Abstract
Background:
Multimorbidity (> 2 conditions) increases the risk of adverse outcomes and challenges health care systems for patients with acute coronary syndrome (ACS). These complications may be partially attributed to ACS clinical care which is driven by single-disease-based practice guidelines; current guidelines do not consider multimorbidity.
Objectives:
To identify multimorbidity phenotypes (combinations of conditions) with suspected ACS. We hypothesized that: 1) subgroups of patients with similar multimorbidity phenotypes could be identified, 2) classes would differ according to diagnosis, and 3) class membership would differ by sex, age, functional status, family history, and discharge diagnosis.
Methods:
This was a secondary analysis of data from a large multi-site clinical study of patients with suspected ACS. Conditions were determined by items on the Charlson Comorbidity Index and the ACS Patient Information Questionnaire. Latent class analysis was used to identify phenotypes.
Results:
The sample (n=935) was predominantly male (68%) and middle-aged (mean= 59 years). Four multimorbidity phenotypes were identified: 1) high multimorbidity (Class 1) included hyperlipidemia, hypertension (HTN), obesity, diabetes, and respiratory disorders (COPD or asthma); 2) low multimorbidity (Class 2) included only obesity; 3) cardiovascular multimorbidity (Class 3) included HTN, hyperlipidemia, and coronary heart disease; and 4) cardio-oncology multimorbidity (Class 4) included HTN, hyperlipidemia, and cancer. Patients ruled-in for ACS primarily clustered in Classes 3 and 4 (OR 2.82, 95% CI 1.95–4.05, p = 0.001 and OR 1.76, 95% CI 1.13–2.74, p = 0.01).
Conclusion:
Identifying and understanding multimorbidity phenotypes may assist with risk-stratification and better triage of high-risk patients in the emergency department.
Keywords: Multimorbidity, ACS, Latent Class Analysis, Multimorbidity Phenotypes, Cardiovascular-Oncology
Introduction
Emergency departments (EDs) evaluate 5.5 million patients for acute coronary syndrome (ACS) annually in the United States—but only 13.5% of these patients are ultimately diagnosed (ruled-in) with ACS.1 Accurate risk stratification and diagnostic testing are critical for the time-dependent therapies required for restoring blood flow to the myocardium, thereby reducing cardiovascular morbidity and mortality.2 Still, ACS diagnosis remains challenging, as no single risk stratification model (e.g., Thrombolysis in Myocardial Infarction [TIMI] risk score; history, ECG, age, risk factors, Troponin [HEART] score) or diagnostic strategy identifies all ACS cases accurately and no clear reference standard exists.3 Risk stratification models have yet to explore the utility of multimorbidity (examining the presence of >2 chronic conditions) to identify patients with a high risk for ACS. Previous studies examined the association between ACS and individual chronic conditions, including chronic obstructive pulmonary disease (COPD),4,5 diabetes,6,7 and heart failure (HF),8–10 on outcome variables such as mortality and hospital readmission rates after an ACS diagnosis. Multimorbidity and ACS, however, are under-studied phenomena, and there is a need to determine the different multimorbidity phenotypes that health care providers will encounter in the emergency department.4
Multimorbid patients who receive an ACS diagnosis experience elevated risks for suboptimal care as they frequently receive lower rates of revascularization6,7 and evidence-based pharmacologic treatments.4,6,7 Multimorbidity increases the rate of in-hospital complications, such as mortality, length of stay, and post-procedural bleeding.4,11 These disparities and complications may be partially attributed to ACS clinical care which is driven by single-disease-based practice guidelines aimed at diagnosis, management, and decision-making; current practice guidelines do not consider multimorbidity.12 Clinical guidelines are based on randomized clinical trials that exclude multimorbid patients, limiting applicability to complex patients.12 Conversely, most patients evaluated in the ED for ACS are ultimately ruled-out and remain understudied because most investigations focus on patients ruled-in for ACS.1 Therefore, the aims of this study were to examine the presence of multimorbidity phenotypes across a range of pre-existing chronic conditions and to determine whether these differed by diagnosis (ruled-in versus ruled-out for ACS). We hypothesized that: 1) subgroups of patients with similar multimorbidity phenotype classes (latent classes) could be identified and 2) these classes would differ according to diagnosis. An exploratory aim was to examine if class membership would vary based on demographic variables such as age, sex, educational level, household income, race, functional status, tobacco use (current, former, and never), and family history of sudden cardiac death at < 55 years of age.
Methods
Study Design
The study was a secondary data analysis of de-identified data from the Think Symptoms study.14 The parent study was approved by the Institutional Review Board at the sponsoring institution, and all participating clinical sites. This analysis received an exemption from the IRB. All human subject involvement, characteristics, potential risks, benefits, strategies to minimize risks and benefits were addressed in the parent study (R01NR012012), and all participants gave written informed consent.
Sample and Setting
The main aim of the parent Think Symptoms study was to characterize the influence of sex on symptoms during ACS.14 Data were collected at five academic medical centers and a large community hospital located in the Midwest, Southwest, Pacific Northwest, and Western regions of the United States. Data were collected between January 2011 and December 2014. Given the exploratory nature of this study conditions were included if 1) they had a frequency of >5% (n=52) of the parent study dataset (n=1064) on either the Charlson Comorbidities index and ACS Patient Information Questionnaire that were present in >5% or 2) were considered theoretically and clinically relevant (i.e. peripheral vascular disease (n=48) and systemic lupus erythematosus (n=36)).
Patients were included in both the parent study and this analysis if they were high risk for ACS (abnormal electrocardiogram [ECG] or positive troponin), English speaking, ≥ 21 years of age, had telephone access, and had intact cognition. A positive troponin was defined as any value exceeding the institutional reference norm. Cognitive capacity was considered acceptable if the patient understood the purpose of this study and could provide written informed consent. Patients were excluded from the parent study and therefore present analysis if they had a history of HF or were diagnosed during initial evaluation for HF exacerbation (B-type natriuretic peptide > 500 ng/mL), were admitted from a hemodialysis center, or were referred for cardiac dysrhythmia evaluation.
Measures
Charlson Comorbidities Index (CCI).
This 19-item, weighted index is the most extensively studied method of quantifying risk associated with comorbid conditions.15,16 Higher scores represent a greater burden of disease. Studies have demonstrated that the CCI is a valid measure for predicting disability and death following ischemic stroke and heart disease,16 as well as hospital readmission and length of stay, with correlations ranging from 0.35–0.93 (p < 0.001).17,18 The following six conditions were extracted from the self-reported CCI: (1) prior history of MI (CCI-1); (2) vascular disease (CCI-3); 13 stroke/transient ischemic attack (CCI-4); (4) lupus (CCI-7); (5) dyspnea (CCI-2) and asthma (CCI-6) combined (to form a new variable of respiratory disorders), and (6) all cancer-related items (CCI 14, 15, 16, and 18; combined to form the cancer variable in the analysis).
ACS Patient Information Questionnaire.
The demographic and clinical questionnaire was designed using the standardized reporting guidelines for studies evaluating risk stratification of ED patients with potential ACS.10 Criteria were established by the Multidisciplinary Standardized Reporting Criteria Task Force and are supported by the Society for Academic Emergency Medicine, American College of Emergency Physicians, American Heart Association, and American College of Cardiology. Three conditions were measured by the ACS patient information questionnaire for this analysis: hypertension, hyperlipidemia, and kidney disease.
The Duke Activity Status Index.
The Duke Activity Status Index (DASI) is a 12-item instrument that measures functional capacity. Scores range from 0–58.2, with higher scores representing better physical functioning. The items on the scale are weighted to reflect metabolic energy expenditure and correlate highly with peak VO2 (r = .80, p < 0.0001)19 in patients with ACS,20 ischemic heart disease,21 HF,20 and revascularization procedures.22 Concurrent validity was supported by correlations with measures of physical functioning (r=0.69, p<0.05 & r=0.61, p<0.05) 23. Cronbach’s alpha reliability has ranged from 0.76–0.85 19,22. The tool was responsive to change in patients recovering from cardiac surgery (p<0.001) 24.
Medical Records Review Form.
Diagnosis of ACS and obesity were extracted from the medical record. Obesity was defined as a body mass index > 30.
Statistical Analysis
Demographic and baseline characteristics were described according to class membership using numbers and percentages for categorical variables. Means and standard deviations were reported for normally distributed continuous variables, and medians and interquartile ranges for non-normally distributed continuous variables. All tests were two-sided, and statistical significance was defined as p < 0.05. Statistical analyses were performed in STATA version 15 (STATA Corp., College Station, TX) and LatentGOLD version 5.1 (Statistical Innovations Inc., Belmont, MA).
The objective of this analysis was to identify patient groups (latent classes) with similar multimorbidity patterns based on 10 comorbid conditions extracted from the Charlson Comorbidity Index, ACS Patient Information Questionnaire, and the patient’s medical record. Using Latent Gold (version 5.1), a latent class analysis (LCA) was used to classify individuals into groups with similar combinations of conditions. Missing data was handled in a listwise fashion as less than 5% had missing data on any variables included in the present analysis. Patients included in the initial class enumeration analysis (n=1003) had to have complete condition data (no missing data on the CCI or the ACS patient information questionnaire). For the final adjusted analysis (n=935) in addition to condition data patients had to have complete covariate data.
A three-step analytic framework was used. Several class solutions were explored, starting with a one-class model and subsequently increasing the number of classes up to five. The best-fitting model was selected based on an assessment of fit indices: specifically, minimization of the Bayesian information criterion (BIC) and the bootstrap likelihood ratio test (BLRT). 25 The BIC is based on the log-likelihood of a fitted model and includes a penalty for the number of model parameters and sample size. 25 The BLRT test has been demonstrated to be superior to other indices of fit; in simulation studies, the BLRT and BIC performed well.25 However, The BLRT has been found to be the most consistent indicator for selecting the correct number of classes.25 Classification quality was evaluated using the entropy statistic. The theoretical interpretability of the emerging classes was used in combination with the BIC and BLRT to determine the final number of classes.
Next, covariates that likely influence the probability of class membership were tested. Between-cluster differences were explored using a one-way analysis of variance for continuous variables and Chi-square for categorical variables, using Stata. Covariates examined for significance were age, sex, educational level, household income, race, functional status, tobacco use, and family history of sudden cardiac death at < 55 years of age. Statistically significant covariates were included in the final analysis within Latent Gold through a multinomial logistic regression of the categorical latent variable on the covariates. Post hoc BLRT testing was conducted to see if the original condition only models or the covariate adjusted models fit the data better to derive the final model for analysis.
Finally, the classification based on the covariate-adjusted LCA model was exported to Stata, and a logistic regression model was used to determine if the classes were predictors of receiving an ACS diagnosis, defined as unstable angina, non-ST segment elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI).
Results
Demographic and Clinical Characteristics
The sample (n=935) was 38% female with a mean age of 59 (± 14.0) years. Forty-four percent were ruled-in for ACS, with a diagnosis of NSTEMI (24.2%), STEMI (10.7%), and unstable angina (9.5%). Approximately 85% were admitted for observation or full admission. Most patients had decreased functional status, as measured by the DASI (mean score 34.2 ± 19.3). The most prevalent chronic conditions were hypertension, hyperlipidemia, coronary heart disease (CHD), and obesity (64.9%, 55.2%, 44.7%, and 43.2%, respectively). See Table 1.
Table 1.
Demographic and Clinical Characteristics (n=935)
| Characteristic | n = 935 |
|---|---|
| ACS ruled-in (n, %) | 415 (44.4) |
| ACS diagnosis (n, %) | |
| NSTEMI | 226 (24.2) |
| STEMI | 100 (10.7) |
| Unstable Angina | 89 (9.5) |
| Female (n, %) | 355 (38.0) |
| Age (mean, SD) | 59.9 (14.0) |
| BMI (mean, SD) | 30.1 (7.1) |
| Duke Activity Status Index Score (mean, SD) | 34.4(19.2) |
| Family history of SCD < 55 years old (n, %) | 436 (46.6) |
| Current Smoker (n, %) | 194 (21.2) |
| Disposition of patient (n, %) | |
| Full admission | 628 (67.2) |
| Observation | 162 (17.3) |
| Discharge | 135(14.5) |
| Conditions (n, %) | |
| Coronary Heart Disease | 421(44.8) |
| Peripheral Vascular Disease | 69(7.4) |
| COPD or Asthma | 248 (26.4) |
| Diabetes | 262(27.9) |
| Obese | 405(43.1) |
| Cancer | 113(12.0) |
| Cerebrovascular Disease | 79(8.4) |
| Renal Disease | 103(11.0) |
| Systemic Lupus Erythematosus | 61(6.5) |
| Hypertension | 610(65.0) |
| Charlson Comorbidity Index | |
| Weighted score (mean, SD) | 1.8 (1.9) |
Note ACS = acute coronary syndrome;BMI= body mass index; NSTEMI = non-STsegment elevation myocardial infarction; and STEMI = STsegment elevation myocardial infarction.
Latent Class Model Selection
Latent class models were derived from all available cases with comorbid condition data to assess relative fit indices for class enumeration (Table 2). Models with 1, 2, 3, 4, and 5 classes were evaluated to determine the best fit. Results from the BLRT analysis indicated that the 3-class model was better than the 2-class model and the 4-class model was better than the 3-class model, but the 5-class model was not better than the 4-class model. The 4-class model was selected as the final class solution for further analysis based on the fit indices, BLRT testing, and theoretical and clinical interpretability. Models were then adjusted for age, sex, family history of sudden cardiac death aged < 55 years old, and total weighted DASI score (Table 3).
Table 2.
Model Fit Evaluation Information for condition only models (n = 1,003)
| LL | BIC (LL) | Npar | df | Entropy | BLRT | |
|---|---|---|---|---|---|---|
| 1-Class | −5390.5707 | 10857.1596 | 11 | 992 | 1.00 | 0.00 |
| 2-Class | −5203.7200 | 10566.3874 | 23 | 980 | 0.62 | 0.00 |
| 3-Class | −5178.1890 | 10598.2544 | 35 | 968 | 0.52 | 0.00 |
| 4-Class | −5154.3749 | 10633.5552 | 47 | 956 | 0.52 | 0.00 |
| 5-Class | −5140.9376 | 10689.6095 | 59 | 944 | 0.57 | 0.12 |
Note: When assessing BLRT values, one looks for the first non-significant p value (> 0.05) to determine optimal class solution. BIC = Bayesian Information Criterion. BLRT = Bootstrap Likelihood Ratio Test. LL = Log Likelihood. Npar = Number of Parameters. df = Degrees of Freedom.
Table 3.
Covariates and Latent Class Membership (n=935)
| Covariate | Class 1: High Multimorbidity (n = 208, 22.2%) | Class 2: Low Multimorbidity (n = 195, 20.9%) | Class 3: Cardiovascular Multimorbidity (n = 381, 40.8%) | Class 4: Cardio-Onc Multimorbidity (n = 151, 16.1%)_ |
|---|---|---|---|---|
| Age (Mean, SD) | 63.4 (9.5)c,d | 43.4 (9.8)a,c,d | 58.6 (7.7)a,b,d | 80.0 (6.3)a,b,c |
| Female (n, %) | 113 (31.8)a,c | 92 (25.9)b,c,d | 98 (27.5)a,b,d | 53 (14.9)a,c |
| DASI Weighted Score (Mean, SD) | 10.0 (6.0)b,c | 47.7 (15.21)a,d | 42.5 (13.5)a,d | 28.2 (16.2)a,b,c |
| Family History of Sudden Cardiac death before age 55 (n,%) | 106 (51.0)b,c,d | 54 (27.6)a | 224 (58.3)a | 52 (34.4)a |
Note. All differences were statistically significant at p<0.05
Significant difference from class 1
Significant difference from class 2
Significant difference from class 3
Significant difference from class 4
Condition-only models (n=1003) were then compared to covariate-adjusted models (n=935). Listwise deletion was used to eliminate patients from the condition only model that did not have complete data for the covariate adjusted model. The 4-class covariate-adjusted model was found to be superior to the condition-only (unadjusted) 4-class model, with a lower BIC (9710.36 vs. 10633.55), increased amount of variance explained by the model (R2 = 0.62 vs. 0.52), and decreased classification errors (0.20 vs. 0.26). The adjusted models were then evaluated against each other. The 4-class covariate-adjusted model had the lowest BIC (9710.37) compared to 2-, 3-, and 5-class covariate-adjusted models (9787.35, 9723.57, and 9734.33, respectively). Finally, the adjusted 4-class model provided the most meaningful theoretical and clinical interpretation as well as best class separation.
Multimorbidity Phenotype Classes
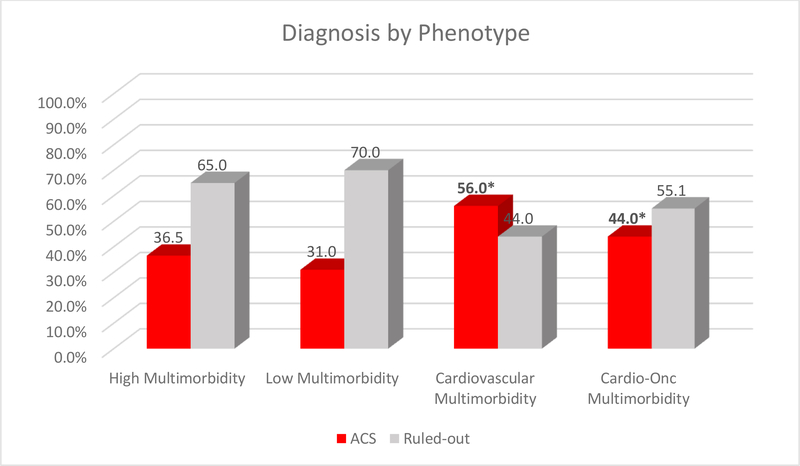
The probability of a specific co-morbid condition being present in a class was defined as high (≥ 0.60–1.0), moderate (≥ 0.30-< 0.60), or low (< 0.30). Figure 1 shows condition probabilities by class. Class 1 was labeled high multimorbidity because it had the greatest number of high-probability conditions (Table 4). Conditions in Class 1 were hyperlipidemia, hypertension, obesity, diabetes, and respiratory disorders (COPD or asthma). Class 2 contained no high-probability conditions, had a moderate probability of obesity, and was labeled low multimorbidity. Class 3 was labeled cardiovascular multimorbidity and included a high probability of CHD, hypertension, and hyperlipidemia. Class 4 was labeled cardiovascular-oncologic (cardio-onc) multimorbidity and included hyperlipidemia, hypertension, and cancer. Class 3 was the largest, with 384 patients, and Class 4 was the smallest, with 151. Patients with four or more individual conditions clustered in Classes 1 (43.2%) and 3 (27.2%).
Figure 1.
Diagnosis by Phenotype (n=935)
Table 4.
Probability of Condition Occurrence by Multimorbidity Class (n=935)
| Chronic Condition N = 935 | Class 1 High N = 208 (22.2%) | Class 2 Low N = 195 (20.9%)\ | Class 3 Cardiovascular N = 381(40.9%) | Class 4 Cardio-Onc N = 151 (16.1%) |
|---|---|---|---|---|
| Diabetes | 64.4~ | 6.9 | 35.0~ | 8.9 |
| Hyperlipidemia | 70.6~ | 17.6 | 71.6~ | 52.9~ |
| Renal | 38.1 | 2.4 | 2.1 | 8.2 |
| Obese (%) | 61.8~ | 30.6~ | 54.3~ | 13.4 |
| Coronary Heart Disease (%) | 54.2~ | 22.3 | 54.1~ | 44.5~ |
| Peripheral vascular disease (%) | 22.0 | 0 | 1.9 | 10.9 |
| Cerebrovascular disease (%) | 19.5 | 1.4 | 3.3 | 15.1 |
| Cancer(%) | 14.0 | 3.3 | 5.0 | 36.5~ |
| Respiratory Disorders (COPD or Asthma) (%) | 52.6~ | 13.3 | 21.8 | 21.9 |
| Systemic Lupus Erythematosus | 10.0 | 3.7 | 4.9 | 9.4 |
| Hypertension (%) | 91.2~ | 19.4 | 82.4~ | 58.0~ |
Bold~ indicates high probability conditions (.6–1) and ~ indicates moderate probability conditions (0.3 to < .60). Models are adjusted for age, Duke Activity Score Index weighted total score, family history of sudden cardiac death < 55 years, and ACS status (rule-in/out).
Results showed that class membership varied by sex, with females most often in Class 1 (31.7%). Classes also differed by age, functional status, and family history of sudden cardiac death aged < 55 years. Patients in Class 4 were the oldest (mean age 80.0 ± 6.3 years), while patients in Class 2 were youngest (mean age 43.4 ± 9.8 years, p < 0.001). Patients in Classes 1 and 4 had lower functional status (mean DASI scores 10.1 and 28.2) than patients in Classes 2 and 3 (mean DASI scores 47.7 and 42.5, p < 0.001). Most patients in Class 3 had a family history of sudden cardiac death aged < 55 years (58.3%). Patients in Class 2 had a lower prevalence of a family history of sudden cardiac death than Classes 1 and 4 (27.5% vs. 51.0% and 34.4%, p < 0.001).
Class membership was associated with ACS diagnosis (ruled-in vs. ruled-out). Patients ruled-in for ACS primarily clustered in Classes 3 and 4 (OR 2.82, 95% CI 1.95–4.05, p = 0.001 and OR 1.76, 95% CI 1.13–2.74, p = 0.01) compared to Class 2. Conversely, Class 1 membership was not significantly associated with ACS diagnosis (OR 1.27 95% CI 0.84–1.92, p=0.251) compared to Class 2 (OR 0.45 95% CI 0.33–0.61, p = 0.000)
Discussion
The two key findings from our analysis were: (1) there were four multimorbidity phenotypes that we labeled: Class 1 High Multimorbidity, Class 2 Low Multimorbidity, Class 3 Cardiovascular Multimorbidity, and Class 4 Cardio-Onc Multimorbidity: and (2) specific phenotypes were associated with being ruled-in or ruled-out for ACS.Phenotype membership differed by age, sex, functional status, and family history of sudden cardiac death before age 55. Average age of our geographically and racially diverse sample was slightly younger (59.9 years) than previous studies examining multimorbidity in the ACS population, where average ages ranged from 67.7 to 79.4 years.4,17,26 The average age was 43.4 years in the Low Multimorbidity phenotype, which had the highest number of patients ruled-out for ACS as would be expected. Females were more likely to cluster in the High Multimorbidity phenotype. Differences in our findings could be attributed to the fact that most prior studies focused on only patients ruled-in for ACS, who tend to be older.4,8,11,27 Patients with lower functional status clustered in the High Multimorbidity and Cardio-Onc Multimorbidity phenotypes, which aligns with previous findings that multimorbidity was associated with lower functional status.11,27 Finally, most patients in the High Multimorbidity and nearly half of the patients in the Cardiovascular Multimorbidity phenotypes had a family history of sudden cardiac death before age 55. This is consistent with previous findings that family history of sudden cardiac death before age 55 is a risk factor for development of CHD,4 earlier onset of CHD,4 and other cardiovascular diseases such as hypertension, cardiomyopathies, and arrhythmias such as atrial fibrillation.28
Our 4-class solution varied from the only prior study that used latent class analysis to examine multimorbidity in the ACS population. Hall et al. found a 3-class solution was optimal.4 However, their sample was larger, only included acute myocardial infarction patients, and was conducted in England and Wales over the course of a decade.4 Our analysis included more conditions (obesity, cancer, lupus, and hyperlipidemia) than the previous study. Obesity was included as a chronic condition, since it was declared a chronic disease by the American Medical Association in 2013.29 Prior studies have either included obesity as a risk factor (covariate) or omitted it from analysis.4 Finally, our classification of patients into phenotypes somewhat differs from the high, medium, and low multimorbidity phenotypes described by Hall et al. 4 Similarly, our analysis found high and low multimorbidity phenotypes; however, we described additional phenotypes (Cardiovascular and Cardio-Onc).
The Cardiovascular Multimorbidity and Cardio-Onc Multimorbidity phenotypes identified in our study are novel additions to previous classifications multimorbidity phenotypes. Each had a distinct clinical profile and increased likelihood of individuals being ruled-in for ACS. However, two conditions were overlapping HTN and HLD. This would be expected given they are known risk factors for ACS.4,30 Cardiovascular Multimorbidity phenotype patients represented 26.2% of our sample, were younger (average age of 58.8 years), were mostly female (51.6% of class membership), reported higher functional status with no significant difference in DASI scores compared to the Low Multimorbidity phenotype, and nearly half had a positive family history of sudden cardiac death before age 55 years. The presence of the Cardiovascular Multimorbidity phenotype in our study is consistent with prior literature examining risk factors for ACS, including hypertension, hyperlipidemia, and CHD,4,11 which were the three conditions highly associated with Cardiovascular Multimorbidity phenotype. The Cardio-Onc Multimorbidity phenotype was smallest, including only 14.5% of our sample. Cardio-Onc Multimorbidity phenotype patients were older, mostly male, had the lowest rate of family history of sudden cardiac death before age 55, and lower functional status. The identification of the Cardio-Onc Multimorbidity phenotype in our sample potentially reflects increasing cancer survivorship rates,31 coupled with the unfavorable cardiovascular risk and cardiovascular disease profile present at diagnosis, resulting from the cancer itself, or from treatment regimens.31
Count based multimorbidity measures may underestimate true burden of multimorbidity in patients evaluated for ACS. Patients in the Cardiovascular Multimorbidity and Cardio-Onc Multimorbidity phenotype exhibited a greater likelihood of being ruled-in for ACS than their Low Multimorbidity phenotype counterparts. This is consistent with prior literature that patients with greater multimorbidity burden are at higher risk for CHD events.4,11 Our finding that the high multimorbidity phenotype was not associated with being ruled-in for ACS supports that multimorbidity phenotypes may provide increased diagnostic utility compared to count based measures.
Strengths and Limitations
Our study had several strengths. Patients ruled-in and ruled-out for ACS were included. Prior studies focused on patients ruled-in for ACS. Because most patients with chest pain and associated symptoms are ruled-out for ACS, it is important to determine similarities and differences between groups to improve diagnostic testing and safe discharge. Latent class analysis provides insight into how chronic conditions cluster, using a data-driven probabilistic modeling approach. Previous studies relied on basic analytical techniques such as correlations and regression modeling, which may not fully capture the impact of multimorbidity in ACS patients. Those techniques have low statistical power and high rates of false positives (type I errors) because conditions are considered independently or additively, or they use all possible combinations of conditions.4
Findings should be interpreted with some degree of caution when trying to generalize our findings to different patient samples and settings (i.e. primary care clinics, cardiac rehab, and inpatient settings) as there were several limitations to our study. These limitations included self-reported data (collected through interviews by trained research assistants) and medical record data, though a previous study32 found a high correlation between self-reported conditions and national health data (96.6%) and that consistency between self-reports and medical data was satisfactory to very good (Kappa = 0.8 and 0.67 for diabetes and hypertension). Unfortunately, as a limitation of the data available for analysis certain CCI conditions, such as systemic lupus erythematosus and peripheral vascular disease were not present in great enough frequency to achieve adequate analysis of the statistical results regarding the loadings of that condition on class membership. We made the choice to include theoretically relevant CCI items such as peripheral vascular disease and lupus because of their association with increased risk of cardiovascular events despite not being present in 5% of the original study population. Also, multimorbidity was determined by the presence or absence of a specific condition; we were unable to account for differences in severity. For example, we were not able to differentiate by current or previous cancer diagnosis nor were we able to discern the type of cancer diagnosis from the CCI. Future studies should incorporate condition severity to capture the multidimensional nature of multimorbidity and its impact on patient outcomes. Also, other outcome measures should be examined to see if the utility of these multimorbidity phenotypes is predictive of other adverse outcomes (mortality, readmission, and increased length of stay), as well as other patient centered outcomes such as medication usage and different types of postdischarge healthcare utilization.
Conclusions
Four distinct multimorbidity phenotypes were identified in patients evaluated in the ED for potential ACS. Having a greater burden of overall multimorbidity may not be as predictive of receiving an ACS diagnosis, rather, specific combinations of chronic conditions may be more diagnostically useful in ACS. Phenotypes may contribute to improved risk-stratification in the ED for patients with symptoms suggestive of ACS. Future research should focus on development of interventions to improve clinical outcomes such as morbidity, health care utilization, and patient-centered outcomes (quality of life and functional status).
Highlights.
Specific Multimorbidity phenotypes were associated with receiving an ACS diagnosis
Cardiovascular and Cardio-onc phenotypes had the greatest risk of ACS
Age, sex, and functional status were predictive of class membership.
Multimorbidity phenotypes may improve current ACS risk-stratification models
Acknowledgements
This work was funded by NINR (R01NR012012, DeVon PI) and the Robert Wood Johnson Foundation Future of Nursing Scholars Program (Scholar Breen). This manuscript was submitted while Dr. Breen was supported as a postdoctoral trainee by the NINR (T32NR012715, S. Dunbar, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Kevin Grandfield, Publication Manager for the UIC Department of Biobehavioral Nursing Science, for editorial assistance.
Footnotes
Conflicts of/competing Interests
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhuiya FA, Pitts SR, McCaig LF. Emergency department visits for chest pain and abdominal pain: United States, 1999–2008. NCHS data brief 2010(43):1–8. [PubMed] [Google Scholar]
- 2.Hess CN, Wang TY, McCoy LA, et al. Unplanned Inpatient and Observation Rehospitalizations After Acute Myocardial Infarction: Insights From the Treatment With Adenosine Diphosphate Receptor Inhibitors: Longitudinal Assessment of Treatment Patterns and Events After Acute Coronary Syndrome (TRANSLATE-ACS) Study. Circulation. 2016;133(5):493–501. [DOI] [PubMed] [Google Scholar]
- 3.Fanaroff AC, Rymer JA, Goldstein SA, Simel DL, Newby LK. Does This Patient With Chest Pain Have Acute Coronary Syndrome?: The Rational Clinical Examination Systematic Review. Jama. 2015;314(18):1955–1965. [DOI] [PubMed] [Google Scholar]
- 4.Hall M, Dondo TB, Yan AT, et al. Multimorbidity and survival for patients with acute myocardial infarction in England and Wales: Latent class analysis of a nationwide population-based cohort. PLoS Med. 2018;15(3):e1002501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothnie KJ, Quint JK. Chronic obstructive pulmonary disease and acute myocardial infarction: effects on presentation, management, and outcomes. European heart journal Quality of care & clinical outcomes. 2016;2(2):81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HY, Saczynski JS, McManus DD, et al. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: a population-based perspective. Clinical epidemiology. 2013;5:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tisminetzky M, Gurwitz JH, Miozzo R, et al. Impact of cardiac- and noncardiac-related conditions on adverse outcomes in patients hospitalized with acute myocardial infarction. J Comorb. 2019;9:2235042×19852499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdulnour J, Doucet E, Brochu M, et al. The effect of the menopausal transition on body composition and cardiometabolic risk factors: a Montreal-Ottawa New Emerging Team group study. Menopause (New York, NY) 2012;19(7):760–767. [DOI] [PubMed] [Google Scholar]
- 9.Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9(5):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeger RV, Pfister O, Radovanovic D, et al. Heart failure in patients admitted for acute coronary syndromes: A report from a large national registry. Clinical cardiology. 2017;40(10):907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canivell S, Muller O, Gencer B, et al. Prognosis of cardiovascular and non-cardiovascular multimorbidity after acute coronary syndrome. PloS one. 2018;13(4):e0195174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman DE, Maurer MS, Boyd C, et al. Multimorbidity in Older Adults With Cardiovascular Disease. J Am Coll Cardiol. 2018;71(19):2149–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. [DOI] [PubMed] [Google Scholar]
- 14.DeVon HA, Burke LA, Vuckovic KM, et al. Symptoms Suggestive of Acute Coronary Syndrome: When Is Sex Important? The Journal of cardiovascular nursing. 2017;32(4):383–392. [DOI] [PubMed] [Google Scholar]
- 15.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. Journal of clinical epidemiology. 2003;56(3):221–229. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein LB, Samsa GP, Matchar DB, Horner RD. Charlson Index comorbidity adjustment for ischemic stroke outcome studies. Stroke. 2004;35(8):1941–1945. [DOI] [PubMed] [Google Scholar]
- 17.Tessier A, Finch L, Daskalopoulou SS, Mayo NE. Validation of the Charlson Comorbidity Index for predicting functional outcome of stroke. Archives of physical medicine and rehabilitation. 2008;89(7):1276–1283. [DOI] [PubMed] [Google Scholar]
- 18.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis and rheumatism. 2003;49(2):156–163. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64(10):651–654. [DOI] [PubMed] [Google Scholar]
- 20.Arena R, Humphrey R, Peberdy MA. Using the Duke Activity Status Index in heart failure. Journal of cardiopulmonary rehabilitation. 2002;22(2):93–95. [DOI] [PubMed] [Google Scholar]
- 21.Nichol G, Llewellyn-Thomas HA, Thiel EC, Naylor CD. The relationship between cardiac functional capacity and patients' symptom-specific utilities for angina: some findings and methodologic lessons. Medical decision making : an international journal of the Society for Medical Decision Making. 1996;16(1):78–85. [DOI] [PubMed] [Google Scholar]
- 22.Coutinho-Myrrha MA, Dias RC, Fernandes AA, et al. Duke Activity Status Index for cardiovascular diseases: validation of the Portuguese translation. Arquivos brasileiros de cardiologia. 2014;102(4):383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LJ, Olson MB, Kip K, et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study. J Am Coll Cardiol. 2006;47(3 Suppl):S36–43. [DOI] [PubMed] [Google Scholar]
- 24.Koch CG, Khandwala F, Cywinski JB, et al. Health-related quality of life after coronary artery bypass grafting: A gender analysis using the Duke Activity Status Index. The Journal of Thoracic and Cardiovascular Surgery. 2004;128(2):284–295. [DOI] [PubMed] [Google Scholar]
- 25.Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling: A multidisciplinary Journal. 2007;14(4):535–569. [Google Scholar]
- 26.Barrabes JA, Bardaji A, Jimenez-Candil J, et al. Characteristics and Outcomes of Patients Hospitalized With Suspected Acute Coronary Syndrome in Whom the Diagnosis is not Confirmed. Am J Cardiol. 2018;122(10):1604–1609. [DOI] [PubMed] [Google Scholar]
- 27.Alfredsson J, Alexander KP. Multiple Chronic Conditions in Older Adults with Acute Coronary Syndromes. Clinics in geriatric medicine. 2016;32(2):291–303. [DOI] [PubMed] [Google Scholar]
- 28.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4(13):256–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollack A. AMA Recognizes Obesity as a Disease. New York Times 2013.
- 30.McManus DD, Nguyen HL, Saczynski JS, Tisminetzky M, Bourell P, Goldberg RJ. Multiple cardiovascular comorbidities and acute myocardial infarction: temporal trends (1990–2007) and impact on death rates at 30 days and 1 year. Clinical epidemiology. 2012;4:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GernaatS AM, Ho PJ, Rijnberg N, et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(3):537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu CJ, Huang HM, Lu TH, Wang YW. National health data linkage and the agreement between self-reports and medical records for middle-aged and older adults in Taiwan. BMC health services research. 2018;18(1):917. [DOI] [PMC free article] [PubMed] [Google Scholar]