Abstract
Introduction:
Half of all postmenopausal women report symptoms of vulvar, vaginal or urinary discomfort with significant impact on sexual function and quality of life; underlying mechanisms leading to symptoms are poorly understood. To examine the possibility that the vaginal microbiota and/or mucosal immune response contribute to the severity of bothersome vaginal symptoms we conducted a substudy of samples from a randomized trial of vaginal treatment for genitourinary syndrome of menopause (GSM) to compare these features between women whose symptoms improved vs. those who did not.
Methods:
This is a secondary analysis of samples collected in a 12-week randomized trial of treatment with vaginal estradiol or moisturizer versus placebo for moderate-severe postmenopausal symptoms of vaginal discomfort. We randomly selected 20 women in each arm with ≥2-point decrease in most bothersome symptom (MBS) severity (responders) and 20 matched controls with ≤1-point decrease (non-responders). At 0, 4, 12 weeks, we characterized vaginal microbiota (16S rRNA gene sequencing), vaginal fluid metabolites (broad-based metabolomic profiling), vaginal fluid soluble immune markers (MesoScale Discovery), pH and vaginal maturation index (VMI). We compared responders versus non-responders at baseline, and across all visits using linear mixed models to evaluate associations with microbiota, metabolites and immune markers, incorporating visit and participant-specific random effects while controlling for treatment arm.
Results:
Women (n=120) were mean age 61 years and primarily White (92%). At enrollment no significant differences were observed between responders and non-responders in age, MBS type or severity, microbiota composition or diversity, Lactobacillus dominance, metabolome or immune markers. There was a significant decrease in diversity of the vaginal microbiota in both responders and non-responders (p<0.001) over 12 weeks. While this change did not differ by responder status, diversity was associated with treatment arm: more women in the estradiol arm (63%) had Lactobacillus-dominant, lower diversity bacterial communities than women in the moisturizer (35%) or dual placebo (23%) arms (p=0.001) at 12 weeks. Metabolome, VMI and the measured immune markers were not associated with responder status over the 12 weeks but varied by treatment arm.
Comment:
Postmenopausal vaginal symptom severity was not significantly associated with vaginal microbiota or mucosal inflammatory markers in this small study. Women receiving vaginal estradiol experienced greater abundance of lactobacilli and lower vaginal pH at end of treatment.
Keywords: genitourinary syndrome of menopause, menopause, vaginal estradiol, vaginal microbiome, vaginal moisturizer
Introduction:
Close to half of postmenopausal women report bothersome symptoms of vulvar, vaginal or urinary discomfort collectively referred to as genitourinary syndrome of menopause (GSM)1,2 which are associated with negative impacts on sexual function and quality of life.3,4 Although all women experience a profound decrease in circulating estrogen after menopause, not all women experience GSM. The pathophysiology underlying symptoms is not well understood, though it is presumed to be related to the loss of circulating estrogen. However, the absence of symptoms in some postmenopausal women, and efficacy of some non-hormonal therapies in relieving symptoms suggests that there may be additional factors modifying the risk for GSM.
In the Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) Vaginal Health Trial, postmenopausal women with moderate-severe vulvovaginal discomfort were randomized to vaginal estradiol tablet (+placebo gel), vaginal moisturizing gel (+placebo tablet) or dual placebo for 12 weeks.5 Although women who received estrogen had greater changes in vaginal pH and vaginal maturation index (VMI), women in all three treatment arms had comparable improvement in vulvovaginal discomfort over the course of the trial. A recent meta-analysis also concluded that treatment with vaginal estrogen was associated with greater improvement in physical findings of GSM compared to non-hormonal therapies, but not greater improvement in vulvovaginal symptoms.6 If symptoms are not due simply to lack of estrogen, what other mechanistic pathways may be involved?
One possible contributing cause of symptoms is the vaginal microbiota and/or mucosal inflammation. In our trial, while mean improvement in women’s most bothersome symptom (MBS) did not significantly vary by treatment arm, some individual women had large improvement while others had no or minimal improvement. To examine the possibility that vaginal microbiota and/or mucosal immune responses contribute to the severity of bothersome vaginal symptoms we conducted a nested substudy comparing women according to symptom improvement. We hypothesized that vaginal microbiota and inflammatory markers (i.e. increased Lactobacillus abundance and decreased inflammation) would differ significantly between women with the largest reductions in MBS severity compared to those women with smaller reductions, regardless of treatment arm.
Methods:
The MsFLASH Vaginal Health Trial was a randomized trial in 302 postmenopausal women with moderate-severe vulvovaginal discomfort, comparing vaginal estradiol tablet 0.01 mg (+placebo gel) nightly for 2 weeks, then 2 times/week (n=102) or vaginal moisturizing gel (+placebo tablet) 3 times/week (n=100) vs. dual placebo (n=100).5 The trial enrolled women in Seattle, WA and Minneapolis, MN. The primary outcome was change over 12 weeks in severity of the MBS selected by the participant at enrollment: vulvovaginal itching, pain, dryness, irritation, or pain with penetration. Symptom severity was rated as 0 to 3, signifying none, mild, moderate or severe. Women with a history of pre-menopausal vaginal discomfort or pain with intercourse (i.e. vulvodynia) were excluded.
Follow-up visits occurred 4 and 12 weeks after randomization. At each visit, vaginal swabs were collected for wet mount, pH measurement, and microbiota analyses, cervicovaginal lavage for soluble immune markers and a vaginal wall spatula for vaginal maturation index. Testing for BV and trichomoniasis was by wet mount, and for yeast by wet mount and culture. Participants were considered eligible for this pilot analysis if genital samples were available from all visits; they used at least 80% of study product doses; and were not diagnosed with infectious vaginitis. We randomly selected 20 eligible participants from each treatment arm who experienced a ≥2-point decrease in MBS severity from enrollment to 12 weeks (called “responders”). We then matched 20 eligible participants with ≤1-point decrease in MBS severity (called “non-responders”), by treatment arm, race (White/people of color), and MBS selection (pain with vaginal penetration/other), and frequency-matched by study site, age, and baseline MBS severity.
The vaginal microbiota was characterized by sequencing of the V3-V4 region of the 16S rRNA gene on the Illumina MiSeq instrument (Illumina, San Diego, CA) as previously described.7,8 Broad-based metabolomic profiling was performed on a liquid-chromatography-mass spectrometry (LC-MS/MS) platform at the Northwest Metabolomics Research Center (NW-MRC) as previously described.9 Soluble immune factors were measured in cervicovaginal lavage fluid using the MesoScale Discovery platform (Rockville, MA). Analytes were selected to represent several potential pathways: pro-inflammatory (IL1β, IL1α, IL2, IL6, IL18), anti-inflammatory (IL10), Th2 (IL9, IL13) and chemoattractant cytokines and chemokines (IL8, IP10, MIP1α, MIP1β, MIP3α). Additional details can be found in the Supplemental Methods.
Statistical methods:
Microbiome and metabolome data were compared between responders and non-responders at baseline to assess pre-intervention predictors of symptom improvement, at 12-weeks to identify features associated with responder status, and as change between 0 and 12 weeks to correlate change in analytes with response. Analyses including data from 4-week or 12-week timepoints, while participants were using study product, were adjusted for treatment arm. Associations between α-diversity (baseline or 12-week) and response status were evaluated using linear regression analysis; the MiRKAT approach was used to evaluate β-diversity by responder status. Baseline α-diversity was compared to 12 weeks using a paired t-test and change in α-diversity over that time compared according to responder status via linear regression; change in β-diversity was embedded as a paired Aitchison distance10 and tested for association with improvement using MiRKAT.11 Associations of individual bacterial taxa with improvement were evaluated via linear regression models of CLR-transformed taxon abundances. Global associations of metabolomics were assessed by kernel machine test with a linear kernel and linear regression of individual metabolites on response status. Linear regression was used to estimate associations between change in individual taxa and metabolites (transformed taxon and metabolite abundances) with responder status. We compared rates of Lactobacillus dominance (relative abundance of any Lactobacillus species (including L. iners) ≥50) by responder status using logistic regression. Change in Lactobacillus-dominant status was evaluated using McNemar test and assessed for association with response via logistic regression. We compared vaginal pH using a Wilcoxon rank-sum test; the association between response and change in pH was evaluated with linear regression. Associations between symptom severity or responder status and soluble immune markers were examined with linear regression. For individual taxon, metabolite and immune marker analyses, we calculated Benjamini-Hochberg false discovery rates (FDR). Otherwise, significance was called at the nominal α=0.05 level.
To further exploit the longitudinal nature of our study, data from enrollment, and weeks 4 and 12 were included in comparisons of α-diversity, the top principal component of β-diversity, top principal component of metabolomic variability, individual taxa and metabolites, and soluble vaginal fluid immune markers according to responder status or symptom severity score. These associations were evaluated using linear mixed models with a random intercept.
Exploratory subset analyses restricted to 1) women whose MBS was pain with penetration or 2) whose MBS was vaginal dryness were conducted in the same manner as above.
Results:
Of 302 women randomized in the parent trial, 165 (55%) met the criteria for inclusion in this sub-study. As in the parent study, most participants were White (92%) and reported a MBS of pain with sex (55%) or vaginal dryness (22%) (Table 1). Mean MBS severity at enrollment was significantly higher in responders compared to non-responders (2.6 vs. 2.4, p=0.02). Although women were required to choose a single MBS, 93% reported more than one moderate to severe symptom of vulvovaginal discomfort at enrollment.
Table 1:
Comparison of demographic characteristics and vulvovaginal symptom severity at enrollment according to symptom improvement during the trial.
| Non-responders (n = 60) | Responders (n = 60) | P value | |
|---|---|---|---|
| Age (years): mean ± SD | 61 ± 4 | 61 ± 4 | * |
| White | 55 (92%) | 55 (92%) | * |
| Sexually active within the past month (yes/no) | 51 (85%) | 47 (78%) | 0.35 |
| MENQOL (1-8): mean ± SD | 3.2 ± 1.0 | 3.4 ± 1.2 | 0.49 |
| MBS type | * | ||
| Dryness | 13 (22%) | 14 (23%) | |
| Pain with penetration | 33 (55%) | 33 (55%) | |
| Itching/Irritation/Soreness | 14 (23%) | 13 (22%) | |
| MBS severity (0-3) | 0.02 | ||
| Mean ± SD | 2.4 ± 0.6 | 2.6 ± 0.53 | |
| Median (IQR) | 2 (2, 3) | 2 (2, 3) | |
| VMI < 5% superficial cells | 48 (94%) | 48 (94%) | 0.72 |
| Vaginal pH (median, IQR) | 7.0 (6.0, 7.5) | 7.5 (7.0, 7.5) | 0.02 |
| Severity of symptoms (whether or not chosen as MBS) | |||
| Pain with penetration | |||
| Mean ± SD | 2.0 ± 1.1 | 2.2 ± 0.9 | 0.36 |
| Median (IQR) | 2 (2, 3) | 2 (2, 3) | |
| Dryness | |||
| Mean ± SD | 2.0 ± 0.8 | 2.2 ± 0.8 | 0.14 |
| Median (IQR) | 2 (2, 3) | 2 (2, 3) | |
| Number of moderate/severe symptoms | |||
| 1 | 6 (10%) | 2 (3%) | 0.41 |
| 2 | 17 (28%) | 16 (27%) | |
| 3 | 16 (27%) | 18 (30%) | |
| 4 | 13 (22%) | 19 (32%) | |
| 5 | 8 (13%) | 5 (8%) | |
Groups were matched on these criteria
SD = standard deviation, MENQOL= Menopause Quality of Life, MBS = most bothersome symptom, IQR = inter-quartile range
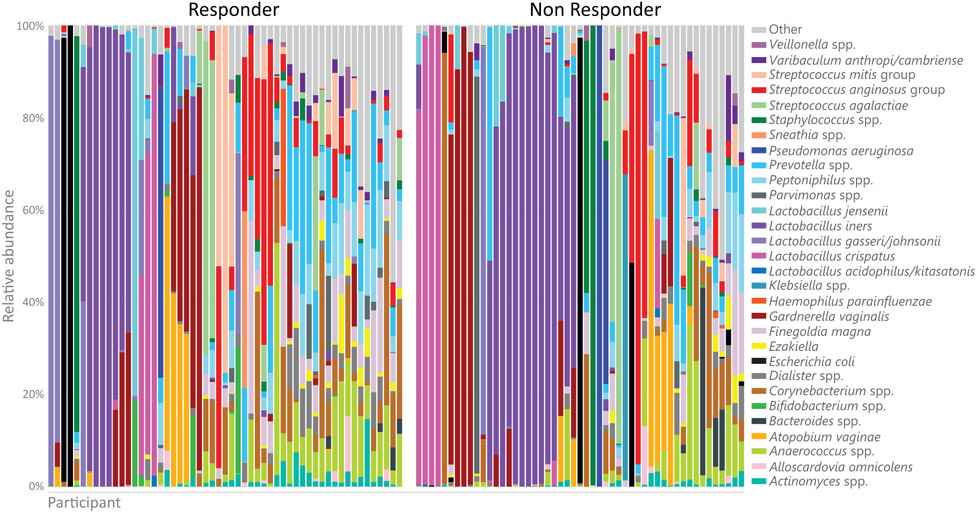
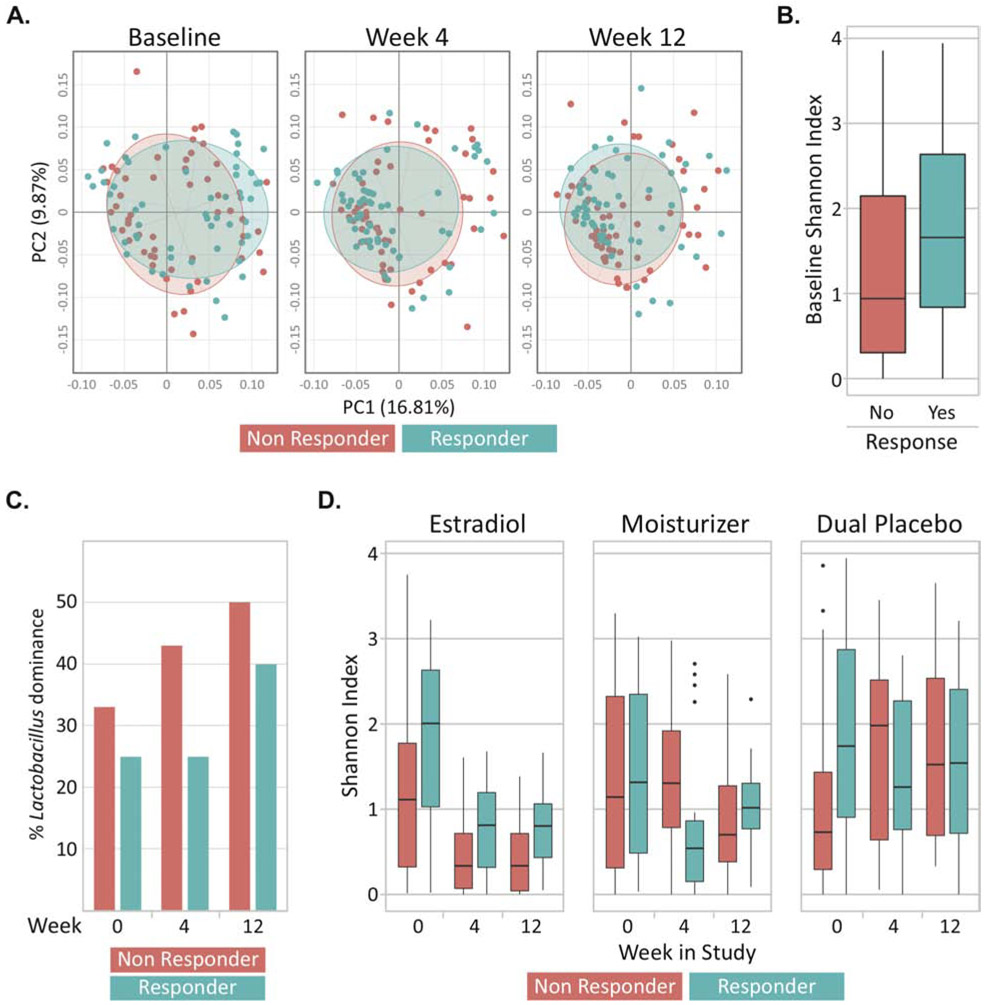
At baseline, the overall composition of the vaginal microbiota of responders and non-responders was similar (Figure 1, Figure 2A) (MiRKAT p=0.44), although responders had a higher Shannon index compared to non-responders (p=0.03; Figure 2B). No individual taxa were differentially abundant between responders and non-responders. There was no significant baseline difference in prevalence of a Lactobacillus-dominant vaginal microbial community: 25% (14/55) responders vs. 33% (17/51) of non-responders (p=0.49) (Figure 2C). Similarly, the overall baseline metabolome was not significantly different between responders and non-responders (Supplementary Figure 1A; p=0.25), but baseline sarcosine (p<0.001, FDR=20%) and alanine (p<0.001, FDR=20%) were lower in responders (Supplementary Figure 1B). The median vaginal pH was significantly higher in responders at baseline (7.5 vs. 7.0, p=0.02). The proportion of women with <5% superficial cells on VMI did not vary significantly between responders and non-responders (Table 1).
Figure 1.
Bacterial composition of vaginal fluid from responders and non-responders at baseline evaluated by 16S rRNA sequencing. Relative abundance of the top 30 taxa shown from enrollment vaginal 16S rRNA sequencing. Bacterial taxa less than 1% abundance were categorized in the “other” group. Responders (n = 60) were defined as women who had a ≥2-point decrease in most bothersome symptom (MBS) severity over 12 weeks of treatment and non-responders (n = 60) were defined as women who had <1-point decrease.
Figure 2.
(A) Beta diversity represented by Principle Coordinate Analysis plots. Each dot represents the bacterial community in a single participant and data is shown across three timepoints; baseline, week 4 and week 12. Axes are the coordinates of maximal variability in the multivariate data with PC1 and PC2 explaining 16.81% and 9.87% of the variation, respectively. The bacterial community was similar between responders (n=58 and 58 at weeks 4 and 12, respectively) and non-responders throughout the trial (n=60 and 59 at weeks 4 and 12). (B) Responders had a higher median Shannon index (α -diversity) at enrollment compared to non-responders (p = 03). (C) There was no statistically significant difference in the proportion of women with Lactobacillus dominance between responders and non-responders at any visit. (D) The Shannon index decreased significantly throughout the trial, especially in the estrogen and moisturizer arms, but was not associated with responder status. Responders were defined as women who had a ≥2-point decrease in most bothersome symptom (MBS) severity over 12 weeks of treatment and non-responders were defined as women who had <1-point decrease.
After 12 weeks of treatment, most non-responders reported moderate (43%) or severe (18%) symptoms, while responders (by definition) reported no (65%) or mild (35%) symptoms. There was no difference in report of sexual activity during follow up between responders (53/60, 88%) and non-responders (58/60, 97%; p = 0.08). Little variability was observed in α-diversity (Figure 2D; p=0.29), beta-diversity (Figure 2A; p=0.16), individual taxa, global metabolome (Supplementary Figure 1A; p=0.43), or individual metabolites by responder status. No significant difference in Lactobacillus dominance was found between responders and non-responders at 12 weeks: 48% vs. 36% (p=0.26) (Figure 2C). Median vaginal pH was similar at 12 weeks in responders vs. non-responders (6.0 vs. 6.0, p=0.64). Similar proportions of responders and non-responders had <5% superficial cells on VMI at 12 weeks: (67% vs. 78%, p=0.19).
The α-diversity decreased significantly from baseline to week 12 in all participants (p<0.001), but this change varied little by responder status (p=0.11; Figure 2D). Paired β-diversity analysis revealed a weak association with responder status (p=0.060), but no changes in individual taxa varied significantly between responders and non-responders (data not shown). There was little association of either change in the global metabolomic profile (p=0.14) or changes in individual metabolites with responder status. Over the course of the study, the proportion of women with Lactobacillus dominance increased from 29% to 42% (p=0.01). Conversion from a non-Lactobacillus dominant community to a Lactobacillus dominant community was not associated with responder status (p=0.73). Longitudinal analysis results (Supplemental Table 1) were similar to the paired analysis, showing that the microbiota and metabolome shifted across visit but not according to response. Additional models adjusted for age and time since menopause were qualitatively similar (results not shown).
The distributions of the absolute quantities of soluble vaginal immune marker values did not significantly vary according to responder status at any of the visits (Supplemental Table 2). The change in marker levels from baseline to week 12 was significantly associated with responder status for IL1β, IL8 and IL10 (Supplemental Table 3). However, the associations were in the opposite direction we hypothesized: responders had an increase in values over the 12 weeks. Across all three visits, mean marker levels were not significantly associated with symptom severity, except for significantly lower IL1α with higher symptom severity (p = 0.003, FDR 0.04) (Figure 3). Summary values for immune markers are listed in Supplemental Table 4.
Figure 3.
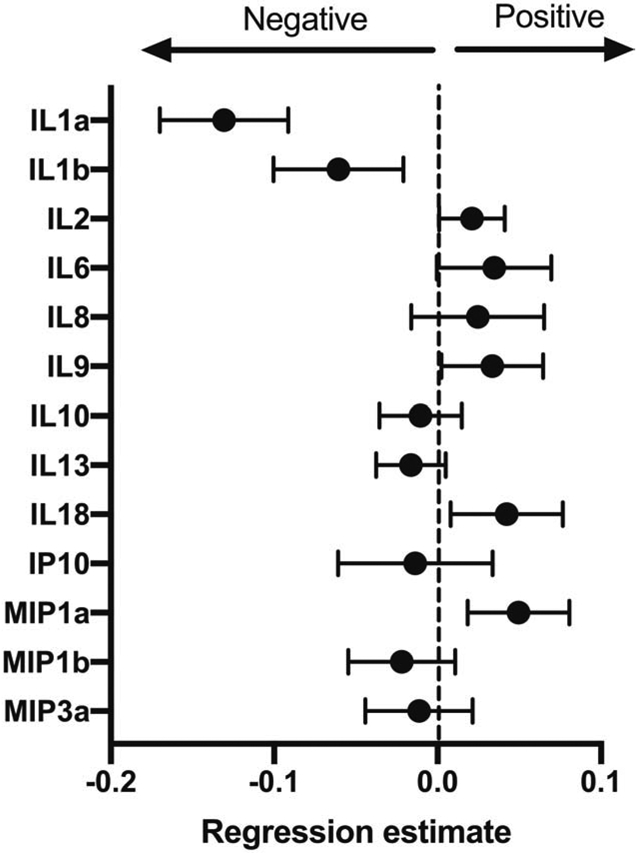
Regression coefficients +/− standard error of linear mixed models evaluating associations (negative or positive) of the concentration of soluble vaginal immune markers at all time points with vulvovaginal symptom severity. A negative correlation means that with higher symptom severity, marker concentration is lower.
In contrast to responder status, treatment arm was significantly associated with both α- and β-diversity of the bacterial community (Figure 2D, Supplemental Table 1). At 12 weeks, a higher proportion of women in the estradiol arm (63%) had Lactobacillus-dominance than women in the moisturizer (35%) or dual placebo (23%) arms (p=0.001). Week 12 median vaginal pH was significantly lower in the estradiol arm vs. the other two arms: 5.0 vs. 6.0, p<0.001.
Subgroup analyses:
Among women who chose pain with penetration as their MBS (n=66), no significant associations were found between responder status and microbial characteristics. However, among women randomized to dual placebo, non-responders had lower α-diversity at enrollment than responders (0.90 vs. 2.10, p=0.03) (Supplemental Figure 2A). Among women who chose vaginal dryness as their MBS (n=27), the Shannon index in the dual placebo arm remained higher in non-responders vs. responders throughout the study period (not statistically significant) (Supplemental Figure 2B).
Principle findings:
One of the most perplexing clinical characteristics of menopause is the variability in women’s experience of symptoms, whether hot flashes or GSM. We used vaginal fluid samples from postmenopausal women enrolled in a randomized trial of treatments for moderate to severe vulvovaginal discomfort to evaluate biological factors that may inform the underlying mechanism and variability of GSM symptoms. In this subset of 120 women from the MsFLASH Vaginal Health Trial, no significant associations were observed between change in MBS severity and vaginal microbiota composition, Lactobacillus dominance, soluble immune markers, VMI or vaginal fluid metabolites. The lack of correlation between our measured biomarkers and participants’ subjective report of symptoms suggests that the markers we measured are either not part of the causal pathway for postmenopausal vaginal discomfort or that sample heterogeneity was too great to detect a signal.
Results:
Our prior cross-sectional analysis of women enrolled in a trial assessing therapies for hot flashes demonstrated little association between microbiota and presence of vulvovaginal symptoms at baseline,12 but Lactobacillus dominance was more common in women whose vulvovaginal symptoms improved during the course of that trial.13 However, the prevalence and severity of vulvovaginal symptoms was low in those participants. Hummelen et al. demonstrated an association between a non-Lactobacillus dominant vaginal community and examiner-rated vaginal dryness, but did not discuss patient-rated symptom severity.14 In our current study, women with no or minimal change in MBS severity were just as likely as women with maximum change in MBS severity to have a Lactobacillus dominant microbiota at the end of the trial. Treatment arm was significantly associated with changes in the microbiota, while symptom improvement was not.
Finding that treatment arm was associated with changes in microbiota and metabolome over time aligns with findings from other studies that have shown that estrogen therapy (ET) (both systemic, with or without progestin, and local) has an impact on the vaginal microbiome.15-17 This is consistent with data from both culture-based and sequencing-based studies showing that after the menopausal transition, many women lose Lactobacillus-dominance of the vaginal microbiota.15,18,19 However, between 20-50% of postmenopausal women will have a Lactobacillus-dominant microbiota.14,16-18
Other studies have shown a concordance between improvement in symptoms and decrease in pH (which is likely a proxy for the presence and abundance of lactobacilli).4,20,21 In the SMART-3 study of oral conjugated estrogen and bazedoxefine for vulvovaginal discomfort, vaginal pH was more strongly associated with Menopause-specific Quality of Life (MENQOL) sexual function scores than either superficial cell or parabasal cell proportion in the VMI.4 In the REJOICE trial, a randomized trial of intravaginal estrogen vs. placebo, change in pH was strongly correlated with changes in the appearance of the vaginal mucosa, and weakly correlated with severity of symptoms at the end of the trial, though the analysis was not adjusted for treatment arm.21 In our study, women with maximal changes in MBS severity were not more likely to have a lower pH than women with no or minimal change in MBS severity, but women randomized to the estradiol arm had significantly lower pH than those in the other arms. Our data, which show that the change in pH and improvement in symptom severity are correlated in the estrogen treatment arm but not in the non-hormonal treatment arms, suggest that estrogen improves symptoms and leads to changes in the microbiota that lower the pH, but that these two things are perhaps not mechanistically linked.
The lack of significant association between soluble chemokines and cytokines and symptom severity or responder status aligns with two previous cross-sectional studies which also found no association between symptom severity and cytokine concentrations.22,23 Of note, these studies and our own include small numbers of participants and may be underpowered. Little is known about soluble vaginal immune markers in post-menopausal women, though some studies show alterations compared to pre-menopausal women.24,25 We chose our cytokine panel to cover many different types of immune signaling, but did not find any significant associations with symptom severity or responder status, with the exception of a negative association between symptoms and IL1α and IL1β. This is the inverse of what we might expect, if superficial inflammation were associated with symptom severity, as the IL1 family of cytokines are key mediators of inflammation. However, symptom severity may be related to deeper features of the vaginal microenvironment not measured with superficial samples like a cervicovaginal lavage, such as mucosal vascularization or elasticity. Of note, in responders, cytokines from both pro- and anti-inflammatory pathways increased over 12 weeks, which could indicate an overall improvement in the health and integrity of the genital tract mucosa.
Clinical implications:
In aggregate, these data demonstrate an association between hormonal status, vaginal microbiota, and the vaginal microenvironment, but suggest that vaginal symptoms have an additional underlying pathophysiology that is not clearly understood. For clinicians, this means that vaginal estrogen treatment alone may not be sufficient to relieve symptoms of vaginal discomfort in all postmenopausal women. Additionally, this suggests that interventions targeted at changing the postmenopausal microbiota or pH are unlikely to alter symptom severity for most women.
Research implications:
Our data demonstrate a lack of understanding in the field about the underlying cause of postmenopausal vaginal discomfort. Additional studies are needed to identify what factors, beyond a drop in circulating serum estrogen levels, contribute to onset of postmenopausal vaginal symptoms, and what local intervention characteristics are associated with symptom improvement. Tissue biopsies to evaluate changes in vasculature, collagen, local immune cells and nerve density may provide important insight into contributing pathways.
Strengths and Limitations:
For this analysis we selected a subset of women from our trial, chosen to have both the best quality data (adherent to medications and follow-up, with no co-occurring infections) and to be at the extremes of symptom change in each study arm, to provide the best chance of detecting differences in biological markers. The placebo gel was chosen to have minimal impact on vaginal bacteria or mucosal inflammatory markers, but had high lubricity consistent with properties of a lubricant and was associated with an improvement in symptoms. We had insufficient numbers to study a single MBS. The relatively small number of women from each treatment arm, in combination with the heterogeneity of the vaginal microbial communities in postmenopausal women and possible heterogeneity in symptom profiles, may have limited our power to detect smaller associations. Additionally, our study population was primarily White, which may limit the generalizability of our results.
Conclusions:
The biological underpinnings of postmenopausal vaginal discomfort are likely complex and may not be easily evaluated with superficial vaginal samples such as vaginal fluid. Our findings suggest that efforts to change superficial features of the vaginal microenvironment, such as pH or Lactobacillus colonization, may not address the primary underlying mechanism that leads to postmenopausal vaginal discomfort and is unlikely to be effective in relieving moderate to severe bothersome symptoms.
Supplementary Material
Supplemental Figure 1: (A) There were no significant association between the profile of metabolites and responder status throughout the trial. Responders were defined as women who had a ≥2-point decrease in most bothersome symptom (MBS) severity over 12 weeks of treatment and non-responders were defined as women who had <1-point decrease. (B) Baseline sarcosine and alanine levels were lower among responders.
Supplemental Figure 2: Subgroup analyses of women who reported an MBS of pain with penetration (A) or vaginal dryness (B) found no significant associations between responder status and Shannon index. Responders were defined as women who had a ≥2-point decrease in most bothersome symptom (MBS) severity over 12 weeks of treatment and non-responders were defined as women who had <1-point decrease.
Condensation: The vaginal microbiome, vaginal cytokines and vaginal fluid metabolites were not different between postmenopausal women with a large vs. no response to treatment for bothersome symptoms.
AJOG at a Glance:
A. Why was this study conducted?
We performed this study to identify microbial, immune or metabolic markers associated with response to topical treatment for genitourinary syndrome of menopause.
B. What are the key findings?
We found no association between treatment response and vaginal microbial community diversity or richness, Lactobacillus dominance, vaginal fluid metabolites or soluble immune markers.
Women randomized to vaginal estrogen had the greatest decrease in microbial diversity and altered vaginal fluid metabolites, whether symptoms improved or not.
C. What does this study add to what is already known?
Our results suggest that the biological markers used to define treatment response for vaginal estradiol may not be useful markers for symptom improvement
Acknowledgements:
We would like to thank the clinical investigators who conducted the MsFLASH VHT trial, all of whom were funded by NIH/NIA (5R01AG048209): Katherine M. Newton, PhD (Kaiser Permanente Washington Health Research Institute, Seattle, WA); Bette J. Caan, DrPH (Division of Research, Kaiser Permanente, Oakland, California); Kristine E. Ensrud, MD; Susan J. Diem, MD (VA Medical Center/ University of Minnesota, Minneapolis, MN).
Funding: This work was funded by NIH/NIA (5R01AG048209). This work was solely conducted by the authors; the funding agency had no role in in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Clinical trials: This is a secondary analysis of samples and data from a randomized clinical trial that was registered on ClinicalTrials.gov: NCT02516202
Presentation: These data were presented in part at the North American Menopause Society Annual Meeting in September 25-28, Chicago, IL
Disclosure statement: Dr. Mitchell reports receiving grant funding from Merck, and has served as a consultant to Scynexis, Inc. Dr. Reed receives research funding from Bayer. Dr. Fredricks receives royalties from BD. Dr. Srinivasan has received speaking honoraria from Lupin Inc. The remaining authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- 1.Huang AJ, Moore EE, Boyko EJ, et al. Vaginal symptoms in postmenopausal women: self-reported severity, natural history, and risk factors. Menopause. 2010;17(1):121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009;6(8):2133–2142. [DOI] [PubMed] [Google Scholar]
- 3.Huang AJ, Gregorich SE, Kuppermann M, et al. Day-to-Day Impact of Vaginal Aging questionnaire: a multidimensional measure of the impact of vaginal symptoms on functioning and well-being in postmenopausal women. Menopause. 2015;22(2):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinkerton JV, Bushmakin AG, Komm BS, Abraham L. Relationship between changes in vulvar-vaginal atrophy and changes in sexual functioning. Maturitas. 2017;100:57–63. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell CM, Reed SD, Diem S, et al. Efficacy of Vaginal Estradiol or Vaginal Moisturizer vs Placebo for Treating Postmenopausal Vulvovaginal Symptoms: A Randomized Clinical Trial. JAMA Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biehl C, Plotsker O, Mirkin S. A systematic review of the efficacy and safety of vaginal estrogen products for the treatment of genitourinary syndrome of menopause. Menopause. 2019;26(4):431–453. [DOI] [PubMed] [Google Scholar]
- 7.Golob JL, Pergam SA, Srinivasan S, et al. Stool Microbiota at Neutrophil Recovery Is Predictive for Severe Acute Graft vs Host Disease After Hematopoietic Cell Transplantation. Clin Infect Dis. 2017;65(12):1984–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell CM, Watson L, Mitchell AJ, et al. Vaginal Microbiota and Mucosal Immune Markers in Women With Vulvovaginal Discomfort. Sex Transm Dis. 2020;47(4):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srinivasan S, Morgan MT, Fiedler TL, et al. Metabolic signatures of bacterial vaginosis. MBio. 2015;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plantinga AM, Chen J, Jenq RR, Wu MC. pldist: ecological dissimilarities for paired and longitudinal microbiome association analysis. Bioinformatics. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao N, Chen J, Carroll IM, et al. Testing in Microbiome-Profiling Studies with MiRKAT, the Microbiome Regression-Based Kernel Association Test. Am J Hum Genet. 2015;96(5):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell CM, Srinivasan S, Zhan X, et al. Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell CM, Srinivasan S, Plantinga A, et al. Associations between improvement in genitourinary symptoms of menopause and changes in the vaginal ecosystem. Menopause. 2018;25(5):500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummelen R, Macklaim JM, Bisanz JE, et al. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS One. 2011;6(11):e26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauci S, Driussi S, De Santo D, et al. Prevalence of bacterial vaginosis and vaginal flora changes in peri- and postmenopausal women. J Clin Microbiol. 2002;40(6):2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillier SL, Lau RJ. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin Infect Dis. 1997;25 Suppl 2:S123–126. [DOI] [PubMed] [Google Scholar]
- 17.Shen J, Song N, Williams CJ, et al. Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci Rep. 2016;6:24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brotman RM, Shardell MD, Gajer P, et al. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause. 2014;21(5):450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang R, Daroczy K, Xiao B, Yu L, Chen R, Liao Q. Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J Med Microbiol. 2012;61(Pt 5):729–739. [DOI] [PubMed] [Google Scholar]
- 20.Kroll R, Archer DF, Lin Y, Sniukiene V, Liu JH. A randomized, multicenter, double-blind study to evaluate the safety and efficacy of estradiol vaginal cream 0.003% in postmenopausal women with dyspareunia as the most bothersome symptom. Menopause. 2018;25(2):133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker KM, Godha K, Mirkin S, Archer DF. Vaginal pH: a simple assessment highly correlated with vaginal morphology and symptoms in postmenopausal women. Menopause. 2018;25(7):762–766. [DOI] [PubMed] [Google Scholar]
- 22.Kollmann Z, Bersinger N, von Wolff M, Thurman AR, Archer DF, Stute P. Vaginal cytokines do not correlate with postmenopausal vulvovaginal symptoms. Gynecol Endocrinol. 2015;31(4):317–321. [DOI] [PubMed] [Google Scholar]
- 23.Stute P, Kollmann Z, Bersinger N, von Wolff M, Thurman AR, Archer DF. Vaginal cytokines do not differ between postmenopausal women with and without symptoms of vulvovaginal irritation. Menopause. 2014;21(8):840–845. [DOI] [PubMed] [Google Scholar]
- 24.Sivro A, Lajoie J, Kimani J, et al. Age and menopause affect the expression of specific cytokines/chemokines in plasma and cervical lavage samples from female sex workers in Nairobi, Kenya. Immun Ageing. 2013;10(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chappell CA, Isaacs CE, Xu W, et al. The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol. 2015;213(2):204 e201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: (A) There were no significant association between the profile of metabolites and responder status throughout the trial. Responders were defined as women who had a ≥2-point decrease in most bothersome symptom (MBS) severity over 12 weeks of treatment and non-responders were defined as women who had <1-point decrease. (B) Baseline sarcosine and alanine levels were lower among responders.
Supplemental Figure 2: Subgroup analyses of women who reported an MBS of pain with penetration (A) or vaginal dryness (B) found no significant associations between responder status and Shannon index. Responders were defined as women who had a ≥2-point decrease in most bothersome symptom (MBS) severity over 12 weeks of treatment and non-responders were defined as women who had <1-point decrease.