Abstract
Mucins are a family of high molecular weight, heavily-glycosylated proteins produced by wet epithelial tissues, including the ocular surface epithelia. Densely-packed O-linked glycan chains added post-translationally confer the biophysical properties of hydration, lubrication, anti-adhesion and repulsion. Membrane-associated mucins (MAMs) are the distinguishing components of the mucosal glycocalyx. At the ocular surface, MAMs maintain wetness, lubricate the blink, stabilize the tear film, and create a physical barrier to the outside world. In addition, it is increasingly appreciated that MAMs function as cell surface receptors that transduce information from the outside to the inside of the cell. Recently, our team published a comprehensive review/perspectives article for molecular scientists on ocular surface MAMs, including previously unpublished data and analyses on two new genes MUC21 and MUC22, new MAM functions and new biological roles, comparing human and mouse (PMID: 31493487). The current article is a refocus for the audience of The Ocular Surface. First, we update the gene and protein information in a more concise form, and include a new section on glycosylation. Next, we discuss biological roles, with some new sections and further updating from our previous review. Finally, we provide a new chapter on MAM involvement in ocular surface disease. We end this with discussion of an emerging mechanism responsible for damage to the epithelia and their mucosal glycocalyces: the unfolded protein response (UPR). The UPR offers a novel target for therapeutic intervention.
Keywords: Ocular surface, mucosal epithelia, glycocalyx, membrane-associated mucin, O-linked glycosylation, barrier function, signal transduction, oxidative stress, unfolded protein response
1. INTRODUCTION
Bodily tissues that face the external environment are frequently exposed to abrasion, desiccation and a wide variety of potential pathogens. One of the functions of “covering” or “coating” epithelia is to protect against such external threats, with specific features adapted to the needs of the specific organ. An example is keratinization, which provides the epidermis with a very effective barrier. However, this is a dry barrier composed of dead and desiccated cells that helps to waterproof the skin [1]. In contrast, most coating epithelia require wetness to fulfill their essential functions. Thus, instead of keratins, the wet epithelia are covered by living cells surfaced by mucins [2].
1.1. Basics of Mucin Structure/Function at the Ocular Surface
Mucins are distinguished by the presence of structural module(s) containing numerous serine and threonine residues that serve as sites for O-linked glycosylation. Usually these modules are composed of tandem amino acid repeat units. The densely-packed glycan chains added enzymatically to these sites by post-translational modification confer the biophysical properties of hydration, lubrication, anti-adhesion and repulsion [3].
There are two types of mucins: secreted and membrane-associated. Secreted mucins, produced by specialized goblet cells, can be soluble or gel-forming. The latter assemble into extremely large oligomeric gels through linkage of protein monomers via disulfide bonds [3]. Secreted mucins create a viscous mucous layer over the epithelia of the tracheobronchial, gastrointestinal and reproductive tracts. In contrast, the aqueous and mucin components of the tear film combine to create a single mucoaqueous gel [4, 5]. A thin lipid layer surfaces this gel, serving to reduce evaporation.
This article focuses on the membrane-associated mucins (MAMs)*. of the ocular surface. MAMs are the defining components of the glycocalyx in all mucosal epithelia. At the ocular surface, they emanate from the plasma membrane of the apical epithelial cells [6], maintaining wetness, lubricating the blink and stabilizing the tear film [2, 5, 7]. In addition, they create a physical barrier against noxious substances [6]. They are by far the largest of ocular surface glycoproteins, with polypeptide chains up to 15,000 amino acids in length [8, 9]. The clustering of O-linked oligosaccharide chains creates steric interactions between carbohydrate and peptide, inducing the peptide backbone to adopt an extended conformation [10]. This results in projection of the MAM well above the cell surface, far beyond other membrane-associated proteins. MAMs would be the first cell-associated molecules encountered by invading pathogens, and are thus well-positioned to shield and protect [6].
Besides their lubricating and barrier functions, it is increasingly appreciated that MAMs also serve an important role as cell surface receptors that sense the extracellular environment and transduce signals intracellularly [11]. In various mucosal tissues, MAMs activate or inhibit intracellular signaling cascades that regulate inflammation, cell-cell interactions, differentiation and cell death [3, 12, 13].
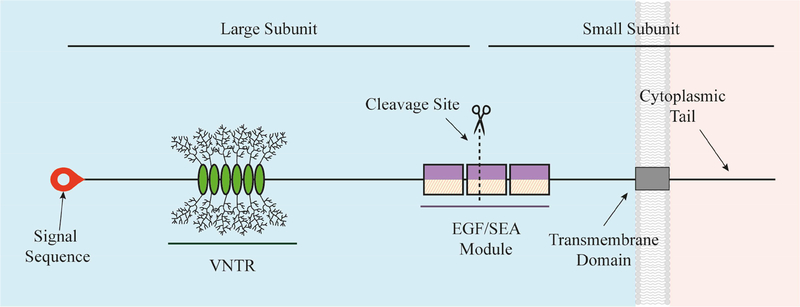
The prototypical MAM is structured much like a classic, single-pass transmembrane immune receptor, as shown in Figure 1. A signal peptide at the N-terminus of the precursor polypeptide chain enables membrane insertion, and may be retained in the final product [14]. The mature protein is composed of two subunits arising from intracellular cleavage, that remain strongly associated via non-covalent interactions [15]. The small subunit consists of a short extracellular region, a single-pass transmembrane domain, and a short cytoplasmic tail (CT). The large subunit, together with the extracellular portion of the small subunit, comprise the extracellular domain (ED).
Figure 1.
Molecular Prototype of a Membrane-Associated Mucin.
The graphic depicts a prototypical MAM, the structure of which is like a classic, single-pass transmembrane immune receptor. A signal peptide is found at the N-terminus of the precursor polypeptide chain to enable its membrane insertion; it may be retained in the mature protein [14]. The mature protein is composed of two subunits that self-associate, arising from intracellular cleavage. The large subunit is entirely extracellular and contains the VNTR module with its tandem repeat that are O-glycosylated. The small subunit consists of a short extracellular region, a single-pass transmembrane domain, and a cytoplasmic tail (CT). The large subunit of the MAM, together with the extracellular portion of the small subunit, comprise the extracellular domain (ED). This domain also contains conserved modules such as the Sperm protein, Enterokinase and Agrin module (SEA) and EGF-like modules.
The tandem amino acid repeat module is located on the ED. The number of tandem repeats for a specific MAM can vary considerably among individuals within a population, thus these repeat-containing module(s) are often called the “VNTR”, for Variable Number of Tandem Repeats [8]. In addition, various conserved sequence modules, such as the Sperm protein, Enterokinase and Agrin module (SEA) and the EGF (epidermal growth factor)-like module, are mixed and matched on the different MAMs.
“Shedding” of the large MAM subunit into extracellular fluids occurs spontaneously or in response to various ligands or stimuli [6]. Ligand binding, shedding of the large subunit, or other external/internal stimuli leads to CT engagement of protein kinases and phosphorylation at specific tyrosine or serine/threonine residues [16]. Phosphorylation initiates signal transduction cascades [11, 17].
1.2. Update on Membrane-Associated Mucins at the Ocular Surface
The Ocular Surface published review/perspectives articles on ocular surface MAMs in 2004 [18], 2010 [19] and 2016 [20]. There have been some more recent reviews on ocular surface mucins that cover both secreted mucins and MAMs [21–24]. Most recently, our team published a comprehensive review/perspectives article for molecular scientists on ocular surface MAMs, including previously unpublished data and analyses on two new genes MUC21 and MUC22, new protein functions, and new biological roles, comparing human and mouse [16]. The current article is a refocus for the audience of The Ocular Surface. First, we update the gene and protein information in a more concise form, and include a new section on glycosylation. Next, we discuss biological roles, with some new sections and further updating from our previous publication. Finally, we provide a new chapter on involvement in human disease. We end this with discussion of an emerging mechanism responsible for damage to the epithelia and their mucosal glycocalyces: the unfolded protein response (UPR). The UPR offers a novel target for therapeutic intervention. These areas of focus are summarized in the graphic if Figure 2, in context of the ocular surface.
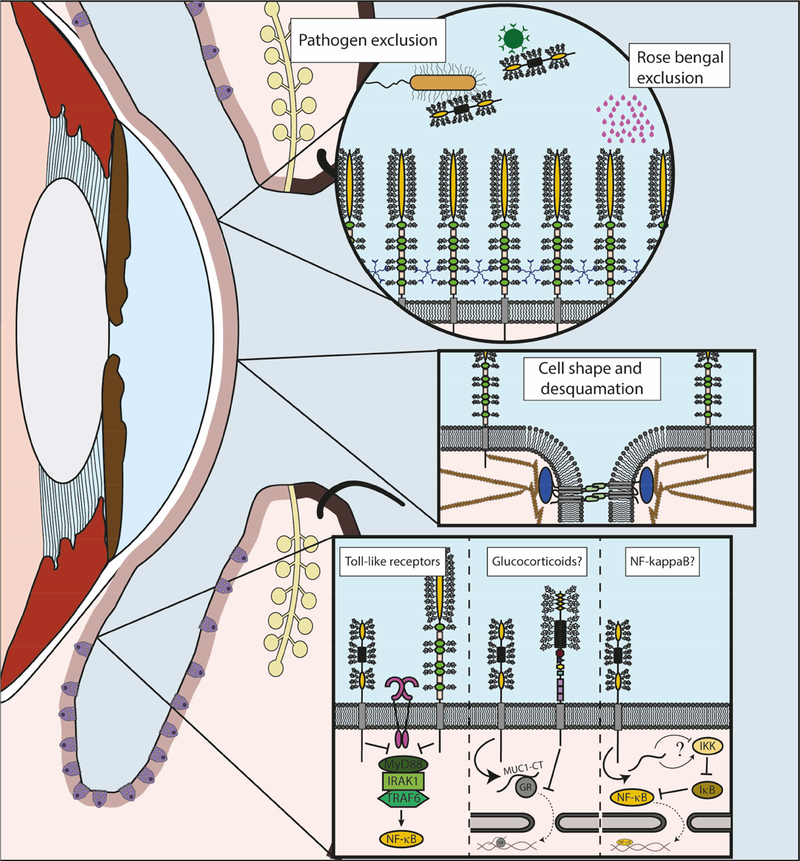
Figure 2.
Functions of Membrane-Associated Mucins at the Human Ocular Surface.
The graphic depicts some of the focus areas of this article.
Left. Sagittal section of the eye and eyelids is represented, with epithelia highlighted in light pink. Goblet cells can be observed in the conjunctival epithelium (purple). Meibomian glands (yellow) can be found at the lids, with their ducts opening at the lid margin.
Top right. Detail of the barrier functions of MAMs. Multiple MUC16 are showed protruding from the plasma membrane as the main elements of the glycocalyx barrier. LGALS3 (galectin-3) pentamers can be found interacting with mucin glycans. This barrier is responsible of the exclusion of different substances, such as the clinical dye rose bengal. Bacteria and viruses can be excluded also by shed mucins, as it is the case of MUC1, represented here.
Mid right. Detail of the interactions of MAMs with elements of the cytoskeleton. MUC16 participates in the formation of membrane microplicae and tight junctions (represented here with the transmembrane protein occluding and ZO1 in blue). Some of these functions have been related to their interaction with actin filaments.
Bottom right. Detail of the immunomodulatory functions of MAMs. A question mark is used to indicate functions that have not been demonstrated in the eye to date. Inhibitory effect on Toll-like receptors (fuchsia) has been described for MUC1 (left) and MUC16 (right) at the ocular surface, reducing the activation of NF-kappaB. Previous studies showed that MUC1 acted by inhibiting recruitment of MyD88. Studies in airways have demonstrated that MUC1-CT interacts with the glucocorticoid receptor (NR3C1), facilitating its migration to the nucleus, while MUC4 (right) can inhibit its translocation. Finally, studies in different systems have described interactions between MUC1-CT and different regulatory elements of the NF-kappaB pathway, although the final result of these interactions is not clear.
1.3. Method of Literature Search
A search in PubMed was performed to review literature for the membrane-associated mucins expressed at the ocular surface. A search with the word string “mucin cornea” OR “mucin ocular surface” in PubMed returned 773 articles published from 1957–2021. Of these, 130 articles were reviews. A search with the term “membrane-associated mucin” in PubMed returned 32 review articles published from 1957–2021. In addition to the three articles published in The Ocular Surface [18–20], the newer review articles cited above [21–24], and our own article [16], we consider the following as most relevant: [2, 25–32]. Finally, we did a search with the string “MUC1 OR MUC4 OR MUC16 OR MUC20 OR MUC21 OR MUC22” and “ocular surface OR cornea” between the years 2015–2021, turning up 160 articles. More targeted searches were done to find disease associations.
2. MOLECULAR BIOLOGY AND BIOCHEMISTRY
2.1. Genes and Gene Expression
Table 1 lists the twenty-one genes currently classified in the human mucin (MUC) gene group by the HUGO gene nomenclature committee. Eleven of these are expressed by the ocular surface epithelia, goblet cells and associated glands.
Table 1.
Human MUC Gene Family
| Gene Symbol | Expressing Tissue(s) at the Ocular Surface | Immunodetected in Tears? |
|---|---|---|
| Secreted Mucins (gel-forming) | ||
| MUC2 | very low in conjunctival epithelium (RT-PCR only) [171, 239]; not detected in lacrimal gland [162] | detected (low level) [51] |
| MUC5AC | conjunctival epithelium [36, 50, 171, 239]; goblet cells [36, 51; lacrimal duct goblet cells [43, 162] | detected [51] |
| MUC5B | conjunctival epithelium (RT-PCR only) [50]; lacrimal gland (RT-PCR only) [42, 162] | not detected [51] |
| MUC6 | not detected in conjunctival epithelium (RT-PCR only) [50, 162]; lacrimal gland (by RT-PCR but not immuno- histochemistry) [162] | N/A |
| MUC19 | corneal and conjunctival epithelia; lacrimal gland [240] | N/A |
| Secreted Mucins (soluble) | ||
| MUC7 | conjunctival epithelium [42, 50, 171]; lacrimal gland (RT-PCR only [42, 162] | not detected [51] |
| MUC8 | not detected in conjunctival epithelium (RT-PCR only) [50] | N/A |
| OVGP1 (MUC9) | very low in conjunctival epithelium (RT-PCR only) [50] | N/A |
| Membrane-Associated Mucins | ||
| MUC1 | corneal & conjunctival epithelia [38, 50, 171]; lacrimal gland [42, 43, 162] | detected [51] |
| MUC3A | very low in conjunctival epithelium (RT-PCR only) [50] | N/A |
| MUC3B | very low in conjunctival epithelium (RT-PCR only) [50] | N/A |
| MUC4 | conjunctival epithelium, corneal epithelium (much less) [36, 37, 50, 171]; lacrimal gland [42] [162] | detected [51] |
| MUC12 | very low in conjunctival epithelium (RT-PCR only) [50] | N/A |
| MUC13 | very low in conjunctival epithelium (RT-PCR only)[ 50, 171] | N/A |
| EMCN (MUC14) | expressed primarily by vascular endothelial cells [33] | N/A |
| MUC15 | conjunctival epithelium (RT-PCR only)[ 50, 171] | N/A |
| MUC16 | corneal & conjunctival epithelia [35, 50, 171]; mucin granules of conjunctival goblet cells [45]; lacrimal gland [44] | detected [51] |
| MUC17 | very low in conjunctival epithelium (RT-PCR only) [50, 171] | N/A |
| MUC20 | corneal & conjunctival epithelia [50] | not detected [50] |
| MUC21 | corneal epithelium [16] | N/A |
| MUC22 | corneal epithelium [16] | N/A |
MUC19 is a secreted mucin (gel-forming), a correction from our previous article [16]; MUC12 was previously called MUC11 [30]; N/A: tear presence has not been examined; EMCN [241] and MUC15 [242] lack the typical mucin tandem repeats, however their extracellular domains share the same characteristics, with long extended sequences devoid of secondary structure, densely modified by O-linked glycan chains.
Eight of the twenty-one total MUC genes encode secretory mucins. Five of these are expressed at the human ocular surface: MUC2, MUC5AC, MUC5B, MUC7 and MUC19. Only the gel-forming MUC5AC, and very low levels of MUC2, have been detected in the tears (reviewed in [30]).
Thirteen of the twenty-one MUC genes encode MAMs. Twelve of these are “epithelial mucins” expressed by mucosal epithelia. The thirteenth – EMCN – is expressed primarily by vascular endothelial cells [33]. Both RNA transcripts and protein products encoded by MUC1, MUC4, MUC16 and MUC20 have been detected at the human ocular surface. Protein products encoded by MUC21 and MUC22 were localized at the ocular surface for the first time by our team [16].
The expression patterns of the different MAMs are not identical across the human ocular surface. MUC1 and MUC16 mRNAs are homogeneously distributed throughout the corneal and conjunctival epithelia [34, 35], while MUC4 mRNA is predominantly expressed in the conjunctival epithelium [36, 37]. MUC21 and MUC22 proteins have been immunolocalized to the human corneal epithelium; their presence in the conjunctiva has not yet been examined [16].
MUC1, MUC4, MUC16, MUC21 and MUC22 proteins accumulate in cells of the apical layer(s) of the corneal/conjunctival epithelia [16, 34, 35, 37–39]. This fits with their functional role as components of the mucosal glycocalyx elaborated at the ocular surface. In apparent paradox, MUC1, MUC4 and MUC16 mRNAs have been localized to cells of all the stratified layers of the corneal/conjunctival epithelia by in situ hybridization [34, 35, 37–39]. One explanation is that the proteins are produced and accumulate throughout the entire epithelia, but mucin glycosylation, and consequently antibody recognition, develops as the cells move up towards the apical layer. An alternative hypothesis supported by the results of studies in rats, is that newly-synthesized MAM proteins are rapidly degraded, becoming stabilized only in the apical cell layer; [39–41]. The question remains unresolved to date.
MUC1, MUC4, MUC16, MUC21 and MUC22 are also expressed at ocular surface locations other than the corneal/conjunctival epithelia. Protein products of all these genes have been immunolocalized to acinar and ductal cells of the lacrimal gland [16, 42–44]. Immunoreactive MUC16 protein has been identified in goblet cells of the conjunctiva, associated with the goblet cell mucin granule membrane [45]. MUC1 is expressed by immune cells that are resident in the ocular surface epithelia, or that infiltrate due to inflammatory or immunological events, including B cells, T cells, monocytes, macrophages and dendritic cells [46–49].
MUC20 protein localization is different from the other MAMs expressed at the ocular surface. It was detected throughout the corneal/conjunctival epithelia, but predominantly within the plasma membrane region of intermediate cell layers rather than the apical layer(s). In the conjunctiva, MUC20 was immunolocalized within the cytoplasm of apical cells, but not in goblet cells [50]. MUC20 expression has not been examined in lacrimal gland.
The shed large subunits of MUC1, MUC4 and MUC16 have been detected in human tear fluid by immunohistochemistry [51]. MUC20 was not found in tears [50]. The presence of MUC21 and MUC22 in tears has not yet been investigated.
2.2. Modular Structure
Table 2 lists the MAMs expressed at the ocular surface, ordered by polypeptide backbone length. These lengths vary dramatically from a maximum of 14,507 amino acids (MUC16) to 484 amino acids (MUC1). MAMs have multiple isoforms, the result of alternative transcription start sites, splice variants, and insertions and deletions. Much of the early interest in MAMs was driven by their pathological roles in cancers, and additional variants may be unique to specific tumors (e.g. MUC1 from a pancreatic tumor [11, 52].
Table 2.
Membrane-Associated Mucins Expressed at the Human Ocular Surface
| Symbol | Total amino acids of protein backbone | Predicted mass of protein backbone | Number of Isoforms listed in NCBI Gene | Number of amino acids in cytoplasmic tail |
|---|---|---|---|---|
| MUC16 | 14,507 | 1,519 kDa | 14 | 31 |
| MUC4 | 7,418 | 734 kDa | 4 | 22 |
| MUC22 | 1,786 | 175 kDa | 3 | 92 |
| MUC20 | 709 | 72 kDa | 4 | N/A* |
| MUC21 | 626 | 60 kDa | 3 | 66 |
| MUC1 | 484** | 50 kDa | 20 | 72 |
Protein data derived from the NCBI Protein database; listed here is the amino acid number of the longest isoform identified in the database
Estimated molecular weight of the protein backbone mass was computed using:https://web.expasy.org/compute_pi/
MUC20 is membrane-associated, but does not appear to be transmembrane
A longer isoform of MUC1, thus far only identified in cancer cells (1255 amino acids) is listed in the UniProtKB database
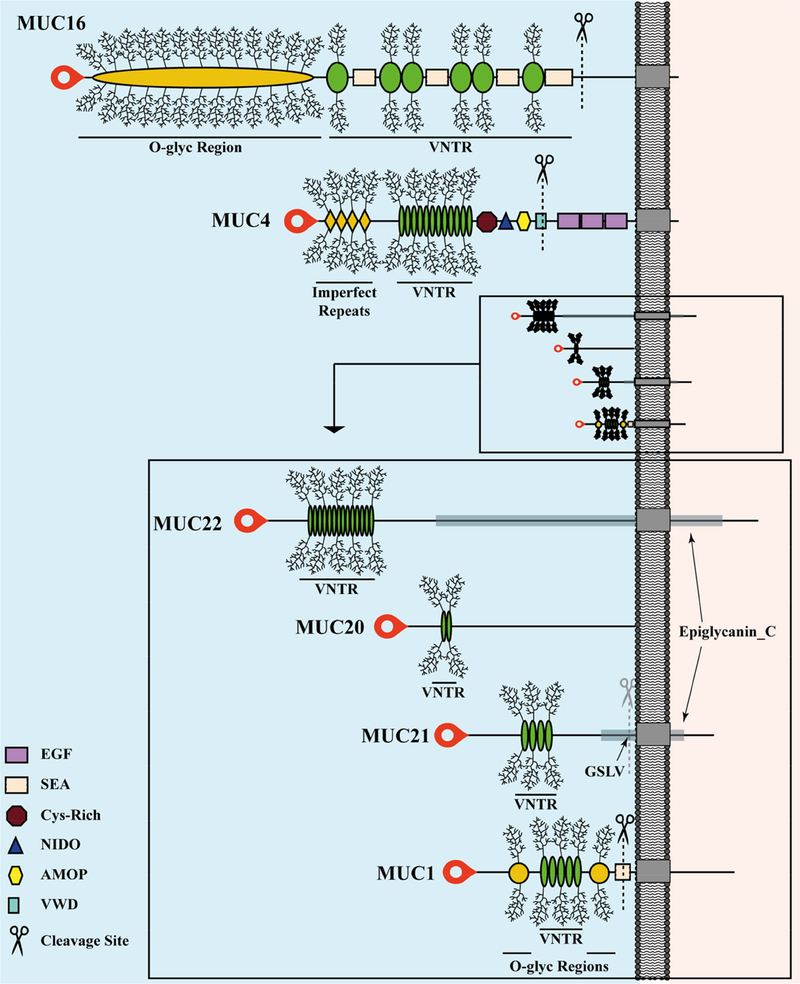
Figure 3 depicts the specific modular architecture of the individual ocular surface MAMs. MUC1, MUC4 and MUC16 are transmembrane proteins that are cleaved during their biosynthesis. MUC1 is cleaved within the SEA module [53], while MUC4 is cleaved within the von Willibrand Domain (vWD) module, carrying a putative cleavage site at the amino acid sequence GDPH. Proteolytic processing of both mucins occurs in the endoplasmic reticulum [54]. MUC16 is cleaved within a juxtamembrane stretch of twelve amino acids located in the ED; this occurs in the Golgi/post-Golgi cellular compartments [55]. Additional MAM cleavages also occur extracellularly and can enhance shedding of the large subunit from the cell surface [56–59].
Figure 3.
Modular Architecture of Membrane-Associated Mucins of the Human Ocular Surface.
The extended conformation of each extracellular domain (ED) is to the left of the plasma membrane (gray bar) and each cytoplasmic tail (CT) to the right, both drawn to scale. The transmembrane domains are indicated as gray boxes embedded in the plasma membrane. MUC20 has been experimentally determined to associate with the plasma membrane, but has no transmembrane domain, and thus no CT has been identified (discussed in the text). Because of the extreme size differences between the large and small MAMs, an expanded view of the ED is shown for the small MAMs. Signal peptides are located at the amino-terminus of each protein (red blobs). The cleavage sites in the EDs are indicated by scissors.
MUC20 contains hydrophobic regions consistent with plasma membrane association [60], but no alpha-helical transmembrane domain has been identified [16]. Nevertheless, several lines of evidence support the idea that MUC20 binds to cell surfaces [60, 61]. In human ocular surface epithelia, immunoreactive MUC20 was detected predominantly in the cell membrane area of intermediate cell layers and was not detected in human tears, [50]. Thus, MUC20 appears to be a non-secreted and non-shed MAM that is retained extrinsically at the plasma membrane.
Sequence analysis of human MUC21 and MUC22 predict that they encode transmembrane proteins. It is not known whether they are cleaved during their biosynthesis. A sequence (GSLV) similar to the putative cleavage site associated with the SEA module in MUC1 is present immediately upstream of the putative transmembrane domain in MUC21, however, this sequence is not conserved in MUC22 [62].
As shown in Table 2, the CTs of individual MAMs are dissimilar in length, ranging from 92 amino acids (MUC22) to 22 amino acids (MUC4). Except for the CTs of MUC21 and MUC22, there is no apparent sequence homology or conserved modules [16]. However, their functional convergence is evidenced by the presence of similar protein binding and phosphorylation motifs located within each of the individual CTs [16]. For example, when activated, receptor tyrosine kinase EGFR binds a short motif in the CT of MUC1 and phosphorylates a tyrosine residue [63]. Large subunit shedding stimulates CT phosphorylation and this is enhanced when cells are treated with EGF [64]. Similarly, in silico analysis predicts that EGFR also phosphorylates a tyrosine residue on the CT of MUC16 [16].
In fact, EGF signaling appears to be a common theme among the MAMs, accomplished in different ways. The large subunit of MUC4 (but not MUC1 or MUC16) has three EGF-like modules located distal to the cleavage site [65]. The one closest to the transmembrane domain is similar in sequence to the EGF-like domain found in ERBB3, a receptor tyrosine kinase of the EGFR family. Rat Muc4 was shown to interact via this EGF-like module with ERBB2, another member of the EGFR family. The protein-protein interaction induced specific phosphorylation of ERBB2 and led to downstream signaling [66].
As another example of functional convergence, the CTs of MUC1 and MUC16 both connect to the cell’s cytoskeleton, but in different ways. MUC1 does this via a binding site for the catenin CTNNB1, which is positioned within the adherens junction that links apical epithelial cells [67]. MUC16 lacks a canonical CTNNB1 binding site, but pull-down experiments suggest that a polybasic amino acid stretch at the proximal end of the CT interacts with ezrin/radixin/moesin (ERM) actin-binding proteins that then interact with the adherens junction [68].
The CTs of MUC1 and MUC16 can also migrate to the nucleus, often initiated by shedding of the large subunit [69–71]. This behavior adds to the possible signal transduction roles by placing the CTs in proximity to transcription factors.
2.3. Glycosylation
Analysis of the carbohydrate content of human ocular surface mucins has revealed that approximately 55% of the mucin mass is carbohydrate [72]. The glycan moieties of MAMs may vary depending on the mucin type, the site of mucin expression, and the physiological or pathological conditions [73]. Glycan structures of ocular surface mucins also display differences among different mammalian species [74].
The synthesis of O-linked glycans is initiated in the Golgi apparatus by the addition of N-acetylgalactosamine to the hydroxyl groups of serine (Ser) or threonine (Thr) amino acids by a group of polypeptide N-acetylgalactosaminyltransferases. The formation of this structure, referred to as the Tn antigen, can be followed by the addition of other carbohydrates such as galactose and N-acetylglucosamine to create core structures, and by the terminal addition of sialic acid, fucose or sulfate. The structures of O-glycan chains found at the ocular surface are relatively simple compared to O-linked glycans in other mucosal secretions, such as the intestinal and respiratory tracts [19]. They are mostly comprised of core 1 which is formed by the addition of galactose in a beta1–3 linkage to N-acetylgalactosamine [75, 76]. The O-linked glycans play an important role in determining the biophysical properties of the MAMs, as well as their extended conformation.
N-linked glycans are also present in the protein backbone of the MAMs. N-glycosylation starts in the endoplasmic reticulum by the transfer of a dolichol oligosaccharide precursor to asparagine (Asp) residues on nascent proteins. At the ocular surface, the N-linked glycans consist primarily of complex-type structures with N-acetyllactosamine. They are not as abundant as the O-linked glycans but play important roles in supporting the stability and function of the mucins [77].
Several biologically important lectins bind the carbohydrate side-chains of MAMs. N-acetyllactosamine, is a preferred ligand for the galectin LGALS3 (galectin-3) [78]. Galectins are involved in barrier function (next chapter), and galectins, as well as selectins and siglecs (sialic acid-binding immunoglobulin-type lectins) are involved in immune responses and immunoregulation [79].
3. BIOLOGICAL ROLES
3.1. Barrier Function
Ocular surface epithelia are continually renewed by division of basal cells at the epithelial basement membrane. As the cells are displaced upwards, they become increasingly flattened and MAMs accumulate in a polarized manner on cells of the apical layer. This creates the transcellular barrier restricting entry into cells. The apical mucosal epithelial cells also develop tight junctions, creating a paracellular barrier that restricts access to lower cell layers [2]. Here we describe two aspects of transcellular barrier function: rose bengal exclusion and pathogen exclusion.
3.1.1. Rose Bengal Exclusion
The study of ocular surface staining by rose bengal, has been instrumental in defining molecular interactions responsible for ocular surface transcellular barrier function. Rose bengal was once commonly used clinically by ophthalmologists as a tool to evaluate ocular surface damage, especially in dry eye [80]. Studies from the Tseng laboratory [81, 82] were the first to show that healthy cells in monolayer culture take up rose bengal, and this can be blocked by mucins. Later, the development of an in vitro model of stratified corneal epithelial cells with mucosal differentiation demonstrated that rose bengal is excluded when cells elaborate a mucosal glycocalyx [83].
MAMs of the ocular surface epithelia are crosslinked by LGALS3 to form an interlocking lattice. Like other members of the galectin family, LGALS3 contains a carbohydrate-binding domain with affinity towards beta-galactosides. Abrogation of interaction between LGALS3 and MAMs results in an increase in rose bengal staining [84].
MUC16 expression is essential for the exclusion of rose bengal [68], as well as both O- and N-linked glycosylation [77, 84]. In contrast, knockdown of MUC1 decreases rose bengal penetrance [85]. This apparent detrimental effect of MUC1 on barrier function was reasoned to be a consequence of the huge difference in size and glycosylated residues compared to MUC16: the large and heavily glycosylated ED of MUC16 dominates over the rest of MAMs, providing a wider surface for MAM-galectin interactions to form a tight structure. The other shorter mucins interspersed in the glycocalyx may contribute to create a more uneven lattice that is less efficient in blocking the passage of substances such as rose bengal [85].
3.1.2. Pathogen Exclusion
Exclusion of pathogens by MAMs has been best studied in relation to MUC1, which has been identified as an adhesion receptor for various pathogenic bacteria (e.g. [86–91]; reviewed in [92]). Rather than a means for the pathogen to enter and infect the cell, binding to MUC1 instead appears to inhibit infection by triggering large subunit shedding [87, 89]. In this way, the large subunit serves as a releasable decoy, promoting bacterial clearance. Consistent with this mechanism, knockdown of MUC1 decreased adherence of Staphylococcus aureus in cultures of stratified corneal epithelial cells with mucosal differentiation [68, 85].
If MUC1 limits pathogen infection, then increasing the amount of MUC1 on the cell surface should be beneficial. In support of this idea, overexpression of MUC1 by transfection of cultured epithelial cells reduced Influenza A viral infection. Similarly, exogenous addition of a mixture of corneal epithelial transmembrane mucins efficiently prevented the interaction of Herpes simplex type 1 viral with a human corneal epithelial cell receptor in vitro [93]. Several reports describe elevated expression of MUC1 when cells in culture are challenged by bacterial and viral pathogens (e.g., [88, 94, 95]. This may be a consequence of upregulated expression of inflammatory cytokines. For example, when airway epithelial cells were exposed to P. aeruginosa, MUC1 upregulation occurred downstream of TNFA induction [88].
Challenge by Respiratory Syncytial Virus also strongly induced MUC21 and MUC22 expression [95]. MUC21 was one of the most upregulated RNAs in bronchoalveolar lavage fluid of children with pneumonia due to severe infections of the atypical bacteria Mycoplasma pneumoniae [96]. Expression of MUC22 was remarkably upregulated by double-stranded RNA in normal human bronchial epithelial cells redifferentiated at the air-liquid interface [97]. These findings suggest that, like MUC1, MUC21 and MUC22 are involved in defense against viral infection.
3.2. Regulation of Inflammation
MAMs regulate the inflammatory response in mucosal epithelial cells via interaction with several different signaling pathways. Here we describe interaction with pathways regulated by NF-kappaB, glucocorticoids, and toll-like receptors.
3.2.1. NF-kappaB
MUC1 is aberrantly overexpressed by diverse carcinomas and certain hematologic malignancies, conferring anchorage-independent growth and tumorigenicity (reviewed in Bafna et al., 2010). This occurs, at least in part, by constitutive activation of the inflammatory pathway regulated by NF-kappaB [98]. The isolated MUC1 CT is sufficient for this effect, when introduced via DNA expression construct into cells that do not express MUC1. The CT of MUC1 interacts with the IKK complex in the cytoplasm, thus releasing NF-kappaB for migration to the nucleus [99]. In contrast, MUC1 expressed at normal levels on mucosal epithelial cells inhibits inflammation due to pathogen exposure by NF-kappaB inhibition [100, 101]. In this case, interaction of the MUC1 CT with IKK prevented NF-kappaB migration to the nucleus [101]. These different results remain to be fully explained.
MUC1 overexpressed by carcinomas is often found in an altered under-glycosylated form. Differences in the glycosylation state of MUC1 can increase its internalization, change its subcellular distribution [102] and alter its interplay with the NF-kappaB pathway [103, 104]. A decrease in the MUC1/A splice variant was observed in dry eye patients [105]. A related study showed some differences between MUC1/A and /B in NF-kappaB activation [106], however, the consequences for the ocular surface are not clear. A possible question for the future is if disease or environment can alter MAMs splicing or glycosylation patterns and affect NF-kappaB activation.
3.2.2. Glucocorticoids
Glucocorticoids are a class of steroid hormones released in response to stress. They activate the glucocorticoid receptor NR3C1, which translocates to the nucleus and binds to the promoters of a battery of genes, activating transcription. Glucocorticoids also inhibit gene expression regulated by inflammatory cytokines and transcription factors AP-1 and NF-kappaB. As part of the natural feedback mechanism that turns down the inflammatory response, glucocorticoid pharmaceuticals are very effective for treating a wide variety of diseases [107].
Glucocorticoids modulate the expression of different MAMs in different tissues [108, 109], including the ocular surface epithelia [110, 111] via response elements located on their transcriptional promoters. The CT of MUC1 interacts with NR3C1, mediating its translocation to the nucleus [112]. Reduced MUC1 expression is associated with resistance to glucocorticoid drugs [113–115]. In contrast, elevated MUC4 expression is associated with resistance to glucocorticoid drugs, due to interaction of the CT of MUC4 with NR3C1, which inhibits its nuclear function [116].
3.2.3. Toll-like Receptors
Toll-like receptors (TLRs) are a family of pattern recognition receptors that respond to danger and pathogen-associated signals, thus triggering an innate immune response through the activation of inflammatory signaling pathways [117]. MAMs have been shown to donw-regulate TLR mediated inflammation. For example, in primary bronchial cells, EGFR phosphorylation of the MUC1 CT results in its association with TLR5, inhibiting the recruitment of MYD88 required for signaling [17]. Exposure of cells to P. aeruginosa also results in MUC1 CT phosphorylation, leading to association with TLR5 and suppression of its signaling [118]. In addition to TLR5, MUC1 can also suppress TLR3-, TLR4-, TLR7- and TLR9-initiated signaling, inhibiting the subsequent activation of NF-kappaB [119] and the NLRP3 inflammasome [120–122]. In a model of corneal epithelial cells in stratified culture with mucosal differentiation, both MUC1 and MUC16 dampen the expression of proinflammatory cytokines after the activation of TLR2 and TLR5 [123].
3.3. Determination of Cell Shape and Desquamation
When the ocular surface is viewed from above by scanning electron microscopy (SEM), the apical epithelial cells of humans and other vertebrates exhibit an intricate pattern of plasma membrane folds called microvillae, microplicae and microridges. MAMs emanate from these processes, which increase the cell surface area and are thought to improve the movement of oxygen, nutrients, and metabolic products across the outer cell membranes [2]. When the magnification is decreased, a contiguous mosaic of polygonal cell shapes with a range of sizes, each having a light, medium, or dark appearance, with the dark reflex cells being the largest [124, 125]. The dark reflex is due to a reduction in the size and number of microprocesses [124, 126]. These cells are thought to be the most mature and are shed fairly rapidly, in a process called desquamation. Complete turnover time of the human corneal epithelium has been estimated to be on the order of 1 week [127].
Mechanisms for desquamation of dead corneocytes from the desiccated superficial layer of the dry epidermis are well-described [128]. In contrast, mechanisms controlling desquamation at the mucosal ocular surface, where the apical cells are wet and still metabolically active, are essentially unknown. It has been suggested that loss of microplicae and the ‘lubricating’ MUC16 may facilitate desquamating cell removal [30]. However, these cells must also detach from the underlying cell layer and actively dissociate themselves from their neighbors to undergo desquamation.
A recent study has causally implicated MAMs in both the elaboration of cell processes at the apical epithelial layer of the ocular surface, and in cell-cell dissociation leading to desquamation [85]. First, the same mosaic of light, medium, and dark cells seen at the ocular surface imaged by SEM was also seen in a surface view of a human corneal whole mount, immunostained with antibodies to MUC1 or MUC16 [85]. The intensity of immunostaining correlated indirectly to the cell surface area, with the largest (and presumably most mature) dark cells staining the least. Thus, the reduction in membrane folds and increase in cell area that occurs with cell maturation was found to correlate with loss of MAMs from the cell surface.
The next experiments were conducted using a model of stratified corneal epithelial cells with mucosal differentiation which express both MUC1 and MUC16 on the apical cells in a polarized manner [129]. Surface microplicae/microridges typical of native apical epithelial cells were observed and immunostaining for MUC16 revealed the same surface pattern of light, medium, and dark cells as seen by SEM [85]. Knockdown of MUC16 reduced surface microplicae and resulted in an increase in surface cell area. Interestingly, MUC1 knockdown did not have this effect. In addition, MUC16, but not MUC1 knockdown decreased tight junction proteins ZO1 and OCLN, as well as transepithelial resistance, a measure of paracellular barrier integrity [85].
Knockdown of MUC16, but not MUC1, was also associated with disruption of the actin cytoskeleton associated with the tight junctions [85]. It was suggested that the CT of MUC16 may mediate this effect by direct interaction with the actin cytoskeleton [85]. Forces originating from cytoskeletal dynamics are posited to generate membrane curvature for the diverse spherical and tubular structures on the cell surface (e.g. [130–132]), thus the CT of MUC16 might also determine microprojections on the cell surface. However, a very recent study provides evidence that the ED of MUC16 would be sufficient [133].
The prototypical MAM ED closely resembles a “brush”, i.e., a well-studied structure in polymer physics, where polymers are grafted on one end to a surface. Polymer brush theory holds that steric interactions in a densely-crowded brush restrict the number of molecular configurations each polymer can explore, thereby reducing entropy and generating pressure on the anchoring surface. Experimental studies with synthetic polymers have confirmed that the pressures generated by these unstructured macromolecules are sufficient to deform flexible lipid membranes (e.g. [134].
To test these hypotheses, a genetically-encoded library of native, semi-synthetic, and rationally designed mucin polymers of varying size, backbone sequence, and membrane anchorage were generated, including the 42 native tandem repeats of MUC1. When expressed at high levels on epithelial cell surfaces, each of the long-chain mucins triggered a dramatic tubularization of the plasma membrane. More rigid, folded protein constructs of comparable size, such as repeating units of highly stable, epidermal growth factor (EGF)-like modules, did not have such an effect. The tubularization phenomenon was relatively insensitive to the length of the mucin polymer domain, provided that the polymers were expressed on the cell surface at moderate to high densities. The results suggested that plasma-membrane morphologies might be predicted simply by the quantity of mucins or other biopolymers on the cell surface [133], including mucosal epithelial cells of the ocular surface.
3.4. Stabilization of Ion Channels
The shed ectodomain of UMOD (Uromodulin) known as Tamm-Horsfall protein, is the most abundant protein in urine, and mutations in this gene cause human kidney disease [135]. Mice expressing UMOD with one of the disease-causing human mutations exhibit less urinary Tamm-Horsfall protein, and they also exhibit hypercalciuria and renal calcium crystals corresponding with reduced immunostaining for the renal calcium channel TRPV5 [136]. In transiently transfected human HEK293 cells, co-expression with UMOD or addition of exogenous Tamm-Horsfall protein increased TRPV5 surface currents, reduced TRPV5 endocytosis and increased TRPV5 cell surface expression. This is consistent with a role for Tamm-Horsfall protein in stabilization of TRPV5 [137].
Because of structural similarities between UMOD and MUC1, this mechanism was also investigated, with similar results. Interestingly, urinary MUC1 is also reduced in patients with calcium nephrolithiasis, a common type of kidney stone. Moreover, cell culture studies revealed that TRPV5 surface expression is also enhanced by binding LGALS3. MUC1 enhancement of TRPV5 surface expression is preceded by LGALS3-dependent crosslinking of O-linked glycans on MUC1 with the N-linked glycan on TRPV5.
Thus, LGALS3 crosslinking of MUC1 with the TRPV5 ion transport channel at the surface of epithelial cells appears to provide a novel mechanism for regulation of function. It seems likely that the MUC1 ED might more broadly enhance surface expression of transient receptor potential (TRP) family ion transport channels by a similar mechanism of crosslinking and maintenance at the cell surface. For example, a large genome-wide association study focused on serum concentrations of cations revealed that the highest association with low serum magnesium levels (hypomagnesemia) was a very common genetic variant of MUC1 (rs4072037) that adds nine amino acids to the extracellular N-terminus of the protein [138].
The findings in kidney suggest possible parallels to the ocular surface. TRP channels have been identified on the ocular surface epithelia, as well as the corneal afferent nerve endings of the ophthalmic branch of the trigeminal nerve [139]. TRPM8 is a cold-sensing receptor activated in ocular surface nerves after evaporation of the tear film to stimulate lacrimal gland secretion [140]. Nerve TRPV1 is activated by hypertonic challenge, which in turn leads to an increased release of pro-inflammatory cytokines [141]. Dysfunction of these ion channels has been suggested as a possible pathophysiological mechanism in dry eye disease [142]. Whether MUC1 can regulate TRP channel activity at the ocular surface will be an interesting question to investigate.
4. DISEASE INVOLVEMENT
Ocular surface diseases are of diverse etiology, but they share a common pathology of damage to the apical layer of the ocular surface epithelia and their glycocalyx, barrier disruption, and cell loss due to programmed cell death. Damage is initiated by internal factors such as meibomian gland dysfunction, blepharitis, rosacea, and by external factors such as ultraviolet light, allergens, pollution, preservatives in glaucoma medication and chemical warfare agents. Ocular surface disease is also seen in Sjögren’s syndrome, mucous membrane pemphigoid, graft-vs-host disease and other autoimmune disorders [143–145]. In this section, we discuss the MAM abnormalities observed in different ocular surface diseases. We then go on to discuss an emerging mechanism responsible for damage to the epithelia and their mucosal glycocalyces that offers a novel target for therapeutic intervention.
4.1. Disease Abnormalities
Ocular surface disease is frequently accompanied by alterations in the mucin component of the tears or the glycocalyx associated with the ocular surface epithelia. Abnormalities in O-glycosylation of MAMS have been identified in many ocular surface disorders where the stability of the tears is compromised (e.g., [18]; reviewed in [19]). Here, we discuss MAM alterations seen in four ocular surface disease with different etiologies: dry eye, contact lens wear pathology, and exposure to allergens and pollution.
4.1.1. Dry Eye
Dry eye is a common disease of the ocular surface with multiple causative factors [146–149]. A reduction in goblet cell density is typically observed. This results in a decrease in secreted mucin and leads to tear dysfunction [22, 24]. Variations in the expression levels and glycosylation pattern of MAMs have also been detected, but no clear pattern has emerged.
In one study on Sjögren’s Syndrome patients, no change was observed in the steady state level of mRNAs for MUC1 or MUC4 mRNA [150]. In contrast, a second study found increased conjunctival MUC1 protein and mRNA in Sjögren’s Syndrome patients, as well as increased immunoreactive protein in the tears [151]. In another study with non-Sjögren’s Syndrome aqueous deficient dry eye, MUC1 and MUC4 gene expression was decreased [152]. MUC16 gene expression was found increased as well as its presence in tears of Sjögren’s Syndrome patients but not in non-Sjögren’s Syndrome patients with dry eye [153]. Conversely, another study found lower MUC16 mRNA in patients with aqueous deficient dry eye [154].
Reduced binding of the monoclonal antibody H185 was observed at the ocular surface of patients with non-Sjögren dry eye as compared to normal [155]. The epitope was later identified as an O-acetylated sialic acid found exclusively on MUC16 [35, 156]. This raised the question (still unanswered) as to whether reduced binding was due to a decrease in MUC16 gene expression, or altered MUC16 glycosylation patterns. Another sialylated epitope, in this case at MUC1, was found increased in both cornea and conjunctiva of dry eye patients, except for the conjunctiva of severe patients [157]. Studies have been directed to the detection of possible changes in glycosyltransferases. While no changes have been observed in dry eye patients [75, 158], different isoforms of polypeptide N-acetylgalactosaminyltransferases and N-acetylglucosaminyltransferases were found altered during ocular surface keratinization [159, 160]. For the polypeptide, N-acetylgalactosaminyltransferases, a change in local distribution but not in their overall expression has been proposed.
Dry eye is age-related and prevalence in the United States population is estimated at nearly twice as high for women as men [161]. However, there is little published on ocular surface MAMs in dry eye with relation to age or sex. In one study, increased amounts of MUC4 was identified in the lacrimal glands of elderly women who received treatment for dry eye [162].
4.1.2. Contact Lens Wear Pathology
Contact lenses artificially separate the tear film into two compartments, thus, challenging its integrity [163–165]. With some frequency, this leads to development of dry eye signs and symptoms. Contact lens wear can reduce goblet cell density and thus, production of MUC5AC, a sign of dry eye [166, 167]. Studies suggest alterations of the ocular surface glycocalyx in contact lens wearers, as evidenced by lower staining with a fluorescein-labeled lectin [168, 169]. Results regarding changes in the expression of MAMs are less consistent [163]. In one study, significant changes in the expression of MUC1, 4 and 16 in contact lens wearers were found [170]. In another study however, changes in different MAMs were detected depending on time and water content of the contact lens [171].
Clinical staining with vital dyes is observed after exposure to contact lenses soaked in certain multipurpose contact lens cleaning solutions (MPS), a phenomenon that has been called solution-induced corneal staining (SICS) [172]. In the first reports, the appearance was described as a ‘classic solution-based toxicity reaction’ [173, 174]. MPS characteristically contain a surfactant cleaner such as Tetronic 1107 [175], a biocide such as polyhexamethylene biguanide (which may also have surfactant properties [176]), and a buffering agent such as borate. Electron microscopic studies revealed frank loss of surface epithelial cells in rabbit eyes exposed to a borate-based MPS while wearing a contact lens, while control eyes with contact lens wear only exhibited occasional loss of surface membranes but retention of intact junctional borders [177]. When the ocular surface of human subjects was observed in white light at high resolution after they wore a pair of contact lenses soaked in a commonly used MPS, distinct grey/white superficial opaque “dots” could be clearly seen over the transparent, unstained cornea, of a size broadly consistent with the known diameter of superficial human corneal epithelial cells [172].
Significantly decreased levels of mRNA for MUC1 and MUC16 at the ocular surface were found in patients that used an MPS containing boric acid [178]. The Fini laboratory observed only a small decrease in metabolic activity in cultures of human stratified ocular surface epithelial cells with mucosal differentiation treated with three different MPS, and MAM mRNA levels were not significantly changed. However, shedding of MUC16 large subunits into the cell culture medium was observed, correlated with an increase in rose-bengal staining [179]. Exposure to MPS also increased uptake of P. aeruginosa uptake by the corneal epithelium of rabbits [177] and cultures of human stratified ocular surface epithelial cells with mucosal differentiation [179].
4.1.3. Allergy
Allergy is a maladaptive immune response to innocuous environmental antigens. Its prevalence has increased in recent years and it is now the sixth cause of chronic illness in the USA [180]. It affects 20–30% of population in industrialized countries, with 50% of affected individuals exhibiting ocular manifestations [181]. Advances in allergy research have changed the way surface epithelia are viewed. They are no longer considered just a barrier. Rather it is now appreciated that they participate actively in the initiation and regulation of immune responses. Activation of pattern-recognition receptors, such as toll-like receptors, on epithelial cells leads to the release of cytokines, chemokines and antimicrobial peptides that attract and activate innate immune cells [182].
Allergic disorders with ocular involvement include seasonal and perennial allergic conjunctivitis and the more severe vernal keratoconjunctivitis and atopic keratoconjunctivitis [183, 184]. Different studies show modifications in the expression of MAMs in these disorders. MUC1 and MUC4 mRNA were elevated in patients with atopic keratoconjunctivitis [185]. In another study of the same group, MUC16 was also found elevated [186]. Similarly, increased MUC1 and MUC4 was observed in patients with atopic keratoconjunctivitis compared to controls, but also when compared to patients with vernal keratoconjunctivitis [187].
Additional knowledge on the MAMs implicated in allergy can be obtained from studies on the airways and other tissues. Bronchial cells from severe asthma patients show a reduced level of the MUC1 CT compared to healthy individuals and this is related to refractivity to glucocorticoid treatment [114]. MUC1 is decreased in patients with allergic rhinitis [188]. A polymorphism of the recently discovered MUC22 is associated with asthma [189]. Elevated MUC1 was observed in long-term smokers, correlating with higher levels of leukocytes, which suggested that the increased MUC1 had an origin in these immune cells [190].
MUC4 overexpression is linked to asthma [116] and eosinophilic esophagitis [191]. In a study of nasal polyp epithelial cells, TLR2 and TLR4 agonists induced the expression of MUC4 [116]. In another study with nasal cells, beta-glucan induced the expression of MUC4 and MUC5B through TLR4 [192]. Also in these cells, diesel particles induced MUC4 expression [193].
Interestingly, allergic response to pollen was abrogated in Myd88−/− and Tlr4 knockout mice. Given that MAMs inhibit MYD88 binding to toll-like receptors, it might be hypothesized that MAMs act to regulate the allergic reaction [181, 194]. This idea remains to be tested.
4.1.4. Pollution
Pollution is a global issue that has severe consequences for health and is responsible for millions of premature deaths every year (reviewed in [195]). Air pollution can affect the eye, causing multiple symptoms (reviewed in [196]). Pollution and cigarette smoke are considered risk factors for dry eye [197]. However, there is still a lack of knowledge on the specific mechanisms of damage by pollutant agents. A few recent studies have investigated alterations to ocular surface MAMs caused by pollutants.
Emissions from sugarcane burning are known to impact on the respiratory health of sugar estate workers and local populations [198]. In a study of the ocular consequences of this practice in Brazil, researchers found alterations in mucin production in workers but also in the residents of neighboring towns [199]. In volcanic settings, airborne, respirable emissions (ash particles, aerosols, and gases) could potentially affect human health [200]. Exposure of conjunctival cells to volcanic ashes induces oxidative stress, an increase in pro-inflammatory cytokines and a decrease in MUC1 [201].
Urban areas, especially those with high levels of traffic, concentrate important amounts of pollutants. In a study with subjects from cities with different levels of pollution levels, goblet cell hyperplasia was detected [202]. Long exposure to traffic pollution, as it is the case for taxi drivers and traffic controllers, causes ocular symptoms and reduces goblet cell density and MUC5AC expression [203]. Diesel exhaust particles increased the expression of IL6 and decreased viability and proliferation of ocular surface epithelial cells. While levels of MUC1 and MUC16 proteins were reduced in corneal cells, they were increased in conjunctival cells [204].
Another common environmental pollutant is cigarette smoke. Passive exposure to E-cigarette smoke for 30 minutes provoked symptoms of discomfort to the eye and airways [205]. Smokers present higher ocular fluorescein [206] and lissamine green staining [207]. Passive cigarette smoke exposure also increased fluorescein and rose bengal staining, which was maintained after 24 hours of exposure [208]. It seems likely that this is due to effects on MAMs, however, this must be investigated.
4.2. Mechanisms of Damage and Therapeutic Protection
Water-soluble “vital” dyes, including fluorescein, rose bengal and lissamine green, have long been used as a clinical tool to evaluate ocular surface damage [209]. Thus, it is rather surprising how little we understand about what these dyes tell us about ocular surface damage on the molecular level [210]. For example, we know that rose bengal exclusion reflects an intact mucosal glycocalyx (see section 3.1.1). However, what exactly changes in ocular surface disease to disrupt this barrier and cause dye update is less clear.
Fluorescein is the parent compound from which rose bengal is derived, thus, the two dyes are structurally related [211]. Nevertheless, they differ in cell uptake properties. Living corneal epithelial cells in monolayer culture take up fluorescein in the same way as rose bengal, but at a lower level, requiring visualization under epifluorescent illumination [82]. Fluorescein concentration by individual corneal epithelial cells was first observed at the rabbit ocular surface in situ [212]. In later studies, individual cells in the superficial epithelial layers of the human ocular surface damaged by dry eye were observed to take up fluorescein, described as “hyperstaining” [213]. Unlike rose bengal, fluorescein uptake by cells was not blocked by mucins [82].
The phenomenon of SICS has provided an opportunity to gain insight into the mechanisms of vital dye staining. In one study, patients wore a pair of contact lenses soaked in an MPS, then their ocular surface was stained sequentially with rose-bengal and fluorescein. Cell-sized pink spots were observed with rose bengal and the same spots were observed with fluorescein. There were some minor differences. With fluorescein, the dots were larger and less sharply-defined, likely due to the well-known diffusibility of this dye. Additionally, some spots of fluorescein staining were observed that did not stain with rose bengal. However, the results suggests that, even if the two dyes identify different types of damage, both are found in most ocular surface cells exposed to MPS [172].
A recent study used cells in monolayer culture to investigate basic mechanisms of fluorescein uptake in cells subjected to damaging stress [214]. While all cells took up fluorescein at a low level, it was found that a small percentage concentrated dye, appearing as hyperfluorescent. Dye uptake was observed to be an active process, inhibited by reducing the temperature or by killing the cells. Application of damaging stress, including hypo- or hyperosmotic stress, exposure to an ophthalmic preservative, or scratch/alkali “wounding” of the cell monolayer greatly increased the number of hyperfluorescent cells. Stressed cells exhibiting high fluorescence intensity also showed characteristics of early apoptosis, whether in monolayer culture, or in the apical epithelial layer of ex vivo rabbit eyes. It was concluded that fluorescein hyperstaining due to damaging stress is an active process of dye concentration by cells that might be compromised, but are still metabolically active [214].
A second team examined fluorescein uptake after MPS-treatment of cells in monolayer culture, with very similar results and conclusions [215]. In a follow-up study, fluorescein uptake in MPS-treated cells was found not to be associated with early apoptotic markers or metabolic compromise, suggesting minimal toxicity [216]. The surfactant Tectronic 1107 alone caused the same fluorescein uptake effects as the complete MPS [216]. Surfactants dissolve plasma membrane lipids, but this may result in only limited plasma membrane compromise.
To more accurately model the ocular surface, we took up the question of dye uptake mechanisms using both monolayer cells and stratified cultures of human ocular surface epithelial cells with mucosal differentiation [217]. We chose oxidative stress as the damaging stress for our study, created by exposing cells to tert-butyl hydroperoxide (tBHP). Numerous reports have implicated oxidative stress as important to the pathophysiology of OcS diseases [218–221]. Cells produce reactive oxygen species (ROS) as a by-product of their normal metabolic processes and ROS serve as 2nd messengers for intracellular signaling and the level of these potentially damaging substances is controlled by antioxidant systems of the cell. However, under some conditions, ROS become elevated, overwhelming the normal checks.
As visualized under epifluorescent illumination, fluorescein stained all cells in monolayer culture, with dye concentrated primarily in the nucleus. A mosaic of scattered individual hyperstained cells was observed. Oxidative stress increased the number of cells hyperstained by fluorescein. Rose-bengal dye uptake was essentially the same as fluorescein, with similar numbers of cells showing dye concentration under both unstressed and stressed conditions. Fluorescein dye uptake (as quantified by plate reader) stimulated by oxidative stress was inhibited to 35% when the culture temperature was reduced to ambient, and to 10% when reduced to 4°C. These results were very similar to those of the previous reports, consistent with the idea that vital dye uptake and concentration is an active process of living cells [217].
To characterize cell damage caused by oxidative stress, we probed for both early (ANXA5 binding assay) and late (TUNEL assay) stages of apoptosis. Phosphatidylserine exposed on the outer leaflet of the membrane surface leads to ANXA5 binding. The percentage of cells that bound ANXA5 was substantially increased by oxidative stress. In contrast, only a small number of cells appeared to be in late stage apoptosis was detected by TUNEL assay performed at the same time point, and there was little if any difference between unstressed and stressed cells. Again, these results agree with the previous reports and support the idea that cell damage due to oxidative stress in our model is primarily sublethal, at least at the time point examined [217].
To repair plasma membrane damage and maintain proteostasis, cells activate remodeling processes that involve endocytosis [222–225]. We hypothesized that fluorescein dye might be taken up into endocytic vesicles of individual cells undergoing such repair. When monolayer cultures were subjected to oxidative stress, both endocytosis and dye uptake were stimulated. Stress-stimulated endocytosis was blocked by three different endocytosis inhibitors. Stress-stimulated fluorescein dye uptake was blocked by genistein, an inhibitor of caveolin-mediated endocytosis, and chlorpromazine, an inhibitor of clathrin-mediated endocytosis. It was also blocked by the small molecule dynasore, which is required for both forms of endocytosis [217]. Interestingly, in a study published about the same time as our own, dynasore was also found to inhibit uptake of fluorescein in MPS-treated cells in monolayer culture [216].
Significantly, when we used stratified cultures with mucosal differentiation, we obtained very different results. We found that oxidative stress did not stimulate uptake of TF (transferrin), a marker for endocytosis, in fact there appeared to be some inhibition. Moreover, dynasore and its more potent analogue dyngo-4a, but not other endocytosis inhibitors, prevented staining with both rose bengal and fluorescein in cells subjected to oxidative stress. These results indicate that the link between vital dye uptake and endocytosis, observed in monolayer cells, does not hold for stratified and differentiated cells, which better model the ocular surface. The fact that dynasore and dyngo-4a inhibit vital dye uptake must thus occur through an alternative mechanism [217].
Our cell culture results were validated using ex vivo mouse eyes exposed to tBHP in organ culture. As in the cell culture studies, dynasore blocked vital dye uptake at the mouse ocular surface when treatment was performed at the same time as eyes were stressed. Significantly however, dynasore had no effect when used after stress was applied and the ocular surface was already damaged. Thus, vital dye uptake cannot be dependent on endocytosis [217].
If it does not directly block dye uptake, then how does dynasore work? Using metabolic and cytotoxicity assays, as well as western blotting, we demonstrated that dynasore directly protects both cells and their mucin barrier. We concluded that dynasore does not block dye entry, but instead, prevents ocular surface barrier damage and disruption, thus precluding dye uptake [217].
Although designed to inhibit endocytosis by targeting the classic dynamin DNM2 [226], dynasore has “off-target” effects, some of them mediated by other members of the dynamin family with no role in endocytosis. Inhibition of dynamin family member DRP1 using the selective small molecule inhibitor, mdivi-1, protected cardiomyocytes against oxidative stress by preventing mitochondrial fragmentation and apoptosis [227–229]. However, in a second study to investigate the protective mechanism of dynasore, we found that mdivi-1 did not protect either the ocular surface cells or their mucin barrier against oxidative stress. Moreover, while damage to mitochondria and the plasma membrane could be prevented with the RIPK1 inhibitor necrostatin-1, damage to the glycocalyx still occurred [230].
Oxidative stress has been linked to endoplasmic reticulum (ER) stress, caused by an insufficient capacity to fold newly-synthesized proteins [231]. This launches the “unfolded protein response” (UPR), a protective mechanism that restores homeostasis. In the presence of misfolded proteins, the molecular chaperone GRP78 releases UPR sensors ATF6, IRE1 and PERK. Each produces a transcription factor launching gene expression programs that result in increased protein-folding capacity and decreased protein load. However, if stress is excessive or chronic, UPR activation is circuited to an alternate PERK branch involving transcription factor CHOP. This initiates programmed cell death [232].
Emerging evidence links ER stress and the UPR with ocular surface disease. UPR activation in the conjunctival epithelia and goblet cells of mice in experimental and genetic forms of dry eye, and in human Sjögren’s syndrome [233]. In a more recent study from the Argüeso lab, UPR activation was observed in the conjunctival epithelium of human patients with ocular cicatricial pemphigoid [234]. It was found that treatment of cultured ocular surface epithelial cells with the proinflammatory cytokine TNFA, stimulated expression of UPR markers, as well as MMP9, a key mediator of OcS damage in DE [235]. Importantly, a chemical chaperone attenuated TNFA-stimulated MMP9 expression suggesting that UPR activation is mechanistically linked to inflammation, MMP9 expression and proteolysis [234].
Investigating this idea in our cell culture model, we found that oxidative stress increased mRNA for sXBP1, a marker of the IRE1 branch of the UPR, and CHOP, a marker of the alternate PERK branch leading to cell death. Oxidative stress also stimulated phosphorylation of eIF2α, the upstream regulator of CHOP. Significantly, dynasore selectively inhibited the increase in PERK branch markers [230].
ER stress disrupts mechanisms of Ca2+ homeostasis, resulting in release of Ca2+ from the ER to the cytosol and to the mitochondria [236, 237], along with UPR activation [238]. In addition to inhibition of PERK branch markers, we found that dynasore also prevented the increase in intracellular Ca2+ due to oxidative stress. The increase in PERK branch markers was further inhibited when cells were treated with the cell permeable Ca2+ chelator, BAPTA-AM [230].
We conclude that dynasore protects cells by inhibiting the intracellular Ca2+ increase responsible, at least in part, for PERK-CHOP pathway activation in cultures of ocular surface epithelial cells subjected to oxidative stress. Whether the same mechanisms are responsible for damage to the mucin barrier subjected to oxidative stress, and its protection by dynasore, is not known yet, but we continue to investigate.
5. SUMMARY AND FUTURE DIRECTIONS
Herein we described a subset of MAM genes expressed at the ocular surface: MUC1, MUC4, MUC16, MUC21, MUC22. Their protein products, heavily modified by addition of O-linked glycan moieties, accumulate at the apical layer of the epithelia covering the cornea and conjunctiva. There, the highly-glycosylated EDs of these MAMs interact with lectins to form the mucosal glycocalyx. These genes are also expressed by conjunctival goblet cells and lacrimal gland. The large subunits of MAMs resulting from cleavage within the ED can be shed into the tears, where they contribute, together with secreted mucin MUC5AC, to formation of a mucoaqueous gel.
It is well-recognized that MAMs, based on their biophysical properties, provide for a stable and transparent refractive surface, maintaining hydration and lubrication, protecting against external challenges and pathogens. However, as discussed herein, it is increasingly appreciated that MAMs play an important role as cell surface receptors that sense the extracellular environment and transduce signals intracellularly. It has been stated that the next big frontier in the MAM field is to expand our knowledge of their function in intracellular signaling [13]. This would be especially important to investigate in relation to disease roles. One that we find most interesting is allergy. As we noted above, allergic response to pollen is abrogated in Myd88 and Tlr4 knockout mice. Given that MAMs inhibit MYD88 binding to toll-like receptors, we hypothesize that MAMs act to regulate the allergic reaction. This mechanism could also be responsible for ocular surface damage due to dry eye. This will be a very interesting area for study in the immediate future. Pollution promotes the development of both allergy and dry eye, and how this interaction will be another important area to study.
We are also following up on the role of the UPR in damage to mucosal epithelial cells and their glycocalyx and the therapeutic potential of dynasore analogues. The generality of this mechanism to ocular surface disease of diverse etiologies will be important to ascertain. If dynasore analogues are to be used therapeutically, it will also be important to determine their molecular target(s).
The VNTR (Variable Number Tandem Repeats) modules and regions of unique sequence that are serine and threonine rich and heavily O-glycosylated (O-glyc Regions) are shown with the O-linked glycan chains depicted as “brushes”.
MUC1: The VNTR module is comprised of tandem repeats of 20 amino acids long, with 25 to 125 repetitions. Flanking the VNTR are two O-glyc Regions.
MUC4: The VNTR module is comprised of tandem repeats of 16 amino acids long, with 145 to 395 repetitions. Proximal to the VNTR is an Imperfect Repeats module, also heavily-O-glycosylated.
MUC16: The VNTR module contains long, only partially-conserved tandem repeats of 156 amino acids, with 10 to 60 repetitions. Proximal to the VNTR is a long O-glyc Region of unique sequence (12,070 amino acids) that is also heavily O-glycosylated.
MUC20: The VNTR module contains tandem repeats of 19 amino acids, with 3–12 repetitions.
MUC21: The VNTR module contains tandem repeats of 15 amino acid, with 32 imperfect repetitions.
MUC22: The VNTR module contains tandem repeats of 10 amino acid, with 124 imperfect repetitions.
Other conserved modules are indicated with various shapes defined in the glossary on the graphic and named as in NCBI Gene:
EGF: Cysteine-rich EGF-like (violet long rectangles)
SEA: Sperm protein, Enterokinase and Agrin (pale pink, long rectangles)
Cys-Rich: contains many cysteines (crimson octahedrons)
NIDO: Nidogen (entactin) (navy triangles)
AMOP: Adhesion-Associated Domain (yellow stretched hexagons)
VWD: von Willebrand factor type D (turquoise tall rectangles)
epiglycanin_C: conserved module found in the CTs of MUC21 and MUC22
GSLV: proposed cleavage site for MUC21 (mirrored in MUC22)
6. ACKNOWLEDGEMENTS
The authors thank Dr. Eric Cox (NCBI, Bethesda, MD) for his consultation on the MAM genes, their RNA transcripts and their protein products.
Funding
Supported by NIH grant R01EY026479 to MEF. Additional support was provided by a grant from the Massachusetts Lions Eye Research Fund and by a challenge grant from Research to Prevent Blindness to the Department of Ophthalmology at Tufts University School of Medicine. The funders had no role in the writing of this article, or in the decision to submit.
Disclosures
RM-C has no disclosures to report. PA is named as an inventor on a patent claiming the use of EMCN as an anti-inflammatory agent. MEF serves as a consultant for Kala Pharmaceuticals, Watertown, MA, receives NIH grant funding through MedChem Partners, LLC, Lexington, MA, and is a co-founder and chief scientific officer for Proteris Biotech, Inc., Glendale, CA.
Footnotes
HUGO nomenclature used for genes and their products. The abbreviations used are: CT: cytoplasmic tail, ED: extracellular domain; ER: endoplasmic reticulum; ERM: ezrin/radixin/moesin; MAM: membrane-associated mucin; MPS: multipurpose contact lens cleaning solutions; NCBI: National Center for Biotechnology Information; SEA: Sperm protein, Enterokinase and Agrin module; SEM: scanning electron microscopy; UPR: unfolded protein response; VNTR: Variable Number of Tandem Repeats; vWD: von Willibrand Domain
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- 1.Madison KC. Barrier function of the skin: “la raison d’etre” of the epidermis. The Journal of investigative dermatology. 2003;121(2):231–41. Epub 2003/07/26. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 2.Gipson IK. The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Investigative ophthalmology & visual science. 2007;48(10):4390; 1–8. Epub 2007/09/28. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nature reviews Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 4.Willcox MDP, Argueso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, et al. TFOS DEWS II Tear Film Report. The ocular surface. 2017;15(3):366–403. doi: 10.1016/j.jtos.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgiev GA, Eftimov P, Yokoi N. Contribution of Mucins towards the Physical Properties of the Tear Film: A Modern Update. International journal of molecular sciences. 2019;20(24). Epub 2019/12/11. doi: 10.3390/ijms20246132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moniaux N, Escande F, Porchet N, Aubert JP, Batra SK. Structural organization and classification of the human mucin genes. Frontiers in bioscience : a journal and virtual library. 2001;6:D1192–206. [DOI] [PubMed] [Google Scholar]
- 7.Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argueso P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Investigative ophthalmology & visual science. 2008;49(1):197–203. Epub 2008/01/04. doi: 10.1167/iovs.07-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gendler SJ, Spicer AP. Epithelial mucin genes. Annual review of physiology. 1995;57:607–34. Epub 1995/01/01. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 9.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annual review of physiology. 2008;70:431–57. Epub 2007/09/14. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 10.Jentoft N. Why are proteins O-glycosylated? Trends in biochemical sciences. 1990;15(8):291–4. [DOI] [PubMed] [Google Scholar]
- 11.Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29(20):2893–904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantinou PE, Danysh BP; Dharmaraj N; Carson DD Transmembrane mucins as novel therapeutic targets. . Expert Rev Endocrinol Metab 2011;6(6): 835–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Putten JPM, Strijbis K. Transmembrane Mucins: Signaling Receptors at the Intersection of Inflammation and Cancer. J Innate Immun 2017;9(3):281–99. doi: 10.1159/000453594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovjazin R, Horn G, Smorodinsky NI, Shapira MY, Carmon L. Cell surface-associated anti-MUC1-derived signal peptide antibodies: implications for cancer diagnostics and therapy. PloS one. 2014;9(1):e85400. Epub 2014/01/15. doi: 10.1371/journal.pone.0085400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi EA, McNeer RR, Price-Schiavi SA, Van den Brande JM, Komatsu M, Thompson JF, et al. Sialomucin complex, a heterodimeric glycoprotein complex. Expression as a soluble, secretable form in lactating mammary gland and colon. The Journal of biological chemistry. 1996;271(52):33476–85. doi: 10.1074/jbc.271.52.33476. [DOI] [PubMed] [Google Scholar]
- 16.Fini ME, Jeong S, Gong H, Martinez-Carrasco R, Laver NMV, Hijikata M, et al. Membrane-associated mucins of the ocular surface: New genes, new protein functions and new biological roles in human and mouse. Progress in retinal and eye research. 2020;75:100777. doi: 10.1016/j.preteyeres.2019.100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, et al. Membrane-tethered MUC1 mucin is phosphorylated by epidermal growth factor receptor in airway epithelial cells and associates with TLR5 to inhibit recruitment of MyD88. Journal of immunology. 2012;188(4):2014–22. doi: 10.4049/jimmunol.1102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. The ocular surface. 2004;2(2):131–48. [DOI] [PubMed] [Google Scholar]
- 19.Guzman-Aranguez A, Argueso P. Structure and biological roles of mucin-type O-glycans at the ocular surface. The ocular surface. 2010;8(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ablamowicz AF, Nichols JJ. Ocular Surface Membrane-Associated Mucins. The ocular surface. 2016;14(3):331–41. doi: 10.1016/j.jtos.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Portal C, Gouyer V, Gottrand F, Desseyn JL. Ocular mucins in dry eye disease. Experimental eye research. 2019;186:107724. Epub 2019/07/22. doi: 10.1016/j.exer.2019.107724. [DOI] [PubMed] [Google Scholar]
- 22.Baudouin C, Rolando M, Benitez Del Castillo JM, Messmer EM, Figueiredo FC, Irkec M, et al. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Progress in retinal and eye research. 2018. doi: 10.1016/j.preteyeres.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Uchino Y. The Ocular Surface Glycocalyx and its Alteration in Dry Eye Disease: A Review. Investigative ophthalmology & visual science. 2018;59(14):DES157–DES62. Epub 2018/11/28. doi: 10.1167/iovs.17-23756. [DOI] [PubMed] [Google Scholar]
- 24.Hori Y. Secreted Mucins on the Ocular Surface. Investigative ophthalmology & visual science. 2018;59(14):DES151–DES6. Epub 2018/11/28. doi: 10.1167/iovs.17-23623. [DOI] [PubMed] [Google Scholar]
- 25.Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8(5):477–83. doi: 10.1097/ACI.0b013e32830e6b04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantelli F, Mauris J, Argueso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 2013;13(5):563–8. doi: 10.1097/ACI.0b013e3283645899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gipson IK, Inatomi T. Cellular origin of mucins of the ocular surface tear film. Advances in experimental medicine and biology. 1998;438:221–7. [DOI] [PubMed] [Google Scholar]
- 28.Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Experimental eye research. 2001;73(3):281–9. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- 29.Gipson IK, Argueso P. Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 2003;231:1–49. [DOI] [PubMed] [Google Scholar]
- 30.Gipson IK. Distribution of mucins at the ocular surface. Experimental eye research. 2004;78(3):379–88. [DOI] [PubMed] [Google Scholar]
- 31.Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Experimental eye research. 2010;90(6):655–63. Epub 2010/03/13. doi: 10.1016/j.exer.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Argueso P. Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol 2013;57(2):150–5. doi: 10.1007/s10384-012-0228-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan SM, Samulowitz U, Darley L, Simmons DL, Vestweber D. Biochemical characterization and molecular cloning of a novel endothelial-specific sialomucin. Blood. 1999;93(1):165–75. [PubMed] [Google Scholar]
- 34.Gipson IK. In situ hybridization techniques for localizing mucin mRNA. Methods in molecular biology. 2000;125:323–36. doi: 10.1385/1-59259-048-9:323. [DOI] [PubMed] [Google Scholar]
- 35.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Investigative ophthalmology & visual science. 2003;44(6):2487–95. [DOI] [PubMed] [Google Scholar]
- 36.Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Investigative ophthalmology & visual science. 1996;37(8):1684–92. [PubMed] [Google Scholar]
- 37.Pflugfelder SC, Liu Z, Monroy D, Li DQ, Carvajal ME, Price-Schiavi SA, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Investigative ophthalmology & visual science. 2000;41(6):1316–26. [PubMed] [Google Scholar]
- 38.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Investigative ophthalmology & visual science. 1995;36(9):1818–27. [PubMed] [Google Scholar]
- 39.Swan JS, Arango ME, Carothers Carraway CA, Carraway KL. An ErbB2-Muc4 complex in rat ocular surface epithelia. Current eye research. 2002;24(5):397–402. [DOI] [PubMed] [Google Scholar]
- 40.Price-Schiavi SA, Zhu X, Aquinin R, Carraway KL. Sialomucin complex (rat Muc4) is regulated by transforming growth factor beta in mammary gland by a novel post-translational mechanism. The Journal of biological chemistry. 2000;275(23):17800–7. doi: 10.1074/jbc.275.23.17800. [DOI] [PubMed] [Google Scholar]
- 41.Lomako J, Lomako WM, Carothers Carraway CA, Carraway KL. Regulation of the membrane mucin Muc4 in corneal epithelial cells by proteosomal degradation and TGF-beta. Journal of cellular physiology. 2010;223(1):209–14. doi: 10.1002/jcp.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jumblatt MM, McKenzie RW, Steele PS, Emberts CG, Jumblatt JE. MUC7 expression in the human lacrimal gland and conjunctiva. Cornea. 2003;22(1):41–5. [DOI] [PubMed] [Google Scholar]
- 43.Paulsen F, Langer G, Hoffmann W, Berry M. Human lacrimal gland mucins. Cell and tissue research. 2004;316(2):167–77. doi: 10.1007/s00441-004-0877-7. [DOI] [PubMed] [Google Scholar]
- 44.Jager K, Wu G, Sel S, Garreis F, Brauer L, Paulsen FP. MUC16 in the lacrimal apparatus. Histochemistry and cell biology. 2007;127(4):433–8. doi: 10.1007/s00418-006-0246-6. [DOI] [PubMed] [Google Scholar]
- 45.Gipson IK, Spurr-Michaud S, Tisdale A. Human conjunctival goblet cells express the membrane associated mucin MUC16: Localization to mucin granules. Experimental eye research. 2016;145:230–4. doi: 10.1016/j.exer.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brugger W, Buhring HJ, Grunebach F, Vogel W, Kaul S, Muller R, et al. Expression of MUC-1 epitopes on normal bone marrow: implications for the detection of micrometastatic tumor cells. J Clin Oncol 1999;17(5):1535–44. doi: 10.1200/JCO.1999.17.5.1535. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal B, Krantz MJ, Parker J, Longenecker BM. Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer research. 1998;58(18):4079–81. [PubMed] [Google Scholar]
- 48.Leong CF, Raudhawati O, Cheong SK, Sivagengei K, Noor Hamidah H. Epithelial membrane antigen (EMA) or MUC1 expression in monocytes and monoblasts. Pathology. 2003;35(5):422–7. [DOI] [PubMed] [Google Scholar]
- 49.Wykes M, MacDonald KP, Tran M, Quin RJ, Xing PX, Gendler SJ, et al. MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. Journal of leukocyte biology. 2002;72(4):692–701. [PubMed] [Google Scholar]
- 50.Woodward AM, Argueso P. Expression Analysis of the Transmembrane Mucin MUC20 in Human Corneal and Conjunctival Epithelia. Investigative ophthalmology & visual science. 2014;55(10):6132–8. Epub 2014/08/30. doi: 10.1167/iovs.14-15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spurr-Michaud S, Argueso P, Gipson I. Assay of mucins in human tear fluid. Experimental eye research. 2007;84(5):939–50. doi: 10.1016/j.exer.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA. Cloning and sequencing of a human pancreatic tumor mucin cDNA. The Journal of biological chemistry. 1990;265(25):15294–9. [PubMed] [Google Scholar]
- 53.Palmai-Pallag T, Khodabukus N, Kinarsky L, Leir SH, Sherman S, Hollingsworth MA, et al. The role of the SEA (sea urchin sperm protein, enterokinase and agrin) module in cleavage of membrane-tethered mucins. The FEBS journal. 2005;272(11):2901–11. doi: 10.1111/j.1742-4658.2005.04711.x. [DOI] [PubMed] [Google Scholar]
- 54.Ligtenberg MJ, Kruijshaar L, Buijs F, van Meijer M, Litvinov SV, Hilkens J. Cell-associated episialin is a complex containing two proteins derived from a common precursor. The Journal of biological chemistry. 1992;267(9):6171–7. [PubMed] [Google Scholar]
- 55.Das S, Majhi PD, Al-Mugotir MH, Rachagani S, Sorgen P, Batra SK. Membrane proximal ectodomain cleavage of MUC16 occurs in the acidifying Golgi/post-Golgi compartments. Scientific reports. 2015;5:9759. doi: 10.1038/srep09759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thathiah A, Blobel CP, Carson DD. Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. The Journal of biological chemistry. 2003;278(5):3386–94. doi: 10.1074/jbc.M208326200. [DOI] [PubMed] [Google Scholar]
- 57.Thathiah A, Carson DD. MT1-MMP mediates MUC1 shedding independent of TACE/ADAM17. The Biochemical journal. 2004;382(Pt 1):363–73. doi: 10.1042/BJ20040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Investigative ophthalmology & visual science. 2008;49(5): 1864–71. Epub 2008/04/26. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argueso P, et al. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PloS one. 2012;7(3):e32418. Epub 2012/03/14. doi: 10.1371/journal.pone.0032418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Higuchi T, Orita T, Nakanishi S, Katsuya K, Watanabe H, Yamasaki Y, et al. Molecular cloning, genomic structure, and expression analysis of MUC20, a novel mucin protein, upregulated in injured kidney. The Journal of biological chemistry. 2004;279(3):1968–79. doi: 10.1074/jbc.M304558200. [DOI] [PubMed] [Google Scholar]
- 61.Higuchi T, Orita T, Katsuya K, Yamasaki Y, Akiyama K, Li H, et al. MUC20 suppresses the hepatocyte growth factor-induced Grb2-Ras pathway by binding to a multifunctional docking site of met. Molecular and cellular biology. 2004;24(17):7456–68. doi: 10.1128/MCB.24.17.7456-7468.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, et al. Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology. 2008;18(1):74–83. Epub 2007/11/06. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 63.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. The Journal of biological chemistry. 2001;276(16):13057–64. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 64.Fendrick JL, Konishi I, Geary SM, Parmley TH, Quirk JG Jr., O’Brien TJ. CA125 phosphorylation is associated with its secretion from the WISH human amnion cell line. Tumour Biol 1997;18(5):278–89. doi: 10.1159/000218041. [DOI] [PubMed] [Google Scholar]
- 65.Hanson RL, Hollingsworth MA. Functional Consequences of Differential O-glycosylation of MUC1, MUC4, and MUC16 (Downstream Effects on Signaling). Biomolecules. 2016;6(3). doi: 10.3390/biom6030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jepson S, Komatsu M, Haq B, Arango ME, Huang D, Carraway CA, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinaseB/Akt pathways. Oncogene. 2002;21(49):7524–32. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- 67.Comamala M, Pinard M, Theriault C, Matte I, Albert A, Boivin M, et al. Downregulation of cell surface CA125/MUC16 induces epithelial-to-mesenchymal transition and restores EGFR signalling in NIH:OVCAR3 ovarian carcinoma cells. Br J Cancer. 2011;104(6):989–99. doi: 10.1038/bjc.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Investigative ophthalmology & visual science. 2007;48(10):4509–18. Epub 2007/09/28. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Kufe D. The Human DF3/MUC1 carcinoma-associated antigen signals nuclear localization of the catenin p120(ctn). Biochemical and biophysical research communications. 2001;281(2):440–3. doi: 10.1006/bbrc.2001.4383. [DOI] [PubMed] [Google Scholar]
- 70.Ren J, Li Y, Kufe D. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. The Journal of biological chemistry. 2002;277(20):17616–22. doi: 10.1074/jbc.M200436200. [DOI] [PubMed] [Google Scholar]
- 71.Kinlough CL, Poland PA, Bruns JB, Harkleroad KL, Hughey RP. MUC1 membrane trafficking is modulated by multiple interactions. The Journal of biological chemistry. 2004;279(51):53071–7. doi: 10.1074/jbc.M409360200. [DOI] [PubMed] [Google Scholar]
- 72.Chao CC, Butala SM, Herp A. Studies on the isolation and composition of human ocular mucin. Exp Eye Res 1988;47(2):185–96. Epub 1988/08/01. doi: 10.1016/0014-4835(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 73.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(4):966–81. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Royle L, Matthews E, Corfield A, Berry M, Rudd PM, Dwek RA, et al. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconjugate journal. 2008;25(8):763–73. Epub 2008/05/10. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 75.Guzman-Aranguez A, Mantelli F, Argueso P. Mucin-type O-glycans in tears of normal subjects and patients with non-Sjogren’s dry eye. Investigative ophthalmology & visual science. 2009;50(10):4581–7. Epub 2009/05/02. doi: 10.1167/iovs.09-3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Berry M, Ellingham RB, Corfield AP. Membrane-associated mucins in normal human conjunctiva. Investigative ophthalmology & visual science. 2000;41(2):398–403. [PubMed] [Google Scholar]
- 77.Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, et al. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. The Journal of biological chemistry. 2017;292(26):11079–90. doi: 10.1074/jbc.M116.770123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Argueso P, Panjwani N. Focus on molecules: galectin-3. Experimental eye research. 2011;92(1):2–3. Epub 2010/11/30. doi: 10.1016/j.exer.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. The Journal of allergy and clinical immunology. 2015;135(3):598–608. doi: 10.1016/j.jaci.2014.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sjögren H. Zur kenntnis der keratoconjunctivitis sicca. Acta ophthalmologica Supplement. 1933;2. [DOI] [PubMed] [Google Scholar]
- 81.Feenstra RP, Tseng SC. What is actually stained by rose bengal? Archives of ophthalmology. 1992;110(7):984–93. [DOI] [PubMed] [Google Scholar]
- 82.Feenstra RP, Tseng SC. Comparison of fluorescein and rose bengal staining. Ophthalmology. 1992;99(4):605–17. [DOI] [PubMed] [Google Scholar]
- 83.Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Investigative ophthalmology & visual science. 2006;47(1):113–9. Epub 2005/12/31. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N. Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. The Journal of biological chemistry. 2009;284(34):23037–45. Epub 2009/06/27. doi: 10.1074/jbc.M109.033332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PloS one. 2014;9(6):e100393. doi: 10.1371/journal.pone.0100393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boll EJ, Ayala-Lujan J, Szabady RL, Louissaint C, Smith RZ, Krogfelt KA, et al. Enteroaggregative Escherichia coli Adherence Fimbriae Drive Inflammatory Cell Recruitment via Interactions with Epithelial MUC1. MBio. 2017;8(3). doi: 10.1128/mBio.00717-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Linden SK, Sheng YH, Every AL, Miles KM, Skoog EC, Florin TH, et al. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog 2009;5(10):e1000617. doi: 10.1371/journal.ppat.1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhar P, Ng GZ, Dunne EM, Sutton P. Mucin 1 protects against severe Streptococcus pneumoniae infection. Virulence. 2017;8(8):1631–42. doi: 10.1080/21505594.2017.1341021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lillehoj EP, Hyun SW, Liu A, Guang W, Verceles AC, Luzina IG, et al. NEU1 Sialidase Regulates Membrane-tethered Mucin (MUC1) Ectodomain Adhesiveness for Pseudomonas aeruginosa and Decoy Receptor Release. The Journal of biological chemistry. 2015;290(30):18316–31. doi: 10.1074/jbc.M115.657114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lillehoj EP, Kim BT, Kim KC. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. American journal of physiology Lung cellular and molecular physiology. 2002;282(4):L751–6. doi: 10.1152/ajplung.00383.2001. [DOI] [PubMed] [Google Scholar]
- 91.McAuley JL, Corcilius L, Tan HX, Payne RJ, McGuckin MA, Brown LE. The cell surface mucin MUC1 limits the severity of influenza A virus infection. Mucosal Immunol 2017;10(6):1581–93. doi: 10.1038/mi.2017.16. [DOI] [PubMed] [Google Scholar]
- 92.Dhar P, McAuley J. The Role of the Cell Surface Mucin MUC1 as a Barrier to Infection and Regulator of Inflammation. Front Cell Infect Microbiol 2019;9:117. doi: 10.3389/fcimb.2019.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Woodward AM, Mauris J, Argueso P. Binding of transmembrane mucins to galectin-3 limits herpesvirus 1 infection of human corneal keratinocytes. Journal of virology. 2013;87(10):5841–7. Epub 2013/03/15. doi: 10.1128/JVI.00166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. American journal of physiology Lung cellular and molecular physiology. 2010;298(4):L558–63. doi: 10.1152/ajplung.00225.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Banos-Lara Mdel R, Piao B, Guerrero-Plata A. Differential mucin expression by respiratory syncytial virus and human metapneumovirus infection in human epithelial cells. Mediators Inflamm 2015;2015:347292. doi: 10.1155/2015/347292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang K, Gao M, Yang M, Meng F, Li D, Lu R, et al. Transcriptome analysis of bronchoalveolar lavage fluid from children with severe Mycoplasma pneumoniae pneumonia reveals novel gene expression and immunodeficiency. Hum Genomics. 2017; 11(1):4. doi: 10.1186/s40246-017-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hijikata M, Matsushita I, Tanaka G, Tsuchiya T, Ito H, Tokunaga K, et al. Molecular cloning of two novel mucin-like genes in the disease-susceptibility locus for diffuse panbronchiolitis. Human genetics. 2011;129(2):117–28. Epub 2010/10/29. doi: 10.1007/s00439-010-0906-4. [DOI] [PubMed] [Google Scholar]
- 98.Ahmad R, Raina D, Trivedi V, Ren J, Rajabi H, Kharbanda S, et al. MUC1 oncoprotein activates the IkappaB kinase beta complex and constitutive NF-kappaB signalling. Nature cell biology. 2007;9(12):1419–27. Epub 2007/11/27. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ahmad R, Raina D, Joshi MD, Kawano T, Ren J, Kharbanda S, et al. MUC1-C oncoprotein functions as a direct activator of the nuclear factor-kappaB p65 transcription factor. Cancer research. 2009;69(17):7013–21. Epub 2009/08/27. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, et al. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. Journal of immunology. 2006;176(7):3890–4. Epub 2006/03/21. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 101.Guang W, Ding H, Czinn SJ, Kim KC, Blanchard TG, Lillehoj EP. Muc1 cell surface mucin attenuates epithelial inflammation in response to a common mucosal pathogen. The Journal of biological chemistry. 2010;285(27):20547–57. Epub 2010/05/01. doi: 10.1074/jbc.M110.121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Altschuler Y, Kinlough CL, Poland PA, Bruns JB, Apodaca G, Weisz OA, et al. Clathrin-mediated endocytosis of MUC1 is modulated by its glycosylation state. Molecular biology of the cell. 2000;11(3):819–31. Epub 2000/03/11. doi: 10.1091/mbc.11.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cascio S, Faylo JL, Sciurba JC, Xue J, Ranganathan S, Lohmueller JJ, et al. Abnormally glycosylated MUC1 establishes a positive feedback circuit of inflammatory cytokines, mediated by NF-kappaB p65 and EzH2, in colitis-associated cancer. Oncotarget 2017;8(62):105284–98. Epub 2017/12/30. doi: 10.18632/oncotarget.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cascio S, Zhang L, Finn OJ. MUC1 protein expression in tumor cells regulates transcription of proinflammatory cytokines by forming a complex with nuclear factor-kappaB p65 and binding to cytokine promoters: importance of extracellular domain. The Journal of biological chemistry. 2011;286(49):42248–56. Epub 2011/10/25. doi: 10.1074/jbc.M111.297630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Imbert Y, Darling DS, Jumblatt MM, Foulks GN, Couzin EG, Steele PS, et al. MUC1 splice variants in human ocular surface tissues: possible differences between dry eye patients and normal controls. Experimental eye research. 2006;83(3):493–501. Epub 2006/04/25. doi: 10.1016/j.exer.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 106.Imbert-Fernandez Y, Radde BN, Teng Y, Young WW Jr., Hu C, Klinge CM. MUC1/A and MUC1/B splice variants differentially regulate inflammatory cytokine expression. Experimental eye research. 2011;93(5):649–57. Epub 2011/08/23. doi: 10.1016/j.exer.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. The New England journal of medicine. 2005;353(16):1711–23. Epub 2005/10/21. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 108.Martinez-Anton A, Debolos C, Garrido M, Roca-Ferrer J, Barranco C, Alobid I, et al. Mucin genes have different expression patterns in healthy and diseased upper airway mucosa. Clin Exp Allergy. 2006;36(4):448–57. Epub 2006/04/25. doi: 10.1111/j.1365-2222.2006.02451.x. [DOI] [PubMed] [Google Scholar]
- 109.Imai M, Hwang HY, Norris JS, Tomlinson S. The effect of dexamethasone on human mucin 1 expression and antibody-dependent complement sensitivity in a prostate cancer cell line in vitro and in vivo. Immunology. 2004;111(3):291–7. Epub 2004/03/11. doi: 10.1111/j.0019-2805.2004.01815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seo KY, Chung SH, Lee JH, Park MY, Kim EK. Regulation of membrane-associated mucins in the human corneal epithelial cells by dexamethasone. Cornea. 2007;26(6):709–14. Epub 2007/06/27. doi: 10.1097/ICO.0b013e31804f5a09. [DOI] [PubMed] [Google Scholar]
- 111.Taniguchi J, Sharma A. Fluorometholone modulates gene expression of ocular surface mucins. Acta Ophthalmol 2019;97(8):e1082–e8. Epub 2019/04/10. doi: 10.1111/aos.14113. [DOI] [PubMed] [Google Scholar]
- 112.Milara J, Peiro T, Armengot M, Frias S, Morell A, Serrano A, et al. Mucin 1 downregulation associates with corticosteroid resistance in chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2015;135(2):470–6. Epub 2014/08/28. doi: 10.1016/j.jaci.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 113.Milara J, Diaz-Platas L, Contreras S, Ribera P, Roger I, Ballester B, et al. MUC1 deficiency mediates corticosteroid resistance in chronic obstructive pulmonary disease. Respir Res 2018;19(1):226. doi: 10.1186/s12931-018-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Milara J, Morell A, de Diego A, Artigues E, Morcillo E, Cortijo J. Mucin 1 deficiency mediates corticosteroid insensitivity in asthma. Allergy. 2019;74(1): 111–21. Epub 2018/07/07. doi: 10.1111/all.13546. [DOI] [PubMed] [Google Scholar]
- 115.Zhang H, Ji J, Liu Q, Xu S. MUC1 downregulation promotes TNF-alpha-induced necroptosis in human bronchial epithelial cells via regulation of the RIPK1/RIPK3 pathway. Journal of cellular physiology. 2019. Epub 2019/01/23. doi: 10.1002/jcp.28148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Milara J, Morell A, Ballester B, Armengot M, Morcillo E, Cortijo J. MUC4 impairs the anti-inflammatory effects of corticosteroids in patients with chronic rhinosinusitis with nasal polyps. The Journal of allergy and clinical immunology. 2017;139(3):855–62 e13. Epub 2016/09/19. doi: 10.1016/j.jaci.2016.06.064. [DOI] [PubMed] [Google Scholar]
- 117.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. American journal of physiology Lung cellular and molecular physiology. 2004;286(5):L887–92. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 118.Kato K, Lillehoj EP, Kim KC. Pseudomonas aeruginosa stimulates tyrosine phosphorylation of and TLR5 association with the MUC1 cytoplasmic tail through EGFR activation. Inflamm Res 2016;65(3):225–33. doi: 10.1007/s00011-015-0908-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, et al. MUC1 mucin is a negative regulator of toll-like receptor signaling. American journal of respiratory cell and molecular biology. 2008;38(3):263–8. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dhar P, Sarkar S, Ng GZ, Kalitsis P, Saeed MA, McGuckin MA, et al. Effect of MUC1 length polymorphisms on the NLRP3 inflammasome response of human macrophages. Hum Immunol. 2019;80(10):878–82. Epub 2019/06/20. doi: 10.1016/j.humimm.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 121.Ng GZ, Sutton P. The MUC1 mucin specifically inhibits activation of the NLRP3 inflammasome. Genes Immun. 2016;17(3):203–6. doi: 10.1038/gene.2016.10. [DOI] [PubMed] [Google Scholar]
- 122.Ng GZ, Menheniott TR, Every AL, Stent A, Judd LM, Chionh YT, et al. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut. 2016;65(7):1087–99. doi: 10.1136/gutjnl-2014-307175. [DOI] [PubMed] [Google Scholar]
- 123.Menon BB, Kaiser-Marko C, Spurr-Michaud S, Tisdale AS, Gipson IK. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol 2015;8(5):1000–8. doi: 0.1038/mi.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pfister RR. The normal surface of corneal epithelium: a scanning electron microscopic study. Investigative ophthalmology. 1973;12(9):654–68. [PubMed] [Google Scholar]
- 125.Doughty MJ. Corneal Surface and Superficial Cells as Viewed by Scanning Electron Microscopy and Impression Cytology Sampling. Cornea. 2016;35(2):243–8. doi: 10.1097/ICO.0000000000000692. [DOI] [PubMed] [Google Scholar]
- 126.Hazlett LD, Spann B, Wells P, Berk RS. Desquamation of the corneal epithelium in the immature mouse: a scanning and transmission microscopy study. Experimental eye research. 1980;31(1):21–30. [DOI] [PubMed] [Google Scholar]
- 127.Hanna C, Bicknell DS, O’Brien JE. Cell turnover in the adult human eye. Archives of ophthalmology. 1961;65:695–8. [DOI] [PubMed] [Google Scholar]
- 128.Milstone LM. Epidermal desquamation. J Dermatol Sci 2004;36(3):131–40. doi: 10.1016/j.jdermsci.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 129.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Investigative ophthalmology & visual science. 2003;44(6):2496–506. [DOI] [PubMed] [Google Scholar]
- 130.Gupton SL, Gertler FB. Filopodia: the fingers that do the walking. Science’s STKE : signal transduction knowledge environment. 2007;2007(400):re5. Epub 2007/08/23. doi: 10.1126/stke.4002007re5. [DOI] [PubMed] [Google Scholar]
- 131.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophysical journal. 2005;89(2):782–95. Epub 2005/05/10. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Footer MJ, Kerssemakers JW, Theriot JA, Dogterom M. Direct measurement of force generation by actin filament polymerization using an optical trap. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2181–6. Epub 2007/02/06. doi: 10.1073/pnas.0607052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shurer CR, Kuo JC, Roberts LM, Gandhi JG, Colville MJ, Enoki TA, et al. Physical Principles of Membrane Shape Regulation by the Glycocalyx. Cell. 2019;177(7):1757–70 e21. Epub 2019/05/06. doi: 10.1016/j.cell.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wald T, Spoutil F, Osickova A, Prochazkova M, Benada O, Kasparek P, et al. Intrinsically disordered proteins drive enamel formation via an evolutionarily conserved self-assembly motif. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(9):E1641–E50. Epub 2017/02/16. doi: 10.1073/pnas.1615334114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Serafini-Cessi F, Malagolini N, Cavallone D. Tamm-Horsfall glycoprotein: biology and clinical relevance. Am J Kidney Dis 2003;42(4):658–76. Epub 2003/10/02. doi: 10.1016/s0272-6386(03)00829-1. [DOI] [PubMed] [Google Scholar]
- 136.Nie M, Bal MS, Yang Z, Liu J, Rivera C, Wenzel A, et al. Mucin-1 Increases Renal TRPV5 Activity In Vitro, and Urinary Level Associates with Calcium Nephrolithiasis in Patients. Journal of the American Society of Nephrology : JASN. 2016;27(11):3447–58. doi: 10.1681/ASN.2015101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wolf MT, Wu XR, Huang CL. Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int 2013;84(1):130–7. Epub 2013/03/08. doi: 10.1038/ki.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Meyer TE, Verwoert GC, Hwang SJ, Glazer NL, Smith AV, van Rooij FJ, et al. Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS genetics. 2010;6(8). doi: 10.1371/journal.pgen.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reinach PS, Mergler S, Okada Y, Saika S. Ocular transient receptor potential channel function in health and disease. BMC Ophthalmol 2015;15 Suppl 1:153. doi: 10.1186/s12886-015-0135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Parra A, Madrid R, Echevarria D, del Olmo S, Morenilla-Palao C, Acosta MC, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nature medicine. 2010;16(12):1396–9. Epub 2010/11/16. doi: 10.1038/nm.2264. [DOI] [PubMed] [Google Scholar]
- 141.Pan Z, Wang Z, Yang H, Zhang F, Reinach PS. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investigative ophthalmology & visual science. 2011;52(1):485–93. Epub 2010/08/27. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, et al. TFOS DEWS II pain and sensation report. The ocular surface. 2017;15(3):404–37. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee J, Koh JH, Kwok SK, Park SH. The EULAR Sjogren’s Syndrome Patient-Reported Index is an independent determinant of health-related utility values of Korean patients with primary Sjogren’s syndrome. Clin Exp Rheumatol 2016;34(4):663–7. [PubMed] [Google Scholar]
- 144.Saboo US, Amparo F, Abud TB, Schaumberg DA, Dana R. Vision-Related Quality of Life in Patients with Ocular Graft-versus-Host Disease. Ophthalmology. 2015;122(8):1669–74. doi: 10.1016/j.ophtha.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun YC, Chai X, Inamoto Y, Pidala J, Martin PJ, Flowers ME, et al. Impact of Ocular Chronic Graft-versus-Host Disease on Quality of Life. Biol Blood Marrow Transplant 2015;21(9):1687–91. doi: 10.1016/j.bbmt.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Clayton JA. Dry Eye. The New England journal of medicine. 2018;378(23):2212–23. doi: 10.1056/NEJMra1407936. [DOI] [PubMed] [Google Scholar]
- 147.Pflugfelder SC, de Paiva CS. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology. 2017;124(11S):S4–S13. doi: 10.1016/j.ophtha.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Archives of ophthalmology. 2012;130(1):90–100. Epub 2012/01/11. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Craig JP, Nelson JD, Azar DT, Belmonte C, Bron AJ, Chauhan SK, et al. TFOS DEWS II Report Executive Summary. The ocular surface. 2017;15(4):802–12. doi: 10.1016/j.jtos.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 150.Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Investigative ophthalmology & visual science. 2002;43(4):1004–11. [PubMed] [Google Scholar]
- 151.Caffery B, Heynen ML, Joyce E, Jones L, Ritter R 3rd, Senchyna M. MUC1 expression in Sjogren’s syndrome, KCS, and control subjects. Molecular vision. 2010;16:1720–7. [PMC free article] [PubMed] [Google Scholar]
- 152.Corrales RM, Narayanan S, Fernandez I, Mayo A, Galarreta DJ, Fuentes-Paez G, et al. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Investigative ophthalmology & visual science. 2011;52(11):8363–9. Epub 2011/09/21. doi: 10.1167/iovs.11-7655. [DOI] [PubMed] [Google Scholar]
- 153.Caffery B, Joyce E, Heynen ML, Jones L, Ritter R 3rd, Gamache DA, et al. MUC16 expression in Sjogren’s syndrome, KCS, and control subjects. Molecular vision. 2008;14:2547–55. [PMC free article] [PubMed] [Google Scholar]
- 154.Shimazaki-Den S, Dogru M, Higa K, Shimazaki J. Symptoms, visual function, and mucin expression of eyes with tear film instability. Cornea. 2013;32(9): 1211–8. Epub 2013/07/13. doi: 10.1097/ICO.0b013e318295a2a5. [DOI] [PubMed] [Google Scholar]
- 155.Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Investigative ophthalmology & visual science. 1998;39(13):2602–9. [PubMed] [Google Scholar]
- 156.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16(12):1219–28. Epub 2006/08/31. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hayashi Y, Kao WW, Kohno N, Nishihara-Hayashi M, Shiraishi A, Uno T, et al. Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Investigative ophthalmology & visual science. 2004;45(7):2212–7. Epub 2004/06/30. doi: 10.1167/iovs.03-0988. [DOI] [PubMed] [Google Scholar]
- 158.Imbert Y, Jumblatt MM, Foulks GN, Couzin EG, Steele PS, Young WW Jr. Expression in human ocular surface tissues of the GalNAc-transferases that initiate mucin-type O-glycosylation. Cornea. 2006;25(10):1193–9. Epub 2006/12/19. doi: 10.1097/01.ico.0000240099.16420.17. [DOI] [PubMed] [Google Scholar]
- 159.Woodward AM, Lehoux S, Mantelli F, Di Zazzo A, Brockhausen I, Bonini S, et al. Inflammatory Stress Causes N-Glycan Processing Deficiency in Ocular Autoimmune Disease. The American journal of pathology. 2019;189(2):283–94. Epub 2018/11/19. doi: 10.1016/j.ajpath.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Investigative ophthalmology & visual science. 2003;44(1):86–92. Epub 2002/12/31. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 161.Matossian C, McDonald M, Donaldson KE, Nichols KK, MacIver S, Gupta PK. Dry Eye Disease: Consideration for Women’s Health. J Womens Health (Larchmt). 2019;28(4):502–14. doi: 10.1089/jwh.2018.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schafer G, Hoffmann W, Berry M, Paulsen F. [Lacrimal gland-associated mucins. Age related production and their role in the pathophysiology of dry eye]. Ophthalmologe 2005;102(2):175–83. Epub 2005/01/29. doi: 10.1007/s00347-004-1075-4. [DOI] [PubMed] [Google Scholar]
- 163.Craig JP, Willcox MD, Argueso P, Maissa C, Stahl U, Tomlinson A, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the contact lens interactions with the tear film subcommittee. Investigative ophthalmology & visual science. 2013;54(11):TFOS123–56. Epub 2013/09/24. doi: 10.1167/iovs.13-13235. [DOI] [PubMed] [Google Scholar]
- 164.Downie LE, Craig JP. Tear film evaluation and management in soft contact lens wear: a systematic approach. Clin Exp Optom 2017;100(5):438–58. Epub 2017/09/25. doi: 10.1111/cxo.12597. [DOI] [PubMed] [Google Scholar]
- 165.Kojima T. Contact Lens-Associated Dry Eye Disease: Recent Advances Worldwide and in Japan. Investigative ophthalmology & visual science. 2018;59(14):DES102–DES8. Epub 2018/11/28. doi: 10.1167/iovs.17-23685. [DOI] [PubMed] [Google Scholar]
- 166.Colorado LH, Alzahrani Y, Pritchard N, Efron N. Time Course of Changes in Goblet Cell Density in Symptomatic and Asymptomatic Contact Lens Wearers. Investigative ophthalmology & visual science. 2016;57(6):2888–94. Epub 2016/05/12. doi: 10.1167/iovs.16-19298. [DOI] [PubMed] [Google Scholar]
- 167.Pisella PJ, Malet F, Lejeune S, Brignole F, Debbasch C, Bara J, et al. Ocular surface changes induced by contact lens wear. Cornea. 2001;20(8):820–5. Epub 2001/10/31. doi: 10.1097/00003226-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 168.Fukui M, Yamada M, Akune Y, Shigeyasu C, Tsubota K. Fluorophotometric Analysis of the Ocular Surface Glycocalyx in Soft Contact Lens Wearers. Current eye research. 2016;41(1):9–14. Epub 2015/01/24. doi: 10.3109/02713683.2014.999948. [DOI] [PubMed] [Google Scholar]
- 169.Read ML, Navascues-Cornago M, Keir N, Maldonado-Codina C, Morgan PB. The impact of contact lens wear on ocular surface mucins using a novel clinical fluorescence imaging system. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2020;43(4):378–88. Epub 2019/08/28. doi: 10.1016/j.clae.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 170.Hori Y, Argueso P, Spurr-Michaud S, Gipson IK. Mucins and contact lens wear. Cornea. 2006;25(2): 176–81. Epub 2005/12/24. doi: 10.1097/01.ico.0000177838.38873.2f. [DOI] [PubMed] [Google Scholar]
- 171.Corrales RM, Galarreta D, Herreras JM, Saez V, Arranz I, Gonzalez MJ, et al. Conjunctival mucin mRNA expression in contact lens wear. Optometry and vision science : official publication of the American Academy of Optometry. 2009;86(9):1051–8. doi: 10.1097/OPX.0b013e3181b4f02e. [DOI] [PubMed] [Google Scholar]
- 172.Maldonado-Codina C, Read ML, Efron N, Dobson CB, Morgan PB. Observation of solution-induced corneal staining with fluorescein, rose bengal and lissamine green. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2013;36(5):267–70. doi: 10.1016/j.clae.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 173.Jones L, Jones D, Houlford M. Clinical comparison of three polyhexanide-preserved multi-purpose contact lens solutions. Contact lens & anterior eye : the journal of the British Contact Lens Association. 1997;20(1):23–30. [DOI] [PubMed] [Google Scholar]
- 174.Jones L, MacDougall N, Sorbara LG. Asymptomatic corneal staining associated with the use of balafilcon silicone-hydrogel contact lenses disinfected with a polyaminopropyl biguanide-preserved care regimen. Optometry and vision science : official publication of the American Academy of Optometry. 2002;79(12):753–61. [DOI] [PubMed] [Google Scholar]
- 175.Mustafi D, Smith CM, Makinen MW, Lee RC. Multi-block poloxamer surfactants suppress aggregation of denatured proteins. Biochimica et biophysica acta 2008;1780(1):7–15. doi: 10.1016/j.bbagen.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 176.Souza AL, Ceridorio LF, Paula GF, Mattoso LH, Oliveira ON Jr. Understanding the biocide action of poly(hexamethylene biguanide) using Langmuir monolayers of dipalmitoyl phosphatidylglycerol. Colloids Surf B Biointerfaces. 2015;132:117–21. doi: 10.1016/j.colsurfb.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 177.Posch LC, Zhu M, Robertson DM. Multipurpose care solution-induced corneal surface disruption and Pseudomonas aeruginosa internalization in the rabbit corneal epithelium. Investigative ophthalmology & visual science. 2014;55(7):4229–37. doi: 10.1167/iovs.14-14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Imayasu M, Hori Y, Cavanagh HD. Effects of multipurpose contact lens care solutions and their ingredients on membrane-associated mucins of human corneal epithelial cells. Eye & contact lens. 2010;36(6):361–6. doi: 10.1097/ICL.0b013e3181faa43e. [DOI] [PubMed] [Google Scholar]
- 179.Gordon GM, Moradshahi N, Jeong S, Lane C, Fini ME. A novel mechanism of increased infections in contact lens wearers. Investigative ophthalmology & visual science. 2011;52(12):9188–94. Epub 2011/11/01. doi: 10.1167/iovs.11-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Michels KR, Lukacs NW, Fonseca W. TLR Activation and Allergic Disease: Early Life Microbiome and Treatment. Curr Allergy Asthma Rep 2018;18(11):61. Epub 2018/09/28. doi: 10.1007/s11882-018-0815-5. [DOI] [PubMed] [Google Scholar]
- 181.Li DQ, Zhang L, Pflugfelder SC, De Paiva CS, Zhang X, Zhao G, et al. Short ragweed pollen triggers allergic inflammation through Toll-like receptor 4-dependent thymic stromal lymphopoietin/OX40 ligand/OX40 signaling pathways. The Journal of allergy and clinical immunology. 2011;128(6):1318–25 e2. Epub 2011/08/09. doi: 10.1016/j.jaci.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nature medicine. 2012;18(5):684–92. Epub 2012/05/09. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 183.Leonardi A, Bogacka E, Fauquert JL, Kowalski ML, Groblewska A, Jedrzejczak-Czechowicz M, et al. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67(11):1327–37. Epub 2012/09/06. doi: 10.1111/all.12009. [DOI] [PubMed] [Google Scholar]
- 184.Mantelli F, Lambiase A, Bonini S, Bonini S. Clinical trials in allergic conjunctivits: a systematic review. Allergy. 2011;66(7):919–24. Epub 2011/01/26. doi: 10.1111/j.1398-9995.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 185.Dogru M, Okada N, Asano-Kato N, Igarashi A, Fukagawa K, Shimazaki J, et al. Alterations of the ocular surface epithelial mucins 1, 2, 4 and the tear functions in patients with atopic keratoconjunctivitis. Clin Exp Allergy. 2006;36(12):1556–65. Epub 2006/12/21. doi: 10.1111/j.1365-2222.2006.02581.x. [DOI] [PubMed] [Google Scholar]
- 186.Dogru M, Matsumoto Y, Okada N, Igarashi A, Fukagawa K, Shimazaki J, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63(10):1324–34. Epub 2008/09/11. doi: 10.1111/j.1398-9995.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- 187.Hu Y, Matsumoto Y, Dogru M, Okada N, Igarashi A, Fukagawa K, et al. The differences of tear function and ocular surface findings in patients with atopic keratoconjunctivitis and vernal keratoconjunctivitis. Allergy. 2007;62(8):917–25. Epub 2007/07/11. doi: 10.1111/j.1398-9995.2007.01414.x. [DOI] [PubMed] [Google Scholar]
- 188.Zhou LB, Zheng YM, Liao WJ, Song LJ, Meng X, Gong X, et al. MUC1 deficiency promotes nasal epithelial barrier dysfunction in subjects with allergic rhinitis. The Journal of allergy and clinical immunology. 2019;144(6):1716–9 e5. Epub 2019/08/20. doi: 10.1016/j.jaci.2019.07.042. [DOI] [PubMed] [Google Scholar]
- 189.Chen JB, Zhang J, Hu HZ, Xue M, Jin YJ. Polymorphisms of TGFB1, TLE4 and MUC22 are associated with childhood asthma in Chinese population. Allergol Immunopathol (Madr). 2017;45(5):432–8. doi: 10.1016/j.aller.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 190.Padra M, Andersson A, Levanen B, Premaratne P, Asgeirsdottir H, Tengvall S, et al. Increased MUC1 plus a larger quantity and complex size for MUC5AC in the peripheral airway lumen of long-term tobacco smokers. Clin Sci (Lond). 2020;134(10):1107–25. Epub 2020/05/14. doi: 10.1042/CS20191085. [DOI] [PubMed] [Google Scholar]
- 191.Arias A, Vicario M, Bernardo D, Olalla JM, Fortea M, Montalban-Arques A, et al. Toll-like receptors-mediated pathways activate inflammatory responses in the esophageal mucosa of adult eosinophilic esophagitis. Clin Transl Gastroenterol 2018;9(4):147. Epub 2018/04/25. doi: 10.1038/s41424-018-0017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim YD, Bae CH, Song SY, Choi YS. Effect of beta-glucan on MUC4 and MUC5B expression in human airway epithelial cells. Int Forum Allergy Rhinol 2015;5(8):708–15. Epub 2015/05/27. doi: 10.1002/alr.21549. [DOI] [PubMed] [Google Scholar]
- 193.Park IH, Kang JH, Kim JA, Shin JM, Lee HM. Diesel Exhaust Particles Enhance MUC4 Expression in NCI-H292 Cells and Nasal Epithelial Cells via the p38/CREB Pathway. Int Arch Allergy Immunol 2016;171(3–4):209–16. Epub 2017/01/04. doi: 10.1159/000453033. [DOI] [PubMed] [Google Scholar]
- 194.Li J, Zhang L, Chen X, Chen D, Hua X, Bian F, et al. Pollen/TLR4 Innate Immunity Signaling Initiates IL-33/ST2/Th2 Pathways in Allergic Inflammation. Scientific reports. 2016;6:36150. Epub 2016/11/01. doi: 10.1038/srep36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Alemayehu YA, Asfaw SL, Terfie TA. Exposure to urban particulate matter and its association with human health risks. Environ Sci Pollut Res Int 2020;27(22):27491–506. Epub 2020/05/16. doi: 10.1007/s11356-020-09132-1. [DOI] [PubMed] [Google Scholar]
- 196.Jung SJ, Mehta JS, Tong L. Effects of environment pollution on the ocular surface. The ocular surface. 2018;16(2):198–205. Epub 2018/03/07. doi: 10.1016/j.jtos.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 197.Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. The ocular surface. 2017;15(3):334–65. Epub 2017/07/25. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 198.Leite MR, Zanetta DMT, Trevisan IB, Burdmann EA, Santos UP. Sugarcane cutting work, risks, and health effects: a literature review. Rev Saude Publica 2018;52:80. Epub 2018/08/30. doi: 10.11606/S1518-8787.2018052000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Matsuda M, Braga ALF, Marquezini MV, Monteiro MLR, Saldiva PHN, de Santos U. Occupational effect of sugarcane biomass burning on the conjunctival mucin profile of harvest workers and residents of an adjacent town - A Brazilian panel study. Experimental eye research. 2020;190:107889. Epub 2019/12/06. doi: 10.1016/j.exer.2019.107889. [DOI] [PubMed] [Google Scholar]
- 200.Mueller W, Cowie H, Horwell CJ, Hurley F, Baxter PJ. Health Impact Assessment of Volcanic Ash Inhalation: A Comparison With Outdoor Air Pollution Methods. Geohealth. 2020;4(7):e2020GH000256. Epub 2020/07/10. doi: 10.1029/2020GH000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tesone AI, Lasagni Vitar RM, Tau J, Maglione GA, Llesuy S, Tasat DR, et al. Volcanic ash from Puyehue-Cordon Caulle Volcanic Complex and Calbuco promote a differential response of pro-inflammatory and oxidative stress mediators on human conjunctival epithelial cells. Environ Res 2018;167:87–97. Epub 2018/07/18. doi: 10.1016/j.envres.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 202.Novaes P, do Nascimento Saldiva PH, Kara-Jose N, Macchione M, Matsuda M, Racca L, et al. Ambient levels of air pollution induce goblet-cell hyperplasia in human conjunctival epithelium. Environ Health Perspect 2007;115(12):1753–6. Epub 2007/12/19. doi: 10.1289/ehp.10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Torricelli AA, Matsuda M, Novaes P, Braga AL, Saldiva PH, Alves MR, et al. Effects of ambient levels of traffic-derived air pollution on the ocular surface: analysis of symptoms, conjunctival goblet cell count and mucin 5AC gene expression. Environ Res 2014;131:59–63. Epub 2014/03/25. doi: 10.1016/j.envres.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 204.Tau J, Novaes P, Matsuda M, Tasat DR, Saldiva PH, Berra A. Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Investigative ophthalmology & visual science. 2013;54(7):4759–65. Epub 2013/06/01. doi: 10.1167/iovs.12-10541. [DOI] [PubMed] [Google Scholar]
- 205.Tzortzi A, Teloniatis S, Matiampa G, Bakelas G, Tzavara C, Vyzikidou VK, et al. Passive exposure of non-smokers to E-Cigarette aerosols: Sensory irritation, timing and association with volatile organic compounds. Environ Res 2020;182:108963. Epub 2019/12/15. doi: 10.1016/j.envres.2019.108963. [DOI] [PubMed] [Google Scholar]
- 206.Matsumoto Y, Dogru M, Goto E, Sasaki Y, Inoue H, Saito I, et al. Alterations of the tear film and ocular surface health in chronic smokers. Eye. 2008;22(7):961–8. Epub 2008/04/22. doi: 10.1038/eye.2008.78. [DOI] [PubMed] [Google Scholar]
- 207.Agin A, Kocabeyoglu S, Colak D, Irkec M. Ocular Surface, Meibomian Gland Alterations, and In Vivo Confocal Microscopy Characteristics of Corneas in Chronic Cigarette Smokers. Graefes Arch Clin Exp Ophthalmol 2020;258(4):835–41. Epub 2019/12/18. doi: 10.1007/s00417-019-04547-0. [DOI] [PubMed] [Google Scholar]
- 208.Rummenie VT, Matsumoto Y, Dogru M, Wang Y, Hu Y, Ward SK, et al. Tear cytokine and ocular surface alterations following brief passive cigarette smoke exposure. Cytokine. 2008;43(2):200–8. Epub 2008/07/08. doi: 10.1016/j.cyto.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 209.Abelson MB, Ingerman A. The Dye-namics of Dry-Eye Diagnosis. Review of Ophthalmology. 2005;https://www.reviewofophthalmology.com/article/the-dye-namics-of-dry-eye-diagnosis. Epub 15 Nov 2005.
- 210.Bron AJ, Argueso P, Irkec M, Bright FV. Clinical staining of the ocular surface: mechanisms and interpretations. Progress in retinal and eye research. 2015;44:36–61. doi: 10.1016/j.preteyeres.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 211.Kim J. The use of vital dyes in corneal disease. Current opinion in ophthalmology. 2000;11(4):241–7. [DOI] [PubMed] [Google Scholar]
- 212.Wilson G, Ren H, Laurent J. Corneal epithelial fluorescein staining. J Am Optom Assoc 1995;66(7):435–41. [PubMed] [Google Scholar]
- 213.Mokhtarzadeh M, Casey R, Glasgow BJ. Fluorescein punctate staining traced to superficial corneal epithelial cells by impression cytology and confocal microscopy. Investigative ophthalmology & visual science. 2011;52(5):2127–35. Epub 2011/01/08. doi: 10.1167/iovs.10-6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bandamwar KL, Papas EB, Garrett Q. Fluorescein staining and physiological state of corneal epithelial cells. Contact lens & anterior eye : the journal of the British Contact Lens Association. 2014;37(3):213–23. doi: 10.1016/j.clae.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 215.Bakkar MM, Hardaker L, March P, Morgan PB, Maldonado-Codina C, Dobson CB. The cellular basis for biocide-induced fluorescein hyperfluorescence in mammalian cell culture. PloS one. 2014;9(1):e84427. doi: 10.1371/journal.pone.0084427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Khan TF, Price BL, Morgan PB, Maldonado-Codina C, Dobson CB. Cellular fluorescein hyperfluorescence is dynamin-dependent and increased by Tetronic 1107 treatment. The international journal of biochemistry & cell biology. 2018;101:54–63. doi: 10.1016/j.biocel.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 217.Webster A, Chintala SK, Kim J, Ngan M, Itakura T, Panjwani N, et al. Dynasore protects the ocular surface against damaging oxidative stress. PloS one. 2018;13(10):e0204288. doi: 10.1371/journal.pone.0204288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dogru M, Kojima T, Simsek C, Tsubota K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investigative ophthalmology & visual science. 2018;59(14):DES163–DES8. Epub 2018/11/28. doi: 10.1167/iovs.17-23402. [DOI] [PubMed] [Google Scholar]
- 219.Cejka C, Cejkova J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxid Med Cell Longev 2015;2015:591530. doi: 10.1155/2015/591530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Seen S, Tong L. Dry eye disease and oxidative stress. Acta Ophthalmol 2017. doi: 10.1111/aos.13526. [DOI] [PubMed] [Google Scholar]
- 221.Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM. Oxidative stress in diseases of the human cornea. Free Radic Biol Med 2008;45(8):1047–55. doi: 10.1016/j.freeradbiomed.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 222.Cavalli V, Vilbois F, Corti M, Marcote MJ, Tamura K, Karin M, et al. The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Molecular cell. 2001;7(2):421–32. [DOI] [PubMed] [Google Scholar]
- 223.Andrews NW, Almeida PE, Corrotte M. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol 2014;24(12):734–42. doi: 10.1016/j.tcb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Clapham DE. The mother of all endocytosis. eLife. 2013;2:e01738. doi: 10.7554/eLife.01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Castro-Gomes T, Corrotte M, Tam C, Andrews NW. Plasma Membrane Repair Is Regulated Extracellularly by Proteases Released from Lysosomes. PloS one. 2016;11(3):e0152583. doi: 10.1371/journal.pone.0152583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–50. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 227.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 228.Sharp WW. Dynamin-related protein 1 as a therapeutic target in cardiac arrest. J Mol Med (Berl). 2015;93(3):243–52. doi: 10.1007/s00109-015-1257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park SW, Kim KY, Lindsey JD, Dai Y, Heo H, Nguyen DH, et al. A selective inhibitor of drp1, mdivi-1, increases retinal ganglion cell survival in acute ischemic mouse retina. Investigative ophthalmology & visual science. 2011;52(5):2837–43. doi: 10.1167/iovs.09-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Martinez-Carrasco R, Argueso P, Fini ME. Dynasore protects ocular surface mucosal epithelia subjected to oxidative stress by maintaining UPR and calcium homeostasis. Free Radic Biol Med 2020;160:57–66. Epub 2020/08/14. doi: 10.1016/j.freeradbiomed.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Chong WC, Shastri MD, Eri R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. International journal of molecular sciences. 2017;18(4). doi: 10.3390/ijms18040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 233.Coursey TG, Tukler Henriksson J, Barbosa FL, de Paiva CS, Pflugfelder SC. Interferon-gamma-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjogren Syndrome. The American journal of pathology. 2016;186(6):1547–58. doi: 10.1016/j.ajpath.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Woodward AM, Di Zazzo A, Bonini S, Argueso P. Endoplasmic reticulum stress promotes inflammation-mediated proteolytic activity at the ocular surface. Scientific reports. 2020;10(1):2216. doi: 10.1038/s41598-020-59237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. The American journal of pathology. 2005;166(1):61–71. Epub 2005/01/06. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, et al. Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. The international journal of biochemistry & cell biology. 2012;44(1):16–20. doi: 10.1016/j.biocel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Xiao WC, Zhang J, Chen SL, Shi YJ, Xiao F, An W. Alleviation of palmitic acid-induced endoplasmic reticulum stress by augmenter of liver regeneration through IP3R-controlled Ca(2+) release. Journal of cellular physiology. 2018;233(8):6148–57. doi: 10.1002/jcp.26463. [DOI] [PubMed] [Google Scholar]
- 238.Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochemical and biophysical research communications. 2015;460(1):114–21. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 239.McKenzie RW, Jumblatt JE, Jumblatt MM. Quantification of MUC2 and MUC5AC transcripts in human conjunctiva. Investigative ophthalmology & visual science. 2000;41(3):703–8. [PubMed] [Google Scholar]
- 240.Yu DF, Chen Y, Han JM, Zhang H, Chen XP, Zou WJ, et al. MUC19 expression in human ocular surface and lacrimal gland and its alteration in Sjogren syndrome patients. Experimental eye research. 2008;86(2):403–11. Epub 2008/01/11. doi: 10.1016/j.exer.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 241.Kinoshita M, Nakamura T, Ihara M, Haraguchi T, Hiraoka Y, Tashiro K, et al. Identification of human endomucin-1 and −2 as membrane-bound O-sialoglycoproteins with anti-adhesive activity. FEBS letters. 2001;499(1–2):121–6. Epub 2001/06/22. doi: 10.1016/s0014-5793(01)02520-0. [DOI] [PubMed] [Google Scholar]
- 242.Pallesen LT, Berglund L, Rasmussen LK, Petersen TE, Rasmussen JT. Isolation and characterization of MUC15, a novel cell membrane-associated mucin. European journal of biochemistry / FEBS. 2002;269(11):2755–63. Epub 2002/06/06. doi: 10.1046/j.1432-1033.2002.02949.x. [DOI] [PubMed] [Google Scholar]