1. Introduction
Family history of substance use disorder (SUD) is a major risk factor for adolescent substance use (Whitesell et al., 2013). Specifically, parental SUD has been linked to SUDs among adolescents (Thatcher & Clark, 2008). Accordingly, studies have analyzed how family history of substance use impacts the brain’s reward circuitry, but an obstacle to interpreting neuroimaging data in addiction is the relative lack of data from youth who have not initiated drug use.
The existing literature suggests that reward processing is highly related to family history of substance use (Cservenka, 2016). These studies commonly utilize the Monetary Incentive Delay task (MID) (Knutson et al., 2000), which measures the expectation and obtainment of rewards and losses (Casey et al., 2018). Stice and Yokum (2014) found enhanced putamen activation in substance-naïve adolescents who were family-history-positive (FHP) for substance use relative to family-history-negative (FHN) adolescents. An fMRI study by Yau et al. (2012) found diminished ventral striatum activation in youth who were FHP for alcohol use disorder compared to controls. Similarly, Andrews and colleagues (2011) found less nucleus accumbens activation during reward anticipation in adults FHP for alcohol abuse, relative to FHN counterparts. Other studies have found no difference in brain activation between FHP and FHN participants (Bjork et al., 2008; Müller et al., 2015).
Thus, previous studies have yielded directionally inconsistent findings. Potential explanations include variable age ranges, sample sizes, and past participant substance use. Collectively, these limitations result in significant confounds and supplementary issues with study comparability. Accordingly, future studies are necessary to clarify the association between family history and reward processing.
These impediments to our understanding of how family history impacts reward processing can be uniquely addressed with the design and statistical power afforded in the Adolescent Brain Cognitive Development (ABCD) Study, a longitudinal study tracking biological and behavioral development in over 10,000 participants across the US, age 9–10 at enrollment (Jernigan & Brown, 2018). Functional-neuroimaging data and other data are made available through the NIMH Data Archive. With these data, we can begin to examine how brain activation differs in drug-naïve children who have, or do not have, a parental history of substance-use problems.
Here we examined brain activation during a reward-processing task, the MID task, focusing on a priori regions of interest (ROIs): the nucleus accumbens (NAcc) (Andrews et al., 2011; Knutson et al., 2001) and the putamen (Pu) (Stice & Yokum, 2014; Knutson et al., 2000). We hypothesized that children who were parent-history-positive (PH+) for substance-use problems would show differential activation in these areas compared to parent-history-negative (PH−) children. Prior findings suggested that activation could be either greater (Stice & Yokum, 2014) or smaller (Yau et al., 2012; Andrews et al., 2011).
2. Methods
2.1. Participants
The data were obtained from the ABCD Study, an observational study that recruited 11,874 children at 21 US study sites (Jernigan & Brown, 2018; National Institutes of Health, 2018). This study follows youth for 10 years, conducting neurocognitive, genetic, and environmental assessments every yearly and mid-year follow-up (Jernigan & Brown, 2018; National Institutes of Health, 2020; Adolescent Brain Cognitive Development Study, n.d.b).
2.2. Measures
Family History Classification
The Family History Assessment evaluates psychopathology and substance use within the family (Adolescent Brain Cognitive Development Study, n.d.a). Biological or adoptive parents are asked whether any biological relative of their child has had problems due to alcohol and/or drugs. Some examples provided include marital separation or divorce, alcohol or drug treatment programs, and others.
The MID task
The MID task (Knutson et al., 2000) is commonly utilized to evaluate reward activation. The task commences with an incentive cue displayed for 2000 milliseconds (ms). There are five displays a participant can encounter: Win $.20, Win $5, Lose $.20, Lose $5, or $0-no money at stake (Casey et al., 2018). This display is succeeded by an anticipation event presented for 1500–4000 ms and a target presented for 150–500 ms. During this time, the participant makes a quick response by pressing a button in the scanner. The next display communicates the result of the trial, and participants win money or avoid losing money when the response is correct during the target duration. The average reaction time for large reward trials with positive feedback for runs 1 and 2 of the task was 273.78 ms (average standard deviation: 34.17 ms). (See supplement for further task information).
2.3. Data Analysis
The data were derived from the publicly available ABCD Study (Data Release 2.0, N = 10,622). 60 total variables were used from the demographic questionnaires, family-history assessment, and the fMRI MID task. fMRI preprocessing was conducted by ABCD’s Informatics Center (Hagler et al., 2019). Data for the current analyses were processed with statsmodels in Python (Seabold & Perktold, 2010). We also excluded fMRI data with null values (n = 25) and data from Philips Scanners due to post-processing errors in this data release (n = 1512). Participants with both usable fMRI baseline data from the MID task, those with and without a family alcohol history (n = 4294), and with and without a family drug history (n = 5882) were included in the analyses.
For children to be included in the PH+ group for the current analyses, their biological parents had to report two or more problems with either alcohol (PH+A; n = 741) or other drugs (PH+D; n = 638). This criterion was adapted from the clinical manual for the National Consortium on Alcohol and Neurodevelopment in Adolescence Study (NCANDA), based on the Collaborative Study on the Genetics of Alcoholism (Rice et al., 1995).
Children who were PH- for alcohol (PH-A) and other drugs (PH-D) were matched to the PH+A children and PH+D children respectively (n = 699 matched those with alcohol histories, 615 matched those with histories with other drugs) based on race/ethnicity, sex assigned at birth, family income, age, parental education, and parental marital status (Supplemental material, Table 1; Table 2). Matching was intended to provide similar sample distributions and to reduce confounding (Rose & van der Laan, 2009). Additionally, participants’ sex was controlled to rule out potential sex differences in reward processing (Warthen et al., 2020).
Group differences in task-related activation during the anticipation of large rewards for the four ROIs (left/right NAcc; left/right Pu) were assessed in four separate ANOVAs in Python with Group as the only predictor. Analyses were conducted for both the alcohol and drug groups (eight ANOVAs total) to evaluate disparities between the PH+ and PH- groups within the alcohol and other drug categories. Brain activity within the ROIs were measured via mean beta weights, and ROIs were taken from FreeSurfer 5.3 (Hagler et al., 2019). Bayesian inference was also used to evaluate the evidence in favor of the null and alternative hypotheses. We used a simple method (Faulkenberry, 2018) to compute the Bayes factor from the ANOVA results. This approach builds upon the work of Masson (2011) and computes the Bayes factor from the Bayes Information Criterion. Importantly, this approach is consistent with the unit information prior (i.e. a normal distribution gathered at the effect size value of the data and continuing over the distribution of the data). A normal prior seems appropriate for data modeled with ANOVA, and it is appropriate to not put much prior probability outside this range (Raftery, 1999). Exploratory analyses during the anticipation of large losses, small losses, and small rewards in the ROIs were also conducted (Supplemental material, Table 3; Table 4).
3. Results
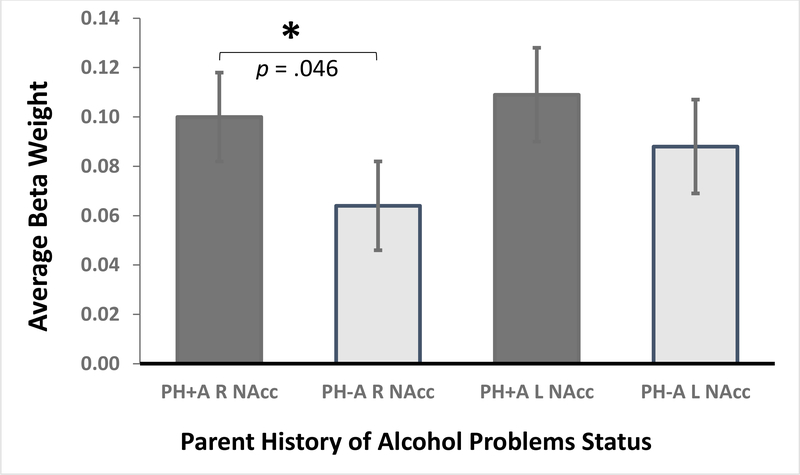
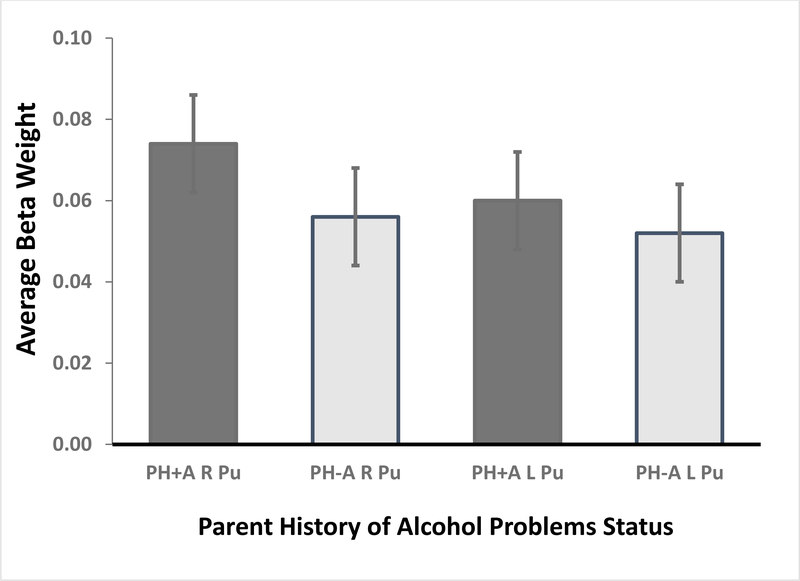
During the anticipation of large-reward trials, PH+A children showed greater activation than PH-A children in the right NAcc, F(1,1440) = 3.92, p = .048, Cohen d =.11 (CI95 .00–.20) (Figure 1). [The estimated Bayes factor (null/alternative) suggested that the data were 5.34:1 in favor of the null hypothesis, or rather, 5.34 times more likely to occur under a model without including an effect of parental history of alcohol problems, rather than a model with it.] Activation did not significantly differ between the groups for the left NAcc, F(1,1440) = 1.16, p = .28, Cohen d = .06 (CI95 −.04 to .16); right Pu, F(1,1440) = 2.25, p = .13, Cohen d = .08 (CI95 −.02 to .18); or left Pu, F(1,1440) = 0.49, p = .49, Cohen d = .04 (CI95 −.06 .14) (Figure 1).
Figure 1.
Monetary Incentive Delay (MID) Task Nucleus Accumbens (NAcc; top) and Putamen (Pu) activation: Anticipation of Large Rewards versus Neutral Response. An analysis of variance showed more right NAcc activation among PH+A subjects compared to their PH-A peers.
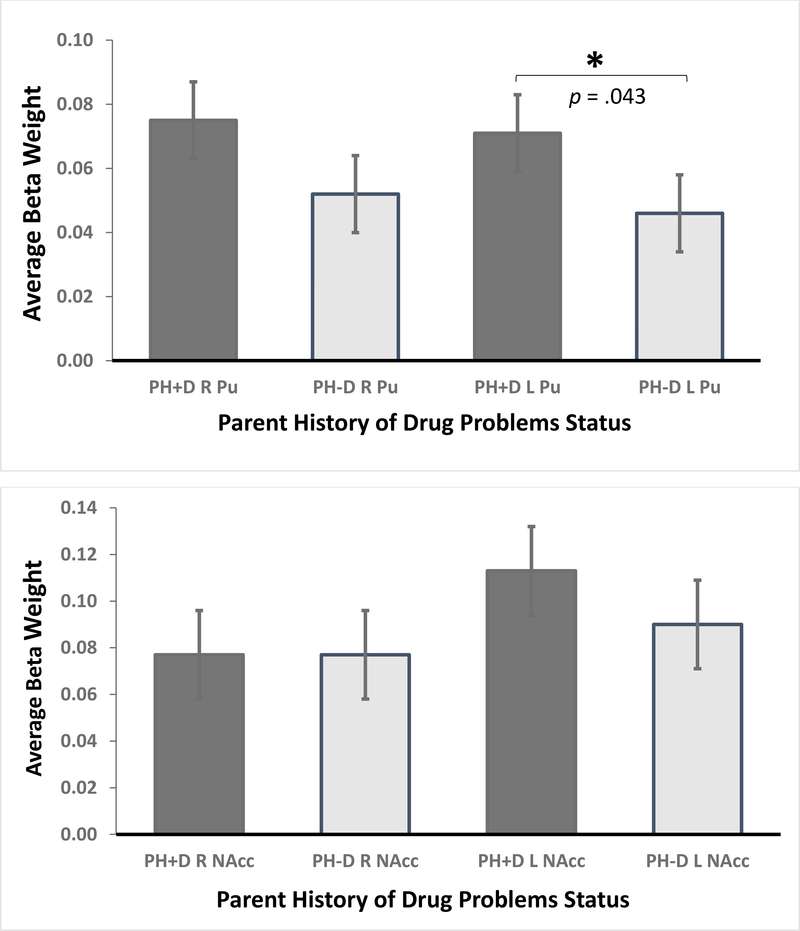
During large-reward trial anticipation, PH+D children showed greater activation than PH-D children in the left Pu, F(1,1253) = 4.25, p = .039, Cohen d = .11 (CI95 −.01–.21) (Figure 2). [The estimated Bayes factor (null/alternative) suggested that the data were 4.22:1 in favor of the null hypothesis, or rather, 4.22 times more likely to occur under a model without including an effect of parental history of drug problems, rather than a model with it.] For each of the other three ROIs, the activation was not statistically significant: right Pu, F(1,1253) = 3.22, p = .07, Cohen d = .10 (CI95 −.01 to .21); left NAcc, F(1,1253) = 1.36, p = .24, Cohen d = .07 (CI95 −.05 to .17); right NAcc, F(1,1253) < 0.001, p >.99, Cohen d = .0007 (CI95 −.09 to .13) (Figure 2).
Figure 2.
Monetary Incentive Delay (MID) Task Nucleus Accumbens (NAcc; top) and Putamen (Pu) activation: Anticipation of Large Rewards versus Neutral Response. An analysis of variance showed enhanced left Pu activation among PH+D youth relative to PH-D youth.
4. Discussion
This study examined neural correlates of reward processing among pre-adolescents whose parents did or did not have histories of substance use. We hypothesized that PH+ youth would show differential NAcc and Pu activation relative to PH- youth. Youth with a parental history of alcohol problems showed greater right NAcc activation during the anticipation of large rewards. In contrast, participants who were PH+ for drug problems showed enhanced left Pu activation during the anticipation of large rewards. Bayesian analyses showed moderate evidence (BF > 3) in favor of the null hypothesis.
These findings suggest that parental substance use history may negligibly influence adolescent reward processing. These alterations can arise from several factors, including genetic and environmental factors (Meyers & Dick, 2010). One particular factor is parenting style, in which the prevalence of child neglect is higher among children with parents with substance-use problems (Kirisci et al., 2001). Another is risk variants in the genomic pool, such as addiction risk variants in dopaminergic receptors important for drug reward processing (Ducci & Goldman, 2012). However, given the positive evidence in support of the null hypothesis in the current analyses, these factors appear to confer little impact on reward processing among substance-naïve youth.
No previous study, as far as the researchers are aware, demonstrates that parental history of substance use is associated with monetary reward processing in substance-naïve children entering early adolescence. The results partly align with prior evidence of enhanced activity in the Pu among FHP youth relative to FHN youth (Stice & Yokum, 2014). However, our results largely differ from previous work. Whereas our findings demonstrate a small effect size of enhanced NAcc activation in youth who were PH+ for alcohol problems, previous studies found diminished NAcc activity (Yau et al., 2012; Andrews et al., 2011) or no difference in NAcc activation (Bjork et al., 2008; Müller et al., 2015) among FHP participants. The differing findings may stem in part from our focus on a younger sample than prior studies.
There are several limitations of this work that will be important to address in future studies. The study does not address the mechanism by which a parental history of substance use may confer risk via alterations in reward processing and cannot rule out potential confounds, such as prenatal exposure. Given this and the low effect sizes found, which serve as a further limitation, future studies should investigate the effects of genetic risk and in utero exposure on reward processing in substance-naïve adolescents, which cannot be disentangled from this study. Additionally, the lack of p-value adjustment in this work is an additional limitation, potentially causing alpha error growth. Interpreting p-values near the 0.05 significance threshold in high-powered designs may result in erroneous claims of evidence for hypotheses with little to no meaningful significance, resulting in misleading study conclusions (Lin et al., 2013). Furthermore, generating a stratified sample among the PH- participants, although executed to address unbalanced sample sizes, may result in diverging results if the analyses are re-conducted.
Future studies utilizing ABCD Study data should also consider evaluating the impact of peer influence on reward processing, given prior evidence that peer contexts can increase neural activity during reward processing (Smith et al., 2018). Additionally, investigations of connectivity will help elucidate more nuanced effects of family history on pre-adolescent reward processing. Prior work suggests alterations in functional networks implicated in substance abuse among individuals with a family history of substance abuse (Just et al., 2019).
Ultimately, findings from this study suggest that pre-adolescents with a parent history of substance abuse show small alterations in reward neurocircuitry. Still, these findings highlight the utility of studying substance-naïve youth, a population that has been understudied. Future research that addresses the impact of parental substance use and delineates mechanisms that underlie risk to adolescents may ultimately inform approaches to prevention or intervention for PH+ individuals, which could promote normative brain development.
Supplementary Material
Highlights.
More accumbens activity was found in youth with a parent history of alcohol abuse.
Greater putamen activity was found among youth with a parent history of drug abuse.
Parent history for substance problems appears to impact reward system activity.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Drug Abuse (NIDA). Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9-10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from Annual Release 2.0., https://doi.org/10.15154/1520712. DOIs can be found at https://nda.nih.gov/study.html?id=1064. The authors thank Dr. David Epstein, Dr. Reagan Wetherill, Dr. Amanda Devoto, Ms. McKenzie Himelein-Wachowiak, Mr. Chase Smitterberg, Ms. Alyssa Lopez, Dr. Rong Zablocki, Ms. Paola Odriozola, Ms. Sadie Zacharek, Dr. BJ Casey, Ms. May Conley, Ms. Syntia Hadis, Mr. Kevin Anderson, Dr. Daniela Cornejo, and Dr. Thomas Ross for their recommendations, amendments, and assistance during the development of this work.
Role of Funding Sources
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Drug Abuse (NIDA). The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolescent Brain Cognitive Development Study. (n.d.). ABCD Protocol Summary Baseline. https://abcdstudy.org/images/Protocol-Brochure-Baseline.pdf
- Adolescent Brain Cognitive Development Study. (n.d.). Protocols. https://abcdstudy.org/scientists/protocols/
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, & Pearlson GD (2011). Individuals family history positive for alcoholism show fMRI differences in reward sensitivity that are related to impulsivity factors. Biological Psychiatry, 69(7), 675–683. 10.1016/j.biopsych.2010.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, & Hommer DW (2008). Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction, 103(8), 1308–1319. 10.1111/j.1360-0443.2008.02250.x [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich M T., Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, … ABCD Imaging Acquisition Workgroup. (2018). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Developmental Cognitive Neuroscience, 32, 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A (2016). Neurobiological phenotypes associated with a family history of alcoholism. Drug and Alcohol Dependence, 158, 8–21. 10.1016/j.drugalcdep.2015.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducci F, & Goldman D (2012). The genetic basis of addictive disorders. The Psychiatric Clinics of North America, 35(2), 495–519. 10.1016/j.psc.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkenberry T. J. (2018). A Simple Method for Teaching Bayesian Hypothesis Testing in the Brain and Behavioral Sciences. Journal of undergraduate neuroscience education: JUNE : a publication of FUN, Faculty for Undergraduate Neuroscience, 16(2), A126–A130. [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ, Hatton SN, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey B, Barch DM, Harms MP, Watts R, Bjork JM, Garavan HP, Hilmer L, Pung CJ, Sicat CS, Kuperman J, Bartsch H, Xue F, … Dale AM (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202, 116091. 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown SA, & ABCD Consortium Coordinators (2018). Introduction. Developmental Cognitive Neuroscience, 32, 1–3. 10.1016/j.dcn.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just AL, Meng C, Smith DG, Bullmore ET, Robbins TW, & Ersche KD (2019). Effects of familial risk and stimulant drug use on the anticipation of monetary reward: an fMRI study. Translational Psychiatry, 9(1), 65. 10.1038/s41398-019-0399-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirisci L, Dunn MG, Mezzich AC, & Tarter RE (2001). Impact of parental substance use disorder and child neglect severity on substance use involvement in male offspring. Prevention Science, 2(4), 241–255. 10.1023/A:1013662132189 [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, & Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 21(16), RC159. 10.1523/JNEUROSCI.21-16-j0002.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage, 12(1), 20–27. 10.1006/nimg.2000.0593 [DOI] [PubMed] [Google Scholar]
- Lin M, Lucas HC Jr., & Shmueli G (2013). Too big to fail: Large samples and the p-value problem. Information Systems Research, 24(4), 906–917. 10.1287/isre.2013.0480 [DOI] [Google Scholar]
- Masson ME (2011). A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behavior Research Methods, 43(3), 679–690. 10.3758/s13428-010-0049-5 [DOI] [PubMed] [Google Scholar]
- Meyers JL, & Dick DM (2010). Genetic and environmental risk factors for adolescent-onset substance use disorders. Child and Adolescent Psychiatric Clinics of North America, 19(3), 465–477. 10.1016/j.chc.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KU, Gan G, Banaschewski T, Barker GJ, Bokde ALW, Büchel C, Conrod P, Fauth-Bühler M, Flor H, Gallinat J, Garavan H, Gowland P, Heinz A, Ittermann B, Lawrence C, Loth E, Mann K, Martinot J-L, Nees F, … IMAGEN Consortium (2015). No differences in ventral striatum responsivity between adolescents with a positive family history of alcoholism and controls. Addiction Biology, 20(3), 534–545. 10.1111/adb.12136 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (2018, December 3). ABCD study completes enrollment, announces opportunities for scientific engagement. https://www.nih.gov/news-events/news-releases/abcd-study-completes-enrollment-announces-opportunities-scientific-engagement
- National Institutes of Health. (2020, April 15). Landmark study of adolescent brain development renews for additional seven years. https://www.nih.gov/news-events/news-releases/landmark-study-adolescent-brain-development-renews-additional-seven-years
- Raftery AE (1999). Bayes factors and BIC: Comment on “A critique of the Bayesian Information Criterion for model selection.” Sociological Methods & Research, 27(3), 411–427. 10.1177/0049124199027003005 [DOI] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Schuckit MA, & Begleiter H (1995). Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research, 19(4), 1018–1023. 10.1111/j.1530-0277.1995.tb00983.x [DOI] [PubMed] [Google Scholar]
- Rose S, & van der Laan MJ (2009). Why match? Investigating matched case-control study designs with causal effect estimation. The International Journal of Biostatistics, 5(1). 10.2202/1557-4679.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold S & Perktold J (2010). “statsmodels: Econometric and statistical modeling with python.” Proceedings of the 9th Python in Science Conference. [Google Scholar]
- Smith AR, Rosenbaum GM, Botdorf MA, Steinberg L, & Chein JM (2018). Peers influence adolescent reward processing, but not response inhibition. Cognitive, Affective, & Behavioral Neuroscience, 18(2), 284–295. 10.3758/s13415-018-0569-5 [DOI] [PubMed] [Google Scholar]
- Stice E, & Yokum S (2014). Brain reward region responsivity of adolescents with and without parental substance use disorders.Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors, 28(3), 805–815. 10.1037/a0034460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher DL, & Clark DB (2008). Adolescents at risk for substance use disorders: role of psychological dysregulation, endophenotypes, and environmental influences. Alcohol research & health : The Journal of the National Institute on Alcohol Abuse and Alcoholism, 31(2), 168–176. [PMC free article] [PubMed] [Google Scholar]
- Warthen KG, Boyse-Peacor A, Jones KG, Sanford B, Love TM, & Mickey BJ (2020). Sex differences in the human reward system: Convergent behavioral, autonomic and neural evidence. Social Cognitive and Affective Neuroscience, 15(7), 789–801. 10.1093/scan/nsaa104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitesell M, Bachand A, Peel J, & Brown M (2013). Familial, social, and individual factors contributing to risk for adolescent substance use. Journal of Addiction, 2013, 579310. 10.1155/2013/579310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau W-YW, Zubieta J-K, Weiland BJ, Samudra PG, Zucker RA, & Heitzeg MM (2012). Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: Relationships with precursive behavioral risk and lifetime alcohol use. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 32(7), 2544–2551. 10.1523/JNEUROSCI.1390-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.