Abstract
Spatial working memory, the ability to temporarily maintain an internal representation of spatial information for use in guiding upcoming decisions, has been shown to be dependent upon a network of brain structures that includes the hippocampus, a region known to be critical for spatial navigation and episodic memory, and the prefrontal cortex (PFC), a region known to be critical for executive function and goal directed behavior. Oscillatory synchronization between the hippocampus and the prefrontal cortex (PFC) is known to increase in situations of high working memory demand. Most of our knowledge about the anatomical connectivity between the PFC and hippocampus comes from the rodent literature. Thus, most of the findings that will be discussed here model human working memory using spatial working memory-dependent maze navigation tasks in rodents. It has been demonstrated that the ventral midline thalamic nucleus reuniens (Re) is reciprocally connected to both the infralimbic and prelimbic subregions of the PFC, collectively referred to as the medial PFC (mPFC), and the hippocampus. Given that the Re serves as a major anatomical route between the mPFC and hippocampus, it is perhaps not surprising that Re has been shown to be critical for spatial working memory. This review will describe the latest findings and ideas on how the Re contributes to prefrontal-hippocampal synchronization and spatial working memory in rodents. The review will conclude with possible future directions that will advance the understanding of the mechanisms that enable the Re to orchestrate long range synchrony in the prefrontal-hippocampal network.
My interest in the ventral midline thalamic nucleus, the nucleus reuniens (Re) began in 2003 when I was writing my dissertation. I was struggling to explain an unexpected finding -- pharmacological inactivation of the anterior cingulate cortex, a subregion of the medial PFC (mPFC), resulted in an elimination of extinction-related suppression of dorsal hippocampal activity, which accompanied an extinction deficit in appetitive Pavlovian conditioning in the rabbit (Griffin & Berry, 2004). In the early 2000’s, Robert Vertes was one of the few researchers studying the ventral midline thalamic nuclei. Before reading Dr. Vertes’ work, I naively thought of the thalamus as strictly a sensory relay and was unaware of the cognitive functions subserved by the nonsensory thalamic nuclei (Pereira de Vasconcelos & Cassel, 2015). Little did I know then that this one small midline thalamic nucleus would later become a main focus of my research program. I was intrigued to learn that although there are little to no direct projections from the medial prefrontal subregions, including anterior cingulate cortex, prelimbic and infralimbic cortices to the hippocampus, there was a prominent projection from mPFC to the Re (Vertes, 2002), which sends projections to the hippocampus and subiculum (Dolleman-Van Der Weel & Witter, 1996; Vertes, Hoover, Do Valle, Sherman, & Rodriguez, 2006; Wouterlood, Saldana, & Witter, 1990) There are, of course, other anatomical routes by which the mPFC and hippocampus can interact (Vertes, 2006; Vertes et al., 2006). What makes the interaction via the Re special and unique is the fact that the same Re neurons that receive input from mPFC also project to the hippocampus (Vertes, Hoover, Szigeti-Buck, & Leranth, 2007). Moreover, there is a small subset (3–9%) of Re neurons that send collaterals to mPFC and hippocampus (Hoover & Vertes, 2007), a finding that was corroborated by Varela and colleagues a few years later (Varela, Kumar, Yang, & Wilson, 2014). In fact, they found that 25% of the Re neurons that project to hippocampus also project to mPFC. The authors suggest that this relatively large number of dual-projecting neurons within the population of hippocampal-projecting Re neurons might enable mPFC activation to co-occur with the activation of hippocampal-projecting Re neurons. This co-activation could enable or facilitate precisely-timed functional connectivity between the hippocampus and mPFC. One other interesting finding from the anatomical studies is that the only direct projection between hippocampus and the mPFC is from the ventral hippocampus to the mPFC. Thus, interactions between dorsal hippocampus, from which most recording studies in rodents are conducted, and mPFC are strictly multi-synaptic. There is evidence that there are anatomical and functional differences along the hippocampal septo-temporal axis. Dorsal hippocampus, which receives strong inputs from polymodal sensory cortices, is linked to cognitive processes such as spatial navigation and learning. By contrast, ventral hippocampus, which receives input from subcortical regions like the amygdala and hypothalamus, is linked to emotional processes such as fear and anxiety (Bannerman et al., 2003; Bannerman et al., 2014; Czerniawski, Yoon, & Otto, 2009; Moser, Moser, & Andersen, 1993; Yoon & Otto, 2007). Because of its link to cognitive processes like spatial working memory, this review will focus on the interactions between dorsal region of hippocampus and the mPFC via the Re. Although this review will focus on Re, I should note that there are other thalamic nuclei that are known to contribute to working memory other than the Re. For example, the anterior thalamus nuclei (ATN) are reciprocally connected to the hippocampal formation, specifically the dorsal subiculum (Christiansen et al., 2016; Meibach & Siegel, 1977; Shibata, 1993; Wright, Erichsen, Vann, O’Mara, & Aggleton, 2010). A recent study found that inhibition of ATN, dorsal subiculum, and the projections between these two regions impaired T-maze alternation. (Nelson, Kinnavane, Amin, O’Mara, & Aggleton, 2020). Another thalamic nucleus, the mediodorsal thalamic nucleus (MD), is reciprocally connected with the mPFC (E. G. Jones, 2007). It was shown that projections from MD to mPFC supporting the maintenance of spatial working memory and the projections from mPFC to MD supporting retrieval. Moreover, optogentically increasing MD excitability during the time period of working memory maintenance enhanced working memory performance (Bolkan et al., 2017).
Although we have known for years now that one of the main anatomical routes by which the mPFC sends information to the dorsal hippocampus is through the Re, it is only in the last few years that we have begun to understand the details of this interaction. I have been fortunate to connect with a community of researchers interested in the limbic thalamus, most of whom are included in this special issue. My fellow “limbic thalamus” researchers and I had an invigorating meeting in the summer of 2018 in the beautiful city of Strasbourg, France. Around that time, there had been somewhat of a surge in interest in the Re. One fascinating discovery was that Re inactivation/activation enhanced/reduced fear memory generalization, respectively (Xu & Sudhof, 2013). Another group found that the Re contains head direction cells (Jankowski et al., 2014). Ito and colleagues had published a paper a few years prior to the meeting that investigated trajectory -dependent firing of single units within the prefrontal-thalamo-hippocampal circuit. (Ito, Zhang, Witter, Moser, & Moser, 2015). Trajectory-dependent firing is defined as single neurons that exhibit different firing rates depending on the trajectory of the animal. This phenomenon is thought to represent the prospective representation of upcoming and/or past spatial trajectories. One of the features of the anatomical connectivity between Re and dorsal hippocampus that is splendid from an experimental standpoint is that Re projects to CA1, but not CA3. This anatomical connectivity gave Ito and colleagues the advantage of being able to compare effects of Re inactivation on CA1 and CA3 trajectory coding. If trajectory dependent firing is a signal that is conveyed from the mPFC to the dorsal hippocampus via the Re, one would expect that trajectory –dependent firing would be less evident in CA3 than CA1 and that inactivation of Re would greatly reduce CA1 trajectory coding. Indeed, that is exactly what Ito et al. (2015) found.
Trajectory dependent firing in dorsal hippocampal neurons was first demonstrated in the early 2000’s by separate labs (Ferbinteanu & Shapiro, 2003; Frank, Brown, & Wilson, 2000; Wood, Dudchenko, Robitsek, & Eichenbaum, 2000) and has recently been shown to emerge quickly and remain stable across multiple days (Kinsky et al., 2020), with the ensemble task representation and task performance remaining stable despite changes in task correlates of individual neurons (Levy, Kinsky, Mau, Sullivan, & Hasselmo, 2021). One puzzling aspect of dorsal hippocampal trajectory dependent firing is that the task used in the Ito et al. 2015 and Wood et al. 2000 studies, continuous spatial alternation, does not depend on the integrity of the dorsal hippocampus. Because continuous spatial alternation only requires that the rat move in a continuous figure eight pattern, we should perhaps not be surprised that the task can be completed successfully in hippocampal-lesioned animals (Ainge, van der Meer, Langston, & Wood, 2007). However, a very subtle change to the task, adding a delay of only a few seconds between trials, renders the task hippocampus-dependent (Ainge, van der Meer, Langston, & Wood, 2007). When Ito and colleagues (2015) introduced a delay into their task, dorsal CA1 and mPFC, but not Re neural ensembles showed retrospective coding during the delay period. They also showed that dorsal CA1, mPFC, and Re showed prospective coding as the rat approached the T-junction (i.e. “choice point”) of the maze. Our lab has compared trajectory dependent firing across various T-maze tasks that seem overtly similar, but yet show dramatic differences in trajectory dependent firing in the dorsal hippocampus and mPFC. As we saw from the Ainge et al. (2007) study mentioned above, just introducing a brief delay into the spatial alternation task dramatically changed the necessity of the hippocampus. Even more interesting, we found that introducing a delay into the task eliminated trajectory coding seen on the continuous version of the task (Hallock & Griffin, 2013).
Our lab has developed a T-maze task that we often use as a control task in our investigations of the neural circuitry that supports spatial working memory. This task, a visuospatial conditional discrimination, requires the rats to make goal arm choices depending on the appearance and texture of a floor insert that is placed in the maze stem prior to each trial. For example, the rat would learn to turn left when the flooring is black mesh and right when the flooring is smooth wood. We discovered that the performance of this task does not require the dorsal hippocampus, but instead depends on the integrity of the dorsal striatum. We also confirmed that the opposite was true for the delayed spatial alternation task – the dorsal hippocampus is required for delayed spatial alternation, whereas the dorsal striatum is not (Hallock, Arreola, Shaw, & Griffin, 2013). Previously, we had discovered that trajectory coding in dorsal hippocampus was not evident during the conditional discrimination task even though the same neural ensembles show robust trajectory coding during continuous spatial alternation (Griffin, Owens, Peters, Adelman, & Cline, 2012). During delayed spatial alternation, the trajectory coding appears to “move” to the delay period (Hallock & Griffin, 2013). Other labs have found that dorsal CA1 neurons are active during the delay period. Pastalkova and colleagues discovered what they called “cell assembly sequences” – dorsal CA1 cells that fired sequentially during the delay period in a sequence that corresponding to an upcoming trajectory (Pastalkova, Itskov, Amarasingham, & Buzsaki, 2008). Gill and colleagues also found dorsal CA1 neurons with “episode fields” – exhibiting differential firing rates depending upon the upcoming trajectory(Gill, Mizumori, & Smith, 2011). Moreover, Kraus and colleagues discovered what they called “time cells” – dorsal hippocampal neurons that exhibited transient increases in firing rate at specific times as rats ran on a treadmill during the delay period, creating a sequence of firing that represents the different paths through the maze(Kraus, Robinson, White, Eichenbaum, & Hasselmo, 2013). Sequential activation of neurons has been observed not only in hippocampus, but also in mPFC, and in Re during the transition from down to up states in a study that recorded local field potentials and single units in Re, mPFC, and hippocampus during slow oscillations in anesthetized rats. Moreover, Re inactivation disrupted sequential firing of mPFC neurons during the down to up state transition and of hippocampal neurons during sharp-wave ripple events, suggesting that Re helps to organize internally generated sequences throughout the prefrontal-hippocampal network (Angulo-Garcia et al., 2020).
One theme that has emerged from the many studies that have investigated the dorsal hippocampal correlates of memory is that just because a specific striking activity pattern is exciting to the researcher, that does not mean that the neurons are coding what we as researchers think is important. For example, apparent encoding of task correlates in a memory sequence task by mPFC neurons was found to be better explained by overt behavioral patterns than by internal cognitive operations(Euston & McNaughton, 2006). As experimenters, we need to be cautious about ascribing neural activity patterns to particular cognitive processes. I learned this lesson during my postdoctoral fellowship. We predicted that trajectory coding would be even more robust in a known hippocampus-dependent task than it was in the continuous alternation task. We selected the commonly-used and hippocampus-dependent delayed nonmatch to position T-maze task. Like delayed spatial alternation, this task requires that rats alternate visits to the left and right goal arms. Unlike delayed spatial alternation, each trial of this task includes two traversals that are separated by a brief delay: a sample traversal in which one of the goal arms is blocked, forcing the rat to choose to enter the opposite goal arm and a choice traversal in which the rat is free to choose a goal arm but only rewarded for choosing the goal arm opposite to the arm visited on the sample traversal. Surprisingly, trajectory coding was very weak in this task. Instead, dorsal hippocampal neurons were sensitive to a sample or choice traversal, with some neurons firing preferentially during sample traversals and some firing preferentially during choice traversals (Griffin, Eichenbaum, & Hasselmo, 2007). One way to decrease the likelihood of misattributing neural activity patterns to cognitive operations is to compare neural activity patterns across tasks that vary only in their reliance on the particular cognitive process of interest. In an attempt to accomplish this for a spatial working memory task, we developed a version of the conditional discrimination task that engages spatial working memory by shortening the length of the floor inserts so that they covered only the first two-thirds of the maze stem. This subtle change in the task made the task much more difficult to learn (Edsall, Gemzik, & Griffin, 2017) and importantly, made the task dependent upon the mPFC (Urban, Layfield, & Griffin, 2014) and the Re (Hallock, Wang, Shaw, & Griffin, 2013). We can now compare neural activity patterns across these two variants of the conditional discrimination task to identify neural firing patterns and cross-regional oscillatory interactions that are selective to working memory by ruling out other possible confounding factors that may be at play. To date, there have been no investigations that have examined neural ensemble sequences in the presence and absence of Re influence in a task with a delay (e.g. delayed nonmatch to position or delayed spatial alternation). Such studies would answer the question of whether/how Re contributes to the prospective and retrospective coding of mPFC and dorsal CA1 ensembles and maybe answer the question of why task correlates vary dramatically with subtle changes to the structure of the task.
In 2015, I wrote a review paper that covered what we knew at the time about prefrontal-hippocampal interactions during spatial working memory and hypothesized that the Re could be critical for meditating these interactions (Griffin, 2015). Briefly, we knew that prefrontal-hippocampal synchronization as measured by mPFC unit phase locking to hippocampal theta and theta coherence between the mPFC and dorsal hippocampus increased during exploration and spatial working memory tasks (Hyman, Zilli, Paley, & Hasselmo, 2005; M. W. Jones & Wilson, 2005; Siapas, Lubenov, & Wilson, 2005) and decreased on error trials (Hyman, Zilli, Paley, & Hasselmo, 2010). We also knew that spatial working memory was dependent upon both the mPFC, the hippocampus, and the connections between them (Churchwell & Kesner, 2011; Floresco, Seamans, & Phillips, 1997) and that the integrity of the Re was important for win/shift performance in a radial arm maze and delayed nonmatch-to-position lever-pressing task, both of which are also impaired by hippocampal and mPFC-lesions (Hembrook & Mair, 2011; Hembrook, Onos, & Mair, 2012). A recent study showed that both spatial working memory and behavioral flexibility were impaired after Re inactivation (Viena, Linley, & Vertes, 2018).
My review paper ended with a question that had been unexplored: Does Re inactivation decrease prefrontal-hippocampal theta synchrony? To answer this question, we performed simultaneous recordings from mPFC and dorsal hippocampus while rats performed the delayed spatial alternation and conditional discrimination tasks. By comparing theta synchronization (coherence, unit phase locking, phase-amplitude coupling) across tasks, we were able to identify times during the task when theta synchrony between the medial PFC and dorsal hippocampus was significantly elevated for the working memory dependent delayed spatial alternation task compared to the working memory independent conditional discrimination task. We found that for the delayed spatial alternation task, theta coherence was elevated at the T-junction of the maze and that mPFC unit phase locking was elevated during the delay period. In a separate group of rats, we then recorded from medial PFC and dorsal hippocampus before and after inactivating the Re with muscimol to investigate whether or not suppressing Re would reduce this spatial working memory specific theta synchrony. As predicted, Re inactivation decreased all of the measures of theta synchrony that were selective to the working memory dependent delayed spatial alternation task. This finding led us to tentatively conclude that Re was the orchestrator of prefrontal-hippocampal synchrony (Hallock, Wang, & Griffin, 2016).
One drawback of the delayed spatial alternation task is that it is impossible to know if deficits in task performance arise due to an inability to encode new information, maintain the information over a delay, or retrieve previously-encoded information at the exact time of a goal arm choice. The Gordon lab has used the delayed nonmatch to position task in mice coupled with optogenetic manipulation to dissociate between the encoding, maintenance, and retrieval of spatial information (Bolkan et al., 2017; O’Neill, Gordon, & Sigurdsson, 2013; Sigurdsson, Stark, Karayiorgou, Gogos, & Gordon, 2010; Spellman et al., 2015; Tamura, Spellman, Rosen, Gogos, & Gordon, 2017). They have found that ventral hippocampal input to mPFC was critical for spatial working memory encoding (Spellman et al., 2015). A later study showed that the mediodorsal thalamus was critical for spatial working memory maintenance (Bolkan et al., 2017). Inspired by this approach, we used the delayed nonmatch to position task in rats to investigate the role of the Re in spatial working memory encoding, maintenance and retrieval. We found that Re optogenetic suppression impaired spatial working memory encoding, leaving maintenance and retrieval unaffected (Maisson, Gemzik, & Griffin, 2018). This finding suggests that Re output, a large majority of which goes to mPFC and hippocampus (Vertes et al., 2007), is necessary for acquiring salient to-be-remembered spatial cues for use in upcoming decisions.
Although we have made a great deal of progress in our understanding of the prefrontal-thalamo-hippocampal circuit in spatial working memory (See Fig. 1), there are many remaining questions:
Is oscillatory synchronization within prefrontal-thalamo-hippocampal circuit specific to spatial working memory, or is spatial working memory just one example of many cognitive functions subserved by this functional connectivity? Recent work, for example, has shown that Re is critical for and trace fear conditioning (Lin, Chiou, & Chang, 2020), contextual fear memory persistence (Quet et al., 2020) and that both Re and mPFC projections to Re are critical for the extinction of contextual fear conditioning (Bouton, Maren, & McNally, 2020; Ramanathan, Jin, Giustino, Payne, & Maren, 2018; Ramanathan & Maren, 2019) and signaled active avoidance (Moscarello, 2020).
Are there times when prefrontal-hippocampal theta synchrony should be low? In other words, would there be situations in which functional connectivity would need to be temporarily inhibited? Perhaps theta synchrony would need to be low during times when it is necessary to pay attention to external cues instead of internally generated information. One hypothesis is that Re bidirectionally modulates prefrontal-hippocampal theta synchrony based on whether new information is being encoded or old information is being retrieved. Future studies could test this hypothesis using a combination of circuit-based suppression techniques (i.e. optogenetics or chemogenetics) combined with high-density multi-site recordings.
What is the functional role of the convergence of inputs to CA1 and mPFC? Re and entorhinal cortex both terminate in stratum lacunosum moleculare of CA1. In a pair of related studies, (Dolleman-Van der Weel, Lopes da Silva, & Witter, 1997, 2017), stimulated Re-CA1 afferents in the anesthetized rat while evoked potentials and unit activity were recorded from CA1. The results showed that Re plays a modulatory role in the excitation of CA1 pyramidal cells and elicits spiking in inhibitory interneurons. Another study stimulated Re and entorhinal cortex simultaneously and found a nonlinear subthreshold response in CA1, indicating that entorhinal cortex and Re synapse on the same cellular compartment (Dolleman-van der Weel et al., 2017). Many testable hypotheses arise from these findings. First, elimination of either Re or entorhinal input to CA1 should have consequences for memory task performance, particularly tasks that rely on interactions within the larger prefrontal-thalmo-hippocampal network. Similarly, task-correlates of dCA1 neurons should be altered as well. Similarly, Re and hippocampal projections both terminate in mPFC layers 1 and 5/6. Little is known about the electrophysiological response profile of these two inputs and the associated behavioral correlates. It should also be noted that entorhinal cortex and Re are densely interconnected and both give rise to terminals that project to the mPFC and hippocampus (Dolleman-van der Weel et al., 2019; Vertes, 2006; Vertes et al., 2006).
What is the role of the Re neurons that send collaterals to the hippocampus and mPFC and how does this small population of Re neurons compare to neurons that project only to either the hippocampus or mPFC? It is possible that these dual-projecting collaterals enhance prefrontal-hippocampal oscillatory synchrony by enabling excitation of mPFC neurons precisely at the same time that projection neurons from Re to hippocampus are active. Targeting the dual-projecting Re population with optogenetic or chemogenetic approaches could partially answer this question. Coupling suppression/activation of this dual-projecting population with multisite recordings along the prefrontal-thalamo-hippocampal network would shed even more “light” on the role of this small but unique subpopulation of Re neurons.
Figure 1.
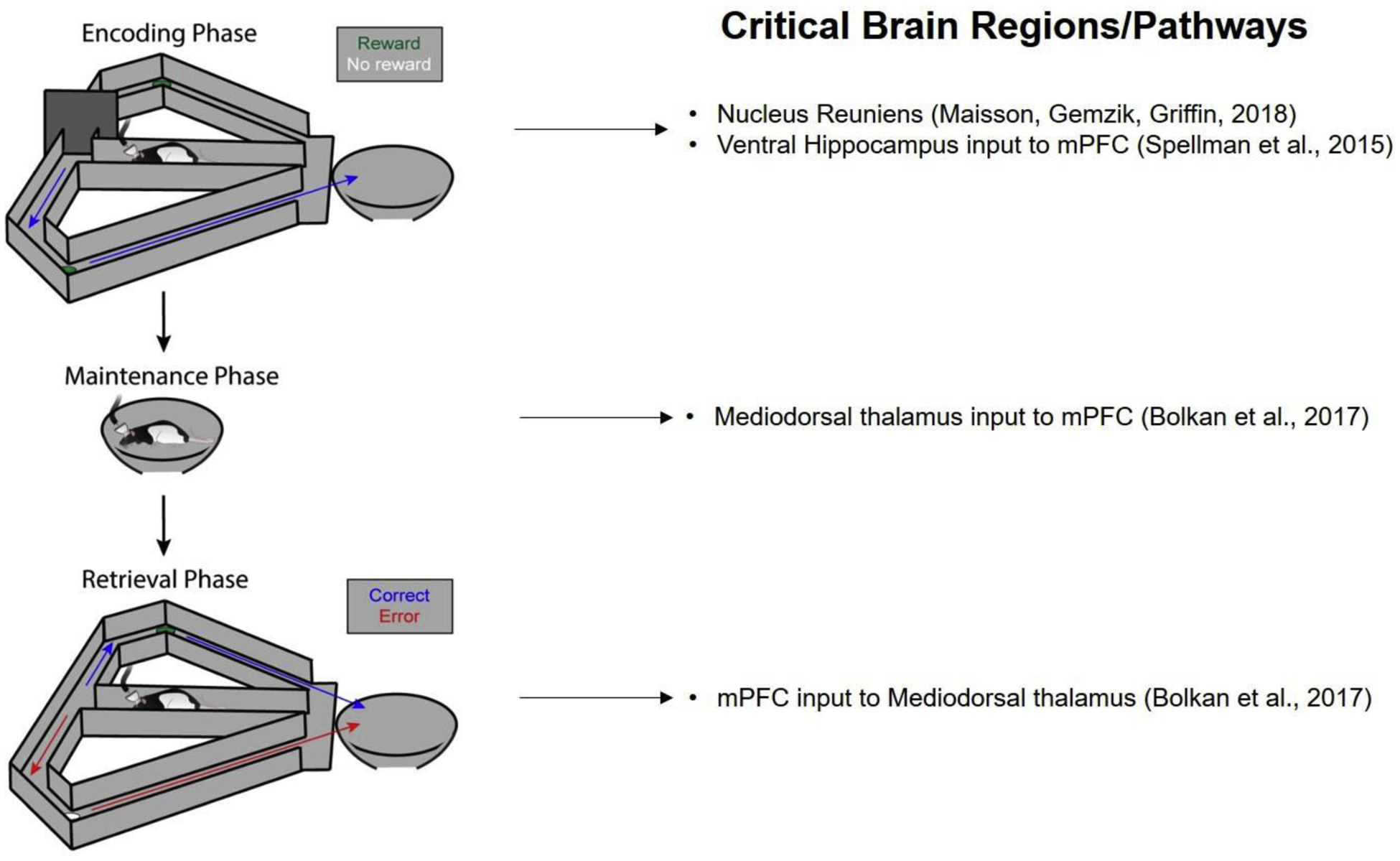
Schematic of the T-maze Delayed Nonmatch to Position task (left) with the brain regions and pathways that have been shown to be critical for task performance (right). The task consists of three phases: an encoding phase in which the rodent is forced to turn into one of the two goal arms, a maintenance phase, in which the rodent is confined to the start box, and a retrieval phase, in which the rodent must chose the opposite goal arm from the previously-visited arm in order to receive reward. Note that Spellman et al., 2015 and Bolkan et al., 2017 used a traditional T-maze in their experiments, instead of the T-maze modified with return arms used in Maisson, Gemzik and Griffin, 2018 that is depicted in the figure.
In conclusion, spatial working memory requires both encoding of task-relevant information at the appropriate time and subsequent retrieval of that information at the exact moment it is needed (Eichenbaum, 2017; Place, Farovik, Brockmann, & Eichenbaum, 2016; Spellman et al., 2015). Working memory deficits may involve a disruption of this precise timing such that information encoding and/or retrieval fail to occur at the appropriate time. We now know that both spatial working memory and prefrontal-hippocampal theta coherence are impaired by Re suppression (Hallock et al., 2016). Working memory impairments are common in various neurodevelopmental and neuropsychiatric disorders, such as schizophrenia and epilepsy and are associated with impaired interactions between the hippocampus and PFC (Dolleman-van der Weel & Witter, 2020). These interactions are known to be reduced in both human clinical populations (Argyelan et al., 2014; Bassett, Nelson, Mueller, Camchong, & Lim, 2012; Lawrie et al., 2002; A. Meyer-Lindenberg et al., 2001; A. S. Meyer-Lindenberg et al., 2005; Venkataraman, Whitford, Westin, Golland, & Kubicki, 2012) and a rodent models of schizophrenia (Dickerson, Wolff, & Bilkey, 2010; Sigurdsson et al., 2010). Interestingly, the Re was recently shown to undergo significant neuron loss in a rodent model of fetal alcohol spectrum disorders (Gursky, Spillman, & Klintsova, 2020). Re neurons were also shown to be hyperexcitable in rodent model of Alzheimer’s disease, which may contribute to hyperexcitability within the prefrontal-hippocampal network (Walsh, Brown, & Randall, 2020). Thus, understanding of how information is exchanged within the prefrontal-thalamo-hippocampal circuit may lead to better treatments and interventions for cognitive dysfunction.
Highlights.
The nucleus reuniens reciprocally connects the hippocampus and prefrontal cortex.
The nucleus reuniens is critical for spatial working memory in rodents.
Inactivation of reuniens disrupts hippocampal-prefrontal synchronization.
Questions remain about communication in the prefrontal-thalamo-hippocampal circuit.
Acknowledgements:
This work is supported by National Institutes of Health R01 MH102394.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- Ainge JA, van der Meer MA, Langston RF, & Wood ER (2007). Exploring the role of context-dependent hippocampal activity in spatial alternation behavior. Hippocampus, 17(10), 988–1002. doi: 10.1002/hipo.20301 [DOI] [PubMed] [Google Scholar]
- Angulo-Garcia D, Ferraris M, Ghestem A, Nallet-Khosrofian L, Bernard C, & Quilichini PP (2020). Cell Assemblies in the Cortico-Hippocampal-Reuniens Network during Slow Oscillations. J Neurosci, 40(43), 8343–8354. doi: 10.1523/JNEUROSCI.0571-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, John M, … Szeszko PR (2014). Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr Bull, 40(1), 100–110. doi: 10.1093/schbul/sbt092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, & Rawlins JN (2003). Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res, 139(1–2), 197–213. doi: 10.1016/s0166-4328(02)00268-1 [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, & Seeburg PH (2014). Hippocampal synaptic plasticity, spatial memory and anxiety. Nat Rev Neurosci, 15(3), 181–192. doi: 10.1038/nrn3677 [DOI] [PubMed] [Google Scholar]
- Bassett DS, Nelson BG, Mueller BA, Camchong J, & Lim KO (2012). Altered resting state complexity in schizophrenia. Neuroimage, 59(3), 2196–2207. doi: 10.1016/j.neuroimage.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI, … Kellendonk C (2017). Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci, 20(7), 987–996. doi: 10.1038/nn.4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Maren S, & McNally GP (2020). Behavioral and Neurobiological Mechanisms of Pavlovian and Instrumental Extinction Learning. Physiol Rev. doi: 10.1152/physrev.00016.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen K, Dillingham CM, Wright NF, Saunders RC, Vann SD, & Aggleton JP (2016). Complementary subicular pathways to the anterior thalamic nuclei and mammillary bodies in the rat and macaque monkey brain. Eur J Neurosci, 43(8), 1044–1061. doi: 10.1111/ejn.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, & Kesner RP (2011). Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res, 225(2), 389–395. doi: 10.1016/j.bbr.2011.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, & Otto T (2009). Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus, 19(1), 20–32. doi: 10.1002/hipo.20469 [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, & Bilkey DK (2010). Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci, 30(37), 12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Griffin AL, Ito HT, Shapiro ML, Witter MP, Vertes RP, & Allen TA (2019). The nucleus reuniens of the thalamus sits at the nexus of a hippocampus and medial prefrontal cortex circuit enabling memory and behavior. Learn Mem, 26(7), 191–205. doi: 10.1101/lm.048389.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-Van der Weel MJ, Lopes da Silva FH, & Witter MP (1997). Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. J Neurosci, 17(14), 5640–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, Lopes da Silva FH, & Witter MP (2017). Interaction of nucleus reuniens and entorhinal cortex projections in hippocampal field CA1 of the rat. Brain Struct Funct, 222(5), 2421–2438. doi: 10.1007/s00429-016-1350-6 [DOI] [PubMed] [Google Scholar]
- Dolleman-Van Der Weel MJ, & Witter MP (1996). Projections from the nucleus reuniens thalami to the entorhinal cortex, hippocampal field CA1, and the subiculum in the rat arise from different populations of neurons. J Comp Neurol, 364(4), 637–650. doi: [DOI] [PubMed] [Google Scholar]
- Dolleman-van der Weel MJ, & Witter MP (2020). The thalamic midline nucleus reuniens: potential relevance for schizophrenia and epilepsy. Neurosci Biobehav Rev, 119, 422–439. doi: 10.1016/j.neubiorev.2020.09.033 [DOI] [PubMed] [Google Scholar]
- Edsall A, Gemzik Z, & Griffin A (2017). A Tactile-visual Conditional Discrimination Task for Testing Spatial Working Memory in Rats. Bio Protoc, 7(10). doi: 10.21769/BioProtoc.2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H (2017). Prefrontal-hippocampal interactions in episodic memory. Nat Rev Neurosci, 18(9), 547–558. doi: 10.1038/nrn.2017.74 [DOI] [PubMed] [Google Scholar]
- Euston DR, & McNaughton BL (2006). Apparent encoding of sequential context in rat medial prefrontal cortex is accounted for by behavioral variability. J Neurosci, 26(51), 13143–13155. doi: 10.1523/JNEUROSCI.3803-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, & Shapiro ML (2003). Prospective and retrospective memory coding in the hippocampus. Neuron, 40(6), 1227–1239. doi: 10.1016/s0896-6273(03)00752-9 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, & Phillips AG (1997). Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci, 17(5), 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, & Wilson M (2000). Trajectory encoding in the hippocampus and entorhinal cortex. Neuron, 27(1), 169–178. [DOI] [PubMed] [Google Scholar]
- Gill PR, Mizumori SJ, & Smith DM (2011). Hippocampal episode fields develop with learning. Hippocampus, 21(11), 1240–1249. doi: 10.1002/hipo.20832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL (2015). Role of the thalamic nucleus reuniens in mediating interactions between the hippocampus and medial prefrontal cortex during spatial working memory. Front Syst Neurosci, 9, 29. doi: 10.3389/fnsys.2015.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, & Berry SD (2004). Inactivation of the anterior cingulate cortex impairs extinction of rabbit jaw movement conditioning and prevents extinction-related inhibition of hippocampal activity. Learn Mem, 11(5), 604–610. doi: 10.1101/lm.78404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, Eichenbaum H, & Hasselmo ME (2007). Spatial representations of hippocampal CA1 neurons are modulated by behavioral context in a hippocampus-dependent memory task. J Neurosci, 27(9), 2416–2423. doi: 10.1523/JNEUROSCI.4083-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AL, Owens CB, Peters GJ, Adelman PC, & Cline KM (2012). Spatial representations in dorsal hippocampal neurons during a tactile-visual conditional discrimination task. Hippocampus, 22(2), 299–308. doi: 10.1002/hipo.20898 [DOI] [PubMed] [Google Scholar]
- Gursky ZH, Spillman EC, & Klintsova AY (2020). Single-day Postnatal Alcohol Exposure Induces Apoptotic Cell Death and Causes long-term Neuron Loss in Rodent Thalamic Nucleus Reuniens. Neuroscience, 435, 124–134. doi: 10.1016/j.neuroscience.2020.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Arreola AC, Shaw CL, & Griffin AL (2013). Dissociable roles of the dorsal striatum and dorsal hippocampus in conditional discrimination and spatial alternation T-maze tasks. Neurobiol Learn Mem, 100, 108–116. doi: 10.1016/j.nlm.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Hallock HL, & Griffin AL (2013). Dynamic coding of dorsal hippocampal neurons between tasks that differ in structure and memory demand. Hippocampus, 23(2), 169–186. doi: 10.1002/hipo.22079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, & Griffin AL (2016). Ventral Midline Thalamus Is Critical for Hippocampal-Prefrontal Synchrony and Spatial Working Memory. J Neurosci, 36(32), 8372–8389. doi: 10.1523/JNEUROSCI.0991-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallock HL, Wang A, Shaw CL, & Griffin AL (2013). Transient inactivation of the thalamic nucleus reuniens and rhomboid nucleus produces deficits of a working-memory dependent tactile-visual conditional discrimination task. Behav Neurosci, 127(6), 860–866. doi: 10.1037/a0034653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, & Mair RG (2011). Lesions of reuniens and rhomboid thalamic nuclei impair radial maze win-shift performance. Hippocampus, 21(8), 815–826. doi: 10.1002/hipo.20797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook JR, Onos KD, & Mair RG (2012). Inactivation of ventral midline thalamus produces selective spatial delayed conditional discrimination impairment in the rat. Hippocampus, 22(4), 853–860. doi: 10.1002/hipo.20945 [DOI] [PubMed] [Google Scholar]
- Hoover WB, & Vertes RP (2007). Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct, 212(2), 149–179. doi: 10.1007/s00429-007-0150-4 [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, & Hasselmo ME (2005). Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus, 15(6), 739–749. doi: 10.1002/hipo.20106 [DOI] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, & Hasselmo ME (2010). Working Memory Performance Correlates with Prefrontal-Hippocampal Theta Interactions but not with Prefrontal Neuron Firing Rates. Front Integr Neurosci, 4, 2. doi: 10.3389/neuro.07.002.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Zhang SJ, Witter MP, Moser EI, & Moser MB (2015). A prefrontal-thalamo-hippocampal circuit for goal-directed spatial navigation. Nature, 522(7554), 50–55. doi: 10.1038/nature14396 [DOI] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP, & O’Mara SM (2014). Nucleus reuniens of the thalamus contains head direction cells. Elife, 3. doi: 10.7554/eLife.03075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG (2007). The Thalamus. 2. New York: Cambridge University Press. [Google Scholar]
- Jones MW, & Wilson MA (2005). Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol, 3(12), e402. doi: 10.1371/journal.pbio.0030402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky NR, Mau W, Sullivan DW, Levy SJ, Ruesch EA, & Hasselmo ME (2020). Trajectory-modulated hippocampal neurons persist throughout memory-guided navigation. Nat Commun, 11(1), 2443. doi: 10.1038/s41467-020-16226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus BJ, Robinson RJ 2nd, White JA, Eichenbaum H, & Hasselmo ME (2013). Hippocampal “time cells”: time versus path integration. Neuron, 78(6), 1090–1101. doi: 10.1016/j.neuron.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, & Johnstone EC (2002). Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry, 51(12), 1008–1011. [DOI] [PubMed] [Google Scholar]
- Levy SJ, Kinsky NR, Mau W, Sullivan DW, & Hasselmo ME (2021). Hippocampal spatial memory representations in mice are heterogeneously stable. Hippocampus, 31(3), 244–260. doi: 10.1002/hipo.23272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YJ, Chiou RJ, & Chang CH (2020). The Reuniens and Rhomboid Nuclei Are Required for Acquisition of Pavlovian Trace Fear Conditioning in Rats. eNeuro. doi: 10.1523/ENEURO.0106-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisson DJ, Gemzik ZM, & Griffin AL (2018). Optogenetic suppression of the nucleus reuniens selectively impairs encoding during spatial working memory. Neurobiol Learn Mem, 155, 78–85. doi: 10.1016/j.nlm.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Meibach RC, & Siegel A (1977). Thalamic projections of the hippocampal formation: evidence for an alternate pathway involving the internal capsule. Brain Res, 134(1), 1–12. doi: 10.1016/0006-8993(77)90921-0 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, & Berman KF (2001). Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry, 158(11), 1809–1817. doi: 10.1176/appi.ajp.158.11.1809 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, & Berman KF (2005). Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry, 62(4), 379–386. doi: 10.1001/archpsyc.62.4.379 [DOI] [PubMed] [Google Scholar]
- Moscarello JM (2020). Prefrontal cortex projections to the nucleus reuniens suppress freezing following two-way signaled avoidance training. Learn Mem, 27(3), 119–123. doi: 10.1101/lm.050377.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Moser MB, & Andersen P (1993). Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci, 13(9), 3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Kinnavane L, Amin E, O’Mara SM, & Aggleton JP (2020). Deconstructing the Direct Reciprocal Hippocampal-Anterior Thalamic Pathways for Spatial Learning. J Neurosci, 40(36), 6978–6990. doi: 10.1523/JNEUROSCI.0874-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA, & Sigurdsson T (2013). Theta oscillations in the medial prefrontal cortex are modulated by spatial working memory and synchronize with the hippocampus through its ventral subregion. J Neurosci, 33(35), 14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, & Buzsaki G (2008). Internally generated cell assembly sequences in the rat hippocampus. Science, 321(5894), 1322–1327. doi: 10.1126/science.1159775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, & Cassel JC (2015). The nonspecific thalamus: A place in a wedding bed for making memories last? Neurosci Biobehav Rev, 54, 175–196. doi: 10.1016/j.neubiorev.2014.10.021 [DOI] [PubMed] [Google Scholar]
- Place R, Farovik A, Brockmann M, & Eichenbaum H (2016). Bidirectional prefrontal-hippocampal interactions support context-guided memory. Nat Neurosci, 19(8), 992–994. doi: 10.1038/nn.4327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quet E, Majchrzak M, Cosquer B, Morvan T, Wolff M, Cassel JC, … Stephan A (2020). The reuniens and rhomboid nuclei are necessary for contextual fear memory persistence in rats. Brain Struct Funct, 225(3), 955–968. doi: 10.1007/s00429-020-02048-z [DOI] [PubMed] [Google Scholar]
- Ramanathan KR, Jin J, Giustino TF, Payne MR, & Maren S (2018). Prefrontal projections to the thalamic nucleus reuniens mediate fear extinction. Nat Commun, 9(1), 4527. doi: 10.1038/s41467-018-06970-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan KR, & Maren S (2019). Nucleus reuniens mediates the extinction of contextual fear conditioning. Behav Brain Res, 374, 112114. doi: 10.1016/j.bbr.2019.112114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata H (1993). Direct projections from the anterior thalamic nuclei to the retrohippocampal region in the rat. J Comp Neurol, 337(3), 431–445. doi: 10.1002/cne.903370307 [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, & Wilson MA (2005). Prefrontal phase locking to hippocampal theta oscillations. Neuron, 46(1), 141–151. doi: 10.1016/j.neuron.2005.02.028 [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, & Gordon JA (2010). Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature, 464(7289), 763–767. doi: 10.1038/nature08855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, & Gordon JA (2015). Hippocampal-prefrontal input supports spatial encoding in working memory. Nature, 522(7556), 309–314. doi: 10.1038/nature14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Spellman TJ, Rosen AM, Gogos JA, & Gordon JA (2017). Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun, 8(1), 2182. doi: 10.1038/s41467-017-02108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban KR, Layfield DM, & Griffin AL (2014). Transient inactivation of the medial prefrontal cortex impairs performance on a working memory-dependent conditional discrimination task. Behav Neurosci, 128(6), 639–643. doi: 10.1037/bne0000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela C, Kumar S, Yang JY, & Wilson MA (2014). Anatomical substrates for direct interactions between hippocampus, medial prefrontal cortex, and the thalamic nucleus reuniens. Brain Struct Funct, 219(3), 911–929. doi: 10.1007/s00429-013-0543-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman A, Whitford TJ, Westin CF, Golland P, & Kubicki M (2012). Whole brain resting state functional connectivity abnormalities in schizophrenia. Schizophr Res, 139(1–3), 7–12. doi: 10.1016/j.schres.2012.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP (2002). Analysis of projections from the medial prefrontal cortex to the thalamus in the rat, with emphasis on nucleus reuniens. J Comp Neurol, 442(2), 163–187. doi: 10.1002/cne.10083 [DOI] [PubMed] [Google Scholar]
- Vertes RP (2006). Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience, 142(1), 1–20. doi: 10.1016/j.neuroscience.2006.06.027 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Do Valle AC, Sherman A, & Rodriguez JJ (2006). Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol, 499(5), 768–796. doi: 10.1002/cne.21135 [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, & Leranth C (2007). Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull, 71(6), 601–609. doi: 10.1016/j.brainresbull.2006.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viena TD, Linley SB, & Vertes RP (2018). Inactivation of nucleus reuniens impairs spatial working memory and behavioral flexibility in the rat. Hippocampus, 28(4), 297–311. doi: 10.1002/hipo.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DA, Brown JT, & Randall AD (2020). Neurophysiological alterations in the nucleus reuniens of a mouse model of Alzheimer’s disease. Neurobiol Aging, 88, 1–10. doi: 10.1016/j.neurobiolaging.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, & Eichenbaum H (2000). Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron, 27(3), 623–633. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, & Witter MP (1990). Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol, 296(2), 179–203. doi: 10.1002/cne.902960202 [DOI] [PubMed] [Google Scholar]
- Wright NF, Erichsen JT, Vann SD, O’Mara SM, & Aggleton JP (2010). Parallel but separate inputs from limbic cortices to the mammillary bodies and anterior thalamic nuclei in the rat. J Comp Neurol, 518(12), 2334–2354. doi: 10.1002/cne.22336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, & Sudhof TC (2013). A neural circuit for memory specificity and generalization. Science, 339(6125), 1290–1295. doi: 10.1126/science.1229534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, & Otto T (2007). Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem, 87(4), 464–475. doi: 10.1016/j.nlm.2006.12.006 [DOI] [PubMed] [Google Scholar]


