Abstract
Background:
The Democratic Republic of the Congo (DRC) remains one of the countries most impacted by malaria despite decades of control efforts, including multiple mass insecticide treated net (ITN) distribution campaigns. The multi-scalar and complex nature of malaria necessitates an understanding of malaria risk factors over time and at multiple levels (e.g., individual, household, community). Surveillance of households in both rural and urban settings over time, coupled with detailed behavioral and geographic data, enables the detection of seasonal trends in malaria prevalence and malaria-associated behaviors as well as the assessment of how the local environments within and surrounding an individual’s household impact malaria outcomes.
Methods:
Participants from seven sites in Kinshasa Province, DRC were followed for over two years. Demographic, behavioral, and spatial information was gathered from enrolled households. Malaria was assessed using both rapid diagnostic tests (RDT) and polymerase chain reaction (PCR) and seasonal trends were assessed. Hierarchical regression modeling tested associations between behavioral and environmental factors and positive RDT and PCR outcomes at individual, household and neighborhood scales.
Results:
Among 1591 enrolled participants, malaria prevalence did not consistently vary seasonally across the sites but did vary by age and ITN usage. Malaria was highest and ITN usage lowest in children ages 6–15 years across study visits and seasons. Having another member of the household test positive for malaria significantly increased the risk of an individual having malaria [RDT: OR= 4.158 (2.86–6.05); PCR: OR= 3.37 (2.41–4.71)], as did higher malaria prevalence in the 250m neighborhood around the household [RDT: OR= 2.711 (1.42–5.17); PCR: OR= 4.056 (2.3–7.16)]. Presence of water within close proximity to the household was also associated with malaria outcomes.
Conclusions:
Taken together, these findings suggest that targeting non-traditional age groups, children >5 years old and teenagers, and deploying household- and neighborhood-focused interventions may be effective strategies for improving malaria outcomes in high-burden countries like the DRC.
Keywords: malaria, longitudinal, Democratic Republic of the Congo, rural, geographic, seasonal, household
Background
Despite decades of effort, malaria infection and death rates in the Democratic Republic of the Congo (DRC) remain some of the highest in the world (Organization, 2016, Mitchell et al., 2020). The majority of the DRC population lives in a high-risk zone for malaria, though within these zones there is high spatial variation in malaria outcomes across small geographic distances (Organization, 2016, Bousema et al., 2010, Bousema et al., 2012, Bejon et al., 2010, Clark et al., 2008, Gaudart et al., 2006, Kreuels et al., 2008, Ferrari et al., 2016). This micro-scale spatial heterogeneity is the result of complex and dynamic human-environment interactions playing out at individual, household and neighborhood scales that are influenced by more distal regional, national, and global forces (Degarege et al., 2019).
For instance, climatic and environmental variation that influences the behavior of Anopheline mosquito vectors, such as seasonality of rainfall or patterns of land use that promote or deter breeding, can alter malaria risk across the spatiotemporal landscape (Bomblies, 2012, Thomson et al., 2005, Thomson et al., 1996, Colón-González et al., 2016, Ferrao et al., 2018, Chuang et al., 2017, Dewald et al., 2016, Kweka et al., 2016, Kabaria et al., 2016, Jedrychowski et al., 2009). At the individual scale, age, occupation and perceptions of malaria risk can alter behaviors, such as the use of insecticide-treated nets (ITNs) or time spent outdoors at certain parts of the day (Storey et al., 2018, Mattern et al., 2016, Ernst et al., 2016, Babalola et al., 2016, Babalola et al., 2018, Moscibrodzki et al., 2018, Moshi et al., 2017, Finda et al., 2019, Monroe et al., 2019, Roberts and Matthews, 2016, Peprah et al., 2019). In households, population size and age composition, wealth, and building materials can impact exposure to mosquitoes and malaria (Ngatu et al., 2019, Bannister-Tyrrell et al., 2017, Mbohou et al., 2019, Guerra et al., 2018, Oguoma et al., 2020, Odufuwa et al., 2020, Were et al., 2018, Okiring et al., 2019, Mpimbaza et al., 2017). Malaria-related behaviors and environmental conditions in other nearby households can further influence mosquito presence and the likelihood of transmission. At larger scales, malaria prevention campaigns designed and carried out by governments and non-governmental organizations change availability of ITNs differentially over time and space. The complexity of this multi-scalar phenomenon necessitates studies that explore spatiotemporal drivers of malaria in ways that incorporate knowledge about behaviors and environments at multiple levels. In order to better understand how and why malaria varies in terms of individual, household and neighborhood characteristics, a prospective, longitudinal study of malaria was implemented across seven rural and urban sites in Kinshasa Province, DRC.
This prospective study allowed for linkage of malaria outcomes, as determined by both rapid diagnostic tests (RDT) and polymerase chain reaction (PCR), with time varying information such as age and ITN usage. Visiting households once per year in the dry season and once per year in the wet season for two years allowed for examination of any seasonal trends in the age distribution of malaria prevalence, as well as how ITN usage varies according to season. At the time of baseline enrollment, we found that malaria prevalence varied strongly by age category, peaking in children older than 5 years of age and in teens, and that these age categories also showed the lowest reported use of ITNs (Author, 2017). These findings confirmed the results of prior cross-sectional studies of malaria and behavior in 5–15 year olds, age groups that are often excluded from national surveys of malaria (Noor et al., 2009, Pinchoff et al., 2015, Pullan et al., 2010, Ross et al., 2006, Walldorf et al., 2015, Deutsch-Feldman et al., 2020). Such studies, however, did not assess whether such patterns were driven by seasonality of rainfall or perceptions of riskier times of the year, which could impact ITN usage behavior.
Additionally, because the social and physical environment in which an individual lives can impact the persistence and transmission of malaria in communities, it is important to determine how malaria among family members and immediate neighbors influences individual risk of malaria. Behavior by family and friends has the potential to either increase or decrease malaria risk, such as through the usage of ITNs or via exposure to parasites during work or other activities that are brought into the household or neighborhood (Hawley et al., 2003, Afrane et al., 2004). Longitudinal surveillance of households with known geographic locations allowed for assessment of household and neighborhood influences on the malaria status of individuals.
To analyze the multi-scalar and time varying nature of malaria, RDT and PCR malaria outcomes were integrated with questionnaire data and spatially derived variables to examine correlates of malaria across seasons and at individual, household and neighborhood levels. The relationship between age and ITN use was explored, with particular focus on variation across rural-urban sites and rainy and dry seasons. The influence of household characteristics, including wealth and household malaria prevalence, on individual malaria outcomes were assessed, as was the role of neighborhood malaria prevalence and neighborhood aquatic environments. Understanding malaria at multiple levels can indicate how current malaria control and eradication efforts can be modified in ways that could more effectively address spaces where transmission is common.
Methods
Individuals residing in seven sites in Kinshasa Province, DRC were sampled at four separate time periods from 2015 to 2016 (Figure 1). The Lingwala and Kimpoko Health Zones were selected based on their year-round accessibility and ability to cover the urban/rural gradient. Health areas that are administrative subdivisions within these two health zones, and villages within these health areas, were selected using pre-specified criteria as previously described (Author, 2017). Detailed methods regarding study sampling choices and participant enrollment are provided in Author, 2017. The baseline visit took place during the rainy period in February- May 2015. Baseline data included information on time-varying individual-level characteristics and behaviors (e.g. recent fever, ITN use) as well as time-invariant household characteristics (e.g. ownership of items, housing construction materials).
Figure 1.
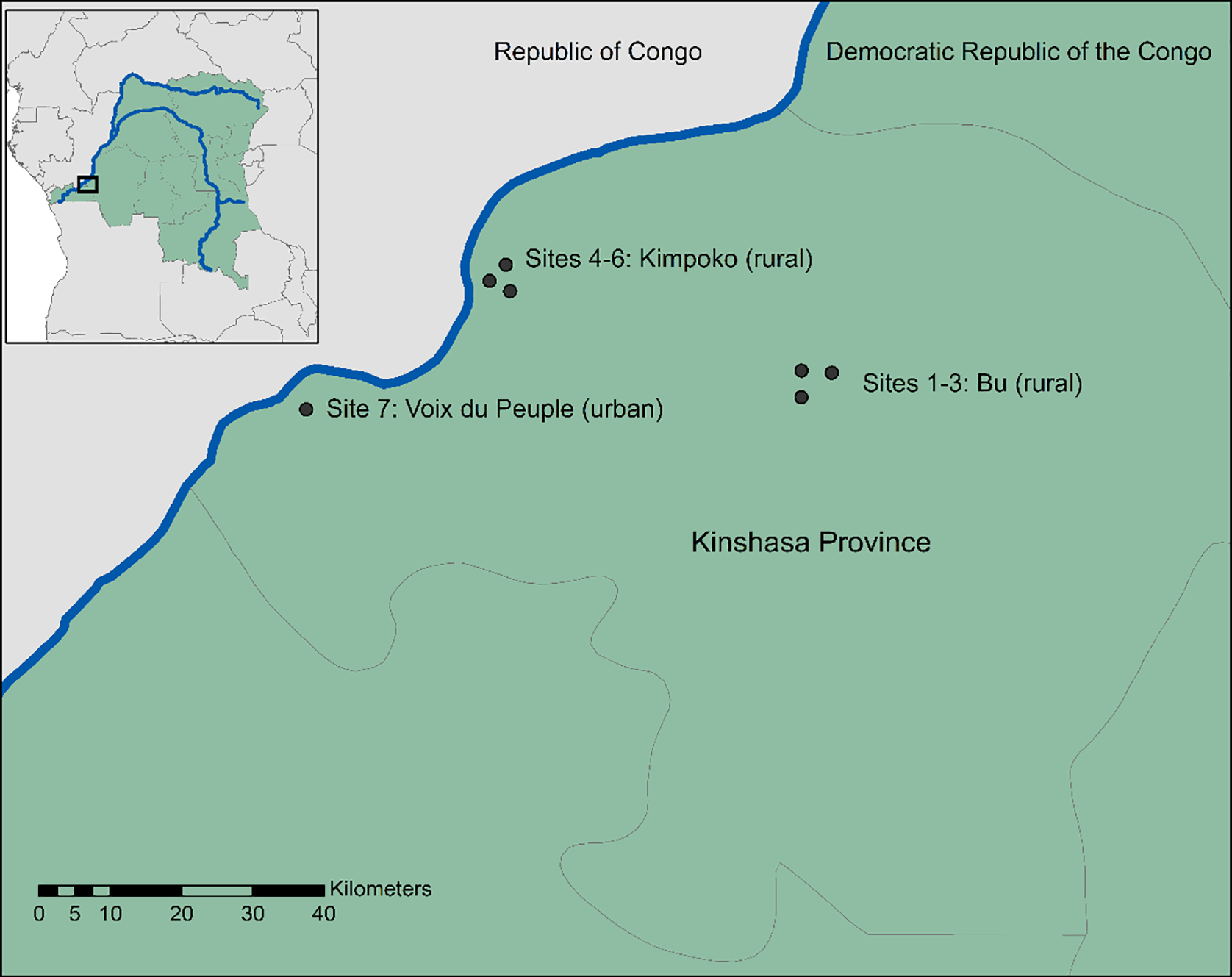
Location of the study sites.
After baseline enrollment of participants, each household was visited three subsequent times over two years and participants were administered a follow-up survey. The follow-up visit schedule was designed to enable detection of seasonal patterns of malaria and associated covariates across the rainy and dry seasons; follow-up visits 1 & 3 were timed to capture dry season malaria patterns (July-October of 2015 and 2016) while follow-up 2 took place during the rainy season (January-April 2016) to augment the data collected during the baseline visit. Follow-up survey questions focused specifically on time-varying malaria-associated covariates that were first assessed during the baseline visit, such as recent fever in participants, ITN use the previous night, and malaria diagnosis in the prior 6 months.
At each of the four scheduled visits, participants had blood drawn via either finger or heel stick, depending on age (young children received a heel stick) and applied to a malaria RDT (SD Bioline Ag P.f./Pan RDT [05FK60], Alere, Gyeonggi-do, Republic of Korea) and results were interpreted according to manufacturer protocol and immediately reported to the participant. All participants who tested positive for P. falciparum via RDT were referred to the local health center for treatment. Dried blood spots (DBS) were also prepared from finger or heel pricks using the protocol described in Author, 2017. DBS were transported to the <School Name Redacted> where DNA was extracted and duplex, quantitative PCR performed targeting the P. falciparum-specific lactate dehydrogenase (pfldh) and human beta-tubulin (HumTuBB) genes. The protocol for DNA extraction, PCR assays and quality control are described in Author, 2017. Treatment according to the National Malaria Control Program guidelines was offered at the local health center for malaria diagnosed via RDT.
At the time of the baseline survey, households reported ownership of various possessions (e.g. radios and lamps) as well as characteristics of the house (e.g. presence of electricity and roof material). The survey items were modeled on those used in Demographic and Healthy Surveys (DHS) to estimate wealth, as were the methods used to generate wealth quintiles for study participant households based on item responses (MEASURE-DHS, Rockville, MD) (Rutstein, 2015). This measure of wealth was then used as a covariate in further analysis.
The baseline survey also indicated that differences in the local environment could be associated with variation in malaria outcomes, namely the type of water present within a two-minute walk of the household. The presence of various types of water bodies (swamps/marshes, puddles, ponds/lakes and streams), providing potential habitat for mosquitoes, was investigated as a risk factor for malaria in individual participants across time (Author, 2017).
Characteristics of participants at baseline and scheduled follow-up visits were summarized and Pearson’s chi-square tests were used to detect differences between RDT- and PCR-positive malaria outcomes. Charts of malaria prevalence by age and at the times of each scheduled visit were generated to explore the consistency of age profiles of malaria across the seven sites that were detected at baseline. The potential for seasonal trends in malaria prevalence and ITN usage were explored. Agreement on malaria outcomes measured via RDT versus PCR, stratified by febrile status, was tested using Cohen’s kappa (Cohen, 1960). Chi-square tests, calculation of Cohen’s kappa and descriptive charts were completed in R using the stats package (CRAN, R Foundation for Statistical Computing, Vienna, Austria).
To detect the potential influence of sharing a household with a malaria-positive person on an individual’s malaria status, a measure of household malaria prevalence exclusive of the individual was created. To explore the potential influence of malaria in neighboring households on an individual’s malaria status, the percentage of individuals with a malaria positive test via RDT or PCR in a 250-meter radius around each household was calculated in ArcMap and R (Esri, Redlands, CA; CRAN, Vienna, Austria). This neighborhood malaria proportion does not include malaria outcomes within the household of residence for the individual, only those in other households. The radii of neighborhoods surrounding households were chosen to detect micro-scale within-site variability and to assess how malaria prevalence among immediate neighbors’ is influential on an individual’s malaria outcome. A distance of 250m has been shown to more fully capture malaria-positive individuals than smaller radii around index households in test and treat studies (Deutsch-Feldman et al., 2018). Larger distances, perhaps reflective of mosquito flying capabilities, would obscure within-site variation as all but one of the study sites are less than 2km across.
A three-level logistic regression model with random intercepts, a special case of a generalized linear mixed model (GLMM), was used to explore the influence of hypothesized covariates on RDT and PCR malaria outcomes for individuals clustered within households within sites. The three-level hierarchical logistic model allows for the estimation of the effects of covariates on the individual-level responses, while accounting for the dependence among binary outcomes from clustered individuals. The intraclass correlation coefficients (ICC) were calculated from the null model (without covariates) to estimate the proportion of variability explained by the differences between households (level 2) and between sites (level 3). The within-subject correlation from the repeated measurements of malaria outcomes obtained from an individual over four occasions were accounted for as a quadratic time trend in the model. All individual- and household-level variables were included in the models as fixed-effects. The parameters were estimated as odds ratios using a maximum likelihood approach based on a Laplace approximation of the marginal log-likelihood.
While every attempt was made to retain participants across all four scheduled study visits, longitudinal studies often suffer from participant loss-to-follow-up; this missingness can lead to a loss of power and robustness of results in regression models (Fitzmaurice et al., 2011). To address problems of missingness in the longitudinal data from baseline across the three follow-up visits, Multiple Imputation (MI) by Fully Conditional Specification (FCS) was used to create imputations of missingness in malaria outcome in follow-up 1–3 and to preserve the uncertainty of missing values (Van Buuren et al., 2006, Patrician, 2002). A total of 25 imputation sets were generated based on the assumption that unobserved responses are missing at random (MAR) and the regression outcomes from these 25 imputation datasets were combined to estimate regression coefficients and standard errors that inherently include the uncertainty. GLMM and FCS MI were conducted in SAS 9.4 (SAS Institute, Cary NC). Residual spatial autocorrelation was assessed using Moran’s I and a neighbor definition of 250m.
The study was approved by Institutional Review Boards at the University of North Carolina- Chapel Hill, the University of Kinshasa and the University of Iowa..
Results
At baseline enrollment, 1591 participants in 242 households across the seven sites joined the study and had RDT results recorded. DBS were available for PCR analysis for 1565 individuals at the baseline visit. Retention in the study was high, 74% of participants had malaria outcomes recorded across all four visits, with an additional 13% recording three of four visits (Supplemental Table 1).
Overall malaria prevalence was highest at follow-up two (37.1% via RDT and 39.6% via PCR) during the rainy months of January-April (Figure 2, Supplemental Tables 2–5, Supplemental Figure 1). Malaria prevalence as measured by PCR was consistently higher than via RDT. The urban Kinshasa site (site 7, Voix du Peuple) had the lowest malaria prevalence in all four time periods, while sites with the highest malaria prevalence varied across visits, ranging as high as 60% via PCR in a rural village in Bu (site 5) during follow-up 2.
Figure 2.
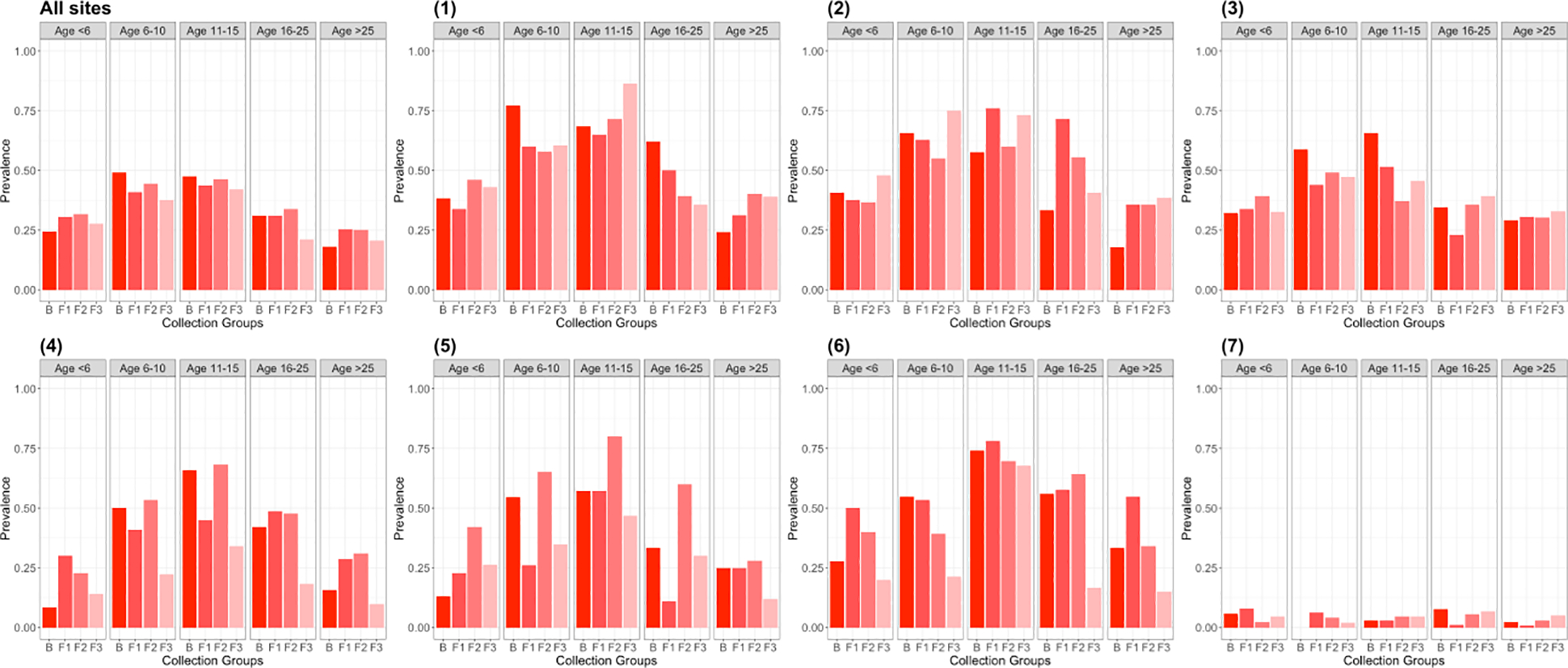
PCR malaria by age group across baseline and follow-up visits for seven sites and summarized across all sites.
Factors such as participant gender, prior malaria outcomes, fever in the prior two weeks and ITN usage were associated with malaria according to Chi-square tests during specific follow-up visits but not consistently across the four visits and RDT and PCR outcomes (Supplemental Tables 2–5). Malaria prevalence by RDT and PCR was higher among those who reported having fever in the prior week than those who did not for all scheduled visits, except for the final visit. Significant variation in both RDT and PCR malaria was observed across wealth categories, with lower malaria prevalence in wealthier households (Supplemental Tables 2–5, Supplemental Figure 2).
Consistent age differences in malaria outcomes were observed at all four visits (Figure 2, Supplemental Tables 2–5, Supplemental Figure 1). The age category of <1 year old was aggregated into the 1–5 year old category for charting purposes because all those children had passed the age of one by follow-up visits 2 and 3. Malaria prevalence was lowest in these young children and in adults age 26 and older. Children ages 6–15 years old had the highest malaria prevalence across all four study visits in all six rural sites.
ITN usage was highest in the youngest and oldest age groups. The 6–15 year-old age group reported consistently low prevalence of ITN usage (Figures 3–4, Supplemental Figure 3). Rural sites 2 & 3 had lower ITN usage than did rural sites 6 all study visits, while urban site 7 had relatively low ITN usage as well (Figure 3).
Figure 3.
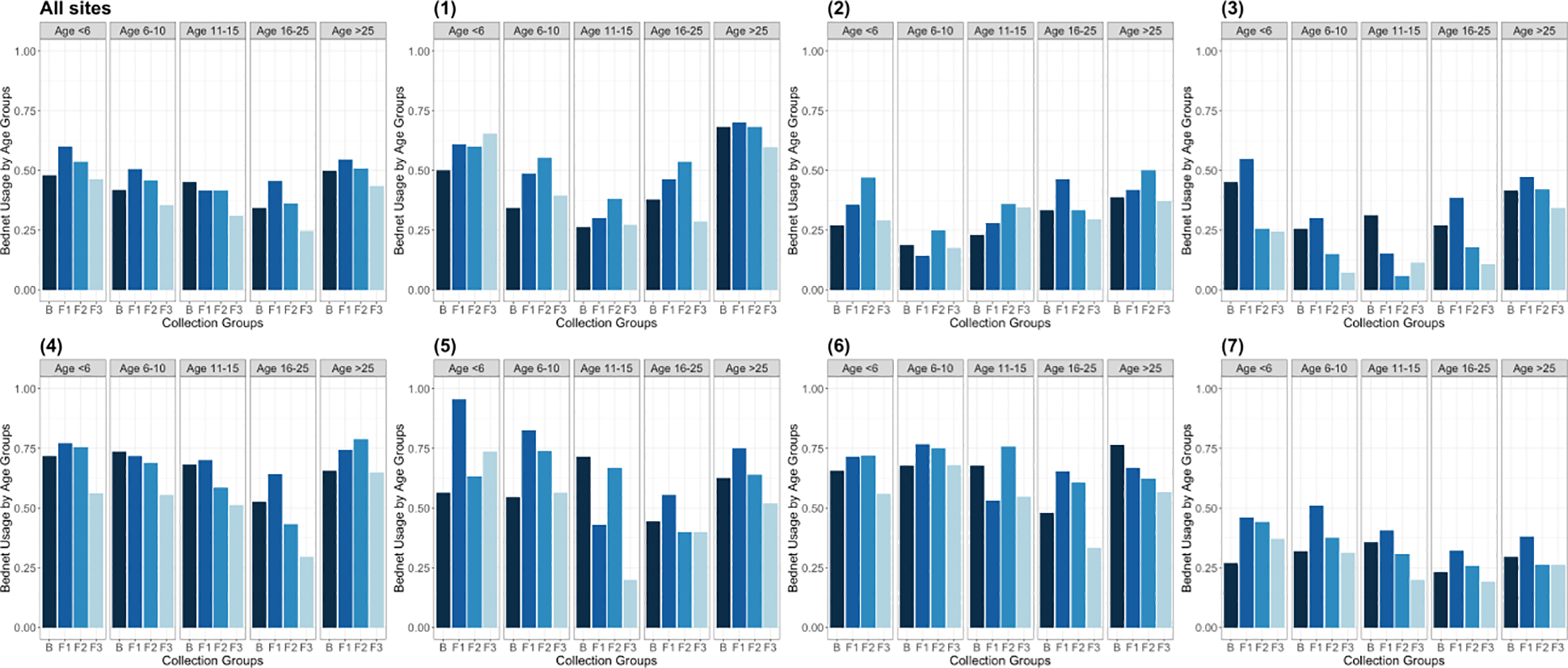
ITN usage by age group across baseline and follow-up visits for seven sites and summarized across all sites.
Figure 4.
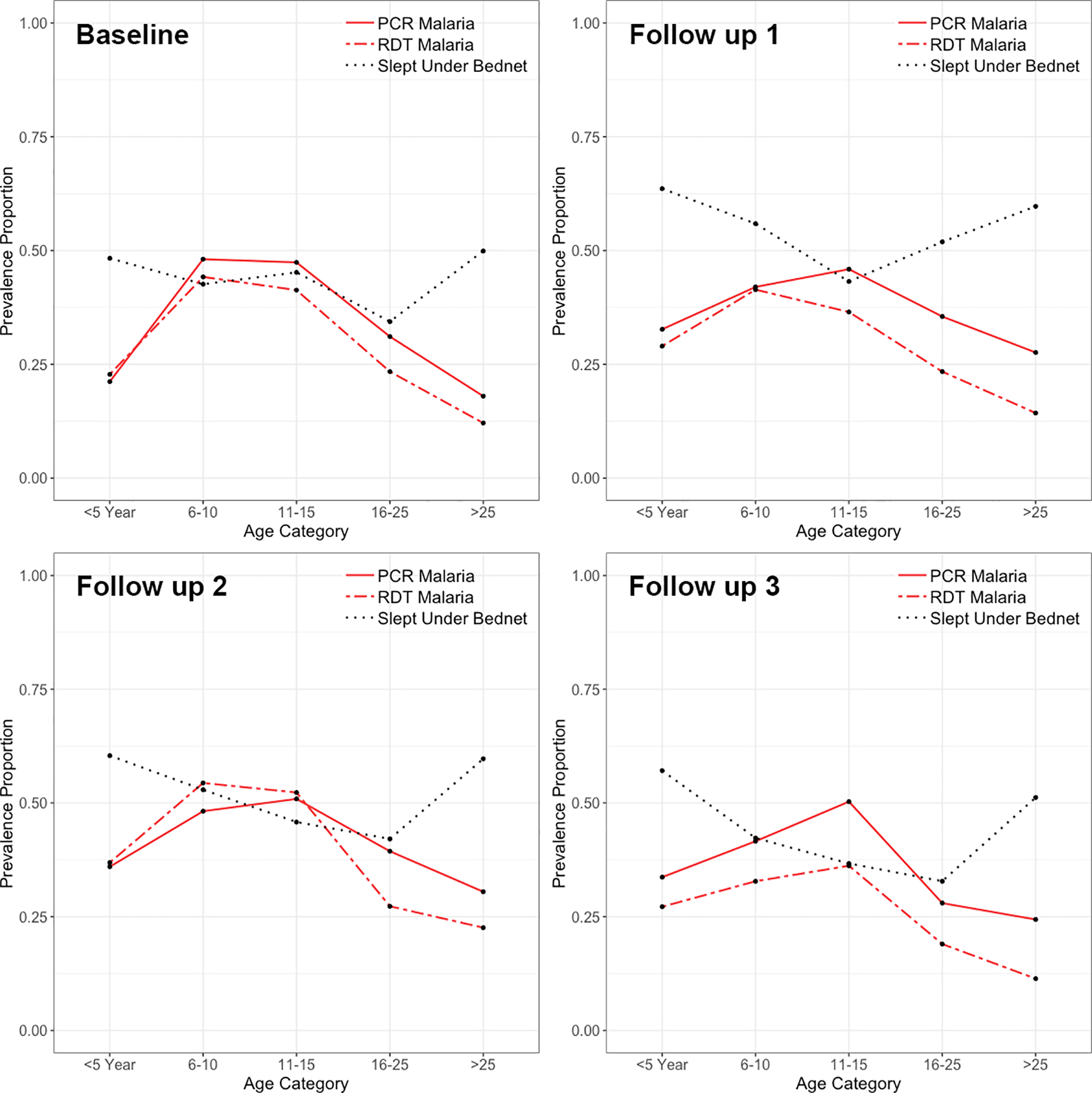
Malaria prevalence according to RDT (dashed red) and PCR (solid red) testing andITN usage (black) in all seven sites across four separate study visits.
No consistent seasonal trends were observed amongst malaria in study participants. In some sites, such as one rural village in Bu (site 1), malaria prevalence appeared relatively stable over time, while in others, it peaked at follow-up 1 or follow-up 2 (Figures 2 & 4). Similarly, ITN usage did not appear to systematically vary according to season (Figure 3, Supplemental Figure 3).
Agreement on malaria status of participants via RDT and PCR at the time of home visits, as measured by Cohen’s kappa, was high. Agreement was higher in those with fever (kappa = 0.74, 95% CI 0.71–0.78) than those without (kappa=0.68, 95% CI 0.66–0.70).
A hierarchical model was used to assess the predictors of RDT and PCR malaria across four scheduled visits. Imputation was used to address the seven different patterns of missing data observed over time (Supplemental Table 1). ICC indicated that a hierarchical model with fixed effects was necessary to avoid overestimation of the significance of malaria predictors. No residual spatial autocorrelation was detected.
Individual Factors
In the hierarchical model using the PCR outcome across four time periods and accounting for correlation in malaria outcomes in individuals across time, the odds of malaria among participants aged 6–10 years, 11–15 years and 16–25 years were 2.373 (1.94–2.91), 3.853 (3.08–4.82) and 1.96 (1.56–2.46) times the odds of malaria among participants ages 5 years or younger, respectively (Table 1). Odds of PCR malaria were lower in adults age 26 and older, but not significantly so. In RDT analysis, malaria odds were similarly higher among participants ages 6–10 and 11–15 than in children 5 or younger, while malaria odds were lower in adults age 26 and older. Odds of malaria in the PCR model were lower for women than men [OR=0.855 (0.75–0.98)]. Sleeping under a ITN was protective in both the RDT and PCR model [RDT: OR=0.825 (0.71–0.96); PCR: OR=0.75 (0.65–0.87)], while reporting fever in the week prior to a visit was associated with increased odds of malaria at a scheduled visit in both RDT and PCR models [RDT: OR= 1.961 (1.65–2.33); PCR: OR= 1.342 (1.13–1.59)].
Table 1.
Results of the hierarchical model, using RDT or PCR outcomes.
| RDT | PCR | |||
|---|---|---|---|---|
| Parameter | Odds Ratio | p-value | Odds Ratio | p-value |
| Age ≤5 | Referent | |||
| Age 6–10 | 2.311 (1.9–2.82) | <.0001 | 2.373 (1.94–2.91) | <.0001 |
| Age 11–15 | 2.607 (2.1–3.23) | <.0001 | 3.853 (3.08–4.82) | <.0001 |
| Age 16– 25 | 1.119 (0.89–1.4) | 0.3347 | 1.960 (1.56–2.46) | <.0001 |
| Age >25 | 0.425 (0.35–0.52) | <.0001 | 0.847 (0.70–1.02) | 0.0785 |
| Male | Referent | |||
| Female | 0.89 (0.78–1.23) | 0.0882 | 0.855 (0.75–0.98) | 0.0199 |
| Slept Under ITN | 0.825 (0.71–0.96) | 0.011 | 0.750 (0.65–0.87) | 0.0001 |
| Fever in Prior Week | 1.961 (1.65–2.33) | <.0001 | 1.342 (1.13–1.59) | 0.0007 |
| Household Malaria Prevalence | 4.158 (2.86–6.05) | <.0001 | 3.370 (2.41–4.71) | <.0001 |
| Malaria Prevalence in 250m | 2.711 (1.42–5.17) | 0.0024 | 4.056 (2.30–7.16) | <.0001 |
| Wealth 1 (Poorest) | Referent | |||
| Wealth 2 | 0.971 (0.77–1.22) | 0.7987 | 1.012 (0.80–1.28) | 0.9210 |
| Wealth 3 | 0.727 (0.57–0.93) | 0.0099 | 0.822 (0.64–1.05) | 0.1169 |
| Wealth 4 | 0.811 (0.62–1.05) | 0.1157 | 0.799 (0.61–1.05) | 0.1055 |
| Wealth 5 (Richest) | 0.518 (0.27–0.98) | 0.0433 | 0.474 (0.25–0.90) | <.0001 |
| No Water in 2 Minutes | Referent | |||
| Stream | 1.036 (0.74–1.46) | 0.8383 | 0.937 (0.67–1.32) | 0.7079 |
| Pond/Lake | 1.205 (0.7–2.09) | 0.5052 | 1.386 (0.79–2.42) | 0.251 |
| Swamp/Marsh | 1.275 (0.82–1.97) | 0.276 | 1.435 (0.91–2.25) | 0.1162 |
| Frequent Puddles | 1.176 (0.93–1.49) | 0.1851 | 1.296 (1.01–1.66) | 0.0413 |
| Variances (null model) | Variance | SE | Variance | SE |
| Household level | 0.91 | 0.095 | 0.756 | 0.076 |
| Site level | 0.726 | 0.4 | 0.811 | 0.443 |
| ICC | ||||
| Household level | 0.332 | 0.323 | ||
| Site level | 0.181 | 0.17 |
Household & Neighborhood Factors
As household prevalence of malaria increases, exclusive of the individual being considered in the model, so does individual malaria risk [RDT: OR= 4.158 (2.86–6.05); PCR: OR= 3.37 (2.41–4.71)]. Higher malaria prevalence in other households within 250m increases an individual’s odds of positive results for both RDT and PCR malaria [RDT: OR= 2.711 (1.42–5.17); PCR: OR= 4.056 (2.3–7.16)].
In comparison to individuals living in the poorest households, residents in the wealthiest category of households had lower odds of malaria for both RDT and PCR outcomes [RDT: OR=0.518 (0.27–0.98); PCR: OR= 0.474 (0.25–0.90)].
Living within a two-minute walk of frequent puddles increased the odds of malaria compared to no residential proximity to water in PCR model [OR=1.296 (1.01–1.66)]. No difference was observed if the water in close residential proximity was a stream or a pond/lake or swamp/marsh.
Discussion
Among the total study population, the prevalence of malaria was highest among children ages 6–15. This was true within the six rural sites, though not in the single urban site (site 7). In addition to older children having the greatest malaria prevalence across rural sites, the patterns of malaria by age also held true across study visits, which spanned multiple years and included both rainy and dry seasons (Figure 4). This age distribution has been reported in several recent studies and was predicted by a recent DRC countrywide model (Walldorf et al., 2015, Pinchoff et al., 2015, Deutsch-Feldman et al., 2020). Lower use of ITNs and greater amounts of time spent outdoors in the evening hours in children age 6–15 are potential explanations, as both could lead to greater exposure to mosquitoes (Babalola et al., 2016, Kateera et al., 2015, Noor et al., 2009, Pullan et al., 2010, Walldorf et al., 2015). This and prior work on the age patterns of malaria indicate that older children are an important reservoir of infection and should be included in malaria surveys and that treatment of school age children could reduce infection and transmission (Cohee et al., 2020).
Previous research indicates that use of ITNs is dependent on perceptions of risk; for instance, seasonal variation in usage, namely lower use in the dry season, is related to lower mosquito presence (Babalola et al., 2016, Afrane et al., 2004, Toé et al., 2009, Adongo et al., 2005, Agyepong and Manderson, 1999, Okrah et al., 2002, Pinchoff et al., 2015). This study, however, did not identify evidence of seasonal trends in ITN usage (Figure 3). Urban site 7 did, however, have consistently low ITN usage in comparison to some of the rural sites (excluding rural site 3 which also had low usage rates), suggesting that perhaps the perception of lower malaria risk in urban environments, in addition to the presence of air conditioning and sealed windows in some households, influences the use of protective strategies.
While fever was significantly predictive of malaria infection in the model, many participants reported no fever despite testing positive for malaria via RDT or PCR at one or more home visit. The absence of fever symptoms despite detectable parasitemia presents challenges for active detection of infection that depends either on febrile status or parasite densities high enough for detection by microscopy (Sturrock et al., 2013a). Agreement on infection status of participants via RDT and PCR was higher in those with fever symptoms than those who were asymptomatic during surveillance visits. As has been previously argued, the identification of individuals with asymptomatic malaria via active surveillance using RDT or PCR is an important component of malaria elimination efforts (Chen et al., 2016, Laishram et al., 2012, Bousema et al., 2014).
If a participant had higher prevalence of malaria among their household members, it significantly increased their odds of a malaria diagnosis (RDT: OR=4.158 (2.86–6.05); PCR: OR=3.37 (2.41–4.71)). Previous analysis (not shown) with a binary indicator of a single other household member with malaria rather than household malaria prevalence also indicated that this significantly increased an individual’s odds of malaria outcome. This finding emphasizes the potential impact of household-focused interventions, given that household parasite prevalence was a striking 78% in the 2013–2014 DRC DHS (Mitchell et al., 2020). Other studies indicate that larger households have greater risk of malaria compared to smaller households and that large households are higher risk transmissions sites than low occupancy households, due at least in part to increased attractiveness to mosquitoes (Kateera et al., 2015, Lwetoijera et al., 2013, Takken and Knols, 1999, Port et al., 1980). The results from this study indicate that in addition to household size, the overall prevalence of malaria amongst household members is an important risk factor for malaria. The importance of household malaria prevalence in our analysis provides support for interventions targeting households, rather than simply targeting specific types of individuals (young children, women of childbearing age) (Stresman et al., 2020).
As the prevalence of malaria within 250 meters of the household increased, so did an individual’s odds of a malaria diagnosis [RDT: OR= 2.711 (1.42–5.17); PCR: OR= 4.056 (2.3–7.16)]. Prior research has indicated that neighborhood and household effects operate over and above those of individual behaviors in influencing malaria risk, but little research has evaluated the two simultaneously or with such granularity (Stresman et al., 2010, Mosha et al., 2013, Sturrock et al., 2013b, Rulisa et al., 2013, Bejon et al., 2014). These studies did, however, indicate that individual risk of malaria was associated with positive malaria tests among household members, though evidence of malaria in neighboring houses at small (e.g., 100 meter) distances were not significantly associated with an individual’s malaria status (Rulisa et al., 2013, Stresman et al., 2010, Sturrock et al., 2013b). However, in contrast to the results presented here, that research often relied solely on diagnosis by RDT or had households that were surveyed days or weeks following the active detection of an index case at a clinic. A test and treat program in a low transmission context found that a radius of 250m rather than 140m more accurately captured the population at risk of infection from an index case (Deutsch-Feldman et al., 2018). The much higher odds of an individual having malaria if there was higher malaria prevalence in the surrounding neighborhood, over and above other malaria in the household and individual behaviors, suggests that the immediate social environment is an important driver of malaria. Findings presented here indicate not only that concurrent household-level testing and treatment of malaria could be beneficial, but that further simultaneous testing and treatment of neighborhoods may be appropriate.
The wealthiest category of households had significantly lower odds of malaria than the poorest households in this study. Indicators of housing construction, along with item ownership, that were measured during the baseline survey were summarized using factor analysis to generate a wealth index. Thus, the wealth index may be masking the direct impacts of housing materials on malaria risk, namely that mud walls, thatched roofs, unscreened windows, and other low-quality, porous materials are associated in many studies with increased exposure to mosquitos and increased malaria risk. Disentangling the contribution of household form to malaria outcomes via either decreased exposure to biting vectors or via increased access to resources that come with higher wealth is challenging. For instance, the majority of households that reported having glass or screen windows were in the urban site 7, and all households with electricity were also in urban site 7. These characteristics contribute to higher wealth calculations in the index and also provide physical barriers to mosquito bites. Site 7 had the lowest malaria prevalence of any of the study sites and also had a high proportion of participants with the highest wealth index (85%).
Households and small neighborhoods are sites of not only diverse behaviors (e.g., ITN usage by age of individual, time spent outside by age or profession or depending on house structure) but also environmental diversity (e.g., puddling of water), potentially contributing to the influence of household and neighborhood malaria on individual outcomes (Clark et al., 2008, Bejon et al., 2010, Peterson et al., 2009). High variability in malaria across small spatial scales is mirrored by variability in mosquito density across space, with the majority of adults within close range of breeding sites (Minakawa et al., 2002, Minakawa et al., 2005). Indeed, self-reporting of frequent puddles within a very short distance (2-minute walk) of a household increased an individual’s risk of PCR-confirmed malaria amongst the study cohort. Such environmental variation and its importance in assessing risk of malaria in individuals needs to be taken into account, alongside household- and neighborhood-level malaria prevalence and associated behaviors, in designing effective malaria treatment and eradication efforts.
Conclusions
The results of this longitudinal study of diverse rural and urban sites in Kinshasa Province, DRC, suggest that malaria interventions that target entire households and neighborhoods rather than specific age or high-risk groups may be beneficial. Targeted vector control interventions beyond ITN distributions, such as indoor residual spraying of insecticides, should also be considered as ways to address malaria drivers above the scale of the individual. Household interventions could also address differences in the malaria outcomes we observed by participant age, as well as differences in ITN usage by age, capturing in particular the high malaria prevalence group of older children. Surveys that target only very young children or pregnant mothers, as is commonplace in large malaria surveys, could underestimate malaria prevalence and ignore important household and neighborhood risk factors. Further, effective interventions in settings like the DRC need to include households and communities living in close proximity to risky environments, such as vector breeding sites. Longitudinal surveillance of households across seasons in the present study provided an opportunity to detect temporally varying and multi-scalar influences on malaria and identified potential gaps in effective malaria prevention efforts. Longitudinal analysis of household, neighborhood, and environmental drivers of individual malaria risk can improve malaria control efforts and identify circumstances when bundled interventions are most impactful.
Supplementary Material
Highlights.
Increased household malaria prevalence significantly increases malaria risk in individuals.
Neighborhood malaria prevalence impacts individual risk of malaria after accounting for household malaria prevalence and individual risk factors.
Children experience high malaria prevalence and report low bednet usage.
Malaria control efforts should target households and neighborhoods rather than individuals.
Acknowledgements
We would like to thank Steven Meshnick posthumously for conceiving the study and for his contributions to the analysis and manuscript. We also thank the study participants, members of the Kinshasa School of Public Health field survey teams who diligently conducted follow-up visits and staff who supported the study, and the staff of the local health centers where symptomatic subjects were assessed. We would also like to thank Lauren Levitz and Mark Janko for their efforts to develop study instruments and the database early in the study.
JBP reports grant support from the World Health Organization and Gilead Sciences, non-financial support from Abbott Diagnostics, and honorarium from Virology Education for educational lectures, all outside the scope of the current study. All other authors declare that they have no competing interests.
Competing interests
JBP reports grant support from the World Health Organization and Gilead Sciences, non-financial support from Abbott Diagnostics, and honorarium from Virology Education for educational lectures, all outside the scope of the current study. All other authors declare that they have no competing interests.
Funding
Authors were supported by NIH R01AI107949, NIH R01AI132547, NIH R01 R01AI129812, NIH P2C HD050924, NSF BCS-1339949 and Gates Grand Challenges Explorations Round 17.
Funders have no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
List of Abbreviations
- DBS
dried blood spot
- DHS
demographic and health survey
- DRC
Democratic Republic of the Congo
- FCS
fully conditional specification
- GLMM
generalized linear mixed model
- HRP2
histidine-rich protein 2
- HumTuBB
human beta-tubulin
- ITN
insecticide-treated net
- MAR
missing at random
- MI
multiple imputation
- PCR
polymerase chain reaction
- pfldh
P. falciparum lactate dehydrogenase
- RDT
rapid diagnostic test
Footnotes
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because they contain information that could compromise research participant privacy/consent but are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics approval for the study was granted by the IRB at the University of North Carolina-Chapel Hill, the University of Kinshasa and the University of Iowa.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ADONGO PB, KIRKWOOD B & KENDALL C 2005. How local community knowledge about malaria affects insecticide-treated net use in northern Ghana. Tropical Medicine & International Health, 10, 366–378. [DOI] [PubMed] [Google Scholar]
- AFRANE YA, KLINKENBERG E, DRECHSEL P, OWUSU-DAAKU K, GARMS R & KRUPPA T 2004. Does irrigated urban agriculture influence the transmission of malaria in the city of Kumasi, Ghana? Acta tropica, 89, 125–134. [DOI] [PubMed] [Google Scholar]
- AGYEPONG IA & MANDERSON L 1999. Mosquito avoidance and bed net use in the Greater Accra Region, Ghana. Journal of Biosocial Science, 31, 79–92. [DOI] [PubMed] [Google Scholar]
- AUTHOR, 2017. [details removed for peer review].
- BABALOLA S, ADEDOKUN ST, MCCARTNEY-MELSTAD A, OKOH M, ASA S, TWEEDIE I & TOMPSETT A 2018. Factors associated with caregivers’ consistency of use of bed nets in Nigeria: a multilevel multinomial analysis of survey data. Malaria journal, 17, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BABALOLA S, RICOTTA E, AWANTANG G, LEWICKY N, KOENKER H & TOSO M 2016. Correlates of intra-household ITN use in Liberia: a multilevel analysis of household survey data. PloS one, 11, e0158331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANNISTER-TYRRELL M, VERDONCK K, HAUSMANN-MUELA S, GRYSEELS C, RIBERA JM & GRIETENS KP 2017. Defining micro-epidemiology for malaria elimination: systematic review and meta-analysis. Malaria journal, 16, 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEJON P, WILLIAMS TN, LILJANDER A, NOOR AM, WAMBUA J, OGADA E, OLOTU A, OSIER FH, HAY SI & FÄRNERT A 2010. Stable and unstable malaria hotspots in longitudinal cohort studies in Kenya. PLoS Med, 7, e1000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEJON P, WILLIAMS TN, NYUNDO C, HAY SI, BENZ D, GETHING PW, OTIENDE M, PESHU J, BASHRAHEIL M & GREENHOUSE B 2014. A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. Elife, 3, e02130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOMBLIES A 2012. Modeling the role of rainfall patterns in seasonal malaria transmission. Climatic change, 112, 673–685. [Google Scholar]
- BOUSEMA T, DRAKELEY C, GESASE S, HASHIM R, MAGESA S, MOSHA F, OTIENO S, CARNEIRO I, COX J & MSUYA E 2010. Identification of hot spots of malaria transmission for targeted malaria control. Journal of Infectious Diseases, 201, 1764–1774. [DOI] [PubMed] [Google Scholar]
- BOUSEMA T, GRIFFIN JT, SAUERWEIN RW, SMITH DL, CHURCHER TS, TAKKEN W, GHANI A, DRAKELEY C & GOSLING R 2012. Hitting Hotspots: Spatial Targeting of Malaria for Control and Elimination. PLoS Medicine, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUSEMA T, OKELL L, FELGER I & DRAKELEY C 2014. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nature reviews. Microbiology, 12, 833. [DOI] [PubMed] [Google Scholar]
- CHEN I, CLARKE SE, GOSLING R, HAMAINZA B, KILLEEN G, MAGILL A, O’MEARA W, PRICE RN & RILEY EM 2016. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS medicine, 13, e1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUANG T-W, SOBLE A, NTSHALINTSHALI N, MKHONTA N, SEYAMA E, MTHETHWA S, PINDOLIA D & KUNENE S 2017. Assessment of climate- driven variations in malaria incidence in Swaziland: toward malaria elimination. Malaria journal, 16, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK TD, GREENHOUSE B, NJAMA-MEYA D, NZARUBARA B, MAITEKI-SEBUGUZI C, STAEDKE SG, SETO E, KAMYA MR, ROSENTHAL PJ & DORSEY G 2008. Factors determining the heterogeneity of malaria incidence in children in Kampala, Uganda. Journal of Infectious Diseases, 198, 393–400. [DOI] [PubMed] [Google Scholar]
- COHEE LM, OPONDO C, CLARKE SE, HALLIDAY KE, CANO J, SHIPPER AG, BARGER-KAMATE B, DJIMDE A, DIARRA S, DOKRAS A, KAMYA MR, LUTUMBA P, LY AB, NANKABIRWA JI, NJAGI JK, MAIGA H, MAITEKI-SEBUGUZI C, MATANGILA J, OKELLO G, ROHNER F, ROSCHNIK N, ROUHANI S, SISSOKO MS, STAEDKE SG, THERA MA, TURNER EL, VAN GEERTRUYDEN JP, ZIMMERMAN MB, JUKES MCH, BROOKER SJ, ALLEN E, LAUFER MK & CHICO RM 2020. Preventive malaria treatment among school-aged children in sub-Saharan Africa: a systematic review and meta-analyses. The Lancet Global Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN J 1960. A Coefficient of Agreement for Nominal Scales. Educational and Psychological Measurement, 20, 37–46. [Google Scholar]
- COLÓN-GONZÁLEZ FJ, TOMPKINS AM, BIONDI R, BIZIMANA JP & NAMANYA DB 2016. Assessing the effects of air temperature and rainfall on malaria incidence: an epidemiological study across Rwanda and Uganda. Geospatial health, 11, 18–37. [DOI] [PubMed] [Google Scholar]
- DEGAREGE A, FENNIE K, DEGAREGE D, CHENNUPATI S & MADHIVANAN P 2019. Improving socioeconomic status may reduce the burden of malaria in sub Saharan Africa: A systematic review and meta-analysis. PloS one, 14, e0211205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH-FELDMAN M, HAMAPUMBU H, LUBINDA J, MUSONDA M, KATOWA B, SEARLE KM, KOBAYASHI T, SHIELDS TM, STEVENSON JC, THUMA PE, MOSS WJ & RESEARCH, F. T. S. A. I. C. O. E. F. M. 2018. Efficiency of a Malaria Reactive Test-and-Treat Program in Southern Zambia: A Prospective, Observational Study. The American Journal of Tropical Medicine and Hygiene, 98, 1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH-FELDMAN M, PARR JB, KEELER C, BRAZEAU NF, GOEL V, EMCH M, EDWARDS JK, KASHAMUKA M, TSHEFU AK & MESHNICK SR 2020. What is the burden of malaria in the DRC? The Journal of Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWALD JR, FULLER DO, MÜLLER GC & BEIER JC 2016. A novel method for mapping village-scale outdoor resting microhabitats of the primary African malaria vector, Anopheles gambiae. Malaria journal, 15, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNST KC, HAYDEN MH, OLSEN H, CAVANAUGH JL, RUBERTO I, AGAWO M & MUNGA S 2016. Comparing ownership and use of bed nets at two sites with differential malaria transmission in western Kenya. Malaria journal, 15, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRAO JL, NIQUISSE S, MENDES JM & PAINHO M 2018. Mapping and modelling malaria risk areas using climate, socio-demographic and clinical variables in Chimoio, Mozambique. International Journal of Environmental Research and Public Health, 15, 795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI G, NTUKU HM, SCHMIDLIN S, DIBOULO E, TSHEFU AK & LENGELER C 2016. A malaria risk map of Kinshasa, Democratic Republic of Congo. Malar J, 15, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINDA MF, MOSHI IR, MONROE A, LIMWAGU AJ, NYONI AP, SWAI JK, NGOWO HS, MINJA EG, TOE LP & KAINDOA EW 2019. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PloS one, 14, e0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZMAURICE G, LAIRD N & WARE J 2011. Missing data and dropout: Multiple imputation and weighting methods. Applied Longitudinal Analysis, 515–550. [Google Scholar]
- GAUDART J, POUDIOUGOU B, DICKO A, RANQUE S, TOURE O, SAGARA I, DIALLO M, DIAWARA S, OUATTARA A & DIAKITE M 2006. Space-time clustering of childhood malaria at the household level: a dynamic cohort in a Mali village. BMC Public Health, 6, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERRA M, DE SOUSA B, NDONG-MABALE N, BERZOSA P & AREZ AP 2018. Malaria determining risk factors at the household level in two rural villages of mainland Equatorial Guinea. Malaria journal, 17, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWLEY WA, PHILLIPS-HOWARD PA, TER KUILE FO, TERLOUW DJ, VULULE JM, OMBOK M, NAHLEN BL, GIMNIG JE, KARIUKI SK, KOLCZAK MS & HIGHTOWER AW 2003. COMMUNITY-WIDE EFFECTS OF PERMETHRIN-TREATED BED NETS ON CHILD MORTALITY AND MALARIA MORBIDITY IN WESTERN KENYA. The American Journal of Tropical Medicine and Hygiene, 68, 121–127. [PubMed] [Google Scholar]
- JEDRYCHOWSKI W, PERERA FP, JANKOWSKI J, MROZEK-BUDZYN D, MROZ E, FLAK E, EDWARDS S, SKARUPA A & LISOWSKA-MISZCZYK I 2009. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology, 32, 270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABARIA CW, MOLTENI F, MANDIKE R, CHACKY F, NOOR AM, SNOW RW & LINARD C 2016. Mapping intra-urban malaria risk using high resolution satellite imagery: a case study of Dar es Salaam. International journal of health geographics, 15, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATEERA F, MENS PF, HAKIZIMANA E, INGABIRE CM, MURAGIJEMARIYA L, KARINDA P, GROBUSCH MP, MUTESA L & VAN VUGT M 2015. Malaria parasite carriage and risk determinants in a rural population: a malariometric survey in Rwanda. Malaria journal, 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREUELS B, KOBBE R, ADJEI S, KREUZBERG C, VON REDEN C, BÄTER K, KLUG S, BUSCH W, ADJEI O & MAY J 2008. Spatial variation of malaria incidence in young children from a geographically homogeneous area with high endemicity. Journal of Infectious Diseases, 197, 85–93. [DOI] [PubMed] [Google Scholar]
- KWEKA EJ, KIMARO EE & MUNGA S 2016. Effect of deforestation and land use changes on mosquito productivity and development in Western Kenya Highlands: implication for malaria risk. Frontiers in public health, 4, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAISHRAM DD, SUTTON PL, NANDA N, SHARMA VL, SOBTI RC, CARLTON JM & JOSHI H 2012. The complexities of malaria disease manifestations with a focus on asymptomatic malaria. Malaria journal, 11,29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWETOIJERA DW, KIWARE SS, MAGENI ZD, DONGUS S, HARRIS C, DEVINE GJ & MAJAMBERE S 2013. A need for better housing to further reduce indoor malaria transmission in areas with high bed net coverage. Parasites & vectors, 6, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTERN C, POURETTE D, RABOANARY E, KESTEMAN T, PIOLA P, RANDRIANARIVELOJOSIA M & ROGIER C 2016. “Tazomoka is not a problem”. Local perspectives on malaria, fever case management and bed net use in Madagascar. PloS one, 11, e0151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MBOHOU CN, FOKO LPK, NYABEYEU HN, TONGA C, NONO LK, KANGAM L, BUNDA GW, MBOU IM, NGO HONDT EO & MBE AJK 2019. Malaria screening at the workplace in Cameroon. PloS one, 14, e0225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAKAWA N, MUNGA S, ATIELI F, MUSHINZIMANA E, ZHOU G, GITHEKO AK & YAN G 2005. Spatial distribution of anopheline larval habitats in Western Kenyan highlands: effects of land cover types and topography. The American journal of tropical medicine and hygiene, 73, 157–165. [PubMed] [Google Scholar]
- MINAKAWA N, SEDA P & YAN G 2002. Influence of host and larval habitat distribution on the abundance of African malaria vectors in western Kenya. The American journal of tropical medicine and hygiene, 67, 32–38. [DOI] [PubMed] [Google Scholar]
- MITCHELL CL, TOPAZIAN HM, BRAZEAU NF, DEUTSCH-FELDMAN M, MUWONGA J, SOMPWE E, TSHEFU AK, MWANDAGALIRWA MK, PARR JB & JULIANO JJ 2020. Household prevalence of P. falciparum, P. vivax, and P. ovale in the Democratic Republic of the Congo, 2013–2014. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONROE A, MOORE S, KOENKER H, LYNCH M & RICOTTA E 2019. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-Saharan Africa: a review of the published literature. Malaria journal, 18, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSCIBRODZKI P, DOBELLE M, STONE J, KALUMUNA C, CHIU Y-HM & HENNIG N 2018. Free versus purchased mosquito net ownership and use in Budondo sub-county, Uganda. Malaria journal, 17, 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSHA JF, STURROCK HJ, GREENHOUSE B, GREENWOOD B, SUTHERLAND CJ, GADALLA N, ATWAL S, DRAKELEY C, KIBIKI G & BOUSEMA T 2013. Epidemiology of subpatent Plasmodium falciparum infection: implications for detection of hotspots with imperfect diagnostics. Malaria journal, 12, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSHI IR, NGOWO H, DILLIP A, MSELLEMU D, MADUMLA EP, OKUMU FO, COETZEE M, MNYONE LL & MANDERSON L 2017. Community perceptions on outdoor malaria transmission in Kilombero Valley, Southern Tanzania. Malaria journal, 16, 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MPIMBAZA A, NDEEZI G, KATAHOIRE A, ROSENTHAL PJ & KARAMAGI C 2017. Demographic, socioeconomic, and geographic factors leading to severe malaria and delayed care seeking in Ugandan children: a case–control study. The American journal of tropical medicine and hygiene, 97, 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGATU NR, KANBARA S, RENZAHO A, WUMBA R, MBELAMBELA EP, MUCHANGA SM, MUZEMBO BA, LEON-KABAMBA N, NATTADECH C & SUZUKI T 2019. Environmental and sociodemographic factors associated with household malaria burden in the Congo. Malaria journal, 18, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOOR AM, KIRUI VC, BROOKER SJ & SNOW RW 2009. The use of insecticide treated nets by age: implications for universal coverage in Africa. BMC Public Health, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ODUFUWA OG, ROSS A, MLACHA YP, JUMA O, MMBAGA S, MSELLEMU D & MOORE S 2020. Household factors associated with access to insecticide- treated nets and house modification in Bagamoyo and Ulanga districts, Tanzania. Malaria journal, 19, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGUOMA VM, ANYASODOR AE, ADELEYE AO, ENEANYA OA & MBANEFO EC 2020. Multilevel modelling of the risk of malaria among children aged under five years in Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene. [DOI] [PubMed] [Google Scholar]
- OKIRING J, OLWOCH P, KAKURU A, OKOU J, OCHOKORU H, OCHIENG TA, KAJUBI R, KAMYA MR, DORSEY G & TUSTING LS 2019. Household and maternal risk factors for malaria in pregnancy in a highly endemic area of Uganda: a prospective cohort study. Malaria journal, 18, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKRAH J, TRAORÉ C, PALÉ A, SOMMERFELD J & MÜLLER O 2002. Community factors associated with malaria prevention by mosquito nets: an exploratory study in rural Burkina Faso. Tropical Medicine & International Health, 7, 240–248. [DOI] [PubMed] [Google Scholar]
- ORGANIZATION, W. H. 2016. World malaria report 2016, Geneva, World Health Organization. [Google Scholar]
- PATRICIAN PA 2002. Multiple imputation for missing data. Research in nursing & health, 25, 76–84. [DOI] [PubMed] [Google Scholar]
- PEPRAH S, TENGE C, GENGA IO, MUMIA M, WERE PA, KUREMU RT, WEKESA WN, SUMBA PO, KINYERA T & OTIM I 2019. A Cross-Sectional Population Study of Geographic, Age-Specific, and Household Risk Factors for Asymptomatic Plasmodium falciparum Malaria Infection in Western Kenya. The American journal of tropical medicine and hygiene, 100, 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON I, BORRELL LN, EL-SADR W & TEKLEHAIMANOT A 2009. Individual and household level factors associated with malaria incidence in a highland region of Ethiopia: a multilevel analysis. The American journal of tropical medicine and hygiene, 80, 103–111. [PubMed] [Google Scholar]
- PINCHOFF J, HAMAPUMBU H, KOBAYASHI T, SIMUBALI L, STEVENSON JC, NORRIS DE, COLANTUONI E, THUMA PE & MOSS WJ 2015. Factors associated with sustained use of long-lasting insecticide-treated nets following a reduction in malaria transmission in southern Zambia. The American journal of tropical medicine and hygiene, 93, 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORT G, BOREHAM P & BRYAN JH 1980. The relationship of host size to feeding by mosquitoes of the Anopheles gambiae Giles complex (Diptera: Culicidae). Bulletin of Entomological Research, 70, 133–144. [Google Scholar]
- PULLAN RL, BUKIRWA H, STAEDKE SG, SNOW RW & BROOKER S 2010. Plasmodium infection and its risk factors in eastern Uganda. Malar j, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS D & MATTHEWS G 2016. Risk factors of malaria in children under the age of five years old in Uganda. Malaria journal, 15, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS A, KILLEEn G & SMITH T 2006. Relationships between host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. Am J Trop Med Hyg, 75, 32–7. [DOI] [PubMed] [Google Scholar]
- rulisa s., kateera f., bizimana j. p., agaba s., dukuzumuremyi j., BAAS L, DE dieu HARELIMANA J, MENS PF, BOER KR & DE VRIES PJ 2013. Malaria prevalence, spatial clustering and risk factors in a low endemic area of Eastern Rwanda: a cross sectional study. PloS one, 8, e69443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTSTEIN SO 2015. Steps to constructing the new DHS wealth index. Rockv MD ICF Int. [Google Scholar]
- STOREY JD, BABALOLA SO, RICOTTA EE, FOX KA, TOSO M, LEWICKY N & KOENKER H 2018. Associations between ideational variables and bed net use in Madagascar, Mali, and Nigeria. BMC public health, 18, 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRESMAN G, WHITTAKER C, SLATER HC, BOUSEMA T & COOK J 2020. Quantifying Plasmodium falciparum infections clustering within households to inform household-based intervention strategies for malaria control programs: An observational study and meta-analysis from 41 malaria-endemic countries. PLoS medicine, 17, e1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRESMAN GH, KAMANGA A, MOONO P, HAMAPUMBU H, MHARAKURWA S, KOBAYASHI T, MOSS WJ & SHIFF C 2010. A method of active case detection to target reservoirs of asymptomatic malaria and gametocyte carriers in a rural area in Southern Province, Zambia. Malaria journal, 9, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STURROCK HJ, HSIANG MS, COHEN JM, SMITH DL, GREENHOUSE B, BOUSEMA T & GOSLING RD 2013a. Targeting asymptomatic malaria infections: active surveillance in control and elimination. PLoS medicine, 10, e1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STURROCK HJ, NOVOTNY JM, KUNENE S, DLAMINI S, ZULU Z, COHEN JM, HSIANG MS, GREENHOUSE B & GOSLING RD 2013b. Reactive case detection for malaria elimination: real-life experience from an ongoing program in Swaziland. PloS one, 8, e63830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKKEN W & KNOLS BG 1999. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annual review of entomology, 44, 131–157. [DOI] [PubMed] [Google Scholar]
- THOMSON M, CONNOR S, MILLIGAN P & FLASSE S 1996. The ecology of malaria—as seen from Earth-observation satellites. Annals of Tropical Medicine & Parasitology, 90, 243–264. [DOI] [PubMed] [Google Scholar]
- THOMSON MC, MASON SJ, PHINDELA T & CONNOR SJ 2005. Use of rainfall and sea surface temperature monitoring for malaria early warning in Botswana. The American journal of tropical medicine and hygiene, 73, 214–221. [PubMed] [Google Scholar]
- TOÉ LP, skovmand O, DABIRÉ KR, DIABATÉ A, DIALLO Y, GUIGUEMDÉ TR, DOANNIO JMC, AKOGBETO M, BALDET T & GRUÉNAIS M-E 2009. Decreased motivation in the use of insecticide-treated nets in a malaria endemic area in Burkina Faso. Malaria journal, 8, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN BUUREN S, BRAND JP, GROOTHUIS-OUDSHOORN CG & RUBIN DB 2006. Fully conditional specification in multivariate imputation. Journal of statistical computation and simulation, 76, 1049–1064. [Google Scholar]
- WALLDORF JA, COHEE LM, COALSON JE, BAULENI A, NKANAUNENA K, KAPITO-TEMBO A, SEYDEL KB, ALI D, MATHANGA D & TAYLOR TE 2015. School-age children are a reservoir of malaria infection in Malawi. PLoS One, 10, e0134061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERE V, BUFF AM, DESAI M, KARIUKI S, SAMUELS A, TER KUILE FO, PHILLIPS-HOWARD PA, KACHUR SP & NIESSEN L 2018. Socioeconomic health inequality in malaria indicators in rural western Kenya: evidence from a household malaria survey on burden and care-seeking behaviour. Malaria journal, 17, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


