Abstract
Background:
It has been proposed that individuals with Generalized Anxiety Disorder (GAD) show dysfunctional computations related to approach-avoidance decision-making. However, few studies have examined the neural basis of this impairment, particularly in adolescents with GAD. The goal of the current study was to address this gap in the literature.
Method:
The study involved 51 adolescents with GAD and 51 typically developing (TD) comparison individuals matched on age (16.10 and 15.75 respective means), gender (30F/21M and 24F/27M) and IQ (103.20 and 103.18 respective means). Participants underwent functional MRI during a Passive Avoidance Task.
Results:
We found a significant Group-by-Reinforcement interaction within reward-related brain regions including the caudate, putamen, mid cingulate/paracentral lobule and superior and middle frontal gyrus. TD adolescents showed a greater differential response to reward versus punishment feedback within these regions relative to adolescents with GAD. In particular, this reflected reduced responses to rewards in the adolescents with GAD. There were no group differences in neural responses when making approach/avoidance responses.
Conclusion:
The results of this study suggest reduced differential responsiveness to reinforcement as a component of the pathophysiology seen in adolescents with GAD. This dysfunction likely underpins decision-making impairments that may exacerbate the participants’ worry.
Keywords: GAD, neuroimaging, fMRI, decision-making, anxiety
INTRODUCTION
Generalized Anxiety Disorder (GAD) is an anxiety disorder from which affected individuals indicate pervasive, sustained, uncontrollable worry as their major symptomatic complaint (Bell & Bell, 2001; Duval, Javanbakht, & Liberzon, 2015). GAD is the most common anxiety disorder in primary care, with a lifetime prevalence rate of up to 6% (Wittchen, 2002). Unfortunately, prognosis is poor as most adult anxiety or depressive disorders are preceded by anxiety disorders in adolescence. (Pine, 2007; Pine, Cohen, Gurley, Brook, & Ma, 1998). GAD is thought to be one of the least successfully treated psychiatric disorders, potentially partly because of the relative lack of knowledge regarding the pathophysiology underpinning this disorder (Li et al., 2020).
It has been proposed that disrupted computations related to approach-avoidance decision-making are implicated in the pathophysiology of GAD (Aupperle & Paulus, 2010; Santiago et al., 2020). GAD may reflect conflicting motivations to approach or avoid anxiogenic situations that also contain potential gains, leading to chronic distress, uncertainty, and use of maladaptive coping mechanisms (i.e., avoidance, worry) (Aupperle & Paulus, 2010). Another, related view of GAD stresses a central role of “intolerance of uncertainty”, a cognitive bias which is thought to interfere with information processing, including decision-making (Ladouceur, Talbot, & Dugas, 1997). Considerable data indicates that self-reports of intolerance of uncertainty are significantly higher in patients with GAD and related conditions relative to typically developing (TD) participants (Gentes & Ruscio, 2011). Uncertainty can occur when a response can result in either reward or punishment (cf. Assaf et al., 2018). This model proposes that excessive emotional responding in uncertain situations contributes to the development and maintenance of worry (Dugas, Gagnon, Ladouceur, & Freeston, 1998).
Both of these views suggest that reinforcement-based decision-making may be disrupted in individuals with GAD. This is largely consistent with previous work. Patients with GAD have been reported to show poorer decision-making (Devido et al., 2009; Dorfman, Rosen, Pine, & Ernst, 2016; though see Mueller, Nguyen, Ray, & Borkovec, 2010) where reinforcement-based decision-making deficits are associated with symptom severity (Devido et al., 2009; Teng et al., 2016). There are also data indicating that rewards are less effective at improving cognitive performance in anxious than in typically developing (TD) adolescents (Hardin, Schroth, Pine, & Ernst, 2007; Jazbec, McClure, Hardin, Pine, & Ernst, 2005)
Related neuroimaging studies have reasonably consistently implicated reported reduced responsiveness to reward within the striatum in patients with GAD in the context of decision-making tasks. The striatum has been consistently shown to respond to reward, relative to punishment in studies with animals and in neuro-imaging studies with typically developing individuals (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000; J. O’Doherty et al., 2004; Schultz, Dayan, & Montague, 1997). However, adolescents with anxiety (mostly GAD), relative to controls, showed reduced striatal responses when anticipating high relative to low rewards (Benson, Guyer, Nelson, Pine, & Ernst, 2015) and during risky relative to safe choices for monetary rewards (Galvan & Peris, 2014). Induced anticipatory anxiety has also been associated with reduced expected value signaling in the ventral striatum in healthy adults (Engelmann, Meyer, Fehr, & Ruff, 2015). Similarly, adults with GAD showed reduced striatal responses to received reward in an instrumental learning task (White et al., 2017) and reduced within-striatum and striatum to frontal cortex correlated activity via connectivity analysis (Dorfman, Benson, Farber, Pine, & Ernst, 2016). In addition, they have been found to show reduced modulation of the striatum and medial frontal cortex when considering rewarding future emotional events (Blair et al., 2017). However, other work, both in adolescents (Guyer et al., 2012) and adults with GAD (Yassa, Hazlett, Stark, & Hoehn-Saric, 2012) did not find reduced striatal responding to reward during a Monetary Incentive Delay task or when making high uncertainty (making a choice between two shapes that had a 50% probability of reward) relative to low uncertainty choices (making a choice between two shapes that had respectively a 100% and 50% probability of reward). It should be noted that the amygdala has also been implicated in this work. Yassa et al. (2012) reported reduced amygdala activity in adults with GAD relative to comparison adults during high uncertainty choices. Similarly, there has been a report that while in comparison adults, the dorsal anterior cingulate cortex (dACC) and amygdala show positive correlated activity during high win frequency blocks and negative correlated activity during high lose frequency blocks, this is significantly reduced in patients with GAD (Assaf et al., 2018). However, a third study with adolescents with GAD and/or Social Anxiety Disorder reported that self-reported intolerance of uncertainty was related to increased amygdala activation during uncertain, or riskier, conditions (Krain et al., 2008).
The goal of the current study was to investigate approach and avoidance decision-making and reinforcement responding in the context of an approach-avoidance instrumental learning task, the passive avoidance (PA) task, in adolescents with GAD relative to typically developing (TD) adolescents. In the PA task (Finger et al., 2011; Kosson et al., 2006; Newman & Kosson, 1986), participants choose to either “approach” (i.e., respond to) stimuli that may engender reward or punishment or to “avoid” responding to the stimulus (leading to the receipt of neither reward nor punishment). The participant’s goal is to learn to distinguish those stimuli probabilistically associated with reward from those probabilistically associated with punishment and choose their approach/avoidance responses accordingly. As such, the task allows a window into both approach-avoidance decision-making and receipt of reward vs. punishment (for exemplar data from this task with an independent sample of TD participants for both phases of the task, see Supplemental Figure 1). While this task has been used in work with adults with GAD (Teng et al., 2016; White et al., 2017), it has not been used previously with adolescents with GAD. On the basis of suggestions that disrupted computations related to approach-avoidance decision-making are implicated in the pathophysiology of GAD (Aupperle & Paulus, 2010), we hypothesized atypical recruitment of regions implicated in avoidance responses (e.g., dorsomedial frontal and anterior insula cortices) in adolescents with GAD (though it should be noted that this was not observed in our previous study with adults with GAD; White et al., 2017). On the basis of our previous work with this task (Teng et al., 2016; White et al., 2017), we hypothesized that adolescents with GAD would show, relative to typically developing adolescents, reduced differential responding to received reward relative to punishment within the striatum and anatomically connected cortical structures such as the ventromedial frontal cortex (vmPFC).
METHODS AND MATERIALS
Participants
Fifty-one adolescents with GAD and 51 TD comparison adolescents participated in the study (age range 11–18 years). The two groups were matched on age, gender and IQ (see Table 1). However, and consistent with the literature (Remes et al., 2018; Shen et al., 2018), 23 of the adolescents with GAD presented with co-morbid major depressive disorder (MDD). Clinical characterization was done through psychiatric interviews by licensed and board-certified psychiatrists with the participants and their parents, to adhere closely to common clinical practice.
Table 1:
Participant characteristics.
| Participants with GAD (N=51) | Participants without GAD (N=51) | p | |
|---|---|---|---|
| Basic Demographics | |||
| Age | 16.10 (SD=1.69) | 15.75 (SD=1.92) | ns |
| Sex | 30F/21M | 24F/27M | ns |
| IQ | 103.20 (SD=4.92) | 103.18 (SD= 10.77) | ns |
| GAD SCARED score | 10.61 (SD=12.36) | 4.50 (SD=3.28) | ns |
| MFQ score | 21.4 (SD=15.254) | 4.98 (SD=5.081) | <0.001 |
| MDD | N=23 (45.1%)4 | -- | <0.001 |
| CD | N=25 (49%) | -- | -- |
| ADHD | N= 37 (72.5%) | -- | -- |
| GAD | N=51 (100%) | -- | -- |
| Antipsychotic medications | 10 (19.6%) | -- | -- |
| SSRIs | 21 (41.2%) | -- | -- |
| Stimulants | 10 (19.6%) | -- | -- |
| Omission errors | 11.02 (SD=8.58) | 10.59 (SD=9.79) | =0.813 |
| Commission errors | 21.31 (SD=11.97) | 16.96 (SD=11.04) | =0.059 |
Key to table. SCARED=Screen for Child Anxiety Related Disorders; MFQ=Moof and Feelings Questionnaire; MDD=Major Depressive Disorder; CD=Conduct Disorder; ADHD=Attentional Deficit Hyperactivity Disorder; GAD=Generalized Anxietey Disorder; SSRI=Selective Serotonin Reuptake Inhibitors
Participants were recruited either shortly after their arrival at a residential care facility (Boys Town) or from the community. The youth at Boys Town are made up of participants with severe internalizing and externalizing pathology (sometimes co-occurring in the same youth). Despite being called Boys Town, this residential facility is home to both males and females, with approximately 40% of the residents being female. Our participant sample reflects this as out of the 51 participants with GAD from Boys Town in the current study, 58% were female.
Community members were recruited via flyers. Participants were excluded if IQ was below 75 assessed with the Wechsler Abbreviated Scale of Intelligence (WASI two-subtest form) (Wechsler, 2011) or if they had medical illnesses that required the use of medication that may have psychotropic effects, such as beta-blockers or steroids. Medications provided for psychiatric disorders (specifically antipsychotic, stimulant or mood stabilizing medications) were not exclusory but participants were asked to withhold stimulants on the day of the scan. Exclusion criteria also included participant’s status as a ward of state, braces, claustrophobia, active substance dependence, pervasive developmental disorder, Tourette’s syndrome, lifetime history of psychosis, neurological disorder, head trauma, non-English speaking, and presence of active safety concerns.
A doctoral level researcher or a member of the clinical research team, who was not part of the youth’s direct clinical care, obtained written informed consent and assent. In all cases, youth had the right to decline participation at any time before or during the study. Consent documents were reviewed with the parent/legal guardians and written permission was obtained 1) at the initial visit for community participants or 2) at the time of intake for youth placed in Boys Town programs. Assent was obtained from the Boys Town youth in a separate session. It was made clear to all participants and their parents that their decision with respect to participation had no influence on their clinical care. The Boys Town National Research Hospital institutional review board approved this study and all forms dealing with consents and assents.
Measures:
Passive Avoidance Task
Participants completed the functional MRI (fMRI) passive avoidance task adapted from previous work (Finger et al., 2011; Teng et al., 2016). On each trial (see Figure 1), one of four shapes was presented for 1500ms. Participants chose whether they wanted to respond to the object or not via button press. After the presentation of the shape, a randomly jittered fixation (1000ms-4000ms) displayed as well as a 1500ms feedback presentation. If the participant responded they would receive one of the following four outcomes: Win $5.00, Win $1.00, Lose $5.00, or Lose $1.00. If they did not respond, they would receive no feedback – the 1500ms feedback presentation would be blank. Each of the four shapes could engender each of the four outcomes, however two of the shapes had an 80% probability of yielding a reward and two of the shapes had an 80% probability of yielding a punishment. Another randomly jittered fixation (1000ms-4000ms) followed each outcome. There were 27 trials for each shape, totaling 108 trials.
Figure 1. Task Illustration.
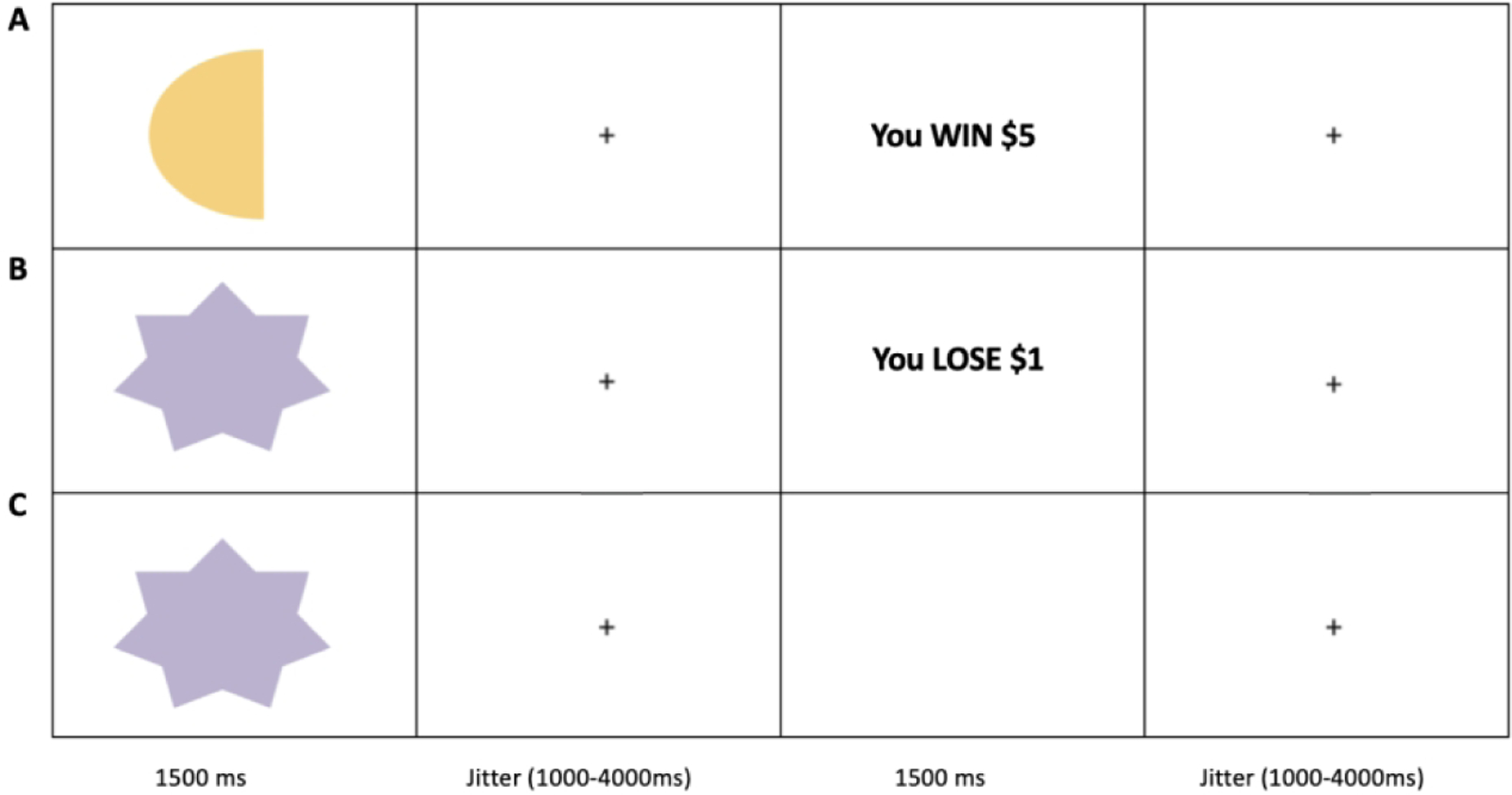
(A) Participant responds to shape and receives reward $5 feedback. (B) Participant responds to shape and receives $1 punishment feedback. (C) Participant does not respond to shape and no feedback is given.
Clinical Measures
SCARED
Screen for Child Anxiety Related Emotional Disorder (SCARED, child version, Birmaher et al., 1997) is a self-report questionnaire that looks at a youth’s potential for having an anxiety disorder. There are five subsets including Generalized Anxiety Disorder (9 questions) Panic Disorder (13 questions), Separation Anxiety Disorder (8 questions), Social Anxiety Disorder (4 questions), and School Anxiety (4 questions). Prior work has indicated that the SCARED shows excellent internal consistency and test-retest reliabilities (α= .921 and r= .782 for random effects model) (Runyon, Chesnut, & Burley, 2018).
MFQ-S
The Mood and Feelings Questionnaire (MFQ, Costello & Angold, 1987) is a self report questionnaire that looks at depressive symptoms in youth and young adults. The MFQ has been shown to have high criterion validity (Rhew et al, 2010) and excellent internal consistency (α = .91 to.93) (Thabrew et al, 2018).
Conners 3 ADHD INDEX-Parent
The Conners 3 ADHD INDEX-Parent (Conners, 2008) is a 10-item parent report scale that assesses ADHD symptoms. It has been shown to have excellent internal consistency and test re-test reliabilities (α = .91 and r=.85) (Kao & Thomas, 2010)
PDS
The Pubertal Development Scale (PDS, Petersen et al, 1988) is a self report measure that looks at pubertal development in male and female youth. Reliability has been shown to be high (α =.77) (Petersen et al, 1988).
fMRI Parameters
Whole-brain blood oxygen level dependent (BOLD) fMRI data were acquired using a 3.0 Tesla Siemens Skyra Magnetic Resonance Scanner. A total of 313 functional images were taken, with a T2* weighted gradient echo planar imaging (EPI) sequence (repetition time =2500 ms, echo time =27ms, flip angle=90°, field-of-view =240 mm). Whole-brain coverage was obtained with 43 axial slices (thickness, 2.5 mm; voxel size 2.6×2.6×2.5 mm3; distance factor 21%). In the same session, a high-resolution T1-weighed anatomical image was acquired to aid with spatial normalization (MP-RAGE, repetition time=2200 ms, echo time=2.48 ms; 230 mm field of view; 8° flip angle; 256×208 matrix) was acquired to register with the EPI dataset. Whole-brain coverage was obtained with 176 axial slices (thickness 1mm; voxel size 0.9×0.9×1 mm3, distance factor 50%).
fMRI Analysis: Data Preprocessing and Individual Level Analysis
Functional MRI data were preprocessed and analyzed using Analysis of Functional NeuroImages (AFNI) software (Cox, 1996) Data from the first four repetitions were collected prior to magnetization equilibrium and were discarded. The anatomical scan for each participant was registered to the Talairach and Tournoux atlas (Talairach & Tournoux, 1988) and each participant’s functional EPI data were registered to their Talairach anatomical scan in AFNI. Functional images were motion corrected and spatially smoothed with a 6-mm full width half maximum Gaussian kernel. The data then underwent time series normalization and these results were multiplied by 100 for each voxel. Therefore, the resultant regression coefficients are representative of a percentage of signal change from the mean.
Following this, regressors depicting each of the response types were created by convolving the train of stimulus events with a gamma-variate haemodynamic response function to account for the slow haemodynamic response. Four regressors were generated: objects chosen, objects refused, reward received, punishment received. Linear regression modelling was then performed using the regressors described above plus regressors to model a fourth order baseline drift function. This produced for each voxel and each regressor, a beta coefficient and its associated t-statistic.
Statistical Analyses
Clinical and behavioral data:
A series of independent sample t-tests were conducted to determine if there were group differences in demographic variables, anxiety (as indexed by the SCARED) and levels of omission and commission errors on the passive avoidance task. Omission errors reflect failures to respond to stimuli probabilistically associated with reward. Commission errors reflect responses to stimuli that were probabilistically associated with punishment.
Movement Data:
Volumes were censored if there was >0.5 mm motion across adjacent volumes. Participants were excluded only if >10% of volumes were censored.Potential group differences in movement were analyzed via three one-way ANOVAs (for average motion per volume, censored volumes and maximum displacement during scanning respectively).
BOLD response data:
We tested our hypotheses via two ANOVAs on the BOLD response data. The first involved a 2 (Group: participants with GAD, participants without GAD) × 2 (Response: approach, avoidance) full factorial design while the second involved a 2 (Group: participants with GAD, participants without GAD) × 2 (Reinforcement: reward, punishment) full factorial design. To facilitate future meta-analytic work, effect sizes (partial eta square [ηp²]) are reported in the Tables.
Follow-up Analyses:
Given the potential influences of medication use, the group-based ANOVA above was re-ran excluding participants using stimulants, antidepressant and antipsychotics. Given the co-morbidity of GAD with MDD, the group-based ANOVA above was re-run excluding participants with MDD.
Four exploratory ANCOVAs were conducted to examine whether there were associations in this population of dysfunction in processing reinforcement with symptom severity. Symptoms examined were those related to GAD and SAD (taken from the SCARED subscales), ADHD (taken from the Conners) and MDD (taken from the MFQ).
For all BOLD response analyses, correction for multiple comparisons was performed using a spatial clustering operation in AFNI’s 3dClustSim utilizing the autocorrelation function (-acf) with 10,000 Monte Carlo simulations for the whole-brain analysis. Spatial autocorrelation was estimated from residuals from the individual-level GLMs. The initial threshold was set at p=0.001. This process yielded an extant threshold of k=20 voxels for the whole brain (multiple comparison corrected p<0.05). Follow-up testing was conducted within the Statistical Package for the Social Sciences (SPSS) version 22.0.0.2 (IBM Corporation, Armonk, NY).
RESULTS
Clinical and Behavioral Data
Participants with GAD and the TD controls did not differ in age, sex or IQ (Table 1). There was expected significant group differences in anxiety as indexed by the SCARED, such that the GAD group reported higher anxiety than the controls (MeanGAD=35.30; MeanTD=13.14; scores above 25 indicate possible anxiety disorder). There were also expected significant group differences in depression as indexed by the MFQ, such that the GAD group reported higher depression than the controls (MeanGAD=21.4; MeanTD=4.98; scores above 27 indicate possible depression. There were no significant correlations of PDS scores and GAD diagnostic status within the females r(45)=-.04, p=.77, males r(43)=-.03, p=.85, or across the whole sample r(90)=-.03, p=.77.
Although there were no group differences in level of omission errors, there was a strong trend for a group difference in commission errors (t(100)=1.91; p=0.06; ηp²=.035). Adolescents with GAD made more commission errors than TD comparison adolescents (see Table 1).
Movement Data
No participant had >10% censored volumes. There were no significant group differences in terms of average motion per volume (F=2.27; p=0.135; ns), censored volumes (F=2.32; p=0.131; ns), or maximum displacement during scanning (F=3.05; p=0.84; ns).
EPI Data
The first ANOVA contrasting group differences in BOLD responses whilst approaching vs. avoiding stimuli revealed no regions showing either significant main effects of Group or Group-by-Response interactions. However, our second ANOVA revealed regions showing significant Group-by-Reinforcement interactions:
Group-by-Reinforcement:
There was a significant Group-by-Reinforcement interaction within regions including the caudate, putamen, mid cingulate/paracentral lobule, and superior and middle frontal gyrus (Table 2 & Figure 2). Within all regions, there was a greater increase to reward relative to punishment in TD controls relative to adolescents with GAD (F(1,100) = 17.16 to 34.34; p<0.001; ηp² range =.146 to .277). Indeed, in all cases, except in the superior frontal gyrus, adolescents with GAD showed reduced responses to reward relative to comparison adolescents (t(100)=2.51 to 3.64; p=0.014 to <0.001).
Table 2.
Significant areas of activation from the 2 (Group: Participants with GAD, participants without GAD) × 2 (Reinforcement: Reward, Punishment). Activations are effects observed in whole brain analyses significant at p<0.001, corrected for multiple comparisons (significant at p<0.05).
| REGION | BA | Voxels | X | Y | Z | F-value | Partial eta squared |
|---|---|---|---|---|---|---|---|
| L Caudate/ Thalamus | -- | 42 | −1 | −4 | 17 | 22.78 | .186 |
| R Putamen | -- | 11 | 20 | 8 | −4 | 18.43 | .156 |
| L Superior Frontal Gyrus | 8 | 24 | −22 | 26 | 50 | 19.62 | .164 |
| L Middle Frontal Gyrus | 6 | 22 | −16 | −7 | 59 | 20.04 | .167 |
| L Medial Frontal Gyrus | 6 | 19 | −1 | −10 | 65 | 17.16 | .146 |
| R Mid Cingulate/Paracentral Lobule | 24/6/5 | 694 | 5 | −31 | 50 | 29.3 | .227 |
| L Superior Temporal Gyrus | 22 | 30 | −55 | 5 | −1 | 21.65 | .178 |
| L Superior Temporal Gyrus | 42 | 21 | −64 | −31 | 11 | 26.26 | .208 |
| L Inferior Occipital Gyrus | 17 | 38 | −19 | −91 | −7 | 23.29 | .189 |
| R Middle Occipital Gyrus | 37 | 19 | 47 | −67 | −4 | 20.62 | .171 |
| R Cuneus | 19 | 62 | 26 | −73 | 29 | 23.24 | .189 |
| R Lingual Gyrus | 18 | 171 | 23 | −76 | −10 | 34.34 | .256 |
| L Anterior Insula Cortex | 47 | 9 | −46 | 29 | −4 | 17.14 | .146 |
Note: coordinates based on the Tournoux & Talairach standard brain template, BA= Brodmann”s Area
Figure 2. Interactions of Group-by-Reinforcement Condition.
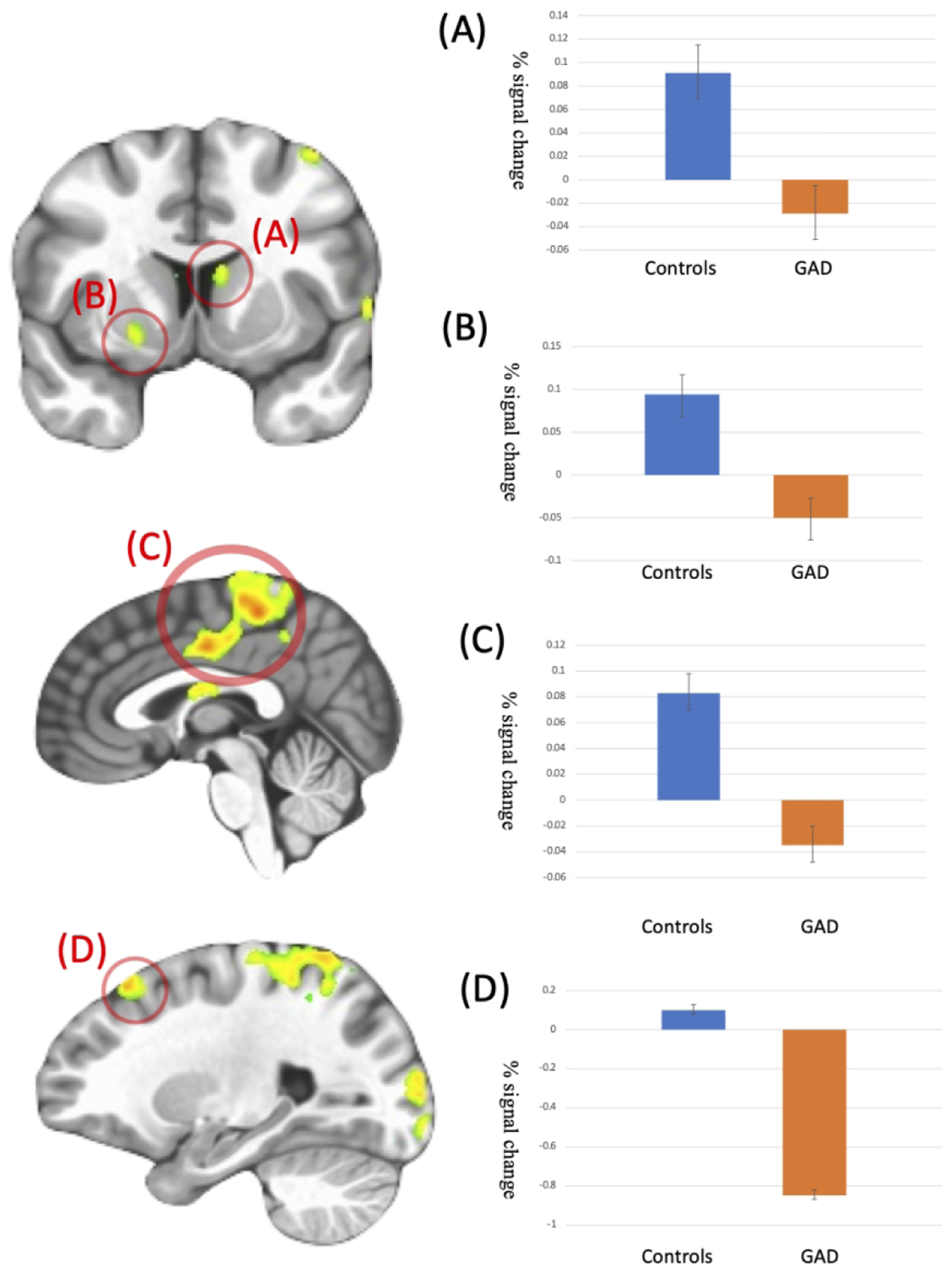
Patients with GAD showed decreased activation to Reward relative to Punishment trials compared to the TD comparison individuals in (A) L caudate/thalamus (−1, −4, 17); (B) R putamen (x, y, z = 20, 8, −4); (C) R mid cingulate/paracentral lobule (x, y, z = 5, −31, 50); (D) L superior frontal gyrus (x, y, z = −22, 26, 50)
Follow-up Analyses:
1. Excluding participants with MDD:
Given the co-morbidity of GAD with MDD (N=24 in the current sample), the group-based ANOVA was re-ran excluding participants with MDD (See Table 1). Regions identified via the Group-by-Reinforcement were proximal to those seen in the main analysis (See Table S1)
2. Excluding participants using medications:
A subset of participants with GAD were prescribed antipsychotic medications (n=10; 19.6%), SSRIs (n=21; 41.2%) and stimulants (n=10; 19.6%) (overall participants on medication N=26; 50.98%). Given the potential influences of medication use, the group-based ANOVA above was re-ran excluding participants on these medications (See Tables S2 to S4). The results of these analyses largely mirrored those obtained in our main analysis.
3. Symptom severity ANCOVAs:
All four symptom severity covariates (GAD & SAD subscales from the SCARED, MFQ and Conners) failed to identify regions showing Reinforcement-by-Symptom interactions that survived multiple comparison correction.
DISCUSSION
The goal of this study was to examine approach and avoidance decision-making and reinforcement responding in the context of a passive avoidance learning task in adolescents with GAD relative to typically developing adolescents. While there were no group differences in the BOLD response data during approach/avoidance decision-making, adolescents with GAD showed, relative to TD adolescents, reduced differential responding to received reward relative to punishment within the caudate, putamen, mid cingulate/paracentral lobule and superior and middle frontal gyrus.
It has been claimed that disrupted computations related to approach-avoidance decision-making are implicated in the pathophysiology of GAD (Aupperle & Paulus, 2010; Santiago et al., 2020). However, we found no regions showing significant atypical activity during approach-avoidance decisions in the adolescents with GAD relative to the TD adolescents. This was also seen in adults with GAD in our previous work using a very similar task (White et al., 2017). Despite this, there were indications of impaired approach avoidance decision making (greater commission errors – i.e., incorrect response to stimuli probabilistically associated with punishment). This was seen in our previous behavioral work with this task with adults with GAD (Teng et al., 2016; though not in our previous fMRI study with adults with GAD - White et al., 2017). As such, there are grounds for considering that approach-avoidance decision-making is disrupted in patients with GAD. This is particularly plausible given that performance on other forms of reinforcement-based decision making tasks appears disrupted in GAD (Devido et al., 2009; Dorfman, Rosen, et al., 2016; though see Mueller et al., 2010) and that rewards are less effective at improving cognitive performance in anxious than in typically developing (TD) adolescents (Hardin et al., 2007; Jazbec et al., 2005).
We believe that this last-mentioned behavioral impairment, and the current data, reflect the primary impairment underpinning the reinforcement-based decision-making difficulties faced by patients with GAD; i.e., dysfunction in responding appropriately to, and thus using, reward information. Adolescents with GAD, relative to comparison adolescents, showed reduced differential responding to received reward relative to punishment (manifesting as reduced responses to reward) within caudate, putamen, mid cingulate/paracentral lobule and superior and middle frontal gyrus. This is compatible with previous work with adolescents with GAD reporting reduced responses to reward within caudate/putamen (Benson et al., 2015), reduced ventral striatal activity relative to controls during risky relative to safe choices for monetary rewards (Galvan & Peris, 2014) and reduced within-striatum and striatum to frontal cortex correlated activity via connectivity analysis (Dorfman, Benson, et al., 2016). Notably, the results are similar to our previous findings with adults with GAD and comparison individuals using a very similar task (White et al., 2017). This latter study reported reduced reinforcement signaling within proximal regions of dorsomedial frontal cortex, ventral striatum, anterior insula cortex and precentral gyrus as seen here. The main differences between the current results, and those of White et al (2017), were that: (i) White et al al (2017) reported main effects of Group in both vmPFC and PCC. There was a Group-by-Reinforcement interaction within vmPFC (but not PCC) in the current study but only at a very lenient statistical threshold (p<0.02) even though the region was sensitive to reward-punishment across the sample as a whole (see Supplemental Figure 1B); and (ii) the regions showing group-by-reinforcement interactions in the current study showed main effects of group in White et al (2017); i.e., ventral striatum, dorsomedial frontal cortex, anterior insula cortex and precentral gyrus. Potentially, these regions show a reduced differential response to reward relative to punishment as well as reduced responses to both reward and punishment prediction errors in patients with GAD. They are certainly compatible with a view that reinforcement signaling is compromised in both adolescent and adult patients with GAD.
Two previous studies did not find indications of reduced striatal responding with respect to reward (Guyer et al., 2012; Yassa, Hazlett, Stark, & Hoehn-Saric, 2012). However, it is perhaps worth noting that both of these studies involved paradigms where the receipt of reinforcement was not influenced by prior learning. The Guyer et al. (2012) study utilized the Monetary Inventive Delay task where reward is received if the participant responds sufficiently fast to a probe stimulus – no learning about the probe stimulus is required. Similarly, while the Yassa et al. (2012) study involved a task that did require some learning, the core contrast was between trials where the feedback was random (both cues were associated with a 50% probability of reward) and the presence of easy discrimination trials (one object was associated with a 100% reward contingency, the other a 50% reward contingency). As such, it is possible that the reduced sensitivity to reinforcement information in patients with GAD is particularly clear in the context of instrumental learning.
One interesting feature of the current results, when examining them in the context of the data from the independent sample of TD participants (see Supplemental Figure 1), is worth noting. As noted, the current data indicated that patients with GAD showed reduced responses within the ventral and dorsal striatum to reward (Figure 1A & B). Supplemental Figures 1B & C shows responses within these regions to reward within the independent sample of TD participants. Moreover, considerable prior data indicates the role of the striatum in responding to reward information (Daniel & Pollmann, 2014; J. P. O’Doherty, Cockburn, & Pauli, 2017). Yet, both the ventral and dorsal striatum showed significant responses during approach vs. avoidance decision-making (see Supplemental Figure 1A). While much of this activity may represent the organization of the motor response during “approach” responses, the data are suggestive that the difficulty faced by patients with GAD is less about recruitment of striatal activity generally and more about recruiting the striatum in response to reward; i.e., there is a functional specification to the impairment.
Four caveats to the current study should be noted. First, some of our participants with GAD were on prescribed medications (i.e., stimulants, SSRIs, and/or anti-psychotics). However, our follow-up analyses, which excluded participants on medications, yielded similar results to our main findings. As such, it is unlikely that our findings can be attributed to these prescribed medications. Second, and unsurprisingly given high GAD-MDD comorbidity (Remes et al., 2018; Shen et al., 2018), some of our participants with GAD were co-morbid with MDD. MDD is also associated with reduced reward processing (Admon & Pizzagalli, 2015; Stringaris et al., 2015). However, the current results cannot be attributed to these comorbid cases. Group differences were present even after the exclusion of these co-morbid cases. Of course, this does not preclude the possibility, which we indeed consider likely, that reduced reward processing contributes to the presentation of both GAD and MDD symptoms with perhaps other forms of neuro-cognitive dysfunction/ compensation determining the exact symptom profile. Third, no regions were identified showing significant Reinforcement-by-GAD severity (as measured by the SCARED subscale). These findings indicate either that the GAD subscale is not an ideal assessment of GAD severity or that while patients with GAD as a group show impairment in reinforcement processing, the severity of this impairment is unrelated to the severity of their GAD. It should be noted that regions were not identified showing Reinforcement-by-severity interactions for three other conditions assessed (SAD, ADHD and MDD). As such, these data do not support a suggestion that while patients with GAD show dysfunction in reinforcement processing, severity of this dysfunction relates to symptom severity in one of psychiatric conditions commonly comorbid with GAD. Fourth, it will be important in future work to examine the association of GAD with neural computations of functions related to reinforcement-based learning (e.g., prediction error signaling). The current study revealed dysfunction in the differential response to received rewards and punishments. It did not investigate responses to rewards and punishment as a function of expectations based on previous reinforcement history (i.e., prediction error signaling). However, pilot analyses of BOLD response data on the current PA task revealed this task implementation was not optimized to reveal a strong prediction error signal. Future computational modeling-based work with other tasks will investigate this issue. Fifth, Boys Town is a residential treatment setting that caters to many disruptive youth. As such, it could be argued that our population of adolescents with GAD showed atypical levels of externalizing behavior and that this might be driving the results. However, it should be noted that externalizing behavior is not atypical in adolescents with GAD; 20%–63% of children with GAD also meet criteria for ODD or CD (Garland & Garland, 2001; Masi et al., 2004).
In conclusion, our study revealed reduced differential reward-punishment responding in adolescents with GAD relative to typically developing adolescents. This dysfunction reflected reduced reward responding. This difficulty likely contributes to non-optimal real-world decision-making that potentially exacerbates worry and anxiety. It is certainly likely to disrupt approach-avoidance decision-making – an impairment that has been implicated in the pathophysiology of GAD (Aupperle & Paulus, 2010; Santiago et al., 2020). Indeed, the adolescents showed some degree in approach/avoidance decision-making on the current task; the participants with GAD were more likely to respond to the stimuli probabilistically associated with punishment (commission errors) than the typically developing participants. As such reward processing might be considered a treatment target for intervention studies with patients with GAD.
Supplementary Material
Figure S1 shows the overlapping activations regions showing a: (A) main effect of Response; (B) main effect of Reinforcement; (C) Response to reward relative to baseline; and (D) Response to punishment relative to baseline. Core regions implicated in all of these results include the ventromedial, dorsomedial frontal and anterior insula cortices as well as striatum.
ACKNOWLEDGMENTS
This research was in part supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number 5P20GM109023-05 (KB) and the National Institute of Mental Health under award number K22-MH109558 (JB). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to IRB restrictions.
REFERENCES
- Admon R, & Pizzagalli DA (2015). Dysfunctional Reward Processing in Depression. Curr Opin Psychol, 4, 114–118. doi: 10.1016/j.copsyc.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf M, Rabany L, Zertuche L, Bragdon L, Tolin D, Goethe J, & Diefenbach G (2018). Neural functional architecture and modulation during decision making under uncertainty in individuals with generalized anxiety disorder. Brain Behav, 8(8), e01015. doi: 10.1002/brb3.1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, & Paulus MP (2010). Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci, 12(4), 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell CC, & Bell CC (2001). Diagnostic and statistical manual of mental disorders, fourth edition, text revision: DSM-IV-TR quick reference to the diagnostic criteria from DSM-IV-TR. Journal of the American Medical Association, 285(6), 811–812. [Google Scholar]
- Benson BE, Guyer AE, Nelson EE, Pine DS, & Ernst M (2015). Role of contingency in striatal response to incentive in adolescents with anxiety. Cogn Affect Behav Neurosci, 15(1), 155–168. doi: 10.3758/s13415-014-0307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Otero M, Teng C, Geraci M, Ernst M, Blair RJ, … Grillon C (2017). Reduced optimism and a heightened neural response to everyday worries are specific to generalized anxiety disorder, and not seen in social anxiety. Psychol Med, 1–10. doi: 10.1017/s0033291717000265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Daniel R, & Pollmann S (2014). A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem, 114, 90–100. doi: 10.1016/j.nlm.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, & Fiez JA (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84(6), 3072. Retrieved from http://nihlibrarysfx.nih.gov:9003/sfx_local?sid=Entrez%3APubMed;id=pmid%3A11110834 [DOI] [PubMed] [Google Scholar]
- Devido G, Jones M, Geraci M, Hollon N, Blair RJ, Pine DS, & Blair K (2009). Stimulus-reinforcement-based decision making and anxiety: impairment in generalized anxiety disorder (GAD) but not in generalized social phobia (GSP). Psychological Medicine, 39(7), 1153–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman J, Benson B, Farber M, Pine D, & Ernst M (2016). Altered striatal intrinsic functional connectivity in pediatric anxiety. Neuropsychologia, 85, 159–168. doi: 10.1016/j.neuropsychologia.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman J, Rosen D, Pine D, & Ernst M (2016). Anxiety and Gender Influence Reward-Related Processes in Children and Adolescents. J Child Adolesc Psychopharmacol, 26(4), 380–390. doi: 10.1089/cap.2015.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas MJ, Gagnon F, Ladouceur R, & Freeston MH (1998). Generalized anxiety disorder: a preliminary test of a conceptual model. Behav Res Ther, 36(2), 215–226. doi: 10.1016/s0005-7967(97)00070-3 [DOI] [PubMed] [Google Scholar]
- Duval ER, Javanbakht A, & Liberzon I (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management, 11, 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Meyer F, Fehr E, & Ruff CC (2015). Anticipatory anxiety disrupts neural valuation during risky choice. Journal of Neuroscience, 35(7), 3085–3099. doi: 10.1523/JNEUROSCI.2880-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, … Blair RJR (2011). Disrupted reinforcement signaling in the orbital frontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. American Journal of Psychiatry, 168(2), 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, & Peris TS (2014). Neural correlates of risky decision making in anxious youth and healthy controls. Depress Anxiety, 31(7), 591–598. doi: 10.1002/da.22276 [DOI] [PubMed] [Google Scholar]
- Garland EJ, & Garland OM (2001). Correlation between anxiety and oppositionality in a children’s mood and anxiety disorder clinic. Can J Psychiatry, 46(10), 953–958. doi: 10.1177/070674370104601008 [DOI] [PubMed] [Google Scholar]
- Gentes EL, & Ruscio AM (2011). A meta-analysis of the relation of intolerance of uncertainty to symptoms of generalized anxiety disorder, major depressive disorder, and obsessive-compulsive disorder. Clin Psychol Rev, 31(6), 923–933. doi: 10.1016/j.cpr.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, … Ernst M (2012). Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am J Psychiatry, 169(2), 205–212. doi: 10.1176/appi.ajp.2011.11010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, & Ernst M (2007). Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J Child Psychol Psychiatry, 48(5), 446–454. doi: 10.1111/j.1469-7610.2006.01722.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, & Ernst M (2005). Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry, 58(8), 632–639. doi: 10.1016/j.biopsych.2005.04.010 [DOI] [PubMed] [Google Scholar]
- Kosson DS, Budhani S, Nakic M, Chen G, Saad ZS, Vythilingam M, … Blair RJ (2006). The role of the amygdala and rostral anterior cingulate in encoding expected outcomes during learning. Neuroimage, 29(4), 1161–1172. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16387514 [DOI] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, & Milham MP (2008). A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry, 63(6), 563–568. doi: 10.1016/j.biopsych.2007.06.011 [DOI] [PubMed] [Google Scholar]
- Ladouceur R, Talbot F, & Dugas MJ (1997). Behavioral expressions of intolerance of uncertainty in worry. Experimental findings. Behav Modif, 21(3), 355–371. doi: 10.1177/01454455970213006 [DOI] [PubMed] [Google Scholar]
- Li J, Zhong Y, Ma Z, Wu Y, Pang M, Wang C, … Zhang N (2020). Emotion reactivity-related brain network analysis in generalized anxiety disorder: a task fMRI study. BMC Psychiatry, 20(1), 429. doi: 10.1186/s12888-020-02831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi G, Millepiedi S, Mucci M, Poli P, Bertini N, & Milantoni L (2004). Generalized anxiety disorder in referred children and adolescents. J Am Acad Child Adolesc Psychiatry, 43(6), 752–760. doi: 10.1097/01.chi.0000121065.29744.d3 [DOI] [PubMed] [Google Scholar]
- Mueller EM, Nguyen J, Ray WJ, & Borkovec TD (2010). Future oriented decision-making in Generalized Anxiety Disorder is evident across different versions of the Iowa Gambling Task. Journal of Behavioral Therapy and Experimental Psychiatry, 41, 165–171. [DOI] [PubMed] [Google Scholar]
- Newman JP, & Kosson DS (1986). Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology, 95, 252–256. [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, & Dolan RJ (2004). Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304, 452–454. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Cockburn J, & Pauli WM (2017). Learning, Reward, and Decision Making. Annu Rev Psychol, 68, 73–100. doi: 10.1146/annurev-psych-010416-044216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS (2007). Research review: a neuroscience framework for pediatric anxiety disorders. J Child Psychol Psychiatry, 48(7), 631–648. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17593144 [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, & Ma Y (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry 55, 6–64. [DOI] [PubMed] [Google Scholar]
- Remes O, Wainwright N, Surtees P, Lafortune L, Khaw KT, & Brayne C (2018). Generalised anxiety disorder and hospital admissions: findings from a large, population cohort study. BMJ Open, 8(10), e018539. doi: 10.1136/bmjopen-2017-018539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyon K, Chesnut SR, & Burley H (2018). Screening for childhood anxiety: A meta-analysis of the screen for child anxiety related emotional disorders. Journal of Affective Disorders, 240, 220–229. doi: 10.1016/j.jad.2018.07.049 [DOI] [PubMed] [Google Scholar]
- Santiago J, Akeman E, Kirlic N, Clausen AN, Cosgrove KT, McDermott TJ, … Aupperle RL (2020). Protocol for a randomized controlled trial examining multilevel prediction of response to behavioral activation and exposure-based therapy for generalized anxiety disorder. Trials, 21(1), 17. doi: 10.1186/s13063-019-3802-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, & Montague PR (1997). A neural substrate of prediction and reward. Science, 275(5306), 1593–1599. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9054347 [DOI] [PubMed] [Google Scholar]
- Shen YM, Chan BSM, Liu JB, Zhou YY, Cui XL, He YQ, … Luo XR (2018). The prevalence of psychiatric disorders among students aged 6~ 16 years old in central Hunan, China. BMC Psychiatry, 18(1), 243. doi: 10.1186/s12888-018-1823-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringaris A, Vidal-Ribas Belil P, Artiges E, Lemaitre H, Gollier-Briant F, Wolke S, … Paillere-Martinot ML (2015). The Brain’s Response to Reward Anticipation and Depression in Adolescence: Dimensionality, Specificity, and Longitudinal Predictions in a Community-Based Sample. Am J Psychiatry, 172(12), 1215–1223. doi: 10.1176/appi.ajp.2015.14101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P (1988). Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme. [Google Scholar]
- Teng C, Otero M, Geraci M, Blair RJ, Pine DS, Grillon C, & Blair KS (2016). Abnormal decision-making in generalized anxiety disorder: Aversion of risk or stimulus-reinforcement impairment? Psychiatry Res, 237, 351–356. doi: 10.1016/j.psychres.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2011). Wechsler Abbreviated Scale of Intelligence–Second Edition (WASI-II). San Antonio, TX: NCS Pearson. [Google Scholar]
- White SF, Geraci M, Lewis E, Leshin J, Teng C, Averbeck B, … Blair KS (2017). Prediction Error Representation in Individuals With Generalized Anxiety Disorder During Passive Avoidance. Am J Psychiatry, 174(2), 110–117. doi: 10.1176/appi.ajp.2016.15111410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU (2002). Generalized anxiety disorder: prevalence, burden, and cost to society. Depression and Anxiety, 16(4), 162–171. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Hazlett RL, Stark CE, & Hoehn-Saric R (2012). Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J Psychiatr Res, 46(8), 1045–1052. doi: 10.1016/j.jpsychires.2012.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 shows the overlapping activations regions showing a: (A) main effect of Response; (B) main effect of Reinforcement; (C) Response to reward relative to baseline; and (D) Response to punishment relative to baseline. Core regions implicated in all of these results include the ventromedial, dorsomedial frontal and anterior insula cortices as well as striatum.


