Abstract
Objective
Mental illnesses may be caused by genetic and environmental factors. Recent studies reported that mental illnesses were accompanied by higher oxidative stress level. However, the results were inconsistent. Thus, present meta-analysis aimed to analyse the association between oxidative DNA damage indicated by 8-hydroxy-2’-deoxyguanosine (8-OHdG) or 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG), which has been widely used as biomarker of oxidative stress, and mental illnesses, including schizophrenia, bipolar disorder and depression. As oxidative DNA damage is caused by reactive oxygen species (ROS), systematic review and meta-analysis were also conducted to analyse the relationship between ROS and these three mental illnesses.
Methods
Studies from 1964 to 2020 (for oxidative DNA damage) and from 1907 to 2021 (for ROS) in Pubmed and Scopus databases were selected and analysed using Comprehensive Meta-Analysis version 2 respectively. Data were subjected to meta-analysis for examining the effect sizes of the results. Publication bias assessments, heterogeneity assessments and subgroup analyses based on biological specimens, patient status, illness duration and medication history were also conducted.
Results
This meta-analysis revealed that oxidative DNA damage was significantly higher in patients with schizophrenia and bipolar disorder based on random-effects models whereas in depressed patients, the level was not significant. Since heterogeneity was present, results based on random-effects model was preferred. Our results also showed that oxidative DNA damage level was significantly higher in lymphocyte and urine of patients with schizophrenia and bipolar disorder respectively. Besides, larger effect size was observed in inpatients and those with longer illness duration and medication history. Significant higher ROS was also observed in schizophrenic patients but not in depressive patients.
Conclusion
The present meta-analysis found that oxidative DNA damage was significantly higher in schizophrenia and bipolar disorder but not in depression. The significant association between deoxyguanosines and mental illnesses suggested the possibility of using 8-OHdG or 8-oxodG as biomarker in measurement of oxidative DNA damage and oxidative stress. Higher ROS level indicated the involvement of oxidative stress in schizophrenia. The information from this study may provide better understanding on pathophysiology of mental illnesses.
Keywords: Mental illness, Schizophrenia, Bipolar disorder, Depression, DNA damage, Meta-analysis, 8-OHdG
INTRODUCTION
Mental illnesses have been affecting 970 million of people all over the world in recent decades [1]. The prevalence (point estimate per 100,000) of severe mental illnesses such as schizophrenia, bipolar disorder and depression is 282, 594, and 3,627 respectively [2]. These disorders had been also listed as main contributors of burden of disability with the percentage of 2.8% in schizophrenia, 2.4% in bipolar disorder and 11.8% in depression [3]. However, the underlying pathophysiology of these mental illnesses remains unknown. Lately, rising evidence postulate the involvement of oxidative stress in developing mental illnesses as brain is vulnerable to oxidative stress due to its structures (i.e., rich with lipid) and functions (i.e., high consumption of oxygen, moderate antioxidant system, presence of reducing agents such as iron and copper, and presence of neurotransmitters with reduced capacity) [4-6].
Oxidative stress occurs when there is imbalance between oxidative and antioxidative systems, leading to the damage to cells, lipids, deoxyribonucleic acids (DNAs) or other components [7,8]. This imbalance occurs when reactive oxygen species (ROS) such as superoxide radical, hydroxyl radical, and hydrogen peroxide (H2O2) are overproduced. Due to shorter lifespan and higher reactivity, the direct measurement of ROS may not be accurate. Thus, indirect measurement of oxidative stress using parameter of oxidative damage becomes the substitution [9].
Oxidative DNA damage which is one of the consequences of oxidative stress usually occurs on guanine nucleobase as it is easier to be oxidised compared to others [10]. The excess ROS will hydroxylate guanine bases and form 8-hydroxyguanine (8-OHGua), producing 8-hydroxy-2’-deoxyguanosine (8-OHdG) through electron abstraction or 8-oxo-7,8-dihydro-2’-deoxyguanosine (8-oxodG) via keto-enol tautomerism of 8-OHdG. However, the terms of 8-OHdG and 8-oxodG are usually used as the same compound in most of the literatures. This production results in oxidative DNA damage, one of the main forms of DNA damage leading to DNA modification with altered functions [11,12]. Since these mutagenic deoxyguanosines can cross the cell membrane easily, they are more often referred as the indicator of oxidative damage [13] and further classified as a biomarker in early detection of various diseases [14].
Weakened antioxidative defence system including decreased antioxidants and antioxidant enhancing enzymes is involved in mental illnesses [15]. Besides, the association between higher level of oxidative stress markers with mental illnesses further suggests the involvement of oxidative stress in pathophysiology of mental illnesses [8,16,17]. Increased oxidants and decreased antioxidants have been reported in brain and peripheral tissues of patients [15]. Oxidative damage has been strongly associated with mental illnesses. Both genetic and environmental factors may cause defects in DNA repair system, thus causing the accumulation of DNA damage which will further lead to changes in neuronal structures and functions [11]. This relationship was also reported by Christensen et al. [18], in which 8-oxodG level was significantly higher in urine and cerebrospinal fluid of deceased patients with severe mental illnesses, thus indicating oxidative DNA damage was the major oxidative damage.
There are now several studies published that address oxidative DNA damage in patients with mental illnesses using various samples. Due to different methodological approaches and perspectives, these studies come to inconsistent conclusions. Hence, this present meta-analysis study aimed to analyse the association between oxidative DNA damage indicated by 8-OHdG or 8-oxodG level in patients with mental illnesses, particularly schizophrenia, bipolar disorder and depression. Furthermore, as oxidative DNA damage is caused by excess ROS, we also aimed to systematically review and analyse the association between ROS and each mental illness for further evidence of oxidative stress in each mental illness.
METHODS
Study search and selection
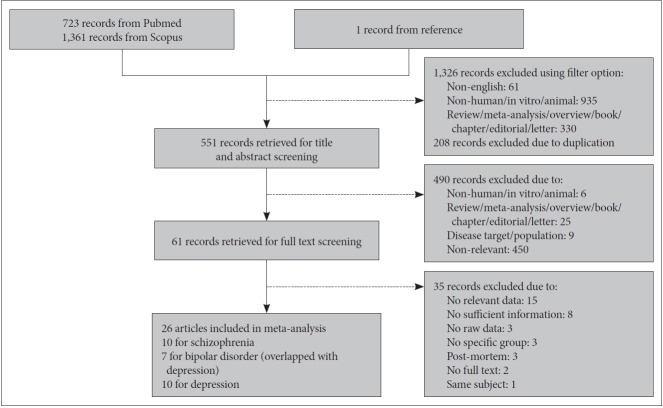
Databases of PubMed and Scopus were used to search for studies. For meta-analysis of oxidative DNA damage, studies published between 1964 and August 2020 were selected, using keywords of (“8-hydroxy-2’-deoxyguanosine” OR “8-OHdG” OR “oxidative damage” OR “DNA damage”) AND (“major mental illness” OR “schizophrenia” OR “bipolar disorder” OR “depression” OR “depressive disorder”). Meanwhile for systematic review and meta-analysis of ROS, keywords of (“reactive oxygen species” OR “ROS” OR “hydrogen peroxide” OR “superoxide radical” OR “hydroxyl radical”) AND (“major mental illness” OR “schizophrenia” OR “bipolar disorder” OR “depression” OR “depressive disorder”) were used to search for studies published between 1907 and March 2021. Articles of non-English language, non-human subjects such as in vitro or animal studies, and non-research such as review, meta-analysis, overview, book, chapter, editorial or letter were excluded using filter option of database. The studies met the following inclusion criteria were selected: 1) articles were published in peer-reviewed journal, 2) diagnostic methods were reported, 3) both cohort and healthy subjects were included, 4) level of 8-OHdG or 8-oxodG or ROS in living subjects was presented, and 5) enough data to calculate effect size were provided (for meta-analysis only). The flow diagrams of study selection for oxidative DNA damage and ROS are shown in Figures 1 and 2 respectively.
Figure 1.
Flow diagram of study selection for meta-analysis of oxidative DNA damage.
Figure 2.
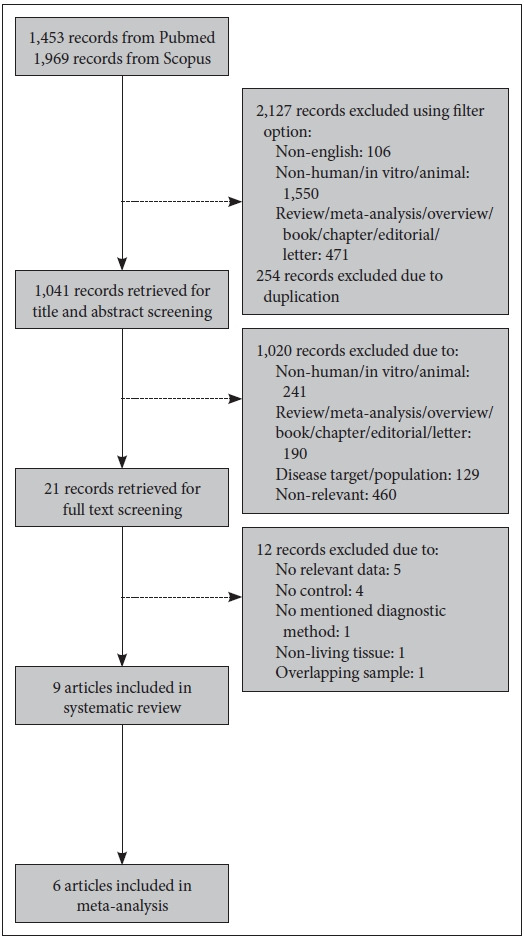
Flow diagram of study selection for systematic review and meta-analysis of reactive oxygen species (ROS).
Data extraction and analysis
First author’s name, publication year, demographic characteristics (age and gender), diagnostic method, disease type, patient status, illness duration, medication and specimen were extracted from each study. The mean and standard deviation or other data forms of 8-OHdG or 8-oxodG and ROS levels were extracted to calculate each effect size respectively. The characteristics of studies included in analysis of oxidative DNA damage and ROS are shown in Tables 1 and 2 respectively.
Table 1.
Study characteristics of meta-analysis of oxidative DNA damage
| Author (year) | Sample size (N) |
Mean age (SD) (years) |
Gender (male/female) |
Diagnostic method | Disease type | Patient status | Illness duration (years/months) | Medication | Specimen | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Control | Patient | Control | Patient | Control | |||||||
| Chestkov et al. [23] (2018) | 55 | 30 | 38(13) | 37(12) | 55 males | 30 males | ICD-10; DSM-IV | Paranoid | Outpatients | <3 years (N=24); 3–10 years (N=20); >10 years (N=66) | No medication | Lymphocyte |
| Ershova et al. [24] (2017) | 69 | 30 | Paranoid=41.5 (10.2); schizophreniform psychotic disorder= 41.8 (10.3) | 41 (12) | 69 males | 30 males | ICD-10 | Paranoid (N=58); schizophreniform psychotic disorder (N=11) | Inpatients | 1–3 years (N=20); 3–10 years (N=15); 10–20 years (N=23) | No medication for at least one week prior to hospitalization | Lymphocyte/plasma |
| Shmarina et al. [25] (2020) | 40 | 25 | 40.4 (9.8) | 18-63 | 40 males | 25 males | ICD-10; DSM-IV | First episode of paranoid | Inpatients | NA | No appropriate treatment | Mononuclear cells |
| Jorgensen et al. [26] (2013) | 40 | 40 | 33.0 (10.7) | 31.4 (9.8) | 20/20 | 20/20 | ICD-10 | Schizophrenia | Inpatients (60%) & outpatients | 76 (36–189) months | 40 patients on second-generation antipsychotics | Urine |
| Nordholm et al. [27] (2016) | 35 | 30 | 23.3 (5.6) | 24.7 (4.9) | 18 males | 19 males | ICD-10 | First-episode schizophrenia | Outpatients | NA | Antipsychotics naïve | Urine |
| Ibrahim et al. [28] (2020) | 150 | 150 | Chronic schizophrenia with acute ischemic stroke=53.1 (3.9); chronic schizophrenia without acute ischemic stroke=51.6 (3.99) | 51.5 (3.76) | 150 males | 150 males | DSM-IV | Chronic schizophrenia with acute ischemic stroke (N=150); chronic schizophrenia without acute ischemic stroke (N=150) | Inpatients | Chronic schizophrenia with acute ischemic stroke=13.8 (1.66) years; chronic schizophrenia without acute ischemic stroke=14.5 (2) years | Patients on antipsychotics | Serum |
| Ma et al. [29] (2020) | 44 | 45 | <25=4; 25–29=10; 30–34=10; ≥35=20 | <25=4; 25–29=18; 30–34=10; ≥35=13 | 15/29 | 9/36 | ICD-10 | Schizophrenia | NA | 100.9 (92.8) months | 18 drug naïve; 26 medicated | Serum |
| Copoglu et al. [30] (2015) | 64 | 80 | Symptomatic remission=34.69 (7.69); without symptomatic remission=35.61 (10.2) | NA | 42/22 | NA | DSM-IV | Symptomatic remission (N=26); without symptomatic remission (N=38) | Outpatients | Symptomatic remission=12.08 (8.70); without symptomatic remission=11.84 (8.98) | All patients on antipsychotics | Serum |
| Şimşek et al. [31] (2016) | 20 | 20 | 14.5 (1.6) | 14.4 (1.5) | 8/12 | 8/12 | DSM-IV | First-episode psychosis | NA | 13.1 (14.3) months | Antipsychotics naïve | Serum |
| Miyaoka et al. [32] (2015) | 29 | 30 | 47.76 (12.50) | 42.43 (14.42) | 13/16 | 11/19 | DSM-IV | Chronic schizophrenia | Inpatients & outpatients | 17.76 (10.25) years | 25 on antipsychotics; 4 no medication | Urine |
| Jacoby et al. [33] (2016) | 148 | 70 | 41.9 (11.9) | 36.7 (11.6) | 32/22 | 20/15 | ICD-10 | Euthymic (N=77); depressive state (N=18); manic state (N=43); mixed state (N=10) | Inpatients & outpatients | 15 (11.0) years | Patients on lithium, anticonvulsants, antipsychotics, selective serotonin reuptake inhibitor (SSRI) | Urine |
| Munkholm et al. [34] (2015) | 168 | 80 | 40.9 (12.3) | 36.3 (12.5) | 25/12 | 23/17 | DSM-IV | Euthymic (N=75); depressive state (N=63); manic state (N=24); mixed state (N=6) | Inpatients & outpatients | 21.2 (13.0) years | Patients on lithium, anticonvulsants, antipsychotics, SSRI | Urine |
| Knorr et al. [35] (2019) | 86 | 44 | 33 (25–42) | 31 (24–41) | 86 females | 44 females | ICD-10; DSM-IV | 49 bipolar type I; 37 bipolar type II | Outpatients | 12.4 (9.8) years | Patients on antipsychotics, anticonvulsants, antidepressants, benzodiazepines | Urine |
| Soeiro-de- Souza et al. [36] (2013) | 50 | 50 | 26.8 (4.5) | 26.0 (4.0) | 17/33 | 25/25 | DSM-IV | Bipolar type I 26 in mania 24 in depressive | NA | 5.0 (3.7) years | Pre-treatment | Plasma |
| Tsai and Huang[37] (2015) | 23 | 40 | 41.3 (12.0) | 30.4 (5.0) | 13/10 | 20/20 | DSM-IV | Bipolar type I (manic phase) | Inpatients | NA | Pre-treatment | Serum |
| Huzayyin et al. [38] (2014) | 14 | 16 | 44.7 (10.9) | 45.8 (13.5) | 8/6 | 10/6 | DSM-IV | 10 bipolar type I; 4 bipolar type II | NA | NA | Lithium treatment | Lymphocyte |
| Ceylan et al. [39] (2020) | 24 | 61 | 32.88 (7.80) | 27.92 (8.21) | 70.8% females | 61.3% females | DSM-IV | Bipolar depression | Outpatients | NA | 26% were drug free. Some patients on antidepressants, stabilizers, antipsychotics | Urine |
| Yi et al. [40] (2012) | 179 | 332 | Male=43.4 (10.3); female=41.5 (10.0) | Male=44.4 (11.1); female=40.4 (10.7) | 105/74 | 196/136 | CES-D | Depressive symptoms | Outpatients | NA | NA | Urine |
| Black et al. [41] (2017) | 107 | 562 | Remitted major depressive disorder=44.4 (12.9); current major depressive disorder=41.2 (12.4) | 41.1 (14.8) | Remitted major depressive disorder=69.7% females; current major depressive disorder=67.1% females | 60.9% females | CIDI | Remitted major depressive disorder (N=107); current major depressive disorder (N=316) | Outpatients | NA | Majority patients (remitted=86.6%; current=61.6%) no medication. Some on antidepressants, selective serotonin reuptake inhibitor (SSRI), tricyclic antidepressant (TCA) | Plasma |
| van Velzen et al. [42] (2017) | 66 | 231 | 37.73 (10.06) | NA | 33% males | NA | DSM-IV | Major depressive disorder and/or anxiety | Outpatients | NA | 22% on SSRI | Plasma |
| Lindqvist et al. [43] (2017) | 49 | 55 | 39.6 (14.7) | 37.6 (13.9) | 54% females | 60% females | DSM-IV | Major depressive disorder | Outpatients | NA | Pre-treatment | Plasma |
| Forlenza and Miller [44] (2006) | 84 | 85 | 28.7 (9.0) | 28.9 (8.9) | 16/68 | 16/69 | DSM-IV | Depression | Outpatients | NA | No medication | Serum |
| Tsai and Huang [45] (2016) | 21 | 40 | 49.6 (7.0) | 33.0 (5.7) | 4/17 | 10/30 | DSM-IV | Major depressive disorder | Inpatients | NA | NA | Serum |
| Wei et al. [46] (2009) | 52 | 30 | 55.95 (13.90) | NA | 1.26 (0.46) male/female ratio | NA | HAMD, SDS, SAS, SCL-90 | Colorectal adenocarcinoma with depression | NA | NA | Pre-treatment | Serum |
| Wei et al. [47] (2009) | 63 | 63 | 54.94 (14.67) | NA | 1.33 (0.50) male/female ratio | NA | HAMD, SDS, SAS, SCL-90 | Gastric adenocarcinoma with depression | NA | NA | Pre-treatment | Serum |
| Liu et al. [48] (2014) | 17 | 46 | 54.71 (8.10) | 51.22 (8.32) | 10/7 | 19/27 | HADS-D screening | Type II diabetes mellitus with depression | NA | NA | NA | Leukocyte DNA |
ICD: International Classification of Diseases, DSM: Diagnostic and Statistical Manual of Mental Disorders, CES-D: Center for Epidemiological Studies-Depression Scale, HAMD: Hamilton Depression Rating Scale, SDS: Self-Rating Depression Scale, SAS: Self-Rating Anxiety Scale, SCL-90: Symptom Checklist-90, HADS-D: Hospital Anxiety and Depression Scale, SSRI: selective serotonin reuptake inhibitor, TCA: tricyclic antidepressant, DNA: deoxyribonucleic acid, N: number of patients, SD: standard deviation, NA: not available
Table 2.
Study characteristics and outcomes of systematic review and meta-analysis of ROS
| Author (year) | Sample size (N) |
Mean age (SD) (years) |
Gender (male/female) |
Diagnostic method | Disease type | Patient status | Illness duration (years/months) | Medication | Specimen (parameter) | Study outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Control | Patient | Control | Patient | Control | ||||||||
| Dietrich-Muszalska et al. [49] (2005) | 31 | 14 | 18–36 | NA | NA | NA | DSM-IV | Paranoid schizophrenia | Inpatients | 4 years | Patients were treated with atypical antipsychotics (risperidone, quetiapine, olanzapine) | Platelets (ROS: superoxide anion, H2O2, singlet oxygen, organic radicals) | ROS level in resting platelets was significantly higher in patients compared to healthy controls (p<0.05). After stimulated with thrombin, platelets from patients (increased 53%) showed decreased chemiluminescence compared to healthy controls (increased 101.5%). |
| Dietrich-Muszalska and Olas [50] (2009) | 35 | 25 | 31.4 (7.3) | 31.0 (6.8) | 21/14 | 15/10 | DSM-IV | Paranoid schizophrenia | Inpatients | 8.9 (4.5) years | Patients were treated with atypical antipsychotics (clozapine, risperidone, olanzapine, quetiapine) | Superoxide anion radicals level was lower in patients compared to healthy controls (p=0.05). They suggested that the reduced level could be due to the formation of RNS. | Superoxide anion radicals level was lower in patients compared to healthy controls (p=0.05). They suggested that the reduced level could be due to the formation of RNS. |
| Melamed et al. [51] (1998) | 29 | 17 | 39.1 (6.3) | 39.4 (6.1) | 19/10 | 9/8 | DSM-III | Schizophrenia | NA | 9.5 (2.4) years | The patients did not take antipsychotics prior to hospitalization | Superoxide anion production was significantly higher (with 34% more production) in patients in comparison to healthy controls (p=0.003). | Superoxide anion production was significantly higher (with 34% more production) in patients in comparison to healthy controls (p=0.003). |
| Sirota et al. [52] (2003) | 18 | 15 | 36.2 (5.8) | 36.8 (5.6) | 15/3 | 12/3 | DSM-IV | Schizophrenia | NA | 11 (9) years | The patients had not taken anti-psychotic medications prior to hospitalization for at least 3 months | Superoxide anion production was significantly higher in patients compared to healthy controls (p=0.012). | Superoxide anion production was significantly higher in patients compared to healthy controls (p=0.012). |
| Szuster-Ciesielska et al. [53] (2008) | 29 | 30 | 48.2 (11.2) | 41.3 (4.4) | 14/15 | 15/15 | DSM-IV | Unipolar depression & bipolar depression | NA | NA | Most of the patients had not taken medication for 3–7 days before the examination; 4 patients received different combinations of amitriptyline, sulpiride, clomipramine, perazine, levomepromazine, or imipramine | Superoxide anion (p<0.05) and H2O2 (p<0.05) productions were significantly higher in patients compared to healthy controls. | Superoxide anion (p<0.05) and H2O2 (p<0.05) productions were significantly higher in patients compared to healthy controls. |
| Alcocer-Gómez et al. [54] (2014) | 40 | 20 | Non-treated=54.4 (10.1); amitriptyline=53.5 (9.8) | 57 (3) | Non-treated=2/18; amitriptyline=1/19 | 2/18 | DSM-IV | Melancholic depression | Outpatients | NA | 20 patients were without treatment; 20 were treated with amitriptyline for 12.1 (2.3) months | Peripheral blood mononuclear cells (mitochondrial superoxide) | Mitochondrial superoxide production was significantly higher in unmedicated patients in comparison to healthy controls (p<0.001). Significantly increased production was also observed in patients treated with amitriptyline (p<0.05 between unmedicated and medicated patients; p<0.001 between medicated patients and healthy controls). |
| Atanackovic et al. [55] (2004) | 10 | 11 | 33.0 (7.4) | 28.0 (4.2) | 2/8 | 4/7 | ICD-10 | Minor depression | Inpatients | NA | Patients were on psychotherapy but not on psychopharmacological treatment | Phagocytes (ROS) | No significant difference in ROS production was observed between patients and healthy controls. |
| Chang et al. [56] (2015) | 35 | 35 | 39.14 (10.23) | 39.37 (8.5) | 13/22 | 11/24 | DSM-IV | First-episode major depressive disorder | Outpatients | NA | Patients were under sertraline treatment for 12 weeks | Blood (superoxide radicals and hydroxyl radicals) | No significant differences in superoxide radicals (p=0.38) and hydroxyl radicals (p=0.26) levels were observed between patients before treatment of sertraline and healthy controls. However, both levels were significantly decreased after treatment of sertraline for 12 weeks (p<0.001). |
| Rybka et al. [57] (2013) | 15 | 19 | 59.7 (1.91) | 62.3 (2.84) | NA | NA | ICD-10 | Recurrent depressive disorder | NA | NA | 60% were treated with selective serotonin reuptake inhibitor (SSRIs), 35% with serotonin-norepinephrine reuptake inhibitor (SNRIs), and 15% with tricyclic antidepressant (TCAs), either as monotherapies or in various combinations | Plasma (H2O2) | Plasma H2O2 concentration was significantly higher in patients in comparison to healthy controls (p=0.005). |
DSM: Diagnostic and Statistical Manual of Mental Disorders, ICD: International Classification of Diseases, ROS: reactive oxygen species, H2O2: hydrogen peroxide, N: number of patients, SD: standard deviation
Comparison of 8-OHdG or 8-oxodG and ROS levels between patients with mental illnesses (schizophrenia, bipolar disorder and depression respectively) and healthy controls were analysed in present study using Comprehensive Meta-Analysis (CMA) version 2 (Biostat, Englewood, NJ, USA). Effect sizes (Hedges’ g) and 95% confidence intervals were pooled using fixedeffect and random-effects models. A random-effects model was preferred when high heterogeneity was present [19], whereas a fixed-effect model was preferred for analysing small number of studies [20]. A p-value of less than 0.05 (p<0.05) was considered significant. The heterogeneity was tested using Q statistic value and I-squared (I2), in which I2 ≤25%, 25%<I2 ≤50% and high I2>50% indicating low, moderate and high heterogeneity respectively. Publication bias was checked using Egger’s test [21] and it was adjusted using trim-and-fill test [22] if bias was present. Besides, subgroup analyses were performed in order to examine the 8-OHdG or 8-oxodG level according to specimens (lymphocyte, plasma/serum and urine), patient status (inpatients and outpatients), illness duration (less than 10 years and below, more than 10 years) and medication (no medication and on medication) in each illness, with minimum of two studies for each category. Subgroup analysis was also performed to examine ROS level according to medication history.
RESULTS
Meta-analysis of oxidative DNA damage
Oxidative DNA damage in schizophrenia
After screening, 10 studies with total of 1,026 subjects (546 patients and 480 controls) were included in the schizophrenic group. The 8-OHdG or 8-oxodG level was significantly higher (random-effects, Hedges’s g=1.310, 95% CI=0.491 to 2.130, p=0.002) in schizophrenic patients compared to healthy controls (Figure 3A) with the heterogeneity I2 of 97.7% [Q=609.08; df(Q)=14; p<0.001]. Publication bias tested using Egger’s test was not significant (p=0.967).
Figure 3.
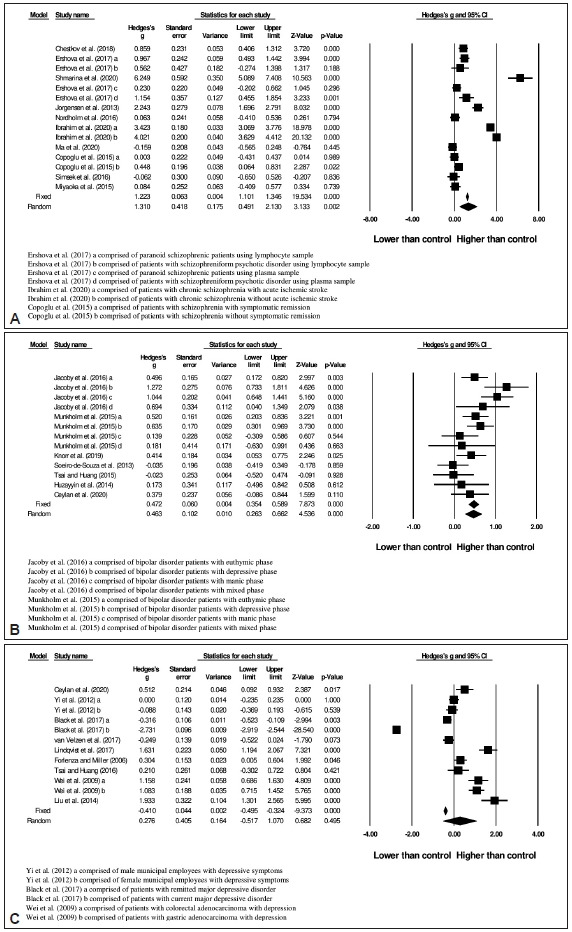
Meta-analysis of oxidative DNA damage (8-OHdG or 8-oxodG) in (A) schizophrenia, (B) bipolar disorder, and (C) depression.[23-48]
Oxidative DNA damage in bipolar disorder
The literature search produced 7 studies with total subjects of 874, consisting of 513 patients and 361 controls. The oxidative DNA damage level was significantly increased (randomeffects, Hedges’s g=0.463, 95% CI=0.263 to 0.622, p<0.001) in patients with bipolar disorder (Figure 3B). The heterogeneity I2 was 62.6% [Q=32.08; df(Q)=12; p=0.001]. In addition, there was no evidence of publication bias (p=0.771).
Oxidative DNA damage in depression
A total of 10 studies were included in present analysis, with 2,176 total subjects (671 patients and 1,505 controls). Mean effect size (Hedges’ g) on oxidative DNA damage was -0.410 (95% CI=-0.495 to -0.324) in the fixed-effects model with no significant difference in the random-effects model (Figure 3C). Due to high heterogeneity [I2 =98.8%, Q=895.45; df(Q)=11; p<0.001], the random-effects model was chosen. Publication bias was significant (p=0.007). The funnel plot was asymmetric, and it was suggested that 5 studies were missing at the left side using Trim-and-Fill test (Figure 4). After adjustment, the imputed point estimate was -0.658 (95% CI=-0.737 to 0.579) based on the fixed-effect model whereas the imputed point estimate was -0.414 (95% CI=-1.088 to 0.260) based on the random-effects model.
Figure 4.
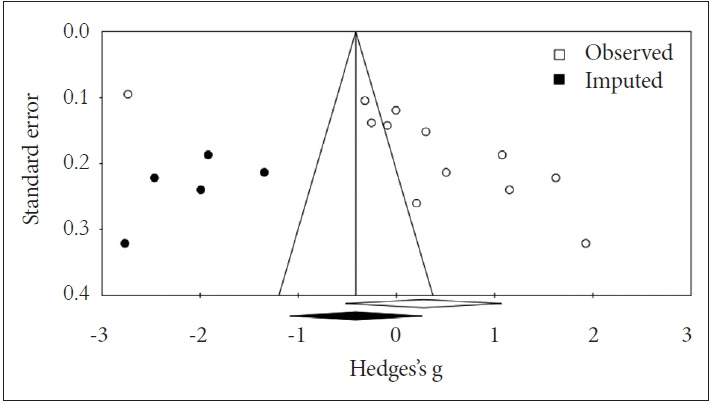
Funnel plot of oxidative DNA damage in depression (random-effects model).
Subgroup analyses
The results of all subgroup analyses were summarised in Table 3.
Table 3.
Subgroup analyses for oxidative DNA damage in each illness
| Subgroup | Category | Effect model | Schizophrenia |
Bipolar Disorder |
Depression |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Hedges’ g (95% CI) | p-value | I2 (%) | Number of studies | Hedges’ g (95% CI) | p-value | I2 (%) | Number of studies | Hedges’ g (95% CI) | p-value | I2 (%) | |||
| Specimen | Lymphocyte | Random | 3 | 2.073 (0.441 to 3.704) | 0.013* | 96.2 | - | - | - | - | - | - | - | - |
| Plasma/serum | Random | 5 | 1.136 (-0.127 to 2.399) | 0.078 | 98.5 | 2 | -0.030 (-0.440 to 0.381) | 0.887 | 0 | 7 | 0.128 (-0.864 to 1.120) | 0.801 | 99.1 | |
| Urine | Random | 3 | 0.791 (-0.564 to 2.146) | 0.253 | 95.4 | 4 | 0.581 (0.397 to 0.764) | <0.001* | 51.1 | 2 | 0.140 (-1.478 to 1.757) | 0.866 | 65.2 | |
| Patient status | Inpatients | Random | 4 | 2.312 (1.341 to 3.283) | <0.001* | 97.7 | 3 | 0.564 (0.332 to 0.796) | <0.001* | 64.5 | - | - | - | - |
| Outpatients | Random | 3 | 0.343 (-1.008 to 1.695) | 0.619 | 66.6 | 2 | 0.398 (-0.072 to 0.868) | 0.097 | 0 | 6 | -0.124 (-1.070 to 0.822) | 0.798 | 99.0 | |
| Duration of illness | 10 years and below | Random | 5 | 0.723 (-0.312 to 1.758) | 0.171 | 88.6 | - | - | - | - | - | - | - | - |
| More than 10 years | Random | 3 | 1.601 (0.304 to 2.899) | 0.016* | 98.9 | 3 | 0.603 (0.393 to 0.813) | <0.001* | 54.7 | - | - | - | - | |
| Medication history | No medication | Random | 4 | 0.538 (-0.628 to 1.703) | 0.366 | 66.3 | 2 | -0.030 (-0.440 to 0.381) | 0.887 | 0 | 6 | 0.119 (-1.102 to 1.339) | 0.849 | 99.2 |
| On medication | Random | 6 | 1.982 (0.893 to 3.071) | <0.001* | 98.6 | 5 | 0.559 (0.380 to 0.737) | <0.001* | 49.3 | - | - | - | - | |
p<0.05 indicating significant result.
CI: confidence interval, I2: I-square
Biological specimens
Oxidative DNA damage levels in lymphocyte, plasma/serum and urine were analysed as there were minimum of two studies for each specimen. Due to expected heterogeneity, a random-effects model was adopted for subgroup analyses. It can be seen that level of 8-OHdG or 8-oxodG was significantly increased in lymphocyte (p=0.013) of schizophrenic patients. Meanwhile in bipolar disorder, significant higher oxidative DNA damage level was observed in urine specimen only (p<0.001). In depressed patients, no significant changes of 8-OHdG or 8-oxodG level were detected in their plasma/serum or urine.
Patient status
Based on this subgroup analysis, significant oxidative DNA damage occurred only in the inpatients with schizophrenia and bipolar disorder (p<0.001 for both). There was no subgroup analysis for inpatients of depression as there was only one study.
Illness duration
Patients with schizophrenia (p=0.016) and bipolar disorder (p<0.001) for more than 10 years showed significantly larger effect size in oxidative DNA damage. No subgroup analysis was carried out for bipolar disorder patients with illness duration of 10 years and below as there was only one study.
Medication history
Schizophrenia patients, as well as bipolar disorder patients, who received medication showed significantly higher (p<0.001) oxidative DNA damage. No significant association was found in those without medication. In this subgroup analysis, only unmedicated depressed patients were included, as there was only one study that reported medicated patients.
Systematic review and meta-analysis of ROS
A total of 9 articles were included in present systematic review after screening. As 3 articles were not providing raw data [49,50,54], thus only 6 articles were included in meta-analysis. The study outcomes are shown in Table 2.
In schizophrenia, ROS was significantly higher in patients in comparison to healthy controls (fixed-effect, Hedges’s g=0.712, 95% CI=0.269 to 1.154, p=0.002) (Figure 5A). As heterogeneity was low, thus fixed-effect model was chosen [I2 =16.2%, Q=1.193; df(Q)=1; p=0.275].
Figure 5.
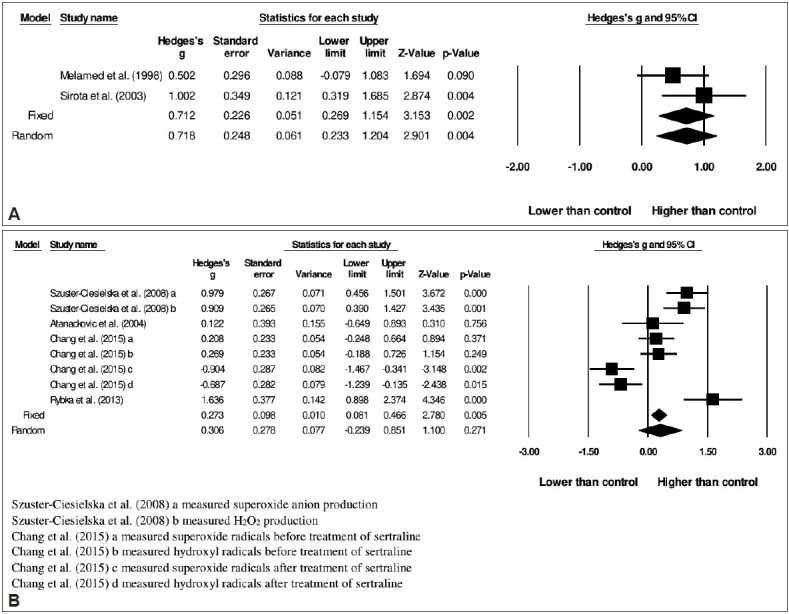
Meta-analysis of reactive oxygen species in (A) schizophrenia and (B) depression.[49-57]
In depression, ROS was not significantly different between patients compared to healthy controls (random-effects, Hedges’s g=0.306, 95% CI=-0.239 to 0.851, p=0.271) (Figure 5B). The heterogeneity I2 was 87.2% [Q=54.527; df(Q)=7; p<0.001]. Publication bias tested using Egger’s test was not significant (p=0.751).
As schizophrenia group was only comprised of unmedicated patients, thus subgroup analysis based on medication history was only conducted in depression group (Table 4). However, no significant difference was observed in both medicated and unmedicated depressive patients.
Table 4.
Subgroup analyses for reactive oxygen species in depression
| Subgroup | Category | Effect model | Depression |
|||
|---|---|---|---|---|---|---|
| Number of studies | Hedges’ g (95% CI) | p-value | I2 (%) | |||
| Medication history | No medication | Random | 3 | 0.504 (-0.143 to 1.152) | 0.127 | 56.0 |
| On medication | Random | 2 | -0.040 (-0.892 to 0.812) | 0.926 | 93.9 | |
CI: confidence interval, I2: I-square
DISCUSSION
The present meta-analysis found enhanced oxidative DNA damage indicated by 8-OHdG or 8-oxodG level in both schizophrenia and bipolar disorder patients. Our results were in agreement with the findings of previous meta-analyses on oxidative stress [8,16,58-60]. To the best of our knowledge, this is the first meta-analysis study of the association between 8-OHdG or 8-oxodG level with schizophrenia. Redox imbalances, oxidative stress and impaired DNA damage repair, which may initiate cellular stress responses, have been observed in schizophrenia patients [30,61], especially those with a family history of schizophrenia [62]. This high neuronal oxidative stress may cause poor prognosis by disturbing metabolic activity, gene expression and cellular dysfunction. Besides, association between oxidative DNA damage and schizophrenia was also supported by previous post-mortem finding, that increased 8-OHdG was found in neuronal cytoplasm in hippocampus of elderly schizophrenic patients with poorer outcome [63].
Bipolar disorder is the mental illness characterised by periodic episodes of mania (bipolar type I) or hypomania (bipolar type II) and depression [64]. The symptom severity was found to be correlated with DNA damage [65]. In a twin-case report, higher level of oxidative stress markers which may lead to lipid and protein oxidation with consequence of increased DNA damage was observed [66]. Increased DNA damage was also found in twins as a trait because the lesion remained higher in comparison to healthy controls even after treatment of mood stabilization. Previous meta-analysis [16] had first reported a larger effect size of DNA and RNA damage than our current study, due to their inclusion of both peripheral blood samples and post-mortem brain samples as well as measurements other than 8-OHdG or 8-oxodG. Post-mortem studies also demonstrated the involvement of DNA damage in bipolar disorder, particularly DNA fragmentation in anterior cingulate cortex [67] as well as in frontal cortex, pons, medulla, and thalamus [68]. From our result, it can be seen that the effect size of bipolar disorder was smaller than that of schizophrenia. This could be due to the occurrence of peripheral oxidative imbalance in the active phases of bipolar disorder only [69].
It has been hypothesized that stressful life events leading to oxidative stress that involved in the pathophysiology of depression [70,71]. Previous meta-analyses found that increased oxidative stress and decreased antioxidant level were associated with greater depressive symptoms [17,72,73]. As we discussed before, high oxidative stress level is associating with oxidative DNA damage. However, no association between oxidative DNA damage with depression was found in present study according to random-effects model. It contrasted with the findings of previous meta-analysis study [74]. This may be due to the different inclusion criteria used. In previous study, bipolar disorder, major depressive disorder and depression were classified as the same category, whereas we excluded bipolar disorder in the present analysis. Among the patients with major depressive disorder, the antidepressant users contrarily showed lower oxidative DNA damage. However, the precise roles of antidepressants in anti-inflammation and antioxidative action are not fully understood [41].
Due to the presence of heterogeneity and the wide distribution of data in forest plot, results obtained with random-effects model were preferred. High heterogeneity found in the present analysis may be due to several reasons such as clinical differences and single study designs [75]. The different effect sizes found in subgroup analyses may also indicate the heterogeneity. On the other hand, since publication bias was not significant in the analyses of schizophrenia and bipolar disorder, thus it was not the reason causing high heterogeneity. However, significant publication bias may contribute certain extents to high heterogeneity in the analysis of depression. This publication bias may be due to the design of single studies, submission of results by researchers, rejection of negative studies by journals, source funding of studies and design of reviews or meta-analyses [76]. The adjustment using Trim-and-Fill test showed that studies were missing at the left side of funnel plot. Thus, this significant publication bias might increase awareness in future to publish any results especially negative results (no effect or opposite effects) which are failed to prove their hypotheses [77].
Subgroup analyses were performed to determine the oxidative DNA damage in different biological specimens, patient status, illness duration and medication history for each mental illness. We found significant oxidative DNA damage in lymphocyte of schizophrenia patients and in urine of bipolar disorder patients. It may reflect the possibility of different mechanisms of oxidative DNA damage in each mental illness. As the major product of oxidative DNA damage, 8-OHdG or 8-oxodG will be released into bloodstream after being repaired and then be excreted by kidneys into urine. Hence, the plasma level of these deoxyguanosines may indicate how much oxidative stress has been exposed. It may represent the balance between DNA repair and clearance into urine within a period of time. However, some claimed that non-invasive urinary 8-oxodG originated from DNA of various tissues is better alternative in reflecting general oxidative stress as it is not dependent on changes of DNA repair and plasma clearance [78,79]. On the other hand, peripheral lymphocytes are also being suggested in reflecting overall oxidative stress status of a whole organism because of their circulation in the body [80]. Since many are questioning about the most favourable body samples to be selected in determining the level of deoxyguanosines [81], our findings may help future studies in choosing specific samples for each mental illness.
In this study, only inpatients of schizophrenia and bipolar disorder had significant oxidative DNA damage whereas no significant association was found in depression. The higher oxidative DNA damage among inpatients was accordance with the previous meta-analysis [59] that chronic schizophrenia inpatients had lower antioxidants and higher oxidants whereas higher concentration of antioxidants was found in the stable medicated outpatients even though their lipid peroxidation was high. It was also suggested that longer illness duration might have cumulative effect in oxidative stress [82-84]. Our subgroup analyses also showed that higher oxidative DNA damage was significantly evident in both schizophrenic and bipolar disorder patients with illness duration of more than 10 years. Interestingly, medicated schizophrenic and bipolar disorder patients showed more oxidative DNA damage. This is evidenced by the role of clozapine in increasing oxidative stress and DNA damage due to long-term use of high doses, accompanied with reduced glutathione (GSH) level and glutathione peroxidase (GSH-Px) activity that function as ROS scavengers [85]. In addition, N-methyl-D-aspartate receptor (NMDAR) hypofunction may be inversely associated with oxidative stress in pathophysiology of schizophrenia. To enhance NMDAR efficacy, the inhibition of D-amino acid oxidase (DAAO) activity was suggested [86]. It is known that the metabolism of D-amino acids by DAAO generates hydrogen peroxide which may result in oxidative damage [87]. The application of sodium benzoate as DAAO inhibitor in clozapine-resistant schizophrenia increased catalase activity in decomposing hydrogen peroxide [88]. Among antipsychotic medications, only blonanserin and risperidone owned stronger DAAO inhibitory effects based on in vitro study [89]. Besides, animal study also demonstrated that increased oxidative damage in rat brain was induced by haloperidol and clozapine but not by olanzapine and aripiprazole [90]. Antidepressant hydrazines such as isocarboxazid and phenelzine were also found to have a role in inactivating DNA when they react with oxygen and produce hydrogen peroxide [91]. Therefore, it can be assumed that the positive association between oxidative DNA damage and medication was correlated with patient status and illness duration. This could be due to the higher usage of antipsychotics in inpatients compared to outpatients [92]. Patients with longer illness duration were also assumed in taking more medications. No significant result was observed in depression as most of the patients were not hospitalized and not on medication.
As discussed, oxidative DNA damage is the consequence of oxidative stress that involves excessive ROS or RNS in impaired antioxidant mechanism [93]. To the best of our knowledge, there was no systematic review or meta-analysis done on ROS in schizophrenia, bipolar disorder and depression yet. The present study revealed that higher ROS level was found in particularly unmedicated schizophrenic patients. However, studies [49,50] also suggested the involvement of higher RNS level in schizophrenic patients treated with atypical antipsychotics. This was consistent with previous meta-analysis [8] which found increased nitric oxide (NO) which is one of the RNS in medicated schizophrenic patients. Hence, these excess ROS/RNS levels may be correlated with increased oxidative DNA damage in schizophrenia found in present meta-analysis. On the other hand, direct measurement of ROS has been less studied compared with NO [16] in patients with bipolar disorder. Meanwhile the current meta-analysis revealed that in depression, the non-significant differences in ROS level and oxidative DNA damage might be contributed by medication. Previous meta-analysis [17] also reported that there was no significant difference in NO level between depressed patients and healthy controls. Therefore, we can postulate that different types of antidepressant may affect oxidative stress level, for instance amitriptyline increased ROS production [54], and sertraline decreased levels of superoxide radicals and hydroxyl radicals [56]. This could be also supported by in vitro study which found tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitor (SSRIs) induced oxidative stress by increasing production of ROS and reducing glutathione level [94].
Our present study has several limitations. First, some studies were excluded in present analysis as the information was insufficient or raw data were not provided. Thus, there might be certain influences on the overall result. Second, the association between oxidative DNA damage and other risk factors such as age [95] or smoking status [96] which have been found to have positive correlation with the damage was not analysed. We cannot rule out the possible effects of external factors such as diet or lifestyle on oxidative DNA damage to determine whether the damage is formed as the consequence of the illness. Besides, we were unable to evaluate the effect of specific antipsychotics drug as the subjects in studies were treated with variety of treatment. Furthermore, as there was insufficient number of studies, we were unable to conduct analysis of ROS level in bipolar disorder.
In conclusion, the present meta-analysis revealed that schizophrenia with the largest effect size and bipolar disorder were associated with increased oxidative DNA damage whereas no association between oxidative DNA damage and depression was observed. Subgroup analyses of oxidative DNA damage level in different biological specimens may indicate different mechanisms of lesion occurrence in different mental illness and may help future studies in choosing specific specimen for a particular mental illness. Other factors such as patient status, illness duration and medication history that may contribute certain effects on oxidative DNA damage need further investigation. The present study also revealed the relationship between high ROS level and schizophrenia but not in depression. More association studies between direct measurement ROS and oxidative DNA damage can be conducted in future to further confirm that 8-OHdG or 8-oxodG may have the potential to be used as biomarker of oxidative DNA damage and oxidative stress in mental illness, as well as to help in understanding the underlying pathophysiology of mental illnesses.
Acknowledgments
None.
Footnotes
The authors have no potential conflicts of interest to disclose.
Authors’ contribution
Conceptualization: all authors. Data curation: Xue Xin Goh. Formal analysis: Xue Xin Goh. Funding acquisition: Shiau Foon Tee. Investigation: all authors. Methodology: Xue Xin Goh. Project administration: Pek Yee Tang, Shiau Foon Tee. Resources: Pek Yee Tang, Shiau Foon Tee. Supervision: Pek Yee Tang, Shiau Foon Tee. Validation: Pek Yee Tang, Shiau Foon Tee. Writing—original draft: Xue Xin Goh. Writing—review & editing: Pek Yee Tang, Shiau Foon Tee.
REFERENCES
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 Diseases and Injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21:10. doi: 10.1007/s11920-019-0997-0. [DOI] [PubMed] [Google Scholar]
- 3.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. Global Burden of Mental, Neurological, and Substance Use Disorders: An Analysis from the Global Burden of Disease Study 2010 (3rd Ed) In: Patel V, Chisholm D, Dua T, Laxminarayan R, Medina-Mora ME, editors. Mental, Neurological, and Substance Use Disorders: Disease Control Priorities. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; 2016. [PubMed] [Google Scholar]
- 4.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 5.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 6.Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegaliński E, et al. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep. 2015;67:569–580. doi: 10.1016/j.pharep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Bulbul F, Virit O, Alpak G, Unal A, Bulut M, Kaya MC, et al. Are oxidative stress markers useful to distinguish schizoaffective disorder from schizophrenia and bipolar disorder? Acta Neuropsychiatr. 2014;26:120–124. doi: 10.1017/neu.2013.44. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Zhao ZM, He L, Wan CL. A meta-analysis of oxidative stress markers in schizophrenia. Sci China Life Sci. 2010;53:112–124. doi: 10.1007/s11427-010-0013-8. [DOI] [PubMed] [Google Scholar]
- 9.Katerji M, Filippova M, Duerksen-Hughes P. Approaches and methods to measure oxidative stress in clinical samples: research applications in the cancer field. Oxid Med Cell Longev. 2019;2019:1279250. doi: 10.1155/2019/1279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickerson RP, Prat F, Muller JG, Foote CS, Burrows CJ. Sequence and stacking dependence of 8-oxoguanine oxidation: comparison of oneelectron vs singlet oxygen mechanisms. J Am Chem Soc. 1999;121:9423–9428. [Google Scholar]
- 11.Raza MU, Tufan T, Wang Y, Hill C, Zhu MY. DNA damage in major psychiatric diseases. Neurotox Res. 2016;30:251–267. doi: 10.1007/s12640-016-9621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2’-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27:120–139. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 13.Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-hydroxydeoxyguanosine: Not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol. 2012;18:302–308. doi: 10.3748/wjg.v18.i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korkmaz KS, Butuner BD, Roggenbuck D. Detection of 8-OHdG as a diagnostic biomarker. J Lab Precis Med. 2018;3:95. [Google Scholar]
- 15.Salim S. Oxidative stress and psychological disorders. Curr Neuropharmacol. 2014;12:140–147. doi: 10.2174/1570159X11666131120230309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown NC, Andreazza AC, Young LT. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–68. doi: 10.1016/j.psychres.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Zhong S, Liao X, Chen J, He T, Lai S, et al. A meta-analysis of oxidative stress markers in depression. PLoS One. 2015;10:e0138904. doi: 10.1371/journal.pone.0138904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christensen MR, Poulsen HE, Henriksen T, Weimann A, Ellervik C, Lynnerup N, et al. Elevated levels of 8-oxoGuo and 8-oxodG in individuals with severe mental illness - An autopsy-based study. Free Radic Biol Med. 2018;126:372–378. doi: 10.1016/j.freeradbiomed.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 19.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 Index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Lin E, Tong T, Chen Y, Wang Y. Fixed-effects model: the most convincing model for meta-analysis with few studies. Preprint arXiv. :2002.04211. [Google Scholar]
- 21.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23.Chestkov IV, Jestkova EM, Ershova ES, Golimbet VG, Lezheiko TV, Kolesina NY, et al. ROS-induced DNA damage associates with abundance of mitochondrial DNA in white blood cells of the untreated schizophrenic patients. Oxid Med Cell Longev. 2018;2018:8587475. doi: 10.1155/2018/8587475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ershova ES, Jestkova EM, Chestkov IV, Porokhovnik LN, Izevskaya VL, Kutsev SI, et al. Quantification of cell-free DNA in blood plasma and DNA damage degree in lymphocytes to evaluate dysregulation of apoptosis in schizophrenia patients. J Psychiatr Res. 2017;87:15–22. doi: 10.1016/j.jpsychires.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Shmarina GV, Orlova MD, Ershova ES, Jestkova EM, Martynov AV, Veiko NN, et al. NRF2 and HMOX1 gene expression against the background of systemic oxidative stress in patients with acute psychosis. Russ J Genet. 2020;56:96–102. [Google Scholar]
- 26.Jorgensen A, Broedbaek K, Fink-Jensen A, Knorr U, Soendergaard MG, Henriksen T, et al. Increased systemic oxidatively generated DNA and RNA damage in schizophrenia. Psychiatry Res. 2013;209:417–423. doi: 10.1016/j.psychres.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Nordholm D, Poulsen HE, Hjorthøj C, Randers L, Nielsen MØ, Wulff S, et al. Systemic oxidative DNA and RNA damage are not increased during early phases of psychosis: a case control study. Psychiatry Res. 2016;241:201?206. doi: 10.1016/j.psychres.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim RR, Amer RA, Abozeid AA, Elsharaby RM, Shafik NM. Micro RNA 146a gene variant/TNF-α/IL-6/IL-1 β; a cross-link axis in between oxidative stress, endothelial dysfunction and neuro-inflammation in acute ischemic stroke and chronic schizophrenic patients. Arch Biochem. 2020;679:108193. doi: 10.1016/j.abb.2019.108193. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Yan L, Guo T, Yang S, Ni D, Liu Y, et al. A pilot study of biomarkers of oxidative stress in serum and schizophrenia. Psychiatry Res. 2020;284:112757. doi: 10.1016/j.psychres.2020.112757. [DOI] [PubMed] [Google Scholar]
- 30.Copoglu US, Virit O, Kokacya MH, Orkmez M, Bulbul F, Erbagci AB, et al. Increased oxidative stress and oxidative DNA damage in non-remission schizophrenia patients. Psychiatry Res. 2015;229:200–205. doi: 10.1016/j.psychres.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Şimşek Ş, Gençoğlan S, Yüksel T, Kaplan İ, Alaca R, Aktaş H. Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology. 2016;73:92–97. doi: 10.1159/000444488. [DOI] [PubMed] [Google Scholar]
- 32.Miyaoka T, Ieda M, Hashioka S, Wake R, Furuya M, Liaury K, et al. Analysis of oxidative stress expressed by urinary level of biopyrrins and 8-hydroxydeoxyguanosine in patients with chronic schizophrenia. Psychiatry Clin Neurosci. 2015;69:693–698. doi: 10.1111/pcn.12319. [DOI] [PubMed] [Google Scholar]
- 33.Jacoby AS, Vinberg M, Poulsen HE, Kessing LV, Munkholm K. Increased DNA and RNA damage by oxidation in patients with bipolar I disorder. Transl Psychiatry. 2016;6:e867. doi: 10.1038/tp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munkholm K, Poulsen HE, Kessing LV, Vinberg M. Elevated levels of urinary markers of oxidatively generated DNA and RNA damage in bipolar disorder. Bipolar Disord. 2015;17:257–268. doi: 10.1111/bdi.12245. [DOI] [PubMed] [Google Scholar]
- 35.Knorr U, Simonsen AH, Roos P, Weimann A, Henriksen T, Christensen EM, et al. Cerebrospinal fluid oxidative stress metabolites in patients with bipolar disorder and healthy controls: a longitudinal case-control study. Transl Psychiatry. 2019;9:325. doi: 10.1038/s41398-019-0664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soeiro-de-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar I disorder. Int J Neuropsychopharmacol. 2013;16:1505–1512. doi: 10.1017/S1461145713000047. [DOI] [PubMed] [Google Scholar]
- 37.Tsai MC, Huang TL. Thiobarbituric acid reactive substances (TBARS) is a state biomarker of oxidative stress in bipolar patients in a manic phase. J Affect Disord. 2015;173:22–26. doi: 10.1016/j.jad.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 38.Huzayyin AA, Andreazza AC, Turecki G, Cruceanu C, Rouleau GA, Alda M, et al. Decreased global methylation in patients with bipolar disorder who respond to lithium. Int J Neuropsychopharmacol. 2014;17:561–569. doi: 10.1017/S1461145713001569. [DOI] [PubMed] [Google Scholar]
- 39.Ceylan D, Yılmaz S, Tuna G, Kant M, Er A, Ildız A, et al. Alterations in levels of 8-Oxo-2’-deoxyguanosine and 8-Oxoguanine DNA glycosylase 1 during a current episode and after remission in unipolar and bipolar depression. Psychoneuroendocrinology. 2020;114:104600. doi: 10.1016/j.psyneuen.2020.104600. [DOI] [PubMed] [Google Scholar]
- 40.Yi S, Nanri A, Matsushita Y, Kasai H, Kawai K, Mizoue T. Depressive symptoms and oxidative DNA damage in Japanese municipal employees. Psychiatry Res. 2012;200:318–322. doi: 10.1016/j.psychres.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 41.Black CN, Bot M, Scheffer PG, Penninx BWJH. Oxidative stress in major depressive and anxiety disorders, and the association with antidepressant use; results from a large adult cohort. Psychol Med. 2017;47:936–948. doi: 10.1017/S0033291716002828. [DOI] [PubMed] [Google Scholar]
- 42.van Velzen LS, Wijdeveld M, Black CN, van Tol MJ, van der Wee NJA, Veltman DJ, et al. Oxidative stress and brain morphology in individuals with depression, anxiety and healthy controls. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:140–144. doi: 10.1016/j.pnpbp.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 45.Tsai MC, Huang TL. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 2016;235:38–42. doi: 10.1016/j.psychres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Zhou F, He D, Bai J, Hui L, Wang X, et al. The level of oxidative stress and the expression of genes involved in DNA-damage signaling pathways in depressive patients with colorectal carcinoma. J Psychosom Res. 2009;66:259–266. doi: 10.1016/j.jpsychores.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Wei YC, Zhou FL, He DL, Bai JR, Ding H, Wang XY, et al. Oxidative stress in depressive patients with gastric adenocarcinoma. Int J Neuropsychopharmacol. 2009;12:1089–1096. doi: 10.1017/S1461145709000091. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z, Zhang J, Yan J, Wang Y, Li Y. Leucocyte telomere shortening in relation to newly diagnosed type 2 diabetic patients with depression. Oxid Med Cell Longev. 2014;2014:673959. doi: 10.1155/2014/673959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets. 2005;16:386–391. doi: 10.1080/09537100500128872. [DOI] [PubMed] [Google Scholar]
- 50.Dietrich-Muszalska A, Olas B. Modifications of blood platelet proteins of patients with schizophrenia. Platelets. 2009;20:90–96. doi: 10.1080/09537100802641499. [DOI] [PubMed] [Google Scholar]
- 51.Melamed Y, Sirota P, Dicker DR, Fishman P. Superoxide anion production by neutrophils derived from peripheral blood of schizophrenic patients. Psychiatry Res. 1998;77:29–34. doi: 10.1016/s0165-1781(97)00124-8. [DOI] [PubMed] [Google Scholar]
- 52.Sirota P, Gavrieli R, Wolach B. Overproduction of neutrophil radical oxygen species correlates with negative symptoms in schizophrenic patients: parallel studies on neutrophil chemotaxis, superoxide production and bactericidal activity. Psychiatry Res. 2003;121:123–132. doi: 10.1016/s0165-1781(03)00222-1. [DOI] [PubMed] [Google Scholar]
- 53.Szuster-Ciesielska A, Słotwińska M, Stachura A, MarmurowskaMichałowska H, Dubas-Ślemp H, Bojarska-Junak A, et al. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:686–694. doi: 10.1016/j.pnpbp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 54.Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Atanackovic D, Kröger H, Serke S, Deter HC. Immune parameters in patients with anxiety or depression during psychotherapy. J Affect Disord. 2004;81:201–209. doi: 10.1016/S0165-0327(03)00165-4. [DOI] [PubMed] [Google Scholar]
- 56.Chang CC, Lee CT, Lan TH, Ju PC, Hsieh YH, Lai TJ. Effects of antidepressant treatment on total antioxidant capacity and free radical levels in patients with major depressive disorder. Psychiatry Res. 2015;230:575–580. doi: 10.1016/j.psychres.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Rybka J, Kędziora-Kornatowska K, Banaś-Leżańska P, Majsterek I, Carvalho LA, Cattaneo A, et al. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63:187–194. doi: 10.1016/j.freeradbiomed.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Andreazza AC, Kauer-Sant’Anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a metaanalysis. J Affect Disord. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 59.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowland T, Perry BI, Upthegrove R, Barnes N, Chatterjee J, Gallacher D, et al. Neurotrophins, cytokines, oxidative stress mediators and mood state in bipolar disorder: systematic review and meta-analyses. Br J Psychiatry. 2018;213:514–525. doi: 10.1192/bjp.2018.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markkanen E, Meyer U, Dianov GL. DNA damage and repair in schizophrenia and autism: implications for cancer comorbidity and beyond. Int J Mol Sci. 2016;17:856. doi: 10.3390/ijms17060856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muraleedharan A, Menon V, Rajkumar RP, Chand P. Assessment of DNA damage and repair efficiency in drug naïve schizophrenia using comet assay. J Psychiatr Res. 2015;68:47–53. doi: 10.1016/j.jpsychires.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 63.Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2004;12:167–175. [PubMed] [Google Scholar]
- 64.Anderson IM, Haddad PM, Scott J. Bipolar disorder. BMJ. 2012;345:e8508. doi: 10.1136/bmj.e8508. [DOI] [PubMed] [Google Scholar]
- 65.Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, et al. DNA damage in bipolar disorder. Psychiatry Res. 2007;153:27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 66.Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J, Salvador M, et al. Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:283–285. doi: 10.1016/j.pnpbp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Buttner N, Bhattacharyya S, Walsh J, Benes FM. DNA fragmentation is increased in non-GABAergic neurons in bipolar disorder but not in schizophrenia. Schizophr Res. 2007;93:33–41. doi: 10.1016/j.schres.2007.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mustak MS, Hegde ML, Dinesh A, Britton GB, Berrocal R, Rao KS, et al. Evidence of altered DNA integrity in the brain regions of suicidal victims of bipolar depression. Indian J Psychol Med. 2010;52:220–228. doi: 10.4103/0019-5545.70974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andreazza AC, Cassini C, Rosa AR, Leite MC, de Almeida LMV, Nardin P, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res. 2007;41:523–529. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 70.Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- 71.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxietylike behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment. J Clin Psychiatry. 2015;76:1658–1667. doi: 10.4088/JCP.14r09179. [DOI] [PubMed] [Google Scholar]
- 73.Palta P, Samuel LJ, Miller ER, Szanton SL. Depression and oxidative stress: Results from a meta-analysis of observational studies. Psychosom Med. 2014;76:12–19. doi: 10.1097/PSY.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BWJH. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 75.Fletcher J. What is heterogeneity and is it important? Br Med J. 2007;334:94–96. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–216. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 77.Mlinarić A, Horvat M, Smolčić VŠ. Dealing with the positive publication bias: why you should really publish your negative results. Biochem Med. 2017;27:447–452. doi: 10.11613/BM.2017.030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henriksen T, Hillestrøm PR, Poulsen HE, Weimann A. Automated method for the direct analysis of 8-oxo-guanosine and 8-oxo-2’-deoxyguanosine in human urine using ultraperformance liquid chromatography and tandem mass spectrometry. Free Radic Biol Med. 2009;47:629–635. doi: 10.1016/j.freeradbiomed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 79.Poulsen HE, Nadal LL, Broedbaek K, Nielsen PE, Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim Biophys Acta. 2014;1840:801–808. doi: 10.1016/j.bbagen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Collins AR, Oscoz AA, Brunborg G, Gaivão I, Giovannelli L, Kruszewski M, et al. The comet assay: topical issues. Mutagenesis. 2008;23:143–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- 81.Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr. 2000;72:1082–1087. doi: 10.1093/ajcn/72.5.1082. [DOI] [PubMed] [Google Scholar]
- 82.Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neurosci Biobehav Rev. 2008;32:675–692. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Kapczinski F, Dias VV, Kauer-Sant’Anna M, Brietzke E, Vázquez GH, Vieta E, et al. The potential use of biomarkers as an adjunctive tool for staging bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1366–1371. doi: 10.1016/j.pnpbp.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen TT, Eyler LT, Jeste DV. Systemic biomarkers of accelerated aging in schizophrenia: a critical review and future directions. Schizophr Bull. 2018;44:398–408. doi: 10.1093/schbul/sbx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abdel-Wahab BA, Abdalla ME, El-khawanki MM. Does clozapine induce myocarditis, myocardial oxidative stress and DNA damage in rats? Egypt J Forensic Sci. 2014;4:75–82. [Google Scholar]
- 86.Lin CH, Lane HY. Early identification and intervention of schizophrenia: insight from hypotheses of glutamate dysfunction and oxidative stress. Front Psychiatry. 2019;10:93. doi: 10.3389/fpsyt.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Williams RE, Lock EA. Sodium benzoate attenuates D-serine induced nephrotoxicity in the rat. Toxicology. 2005;207:35–48. doi: 10.1016/j.tox.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Lin CH, Lin CH, Chang YC, Huang YJ, Chen PW, Yang HT, et al. Sodium benzoate, a D-amino acid oxidase inhibitor, added to clozapine for the treatment of schizophrenia: a randomized, double-blind, placebo-controlled trial. Biol Psychiatry. 2018;84:422–432. doi: 10.1016/j.biopsych.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 89.Shishikura M, Hakariya H, Iwasa S, Yoshio T, Ichiba H, Yorita K, et al. Evaluation of human D-amino acid oxidase inhibition by anti-psychotic drugs in vitro. Biosci Trends. 2014;8:149–154. doi: 10.5582/bst.2014.01034. [DOI] [PubMed] [Google Scholar]
- 90.Martins MR, Petronilho FC, Gomes KM, Dal-Pizzol F, Streck EL, Quevedo J. Antipsychotic-induced oxidative stress in rat brain. Neurotox Res. 2008;13:63–69. doi: 10.1007/BF03033368. [DOI] [PubMed] [Google Scholar]
- 91.Freese E, Sklarow S, Freese EB. DNA damage caused by antidepressant hydrazines and related drugs. Mutat Res. 1968;5:343–348. doi: 10.1016/0027-5107(68)90004-3. [DOI] [PubMed] [Google Scholar]
- 92.Alosaimi FD, Alhabbad A, Abalhassan MF, Fallata EO, Alzain NM, Alassiry MZ, et al. Patterns of psychotropic medication use in inpatient and outpatient psychiatric settings in Saudi Arabia. Neuropsychiatr Dis Treat. 2016;12:897–907. doi: 10.2147/NDT.S100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smith JA, Park S, Krause JS, Banik NL. Oxidative stress, DNA damage, and the telomeric complex as therapeutic targets in acute neurodegeneration. Neurochem Int. 2013;62:764–75. doi: 10.1016/j.neuint.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Post A, Crochemore C, Uhr M, Holsboer F, Behl C. Differential induction of NF‐κB activity and neural cell death by antidepressants in vitro. Eur J Neurosci. 2000;12:4331–4337. doi: 10.1046/j.0953-816x.2000.01352.x. [DOI] [PubMed] [Google Scholar]
- 95.Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, et al. Does oxidative damage to DNA increase with age? Proc Natl Acad Sci USA. 2001;98:10469–10474. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Graille M, Wild P, Sauvain JJ, Hemmendinger M, Canu IG, Hopf NB. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int J Mol Sci. 2020;21:3743. doi: 10.3390/ijms21113743. [DOI] [PMC free article] [PubMed] [Google Scholar]