Abstract
Sexually dimorphic establishment of the reproductive tract system requires sex-specific regression of the Wolffian duct and Müllerian duct in the mesonephros. In an XX embryo, the Wolffian duct regresses under the control of the mesenchymal transcription factor COUP-TFII. To understand cellular and molecular actions underlying Wolffian duct regression, we performed transcriptomic analyses of XX mesonephroi with or without Coup-tfII and genome-wide analysis of COUP-TFII chromatin occupancy in XX mesonephroi. The integrative analysis of COUP-TFII genome-wide binding and transcriptomic analysis revealed the suppression of muscle differentiation and extracellular matrix genes by COUP-TFII and identified a group of COUP-TFII’s potential transcriptional partners in the mesenchyme that potentially facilitate Wolffian duct regression. These findings provide insights into the molecular action of COUP-TFII in the Wolffian duct mesenchyme and identify a list of biologically relevant candidate genes and pathways for future functional analyses in sexual differentiation of reproductive tracts.
Keywords: Wolffian duct, Sexual differentiation, reproductive tracts, COUP-TFII
Introduction
Sexually dimorphic establishment of reproductive tracts require sex-specific regression of primitive male and female reproductive tracts, also known as Wolffian and Müllerian ducts, respectively [Zhao and Yao, 2019a]. In most mammals, before sexual differentiation, the Wolffian and Müllerian ducts co-exist in the mesonephros of both XX and XY embryos [Shaw and Renfree, 2014]. During sexual differentiation, fetal ovaries in XX embryos do not produce androgens, the predominant hormone that maintains the survival of Wolffian ducts [Jost, 1947, 1953; Murashima et al., 2011]. As a result, the Wolffian ducts regress. Therefore, Wolffian duct regression was thought to be a default and passive process that occurs in the absence of androgen actions [Jost, 1947, 1953]. However, we discovered that Wolffian duct regression in the mouse embryo is promoted by a local mesenchymal transcriptional factor, chicken ovalbumin upstream promoter transcription factor II (COUP-TFII, or NR2F2) [Zhao et al., 2017]. COUP-TFII is expressed in the mesenchymal compartment of a variety of tissues and plays critical roles in embryonic and fetal organ development [Takamoto et al., 2005; Tang et al., 2010; Yu et al., 2012]. Absence of Coup-tfII in the mesenchyme of the mesonephros led to ectopic maintenance of Wolffian ducts in XX embryos independent of androgen actions [Zhao et al., 2017]. This phenotype is similar to the retention of Wolffian duct remnants (also known as mesonephric duct remnants or Gartners’ ducts) in XX patients. Although retention of Wolffian duct remnants in XX individuals are clinically asymptomatic on most occasions, residual tissues can undergo malignant transformations, developing cystic masses [Bats et al., 2009; Tiwari et al., 2014; Davidson and Barber, 2017].
In this study, we identified potential factors responsible for Wolffian duct regression by comparing the transcriptomes between control XX (proper Wolffian duct regression) and Coup-tfII knockout XX (Wolffian duct retention) without the complication of androgen action. Since COUP-TFII is a transcriptional factor, we also sought its genome-wide bound chromatin regions and the potential direct targets of COUP-TFII by chromatin immunoprecipitation followed by sequencing (ChIP-seq) assays in XX mouse mesonephroi. These strategies yielded a list of biologically relevant genes and pathways that serve as an entry point for future functional analyses and understanding of the etiologies of human disorders of sex development.
Materials and Methods
Mouse models
Wt1CreERT2 knock-in mice (Stock # 010912) in mixed genetic backgrounds (C57BL/6, Swiss Webster, and B6129SF1/J) was purchased from Jackson Laboratories (Bar Harbor, ME). Generation of the floxed Coup-tfII mice was described in our previous work [Takamoto et al., 2005]. Timed mating was produced by housing two females with a male. Vaginal plugs were checked daily, and the day when the vaginal plug was found was designated as embryonic day E0.5.
Wt1CreERT2;Coup-tfIIf/f mouse was used for Coup-tfII knockout in the mesonephros as previously described [Zhao et al., 2017]. CreERT2 activity in the fetal embryos was induced by intraperitoneal injection of 2.5 mg tamoxifen (T-5648, Sigma) per dam once daily on E11.5 and E12.5. The Wt1Cre- ;Coup-tfIIf/f and Wt1Cre+;Coup-tfIIf/f embryos were designated as the control and knockout, respectively. Mesonephroi were collected on E14.5, the time of initiation of sexual differentiation of reproductive tracts.
All animal procedures were approved by the National Institute of Environmental Health Sciences (NIEHS) Animal Care and Use Committee and are in compliance with a NIEHS-approved animal study proposal and public laws.
Microarray analysis
Gene expression analysis was conducted using GeneChip® Mouse Transcriptome Array 1.0 (Affymetrix, Santa Clara, CA) on four biological replicates (one pair of E14.5 mesonephros) for each genotype. One hundred ng of total RNA was amplified as instructed in the Affymetrix 3’ IVT Express kit protocol. Amplified biotin-aRNAs (12.5 μg) were fragmented and 10 μg were hybridized to each array for 16 hours at 45°C in a rotating hybridization oven using the Affymetrix Eukaryotic Target Hybridization Controls and protocol. Array slides were stained with streptavidin/phycoerythrin utilizing a double-antibody staining procedure and then washed for antibody amplification according to the GeneChip Hybridization, Wash and Stain Kit and user manual. Arrays were scanned in an Affymetrix Scanner 3000 and data were obtained using the GeneChip® Expression Console Software (AGCC; Version 1.2) using the MAS5 algorithm. The resulting data were processed using Partek Genomic Suite Software. The cutoff for identifying differentially expressed genes is fold-change >1.2 and P < 0.05.
ChIP-seq assays and analysis
XX mesonephroi from E14.5 CD1 embryos were snap-frozen and stored at −80°C. Two independent ChIP-seq experiments were performed by Active Motif Inc. (Carlsbad, CA) using 20–30 μg of sheared chromatin from pooled embryonic mesonephroi (n = 80–100 mesonephroi/ChIP), and 4 μg of COUP-TFII rabbit antibody (Active Motif cat # 61213). ChIP-seq libraries were sequenced as single-end 75-bp with an Illumina NextSeq 500, then filtered to retain only reads with average base quality score > 20. Adapter sequence was trimmed from the ends of reads with Cutadapt v1.2 [Martin, 2011] with parameters ‘-a AGATCGGAAGAG -O 5 -q 0’; reads less than 30bp in length after trimming were excluded from further analysis. Filtered and trimmed reads were mapped against the mouse mm10 reference genome using Bowtie v1.2 (61) with parameter ‘-m 1’ to collect only uniquely-mapped hits. Duplicate mapped reads were removed using Picard tools MarkDuplicates.jar (v1.110). The number of uniquely-mapped non-duplicate reads for each biological replicate was 25,975,313 and 27,234,399. After merging the replicates, binding regions were identified by peak calling using HOMER v.10.3 (62) with parameters ‘-style factor -fdr 0.00001’. Called peaks were subsequently redefined as 300bp centered on the called peak midpoints. Enriched motifs were identified using HOMER findMotifsGenome.pl known motif analysis with parameter ‘-size given’.
The genomic context of COUP-TFII peaks was determined by finding if peaks overlapped the following regions, defined by Gencode VM18 gene models, Upstream = from −10kb to −1kb from TSS; TSS proximal = from −1kb to the TSS; Gene body = from TSS to TES; Intergenic = everything that does not fall in any of the above categories. In cases where a peak overlapped more than one type of area, the assignment of context was made based on the priority order of TSS proximal > upstream > gene body > intergenic. For the purposes of integration with transcriptomic analysis, TSS proximal peaks were assigned to the nearest gene based on distance to TSS with maximum distance 1kb. The ChIP-seq data are available in GEO under accession GSE157836 (http://www.ncbi.nlm.nih.gov/geo/).
Real-time PCR
Collected E14.5 mesonephroi were snap-frozen. RNA extraction and first strand cDNA synthesis from 150 ng RNA were performed using PicoPure RNA Isolation kit (Life technologies, USA) and the Superscript II cDNA synthesis kit (Invitrogen, USA), respectively, according to manufacturer’s protocols. SYBR primer pairs (Supplementary table 1) were used to run thermal cycles in the Bio-Rad CFX96 Real-Time PCR Detection system. Taqman gene-expression probes (Nr2f2, Mm00772789_m1 and Gapdh, mm99999915_g1) were used for examining the relative Nr2f2 expression. All samples were analyzed in duplicate and normalized to the housekeeping gene Gapdh. The relative expression was reported as a ratio of the expression of genes in knockout mice relative to those of wild type mice.
Immunofluorescence
Mesonephros and gonad complexes were fixed in 4% paraformaldehyde at 4°C overnight. Tissues and sections were processed for immunostaining as previously described [Zhao et al., 2017]. Tissues were dehydrated, embedded, and cyrosectioned at 10 μm. The sections were subjected for antigen retrieval (for detection COUP-TFII and TWIST1) using commercial antigen unmasking solution (H-3300, VECTOR) and underwent immunostaining procedures. The following primary antibodies were used: mouse anti-COUP-TFII (1:300, PP-H7147–00, R&D systems) and mouse anti-TWIST1 (1:200, ab50887, Abcam). The secondary antibodies conjugated with different fluorescent dyes were used (1:200): Alexa Fluor@ 488 and 568 donkey anti-mouse IgG. All the results were imaged under Leica DMI4000 confocal microscope.
Statistical analysis
Quantitative data is presented as mean±SEM. Sample sizes are N=4 in each group in RT-PCR results. A minimum of three biological replicates were used in immunofluorescence. Two-tail Student’s t test was used for evaluating significant differences. The significance level was set at p<0.05.
Results
Transcriptomic changes in the Wolffian ducts of XX mesonephroi in the absence of COUP-TFII
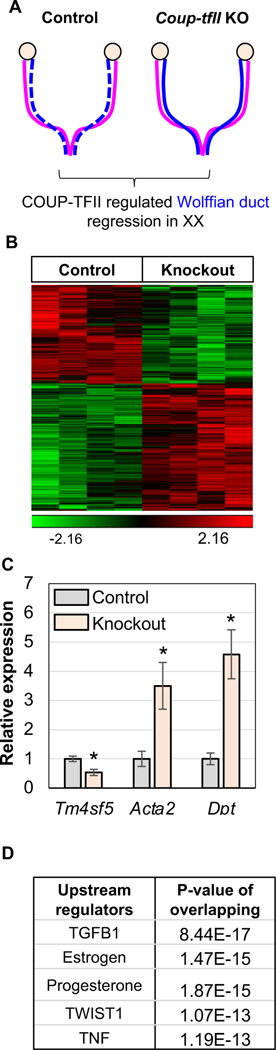
Wolffian duct regression in the normal XX mesonephros is promoted by the transcriptional factor COUP-TFII in the mesenchyme [Zhao et al., 2017]. To identify potential factors and signaling pathways downstream of COUP-TFII action in Wolffian duct regression, we compared the transcriptomes between control and Coup-tfII knockout XX mesonephroi at E14.5 when Wolffian ducts regression starts (Fig. 1A). In the absence of COUP-TFII action, expression of 526 genes was altered in the XX mesonephros, with 228 downregulated and 298 upregulated (Fig. 1B). Coup-tfII (or Nr2f2; FC=−2.51) was the most downregulated gene, confirming the effective Coup-tfII ablation in this mouse model. The most upregulated gene (FC=3.53) in the absence of Coup-tfII was an extracellular matrix protein Dpt (Dermatopontin). To further validate our microarray results, we performed RT-PCR for the top 20 down- and up-regulated genes (Supplementary tables. 2 & 3), including the upregulated genes Dpt and Acta2 and the downregulated gene Tm4sf5 (Fig. 1C and supplementary figure 1). We confirmed most of these differentially expressed genes identified in our microarray (Supplementary figure. 1). Of note, Acta2 was one of the top upregulated genes, known to be suppressed by COUP-TFII in vitro [Bailey et al., 1998; Xie et al., 2011] and in vivo [Lee et al., 2017].
Figure 1:
Transcriptomic comparison between control and Coup-tfII knockout mesonephroi reveals genes and signaling pathways associated with the regression of Wolffian ducts. (A) The experimental model used for revealing molecular actions underlying Wolffian duct regression. (B) Heatmap of differentially expressed genes between control and Coup-tfII knockout. (C) Validation of representative differentially expressed genes by qPCR. t-test; mean±SEM (n=4); P<0.05. (D) Top upstream regulators for differentially expressed genes by IPA analysis.
To identify signaling pathways that may be involved in COUP-TFII mediated Wolffian duct regression, we performed IPA analysis to uncover upstream regulators which may control the observed expression changes upon Coup-tfII ablation [Kramer et al., 2014]. The analysis suggested TGFB1, estrogen, progesterone, TNF, and TWIST1 signaling were enriched in the differential genes (Fig. 1D and supplementary table 4). TGFB1 signaling is known to be involved in Wolffian duct development [Hannema and Hughes, 2007]. Estrogen [Kurihara et al., 2007], progesterone [Kurihara et al., 2007], and TNF signaling [Li et al., 2013] were documented to interact with COUP-TFII in stromal compartment of adult female reproductive tract system. TWIST1 is a mesoderm associated transcriptional factor critical for organogenesis [Barnes and Firulli, 2009]; however, its association with COUP-TFII in the female reproductive tract system has not been reported. We performed immunostaining of COUP-TFII and TWIST1 on two consecutive sections (co-staining of both antibodies in one section was not feasible due to antibody incompatibility). We found these two transcription factors partially colocalized in the mesenchymal cells of mesonephros (Supplementary figure 2). Taken together, analysis of transcriptomic changes between control and Coup-tfII knockout XX mesonephroi identified potential factors and signaling pathways involved in Wolffian duct regression.
Identification of potential direct target genes of COUP-TFII in Wolffian duct regression
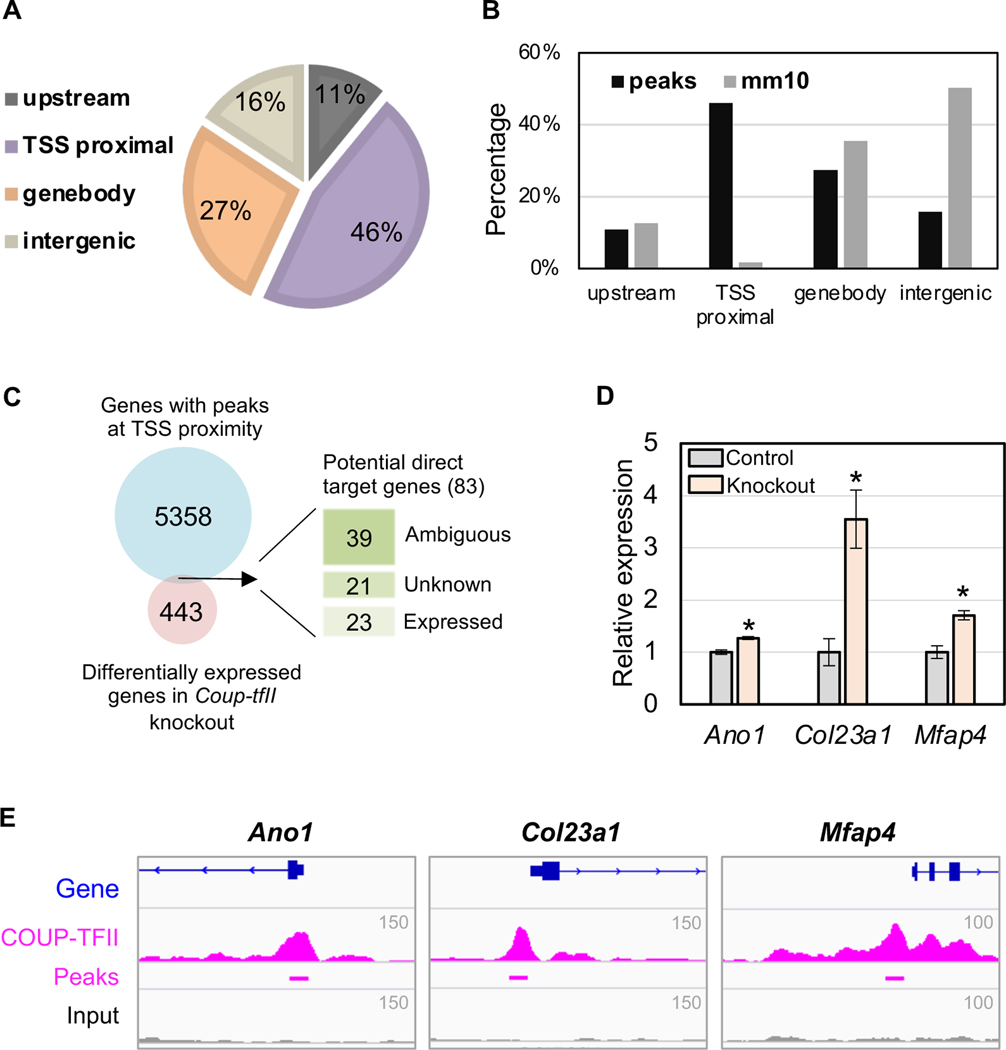
To further understand COUP-TFII transcriptional regulation, we performed COUP-TFII ChIP-seq on E14.5 XX mesonephros in two replicates. The replicates had high correlation (R=0.904), indicating the reproducibility of our COUP-TFII ChIP-seq experiments. The ChIP-seq analysis identified 11,175 peaks, among which 46% were localized at transcription starting site (TSS) proximal regions (−1kb-TSS), 27% in the gene body, 16% in the intergenic regions and 11% upstream (Fig. 2A). The percentage of peaks at TSS proximal regions in ChIP-seq was higher than that of genome-wide GRCm38/mm10 annotations, which specify localizations of transcription starting sites, indicating that COUP-TFII preferentially bound to TSS regions (Fig. 2B).
Figure 2:
Integrative analyses of microarray and COUP-TFII ChIP-seq reveal gene candidates for direct regulation by COUP-TFII in the Wolffian duct mesenchyme. (A) Genomic context of COUP-TFII binding peaks in E14.5 XX mesonephroi. (B) Percentages of COUP-TFII binding peaks and annotation in mm10 localized in the upstream, TSS proximal, gene body, intergenic regions. (C) Comparison of genes with peaks at TSS proximity and differentially expressed genes, and the expression of their overlapped genes in the mesonephros. (D) Validation of three COUP-TFII direct target genes by qPCR. t-test; mean±SEM (n=4); P<0.05. (E) Genome browser view of COUP-TFII-bound regions near Ano1, Col23a1, and Mfap4.
We assigned COUP-TFII binding peaks to the nearest genes, resulting in 5,441 potential target genes. Overlapping these genes with the 526 genes that were changed by the loss of Coup-tfII in Fig. 1B allowed us to identify 83 potential direct target genes of COUP-TFII in the mesonephros (Fig. 2C). We then queried the expression of patterns of these 83 genes using online gene expression databases [Bult et al., 2019] and GUDMAP [McMahon et al., 2008; Harding et al., 2011] that include in situ hybridization and transcriptomic data for various cell types in the mesonephros. We found that expression of 39 genes were ambiguous in mesonephros, 21 genes were not examined (Supplementary table 5), and 23 genes (Supplementary table 6) were detected in mesonephros (Fig. 2C). Among these 23 genes, 4 genes (Ano1, Col23a1, Enc1, and Mfap4) were expressed in the mesenchyme, where COUP-TFII is present. We confirmed the differential expression of Ano1 (Anoctamin-1), Col23a1 (Collagen, type XXIII, alpha 1) and Mfap4 (microfibrillar-associated protein 4) in the Coup-tfII knockout tissue by RT-PCR (Fig. 2D). Ano1, Col23a1 and Mfap4 were all upregulated by the loss of Coup-tfII and their TSS proximal regions had distinct COUP-TFII binding signatures (Fig. 2E), implicating that they were direct target genes of COUP-TFII transcriptional suppression. Knockout mice were generated for Mfap4 [Holm et al., 2015] and Ano1[Rock et al., 2008] but the functional significance of these genes in Wolffian duct has not been examined.
COUP-TFII associated transcriptional factors in the mesonephros
We next performed HOMER motif analysis to determine which known transcription factor binding motifs were enriched in COUP-TFII binding peaks [Heinz et al., 2010]. We categorized peaks by their locations, the upstream (>1kb TSS) or TSS regions (<1kb TSS), to identify potential transcriptional factors associated with COUP-TFII at the enhancer or promoter regions, respectively. The sequence of COUP-TFs’ response element is similar to multiple members in nuclear factor family [Pawlak et al., 2012]. As expected, the top five enriched motifs were nuclear receptors, COUP-TFs (NR2F1, NR2F1), EAR2 (NR2F6), TR4 (NR2C2), RARa (NR1B1), and THRb (NR1A2) (Table 1). Other than nuclear receptors, we were also interested in transcription factors that share a similar expression pattern with COUP-TFF in the mesonephros based on online gene expression databases MGI and GUDMAP [McMahon et al., 2008; Harding et al., 2011; Bult et al., 2019]. There were 22 transcription factors of this kind, with 13 and 9 motifs enriched in TSS and upstream COUP-TFII peaks (Table 2), respectively. Among these transcription factors, 8 of them (cMYC, HIF-1A, SRF, TCF4, TCF21, FOXK1, and MYOG) were essential for muscle differentiation and function based on their knockout phenotypes indicated in the Mouse Genome Database [Bult et al., 2019]. This observation is consistent with the suggested role of COUP-TFII in myogenic differentiation in the mesonephric mesenchyme (Fig. 1C). Of note, MITF and HOXC9 motifs were enriched in both TSS and upstream COUP-TFII peaks (Table 2). MITF (melanocyte-inducing transcription factor) is a COUP-TFII-regulated transcriptional factor in retinal pigmented epithelium [Tang et al., 2010]. HOXC9 is expressed in the proximal region of Wolffian ducts during sexual differentiation [Snyder et al., 2010]. These observations indicate that MITF and HOXC9 may act as COUP-TFII transcriptional partners in the mesonephric mesenchyme in Wolffian duct regression.
Table 1:
Top enriched motifs in COUP-TFII binding sites
| Homer known motif enrichment (Top five) | ||||
|---|---|---|---|---|
| Upstream (>1kb TSS) (Total Target Sequences=1220, Total Background Sequences=48350) | ||||
| Rank | Motif | Name | log P-value | % of Targets (Background) Sequences with Motif |
| 1 | EAR2(NR)/K562-NR2F6-ChIP-Seq(Encode)/Homer | −3.374e+02 | 52.95% (19.62%) | |
| 2 | COUP-TFII(NR)/Artia-Nr2f2-ChIP-Seq(GSE46497)/Homer | −3.275e+02 | 60.25% (25.65%) | |
| 3 | COUP-TFII(NR)/K562-NR2F1-ChIP-Seq(Encode)/Homer | −3.044e+02 | 53.11% (21.08%) | |
| 4 | TR4(NR),DR1/Hela-TR4-ChIP-Seq(GSE24685)/Homer | −3.036e+02 | 16.97% (1.75%) | |
| 5 | RARa(NR)/K562-RARa-ChIP-Seq(Encode)/Homer | −1.772e+02 | 62.13% (35.81%) | |
| TSS (<1kb TSS) (Total Target Sequences=5140, Total Background Sequences=43503) | ||||
|---|---|---|---|---|
| Rank | Motif | Name | log P-value | % of Targets (Background) Sequences with Motif |
| 1 | COUP-TFII(NR)/Artia-Nr2f2-ChIP-Seq(GSE46497)/Homer | −5.340e+02 | 42.28% (21.94%) | |
| 2 | EAR2(NR)/K562-NR2F6-ChIP-Seq(Encode)/Homer | −4.737e+02 | 33.43% (16.01%) | |
| 3 | COUP-TFII(NR)/K562-NR2F1-ChIP-Seq(Encode)/Homer | −4.561e+02 | 34.54% (17.12%) | |
| 4 | RARa(NR)/K562-RARa-ChIP-Seq(Encode)/Homer | −2.502e+02 | 42.87% (28.34%) | |
| 5 | THRb(NR)/Liver-NR1A2-ChIP-Seq(GSE52613)/Homer | −2.127e+02 | 60.83% (46.61%) | |
Table 2:
Potential transcriptional partners of COUP-TFII in the mesonephros identified by ChIP-seq motif
| Motifs in COUP-TFII ChIP-seq peaks These transcription factors (TF) are expressed in the mesonephros | |||||
|---|---|---|---|---|---|
| TSS (<1kb TSS) | Upstream (>1kb TSS) | ||||
| Motif | Log p-value | Ratio | Motif | Log p-value | Ratio |
| ELK4 | −181.70 | 1.55 | TCF41 | −19.89 | 1.34 |
| ETS1 | −91.27 | 1.39 | HOXC92 | −16.31 | 1.98 |
| MITF2 | −52.09 | 1.40 | TCF21 | −13.98 | 1.34 |
| ATF7 | −36.22 | 1.48 | TFAP4 | −10.88 | 1.25 |
| E2F4 | −30.50 | 1.21 | MITF2 | −9.45 | 1.30 |
| cMYC1 | −28.04 | 1.21 | FOXK11 | −6.25 | 1.32 |
| HIF-1B | −26.34 | 1.18 | WT11 | −5.38 | 1.19 |
| RFX3 | −15.55 | 1.52 | GATA2 | −5.01 | 1.34 |
| HIF-1A1 | −9.14 | 1.21 | MYOG1 | −4.63 | 1.14 |
| PKNOX1 | −8.91 | 1.29 | |||
| SRF1 | −7.82 | 1.35 | |||
| E2F1 | −5.70 | 1.11 | |||
| HOXC92 | −4.78 | 1.26 | |||
Enriched TF motifs that are critical for muscle development
Enriched TF motifs for both upstream and TSS regions
Discussion:
Sexually dimorphic establishment of the reproductive tract system requires the elimination of Wolffian ducts in XX and Müllerian ducts in XY, which are controlled by COUP-TFII and AMH/AMHR2 mediated mesenchymal signaling, respectively. In this study, to understand molecular actions underlying Wolffian duct regression, we determined transcriptomic changes upon Coup-tfII ablation and the genome-wide binding of COUP-TFII in XX mesonephroi. We uncovered actions of mesenchymal COUP-TFII in suppressing muscle differentiation and specific extracellular matrix genes (Figs. 1C & 2D; Table 2), and identified COUP-TFII’s potential direct target genes and partners in its transcription function (Fig. 2C; Tables. 2, S5 and S6). A number of potential target genes display expression changes but lack COUP-TFII binding, suggesting these may be regulated indirectly by COUP-TFII (Fig. 2C).
Towards the understanding of COUP-TFII functions in the mesenchyme and how mesenchymal changes in the absence of Coup-tfII leads to Wolffian duct Maintenance in XX embryos
Our study reveals two potential actions of COUP-TFII in mesenchymal cells in the mesonephros: suppressing myogenic differentiation and extracellular matrix genes, which are evidenced by increased expression of myogenic marker Acta2 and extracellular matrix genes Col23a1, Mfap4 and Lama1 in the absence of Coup-tfII. COUP-TFII is widely expressed in the mesenchymal compartment of developing organs [Lin et al., 2011] and acts as a transcriptional suppressor in organ development [You et al., 2005; Chen et al., 2015]. The transcriptional repression of mesenchymal differentiation to muscular tissue by COUP-TFII had been documented in vitro [Bailey et al., 1998; Xie et al., 2011] and in vivo [Lee et al., 2017]. Inactivation of Coup-tfII in mouse mesenchymal cell line caused the preferential differentiation to muscular cell types, osteoblasts and myoblasts [Xie et al., 2011]. Coup-tfII haplodeficiency or overexpression in mice resulted in enhanced or inefficient muscle development, respectively [Xie et al., 2011; Lee et al., 2017]. One of the potential molecular mechanisms is that COUP-TFII represses activity/function of MyoD, a basic helix-loop-helix transcriptional regulator for muscle differentiation, through competitively binding to the MyoD coactivator [Bailey et al., 1998]. In situ hybridization data available in the online gene expression database [McMahon et al., 2008; Harding et al., 2011] did not detect MyoD expression in the mesonephros. However, MyoG, a member of the MyoD family of transcription factors is expressed in the mesonephros and the motif for MyoG was enriched in COUP-TFII binding peaks (Table. 2). These results suggest that COUP-TFII might modulate the transcriptional activity of MyoG in suppressing myogenic differentiation in the mesonephros. Besides, COUP-TFII’s suppression of extracellular matrix genes, such as collagens and Lama1 (table. S6), was consistent with an earlier study on COUP-TFII functions in human endometrial stromal cells [Li et al., 2013].
It remains to be determined how muscle related genes and extracellular matrix genes of the mesenchyme influence the regression/maintenance of Wolffian duct epithelium. Of note, both smooth muscle actin and extracellular matrix play integral roles in regulating the development of cellular mechanical tension and properties [Gunst and Zhang, 2008]. The mechanical forces and properties of the mesenchyme can regulate epithelial fate and differentiation as demonstrated in development of mammary gland and tooth [Kass et al., 2007; Calamari et al., 2018]. Therefore, it is possible that mechanical properties of the mesenchyme were altered in the absence of Coup-tfII in such a way that the mesenchyme promotes the maintenance of Wolffian duct in XX embryos. Wolffian ducts can be isolated devoid of any mesenchymal cells for ex vivo culture [Maeshima et al., 2007]. It would be interesting to examine the fate and differentiation of Wolffian duct epithelium in the culture platforms of different extracellular matrices and mechanical properties.
Identifying COUP-TFII related transcriptional factors in the mesenchyme that potentially control the regression/maintenance of Wolffian ducts
Our microarray and ChIP-seq analyses highlight a list of transcription factors in the mesenchyme that potentially are involved in mesenchymal differentiation and regulation of Wolffian duct regression (Fig. 1D & Table 2). Of note, TWIST1 is a master transcriptional factor regulating phenotypes and behaviors of mesenchymal cells during morphogenesis [Chen and Behringer, 1995] and directing epithelial-mesenchymal transition in the conversion of early stage tumors into invasive malignancies [Yang et al., 2004]. TWIST1 expression was reported in the mesonephric mesenchyme [Stewart et al., 2013] but its functional roles in sexual differentiation of reproductive tracts remained unclear.
Notably, motif analysis of COUP-TFII binding peaks did not reveal androgen receptor (AR), which is the predominant transcriptional factor in the mesenchyme for maintaining Wolffian ducts in the XY mice [Gaspar et al., 1991; Yeh et al., 2002; Murashima et al., 2011]. Since COUP-TFII suppresses the survival of Wolffian ducts in the XX embryos, androgen receptor actions in XY embryos must antagonize COUP-TFII actions, therefore allowing Wolffian ducts to survive. Because AR and COUP-TFII belong to different subfamilies (i.e. steroid receptor and orphan nuclear receptor subfamily respectively) in the nuclear receptor family[Mazaira et al., 2018] and have different DNA response elements [Pawlak et al., 2012], AR may bind to differential sets of regulatory regions and target genes to maintain the survival of Wolffian ducts. The actions of mesenchymal AR in promoting Wolffian duct survival was suggested to act through the induction of epidermal growth factors (EGFs) [Gupta, 1996; Gupta and Singh, 1996] while mesenchymal COUP-TFII in stunting Wolffian duct survival was partly through suppression of fibroblast growth factors (FGFs) [Zhao et al., 2017]. EGFs and FGFs bind to their own membrane receptor tyrosine kinases, EGFR and FGFR2 in the Wolffian duct epithelium, activating p-ERK mediated intracellular survival signaling [Zhao and Yao, 2019b]. Therefore, we hypothesize that AR induces a different survival signaling pathway (EGFs) to antagonize COUP-TFII suppression of the survival factor FGFs for the maintenance of Wolffian ducts.
In conclusion, our study provides insights into molecular actions in the mesenchyme for the regression of Wolffian ducts in XX embryos. Mesenchymal COUP-TFII suppresses muscle differentiation, extracellular matrix genes and expression of fibroblast growth factors. Those genes and pathways identified in our study are potential candidates for future functional analyses in sexual differentiation of reproductive tracts.
Supplementary Material
Acknowledgements:
We are thankful to Drs. Ming-Jer Tsai and Sophia Tsai in Baylor College of Medicine for providing us Coup-tfII flox mice. We are grateful to the NIEHS Molecular Genomics Core for the microarray analyses, Comparative Medicine Branch for mouse colony maintenance, and Paula Brown and Karina Rodriguez in Dr. Yao’s lab for their help with packaging and shipping samples. We are grateful to Robert Lipinski’s lab at the University of Wisconsin-Madison for the use of the Bio-Rad CFX96 Real-Time PCR Detection system and to Shuai Jia at the University of Wisconsin-Madison for his help with primer preparations.
Funding Sources:
This work was supported by the Intramural Research Program of National Institute of Environmental Health Sciences (Z01-ES102965 for HHCY) and National Institute of Child Health and Development (R00-HD096051 for FZ).
Footnotes
Statement of Ethics:
All animal procedures were approved by the National Institute of Environmental Health Sciences (NIEHS) Animal Care and Use Committee and are in compliance with a NIEHS-approved animal study proposal and public laws. The approved Animal Study Protocol (ASP) number is 2010–0016 RDBL.
Conflict of Interest Statement:
The authors have no conflicts of interest to declare.
References
- Bailey P, Sartorelli V, Hamamori Y, Muscat GE: The orphan nuclear receptor, COUP-TF II, inhibits myogenesis by post-transcriptional regulation of MyoD function: COUP-TF II directly interacts with p300 and myoD. Nucleic Acids Res 26:5501–5510 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes RM, Firulli AB: A twist of insight - the role of Twist-family bHLH factors in development. Int J Dev Biol 53:909–924 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bats AS, Metzger U, Le Frere-Belda MA, Brisa M, Lecuru F: Malignant transformation of Gartner cyst. Int J Gynecol Cancer 19:1655–1657 (2009). [DOI] [PubMed] [Google Scholar]
- Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE, Mouse Genome Database G: Mouse Genome Database (MGD) 2019. Nucleic Acids Res 47:D801–D806 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamari ZT, Hu JK, Klein OD: Tissue Mechanical Forces and Evolutionary Developmental Changes Act Through Space and Time to Shape Tooth Morphology and Function. Bioessays 40:e1800140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Wei SY, Yang WS, Wu TT, Li HY, Ho HN, Yang YS, Chen PL: Concurrent exome-targeted next-generation sequencing and single nucleotide polymorphism array to identify the causative genetic aberrations of isolated Mayer-Rokitansky-Kuster-Hauser syndrome. Hum Reprod 30:1732–1742 (2015). [DOI] [PubMed] [Google Scholar]
- Chen ZF, Behringer RR: twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev 9:686–699 (1995). [DOI] [PubMed] [Google Scholar]
- Davidson ERW, Barber MD: A Gartner Duct Cyst Masquerading as Anterior Vaginal Prolapse. Obstet Gynecol 130:1039–1041 (2017). [DOI] [PubMed] [Google Scholar]
- Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M: A single base deletion in the Tfm androgen receptor gene creates a short-lived messenger RNA that directs internal translation initiation. Proc Natl Acad Sci U S A 88:8606–8610 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunst SJ, Zhang W: Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 295:C576–587 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C: The role of epidermal growth factor receptor (EGFR) in male reproductive tract differentiation: stimulation of EGFR expression and inhibition of Wolffian duct differentiation with anti-EGFR antibody. Endocrinology 137:905–910 (1996). [DOI] [PubMed] [Google Scholar]
- Gupta C, Singh M: Stimulation of epidermal growth factor gene expression during the fetal mouse reproductive tract differentiation: role of androgen and its receptor. Endocrinology 137:705–711 (1996). [DOI] [PubMed] [Google Scholar]
- Hannema SE, Hughes IA: Regulation of Wolffian duct development. Horm Res 67:142–151 (2007). [DOI] [PubMed] [Google Scholar]
- Harding SD, Armit C, Armstrong J, Brennan J, Cheng Y, Haggarty B, Houghton D, Lloyd-MacGilp S, Pi X, Roochun Y, Sharghi M, Tindal C, McMahon AP, Gottesman B, Little MH, Georgas K, Aronow BJ, Potter SS, Brunskill EW, Southard-Smith EM, Mendelsohn C, Baldock RA, Davies JA, Davidson D: The GUDMAP database--an online resource for genitourinary research. Development 138:2845–2853 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK: Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38:576–589 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm AT, Wulf-Johansson H, Hvidsten S, Jorgensen PT, Schlosser A, Pilecki B, Ormhoj M, Moeller JB, Johannsen C, Baun C, Andersen T, Schneider JP, Hegermann J, Ochs M, Gotz AA, Schulz H, de Angelis MH, Vestbo J, Holmskov U, Sorensen GL: Characterization of spontaneous air space enlargement in mice lacking microfibrillar-associated protein 4. Am J Physiol Lung Cell Mol Physiol 308:L1114–1124 (2015). [DOI] [PubMed] [Google Scholar]
- Jost A: *Recherches Sur La Differenciation Sexuelle De Lembryon De Lapin .1. Introduction Et Embryologie Genitale Normale. Arch Anat Microsc Mo 36:151–200 (1947). [Google Scholar]
- Jost A: Problems of Fetal Endocrinology - the Gonadal and Hypophyseal Hormones. Recent Prog Horm Res 8:379–418 (1953). [DOI] [PubMed] [Google Scholar]
- Kass L, Erler JT, Dembo M, Weaver VM: Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol 39:1987–1994 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer A, Green J, Pollard J, Jr., Tugendreich S: Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30:523–530 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY: COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet 3:e102 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kao CY, Lin SC, Xu M, Xie X, Tsai SY, Tsai MJ: Dysregulation of nuclear receptor COUP-TFII impairs skeletal muscle development. Sci Rep 7:3136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Large MJ, Creighton CJ, Lanz RB, Jeong JW, Young SL, Lessey BA, Palomino WA, Tsai SY, Demayo FJ: COUP-TFII regulates human endometrial stromal genes involved in inflammation. Mol Endocrinol 27:2041–2054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ: Coup d’Etat: an orphan takes control. Endocr Rev 32:404–421 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima A, Sakurai H, Choi Y, Kitamura S, Vaughn DA, Tee JB, Nigam SK: Glial cell-derived neurotrophic factor independent ureteric bud outgrowth from the Wolffian duct. J Am Soc Nephrol 18:3147–3155 (2007). [DOI] [PubMed] [Google Scholar]
- Martin M: Cutadapt removes adapter sequences from high-throughput sequencing reads. 2011 17:3 (2011). [Google Scholar]
- Mazaira GI, Zgajnar NR, Lotufo CM, Daneri-Becerra C, Sivils JC, Soto OB, Cox MB, Galigniana MD: The Nuclear Receptor Field: A Historical Overview and Future Challenges. Nucl Receptor Res 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AP, Aronow BJ, Davidson DR, Davies JA, Gaido KW, Grimmond S, Lessard JL, Little MH, Potter SS, Wilder EL, Zhang P, project G: GUDMAP: the genitourinary developmental molecular anatomy project. J Am Soc Nephrol 19:667–671 (2008). [DOI] [PubMed] [Google Scholar]
- Murashima A, Miyagawa S, Ogino Y, Nishida-Fukuda H, Araki K, Matsumoto T, Kaneko T, Yoshinaga K, Yamamura K, Kurita T, Kato S, Moon AM, Yamada G: Essential roles of androgen signaling in Wolffian duct stabilization and epididymal cell differentiation. Endocrinology 152:1640–1651 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M, Lefebvre P, Staels B: General molecular biology and architecture of nuclear receptors. Curr Top Med Chem 12:486–504 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD: The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol 321:141–149 (2008). [DOI] [PubMed] [Google Scholar]
- Shaw G, Renfree MB: Wolffian duct development. Sex Dev 8:273–280 (2014). [DOI] [PubMed] [Google Scholar]
- Snyder EM, Small CL, Bomgardner D, Xu B, Evanoff R, Griswold MD, Hinton BT: Gene expression in the efferent ducts, epididymis, and vas deferens during embryonic development of the mouse. Dev Dyn 239:2479–2491 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CA, Wang Y, Bonilla-Claudio M, Martin JF, Gonzalez G, Taketo MM, Behringer RR: CTNNB1 in mesenchyme regulates epithelial cell differentiation during Mullerian duct and postnatal uterine development. Mol Endocrinol 27:1442–1454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamoto N, You LR, Moses K, Chiang C, Zimmer WE, Schwartz RJ, DeMayo FJ, Tsai MJ, Tsai SY: COUP-TFII is essential for radial and anteroposterior patterning of the stomach. Development 132:2179–2189 (2005). [DOI] [PubMed] [Google Scholar]
- Tang K, Xie X, Park JI, Jamrich M, Tsai S, Tsai MJ: COUP-TFs regulate eye development by controlling factors essential for optic vesicle morphogenesis. Development 137:725–734 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari U, Relia N, Shailesh F, Kaushik C: Gartner Duct Cyst: CT and MRI Findings. J Obstet Gynaecol India 64:150–151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Qin J, Lin SH, Tsai SY, Tsai MJ: Nuclear receptor chicken ovalbumin upstream promotertranscription factor II (COUP-TFII) modulates mesenchymal cell commitment and differentiation. Proc Natl Acad Sci U S A 108:14843–14848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA: Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939 (2004). [DOI] [PubMed] [Google Scholar]
- Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, Xing L, Boyce BF, Hung MC, Zhang S, Gan L, Chang C: Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A 99:13498–13503 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY: Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435:98–104 (2005). [DOI] [PubMed] [Google Scholar]
- Yu CT, Tang K, Suh JM, Jiang R, Tsai SY, Tsai MJ: COUP-TFII is essential for metanephric mesenchyme formation and kidney precursor cell survival. Development 139:2330–2339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Franco HL, Rodriguez KF, Brown PR, Tsai MJ, Tsai SY, Yao HH: Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science 357:717–720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Yao HH: A Tale of Two Tracts: history, current advances and future directions of research on sexual differentiation of reproductive tracts. Biol Reprod (2019a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Yao HH: A tale of two tracts: history, current advances, and future directions of research on sexual differentiation of reproductive tractsdagger. Biol Reprod 101:602–616 (2019b). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.