Abstract
Purpose
To describe dome-shaped macula and associated clinical findings in premature infants.
Methods
This prospective, observational cohort study included a consecutive sample of premature infants screened for retinopathy of prematurity (ROP) with 9-month follow-up. Handheld spectral domain optical coherence tomography (SD-OCT) was performed at the time of ROP screening. Images were assessed for dome-shaped macula, cystoid macular edema, epiretinal membrane, vitreous bands, and punctate hyperreflective vitreous opacities. Dome height measurements were performed in a subset of images. Teller visual acuity and cycloplegic refraction were performed at an adjusted age of 8-10 months.
Results
Of 37 infants (74 eyes; 49% male; mean gestational age 27.8 ± 3.2 weeks; mean birth weight 949 ± 284 g), 24/37 (65%) demonstrated dome-shaped macula in at least one eye (13 both eyes, 5 right eye only, and 6 left eye only). Of the 74 eyes, 26 (35%) could be reliably measured, with a mean dome height of 139.0 ± 72.3 μm (range, 54–369 μm). Presence of dome-shaped macula was associated with a diagnosis of ROP (P = 0.02; OR, 3.03; 95% CI, 1.18-7.82) and pre-plus or plus disease (P = 0.02; OR, 4.20; 95% CI, 1.05-16.78). Infants with dome-shaped macula had lower birth weight compared with those without (877 vs 1081 g; P = 0.04). No associations with other demographics, OCT findings, and 9-month refractive outcomes were found.
Conclusions
Dome-shaped macula was frequently identified by handheld SD-OCT in premature infants, especially those with lower birth weight and severe ROP. The long-term clinical significance of this finding is unknown.
Retinopathy of prematurity (ROP) is a vasoproliferative disease of the developing retina among premature infants and a major cause of childhood vision loss worldwide.1 Sequelae can range from mild visual impairment to retinal detachment and blindness. Other long-term negative outcomes include high myopia, amblyopia, and strabismus.2 ROP is traditionally diagnosed and monitored by indirect ophthalmoscopy or wide-field fundus photography.
Handheld spectral domain optical coherence tomography (SD-OCT) imaging in premature neonates has identified numerous vitreoretinal changes not visible by indirect ophthalmoscopy, including foveal immaturity,3,4 cystoid macular edema,3 retinoschisis,5,6 retinal neovascularization,5 epiretinal membrane,6 punctate hyperreflective vitreous opacities,7,8 and vitreous bands.7,8
Dome-shaped macula was first described in OCT images by Gaucher and colleagues9 as a macular convexity in the setting of posterior staphyloma in highly myopic adults. Subsequent studies have identified this condition with or without staphyloma in 9%-20% of highly myopic eyes, and it has been associated with serous retinal detachment, retinal pigment epithelial atrophy, extrafoveal retinoschisis, and choroidal neovascularization.9–15 Even without these additional pathologies, dome-shaped macula has been linked to vision loss and metamorphopsia.9,16 Though primarily described in high myopia, dome-shaped macula has also been reported in emmetropic and hypermetropic eyes.17 Its prevalence in healthy eyes is unknown.
The majority of dome-shaped macula has been described in adults commonly between 40-60 years of age, however there are rare reports of dome-shaped macula in young adults and children.17,18 Recently, Xu and colleagues19 reported macular elevations resembling dome-shaped maculae in 11 pediatric patients with high myopia. These elevations were called “ridge-shaped” because they were only observed in the vertical OCT orientation (ie, the ridges ran in a horizontal direction). In this study, a cohort of premature infants screened for ROP underwent retinal imaging by handheld SD-OCT. Those with dome-shaped maculae were compared to those without this configuration for ocular and systemic characteristics, including early visual outcomes.
Subjects and Methods
This prospective, observational study included infants who underwent routine ROP screening in the University of Washington and Seattle Children’s Hospital neonatal intensive care units (NICUs). Informed consent was obtained from the legal guardians prior to the initial imaging session. The study was approved by the University of Washington and Seattle Children’s Hospital Institutional Review Board, conformed to the requirements of the US Health Insurance Portability and Accountability Act of 1996 and is adherent to the tenets of the Declaration of Helsinki.
Eligible patients were defined as those infants undergoing routine ROP screening examinations. This group included infants of gestational age of ≤30 weeks or birth weight of ≤1,500 g. Infants who were too medically unstable for SD-OCT imaging or routine screening examinations, as determined by the NICU medical team, were excluded from the study. Additional patients were excluded from analysis if they did not return for their 9-month clinical follow-up examination. Pupils were dilated with phenylephrine hydrochloride 1% and cyclopentolate ophthalmic 0.2% solution prior to the ROP screening examination and imaging sessions.
SD-OCT Imaging
Imaging was performed on the same day as routine ROP examination using the Envisu C2300 handheld SD-OCT device (Leica Microsystems, Wetzlar, Germany), as previously described.20 Imaging was repeated every time an enrolled infant required a routine ROP screening examination. Infants were imaged supine and without sedation by one of two trained imagers (AS, LG), who used their fingers to gently retract the upper and lower eyelids. The maximum duration of the imaging session of both eyes was 15 minutes, during which attempts were made to acquire high-quality OCT images of the fovea and optic nerve. Imaging was mostly obtained in a vertical orientation (vertical B-scans). Occasionally, other imaging orientations were used because of challenging imaging conditions, such as the presence of continuous positive airway pressure masks; however, imaging orientation was not recorded.
Each eye’s OCT volume scans were reviewed using the device’s included software (Envivovue; Leica Microsystems) by two trained graders (ATL, YM), each of whom was masked to the other’s assessments as well as to demographic information and clinical examination results. Each volume scan that included the fovea was subjectively reviewed for the presence of a dome-shaped, chorioretinal convexity of the macula, in accordance with previous definitions of dome-shaped macula.17,18,21,22 Almost all imaging sessions were obtained in a single orientation, therefore the possibility that macular elevations were ridge-shaped rather than dome-shaped in three-dimensions could not be ruled out. For simplicity, the nomenclature “dome-shaped macula” is used for all elevations described in this study. Images of inadequate quality or incomplete visibility of the parafoveal retina to identify contour on both sides of the fovea were considered “indeterminate” and excluded from the analysis. Images with visible concave inflection points on both sides of the macular dome, such that a single line could connect the two inflections tangentially, were assessed for dome height using Envivovue software calipers in accordance with accepted methods.12,15 Each volumetric scan was assessed by two additional trained graders (AS, EMZ) for cystoid macular edema, epiretinal membrane, vitreous bands, and punctate hyperreflective vitreous opacities, as described in a previous publication.7 Disagreements between the grader pairs were mediated by a third trained grader (MTC).
Clinical Examinations
ROP examinations were performed by one of three pediatric ophthalmologists (including MTC and KT-H) in the NICU on the same day as SD-OCT imaging. The ophthalmologists were masked to the SD-OCT imaging findings at the time of their examinations. The examinations consisted of indirect ophthalmoscopy with scleral depression, with determination of follow-up and treatment according to published standard guidelines.23 Follow-up examinations were conducted at the Seattle Children’s Hospital outpatient ophthalmology clinic between 8 and 10 months’ adjusted age, at which time each participant received a complete ophthalmologic examination, including Teller visual acuity and cycloplegic refraction, without OCT imaging. Each patient’s medical record was reviewed for sex, race, gestational age, birth weight, clinical indirect ophthalmoscopic results (ROP stage, zone, and plus disease), and history of ophthalmic injections or laser treatments.
Statistical Analysis
For the primary outcome, eyes that never demonstrated dome-shaped macula were compared with eyes that demonstrated dome-shaped macula at least once with regard to worst clinical diagnosis of ROP in that eye (worst, ROP stage, presence of ROP, type 1 ROP, plus or pre-plus disease). For secondary outcomes, infants who never demonstrated dome-shaped macula in either eye were compared with those who exhibited dome-shaped macula at least once in at least one eye with regard to sex, race, gestational age, and birth weight. Eyes that never demonstrated dome-shaped macula were compared to eyes that demonstrated dome-shaped macula at least once with regard to 9-month cycloplegic spherical equivalence and 9-month logMAR visual acuity.
As a convenience sample, right eyes that never demonstrated dome-shaped macula were compared with right eyes that demonstrated dome-shaped macula at least once with regard to other SD-OCT findings in that eye (presence of cystoid macular edema, epiretinal membrane, vitreous bands, punctate hyperreflective vitreous opacities).
Generalized linear mixed models were used to adjust for two eyes and multiple imaging sessions from the same infant. Demographic comparisons between infants were performed using the nonparametric Mann-Whitney U test for continuous variables. All categorical data was analyzed using either the χ2 test or the Fisher exact test. The unweighted Cohen’s kappa statistic was applied to assess the strength of inter-observer agreement. Statistical analyses were performed using SAS version 9.4 (SAS institute Inc, Cary, NC), with a P value of <0.05 considered statistically significant.
Results
A total of 49 premature infants were enrolled in the study. Of these, 74 eyes of 37 infants (18 males [49%]) were included, and 12 infants were excluded because of lack of adequate image quality in both eyes. Mean birth weight was 949 ± 284 g, and mean gestational age was 27.8 ± 3.2 weeks. Of the 37 infants, 20 (54%) were white, 7 (19%) were Hispanic, 3 (8%) were black, 1 (3%) was Asian, 1 (3%) was Native American, and 5 (13%) were other. On clinical examination, 36 of 74 eyes (49%) were diagnosed with ROP, and stage 3 was diagnosed in 18 eyes (24%). Thirteen eyes (18%) demonstrated pre-plus or plus disease, and 7 (9%) were diagnosed with type 1 ROP. Of 7 eyes with type 1 ROP, 6 (86%) were treated with laser photocoagulation; the remaining eye was treated with intravitreal bevacizumab injection.
Dome-Shaped Macula on Handheld SD-OCT
The interobserver agreement on the presence of dome-shaped macula in all eyes was 315 of 329 scans (96%; Cohen’s κ = 0.94). Of the 14 disagreements, 12 (86%) resulted from one observer concluding that the scan was “indeterminate” with regard to the presence of dome-shaped macula, and 2 (14%) had frank disagreement on the presence versus absence of dome-shaped macula.
Following mediation of all disagreements by a third trained grader, 95 of 329 scans (29%) were designated as indeterminate regarding the presence of dome-shaped macula. All 37 infants had at least one imaging session in each eye (range, 1-11) that was adequate to determine the presence or absence of dome-shaped macula. Among these scans, dome-shaped macula was observed in at least one eye in 24 of the 37 infants (65%). See Figure 1. Of these infants, 13 (54%) demonstrated evidence of bilateral elevation, and 11 (46%) were unilateral (right eye, 5; left eye, 6). Of 37 eyes with evidence of dome-shaped macula, the elevation was consistently seen throughout all subsequent scans in 33 eyes (89%). In the remaining 4 eyes (11%), the dome-shaped macula appeared absent in one or more scans following its initial identification. Dome height was measurable in 26 of 74 eyes (35%) in 18 infants. Mean maximum dome height in these eyes was 139.0 ±72.3 μm (range, 54–369 μm).
FIG 1.
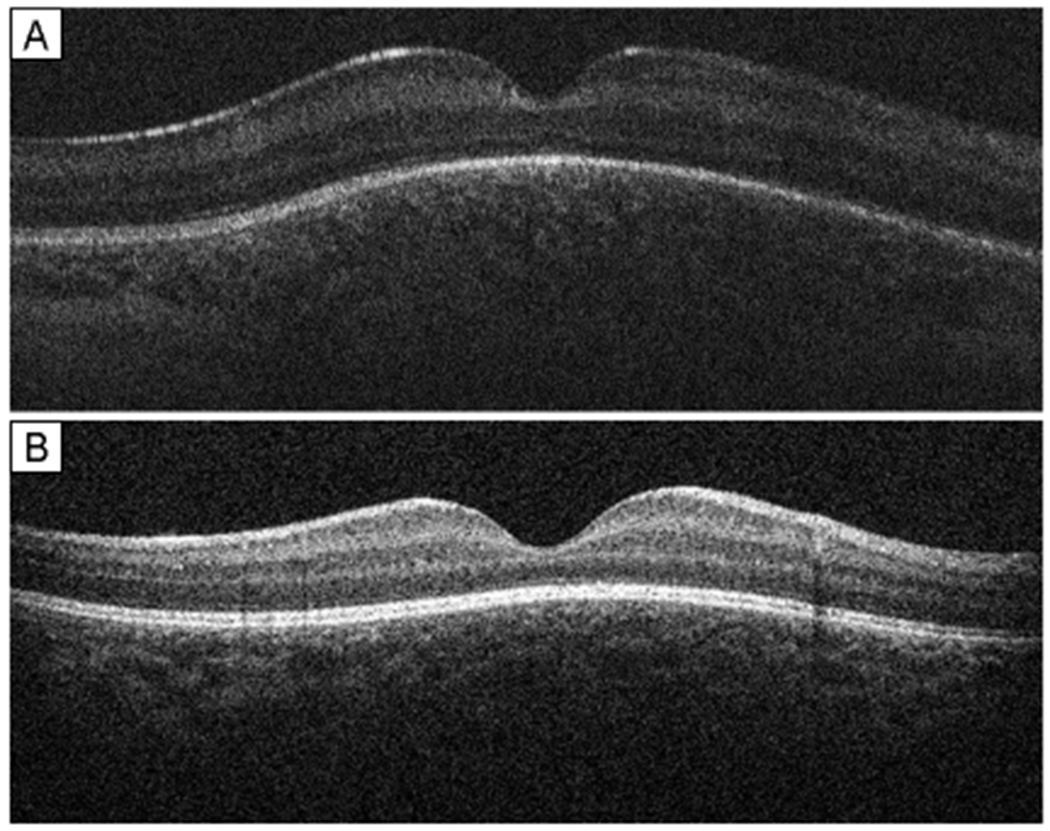
Handheld spectral-domain optical coherence tomography of dome-shaped macula: examples of dome-shaped macula in premature infants. A, right eye of premature male infant (birth weight [BW], 691 g; gestational age [GA], 25 0/7 weeks) imaged at postmenstrual age (PMA) of 39 2/7 weeks with zone 2, stage 2 retinopathy of prematurity. B, mature right eye of premature female infant (BW, 711 g; GA, 24 4/7 weeks) imaged at PMA 48 5/7 weeks. Dome-shaped macula was defined as the presence of a dome-shaped, chorioretinal convexity of the macula seen on optical coherence tomography B-scan.
Comparing 24 (65%) infants with evidence of dome-shaped macula in either eye with 13 infants (35%) without, birth weight was significantly lower in the group with dome-shaped macula (877 g vs 1,081 g; P = 0.04). The presence of dome-shaped macula was associated with borderline but not statistically significantly lower gestational age at birth (27.1 weeks vs 29.1 weeks; P = 0.06). No difference in sex or race/ethnicity was identified between the two groups (see Table 1).
Table 1.
Dome-shaped macula and associated demographic characteristics in premature infants screened for retinopathy of prematurity
| Characteristic | No evidence of dome-shaped macula (N = 13 infants) |
Evidence of dome-shaped maculaa (N = 24 infants) |
P value |
|---|---|---|---|
| Demographics | |||
| Male, no. (%) | 7 (54) | 11 (46) | 0.74 |
| Birth weight, g, mean ± SD | 1081 ± 287 | 877 ± 261 | 0.04 |
| Gestational age, weeks, mean ± SD | 29.1 ± 4.0 | 27.1 ± 2.5 | 0.06 |
| Race/ethnicity. no. (%) | |||
| White | 9 (69) | 11 (46) | 0.38 |
| Hispanic | 2 (15) | 5 (21) | |
| Native American | 0 (0) | 1 (4) | |
| Black | 0 (0) | 3 (13) | |
| Asian | 0 (0) | 1 (4) | |
| Otherb | 2 (15) | 3 (13) | |
SD, standard deviation; SD-OCT, spectral-domain optical coherence tomography.
Dome-shaped macula identified by handheld SD-OCT at least once during newborn screening examinations.
Race/ethnicity no listed in medical records.
A diagnosis of ROP was made in 23 of 37 eyes (62%) with dome-shaped macula and 13 of 37 eyes (35%) without (P = 0.02; OR = 3.03; 95% CI, 1.18-7.82). Pre-plus or plus disease was present in 10 of 37 eyes (27%) with dome-shaped macula and 3 of 37 eyes (8%) without (P = 0.02; OR = 4.20; 95% CI, 1.05-16.78). Thirteen of 37 eyes (35%) that demonstrated dome-shaped macula reached stage 3 ROP compared with 5 of 37 eyes (14%) without dome-shaped macula (P = 0.36). Handheld SD-OCT imaging revealed no difference between groups with respect to the presence of cystoid macular edema, epiretinal membrane, vitreous bands, or punctate hyperreflective vitreous opacities in the same eye (see Table 2).
Table 2.
Dome-shaped macula and associated ocular characteristics of premature infants screened for)retinopathy of prematurity
| Characteristic | No evidence of dome-shaped macula, no. (%) | Evidence of dome-shaped macula,a no. (%) | P valueb |
|---|---|---|---|
| Indirect ophthalmoscopic examination findingsc | 37 eyes | 37 eyes | |
| Ever diagnosed with ROP | 13 (35) | 23 (62) | 0.02 |
| ROP stage 0 | 24 (65) | 14 (38) | 0.36 |
| ROP stage 1 | 3 (8) | 4 (11) | |
| ROP stage 2 | 5 (14) | 6 (16) | |
| ROP stage 3 | 5 (14) | 13 (35) | |
| Pre-plus or plus disease | 3 (8) | 10 (27) | 0.02 |
| Type 1 ROP | 3 (8) | 4 (11) | 0.76 |
| Nine-month visual outcomes | |||
| BCVA, logMAR, mean ± SD [Snellen] | 0.66 ± 0.17 [20/91] | 0.78 ± 0.21 [20/121] | 0.66 |
| Refractive error, D, mean ± SD | 1.68 ± 2.32 | 1.78 ± 2.34 | 0.71 |
| SD-OCT findingsd | 19 eyes | 18 eyes | |
| Cystoid macular edema | 5 (26) | 10 (56) | 0.10 |
| Epiretinal membrane | 6 (32) | 8 (44) | 0.51 |
| Vitreous bands | 8 (42) | 7 (39) | 0.84 |
| Punctate vitreous opacities | 7 (37) | 8 (44) | 0.74 |
BCVA, best-corrected visual acuity; ROP, retinopathy of prematurity; SD-OCT, spectral-domain optical coherence tomography; SD, standard deviation.
Dome-shaped macula identified by handheld SD-OCT at least once during newborn screening examinations.
Generalized linear mixed models were used to adjust for two eyes and multiple imaging sessions from the same infant.
Worst-documented stage of ROP and worst diagnosis of pre-plus or plus disease throughout clinical course are shown.
Comparison of other SD-OCT findings includes only right eyes.
Nine-month follow-up examination revealed no difference in visual acuity in eyes that had dome-shaped macula in infancy compared with those that never had dome-shaped macula (mean logMAR 0.78 [Snellen 20/121] vs 0.66 [Snellen 20/91]; P = 0.66) and no difference in refractive error (spherical equivalence, 1.78 D vs 1.70 D; P = 0.71). See Table 2.
Discussion
In this study, dome-shaped macula was identified by handheld SD-OCT imaging of premature infants screened for ROP. It was associated with lower birth weight (P = 0.04), ROP (P = 0.02), and pre-plus or plus disease (P = 0.02) in the newborn period, without significant associations with macular edema or other OCT pathology. To our knowledge, this finding has not been previously described in this population, although careful review of published images from other studies reveals similar morphology in some premature infants.3,4
“Ridge-shaped” macula was described by Xu and colleagues19 in 2020 in a pediatric population (age 4-19 years) with high myopia. This morphology was labeled ridge- (as opposed to dome-) shaped macula due to its horizontal orientation (ie, it appeared as a fold in the retina only visible in vertical OCT section but not in horizontal section). The authors described the differences from dome-shaped macula but also acknowledged that dome- and ridge-shaped macula may be one and the same. In fact, reports on dome-shaped macula in adults have revealed that many domes are actually ridge-shaped (or “oval-shaped”) and more commonly in a horizontal orientation.12,14–16,21 B-scans obtained in parallel to oval-shaped maculae in adults often failed to capture their presence. 12,15,16,21 In the present study, variability in OCT probe orientation may explain the few cases where dome-shaped macula was not detected after initial identification. However, it is unknown what proportion of cases in this study were ridge shaped.
The pathophysiology of dome-shaped macula is unclear. Although adult dome-shaped macula has been strongly associated with high myopia, its prevalence in the normal population is unknown and may be a more frequent finding than previously considered (this study did not include a full-term normal control group). Alternatively, these newborn dome-shaped maculae may represent a stage of normal development that resolves over time. For example, the premature infant retina and sclera may differ in size, resulting in a bend at this location. A similar theory was suggested by Dubis and colleagues4 to explain a “temporal divot” seen in premature infant retinas using handheld SD-OCT. Finally, a pressure gradient between intraocular pressure and posterior pressure may affect the shape of the retinal pigment epithelium and Bruch’s membrane at the macula, analogous to known peripapillary changes due to elevated intracranial pressure.24,25 Regardless of potentially varying etiologies in this population and others, dome-shaped macula was associated with ROP severity in this study, suggesting that prematurity also plays a role here. Similar to retinal vascular dilation and tortuosity, dome-shaped macula may be a helpful ROP biomarker even though it is not exclusively found in premature infants. Further research is necessary to determine the clinical utility of this finding.
While retinoscopy was not performed in the newborn period, the results of the present study raise the possibility that at least some previously-reported dome-shaped maculae started at birth and may contribute to later pathology. Imamura and colleagues11 hypothesized that regional defocus due to dome-shaped macula may lead to impaired emmetropization and myopia. In the present study, subjects with neonatal dome-shaped maculae demonstrated no difference in refractive error and visual acuity compared to those without dome-shaped maculae at 9 months of age; however, longer follow-up may be necessary to capture these differences.
Among eyes with measurable dome height, 100% exceeded the minimum threshold definition of dome-shaped macula (50 microns), as defined by Ellabban and colleagues.12 However, measurement accuracy was limited by variable lateral resolution, refractive index, and scan length in this awake infant population. The authors were therefore unable to accurately measure dome height in most infants and thus elected to use a qualitative definition of dome-shaped macula for the overall analysis based on existing literature.17,18,21,22 Measurement limitations using this handheld device also prevented accurate assessment of local choroidal and scleral thickness in this study, previously associated with dome-shaped macula in highly myopic adults.11,13,15 The authors acknowledge some imaging error; nevertheless, the elevations are felt to represent a true anatomical finding rather than imaging artifact, given that they were invariably centered at the fovea, and seen consistently over time in 33 of 37 eyes (89%).
This study identified dome-shaped macula with high frequency among premature infants, associated with low birth weight, ROP presence, and ROP severity. Recent studies have attempted to identify vitreoretinal biomarkers of ROP using handheld OCT in premature infants.7,8,26 Dome-shaped macula should be explored as an additional ROP severity biomarker. Presence of this morphology in an infant population raises intriguing questions regarding the pathophysiology of dome-shaped macula and its relationship to myopia and prematurity. Regardless of the underlying etiology, this study contributes to our understanding of expected OCT morphology in the preterm infant eye, informing clinicians who may be using handheld OCT to diagnose retinal pathology in this population.
Financial support:
Supported by The Knights Templar Eye Foundation (MTC), the Latham Vision Research Innovation Award (MTC), NEI K23EY024921 (CSL), NEI K23EY029246 (AYL), and unrestricted grants from Research to Prevent Blindness and the NIH CORE Grant EY001730. The funding organizations had no role in study design, data collection and analysis, or in the preparation of the manuscript.
Dr. Cecilia Lee reports NIA grant R01AG060942; Dr. Aaron Lee reports personal fees from Genentech, Verana Health, and Topcon, and grants from Novartis, Carl Zeiss Meditec, and Santen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as a poster presentation at the 45th Annual Meeting of the American Association for Pediatric Ophthalmology and Strabismus, San Diego, California, March 27-31, 2019.
Literature Search
PubMed was searched on August 8, 2020, without language or date restriction, using the following terms: dome AND macula; ridge AND macula.
References
- 1.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013;74 Suppl 1:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielder A, Blencowe H, O’Connor A, Gilbert C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch Dis Child Fetal Neonatal Ed 2015;100:F179–84. [DOI] [PubMed] [Google Scholar]
- 3.Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology 2013;120:1665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubis AM, Costakos DM, Subramaniam CD, et al. Evaluation of normal human foveal development using optical coherence tomography and histologic examination. Arch Ophthalmol 2012;130:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology 2009;116:2448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina 2011;31:1470–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zepeda EM, Shariff A, Gillette TB, et al. Vitreous bands identified by handheld spectral-domain optical coherence tomography among premature infants. JAMA ophthalmology 2018;136:753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Legocki AT, Zepeda EM, Gillette TB, et al. Vitreous findings by handheld spectral-domain OCT correlate with retinopathy of prematurity severity. Ophthalmol Retina 2020;4:1008–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaucher D, Erginay A, Lecleire-Collet A, et al. Dome-shaped macula in eyes with myopic posterior staphyloma. Am J Ophthalmol 2008;145:909–14. [DOI] [PubMed] [Google Scholar]
- 10.Ohsugi H, Ikuno Y, Oshima K, Yamauchi T, Tabuchi H. Morphologic characteristics of macular complications of a dome-shaped macula determined by swept-source optical coherence tomography. Am J Ophthalmol 2014;158:162–70.e1. [DOI] [PubMed] [Google Scholar]
- 11.Imamura Y, Iida T, Maruko I, Zweifel SA, Spaide RF. Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. Am J Ophthalmol 2011;151:297–302. [DOI] [PubMed] [Google Scholar]
- 12.Ellabban AA, Tsujikawa A, Matsumoto A, et al. Three-dimensional tomographic features of dome-shaped macula by swept-source optical coherence tomography. Am J Ophthalmol 2013;155:320–28.e2. [DOI] [PubMed] [Google Scholar]
- 13.Liang IC, Shimada N, Tanaka Y, et al. Comparison of clinical features in highly myopic eyes with and without a dome-shaped macula. Ophthalmology 2015;122:1591–600. [DOI] [PubMed] [Google Scholar]
- 14.Ellabban AA, Tsujikawa A, Muraoka Y, et al. Dome-shaped macular configuration: longitudinal changes in the sclera and choroid by swept-source optical coherence tomography over two years. Am J Ophthalmol 2014;158:1062–70. [DOI] [PubMed] [Google Scholar]
- 15.Caillaux V, Gaucher D, Gualino V, Massin P, Tadayoni R, Gaudric A. Morphologic characterization of dome-shaped macula in myopic eyes with serous macular detachment. Am J Ophthalmol 2013;156:958–67.e1. [DOI] [PubMed] [Google Scholar]
- 16.Coco RM, Sanabria MR, Alegria J. Pathology associated with optical coherence tomography macular bending due to either dome-shaped macula or inferior staphyloma in myopic patients. Ophthalmologica 2012;228:7–12. [DOI] [PubMed] [Google Scholar]
- 17.Errera MH, Michaelides M, Keane PA, et al. The extended clinical phenotype of dome-shaped macula. Graefes Arch Clin Exp Ophthalmol 2014;252:499–508. [DOI] [PubMed] [Google Scholar]
- 18.Cebeci Z, Kir N. Bilateral dome-shaped macula with serous macular detachment in a child. Case Rep Ophthalmol Med 2015;2015:213968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Fang Y, Jonas JB, et al. Ridge-shaped macula in young myopic patients and its differentiation from typical dome-shaped macula in elderly myopic patients. Retina 2020;40:225–32. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci 2010;51:2678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenzo D, Arias L, Choudhry N, et al. Dome-shaped macula in myopic eyes: twelve-month follow-up. Retina 2017;37:680–86. [DOI] [PubMed] [Google Scholar]
- 22.Ceklic L, Wolf-Schnurrbusch U, Gekkieva M, Wolf S. Visual acuity outcome in RADIANCE study patients with dome-shaped macular features. Ophthalmology 2014;121:2288–9. [DOI] [PubMed] [Google Scholar]
- 23.Fierson WM. Screening examination of premature infants for retinopathy of prematurity. Pediatrics 2013;131:189–95. [DOI] [PubMed] [Google Scholar]
- 24.Wang JK, Kardon RH, Ledolter J, et al. Peripapillary retinal pigment epithelium layer shape changes from acetazolamide treatment in the Idiopathic Intracranial Hypertension Treatment Trial. Invest Ophthalmol Vis Sci 2017;58:2554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci 2011;52:7987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maldonado RS, Yuan E, Tran-Viet D, et al. Three-dimensional assessment of vascular and perivascular characteristics in subjects with retinopathy of prematurity. Ophthalmology 2014;121:1289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]


