ||
| mean | 3.22 | 85.47166667 | 11.005 | 0.303333333 | |
| SD | 2.164070239 | 4.565397756 | 3.536986005 | 0.189384969 | |
| CD3+CD4+CD183+CD196- | CD3+CD4+CD183+CD196+ | CD3+CD4+CD183-CD196+ | CD3+CD4+CD183-CD196- | ||
| 13.83 | 51.71 | 24.07 | 10.39 | ||
| 7.44 | 74.06 | 13.35 | 5.15 | ||
| 5.56 | 62.31 | 26.31 | 5.82 | ||
| SCI(VX-765) | 15.21 | 75.35 | 1.36 | 8.08 | |
| 5.57 | 68.97 | 20.05 | 5.41 | ||
| 0 | 49.19 | 44.61 | 6.2 | ||
| mean | 7.935 | 63.59833333 | 21.625 | 6.841666667 | |
| SD | 5.694203193 | 11.19817202 | 14.40738665 | 2.024405262 | |
Additional file 9: Original data of Figure 9.
IHF quantitative results of Treg in injured spinal cord
| CD4+FoxP3+ cells/mm2 | |||
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 9 | 73 | 54 | |
| 5 | 54 | 64 | |
| 13 | 79 | 82 | |
| 10 | 78 | 67 | |
| 9 | 73 | 88 | |
| 8 | 59 | 56 | |
| mean | 9 | 69.33333333 | 68.5 |
| SD | 2.607680962 | 10.36661308 | 13.7949266 |
| FCM results of Treg in injured spinal cord | |||
| Treg (%) | |||
| sham | SCI (DMSO) | SCI (VX-765) | |
| 0 | 6.27 | 13.6 | |
| 0 | 11.3 | 21.2 | |
| 0 | 10.2 | 22.7 | |
| 0 | 6.78 | 15.2 | |
| 0 | 6.2 | 10.5 | |
| 0 | 4.5 | 12.56 | |
| mean | 0 | 7.541666667 | 15.96 |
| SD | 0 | 2.624533609 | 4.907219172 |
Additional file 10: Original data of Figure 10.
IHF quantitative results of Tc in injured spinal cord
| CD8+CD28+ cells/mm2 | |||
|---|---|---|---|
| Sham | SCI(DMSO) | SCI(VX-765) | |
| 13 | 367 | 238 | |
| 15 | 335 | 261 | |
| 22 | 386 | 221 | |
| 16 | 297 | 228 | |
| 28 | 318 | 257 | |
| 22 | 330 | 248 | |
| mean | 19.33333333 | 338.8333333 | 242.1666667 |
| SD | 5.645056835 | 32.54176803 | 15.96767568 |
| FCM quantitative results of Tc in injured spinal cord | |||
| Tc (%) | |||
| sham | SCI (DMSO) | SCI (VX-765) | |
| 0 | 97.6 | 96.7 | |
| 0 | 96.8 | 92.8 | |
| 0 | 97.8 | 96 | |
| 0 | 95.6 | 96.2 | |
| 0 | 93.7 | 94.5 | |
| 0 | 98.8 | 98 | |
| mean | 0 | 96.71666667 | 95.7 |
| SD | 0 | 1.824737424 | 1.815488915 |
Additional file 11: Original data of Figure 11.
Quantitative results of histopathology and behavior
| Lesion area to total | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance from epicenter | SCI(DMSO) | Mean | SD | SCI(VX-765) | Mean | SD | |||||||||||
| -1.5 | 0.020934972 | 0.198672436 | 0.072790279 | 0.0421459 | 0.070142325 | 0.074313363 | 0.079833213 | 0.06194527 | 0.008040479 | 0.109652717 | 0.09122246 | 0.031848086 | 0.148935905 | 0.054825342 | 0.074087498 | 0.052303314 | |
| -1 | 0.121614676 | 0.327233346 | 0.223957284 | 0.235502816 | 0.266272026 | 0.233989264 | 0.234761569 | 0.067028406 | 0.194124451 | 0.207606515 | 0.161656759 | 0.121239216 | 0.244912092 | 0.226950049 | 0.192748181 | 0.04515244 | |
| -0.5 | 0.183455386 | 0.497416006 | 0.373046569 | 0.344640358 | 0.406002782 | 0.369580948 | 0.362357008 | 0.102598254 | 0.406444137 | 0.350279479 | 0.267345389 | 0.22573113 | 0.30295254 | 0.36252986 | 0.319213756 | 0.06654962 | |
| -0.2 | 0.404871143 | 0.560123618 | 0.573021289 | 0.492982405 | 0.466293214 | 0.461224099 | 0.493085961 | 0.063872683 | 0.446932388 | 0.438074876 | 0.403895513 | 0.408224727 | 0.391682887 | 0.365713498 | 0.409087315 | 0.029948053 | |
| HE | 0 | 0.566450781 | 0.667543714 | 0.624096417 | 0.543782021 | 0.567033142 | 0.575636801 | 0.590757146 | 0.04603607 | 0.520213838 | 0.514440653 | 0.462149192 | 0.429984932 | 0.452847603 | 0.417748096 | 0.466230719 | 0.042657856 |
| 0.25 | 0.404532647 | 0.575882653 | 0.609305031 | 0.452777047 | 0.458449097 | 0.51087368 | 0.501970026 | 0.078576355 | 0.421355325 | 0.406510482 | 0.407645578 | 0.419734322 | 0.357829482 | 0.375977526 | 0.398175453 | 0.025619495 | |
| 0.5 | 0.235246608 | 0.383492388 | 0.405073129 | 0.310551853 | 0.334126376 | 0.401164851 | 0.344942534 | 0.065720063 | 0.363224541 | 0.294904199 | 0.243526245 | 0.29242032 | 0.319893854 | 0.30843719 | 0.303734391 | 0.039123711 | |
| 1 | 0.10300629 | 0.278985617 | 0.376672514 | 0.234993661 | 0.24298161 | 0.293184081 | 0.254970629 | 0.089982568 | 0.155089706 | 0.238256795 | 0.125708351 | 0.198799534 | 0.255819304 | 0.105663774 | 0.179889577 | 0.060974996 | |
| 1.5 | 0.036569092 | 0.154128774 | 0.259476982 | 0.077567317 | 0.040334827 | 0.06158069 | 0.104942947 | 0.08692401 | 0.017566131 | 0.087522688 | 0.067217507 | 0.039525132 | 0.085362316 | 0.05753885 | 0.059122104 | 0.027107653 | |
| LFB-postive area to total | |||||||||||||||||
| Distance from epicenter | SCI(DMSO) | Mean | SD | SCI(VX-765) | Mean | SD | |||||||||||
| 1.5 | 52.7 | 56.7 | 52.3 | 53.6 | 52.5 | 55.9 | 53.95 | 1.89076704 | 56.8 | 57.1 | 55.1 | 53.2 | 55 | 54.8 | 55.33333333 | 1.433410851 | |
| -1 | 50.5 | 48.5 | 47.1 | 47.2 | 48.5 | 51.3 | 48.85 | 1.71784749 | 52.6 | 54.6 | 47.3 | 48.3 | 53.2 | 53.6 | 51.6 | 3.031171391 | |
| -0.5 | 30.3 | 34.8 | 33.2 | 31.5 | 32.8 | 35.5 | 33.01666667 | 1.954908352 | 37.5 | 34.8 | 35.7 | 35.5 | 38.2 | 34.7 | 36.06666667 | 1.45143607 | |
| LFB | -0.5 | 30.3 | 34.8 | 33.2 | 31.5 | 32.8 | 35.5 | 33.01666667 | 1.954908352 | 37.5 | 34.8 | 35.7 | 35.5 | 38.2 | 34.7 | 36.06666667 | 1.45143607 |
| 0 | 13.3 | 16.7 | 19.7 | 11.6 | 15.7 | 20.1 | 16.18333333 | 3.392000393 | 19.2 | 20.3 | 24.1 | 19.2 | 21.7 | 18.6 | 20.51666667 | 2.070185177 | |
| 0.25 | 21.7 | 18.5 | 26.7 | 19.8 | 21.5 | 29.8 | 23 | 4.344191524 | 24.6 | 31.5 | 34.5 | 28.5 | 33.2 | 27.8 | 30.01666667 | 3.714521055 | |
| 0.5 | 31.4 | 20.3 | 31.3 | 31.5 | 30.8 | 33.7 | 29.83333333 | 4.778144689 | 36.9 | 35.4 | 33.4 | 34.5 | 35.3 | 29.5 | 34.16666667 | 2.559427019 | |
| 1 | 42.3 | 42.6 | 45.2 | 44.2 | 45.5 | 47.3 | 44.51666667 | 1.890414417 | 44.2 | 47.1 | 40.3 | 47.9 | 50.1 | 40.7 | 45.05 | 4.000874904 | |
| 1.5 | 57.4 | 56.8 | 54.1 | 56.2 | 54.1 | 57.3 | 55.98333333 | 1.519758753 | 48.3 | 50.5 | 54.4 | 56.6 | 55.2 | 51.2 | 52.7 | 3.18747549 | |
| Number of neurons | |||||||||||||||||
| Distance from epicenter | SCI(DMSO) | Mean | SD | SCI(VX-765) | Mean | SD | |||||||||||
| -1.5 | 23 | 27 | 23 | 25 | 23 | 20 | 24.2 | 2.34520788 | 31 | 26 | 27 | 26 | 26 | 27 | 27.16666667 | 1.940790217 | |
| -1 | 18 | 24 | 19 | 19 | 15 | 15 | 19 | 3.326659987 | 25 | 20 | 22 | 23 | 23 | 23 | 22.66666667 | 1.632993162 | |
| -0.5 | 7 | 12 | 7 | 9 | 8 | 9 | 8.6 | 1.861898673 | 10 | 8 | 9 | 9 | 7 | 8 | 8.5 | 1.048808848 | |
| -0.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Niss | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0.5 | 5 | 10 | 7 | 6 | 8 | 7 | 7.2 | 1.722401424 | 7 | 8 | 6 | 11 | 10 | 9 | 8.5 | 1.870828693 | |
| 1 | 10 | 20 | 12 | 12 | 15 | 18 | 13.8 | 3.885871846 | 18 | 24 | 19 | 18 | 20 | 20 | 19.83333333 | 2.228601953 | |
| 1.5 | 22 | 22 | 23 | 20 | 21 | 22 | 21.6 | 1.032795559 | 28 | 29 | 28 | 26 | 27 | 28 | 27.66666667 | 1.032795559 | |
| Groups | mean | SD | |||||||||||||||
| Pre-injury | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 1 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 3 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 7 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| sham | 14 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | ||||
| 21 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 28 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 35 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 42 dpi | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| Pre-injury | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 1 dpi | 0.5 | 1.5 | 1.5 | 0 | 0.5 | 1 | 0.5 | 1.5 | 0 | 1 | 0.8 | 0.586893895 | |||||
| 3 dpi | 1.5 | 1.5 | 1.5 | 0.5 | 1 | 1.5 | 1 | 2 | 0.5 | 1.5 | 1.25 | 0.485912658 | |||||
| BMS | 7 dpi | 4.5 | 4 | 4 | 2 | 4 | 4.5 | 4 | 4.5 | 2 | 4 | 3.75 | 0.950146188 | ||||
| SCI(DMSO) | 14 dpi | 5.5 | 6 | 6 | 4.75 | 5 | 6 | 5 | 6 | 4 | 5 | 5.325 | 0.687689368 | ||||
| 21 dpi | 5.5 | 6.25 | 5.5 | 5.75 | 5.5 | 6.5 | 5 | 6.5 | 5 | 5.5 | 5.7 | 0.550252467 | |||||
| 28 dpi | 5.5 | 6.5 | 6 | 6 | 6 | 6.5 | 5.5 | 6.5 | 5.5 | 6 | 6 | 0.40824829 | |||||
| 35 dpi | 6 | 6.5 | 6.25 | 6 | 6 | 6.5 | 6 | 6.5 | 6 | 6 | 6.175 | 0.237170825 | |||||
| 42 dpi | 6 | 6.5 | 6.5 | 6 | 6 | 6.5 | 6 | 6.5 | 6 | 6 | 6.2 | 0.25819889 | |||||
| Pre-injury | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 9 | 0 | |||||
| 1 dpi | 0.5 | 1 | 0 | 1.5 | 0.5 | 0.5 | 1 | 1 | 0 | 1 | 0.7 | 0.483045892 | |||||
| 3 dpi | 1 | 1.5 | 1.5 | 1.5 | 1 | 1 | 1.5 | 1.5 | 1.5 | 1.5 | 1.35 | 0.241522946 | |||||
| 7 dpi | 3 | 3 | 5 | 4.75 | 3.5 | 4.5 | 4 | 5 | 4 | 4.5 | 4.125 | 0.756912589 | |||||
| SCI(VX765) | 14 dpi | 6 | 7 | 6 | 6 | 5.5 | 6 | 5.5 | 7 | 5.5 | 6 | 6.05 | 0.550252467 | ||||
| 21 dpi | 6.5 | 7.5 | 6.5 | 7 | 6.5 | 6.5 | 6 | 7 | 6 | 6.5 | 6.6 | 0.459468292 | |||||
| 28 dpi | 7 | 7.5 | 7 | 7 | 6.5 | 6.5 | 6 | 7 | 6 | 7 | 6.75 | 0.485912658 | |||||
| 35 dpi | 7 | 7.5 | 7 | 7 | 7 | 7 | 6.5 | 7 | 6 | 7 | 6.9 | 0.394405319 | |||||
| 42 dpi | 7 | 7.5 | 7 | 7 | 7 | 7 | 6.5 | 7 | 6 | 7 | 6.9 | 0.394405319 | |||||
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interests.
Financial support: The study was supported by the National Natural Science Foundation of China, Nos. 81772321, 82072416 (both to HZL). The funders had no roles in the study design, conduction of experiment, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Animal Care Ethics Committee of Bengbu Medical College (approval No. 2017-037) on February 23, 2017.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Sidharth Mehan, Indo Soviet Friendship College of Pharmacy, India.
P-Reviewer: Mehan S; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Gardner B, Yu J, Song LP; T-Editor: Jia Y
Funding: The study was supported by the National Natural Science Foundation of China, Nos. 81772321, 82072416 (both to HZL).
References
- 1.Aceves M, Terminel MN, Okoreeh A, Aceves AR, Gong YM, Polanco A, Sohrabji F, Hook MA. Morphine increases macrophages at the lesion site following spinal cord injury: Protective effects of minocycline. Brain Behav Immun. 2019;79:125–138. doi: 10.1016/j.bbi.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Adornetto A, Russo R, Parisi V. Neuroinflammation as a target for glaucoma therapy. Neural Regen Res. 2019;14:391–394. doi: 10.4103/1673-5374.245465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed A, Patil AA, Agrawal DK. Immunobiology of spinal cord injuries and potential therapeutic approaches. Mol Cell Biochem. 2018;441:181–189. doi: 10.1007/s11010-017-3184-9. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 5.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 6.Chai L, Lü JL, Hu JT, Hu HH, Xu QJ, Yu JW, Quan RF. Signal pathway variation after induction of inflammatory response in rats with acute spinal cord injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2021;25:1218–1223. [Google Scholar]
- 7.Chen AC, Tran HB, Xi Y, Yerkovich ST, Baines KJ, Pizzutto SJ, Carroll M, Robertson AAB, Cooper MA, Schroder K, Simpson JL, Gibson PG, Hodge G, Masters IB, Buntain HM, Petsky HL, Prime SJ, Chang AB, Hodge S, Upham JW. Multiple inflammasomes may regulate the interleukin-1-driven inflammation in protracted bacterial bronchitis. ERJ Open Res. 2018;4:00130–02017. doi: 10.1183/23120541.00130-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Chen YQ, Wang SN, Duan FX, Shi YJ, Ding SQ, Hu JG, Lü HZ. Effect of VX 765 on the transcriptome profile of mice spinal cords with acute injury. Mol Med Rep. 2020a;22:33–42. doi: 10.3892/mmr.2020.11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Yao L, Huang P, He Q, Guan H, Luo Y, Zou Z, Wei S, Peng G, Yan J, Chen R, Zhang Q, Tao A. Blockade of the NLRP3/Caspase-1 axis ameliorates airway neutrophilic inflammation in a toluene diisocyanate-induced murine asthma model. Toxicol Sci. 2019;170:462–475. doi: 10.1093/toxsci/kfz099. [DOI] [PubMed] [Google Scholar]
- 10.Chen YJ, Zhu H, Zhang N, Shen L, Wang R, Zhou JS, Hu JG, Lü HZ. Temporal kinetics of macrophage polarization in the injured rat spinal cord. J Neurosci Res. 2015;93:1526–1533. doi: 10.1002/jnr.23612. [DOI] [PubMed] [Google Scholar]
- 11.Chen YQ, Wang SN, Shi YJ, Chen J, Ding SQ, Tang J, Shen L, Wang R, Ding H, Hu JG, Lü HZ. CRID3, a blocker of apoptosis associated speck like protein containing a card, ameliorates murine spinal cord injury by improving local immune microenvironment. J Neuroinflammation. 2020b;17:255. doi: 10.1186/s12974-020-01937-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christgen S, Place DE, Kanneganti TD. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020;30:315–327. doi: 10.1038/s41422-020-0295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehlings MG, Wilson JR, Tetreault LA, Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Kwon BK, Marino RJ, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: Recommendations on the use of methylprednisolone sodium succinate. Global Spine J. 2017;7:203s–211s. doi: 10.1177/2192568217703085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flores J, Noël A, Foveau B, Beauchet O, LeBlanc AC. Pre-symptomatic Caspase-1 inhibitor delays cognitive decline in a mouse model of Alzheimer disease and aging. Nat Commun. 2020;11:4571. doi: 10.1038/s41467-020-18405-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores J, Noël A, Foveau B, Lynham J, Lecrux C, LeBlanc AC. Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun. 2018;9:3916. doi: 10.1038/s41467-018-06449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu KY, Tan YH, Sung B, Mao J. Peripheral formalin injection induces unique spinal cord microglial phenotypic changes. Neurosci Lett. 2009;449:234–239. doi: 10.1016/j.neulet.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galanopoulou AS, Mowrey WB, Liu W, Li Q, Shandra O, Moshé SL. Preclinical screening for treatments for infantile spasms in the multiple hit rat model of infantile spasms: An update. Neurochem Res. 2017;42:1949–1961. doi: 10.1007/s11064-017-2282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosselin A, Monteiro P, Chomont N, Diaz-Griffero F, Said EA, Fonseca S, Wacleche V, El-Far M, Boulassel MR, Routy JP, Sekaly RP, Ancuta P. Peripheral blood CCR4+CCR6+ and CXCR3+CCR6+CD4+ T cells are highly permissive to HIV-1 infection. J Immunol. 2010;184:1604–1616. doi: 10.4049/jimmunol.0903058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greaves DR, Gordon S. Macrophage-specific gene expression: current paradigms and future challenges. Int J Hematol. 2002;76:6–15. doi: 10.1007/BF02982713. [DOI] [PubMed] [Google Scholar]
- 20.Hayta E, Elden H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2018;87:25–31. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 22.Horiuchi H, Oshima Y, Ogata T, Morino T, Matsuda S, Miura H, Imamura T. Evaluation of injured axons using two-photon excited fluorescence microscopy after spinal cord contusion injury in YFP-H line mice. Int J Mol Sci. 2015;16:15785–15799. doi: 10.3390/ijms160715785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu JG, Shen L, Wang R, Wang QY, Zhang C, Xi J, Ma SF, Zhou JS, Lü HZ. Effects of Olig2-overexpressing neural stem cells and myelin basic protein-activated T cells on recovery from spinal cord injury. Neurotherapeutics. 2012;9:422–445. doi: 10.1007/s13311-011-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu JG, Shi LL, Chen YJ, Xie XM, Zhang N, Zhu AY, Jiang ZS, Feng YF, Zhang C, Xi J, Lü HZ. Differential effects of myelin basic protein-activated Th1 and Th2 cells on the local immune microenvironment of injured spinal cord. Exp Neurol. 2016a;277:190–201. doi: 10.1016/j.expneurol.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Liu X, Moisan J, Wang Y, Lesch CA, Spooner C, Morgan RW, Zawidzka EM, Mertz D, Bousley D, Majchrzak K, Kryczek I, Taylor C, Van Huis C, Skalitzky D, Hurd A, Aicher TD, Toogood PL, Glick GD, Paulos CM, et al. Synthetic RORγ agonists regulate multiple pathways to enhance antitumor immunity. Oncoimmunology. 2016b;5:e1254854. doi: 10.1080/2162402X.2016.1254854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc Res Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Jamilloux Y, Martinon F. Cell-free assay for inflammasome activation. Methods Mol Biol. 2016;1417:207–215. doi: 10.1007/978-1-4939-3566-6_14. [DOI] [PubMed] [Google Scholar]
- 28.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karsy M, Hawryluk G. Modern medical management of spinal cord injury. Curr Neurol Neurosci Rep. 2019;19:65. doi: 10.1007/s11910-019-0984-1. [DOI] [PubMed] [Google Scholar]
- 30.Kawahara Y, Kaneko T, Yoshinaga Y, Arita Y, Nakamura K, Koga C, Yoshimura A, Sakagami R. Effects of sulfonylureas on periodontopathic bacteria-induced inflammation. J Dent Res. 2020;99:830–838. doi: 10.1177/0022034520913250. [DOI] [PubMed] [Google Scholar]
- 31.Kitamura K, Nagoshi N, Tsuji O, Matsumoto M, Okano H, Nakamura M. Application of hepatocyte growth factor for acute spinal cord injury: The road from basic studies to human treatment. Int J Mol Sci. 2019;20:1054. doi: 10.3390/ijms20051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Dai Z, Cao Y, Wang L. Caspase-1 inhibition mediates neuroprotection in experimental stroke by polarizing M2 microglia/macrophage and suppressing NF-κB activation. Biochem Biophys Res Commun. 2019;513:479–485. doi: 10.1016/j.bbrc.2019.03.202. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Niu X, Xu H, Li Q, Meng L, He M, Zhang J, Zhang Z, Zhang Z. VX-765 attenuates atherosclerosis in ApoE deficient mice by modulating VSMCs pyroptosis. Exp Cell Res. 2020;389:111847. doi: 10.1016/j.yexcr.2020.111847. [DOI] [PubMed] [Google Scholar]
- 35.Lin YH, Wu Y, Wang Y, Yao ZF, Tang J, Wang R, Shen L, Ding SQ, Hu JG, Lü HZ. Spatio-temporal expression of Hexokinase-3 in the injured female rat spinal cords. Neurochem Int. 2018;113:23–33. doi: 10.1016/j.neuint.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Liu S, Xie YY, Wang B. Role and prospects of regenerative biomaterials in the repair of spinal cord injury. Neural Regen Res. 2019a;14:1352–1363. doi: 10.4103/1673-5374.253512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Yang Y, He L, Pang M, Luo C, Liu B, Rong L. High-dose methylprednisolone for acute traumatic spinal cord injury: A meta-analysis. Neurology. 2019b;93:e841–850. doi: 10.1212/WNL.0000000000007998. [DOI] [PubMed] [Google Scholar]
- 38.Ma SF, Chen YJ, Zhang JX, Shen L, Wang R, Zhou JS, Hu JG, Lü HZ. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Marchesan JT, Girnary MS, Moss K, Monaghan ET, Egnatz GJ, Jiao Y, Zhang S, Beck J, Swanson KV. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol 2000. 2020;82:93–114. doi: 10.1111/prd.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, Vezzani A. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin E, El-Behi M, Fontaine B, Delarasse C. Analysis of microglia and monocyte-derived macrophages from the central nervous system by flow cytometry. J Vis Exp. 2017:55781. doi: 10.3791/55781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 43.McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO, Lu JQ, Branton WG, Power C. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci U S A. 2018;115:E6065–6074. doi: 10.1073/pnas.1722041115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, Mathian A, Nakahata T, Yamaguchi T, Nomura T, Ono M, Amoura Z, Gorochov G, Sakaguchi S. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Mortezaee K, Khanlarkhani N, Beyer C, Zendedel A. Inflammasome: Its role in traumatic brain and spinal cord injury. J Cell Physiol. 2018;233:5160–5169. doi: 10.1002/jcp.26287. [DOI] [PubMed] [Google Scholar]
- 46.Nikitina IY, Panteleev AV, Kosmiadi GA, Serdyuk YV, Nenasheva TA, Nikolaev AA, Gorelova LA, Radaeva TV, Kiseleva YY, Bozhenko VK, Lyadova Th1, Th17, and Th1Th17 lymphocytes during tuberculosis: Th1 lymphocytes predominate and appear as low-differentiated CXCR3(+)CCR6(+) cells in the blood and highly differentiated CXCR3(+/-)CCR6(-) cells in the lungs. J Immunol. 2018;200:2090–2103. doi: 10.4049/jimmunol.1701424. [DOI] [PubMed] [Google Scholar]
- 47.Noe FM, Polascheck N, Frigerio F, Bankstahl M, Ravizza T, Marchini S, Beltrame L, Banderó CR, Löscher W, Vezzani A. Pharmacological blockade of IL-1β/IL-1 receptor type 1 axis during epileptogenesis provides neuroprotection in two rat models of temporal lobe epilepsy. Neurobiol Dis. 2013;59:183–193. doi: 10.1016/j.nbd.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 48.Popovich PG. Neuroimmunology of traumatic spinal cord injury: a brief history and overview. Exp Neurol. 2014;258:1–4. doi: 10.1016/j.expneurol.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 50.Rose-John S. Interleukin-6 family cytokines. Cold Spring Harb Perspect Biol. 2018;10:a028415. doi: 10.1101/cshperspect.a028415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic spinal cord injury: current concepts and treatment update. Arq Neuropsiquiatr. 2017;75:387–393. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 52.Rubiano AM, Carney N, Chesnut R, Puyana JC. Global neurotrauma research challenges and opportunities. Nature. 2015;527:S193–197. doi: 10.1038/nature16035. [DOI] [PubMed] [Google Scholar]
- 53.Sedgwick JD, Ford AL, Foulcher E, Airriess R. Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol. 1998;160:5320–5330. [PubMed] [Google Scholar]
- 54.Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stack JH, Beaumont K, Larsen PD, Straley KS, Henkel GW, Randle JC, Hoffman HM. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol. 2005;175:2630–2634. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 57.Sun X, Jones ZB, Chen XM, Zhou L, So KF, Ren Y. Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship. J Neuroinflammation. 2016;13:260. doi: 10.1186/s12974-016-0736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng YD, Mocchetti I, Wrathall JR. Basic and acidic fibroblast growth factors protect spinal motor neurones in vivo after experimental spinal cord injury. Eur J Neurosci. 1998;10:798–802. doi: 10.1046/j.1460-9568.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- 59.Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 60.Thompson CD, Zurko JC, Hanna BF, Hellenbrand DJ, Hanna A. The therapeutic role of interleukin-10 after spinal cord injury. J Neurotrauma. 2013;30:1311–1324. doi: 10.1089/neu.2012.2651. [DOI] [PubMed] [Google Scholar]
- 61.Wannamaker W, Davies R, Namchuk M, Pollard J, Ford P, Ku G, Decker C, Charifson P, Weber P, Germann UA, Kuida K, Randle JC. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3, 3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R, 3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther. 2007;321:509–516. doi: 10.1124/jpet.106.111344. [DOI] [PubMed] [Google Scholar]
- 62.Wu J, Zhao Z, Kumar A, Lipinski MM, Loane DJ, Stoica BA, Faden AI. Endoplasmic reticulum stress and disrupted neurogenesis in the brain are associated with cognitive impairment and depressive-like behavior after spinal cord injury. J Neurotrauma. 2016;33:1919–1935. doi: 10.1089/neu.2015.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y, Lin YH, Shi LL, Yao ZF, Xie XM, Jiang ZS, Tang J, Hu JG, Lü HZ. Temporal kinetics of CD8(+) CD28(+) and CD8(+) CD28(-) T lymphocytes in the injured rat spinal cord. J Neurosci Res. 2017;95:1666–1676. doi: 10.1002/jnr.23993. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y, Shen L, Wang R, Tang J, Ding SQ, Wang SN, Guo XY, Hu JG, Lü HZ. Increased ceruloplasmin expression caused by infiltrated leukocytes, activated microglia, and astrocytes in injured female rat spinal cords. J Neurosci Res. 2018;96:1265–1276. doi: 10.1002/jnr.24221. [DOI] [PubMed] [Google Scholar]
- 65.Yang XM, Downey JM, Cohen MV, Housley NA, Alvarez DF, Audia JP. The highly selective caspase-1 inhibitor VX-765 provides additive protection against myocardial infarction in rat hearts when combined with a platelet inhibitor. J Cardiovasc Pharmacol Ther. 2017;22:574–578. doi: 10.1177/1074248417702890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu N, Li X, Song W, Li D, Yu D, Zeng X, Li M, Leng X, Li X. CD4(+)CD25 (+)CD127 (low/-) T cells: a more specific Treg population in human peripheral blood. Inflammation. 2012;35:1773–1780. doi: 10.1007/s10753-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y, Zheng Y. Effects and mechanisms of potent caspase-1 inhibitor VX765 treatment on collagen-induced arthritis in mice. Clin Exp Rheumatol. 2016;34:111–118. [PubMed] [Google Scholar]
- 68.Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15–44. doi: 10.1007/978-94-017-9487-9_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.