Abstract
Purpose:
The molecular basis of the tear film and lipid layer alterations in meibomian gland dysfunction (MGD) is unknown. This study aimed to identify and compare (O-acyl)-omega-hydroxy fatty acids (OAHFAs) derived from human meibum and tears in MGD.
Methods:
Of 195 eligible subjects (18–84 years, 62.6% female), 183 and 174 provided samples for tears and meibum, respectively. Subjects were classified into four groups: Normal, Asymptomatic MGD, MGD, and Mixed. Samples from the right eye of each subject were infused into the SCIEX 5600 TripleTOF mass spectrometer in negative ion mode. Lipid intensities identified with Analyst1.7TF and SCIEX LipidView1.3 were normalized by an internal standard and total ion current, then statistically compared in MetaboAnalyst 4.0.
Results:
In meibum and tears, 76 and 78 unique OAHFAs were identified, respectively. The five most frequent and abundant OAHFAs were 18:2/16:2, 18:1/32:1, 18:1/30:1, 18:2/32:1, and 18:1/34:1. Two OAHFAs, 18:2/20:2 and 18:2/20:1, were identified only in tears. Initial univariate analysis revealed three differently regulated OAHFAs in meibum and eight in tears. Partial Least Square Discriminant Analysis showed 18:1/32:1, 18:2/16:2, 18:1/34:1 and 18:0/32:1 in tears, and 18:2/16:2, 18:1/32:1 and 18:2/32:2 in meibum, had variable importance in projection scores >1.5 and contributed the most to the separation of groups. In both meibum and tears, all OAHFAS except 18:2/16:2 were reduced in MGD compared to the normal group.
Conclusion:
MGD is accompanied by differential expression of specific OAHFAs in meibum and tears. These results suggest OAHFAs play a role in the altered biochemical profile of the tear film lipid layer in humans with MGD.
Keywords: Tear film, (o-acyl)-omega-hydroxy fatty acids, lipids, meibum, meibomian glands, dry eye disease
1. Introduction
Normally functioning meibomian glands are a key component of the ocular surface functional unit that maintains tear film (TF) homeostasis. These holocrine glands are imbedded within the tarsal plates, and through their orifices on the eyelid margin, secrete waxy lipid-rich meibum that admixes with the aqueous lacrimal secretion and glycocalyx, and together they form the semi-viscous TF over the ocular surface [1]. In meibomian gland dysfunction (MGD), terminal duct obstruction or qualitative or quantitative changes in glandular secretion occur [2]. Consequently, these pathophysiological alterations in meibum cause TF instability, resulting in symptoms of discomfort and ocular surface diseases, including dry eye disease (DED), a common ocular condition affecting millions worldwide [3,4]. MGD is recognized as a major cause of evaporative dry eye, which exists on an overlapping continuum with aqueous deficient dry eye [5]. Estimates from population-based epidemiological studies indicate a prevalence of MGD as high as 69% and significant comorbidity with DED [6,7], with several clinical and population-based studies reporting positive associations of signs and symptoms and common risk factors between the two disease states [8–10].
The structural and functional properties of the TF are largely determined by the chemical composition of its constituents [11]. The human TF exists as a relatively thin layer (~3–5 μm) [12,13], yet it comprises lipids, proteins, mucins, and electrolytes, and serves several vital functions, including protection against pathogens, lubrication of the ocular surface, and formation of a smooth refractive surface for optimal vision [14,15]. Contrary to the previous view that the human TF is a three-layer sandwich of mucin, aqueous, and lipid components [16], recent evidence points to a two-layer gradient model of the TF [17]. In this model, highly soluble mucins and proteins mix with the aqueous layer containing salts and proteins and form a muco-aqueous phase that occupies the bulk of the TF [18]. Overlying and in part integrated with the muco-aqueous layer is the outermost tear film lipid layer (TFLL) of approximately 40 nm thickness [19].
While a detailed biochemical composition of the TFLL has yet to be established, studies show that the TFLL largely consists of a complex mixture of polar and non-polar lipids [20–22]. In this duplex structure, non-polar lipids (e.g., wax esters, cholesteryl esters, and diesters) form a lipophilic layer at the outermost air-lipid interface, while polar lipids (e.g., phospholipids, sphingolipids, OAHFAs) separate the TFLL from the underlying polar aqueous interface [17,23]. The non-polar lipids of the TFLL primarily function to retard the evaporation of the aqueous tears and may also form a protective barrier against microbes and organic matters [2,24]. The polar lipids likely enhance the spread of hydrophobic/non-polar lipids across the aqueous tears by reducing the surface tension, either directly or through interaction among proteins (e.g., lipocalin, lysozyme, and surfactant proteins) that are intercalated in the outer lipid layer [25–28]. While the lipid composition of meibum and TF is similar in terms of ratios and classes of lipids [29–31], the absence of phospholipids in meibum, in addition to the low quantity of phospholipids in TF has led to the search for other classes of non-polar lipids [32].
Omega-acyl hydroxy fatty acids (OAHFAs) are a more recently identified class of amphiphilic lipids that are structurally similar to wax esters but with an additional carboxyl group and compose 4.4% of total lipids in tears and 3.5% of total lipids in meibum [31,33–35]. It has been proposed that OAHFAs may form a surfactant lipid sublayer in the TFLL and may offer greater stability at the lipid and muco-aqueous interface, as they have a relatively higher abundance in TF than phospholipids [17]. OAHFAs are found in the meibum and TFLL, and they have been shown in in-vitro experiments to induce a polarity gradient at the surface [16,31,36,37]. In support of this notion, in-vitro experiments have shown that OAHFAs readily spread over large surface areas of a muco-aqueous subphase [36–39]. It has also been shown that the relative amount of OAHFAs decreases with an increase in the severity of DED, but that eyelid warming also increased their presence in patients with MGD [34,40]. While the presence of OAHFAs in human meibum and TFLL is well documented [31,34,41–43], and in-vitro experiments point toward their physiological role, the exact in-vivo physiological function of these lipids and their role in the structural and pathophysiological alterations in MGD are still unclear. Furthermore, the precise molecular composition of the TFLL and the underlying biochemical basis of the TFLL changes in MGD are as yet poorly understood in humans.
This study aimed to investigate the association between the presence and quantity of OAHFAs and meibomian gland dysfunction. Recently, we demonstrated the methodology of detecting and quantifying OAHFAs in small volumes of the human TF using high-resolution, direct infusion electrospray ionization-mass spectrometry in negative ion mode [44]. In the present work, this lipidomics methodology was used to identify and compare the abundance of OAHFAs in meibum and TF of human subjects with and without MGD.
2. Materials and Methods
All procedures in the study were conducted according to the principles of the Declaration of Helsinki. Ethical approval was obtained from the University of Alabama at Birmingham Institutional Review Board, and informed consent was obtained from subjects in writing prior to study participation.
2.1. Nomenclature
OAHFAs are large molecular weight molecules of a fatty acid esterified with an omega-hydroxy fatty acid. When these carboxylic acids with long aliphatic chain molecules contain double bonds, they are referred to as being unsaturated. When multiple double bonds are present in the chain these molecules are referred to as polyunsaturated fatty acids. The nomenclature for OAHFAs used throughout the study is based on previous recommendations [45,46]. In brief, the sum composition of OAHFAs is described as OAHFA A: B, where A denotes the total number of carbons and B denotes the degree of unsaturation (total number of double bonds). If the stoichiometry of the fatty acid and hydroxy fatty acid chains are known, OAHFAs are described as OAHFA A1:B1/A2:B2, where A1:B1 denotes the number of carbons and the degree of unsaturation (double bonds) in the fatty acid and A2:B2 denotes the number of carbons and the degree of unsaturation (double bonds) in the ω-hydroxy fatty acid. As an example, Figure 1 illustrates the structure of OAHFA 18:1/32:1 which contains 18 carbons in the fatty acid chain and 32 carbons in the ω-hydroxy fatty acid chain, with one double bond in each chain.
Figure 1.
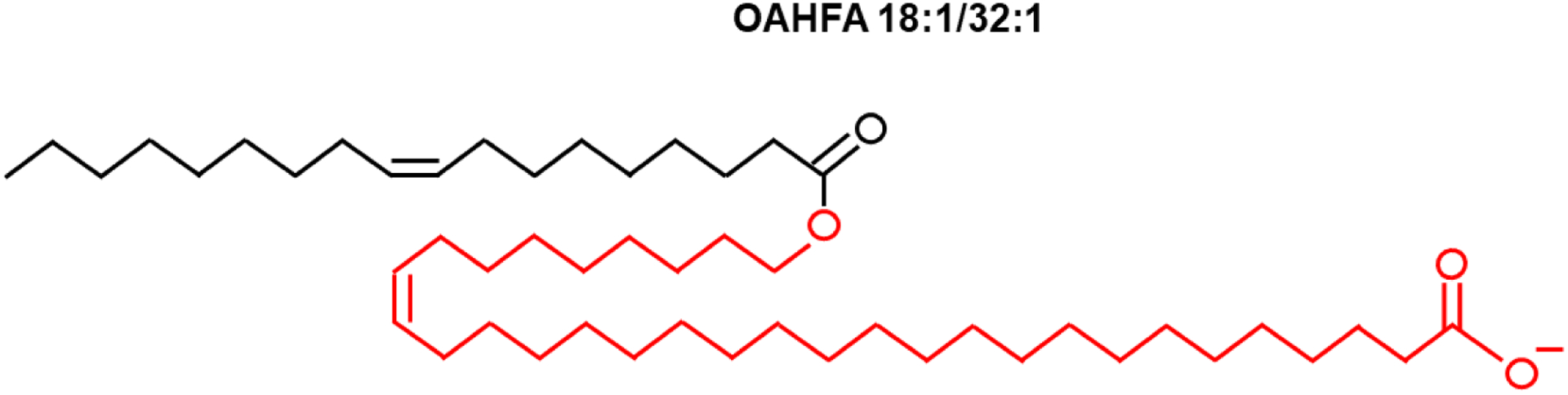
Schematic illustration of the structure of (O-Acyl)-ω-hydroxy fatty acid (OAHFA) 18:1/32:1. OAHFA 18:1/32:1 contains 18 carbons in the fatty acid chain (black) and 32 carbons in the hydroxyl fatty acid chain (red), with one double bond in each chain.
2.2. Human subject recruitment
Subjects were pre-screened for eligibility prior to informed consent and included if they were at least 18 years of age, but were excluded if they were receiving urgent eyecare for ocular diseases except for MGD or dry eye, using any topical or ophthalmic medications, concurrently enrolled in any other ophthalmic clinical research study, or were wearing contact lenses. Based on pre-screening, a total of 222 human subjects were consented from the University of Alabama at Birmingham Academic Medical Center and the University of Alabama at Birmingham Clinical Eye Research Facility IRB approved recruitment database.
2.3. Clinical protocol
All eligible subjects attended a single study visit at the University of Alabama at Birmingham Clinical Eye Research Facility. Following informed consent, a detailed ocular and medical history was taken, and each subject was administered the Ocular Surface Disease Index (OSDI) questionnaire [47]. Subjects then underwent a clinical examination, which included a battery of clinical tests, including visual acuity, slit-lamp examination, meibomian gland assessment, non-invasive Keratograph tear break-up time evaluation, eyelid margin imaging, meibography, lid wiper epitheliopathy, and corneal esthesiometry. Meibomian gland evaluation of each eyelid included infrared meibography using the OCULUS Keratograph 5M, gland expressibility and quality, and lid margin injection. Firm digital pressure was applied to the lower eyelid to determine the expressibility and quality of meibum from the gland orifices. Each gland was then graded in a scale of 0 to 3, with 0 representing clear fluid, 1 representing cloudy liquid, 2 representing cloudy with debris, and 3 representing toothpaste-like thick secretion [48].
The sum of the grades from the central 8 glands was taken as the clinical meibum grade score, which ranged from 0–24. The OSDI and clinical meibum grade scores were used to classify subjects into four study groups similar to the recommendation of the TFOS International Workshop on Meibomian Gland Dysfunction based on the following general criteria: Normal (OSDI<13 and meibum grade <10), MGD (OSDI≥13 and meibum grade ≥10), Asymptomatic MGD (OSDI<13 and meibum grade ≥10), and Mixed (OSDI≥13 and meibum grade <10) [49]. After the completion of the clinical examination, tears and meibum samples were collected and stored dry and frozen in collection tubes before being processed and analyzed using a mass spectrometer. These procedures, including sample collection methods, extraction, and mass spectrometry analyses and protocols have been described extensively in previous papers [35,43,44,50–52] and are not repeated here. A summary description of these protocols is available in the supplementary section (see Supplementary material 1).
2.4. Sample size
The overall sample size estimate for this study was determined on differences in OAHFA abundance based on functional interferometric measures of TF thinning, with the prediction that a reduction in the overall abundance of the OAHFA class of lipids is ultimately associated with an increase in TF thinning (e.g., evaporation). While the interferometric measures are being reported elsewhere, the original sample size calculations revealed that for a 38% reduction in OAHFA abundance and doubling of the TF thinning, using two-sample sample size calculations (power = 80%, alpha = 0.05) showed that 87 subjects were needed per group for two-group comparisons (~190 in total).
2.5. Data analyses
Peak intensities corresponding to lipids identified in meibum and tears after MS data acquisition and processing were exported to Excel spreadsheets. Lipid samples with undetected internal standard were removed from the analyses (meibum, n=8). In each sample, the peak intensity of individual OAHFAs was normalized by the internal standard intensity to obtain standard-corrected intensity. Distribution and relative abundance of OAHFAs in the entire study population and between tears and meibum samples were assessed using the sum total of the normalized intensity. Lipid peak list and intensities were uploaded to MetaboAnalyst 4.0 (www.metaboanalyst.ca, [53]) for data transformation and comparison of OAHFAs across the four study disease classifications. To avoid issues in downstream analyses, zero values were dealt with in a two-step procedure. First, lipids that were not observed in at least 20% of samples in any of the four groups were excluded. Second, all zero values were replaced with a small imputed value (half of the minimum peak intensity). Data corrected by internal standard were then normalized by the total ion current, mean-centered and subjected to Pareto scaling. This data transformation procedure resulted in an approximately normal distribution.
Differences in OAHFAs among the groups were assessed visually using graphical illustrations. In addition, lipidomics data were compared among the groups using both univariate and multivariate methods. Univariate analysis was carried out using a one-way analysis of variance (ANOVA) with a false discovery rate of 0.05 and Fisher Least Significant Difference post-hoc tests for multiple comparisons. Multivariate analyses were performed to describe the separation among groups using the unsupervised method of Principal Component Analysis (PCA) and the supervised method of Partial Least Square Discriminant Analysis (PLSDA). Statistical analyses were carried out in GraphPad Prism (v8.3.0, GraphPad software, San Diego, CA). Data are presented as mean ± standard deviation (SD) unless stated otherwise. P-values less than 0.05 were considered statistically significant.
3. Results
Out of 195 eligible subjects enrolled in the study, 73 subjects (37.4%) were male and 122 subjects (62.6%) were female. The age range of subjects was 18 – 84 years, with a mean age of 39.3 ± 14.3 years. The majority of subjects were Black or African American (50.8%), followed by White or Caucasian (35.9%) and Asian (11.8%). All subjects, except one, were of non-Hispanic ethnicity.
3.1. OAHFA descriptors
Of 195 eligible subjects, OAHFAs were detected in the tears of 183 subjects and the meibum of 174 subjects. For both tear and meibum samples from which no OAFHAs were detected, those subjects were excluded from subsequent analyses. A total of 78 unique OAHFA species were identified in tears and 76 in meibum across all subject samples. Among these lipids, two OAHFAs, 18:2/20:2 and 18:2/20:1, were identified only in tears. Table 1 shows the distribution of the OAHFAs in tears and meibum for the entire subject cohort. In tears, the five most frequently observed OAHFAs were 18:2/16:2, 18:1/32:1, 18:1/30:1, 18:1/34:1, 18:2/32:1. These species also had the highest summed normalized intensities and were the most abundant OAHFAs. In meibum, the five most frequent and abundant OAHFAs were 18:2/16:2, 18:1/32:1, 18:1/30:1, 18:2/32:1, and 18:1/34:1. Compared with the meibum samples, tear samples had a relatively fewer proportion of the total OAHFAs (Figure 2). For example, greater than 50% of tear samples had 21 OAHFAs, whereas an equivalent proportion of meibum samples had 38 OAHFAs. Similarly, 50% or more OAHFAs were detected in 21 tear samples but 100 meibum samples. A total of 48 OAHFAs were detected in 50 or more meibum samples, whereas only 26 OAHFAs were detected in 50 or more tear samples (Figure 2C). In addition, OAHFAs were relatively more abundant in the meibum samples than in the tear samples (Figure 3). When the peak intensities of OAHFAs were compared between meibum and tears in subjects that had OAHFAs present in both samples, the summed normalized intensities of OAHFA species was significantly greater in meibum than in tears (p <0.0001, Figure 4).
Table 1:
Distribution of OAHFAs in tears and meibum.
| Tears (n = 183 subjects) | Meibum (n = 174 subjects) | |
|---|---|---|
| Total OAHFAs | 78 | 76 |
| No. of OAHFAs in at least 50% subjects | 21 | 39 |
| Five most frequent OAHFAs | 18:2/16:2, 18:1/32:1, 18:1/30:1, 18:1/34:1, 18:2/32:1 | 18:2/16:2, 18:1/32:1, 18:1/30:1, 18:2/32:1, 18:1/34:1 |
| Five least frequent OAHFAs | 18:2/28:3, 18:2/25:1, 18:1/23:1, 18:2/18:2, 18:0/31:0 | 16:0/16:1, 16:1/18:1, 18:0/20:0, 18:1/22:2, 18:1/20:1 |
| Five most abundant OAHFAs | 18:2/16:2, 18:1/32:1, 18:1/30:1, 18:1/34:1, 18:2/32:1 | 18:1/32:1, 18:1/30:1, 18:1/34:1, 18:2/32:1, 18:2/16:2 |
| Five least abundant OAHFAs | 18:2/28:3, 18:2/18:2, 18:1/23:1, 18:2/25:2, 18:2/25:1 | 18:1/22:2, 18:1/20:1, 16:0/16:1, 16:1/18:1, 18:1/23:1 |
Note: Abundance is based on sum total of intensity corrected for internal standard
Figure 2.
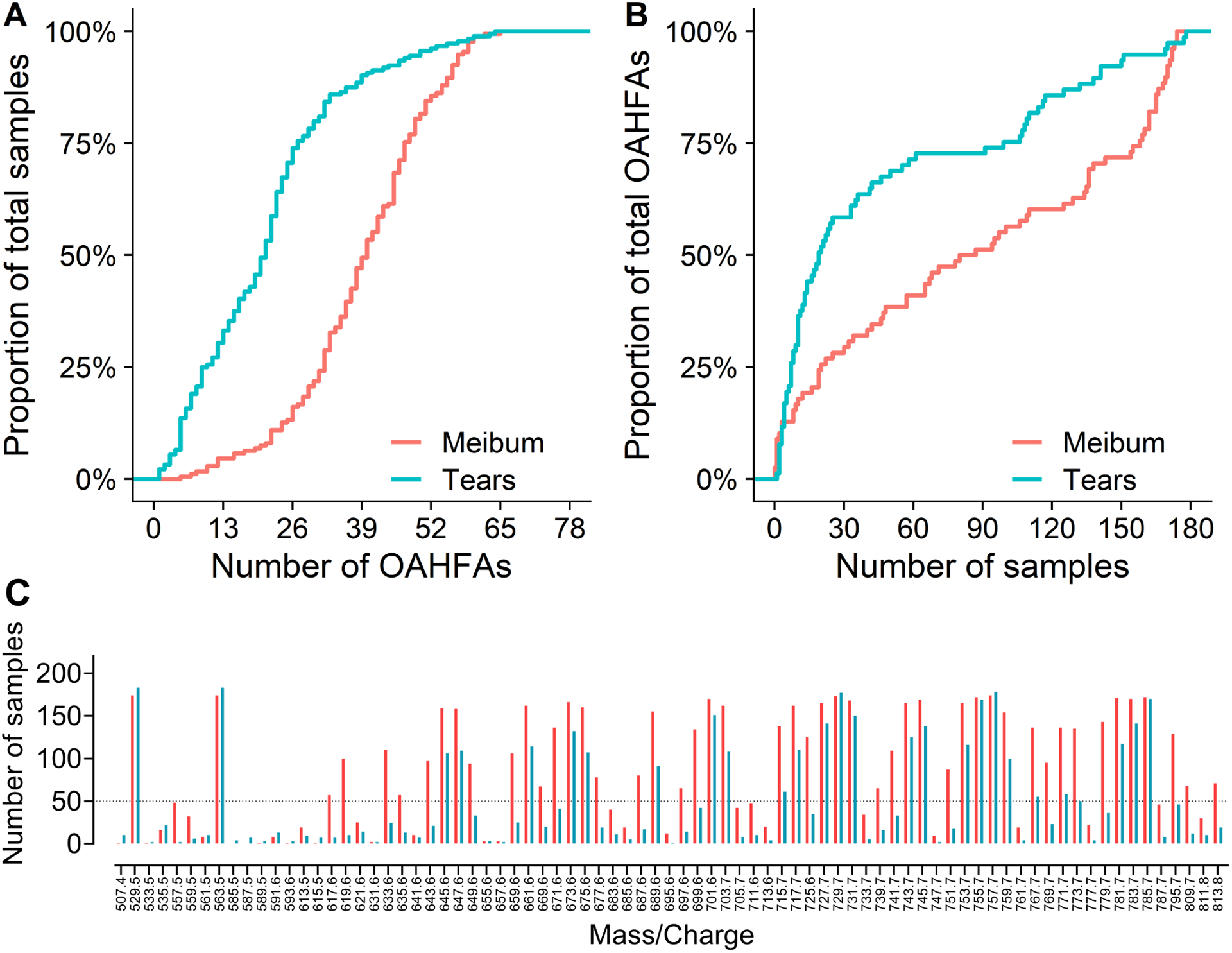
A) Proportion of samples as a function of total number of (O-Acyl)-ω-hydroxy fatty acids (OAHFAs), B) Proportion of OAHFAs as a function of total number of samples, and C) individual OAHFA species in tears (cyan) and meibum (orange). The m/z 563.5 ion in Figure 3 denotes the internal standard. The dotted line represents 50 samples. Tear samples had a relatively fewer OAHFA species than meibum samples. For example, greater than 50% of tear samples had 21 OAHFAs, whereas an equivalent proportion of meibum samples had 38 OAHFAs. Similarly, 50% or more OAHFAs were detected in 21 tear samples but in 100 meibum samples. A total of 48 OAHFAs were detected in 50 or more meibum samples, whereas only 26 OAHFAs were detected in 50 or more tear samples.
Figure 3.
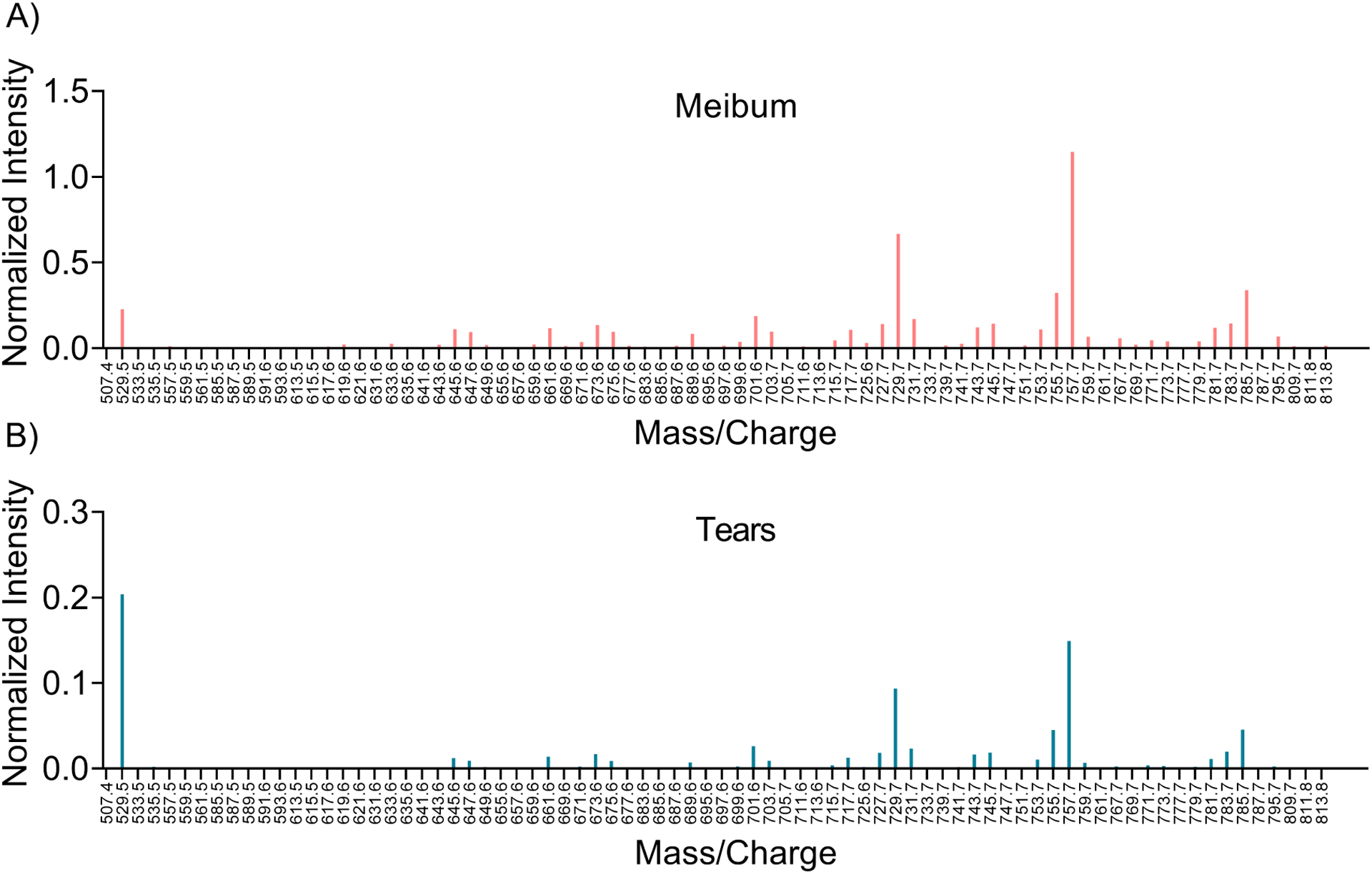
Average intensity profile of the mass spectrometry (MS) peaks identified in meibum and tears samples. Each peak intensity is normalized to the internal standard. The (O-Acyl)-ω-hydroxy fatty acid (OAHFA) peaks associated with meibum samples were stronger than the peaks associated with tears samples, with an approximately 5-fold increase in the scale of mean normalized intensity in meibum compared with tears, but a very similar profile.
Figure 4.
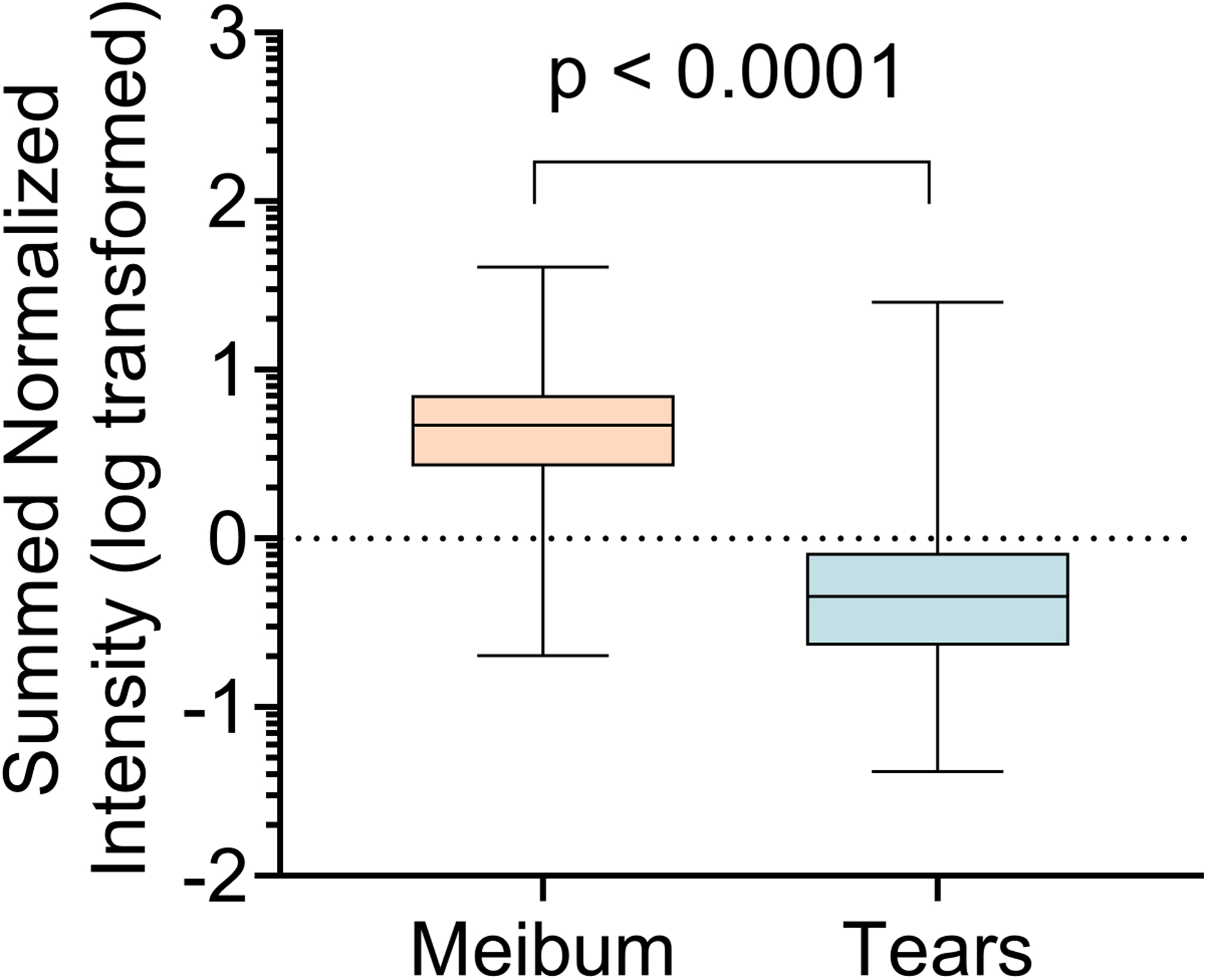
Comparison of the abundance of ω-acyl hydroxy fatty acids (OAHFAs) in tears and meibum samples (n = 167). Abundance in a particular sample is expressed as the total of the individual OAHFA intensities corrected by the internal standard. The abundance of OAHFAs in tears was significantly lower than that in meibum. Data are log-transformed and compared with Wilcoxon matched-pairs signed-rank test.
3.2. Group characteristics
Table 2 describes the demographic and clinical characteristics of the four study groups: Normal, Asymptomatic MGD, Mixed, and MGD. Subjects in the MGD group were slightly older than the other three groups. There was a female preponderance across all groups. The volumes of samples collected from subjects were well matched across the groups (tears: F (3, 179) = 1.14, p = 0.334; meibum: F (3, 170) = 2.36, p = 0.073).
Table 2.
Characteristics of tears and meibum samples in each study group.
| Tears | Meibum | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Mixed | Asx_MGD | MGD | Normal | Mixed | Asx_MGD | MGD | |
| Number of samples | 59 | 46 | 28 | 50 | 55 | 46 | 25 | 48 |
| Male/Female | 19/40 | 17/29 | 11/17 | 22/28 | 19/36 | 17/29 | 12/13 | 21/27 |
| Age, years Mean (SD) | 32.5 (12.2) | 39.5 (13.1) | 39.2 (12.4) | 47.6 (14.7) | 33.3 (13.2) | 39.5 (13.1) | 37.8 (12.1) | 46.7 (14.5) |
| Volume, μL Mean (SD) | 0.029 (0.010) | 0.027 (0.013) | 0.030 (0.009) | 0.026 (0.011) | 0.032 (0.014) | 0.026 (0.010) | 0.026 (0.021) | 0.025 (0.016) |
| OSDI Mean (SD) | 1.3 (2.7) | 47.0 (20.5) | 1.9 (3.1) | 48.8 (20.8) | 1.3 (3.0) | 46.9 (20.4) | 1.9 (3.2) | 49.6 (20.7) |
| Meibum grade Mean (SD) | 4.8 (3.7) | 6.0 (2.9) | 14.3 (2.8) | 16.0 (4.0) | 4.5 (3.7) | 6.0 (2.9) | 14.2 (2.8) | 15.7 (3.9) |
Asx_MGD: Asymptomatic MGD; MGD: Meibomian Gland Dysfunction; OSDI: Ocular Surface Disease Index
3.3. Comparison of OAHFAs across disease classifications
As illustrated in Figure 1, the proportion of total OAHFAs detected in both tears and meibum samples were highly variable across samples. In addition, the frequency and abundance (Figure 3) of OAHFAs in tear samples were less than in the meibum samples. The frequency distribution of the OAHFAs peaked at approximately 25 samples for tears and 45 samples for meibum. This resulted in a significant number of undetected OAHFA species in the majority of the samples. An empirical data filtering technique was employed to deal with zero values, which could be problematic while performing univariate and multivariate analyses. A 20% cut-off criterion was defined for the inclusion of the identified features: OAHFAs present in not more than 20% of the samples in all four groups were deemed as unlikely to be of use in the subsequent models and therefore not considered for further analysis. When the data were filtered based on this criterion, 38 OAHFAs were present in the tears samples and 55 OAHFAs were present in the meibum samples. The analyses in the following sections are based on this filtered data of peak signal intensities from 38 TF-derived and 55 meibum-derived OAHFAs.
An initial analysis performed using univariate ANOVA with a false discovery rate of 0.05 identified three features in the meibum sample and eight features in the tears sample that could be potentially important in discriminating the four disease classification groups. Table 3 shows the details of these OAHFAs. The three meibum-derived OAHFAs that were significantly different across groups were 18:0/23:0, 18:2/16:2, and 18:0/30:1. Among these lipids, the former two were more abundant in the MGD group than the Normal and Mixed groups, whereas 18:0/30:1 was the least abundant in the MGD group. Similarly, the eight TF-derived OAHFAs that were significantly different across the groups were 18:2/33:2, 18:2/16:2, 18:2/35:2, 18:1/32:1, 18:2/33:0, 18:1/33:0, 18:1/34:1, and 18:2/26:1. Among these lipids, 18:2/33:2, 18:2/16:2, 18:2/35:2, 18:1/32:1, 18:2/33:0, and 18:1/34:1, one differed in abundance between the MGD and Normal groups. Out of these six OAHFAs, all except 18:2/16:2 had lower mean intensity in the MGD group than in the Normal group (five of the six OAHFAs were lower in the MGD group compared with the Normal group).
Table 3.
(O-Acyl)-ω-hydroxy fatty acids (OAHFAs) with a significant difference in abundance among the groups as identified by univariate analysis.
| OAHFA | Asymptomatic MGD (A) | MGD (B) | Mixed (C) | Normal (D) | F-value | p-value* | Significant pairs (Fisher’s LSD) |
|---|---|---|---|---|---|---|---|
| Tears | |||||||
| 18:2/33:2 | 0.86 ± 2.00 | −0.64 ± 1.65 | −0.63 ± 1.64 | 0.63 ± 2.02 | 8.24 | <0.001 | A-B; A-C; B-D; C-D |
| 18:2/16:2 | −5.09 ± 10.19 | 3.1 ± 16.19 | 5.28 ± 15.99 | −4.33 ± 8.82 | 6.77 | <0.001 | A-B; A-C; B-D; C-D |
| 18:2/35:2 | 1.01 ± 2.11 | −0.6 ± 1.91 | −0.55 ± 1.84 | 0.45 ± 2.21 | 5.87 | <0.001 | A-B; A-C; B-D |
| 18:1/32:1 | 1.72 ± 6.91 | −1.82 ± 7.45 | −2.09 ± 7.7 | 2.35 ± 6.19 | 5.21 | 0.002 | B-D; C-D |
| 18:2/33:0 | 0.22 ± 1.66 | −0.36 ± 1.94 | −0.64 ± 1.86 | 0.7 ± 2.1 | 4.89 | 0.003 | B-D; C-D |
| 18:1/33:0 | 0.09 ± 1.68 | −0.23 ± 1.76 | −0.65 ± 1.76 | 0.65 ± 2.05 | 4.61 | 0.004 | C-D |
| 18:1/34:1 | 0.78 ± 3.86 | −0.97 ± 5.05 | −1.25 ± 4.71 | 1.43 ± 4.26 | 4.16 | 0.007 | B-D; C-D |
| 18:2/26:1 | 0.48 ± 1.82 | −0.33 ± 1.6 | −0.53 ± 1.69 | 0.47 ± 1.83 | 4.15 | 0.007 | C-D |
| Meibum | |||||||
| 18:0/23:0 | 0.06 ± 1.45 | 0.63 ± 1.52 | −0.46 ± 1.07 | −0.19 ± 1.19 | 6.05 | <0.001 | B-C; B-D |
| 18:2/16:2 | −0.57 ± 7.21 | 3.41 ± 9.27 | −0.95 ± 4.23 | −1.93 ± 6.33 | 5.58 | 0.001 | A-B; B-C; B-D |
| 18:0/30:1 | 0.89 ± 2.38 | −1.15 ± 3.17 | −0.02 ± 2.24 | 0.61 ± 2.57 | 4.91 | 0.003 | A-B; B-C; B-D |
OAHFA: (O-Acyl)-ω-hydroxy fatty acids;
One-way ANOVA with false discovery rate of 0.05.
The exploratory univariate analyses were followed by both unsupervised and supervised multivariate analyses. Figure 4 illustrates the results of the multivariate analyses for the tears samples. In the absence of any information about the groups, PCA analysis showed that when the features were combined linearly, the first and second components explained approximately 72% of the variation in the data. The biplot in Figure 5A showed that, while most samples projected onto a narrow region forming a herd indicating that the samples were unlikely to be dissimilar, a few samples were projected relatively far apart from the herd. Likewise, at least three features were visible with relatively longer vector lengths, indicating that their contributions to the principal components were comparatively greater than other features. In the supervised method, PLS-DA confirmed that the OAHFAs contributed to the slight separation among the groups, as shown in the 3D score plot in Figure 5C. However, there was a relatively large area of overlap across the groups, indicating a high degree of similarity within the clusters. Nonetheless, variable importance in projection (VIP) scores highlighted some OAHFAs that contributed the most to the separation of the groups (Figure 5B). Four OAHFAs had VIP scores greater than 1.5. These four OAHFAs, 18:1/32:1, 18:2/16:2, 18:1/34:1, and 18:0/32:1, were further analyzed for differences between groups in ANOVA models with post-hoc tests. OAHFAs 18:1/32:1 and 18:1/34:1 were reduced in the MGD and Mixed groups compared with the Asymptomatic MGD and Normal groups, whereas OAHFA 18:2/16:2 showed an opposite trend, with a reduction in the Asymptomatic MGD and Normal groups (Figure 6). By contrast, OAHFA 18:0/32:1 showed a trend of reduction in intensity in all three MGD groups relative to the Normal subjects.
Figure 5.
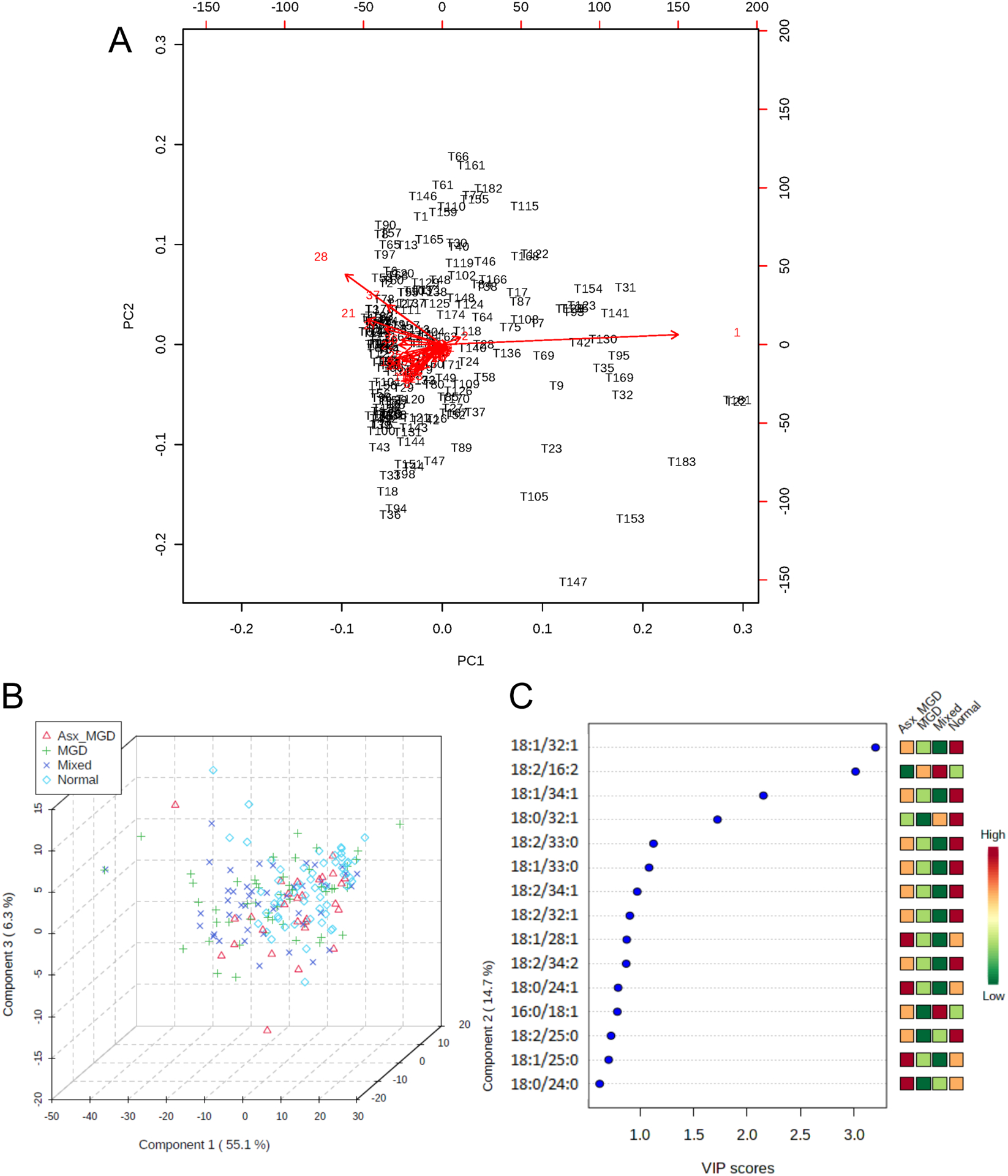
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) of tear film (TF)-derived (O-Acyl)-ω-hydroxy fatty acids (OAHFAs). A) PCA biplot showing samples as black labels and features as red vector trajectories. B) Three-dimensional plot of PLS-DA scores for the first three components (Component 1, Component 2, and Component 3) in the study groups. The proportion of variance accounted for by each component is shown inside brackets in the corresponding axes. C) Variable Importance in Projection (VIP) scores for the top 10 features identified by PLS-DA. Features are ranked by the VIP scores. The color bar on the right illustrates the relative abundance of each feature in the four study groups.
Figure 6.
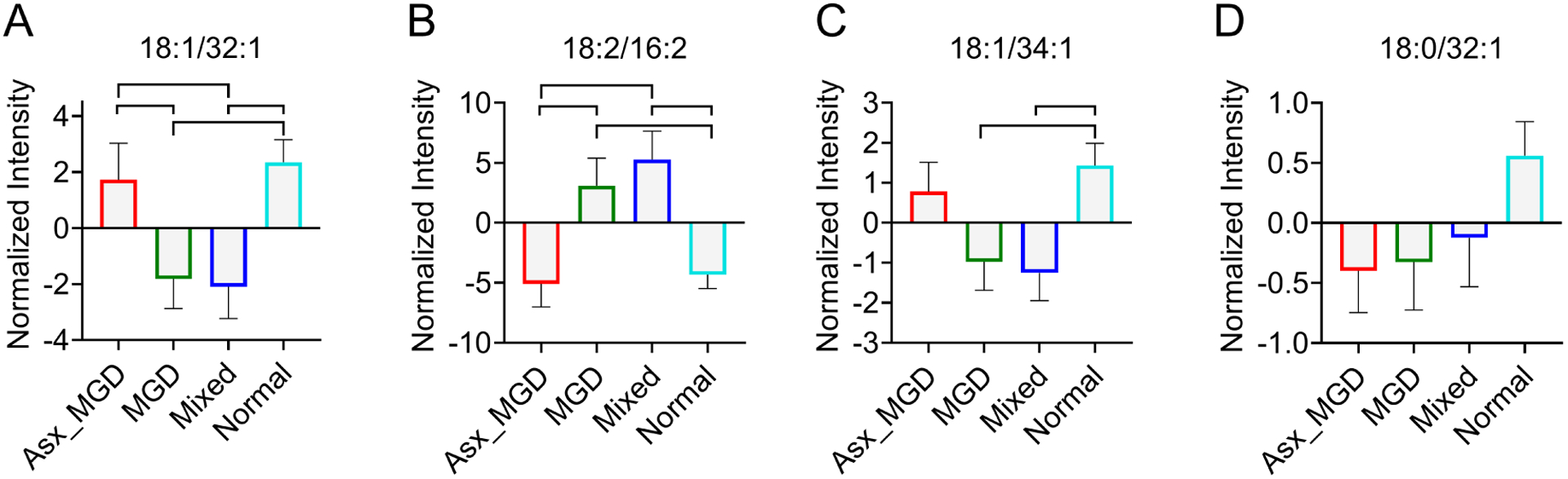
Comparisons of tear film (TF)-derived (O-Acyl)-ω-hydroxy fatty acids (OAHFAs) (A. 18:1/32:1; B. 18:2/16:1; C. 18:1/34:1; D. 18:0/32:1) that have variable importance in projection scores greater than 1.5 in partial least squares discriminant analysis (PLS-DA). Group-pairs with statistically significant differences in intensities are indicated with shapes.
Multivariate analyses for meibum samples revealed a similar pattern of results as in the tears samples. PCA showed that the first two components captured nearly half of the variance in the data. Like the biplot for the tears data, the biplot for the meibum data (Figure 7A) showed a similar narrow grouping of samples, although a relatively larger number of samples showed a tendency to separate from the herd compared with the data for tears. A few features were again visible as the ones contributing greater than other features to the principal components. When group information was provided to the multiple regression model, PLS-DA again exhibited a largely similar pattern of clustering, similar to the TF sample data. The OAHFAs contributed to some separation among the groups, but there was a relatively large area of overlap across the groups, suggesting a high degree of similarity within the clusters. However, the MGD group showed a much greater separation from the other three clusters, as visible in the 3D score plot in Figure 7B. Altogether, three features were identified as having VIP scores greater than 1.5 and likely contributing the most to the separation among the groups (Figure 7C). These OAHFAs were 18:2/16:2, 18:1/32:1, and 18:2/32:2. OAHFA 18:2/16:2 showed an increase in the MGD group relative to the Mixed and Normal groups, while the latter two OAHFAs, 18:1/32:1, and 18:2/32:2, were significantly reduced in the MGD group compared with the Normal subjects (Figure 8).
Figure 7.
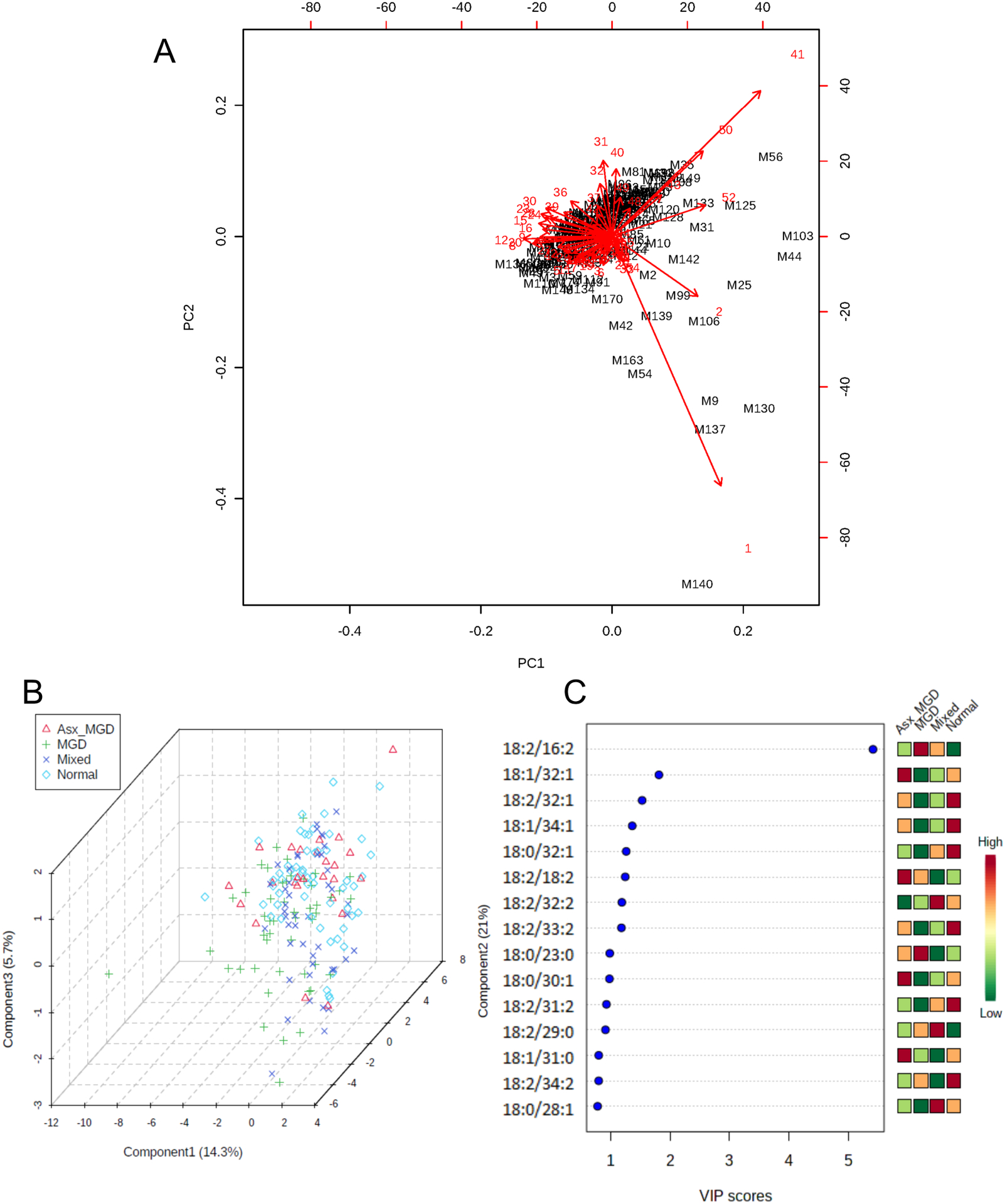
Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) of meibum-derived (O-Acyl)-ω-hydroxy fatty acids (OAHFAs). A) PCA biplot showing samples as black labels and features as red vector trajectories. B) Three-dimensional plot of PLS-DA scores for the first three components (Component 1, Component 2, and Component 3) in the study groups. The proportion of variance accounted for by each component is shown inside brackets in the corresponding axes. C) Variable Importance in Projection (VIP) scores for the top 10 features identified by PLS-DA. Features are ranked by the VIP scores. The color bar on the right illustrates the relative abundance of each feature in the four study groups.
Figure 8.
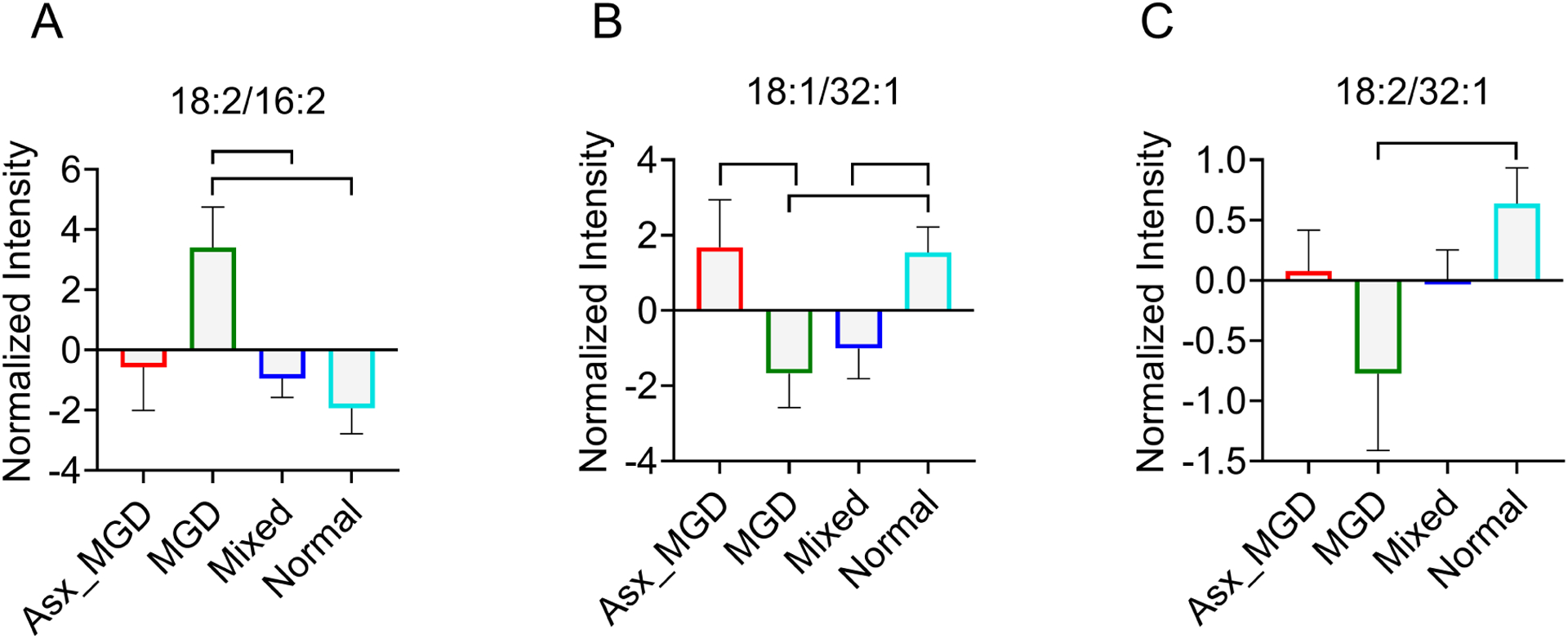
Comparisons of meibum-derived (O-Acyl)-ω-hydroxy fatty acids (OAHFAs) (A. 18:2/16:2; B. 18:1/32:1; C. 18:2/32:1) that have variable importance in projection scores greater than 1.5 in partial least squares discriminant analysis (PLS-DA). Group-pairs with statistically significant differences in intensities are indicated with shapes.
4. Discussion
Lipids derived from meibomian gland secretion (meibum) compose the outermost TFLL and play a critical role in retarding evaporation and preventing destabilization of the TF leading to DED. This research examined the relative abundance of the polar OAHFA lipids in meibum and TF in healthy subjects and those with MGD so as to explore the role of the OAHFAs in the structure and function of the TFLL. The results of the study show that dysfunction of the meibomian glands is associated with differential regulation of several specific OAHFAs in both tears and meibum. These findings suggest that OAHFAs may potentially underlie the altered biochemical profile of the TFLL in MGD, perhaps resulting in physical alterations of the TF leading to disease.
It is well established that qualitative and quantitative alterations of lipid-enriched meibomian gland secretion account for the vast majority of evaporative dry eye disease that is characterized by the destabilization of the TF and excessive tear evaporation leading to loss of ocular surface homeostasis [54]. Studies have previously shown a reduction in the abundance of several polar and non-polar lipids in MGD and DED [34,55–57]. However, the functional consequences of these biochemical changes remain to be reproducibly established. There remains a paucity of evidence on whether the alterations of these lipid classes translate to impaired functionality of the TFLL as a barrier between the environment and aqueous tears to prevent tear evaporation. Recently, it has become evident that, despite occurring in considerably less abundance than non-polar lipids [34,52,58], polar lipids OAHFAs may provide stability to TF by creating an interface between the non-polar lipid sublayer and muco-aqueous phase [33]. More recent studies have shown that OAHFAs form a stable monolayer to reduce surface tension at the muco-aqueous interface and may primarily contribute to suppress excessive evaporation of water from the TF [36,37,39]. This may not necessarily be due to the action of OAHFAs in stabilizing the non-polar lipid layer in the TF, but “through a direct evaporation resistant effect” [39].
Some evidence for a role of OAHFAs in maintaining TF structure and function comes from a recent animal study that shows mice deficient in FA ω-hydroxylase Cyp4f39, which contributes to OAHFA production, exhibit dry eye accompanied by meibomian gland obstruction, TF destabilization, in addition to the reduction of OAHFAs and their derivatives [36]. It has also been shown in humans that eyelid warming treatment in MGD patients for 12 weeks leads to an appreciable increase in OAHFAs in tear fluid [40]. Our results corroborate these indirect findings in suggesting a potentially critical role of OAHFAs in maintaining the biochemical homeostasis of the TFLL. When this homeostasis was altered, for example in MGD, several OAHFAs were found to be differentially regulated. Both supervised and unsupervised analytical methods revealed that the relative abundance of OAHFAs contributed to some separation of the MGD study groups from normal healthy subjects. The TF-derived OAHFAs contributing the most to the separation among groups were identified as 18:1/32:1, 18:1/34:1, 18:2/16:2, and 18:0/32:1. The former two OAHFAs were reduced in MGD patients, while the latter two were increased relative to their abundance in normal subjects. Similarly, the meibum-derived OAHFAs contributing the most to the separation among groups were 18:2/16:2, 18:1/32:1, and 18:2/32:2. OAHFA 18:2/16:2 was again increased in the MGD group, while the latter two OAHFA were reduced. Because OAHFA 18:1/32:1 and 18:2/16:2 showed similar trends regardless of the type of sample (i.e., TF or meibum), these OAHFAs could serve as potential biomarkers for the altered biochemical composition of the TFLL. Despite having similar meibum scores, the asymptomatic MGD group had a significantly greater abundance of OAHFA 18:1/32:1 in both tears and meibum but a significantly lower abundance of OAHFA 18:2/16:2 than the MGD group. The contrasting profile of OAHFA abundance between the two MGD groups suggests that perhaps the concentration of OAHFA varies with neurotrophic factors that may cause a dysfunctional sensation in MGD. Interestingly, all of the candidate OAHFAs identified in this study are unsaturated lipids, suggesting that the presence of one or more double bonds, resulting in greater solubility and lower melting point, may be important features of OAHFAs involved in regulating TFLL function. For instance, the saturated OAHFAs with long chains may interdigitate better between the muco-aqueous phase and the non-polar lipid layer than the unsaturated OAHFAs with one or more double bonds. This interdigitating connection may become weak in conditions where tear film instability is compromised, such as MGD.
Using a global mass spectrometry lipidomics approach, this study identified 78 unique OAHFAs in TF and 76 in meibum. Although the numbers of TF and meibum-derived OAHFAs in this study agree well with some previous reports [31,41], other studies have identified unique OAHFAs to a considerably less extent [30,34,40,55,59]. The exact reason for the discrepancy across studies is difficult to ascertain but could be due to the inherent biological variability across samples. Alternatively, the discrepancy could be brought about by the difference in analytical approaches. To extract and identify lipids from human meibum and TF, previous studies have used a wide range of methods, including electrospray ionization-multiple reaction monitoring either with a direct infusion of samples into mass spectrometer [31] or combined with high-performance liquid chromatography [55]. A high number of unique OAHFAs in this study is not surprising, however, because a comprehensive approach was utilized to elucidate as many lipids as possible. This approach is known to yield a large number of lipids, as illustrated by a previous study that had identified a considerably large number of OAHFAs (>190) in human meibum [43]. Despite the differences in the absolute number of OAHFAs, there is some concordance regarding the abundance of OAHFAs observed in our study with published reports. As observed in this study, several previous studies have identified 18:1/32:1 and 18:1/30:1, and 18:1/34:1 as the most abundant OAHFAs in meibum and tear fluid [31,55,59].
Consistent with the finding from a previous study [50], OAHFAs derived from meibum samples demonstrated significantly stronger intensities than the tear samples, with approximately a 5-fold difference in intensity. In addition, meibum consistently exhibited a greater number of OAHFAs than tears. For instance, 48 OAHFAs were detected in 50 or more meibum compared with only 26 in 50 or more tears. This is slightly surprising given that both meibum and tear samples exhibited an approximately equal number of unique OAHFAs. Also, the lipidomic profile of human meibum and TF is similar, except for phospholipids, which have relatively more abundance in tear fluid [31].
While the results of this study suggest that alterations in OAHFAs are associated with meibomian gland function and may potentially serve a physiological role in mediating the biochemical changes in the TFLL patients with MGD, the findings need to be interpreted in the context of its design. For example, although the implementation of the direct infusion approach facilitates characterization and quantification of OAHFAs on a large scale with stability and reproducibility [44], the 1:1 signal to noise ratio used in this study may have inadvertently led to an increased possibility of false positives. Therefore, further confirmation through approaches such as multiple reaction monitoring MS analyses seems necessary. It has also been suggested that combining liquid chromatography with MS may improve the identification and quantification of lipids and that the implementation of MS without a prior chromatographic step may lead to dilution of signals from OAHFAs with signals of their cholesterol esters [42]. However, the optimal approach remains a matter of debate [60], as high-resolution untargeted MS with direct infusion positive and negative ion modes per se have been reported to yield reliable and highly sensitive assessments of human tear and meibum-derived lipids, including OAHFAs [43,44,50], given their ‘softer’ ionization. It is also important to note that, although the study identified 78 unique OAHFAs in tears and 76 in meibum, the univariate and multivariate group comparisons included only those OAHFAs that were present in at least 20% of the samples in the four groups so as to avoid downstream issues while performing robust unsupervised and supervised statistical analyses. Nonetheless, this is one of the largest studies to date evaluating the lipidomics of the TF and meibum in normal and MGD patients, and therefore provides further insight into the physiological role of OAHFAs in TF structure and function including the molecular basis of TF alterations in MGD related DED. Further analysis of lipid components and lipid-lipid interactions are warranted to further advance this important aspect of ocular surface research.
Supplementary Material
5. Acknowledgments
The authors acknowledge all individuals involved for their contribution in participant recruitment, data collection, and data management for this study in addition to the participants who provided their time to participate in the clinical visit.
6. Financial Disclosures
Safal Khanal: None
William Ngo: William Ngo is an employee of the Centre for Research & Education (CORE). Over the past three years, members of CORE have received funding from the following companies: Alcon, Allergan, CooperVision, GL Chemtec, IMedPharma, Johnson & Johnson Vision, Lubris, Menicon, Nature’s Way, Novartis, Oté, PS Therapy, Safilens, Santen, Shire, SightGlass, Visioneering, and Visu.
Kelly K. Nichols: In the past 12 months, Dr. Nichols has consulted for and received honorarium from: Bruder, Dompe, Kala, Novartis/Shire (Medical Exchange International), Osmotica, Oyster Point, Sight Sciences, Tear Film Innovations/Alcon/Acquiom, Thea, Tarsus, and TopiVert. Research funding has been received from: Allergan, Kala, and Tear Science, Also, Dr. Jason Nichols is the spouse of Dr. Kelly Nichols, extending her declarations to him.
Landon Wilson: None
Stephen Barnes: None
Jason J. Nichols: In 2019 and 2020, Dr. Jason J. Nichols has received honoraria from Paragon Vision Sciences and Coopervision. He has also received research funding from Alcon, Bruder, Johnson and Johnson Vision, and Mallinckrodt over the last 3 years. Also, Dr. Kelly Nichols is the spouse of Dr. Jason Nichols, extending her declarations to him.
Funding support:
This work was funded by NIH/NEI R01EY026947, partially supported by NIH/NCATs UL1 TR003096. The mass spectrometer was purchased from funding by a NIH Shared Instrumentation Grant S10 RR027822 to SB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 2011;52:1938–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci 2011;52:1922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stapleton F, Alves M, Bunya VY, Jalbert I, Lekhanont K, Malet F, et al. TFOS DEWS II Epidemiology Report. Ocul Surf 2017;15:334–65. [DOI] [PubMed] [Google Scholar]
- [4].Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 2011;52:1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nelson JD, Craig JP, Akpek EK, Azar DT, Belmonte C, Bron AJ, et al. TFOS DEWS II Introduction. Ocul Surf 2017;15:269–75. [DOI] [PubMed] [Google Scholar]
- [6].Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52:1994–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf 2009;7:S1–14. [DOI] [PubMed] [Google Scholar]
- [8].Viso E, Gude F, Rodríguez-Ares MT. The association of meibomian gland dysfunction and other common ocular diseases with dry eye: a population-based study in Spain. Cornea 2011;30:1–6. [DOI] [PubMed] [Google Scholar]
- [9].Tong L, Chaurasia SS, Mehta JS, Beuerman RW. Screening for meibomian gland disease: its relation to dry eye subtypes and symptoms in a tertiary referral clinic in singapore. Invest Ophthalmol Vis Sci 2010;51:3449–54. [DOI] [PubMed] [Google Scholar]
- [10].Rosman M, Wong TY, Tay W-T, Tong L, Saw S-M. Prevalence and Risk Factors of Undercorrected Refractive Errors among Singaporean Malay Adults: The Singapore Malay Eye Study. Investigative Opthalmology & Visual Science 2009;50:3621. 10.1167/iovs.08-2788. [DOI] [PubMed] [Google Scholar]
- [11].Cwiklik L. Tear film lipid layer: A molecular level view. Biochim Biophys Acta 2016;1858:2421–30. [DOI] [PubMed] [Google Scholar]
- [12].Azartash K, Kwan J, Paugh JR, Nguyen AL, Jester JV, Gratton E. Pre-corneal tear film thickness in humans measured with a novel technique. Mol Vis 2011;17:756–67. [PMC free article] [PubMed] [Google Scholar]
- [13].Werkmeister RM, Alex A, Kaya S, Unterhuber A, Hofer B, Riedl J, et al. Measurement of tear film thickness using ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 2013;54:5578–83. [DOI] [PubMed] [Google Scholar]
- [14].Bron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Exp Eye Res 2004;78:347–60. [DOI] [PubMed] [Google Scholar]
- [15].Pflugfelder SC, Stern ME. Biological functions of tear film. Exp Eye Res 2020;197:108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Tear Film Lipids and Lipid–Protein Interactions in Health and Disease. Invest Ophthalmol Vis Sci 2011;52:1979–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Willcox MDP, Argüeso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, et al. TFOS DEWS II Tear Film Report. Ocul Surf 2017;15:366–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res 2004;78:379–88. [DOI] [PubMed] [Google Scholar]
- [19].King-Smith PE, Hinel EA, Nichols JJ. Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci 2010;51:2418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Butovich IA. Lipidomics of human Meibomian gland secretions: Chemistry, biophysics, and physiological role of Meibomian lipids. Prog Lipid Res 2011;50:278–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen J, Nichols KK. Erratum: Comprehensive shotgun lipidomics of human meibomian gland secretions using MS/MSall with successive switching between acquisition polarity modes. J Lipid Res 2019;60:1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rantamäki AH, Seppänen-Laakso T, Oresic M, Jauhiainen M, Holopainen JM. Human tear fluid lipidome: from composition to function. PLoS One 2011;6:e19553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res 2003;26:89–94. [DOI] [PubMed] [Google Scholar]
- [24].Craig JP, Tomlinson A. Importance of the lipid layer in human tear film stability and evaporation. Optom Vis Sci 1997;74:8–13. [DOI] [PubMed] [Google Scholar]
- [25].Rantamäki AH, Holopainen JM. The Effect of Phospholipids on Tear Film Lipid Layer Surface Activity. Invest Ophthalmol Vis Sci 2017;58:149–54. [DOI] [PubMed] [Google Scholar]
- [26].Millar TJ, Mudgil P, Butovich IA, Palaniappan CK. Adsorption of human tear lipocalin to human meibomian lipid films. Invest Ophthalmol Vis Sci 2009;50:140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mudgil P, Millar TJ. Adsorption of apo- and holo-tear lipocalin to a bovine Meibomian lipid film. Exp Eye Res 2008;86:622–8. [DOI] [PubMed] [Google Scholar]
- [28].Miano F, Calcara M, Millar TJ, Enea V. Insertion of tear proteins into a meibomian lipids film. Colloids Surf B Biointerfaces 2005;44:49–55. [DOI] [PubMed] [Google Scholar]
- [29].Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film--A review. Exp Eye Res 2015;137:125–38. [DOI] [PubMed] [Google Scholar]
- [30].Lam SM, Tong L, Reux B, Duan X, Petznick A, Yong SS, et al. Lipidomic analysis of human tear fluid reveals structure-specific lipid alterations in dry eye syndrome. J Lipid Res 2014;55:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brown SHJ, Kunnen CME, Duchoslav E, Dolla NK, Kelso MJ, Papas EB, et al. A comparison of patient matched meibum and tear lipidomes. Invest Ophthalmol Vis Sci 2013;54:7417–24. [DOI] [PubMed] [Google Scholar]
- [32].Butovich IA. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res 2009;28:483–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res 2009;50:2471–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lam SM, Tong L, Yong SS, Li B, Chaurasia SS, Shui G, et al. Meibum lipid composition in Asians with dry eye disease. PLoS One 2011;6:e24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci 2010;51:6220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Miyamoto M, Sassa T, Sawai M, Kihara A. Lipid polarity gradient formed by ω-hydroxy lipids in tear film prevents dry eye disease. Elife 2020;9. 10.7554/eLife.53582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bland HC, Moilanen JA, Ekholm FS, Paananen RO. Investigating the role of specific tear film lipids connected to dry eye syndrome: A study on O-acyl-ω-hydroxy fatty acids and diesters. Langmuir 2019;35:3545–52. [DOI] [PubMed] [Google Scholar]
- [38].Schuett BS, Millar TJ. An investigation of the likely role of (O-acyl) ω-hydroxy fatty acids in meibomian lipid films using (O-oleyl) ω-hydroxy palmitic acid as a model. Exp Eye Res 2013;115:57–64. [DOI] [PubMed] [Google Scholar]
- [39].Paananen RO, Viitaja T, Olżyńska A, Ekholm FS, Moilanen J, Cwiklik L. Interactions of polar lipids with cholesteryl ester multilayers elucidate tear film lipid layer structure. Ocul Surf 2020;18:545–53. [DOI] [PubMed] [Google Scholar]
- [40].Lam SM, Tong L, Duan X, Acharya UR, Tan JH, Petznick A, et al. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. J Lipid Res 2014;55:1959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mori N, Fukano Y, Arita R, Shirakawa R, Kawazu K, Nakamura M, et al. Rapid identification of fatty acids and (O-acyl)-ω-hydroxy fatty acids in human meibum by liquid chromatography/high-resolution mass spectrometry. J Chromatogr A 2014;1347:129–36. [DOI] [PubMed] [Google Scholar]
- [42].Butovich IA. On the presence of (O-acyl)-omega-hydroxy fatty acids and of their esters in human meibomian gland secretions. Invest Ophthalmol Vis Sci 2011;52:639–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen J, Nichols KK. Comprehensive shotgun lipidomics of human meibomian gland secretions using MS/MSall with successive switching between acquisition polarity modes. Journal of Lipid Research 2018;59:2223–36. 10.1194/jlr.d088138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chen J, Nichols KK, Wilson L, Barnes S, Nichols JJ. Untargeted lipidomic analysis of human tears: A new approach for quantification of O-acyl-omega hydroxy fatty acids. Ocul Surf 2019;17:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liebisch G, Vizcaíno JA, Köfeler H, Trötzmüller M, Griffiths WJ, Schmitz G, et al. Shorthand notation for lipid structures derived from mass spectrometry. J Lipid Res 2013;54:1523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Marshall DL, Saville JT, Maccarone AT, Ailuri R, Kelso MJ, Mitchell TW, et al. Determination of ester position in isomeric (O-acyl)-hydroxy fatty acids by ion trap mass spectrometry. Rapid Commun Mass Spectrom 2016;30:2351–9. [DOI] [PubMed] [Google Scholar]
- [47].Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118:615–21. [DOI] [PubMed] [Google Scholar]
- [48].Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. EYE 1991;5 (Pt 4):395–411. [DOI] [PubMed] [Google Scholar]
- [49].Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci 2011;52:2006–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ngo W, Chen J, Panthi S, Nichols KK, Nichols JJ. Comparison of collection methods for the measure of human meibum and tear film-derived lipids using mass spectrometry. Curr Eye Res 2018;43:1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Haworth KM, Nichols JJ, Thangavelu M, Sinnott LT, Nichols KK. Examination of human meibum collection and extraction techniques. Optom Vis Sci 2011;88:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chen J, Green KB, Nichols KK. Quantitative profiling of major neutral lipid classes in human meibum by direct infusion electrospray ionization mass spectrometry. Invest Ophthalmol Vis Sci 2013;54:5730–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, et al. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 2018;46:W486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf 2017;15:438–510. [DOI] [PubMed] [Google Scholar]
- [55].Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles. J Lipid Res 2014;55:289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Borchman D, Ramasubramanian A, Foulks GN. Human meibum cholesteryl and wax ester variability with age, sex, and meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2019;60:2286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen J, Keirsey JK, Green KB, Nichols KK. Expression profiling of nonpolar lipids in meibum from patients with dry eye: A pilot study. Invest Ophthalmol Vis Sci 2017;58:2266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Butovich IA. Meibomian glands, meibum, and meibogenesis. Exp Eye Res 2017;163:2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hancock SE, Ailuri R, Marshall DL, Brown SHJ, Saville JT, Narreddula VR, et al. Mass spectrometry-directed structure elucidation and total synthesis of ultra-long chain (O-acyl)-ω-hydroxy fatty acids. J Lipid Res 2018;59:1510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen J, Green-Church KB, Nichols KK. Author response: On the presence of (O-acyl)-omega-hydroxy fatty acids and their esters in human meibomian gland secretions. Invest Ophthalmol Vis Sci 2011;52:1894–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


