Abstract
Medicine has been a great beneficiary of the nanotechnology revolution. Nanotechnology involves the synthesis of functional materials with at least one size dimension between 1 and 100 nanometers. Advances in the field have enabled the synthesis of bio-nanoparticles that can interface with physiological systems to modulate fundamental cellular processes. One example of a diverse acting nanoparticle-based therapeutic is synthetic high-density lipoprotein (HDL) nanoparticles (NP), which have great potential for treating diseases of the ocular surface. Our group has developed a spherical HDL NP using a gold nanoparticle core. HDL NPs: (i) closely mimic the physical and chemical features of natural HDLs; (ii) contain apoA-I; (iii) bind with high-affinity to SR-B1, which is the major receptor through which HDL modulates cell cholesterol metabolism and controls the selective uptake of HDL cargo into cells; (iv) are non-toxic to cells and tissues; and (v) can be chemically engineered to display nearly any surface or core composition desired. With respect to the ocular surface, topical application of HDL NPs accelerates re-epithelization of the cornea following wounding, attenuates inflammation resulting from chemical burns and/or other stresses, and effectively delivers microRNAs with biological activity to corneal cells and tissues. HDL NPs will be the foundation of a new class of topical eye drops with great translational potential and exemplify the impact that nanoparticles can have in medicine.
Keywords: chemical burn, cholesterol, cornea, eye drop, inflammation, lipoprotein, microRNA, nanotechnology, wound healing
1. PAST
Nanotechnology and nanoparticles are part of our everyday lexicon; however, sixty years ago these terms were at best only hypotheses. The concept of nanotechnology is ascribed to the Noble Laureate, Richard Feynman, who, on New Year’s Eve in 1959 postulated that extraordinarily small functional materials could be constructed through the controlled assembly of individual atoms. In an iconic speech entitled, “There’s Plenty of Room at the Bottom,” Feynman discussed powerful opportunities and potential uses for such materials. For instance in biomedicine, he suggested a new approach to synthesizing drugs targeted to the site of disease that, upon arrival, would perform therapeutic tasks at the molecular level [1]. Fifteen years later, the term “nanotechnology” was first used and defined as the processing or separation, consolidation, and deformation of materials by one atom or one molecule [2]. Generally, the goals of scientists in this new field were to synthesize, characterize, assemble and ascribe function to nanoscale materials. A nanoparticle is customarily defined as a particle of matter that has at least one size dimension between 1 and 100 nanometers (nm) [3]. Because the radii of individual atoms are measured on the picometer (10−12 m) or, more commonly, ångström (10−10m) scale, one can conceptualize that rationally assembling atoms results in nanoscale (10−9m) materials.
Owing to the extraordinarily small size, the development and improvement of scientific tools was critical and, often, preceded or occurred simultaneously with important advances in nanotechnology. Interestingly, prior to the invention of new instruments, some of the novel properties of nanoparticles were appreciated as far back as the fourth century AD. Nanoparticles made of gold and silver, for instance, were used in the Lycurgus Cup and in stained glass windows because of their brilliant color and stability to photobleaching [4]. It was not until the mid-to-late 1980s, using scanning tunneling (STM) and atomic force (AFM) microscopes that silicon, xenon and carbon atoms were resolved in the form of stable spheres (see [5] and references therein). The impact of this work is exemplified by the fact that Richard Smalley, Robert F. Curl and Sir Harold W. Kroto were awarded the Nobel Prize in Chemistry in 1996 - the first for nanotechnology - for their, along with Jim Heath and Sean O’Brien, discovery of a carbon allotrope containing 60 atoms (C60) called buckminsterfullerene [6, 7].
By 2000, nanotechnology was impacting a wide range of disciplines including physics, chemistry, biology, computer science and engineering, though medicine has been the area of the most significant advances in nanotechnology [5]. Within the medical field, nanotechnology has impacted a number of areas; however, with differences in the pace of progress. Advances in miniaturizing the dimensions of existing technologies, a common approach across nanotechnology disciplines, led to significant advances in the performance and throughput capabilities of nucleic acid sequencing, drug screening and many other examples in basic and applied molecular diagnostics [8-11]. Furthermore, the area of nanodiagnostics benefited by new approaches combining biological molecules (e.g. nucleic acids and proteins) with nanoparticles (e.g. colloidal gold nanoparticles and semiconductor quantum dots) such that the conjugates specifically bound to their molecular counterpart and the nanoparticles, in turn, imparted signal transduction opportunities for realizing next generation molecular diagnostic assays [12, 13]. Finally, the brilliant physical properties of nanoparticles have been used to develop probes for passive and targeted in vitro and in vivo imaging applications, including clinical imaging modalities such as magnetic resonance imaging (MRI) [14, 15]. Overall, the impact of nanomaterials and nanotechnology has, and continues to, contribute greatly to molecular detection and medical diagnostics.
The introduction and application of nanotechnology for developing medicines to treat human disease, even though this process is not new, requires increased resources and takes time. Much of the early work to develop new nanotherapies was focused on cancer. To some degree, this was not by chance as the National Cancer Institute (NCI) set aside funds for designated academic Centers of Cancer Nanotechnology Excellence (CCNE). The CCNE program lasted for three funding cycles, 5 years/ cycle, and started in 2005. The ending of the CCNE program in 2020 resulted in retrospectives and commentary regarding its success [16-18]. Certainly, over this time a number of nanomedicines gained approval and an increasing number are currently in human clinical trials [19]. Drawing on lessons learned from the last 20 years, there is an optimism that novel nanotherapies with increasing sophistication will take more prominent positions in pharmaceutical developmental pipelines for an expanding number of clinical indications in, and outside of, the cancer field [20] which is a topic of significant current national interest [21].
Overall, nanomaterials and nanoparticles can be used to synthesize higher order supramolecular materials that seamlessly interface with biological systems to modulate fundamental biologic processes such as cell proliferation, migration, inflammation, autophagy and metabolism [5, 22]. Our multidisciplinary group is particularly interested in developing new topical and molecularly targeted therapies for ophthalmology, which is a clinical area that has heretofore received little attention. We have demonstrated and proposed a mono-therapeutic nanoparticle platform that exemplifies diversity of action in the form of synthetic high-density lipoprotein (HDL) nanoparticles (NP) [23], which will be the focus of this review.
2. PRESENT
2.1. HDL NPs.
High-density lipoproteins (HDLs) are natural nanoparticles, ranging in size from 7-13 nm in diameter, that circulate in the bloodstream and move into and out of human tissues. HDLs are responsible for targeted lipid, mainly cholesterol and cholesterol ester, transport [24-26]. Apolipoprotein A-1 (apoA-I) is the most abundant and important protein of HDL and is responsible for HDL size, shape, and molecular targeting. In addition to apoA-I, the HDL particle is typically modeled as being comprised of cholesterol, cholesterol esters, and phospholipids. Mature, cholesterol-ester-rich, spherical HDL particles target their high-affinity ligand, scavenger receptor type B1 (SR-B1) [27]. Upon binding the receptor, diffusion of free cholesterol between the cell and the surface of the HDL particle is initiated, and the delivery of cholesterol esters from the core of the HDL particle to the cell occurs. The synthesis of immature versions of HDLs consisting of mixtures of apoA-I and phospholipids is commonplace [28]; however, because of the chemical complexity of cholesterol-rich spherical HDLs, and the biological/ enzymatic steps required for biosynthesis, little success had been achieved in developing reliable and scalable methods of producing spherical forms of HDL [29]. Major breakthroughs in the synthetic HDL bioengineering field came with the development by the Thaxton Lab of a spherical HDL biomimetic synthesized using a gold nanoparticle (NP) core as a size and shape-restrictive template for apoA-I and lipid assembly, whereupon the conjugates tightly bind and regulate cell cholesterol [30-32] (Fig. 1). A similar synthetic approach was used by others to synthesize HDL-like nanoparticle imaging agents [33]. As therapeutics, HDL NPs: (i) are endowed with a surface chemistry that closely mimics the physical and chemical features of natural HDLs; (ii) contain apoA-I; (iii) bind with high-affinity to SR-B1; (iv) are not toxic to healthy cells in vitro or in vivo; (v) and can be chemically engineered to display nearly any surface or core composition desired. [30]. Another core property of native HDLs is their ability to transport cholesterol and other small molecules and nucleic acids and deliver these payloads to cells expressing SR-B1 [34-38]. Because of their targeted nature, the inherent functions of native HDLs, the ability to carry precious cargo for delivery and their biocompatibility, HDLs are an intriguing natural nanomaterial to synthetically harness.
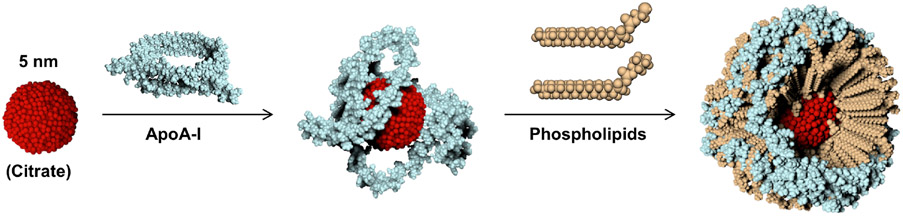
Figure 1: Bioengineered synthetic HDL NPs using inorganic (gold) core scaffold.
Synthesis scheme for HDL NPs made using a 5 nm diameter citrate stabilized gold nanoparticle scaffold (red) surface-functionalized with apoA-I (blue) and a phospholipid layer (tan).
2.2. HDL and the Cornea.
It is recognized that HDL protects against cardiovascular disease by regulating cholesterol efflux from tissues and reducing inflammation [39, 40]. Other positive effects of HDL are their antioxidant, antifibrotic, vasoprotective, and antithrombotic properties [39, 40]. There have also been reports that HDL can maintain the integrity of the endothelium [41]. Overall, data support that HDLs play a powerful role in maintaining epi- and endo-thelial cell homeostasis and can potently reduce inflammation. To our knowledge, materials with inherent targeting ability and tunable structure-function properties, like synthetic HDL NPs, have not been considered as a therapeutic agent by the ophthalmology community. With this in mind, we have reported on the effects of HDL NPs on corneal epithelial regeneration and inflammation [23].
2.3. HDL NPs enter cells and tissues of the cornea.
SR-B1 is the major receptor that controls the selective uptake of HDL cargo into cells [42]. Not surprisingly, single cell RNA sequencing showed that SR-B1 mRNA was detected in all cell populations of the mouse cornea [43]. Consistently, SR-B1 protein was observed on primary human corneal epithelial cells (HCECs) as well as human and mouse corneal and limbal epithelia [23]. SR-B1 was also present on the stromal keratocytes. In a series of in vitro and in vivo experiments, HDL NPs were demonstrated to enter HCECs and intact mouse corneas [23]. Specifically, following the addition of HDL NPs to HCECs, non-membrane-bound gold particles were readily observed, free in the cytoplasm; few if any particles were detected in the nucleus or in the proximity of the cell membrane. This suggested a non-endocytic mechanism of HDL NP transport into HCECs via SR-B1, similar to what has been described in other cells [42, 44, 45]. More importantly, twenty-four hours after a fluorescent HDL NP conjugate in PBS was topically applied to intact mouse corneas, fluorescent signal was readily detected in the superficial and basal corneal epithelial cells as well as in the stromal keratocytes [23].
2.4. HDL NPs accelerate re-epithelialization in vivo.
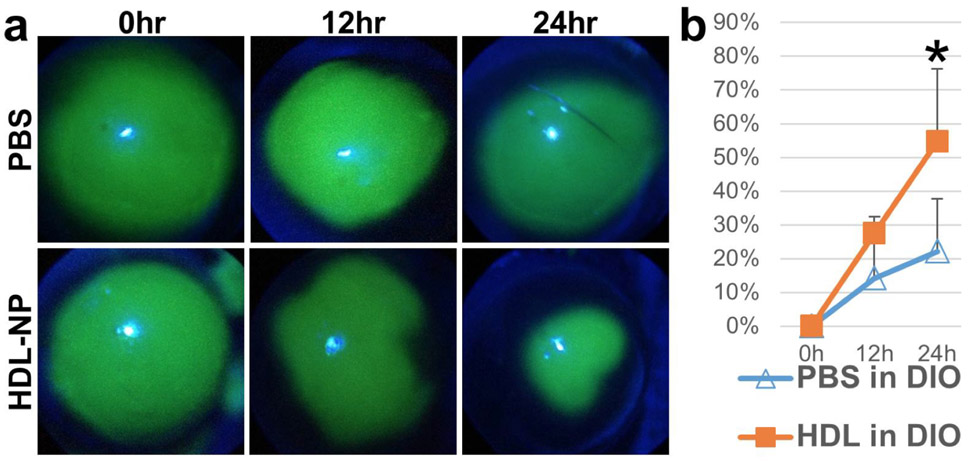
Using a diet-induced obesity (DIO) mouse model, which has an impaired wound healing response [46, 47], HDL NPs applied topically to the corneal surface after a debridement wound sealed wounds significantly faster than controls (Fig. 2; [23]). Such a positive effect on wound closure is due, in part, by HDL NPs up-regulation of the Akt signaling pathway [23], which is involved in re-epithelialization [48]. Furthermore, Akt signaling regulates actin remodeling and cell migration [49]. Thus, it is not surprising that a marked increase in F-actin was observed at the leading edge of the HDL NP-treated migrating cells compared with the control NP-treated cells [23]. This suggests that HDL NPs are positive regulators of F-actin polymerization during the initial migratory phase of cell migration [50]. Finally, phosphorylation of EphA2 at S897 via Akt can signal an increase in cell migration through the reorganization of actin filaments at the leading edge of a migrating sheet [51]. Treatment of cells with HDL NPs resulted in a dramatic increase in p-EphA2-S897 expression compared with control NPs, providing compelling evidence that HDL NPs positively affect cell migration via targeting EphA2 [23]. In the future, it is necessary to understand how Akt/pS897-EphA2 signaling impacts corneal epithelial wound healing when treated with synthetic HDL NPs.
Figure 2: Therapeutic effect of HDL NPs on wound healing of mouse corneal epithelium.
Corneal images (a) and epithelial corneal wound closure percentage (b) in DIO mouse corneas treated with HDL NPs or control. Green fluorescence represents corneal wound. N=8. *p<0.05. The figure is taken from Junyi Wang et al. with permission [23].
2.5. HDL NPs as anti-inflammatory agents.
HDL inhibits inflammatory and oxidative damage that are processes involved in atherogenesis [52], in part, by preventing monocyte recruitment into the arterial wall [53], and by decreasing production and inactivation of neutrophil NADPH [54]. It is now recognized that synthetic HDLs can reduce acute inflammation and oxidative stress in rabbit carotid arteries (in vivo) and in primary human coronary artery endothelial cells [55]. Furthermore, the type of phospholipid used in the reconstituted HDL has significant influence on anti-inflammatory and anti-atherosclerosis properties [56]. Thus, it is apparent that various recombinant ApoA-I proteins and ApoA-I mimetic peptides used for the preparation of synthetic HDLs exhibit properties similar to those of endogenous HDL suggesting potential as anti-inflammatory agents (see [57] and references therein). Our group has demonstrated that synthetic HDL NPs made using inorganic [58] and organic core [59] scaffolds endowed with apoA-I and certain phospholipids potently reduce the in vitro (human cell lines) and ex vivo (primary human cells) inflammatory response induced by exposure to lipopolysaccharide (LPS, Gram-negative bacterial endotoxin). The use of HDL NPs as anti-inflammatory agents in an ocular setting is scant [23].
2.5.1. HDL NPs are effective in treating alkali burn-induced corneal inflammation.
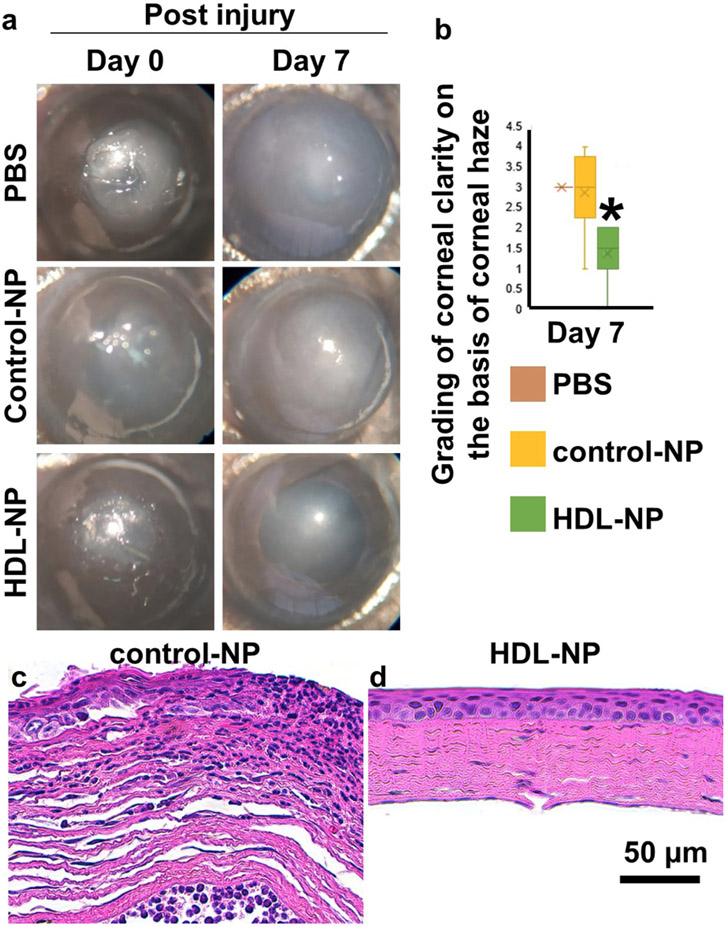
In the alkaline burn model to induce an inflammatory response in mouse corneas, the corneal epithelium, stromal, and inflammatory cells are involved in the injury, repair, and wound healing processes, which are accompanied by the production of numerous cytokines [60, 61]. Following such a wounding protocol, mice were treated daily for 4 days with a topical solution of HDL NPs, control NPs in PBS, or PBS [23]. By day 7 post-wounding, PBS- and control NP-treated corneas remained opaque whereas the HDL NP-treated mice showed a 40-50% (p < 0.05) improvement in corneal opacity and surface integrity (Figs. 3, a, b). Control NP treated corneas displayed a range of thickened and disorganized corneal epithelia as well as a wide spectrum of stromal alterations, ranging from a stroma filled with inflammatory cells (Fig. 3 c) to randomly oriented collagen bundles resulting in a disorganized appearance (Fig. 3 c). In contrast the HDL NP-treated corneas displayed a well-organized stratified epithelium (Fig. 3 d) and a stroma with collagen bundles highly organized in a plywood-like fashion that were relatively devoid of inflammatory cells (Fig. 3 d). In some HDL NP treatment groups, stromal keratocytes were prominent (Fig. 3 d).
Figure 3: HDL NP treatment reduces inflammation after alkali burn.
Mice were treated with HDL NPs, control NPs, or PBS (topically) following 30s alkali burns. (a) Representative images. (b). Degree of haze. (c-d). H&E 7 days post burn. N=8. The figure is taken from Junyi Wang et al. with permission [23].
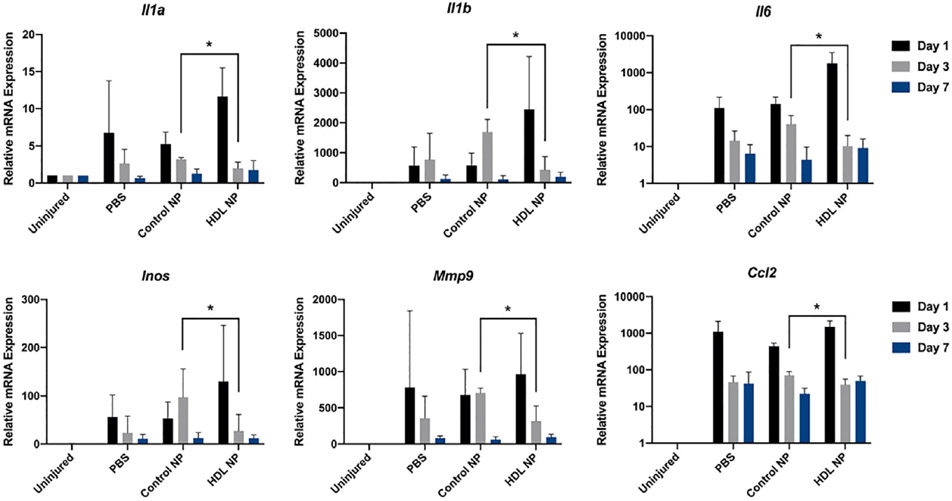
Immune cell recruitment after corneal injury is mediated by proinflammatory cytokines released from epithelial cells and keratocytes at the injured site. IL-1, lL-6 and TNF are important [60, 62] and aid in attracting neutrophils, which are the first cells infiltrating the cornea after injury [63, 64]. Shortly after neutrophils enter the cornea, macrophages extravasate from the limbal vessels, infiltrate the stroma from superficial to deeper layers and migrate towards the center of the cornea. Macrophages aid in corneal wound closure by secreting TGF;β to promote the differentiation of fibroblasts to myofibroblasts. In addition to removal of debris and apoptotic cells, macrophages are essential mediators of angiogenesis after severe and prolonged corneal injury [65]. Several chemokines and their receptors have been identified in the inflamed cornea. CXCL1, CXCL8 and MCP-1/CCL2 mRNA levels were found to be elevated in human inflamed corneas. Additionally, CCR7 and its ligand CCL21 were upregulated in inflamed corneas, mediating MHC II+ cell recruitment [66-68]. Evaluation of the alkali burn-induced cytokine expression pattern after treatment with HDL NPs revealed that day 1 post-injury, Il1a, Il1b, Il6 and Ccl2 were most highly expressed (Fig. 4), which is consistent with an initial stage of inflammation. By day 3, HDL NP treatment significantly reduced the expression levels of Il1a, Il1b, Il6, Inos, Mmp9 and Ccl2 when compared with control NPs (Fig. 4) [23]. Chemokines such as CCL2 play important roles in the recruitment of macrophages to the site of injury during an inflammatory event [69, 70], as well as the inflammatory mediator iNOS, which is associated with activated macrophages [71]. Elevated levels of Gelatinase or MMP-9 are associated with numerous diseases of the cornea and can facilitate corneal ulceration [72]. All genes evaluated, returned to pre-treatment levels by day 7 (Fig. 4). Collectively, these findings strongly indicate that topical application of HDL NPs to the corneal surface following a chemical burn can aid in attenuating the inflammatory response.
Figure 4: HDL NP has anti-inflammation activity.
Filter paper (1 mm) soaked in NaOH (1 m) was placed on the corneal surface of 6 week old WT mice for 30 s and then washed extensively with PBS. Corneas were topically treated with HDL NPs, control NPs (inert AuNP core, passivated with polyethyleneglycol (PEG)), or PBS daily for 7 d. Whole corneal tissues were dissected and total RNAs were isolated for RT-qPCR for inflammation-related genes at post injury day 1, 3, and 7 (N = 8). *p < 0.05. Unpaired t-tests were conducted. The figure is taken from Junyi Wang etal. with permission [23].
2.6. HDL NPs are an effective platform for the delivery of microRNAs to cells.
miRNAs are small (~22 nucleotides in length), “noncoding” or “non-messenger” RNAs that are part of the RNAi silencing machinery [for reviews see [73-76]]. Consequently, miRNAs influence the regulation of a myriad of biological processes in both normal and disease circumstances. Hence, miRNAs hold great promise as potential therapeutic agents. The effective formulation and delivery of therapeutic miRNAs to the cytoplasm of target cells in a biologically active form, has proven to be a major hurdle toward realizing this goal. A conceptual breakthrough to this problem occurred with the demonstration that natural HDLs, isolated from human serum contained miRNAs and that these HDL-bound miRNAs had improved stability compared with naked miRNAs [38]. Furthermore, as mentioned above, native HDLs deliver bound miRNAs to cells that express the high-affinity receptor of HDLs, SR-B1, whereupon the miRNA regulates the expression of the target gene [77]. Capitalizing on these observations, the Thaxton laboratory has demonstrated that the HDL NP platform can be extensively tailored to efficiently deliver any desired individual (i.e. similar to miRNAs) or complementary pairs of RNA strands (e.g. siRNA duplex pairs) on separate HDL NPs or on the same HDL NP for potent target gene regulation [37]. In vitro and in vivo data reveal that the HDL NP platform can be utilized to efficiently target prostate and ovarian cancer [78, 79].
2.6.1. HDL NPs complexed to miR-205 accelerate corneal epithelial cell migration.
In the context of corneal epithelial biology, microRNA-205 (miR-205) has well-established regulatory roles [80-83]. Specifically, miR-205 negatively regulates the lipid phosphatase SHIP2 in epithelial cells resulting in the activation of Akt signaling [83]. Consequently, by suppressing SHIP2, miR-205 promotes epithelial migration via cofilin activation [82]. Because of such easily monitored outcomes, HDL NPs were complexed with miR-205 (HDL NP-miR-205) and this compound was interrogated as a positive effector of corneal epithelial migration [23]. When HCECs were exposed to miR-205-HDL NPs for 48 hours: (i) the complex was readily taken up; (ii) remained stable; (iii) RNA increased; and, (iv) no signs of toxicity were observed [23]. As anticipated, a decrease in SHIP2 and an increase in p-Akt levels were observed compared to control NPs. To determine further the biological activity of the miR-205-HDL NP compound, a series of scratch wound assays using a limbal-derived corneal epithelial cell line (hTCEpi) [84, 85] revealed that miR-205-HDL NP treatment sealed wounds after 6 hrs compared to an 18 hr closure time for the scrambled-miR-HDL NP treatment [23]. Taken together, these findings firmly established that HDL NPs are an effective platform for delivery of biologically active miRNAs to corneal epithelial cells.
2.6.2. miR-146a-HDL NPs reduce NF-kB activity.
As a second proof of concept for the efficacy of HDL NPs to serve as a platform for delivery of miRNAs into cells, miR-146a was evaluated. miR-146a is expressed in response to pro-inflammatory stimuli and can inhibit NF-kB transcriptional activity [86-88]. When miR-146a-HDL NPs were exposed to J774-dual macrophages, which express the secreted alkaline phosphatase (SEAP) gene downstream of the NF-kB consensus transcriptional response element, the signal of LPS-induced SEAP was significantly reduced compared with controls [23]. This is consistent with the miR-205 data indicating that miR-146a complexed with HDL NPs can penetrate cells and retains biological activity.
3. FUTURE
3.1. Organic core scaffolds
One of the major benefits of the synthetic HDL NPs is that the 5nm gold core can be functionalized with phospholipids and apoA-I and targeted to specific cells to modulate cellular lipid metabolism [37, 89]. Another feature of these synthetic HDL NPs is their stability after cell binding [59, 90]. However, this property has a potential downside in ocular tissues since gold particles binding to and interacting with cells that do not rapidly turnover (e.g., keratocytes, endothelial cells) might theoretically impair the passage of light to the lens. Recently we have successfully replaced the inorganic gold nanoparticle with an organic, transparent Lipid-conjugated core (oc) scaffold (Fig. 5) [59]. These ocHDL NPs have the size, shape, surface chemistry composition, protein structure, cholesterol transport properties and targeting properties similar to mature human HDLs [59]. Functionally, they possess cholesterol transport and anti-inflammatory capabilities similar to native HDLs [59]. Delivery of these highly innovative and optically transparent ocHDL NPs in various ocular cells and tissues should pose no problems with light transmission. We envision that such ocular core scaffolds will form the foundation of a unique class of nanoparticle-based products particularly well-suited for the ocular community.
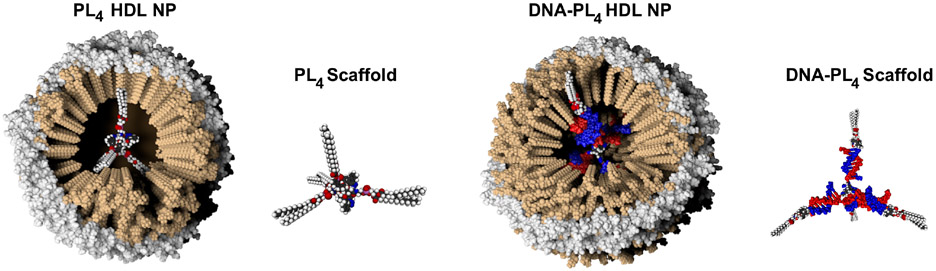
Figure 5: Bioengineered synthetic HDL NPs using organic (PL4 and DNA-PL4) core scaffold.
Organic tetrahedral phospholipid (PL4) or PL4 with bioprogrammable DNA “arms” (DNA-PL4) are used as scaffolds for ocHDL NPs. Synthesis of ocHDL NPs made using organic scaffolds proceeds similar to the ones made using AuNPs.
3.2. Modification of existing synthetic HDL NPs: potential for novel therapeutics.
As mentioned previously, modifying the qualitative and quantitative nature of the phospholipids that comprise the synthetic HDL NPs can have profound effects on the biological nature of these particles [30, 58]. Specifically, HDL NPs endowed with phospholipids that can be metabolized to polyunsaturated fatty acids are particularly appealing. In fact, native HDL binding to macrophages has been reported to stimulate production of pro-resolving lipids [91]. Therefore, adding phospholipids to synthetic HDL NPs that are pro-resolving through metabolism, including certain ω6 and ω3 fatty acids [92-94] as well as cardiolipin, will be potentially more potent activators of wound healing and the resolution of inflammation. Having demonstrated the targeted delivery of microRNA cargo using the HDL NP platform [23], a logical next phase is the generation of targeted HDL NP therapies that incorporate small molecules such as Vitamin D3 (Vit D3).
3.3. Vitamin D3-HDL NP: a potential enhanced anti-inflammatory eye drop.
Vit D3 is well-recognized as an immunomodulator through direct inhibition of NF-kB activation, suppression of TNF-α and iNOS expression, as well as activation of autophagy [95-99]. Vit D3 is converted intracellularly to the active form in macrophages, a critical cell population activated following stress that exacerbates local cellular inflammation. The presence of the Vit D receptor (VDR) was detected in the human corneal epithelium, as well as the corneal endothelium [100]. Additionally, the presence of vitamin D hydroxylases (CYP27B1, CYP27A1, CYP2R1, and CYP24A1) are present in corneal epithelial and endothelial cell lines [101, 102], indicative that these cells have the ability to initiate and regulate Vit D3 metabolism. With respect to corneal inflammation, topical administration of Vit D3 to sutured mouse corneas (a model for inflammation) inhibited Langerhans cell migration and maturation, while delaying neovascularization in the central cornea (see [103] and references therein). Vit D3 protected corneal graft rejection by inhibiting the proinflammatory cytokines IL-1α and TNF-α, in rats [104]. In vitro studies in corneal epithelial cells demonstrated immunomodulatory activity of Vit D3 via attenuation of proinflammatory mediators while increasing antimicrobial peptides and anti-pseudomonas activity [105]. We propose that Vit D3 has potential for treatment of a variety of corneal inflammatory diseases because it targets macrophages and suppresses inflammation without the side effects commonly associated with long-term steroid usage. In addition, Vit D3 can activate autophagy [99], a critical stress response process to differentiate macrophages towards an anti-inflammatory repair phenotype. We have demonstrated that autophagy has an important role in maintaining limbal epithelial stem cell homeostasis [43, 106].
Given the anti-inflammatory properties of HDL NPs as well as Vit D3, we envision development of a “super” HDL NP-Vit D3 eye drop in order to reduce inflammation in the anterior segment. The HDL NP platform affords significant synthetic control and latitude in order to rationalize and develop targeted materials with desired characteristics. Specifically, HDL NPs made with various cores and phospholipids can also be formulated with Vit D3. Work to realize this construct is underway.
4. CONCLUSIONS
It is clear that HDL NPs will be the foundation of a new class of topical eye drops (Fig. 6) that have: (i) stability; (ii) minimal adverse side effects; (iii) tissue regenerative capabilities; and, (iv) anti-inflammatory properties. In addition to its use as a monotherapy, HDL NPs are ideal for incorporating small molecules (e.g. Vit D3) and nucleic acids (e.g. miR-146a) to modulate lipid metabolism and deliver drugs to enhance wound healing and reduce inflammation. These features endow HDL NPs with great translational potential and exemplify the impact that nanoparticles can have in medicine. Specifically, an HDL NP eye drop should be effective in treating dry eye or tear gland insufficiency, whose underlying etiology is inflammation [107]. Since destruction of the stem cell niche due to persistent inflammation is thought to be an underlying cause of limbal stem cell deficiency (see, [108] and references therein), an HDL NP eye drop should be helpful in modulating and/or avoiding limbal stem cell deficiency (LSCD). Nitrogen and sulfur mustards (NM and SM) are devastating compounds that have been used as chemical warfare agents. Recently, it has been shown that Vit D3 treatment protected against SM toxicity and prevented SM-induced mortality [109]. The eye is the most susceptible part of the body to the effects of mustard exposure, particularly SM [110-115]. Following exposure, severe and extensive inflammation is triggered, which also can result in phenotypes mimicking LSCD [116]. We opine that HDL NPs and HDL NP-Vit D3 eye drops have great potential as an extremely innovative therapy to comprehensively address corneal mustard keratopathy (CMK) without the side-effects associated with steroidal treatments. Corneal keratopathy occurs in more than 70% of diabetic patients, which manifested in part, as persistent epithelial defects, and recurrent erosions [117, 118]. Therefore, HDL NP eye drops might have prophylactic value in patients with diabetes. Finally, HDL NPs either alone or complexed with small molecules should be useful in a wide variety of ocular surface diseases such as chemical and thermal injury, long-term contact lens wear, severe chronic rosacea, Stevens-Johnson syndrome, atopic keratoconjunctivitis, bacterial keratitis and graft versus host disease, to name a few.
Figure 6: High-Density Lipoprotein Nanoparticle Eye Drop.
3. ACKNOWLEDGEMENTS
This research is supported by National Institutes of Health Grants EY06769, EY019463, and EY028560 (to R.M.L.); a Dermatology Foundation research grant and Career Development Award (to H.P.); and an Eversight research grant (to H.P.); AR064144 and AR071168 (to K.Q.L.); the Prostate Cancer Foundation (to C.S.T.), the Center for Regenerative Nanomedicine (CRN), and the Nanyang Technological Institute-Northwestern University (NTU-NU) Institute for Nanomedicine (to C.S.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
4. REFERENCES
- [1].Feynman RP. There's plenty of room at the bottom. Eng Sci. 1960;23:22–36. [Google Scholar]
- [2].Taniguchi N, Arakawa C, Kobayashi T. On the basic concept of nano-technology. Proceedings of the International Conference on Production Engineering. Tokyo, Japan1974. p. 26–9. [Google Scholar]
- [3].Vert M, Doi Y, Hellwich K-H, Hess M, Hodge P, Kubisa P, et al. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem. 2012;84:377–410. [Google Scholar]
- [4].Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules. 2019;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lepeltier E, Rijo P, Rizzolio F, Popovtzer R, Petrikaite V, Assaraf YG, et al. Nanomedicine to target multidrug resistant tumors. Drug Resist Updat. 2020;52:100704. [DOI] [PubMed] [Google Scholar]
- [6].25 years of C60. Nat Nanotechnol. 2010;5:691. [DOI] [PubMed] [Google Scholar]
- [7].Kroto HW, Heath Jr, O'Brian SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–3. [Google Scholar]
- [8].Perry JL, Herlihy KP, Napier ME, Desimone JM. PRINT: a novel platform toward shape and size specific nanoparticle theranostics. Acc Chem Res. 2011;44:990–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Krishnaswami V, Kandasamy R, Alagarsamy S, Palanisamy R, Natesan S. Biological macromolecules for ophthalmic drug delivery to treat ocular diseases. Int J Biol Macromol. 2018;110:7–16. [DOI] [PubMed] [Google Scholar]
- [10].Kricka LJ, Park JY, Li SF, Fortina P. Miniaturized detection technology in molecular diagnostics. Expert Rev Mol Diagn. 2005;5:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kiechle FL, Holland CA. Point-of-care testing and molecular diagnostics: miniaturization required. Clin Lab Med. 2009;29:555–60. [DOI] [PubMed] [Google Scholar]
- [12].Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–9. [DOI] [PubMed] [Google Scholar]
- [13].Alivisatos AP, Johnsson KP, Peng X, Wilson TE, Loweth CJ, Bruchez MP Jr., et al. Organization of 'nanocrystal molecules' using DNA. Nature. 1996;382:609–11. [DOI] [PubMed] [Google Scholar]
- [14].Park SM, Aalipour A, Vermesh O, Yu JH, Gambhir SS. Towards clinically translatable in vivo nanodiagnostics. Nat Rev Mater. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu L, Zhang L, Chen J, Lanza GM, Wickline SA. Diffusional mechanisms augment the fluorine MR relaxation in paramagnetic perfluorocarbon nanoparticles that provides a "relaxation switch" for detecting cellular endosomal activation. J Magn Reson Imaging. 2011;34:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park K The beginning of the end of the nanomedicine hype. J Control Release. 2019;305:221–2. [DOI] [PubMed] [Google Scholar]
- [17].Martins JP, das Neves J, de la Fuente M, Celia C, Florindo H, Gunday-Tureli N, et al. The solid progress of nanomedicine. Drug Deliv Transl Res. 2020;10:726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Grodzinski P NCI Centers of Cancer Nanotechnology Excellence (CCNEs) - A full story to set the record straight. J Control Release. 2019;309:341–2. [DOI] [PubMed] [Google Scholar]
- [19].Sanna V, Sechi M. Therapeutic Potential of Targeted Nanoparticles and Perspective on Nanotherapies. ACS Med Chem Lett. 2020;11:1069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nelson BC, Maragh S, Ghiran IC, Jones JC, DeRose PC, Elsheikh E, et al. Measurement and standardization challenges for extracellular vesicle therapeutic delivery vectors. Nanomedicine (Lond). 2020;15:2149–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].A Quadrennial Review of the National Nanotechnology Initiative: Nanoscience, Applications, and Commercialization. Washington (DC)2020. [PubMed] [Google Scholar]
- [22].Cordani M, Somoza A. Targeting autophagy using metallic nanoparticles: a promising strategy for cancer treatment. Cell Mol Life Sci. 2019;76:1215–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang J, Calvert AE, Kaplan N, McMahon KM, Yang W, Lu K, et al. HDL nanoparticles have wound healing and anti-inflammatory properties and can topically deliver miRNAs. Adv Therapeutics. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50 Suppl:S189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rosenson RS, Brewer HB Jr., Davidson WS, Fayad ZA, Fuster V, Goldstein J, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rothblat GH, Phillips MC. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr Opin Lipidol. 2010;21:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–20. [DOI] [PubMed] [Google Scholar]
- [28].Smith JD. Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2010;11:989–96. [PMC free article] [PubMed] [Google Scholar]
- [29].Silva RA, Huang R, Morris J, Fang J, Gracheva EO, Ren G, et al. Structure of apolipoprotein A-I in spherical high density lipoproteins of different sizes. Proc Natl Acad Sci U S A. 2008;105:12176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Luthi AJ, Lyssenko NN, Quach D, McMahon KM, Millar JS, Vickers KC, et al. Robust passive and active efflux of cellular cholesterol to a designer functional mimic of high density lipoprotein. J Lipid Res. 2015;56:972–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Luthi AJ, Zhang H, Kim D, Giljohann DA, Mirkin CA, Thaxton CS. Tailoring of biomimetic high-density lipoprotein nanostructures changes cholesterol binding and efflux. ACS Nano. 2012;6:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thaxton CS, Daniel WL, Giljohann DA, Thomas AD, Mirkin CA. Templated spherical high density lipoprotein nanoparticles. J Am Chem Soc. 2009;131:1384–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, et al. A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nat Commun. 2014;5:3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang H, Cruz W, Chen J, Zheng G. Learning from biology: synthetic lipoproteins for drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Luthi AJ, Patel PC, Ko CH, Mutharasan RK, Mirkin CA, Thaxton CS. Nanotechnology for synthetic high-density lipoproteins. Trends Mol Med. 2010;16:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McMahon KM, Plebanek MP, Thaxton CS. Properties of Native High-Density Lipoproteins Inspire Synthesis of Actively Targeted In Vivo siRNA Delivery Vehicles. Adv Funct Mater. 2016;26:7824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vickers KC, Sethupathy P, Baran-Gale J, Remaley AT. Complexity of microRNA function and the role of isomiRs in lipid homeostasis. J Lipid Res. 2013;54:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Camont L, Chapman MJ, Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol Med. 2011;17:594–603. [DOI] [PubMed] [Google Scholar]
- [40].Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. [DOI] [PubMed] [Google Scholar]
- [41].Kimura T, Sato K, Malchinkhuu E, Tomura H, Tamama K, Kuwabara A, et al. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler Thromb Vasc Biol. 2003;23:1283–8. [DOI] [PubMed] [Google Scholar]
- [42].Rhainds D, Brissette L. The role of scavenger receptor class B type I (SR-BI) in lipid trafficking. defining the rules for lipid traders. Int J Biochem Cell Biol. 2004;36:39–77. [DOI] [PubMed] [Google Scholar]
- [43].Kaplan N, Wang J, Wray B, Patel P, Yang W, Peng H, et al. Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Invest Ophthalmol Vis Sci. 2019;60:3570–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].McMahon KM, Mutharasan RK, Tripathy S, Veliceasa D, Bobeica M, Shumaker DK, et al. Biomimetic high density lipoprotein nanoparticles for nucleic acid delivery. Nano Lett. 2011;11:1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yang M, Jin H, Chen J, Ding L, Ng KK, Lin Q, et al. Efficient cytosolic delivery of siRNA using HDL-mimicking nanoparticles. Small. 2011;7:568–73. [DOI] [PubMed] [Google Scholar]
- [46].Kneer K, Green MB, Meyer J, Rich CB, Minns MS, Trinkaus-Randall V. High fat diet induces pre-type 2 diabetes with regional changes in corneal sensory nerves and altered P2X7 expression and localization. Exp Eye Res. 2018;175:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shen Y, Pfluger T, Ferreira F, Liang J, Navedo MF, Zeng Q, et al. Diabetic cornea wounds produce significantly weaker electric signals that may contribute to impaired healing. Sci Rep. 2016;6:26525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Squarize CH, Castilho RM, Bugge TH, Gutkind JS. Accelerated wound healing by mTOR activation in genetically defined mouse models. PLoS One. 2010;5:e10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kakinuma N, Roy BC, Zhu Y, Wang Y, Kiyama R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J Cell Biol. 2008;181:537–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moftah H, Dias K, Apu EH, Liu L, Uttagomol J, Bergmeier L, et al. Desmoglein 3 regulates membrane trafficking of cadherins, an implication in cell-cell adhesion. Cell Adh Migr. 2017;11:211–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, et al. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell. 2009;16:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tabet F, Rye KA. High-density lipoproteins, inflammation and oxidative stress. Clin Sci (Lond). 2009;116:87–98. [DOI] [PubMed] [Google Scholar]
- [53].Nicholls SJ, Dusting GJ, Cutri B, Bao S, Drummond GR, Rye KA, et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation. 2005;111:1543–50. [DOI] [PubMed] [Google Scholar]
- [54].Kopprasch S, Pietzsch J, Graessler J. The protective effects of HDL and its constituents against neutrophil respiratory burst activation by hypochlorite-oxidized LDL. Mol Cell Biochem. 2004;258:121–7. [DOI] [PubMed] [Google Scholar]
- [55].Tabet F, Remaley AT, Segaliny AI, Millet J, Yan L, Nakhla S, et al. The 5A apolipoprotein A-I mimetic peptide displays antiinflammatory and antioxidant properties in vivo and in vitro. Arterioscler Thromb Vasc Biol. 2010;30:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schwendeman A, Sviridov DO, Yuan W, Guo Y, Morin EE, Yuan Y, et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J Lipid Res. 2015;56:1727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kuai R, Li D, Chen YE, Moon JJ, Schwendeman A. High-Density Lipoproteins: Nature's Multifunctional Nanoparticles. ACS Nano. 2016;10:3015–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Foit L, Thaxton CS. Synthetic high-density lipoprotein-like nanoparticles potently inhibit cell signaling and production of inflammatory mediators induced by lipopolysaccharide binding Toll-like receptor 4. Biomaterials. 2016;100:67–75. [DOI] [PubMed] [Google Scholar]
- [59].Henrich SE, Hong BJ, Rink JS, Nguyen ST, Thaxton CS. Supramolecular Assembly of High-Density Lipoprotein Mimetic Nanoparticles Using Lipid-Conjugated Core Scaffolds. J Am Chem Soc. 2019;141:9753–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sotozono C, He J, Matsumoto Y, Kita M, Imanishi J, Kinoshita S. Cytokine expression in the alkali-burned cornea. Curr Eye Res. 1997;16:670–6. [DOI] [PubMed] [Google Scholar]
- [61].Wolf M, Clay SM, Zheng S, Pan P, Chan MF. MMP12 Inhibits Corneal Neovascularization and Inflammation through Regulation of CCL2. Sci Rep. 2019;9:11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–29. [DOI] [PubMed] [Google Scholar]
- [63].Burns AR, Li Z, Smith CW. Neutrophil migration in the wounded cornea: the role of the keratocyte. Ocul Surf. 2005;3:S173–6. [DOI] [PubMed] [Google Scholar]
- [64].Pfister RR, Haddox JL. A neutrophil chemoattractant is released from cellular and extracellular components of the alkali-degraded cornea and blood. Invest Ophthalmol Vis Sci. 1996;37:230–7. [PubMed] [Google Scholar]
- [65].Saika S Yin and yang in cytokine regulation of corneal wound healing: roles of TNF-alpha. Cornea. 2007;26:S70–4. [DOI] [PubMed] [Google Scholar]
- [66].Jin Y, Shen L, Chong EM, Hamrah P, Zhang Q, Chen L, et al. The chemokine receptor CCR7 mediates corneal antigen-presenting cell trafficking. Mol Vis. 2007;13:626–34. [PMC free article] [PubMed] [Google Scholar]
- [67].Spandau UH, Toksoy A, Verhaart S, Gillitzer R, Kruse FE. High expression of chemokines Gro-alpha (CXCL-1), IL-8 (CXCL-8), and MCP-1 (CCL-2) in inflamed human corneas in vivo. Arch Ophthalmol. 2003;121:825–31. [DOI] [PubMed] [Google Scholar]
- [68].Wallace GR, John Curnow S, Wloka K, Salmon M, Murray PI. The role of chemokines and their receptors in ocular disease. Prog Retin Eye Res. 2004;23:435–48. [DOI] [PubMed] [Google Scholar]
- [69].Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–57. [DOI] [PubMed] [Google Scholar]
- [70].Wilson SE, Mohan RR, Mohan RR, Ambrosio R Jr., Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–37. [DOI] [PubMed] [Google Scholar]
- [71].Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. J Cell Sci. 2004;117:2865–7. [DOI] [PubMed] [Google Scholar]
- [72].Kaufman HE. The practical detection of mmp-9 diagnoses ocular surface disease and may help prevent its complications. Cornea. 2013;32:211–6. [DOI] [PubMed] [Google Scholar]
- [73].Berezikov E Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12:846–60. [DOI] [PubMed] [Google Scholar]
- [74].Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lavker RM, Jia Y, Ryan DG. The tiny world of microRNAs in the cross hairs of the mammalian eye. Hum Genomics. 2009;3:332–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- [77].Hu Z, Shen WJ, Kraemer FB, Azhar S. MicroRNAs 125a and 455 repress lipoprotein-supported steroidogenesis by targeting scavenger receptor class B type I in steroidogenic cells. Mol Cell Biol. 2012;32:5035–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Murmann AE, Gao QQ, Putzbach WE, Patel M, Bartom ET, Law CY, et al. Small interfering RNAs based on huntingtin trinucleotide repeats are highly toxic to cancer cells. EMBO Rep. 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Murmann AE, McMahon KM, Haluck-Kangas A, Ravindran N, Patel M, Law CY, et al. Induction of DISE in ovarian cancer cells in vivo. Oncotarget. 2017;8:84643–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Li J, Bai H, Zhu Y, Wang XY, Wang F, Zhang JW, et al. Antagomir dependent microRNA-205 reduction enhances adhesion ability of human corneal epithelial keratinocytes. Chin Med Sci J. 2010;25:65–70. [DOI] [PubMed] [Google Scholar]
- [81].Lin D, Halilovic A, Yue P, Bellner L, Wang K, Wang L, et al. Inhibition of miR-205 impairs the wound-healing process in human corneal epithelial cells by targeting KIR4.1 (KCNJ10). Invest Ophthalmol Vis Sci. 2013;54:6167–78. [DOI] [PubMed] [Google Scholar]
- [82].Yu J, Peng H, Ruan Q, Fatima A, Getsios S, Lavker RM. MicroRNA-205 promotes keratinocyte migration via the lipid phosphatase SHIP2. FASEB J. 2010;24:3950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Yu J, Ryan DG, Getsios S, Oliveira-Fernandes M, Fatima A, Lavker RM. MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A. 2008;105:19300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Peng H, Kaplan N, Yang W, Getsios S, Lavker RM. FIH-1 disrupts an LRRK1/EGFR complex to positively regulate keratinocyte migration. Am J Pathol. 2014;184:3262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, et al. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–8. [DOI] [PubMed] [Google Scholar]
- [86].Reale M, D'Angelo C, Costantini E, Laus M, Moretti A, Croce A. MicroRNA in Sjogren's Syndrome: Their Potential Roles in Pathogenesis and Diagnosis. J Immunol Res. 2018;2018:7510174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103:12481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Tahamtan A, Teymoori-Rad M, Nakstad B, Salimi V. Anti-Inflammatory MicroRNAs and Their Potential for Inflammatory Diseases Treatment. Front Immunol. 2018;9:1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Plebanek MP, Mutharasan RK, Volpert O, Matov A, Gatlin JC, Thaxton CS. Nanoparticle Targeting and Cholesterol Flux Through Scavenger Receptor Type B-1 Inhibits Cellular Exosome Uptake. Sci Rep. 2015;5:15724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Rink JS, Yang S, Cen O, Taxter T, McMahon KM, Misener S, et al. Rational Targeting of Cellular Cholesterol in Diffuse Large B-Cell Lymphoma (DLBCL) Enabled by Functional Lipoprotein Nanoparticles: A Therapeutic Strategy Dependent on Cell of Origin. Mol Pharm. 2017;14:4042–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tsuda S, Shinohara M, Oshita T, Nagao M, Tanaka N, Mori T, et al. Novel mechanism of regulation of the 5-lipoxygenase/leukotriene B4 pathway by high-density lipoprotein in macrophages. Sci Rep. 2017;7:12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dalli J Does promoting resolution instead of inhibiting inflammation represent the new paradigm in treating infections? Mol Aspects Med. 2017;58:12–20. [DOI] [PubMed] [Google Scholar]
- [93].Hellmann J, Sansbury BE, Wong B, Li X, Singh M, Nuutila K, et al. Biosynthesis of D-Series Resolvins in Skin Provides Insights into their Role in Tissue Repair. J Invest Dermatol. 2018;138:2051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chen Y, Kong J, Sun T, Li G, Szeto FL, Liu W, et al. 1,25-Dihydroxyvitamin D(3) suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-kappaB activation. Arch Biochem Biophys. 2011;507:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–97. [DOI] [PubMed] [Google Scholar]
- [97].Holick MF. Active vitamin D compounds and analogues: a new therapeutic era for dermatology in the 21st century. Mayo Clin Proc. 1993;68:925–7. [DOI] [PubMed] [Google Scholar]
- [98].Lagishetty V, Liu NQ, Hewison M. Vitamin D metabolism and innate immunity. Mol Cell Endocrinol. 2011;347:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Das LM, Binko AM, Traylor ZP, Peng H, Lu KQ. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Johnson JA, Grande JP, Roche PC, Campbell RJ, Kumar R. Immuno-localization of the calcitriol receptor, calbindin-D28k and the plasma membrane calcium pump in the human eye. Curr Eye Res. 1995;14:101–8. [DOI] [PubMed] [Google Scholar]
- [101].Alsalem JA, Patel D, Susarla R, Coca-Prados M, Bland R, Walker EA, et al. Characterization of vitamin D production by human ocular barrier cells. Invest Ophthalmol Vis Sci. 2014;55:2140–7. [DOI] [PubMed] [Google Scholar]
- [102].Yin Z, Pintea V, Lin Y, Hammock BD, Watsky MA. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011;52:7359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Reins RY, McDermott AM. Vitamin D: Implications for ocular disease and therapeutic potential. Exp Eye Res. 2015;134:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].St Dang, Lu XH, Zhou J, Bai L. [Effects of 1alpha, 25-dihydroxyvitamin D3 on the acute immune rejection and corneal neovascularization in high-risk penetrating keratoplasty in rats]. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:892–6, 903. [PubMed] [Google Scholar]
- [105].Reins RY, Baidouri H, McDermott AM. Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation. Invest Ophthalmol Vis Sci. 2015;56:7715–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Park JK, Peng H, Katsnelson J, Yang W, Kaplan N, Dong Y, et al. MicroRNAs-103/107 coordinately regulate macropinocytosis and autophagy. J Cell Biol. 2016;215:667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15:276–83. [DOI] [PubMed] [Google Scholar]
- [108].Deng SX, Borderie V, Chan CC, Dana R, Figueiredo FC, Gomes JAP, et al. Global Consensus on Definition, Classification, Diagnosis, and Staging of Limbal Stem Cell Deficiency. Cornea. 2019;38:364–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Das LM, Binko AM, Traylor ZP, Duesler LR, Dynda SM, Debanne S, et al. Early indicators of survival following exposure to mustard gas: Protective role of 25(OH)D. Toxicol Lett. 2016;248:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Aasted A, Darre E, Wulf HC. Mustard gas: clinical, toxicological, and mutagenic aspects based on modern experience. Ann Plast Surg. 1987;19:330–3. [PubMed] [Google Scholar]
- [111].Baradaran-Rafii A, Eslani M, Tseng SC. Sulfur mustard-induced ocular surface disorders. Ocul Surf. 2011;9:163–78. [DOI] [PubMed] [Google Scholar]
- [112].Ghasemi H, Ghazanfari T, Ghassemi-Broumand M, Javadi MA, Babaei M, Soroush MR, et al. Long-term ocular consequences of sulfur mustard in seriously eye-injured war veterans. Cutan Ocul Toxicol. 2009;28:71–7. [DOI] [PubMed] [Google Scholar]
- [113].Mousavi SS, Vahedi E, Shohrati M, Panahi Y, Parvin S. Nocturnal serum melatonin levels in sulfur mustard exposed patients with sleep disorders. J R Army Med Corps. 2017;163:411–5. [DOI] [PubMed] [Google Scholar]
- [114].Panahi Y, Rajaee SM, Sahebkar A. Ocular Effects of Sulfur Mustard and Therapeutic Approaches. J Cell Biochem. 2017;118:3549–60. [DOI] [PubMed] [Google Scholar]
- [115].Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clin Exp Dermatol. 2006;31:1–5. [DOI] [PubMed] [Google Scholar]
- [116].Kadar T Turetz J, Fishbine E, Sahar R, Chapman S, Amir A. Characterization of acute and delayed ocular lesions induced by sulfur mustard in rabbits. Curr Eye Res. 2001;22:42–53. [DOI] [PubMed] [Google Scholar]
- [117].Misra SL, Braatvedt GD, Patel DV. Impact of diabetes mellitus on the ocular surface: a review. Clin Exp Ophthalmol. 2016;44:278–88. [DOI] [PubMed] [Google Scholar]
- [118].Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7:e251. [DOI] [PMC free article] [PubMed] [Google Scholar]