Abstract
Background
The cortisol awakening response (CAR) is a core biomarker of hypothalamic-pituitary-adrenal (HPA) axis regulation. To date, however, studies of HPA-axis function amongst patients with chronic pain are scarce and show equivocal results. The objectives of this study were to investigate the association between CAR and pain-related outcomes and to investigate potential sex differences in patients with knee osteoarthritis (KOA).
Methods
In this cross-sectional study, KOA patients (N = 96) completed self-report questionnaires assessing pain and psychosocial factors and underwent Quantitative Sensory Testing (QST) to assess pressure pain threshold (PPT). Additionally, salivary cortisol samples (N = 60) were collected to assess HPA-axis function at 6 time points (awakening, 15- and 30-minutes post-awakening, 4PM, 9PM and bedtime). The CAR was calculated by examining increases in salivary cortisol from awakening to 30min post awakening and the total post-awakening cortisol concentration by calculating the lower areas under the curve of cortisol with respect to ground (AUCG).
Results
Patients with a relatively blunted CAR had significantly higher anxiety levels and lower PPT than patients with relatively normal CAR. Similarly, patients with a relatively reduced AUCG had significantly higher pain interference and anxiety levels compared to patients with relatively normal AUCG. PPT was positively correlated with CAR and AUCG and negatively correlated with pain severity and anxiety. Men with KOA had significantly lower anxiety, higher PPT and higher CAR and AUCG than women with KOA. Mediation analysis results revealed a significant indirect effect of PPT on the relationship between sex and AUCG.
Conclusions
The findings of this study suggest that neuroendocrine factors such as CAR and AUCG may contribute to individual differences in pain-related outcomes in patients with KOA. Additionally, our results show sex differences in the magnitude of morning HPA activation and pain-related outcomes. Finally, our findings are suggestive of a sex-dependent relationship between post-awakening cortisol concentrations and pain perception. Future research should examine these associations across various pain populations.
Keywords: cortisol, cortisol awakening response, pain, pain threshold, sex, osteoarthritis
1. Introduction
Cortisol, the primary glucocorticoid regulated by the hypothalamic-pituitary-adrenal axis, is highly integrated in animal and human metabolic, immunologic and stress-response systems. Cortisol levels exhibit profound circadian rhythms, varying substantially across sleep-wake cycles. Abnormal cortisol diurnal profiles have been associated with a wide range of pathologies including autoimmune and atopic inflammatory diseases, immunodeficiency, as well as post-traumatic stress disorder, negative mood affect, and depression (Sapse 1997; van Eck et al. 1996). The cortisol awakening response (CAR), characterized by a consistent steep increase in cortisol activity in the immediate 30 minutes post-awakening, is a reliable biologic marker for individual adrenocortical activity (Pruessner et al. 1997). The diurnal pattern of cortisol secretion is characterized by a sharp fall after the initial CAR and a subsequent gradual decline throughout the day. The availability of free cortisol immediately after awakening predicts mean cortisol levels throughout the day – demonstrating that the CAR can serve as a stable characteristic of basal cortisol levels during the diurnal cycle (Edwards et al. 2001). Even though its exact function has yet to be elucidated, it has been demonstrated that CAR levels might be associated with numerous factors that include age, sex, time of waking, smoking, stress, anxiety and depression (Steptoe and Serwinski 2016). Studies investigating the effect of sex on the CAR show inconsistent findings, showing either higher CAR in women on workdays or no difference between the two sexes (Fries et al. 2009). Further, a blunted cortisol awakening response, has been associated with multiple adverse health outcomes including poorer general health, metabolic profiles, and poorer sleep quality in adults (Lasikiewicz et al. 2008).
Previous reports have demonstrated CAR to be influenced by states of chronic stress (which may include chronic pain states) (Pruessner et al. 1999) but the existing literature remains inconsistent (Lasikiewicz et al. 2008). Collectively, some studies have shown that reduced (i.e., blunted) CAR is associated with chronic pain and fatigue-related disorders including whiplash-associated disorder and fibromyalgia, suggesting that cortisol and hypothalamic-pituitary-adrenal (HPA) axis dysregulation may be useful as potential biomarkers for chronic pain and fatigue-related conditions (Gaab et al. 2005). In an experimental setting, a blunted CAR measured by AUCI (area under the curve with respect to increase) was associated with greater pain intensity and pain unpleasantness during an acute pain task (Fabian et al. 2009). The authors of this study suggested that a blunted CAR may correlate with dysregulation of the HPA-axis, placing individuals at higher risk for acute and chronic pain. In studies with chronic pain patients, both elevated (Carlesso et al. 2016) and lower levels (Riva et al. 2012) of cortisol, as well as dysregulations in diurnal cortisol (Crofford et al. 2004) have been reported. However, in a small study comparing low back pain patients (14 acute, 17 chronic) to healthy individuals, it was found that the cortisol profiles of the two groups did not differ significantly (Sudhaus et al. 2007). Despite these less than perfectly consistent findings, the weight of the evidence suggests that the HPA-axis may be overactive in the acute stages of a pain condition but may spiral into an exhausted state of hypoactivity and blunted circadian rhythms after pain persistence (Nees et al. 2019).
Knee osteoarthritis (KOA) is one of the most prevalent musculoskeletal diseases causing persistent pain and disability worldwide, leading to high direct and indirect financial costs (Cross et al. 2014; Losina et al. 2015; Smith et al. 2014). Although the existing literature has investigated the relationships between disease-related pain and cortisol levels for a number of musculoskeletal disorders, few studies have examined these relationships in patients with osteoarthritis (Carlesso et al. 2016), and the existing literature has been characterized as being of fairly low quality (Villafañe et al. 2020). Additionally, the relationship between chronic pain, comorbid pain symptomatology, sex, and sensory function has rarely been examined. Interestingly, there is some evidence that the HPA-axis regulation system interacts with the nociceptive sensory system (Nees et al. 2019) and a recent review by Villafane et al. suggests a potential influence of sex on the association between pain in patients with OA and cortisol levels (Villafañe et al. 2020), suggesting that the HPA-axis dysregulation may be related to sex differences in pain sensitivity.
The objectives of this study were to investigate the association between the cortisol awakening response (and post-awakening cortisol concentrations) and pain-related outcomes including pain threshold, as well as to delineate potential sex differences in patients with KOA.
2. Methods
2.1. Ethics
The involvement of human subjects in this study was reviewed, approved, and monitored by the Institutional Review Board (IRB) of Brigham & Women’s Hospital (Boston, MA, USA). Informed and written consent was obtained from each participant.
2.2. Study population characteristics
Ninety-six (96) participants with chronic advanced KOA (i.e., Kellgren-Lawrence grade 3+) awaiting unilateral total knee replacement at Brigham & Women’s Hospital (Boston, MA, USA) were recruited. Patients were included if they met the following criteria: 1) they had an age of 50 years or older, 2) met the American College of Rheumatology criteria for KOA, 3) were scheduled to undergo total knee arthroplasty, 4) were fluent in English and able to provide written informed consent, 5) were on stable medication doses for one month before the study. Patients were excluded for the following: 1) they had a cognitive impairment preventing the completion of study assessment procedures, 2) had suffered a myocardial infarction within the past 12 months, 3) the presence of Raynaud’s phenomenon or severe neuropathy, 4) the presence of active vasculitis or severe peripheral vascular disease, 5) had a current infection, 6) were using oral steroids, 7) had a recent history of substance abuse or dependence, 8) were suffering from systemic inflammatory or autoimmune disorders such as rheumatoid arthritis and lupus, 9) were suffering from severe anemia.
2.3. Self-report Assessments
2.3.1. Sociodemographic Data
Sociodemographic information collected included: age, marital, educational, and current occupational status, duration of KOA symptoms and medical comorbidities.
2.3.2. Pain Catastrophizing
The pain catastrophizing scale (PCS) is a widely used self-report measure of catastrophic thinking associated with pain. The PCS has shown good psychometric properties in samples of chronic pain patients and controls. The PCS is a 13-item instrument that uses a five-point Likert scale (“not at all” to “all the time”), with higher scores indicating elevated levels of catastrophizing. The PCS examines three components of catastrophizing: rumination, magnification, and helplessness (Osman et al. 1997).
2.3.3. Pain
To measure clinical pain levels, the Brief Pain Inventory (BPI) was used. It is a 15-item measure that consists of two multi-item sub-scales that measure pain intensity and pain interference with daily activities. The BPI is well-validated in chronic pain population and is frequently recommended as an outcome measure of pain severity and pain interference (Cleeland and Ryan 1994).
2.3.4. Emotional Distress—Anxiety and Depression
Participants completed the Patient-Reported Outcomes Measurement Information System (PROMIS) anxiety and depression short forms, which are widely used and extensively validated in chronic pain populations. The anxiety subscale consists of seven items that ask respondents about the frequency with which they have experienced emotions such as fear, stress, and anxiety (“never” to “always”). The depression subscale consists of eight items in which respondents indicate the frequency with which they have experienced emotions such as worthlessness, hopelessness, and sadness (“never” to “always”). Higher scores indicate more severe symptoms of emotional distress (Cella et al. 2010; Pilkonis et al. 2013).
2.3.5. Sleep
To assess sleep quality, we used the corresponding component from the Pittsburgh Sleep Quality Index (PSQI). Each component can be scored from 0 (no difficulty) to 3 (severe difficulty). Higher scores are indicating worse sleep quality (Buysse et al. 1989).
2.4. Quantitative Sensory Testing (QST)
Mechanical pain thresholds were assessed using a digital pressure algometer (Somedic) bilaterally at two lower-body sites (i.e., the patella and the quadriceps muscle). Mechanical force was applied using a 0.5-cm2 probe covered with polypropylene pressure-transducing material; pressure was slowly increased until the subject indicated that the pressure was first perceived as painful. The pressure pain threshold (PPT) was calculated as the average of the pain thresholds measured on the patella and the quadriceps muscle.
2.5. Cortisol assessment
Similar to prior studies (Fabian et al. 2009; Kudielka et al. 2003), six salivary cortisol samples were obtained over the course of a single day using salivettes in order to assess each participant’s diurnal cortisol rhythm. At predetermined times (upon awakening, 15min and 30min after awakening, and at 1600h, 2100h and right before bedtime), participants placed a sterile cotton pad in their mouth for 2 minutes. The participants were instructed to refrain from toothbrushing or mouthwash, caffeine, dairy products, alcohol, nicotine, medication, or hard food 30 minutes before the collection. Saliva samples were analyzed for cortisol with the expanded range, high sensitivity Salimetrics® Cortisol Enzyme Immunoassay (#1-3002). Salivary cortisol is not influenced by saliva flow rate and reflects the levels of unbound, biologically active cortisol in the blood (Kirschbaum and Hellhammer 1994).
The present study, consistent with numerous prior reports, utilized the AUC model proposed by Pruessner et. al. (Pruessner et al. 2003) to quantify CAR and total post-awakening cortisol concentrations. Using selected time points (i.e., upon awakening and 15min, and 30min post awakening), the CAR was measured by calculating the area under the curve with respect to increase (AUCI). The total post-awakening cortisol concentration (AUCG) by calculating the area under the curve with respect to the ground using the same points. For the CAR, relatively normal and blunted curves (in relation to this patient sample) were defined using a median split calculation based on AUCI (Fabian et al. 2009). For the post-awakening cortisol concentration, participants were then categorized as showing either normal or reduced (in relation to this patient sample) AUCG using a median split calculation based on AUCG.
2.6. Statistical Analyses
Power analyses were performed to determine the optimal sample size and assure an adequate power to detect statistical significance. Based on these calculations we determined that a minimum sample size of n = 40 participants would be necessary to detect the hypothesized correlation and mediation effects. For descriptive purposes, we calculated means and standard deviations (SD) for continuous variables (age and clinical variables) and percentages for dichotomous variables (demographics). A Mann-Whitney U-test was performed to explore group differences (normal vs. blunted CAR and normal vs. reduced post-awakening cortisol). Spearman correlations were calculated to assess relationships between cortisol measures and pain-related variables. Finally, we performed mediation analysis (PROCESS, model 4) to examine whether PPT mediated the relationship between sex and AUCG. A custom written macro (PROCESS; www.processmacro.org) for SPSS (v26, IBM, USA) was used to perform multiple mediation pathway analysis with bias-corrected bootstrapping tests.
3. Results
3.1. Demographic, psychometric and pain-related variables
Ninety-six KOA patients participated in the study, out of which sixty provided complete salivary cortisol samples. Patient demographics and clinical characteristics are depicted on Table 1. PPT was negatively associated with pain severity and anxiety, and positively associated with CAR and AUCG (Table 2).
Table 1.
Sociodemographic and Clinical Variables
| Sociodemographic Variables | n= 95 | |
|---|---|---|
| Age (mean ± SD) | 65.5 ± 7.4 | |
| Female | 57.3% | |
| Married | 72.9% | |
| Education Level (College Degree) | 69.8% | |
| Working Full-Time | 31.3% | |
| Caucasian | 88.5% | |
| African American | 6.3% | |
| Clinical Variables | Scale Score Range | Mean ± SD |
| BPI (Severity) (n=78) | 0–10 | 3.1 ± 1.9 |
| BPI (Interference) (n= 74) | 0–10 | 3.6 ± 2.3 |
| PCS (n=76) | 0–52 | 12.2 ± 10.6 |
| Anxiety (PROMIS) (n= 92) | 4–20 | 13.6 ± 5.2 |
| Depression (PROMIS) (n= 92) | 4–20 | 11.8 ± 9.0 |
| Pain Threshold (n= 87) | 636.4 ± 213.9 | |
| Sleep Quality (PSQI) (n= 76) | 0–3 | 1.0 ± .90 |
| Cortisol 0 min p.a (n=63) | .09–1.55 µg/dL | .19 ± .23 |
| Cortisol 15 min p.a. (n=62) | .26 ± .29 | |
| Cortisol 30 min p.a. (n=62) | .26 ± .27 | |
| Cortisol 4PM (n=61) | .07 ± .08 | |
| Cortisol 9PM (n=62) | ND-.36 µg/dL | .04 ± .03 |
| Cortisol bedtime (n=60) | .06 ± .12 | |
| CAR (n=60)) | .03 ± .09 | |
| AUCG (n= 60 | .13 ± .12 | |
Note: SD: standard deviation; BPI: Brief Pain Inventory; PCS: Pain Catastrophizing Scale; PROMIS: Patient-Reported Outcomes Measurement Information System (T-Score); PSQI: Pittsburgh Sleep Quality Index; p.a.: post awakening; CAR: Cortisol Awakening Response; AUCG: Area under the curve with respect to ground, Total post-awakening cortisol levels
Table 2.
Non-Parametric Correlations
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 BPI Severity | 1 | .66** | .50** | .29* | .17 | −.24 | .38** | −.16 | −.14 |
| 2 BPI Interference | .66** | 1 | .53** | .28* | .25* | −.15 | .42** | .11 | −.09 |
| 3 PCS | .50** | .53** | 1 | .50** | .43** | −.17 | .44** | .02 | −.04 |
| 4 Anxiety (PROMIS) | .30* | .28* | .50** | 1 | .64** | −.26* | .38** | −.12 | −.26 |
| 5 Depression (PROMIS) | .17 | .25* | .43** | .64** | 1 | −.12 | .36** | −.02 | .02 |
| 6 Pain Threshold | −.24* | −.15 | −.17 | −.26* | −.12 | 1 | −.09 | .33* | .30* |
| 7 Sleep Quality | .38** | .42** | .44** | .38** | .36** | −.09 | 1 | .01 | −.05 |
| 8 CAR | −.16 | .11 | .02 | −.12 | −.02 | .33* | .01 | 1 | .52** |
| 9 AUCG | −.14 | −.09 | −.04 | −.26 | .02 | .30* | −.05 | .52** | 1 |
Correlation is significant at the 0.05 level (two-tailed)
Correlation is significant at the 0.01 level (two-tailed)
BPI: Brief Pain Inventory; PCS: Pain Catastrophizing; PROMIS: Patient-Reported Outcomes Measurement Information System (T-Score); PSQI6: Pittsburgh Sleep Quality Index; CAR: Cortisol Awakening Response; AUCG: Area under the curve with respect to ground, Total post-awakening cortisol levels
3.2. Cortisol levels in response to awakening and during the day
Table 1 displays mean free cortisol levels during the first hour after awakening, throughout the day, and the calculated CAR and total post-awakening cortisol concentrations (AUCG). The mean AM cortisol values were low-normal and the PM values were also within the normal range. The cortisol levels largely followed the expected diurnal pattern with AM values increasing in the first 30 minutes after waking and subsequent dropping in the PM hours. A surge of approximately 35–40% (similar to typical surges of approximately 50% (Adam and Kumari 2009)) was observed in the 30 min following awakening.
3.2.1. Blunted vs. normal CAR
Following CAR AUCI calculation, a median split (median= 0.01) was used to separate participants into two groups: “blunted” and “normal” (in relation to this patient sample). The CAR of the participants of the two groups was significantly different (−0.03 ± 0.06 vs. 0.08 ± 0.07; p <.001). Participants with a relatively blunted CAR had significantly higher anxiety levels and lower PPT. The two groups did not differ in their age and other measured variables (Figure 1, Table 3).
Figure 1.
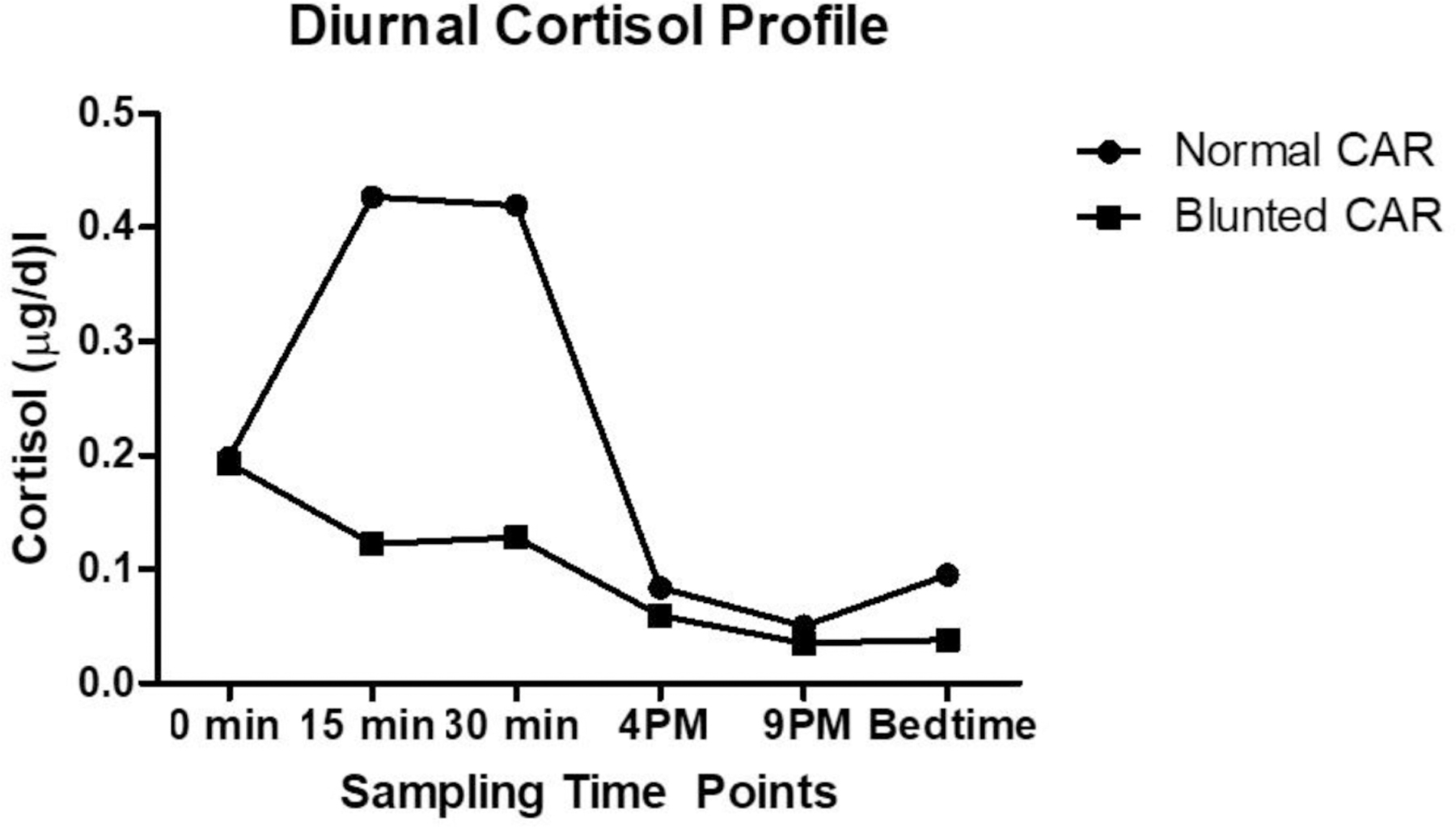
Diurnal cortisol values in participants with “blunted” CAR and “normal” CAR. CAR = Cortisol Awakening Response
Table 3.
Group Differences in CAR and AUCG
| CAR | “Blunted” (n=29) | “Normal” (n=29) | p-value | Cohen’s d |
|---|---|---|---|---|
| BPI Severity | 3.32 ± 1.94 | 2.75 ± 1.96 | .270 | 0.29 |
| BPI Interference | 3.23 ± 2.09 | 3.86 ± 2.51 | .449 | 0.27 |
| PCS | 12.65 ± 8.80 | 12.63 ± 11.83 | .597 | 0.001 |
| Anxiety (PROMIS) | 15.46 ± 5.08 | 12.61 ± 4.86 | .028 | 0.57 |
| Depression (PROMIS) | 12.89 ± 4.65 | 11.04 ± 3.87 | .128 | 0.43 |
| Sleep Quality (PSQI) | 1.00 ± .89 | .97 ± .91 | .851 | 0.03 |
| Pain Threshold | 591.77 ± 204.97 | 714.12 ± 231.21 | .036 | 0.56 |
|
| ||||
| AUCG | “Reduced” (n=31) | “Normal” (n=29) | p-value | Cohen’s d |
| BPI Severity | 3.39 ± 2.06 | 2.56 ± 1.75 | .136 | 0.43 |
| BPI Interference | 4.09 ± 2.28 | 2.98 ± 2.19 | .048 | 0.50 |
| PCS | 13.02 ± 9.47 | 11.70 ± 11.21 | .402 | 0.13 |
| Anxiety (PROMIS) | 15.13 ± 5.16 | 12.50 ± 4.84 | .049 | 0.53 |
| Depression (PROMIS) | 11.77 ± 3.80 | 11.89 ± 4.90 | .806 | 0.03 |
| Sleep Quality (PSQI) | 1.17 ± .96 | .82 ± .82 | .183 | 0.39 |
| Pain Threshold | 601.04 ± 205.89 | 702.61 ± 236.41 | .075 | 0.46 |
Note: BPI: Brief Pain Inventory; PCS: Pain Catastrophizing; PROMIS: Patient-Reported Outcomes Measurement Information System (T-Score); Pittsburgh Sleep Quality Index; CAR: Cortisol Awakening Response; AUCG: Area under the curve with respect to ground, Total post-awakening cortisol levels; “Blunted”, “Reduced” and “Normal” are relative and refer to this particular patient sample.
3.2.2. Reduced vs. normal post-awakening cortisol concentrations (AUCG)
Following AUCG calculation, a median split (median= 0.08) was used to separate participants into two groups: “reduced” and “normal” (in relation to this patient sample).. The AUCG of the participants of the two groups was significantly different (0.03 ± 0.03 vs. 0.22 ± 0.11; p <.001). Participants in the relatively reduced AUCG group, had significantly higher pain interference and anxiety levels. The two groups did not differ in their age, sleep quality and depression. The difference in their PPTs approached but did not reach significance (Table 3).
3.3. Sex differences
Women had significantly higher anxiety levels and lower PPT, compared to men. There were also significant differences in the diurnal cortisol, CAR and AUCG: women exhibited lower cortisol after 15 (p=.03) and 30 minutes (p=.04) post-awakening, as well as lower CAR and AUCG. Pain levels, sleep quality and depression did not differ by sex (Table 4).
Table 4.
Sex Differences (mean ± SD)
| Male (n=39) | Female (n=53) | p-value | Cohen’s d | |
|---|---|---|---|---|
| Age | 66.65 ± 8.32 | 64.67 ± 6.66 | .259 | 0.26 |
| BPI Severity | 2.92 ± 2.02 | 3.33 ± 1.85 | .212 | 0.21 |
| BPI Interference | 3.32 ± 2.18 | 3.77 ± 2.46 | .445 | 0.19 |
| PCS | 10.50 ± 9.32 | 13.49 ± 11.48 | .291 | 0.29 |
| Anxiety (PROMIS) | 12.36 ± 5.7 | 14.45 ± 4.57 | .023 | 0.40 |
| Depression (PROMIS) | 11.44 ± 5.02 | 12.00 ± 3.89 | .167 | 0.12 |
| Pain Threshold | 759.85 ± 231.92 | 554.28 ± 155.76 | .000 | 1.04 |
| Sleep Quality (PSQI) | .94 ± .791 | 1.20 ± .91 | .195 | 0.30 |
| CAR | .05 ±.08 | .01 ± .09 | .029 | 0.47 |
| AUCG | .17 ± .14 | .09 ± .10 | .029 | 0.66 |
Note: PCS: Pain Catastrophizing; BPI: Brief Pain Inventory; PROMIS: Patient-Reported Outcomes Measurement Information System (T-Score); PSQI: Pittsburgh Sleep Quality Index; CAR: Cortisol Awakening Response; AUCG: Area under the curve with respect to ground, Total post-awakening cortisol levels.
3.3.1. Pressure pain threshold (PPT) as a mediator of the relationship between sex and AUCG
Figure 2 depicts the results of the mediation analysis. Controlling for pain severity and sleep quality, the results indicate that PPT mediated the influence of sex on AUCG. The standardized indirect effect was −0.44, CI, 95%, [−.9835, −.0892]. Sex was negatively associated with PPT (path a = −.1.1, p < .001). PPT was significantly positively associated with AUCG (path b = .40, p < .05).
Figure 2.
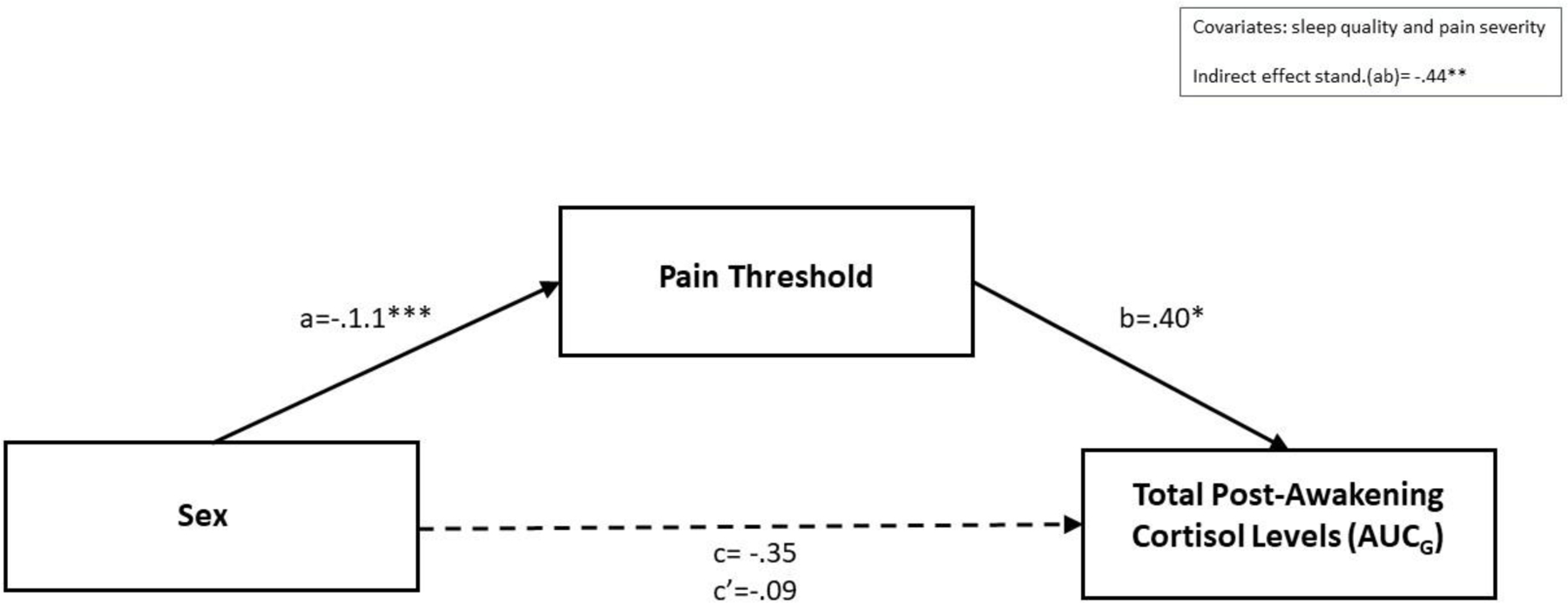
Pressure pain threshold mediating the relationship between sex and total post-awakening cortisol concentrations (AUCG)
4. Discussion
In this study, we aimed to examine the associations between CAR, sex and pain experience, as well as pain-related outcomes in patients with KOA. To the best of our knowledge, this is the first study to investigate these associations in patients with KOA. Our results show a modest surge in absolute CAR values following awakening. When comparing groups (relatively blunted vs. normal), participants with relatively blunted CAR had higher anxiety and lower PPT. Similarly, participants with relatively reduced AUCG had higher pain interference and anxiety compared to participants with relatively normal AUCG. We found no overall relationship between depressive symptoms and CAR, a finding that is consistent with previous studies including patients with (Sudhaus et al. 2007) and without chronic pain (Therrien et al. 2008; Vargas et al. 2017). This result may reflect the inconsistent relationship between depression and CAR, as evidence of a negative association between CAR and depression also exists (Dedovic et al. 2010).
Hypocortisolism in chronic pain has been reported in previous studies (Griep et al. 1998; Heim et al. 1999; Lentjes et al. 1997; Nees et al. 2019). However, it is still unclear whether the hypocortisolemia is the cause or the consequence of chronic pain. Previous work suggests that HPA-dysregulation might be predictive for the development of chronic pain (Turner-Cobb et al. 2010), based on the findings of McBeth and colleagues, who found relatively flattened diurnal cortisol in the pool of generally healthy subjects who later developed chronic widespread pain (McBeth et al. 2005). While our study was not designed to diagnose hypocortisolism, our CAR and total post-awakening cortisol concentration findings are in line with previous observations and are generally indicative of reduced neuroendocrine circadian rhythmicity, with a good deal of inter-patient variation. We did not find a direct correlation between pain severity and cortisol levels/CAR; however, pain interference was higher in the patient group with decreased post-awakening cortisol concentrations. These findings are not surprising, given that the evidence for a relationship between cortisol levels and pain in OA is equivocal (Villafañe et al. 2020).
The identified positive correlations between PPT and both CAR and total post-awakening cortisol are novel findings that are supported by previous research. For example, Kuehl and colleagues have demonstrated heightened sensitivity to noxious stimuli (lower pain threshold) under conditions of induced hypocortisolism, demonstrating a causal relationship between HPA-axis function and acute pain processing. The authors further suggest that chronic hypocortisolism might also play a potential causal role in pain chronicity through an N-methyl-D-aspartate receptor (NMDA-R) and b-endorphin mediated mechanism leading to central sensitization (Kuehl et al. 2010).
Our results indicated sex differences in the magnitude of morning HPA activation and pain-related outcomes: women exhibited lower CAR and post-awakening cortisol concentrations, lower PPT, and higher anxiety. These results suggest that women with KOA may be more neurohormonally perturbed by the experience of chronic pain and may experience a larger HPA-axis downregulation compared to men. As discussed in the introduction, findings related to cortisol profile and sex are inconsistent in the extant literature. Specifically, in patients with chronic pain, sex-differences have only been described in a previous study that found low cortisol levels in male participants compared to female in the afternoon (Turner-Cobb et al. 2010). To our knowledge, this is the first study investigating the CAR in patients with KOA. It is possible that the finding of higher CAR in men in this older patient cohort might be partly related to previous findings showing that CAR increases with age in men, but not in women (Almeida et al. 2009).
Further, we sought to identify the mechanism by which sex is associated with post-awakening cortisol concentrations. Given the links between cortisol and pain sensitivity in musculoskeletal pain (Bonifazi et al. 2006), we hypothesized that the higher PPT in men might be involved in the identified sex differences in CAR. Our mediation analysis revealed that PPT serves as a mediator in the relationship between sex and AUCG. That is, higher pain sensitivity in women with KOA compared to men with KOA may contribute to greater pain-related disruptions of normal functioning, including neurohormonal functioning, among women. Likely, PPT is just one of a number of variables that differ systematically as a function of sex and contribute to sex differences in daily cortisol rhythms; future studies may benefit from considering the importance of pain sensitivity when investigating the multimodal contributors to sex differences in neuroendocrine system functioning.
There are some limitations that need to be taken into consideration when interpreting these findings. First of all, no healthy control comparison group was included for this study, thus not allowing to investigate the differences of HPA-axis function of this patient population compared to healthy individuals. Due to potential intra-individual variability, the assessment of the diurnal cortisol and CAR on a single day poses a further limitation. However, when trait CAR estimates (i.e. sex, age, BMI) are of interest, accounting for the possibility of state covariates related to the sampling day (i.e. time of awakening, sleep duration and quality) should be the highest priority, and if addressed, a smaller amount of number of study is justifiable (Lauche et al. 2013). Additionally, it should be noted that in the absence of specific guidelines on how to define normal and dysregulated CAR, we used the median split to create CAR /AUCG groups. This type of approach has been utilized by previous studies (Fabian et al. 2009; Lutgendorf et al. 2002) and even though the distinction between the two groups might appear arbitrary, the groups were examined for, and demonstrated, a statistical and meaningful distinction.
In summary, to our knowledge this is the first study to explore relationships between CAR, sex, pain-related outcomes, and pain perception in patients with KOA. The findings of this study suggest that neuroendocrine factors such as CAR and AUCG may contribute to individual differences in pain-related outcomes in patients with KOA. Additionally, our findings are suggestive of a sex-dependent relationship between post-awakening cortisol concentrations and pain perception.
Future studies may provide further understanding of the scope of altered HPA-axis activity on changes in pain perception and explore clinical implications for prophylaxis, diagnosis and therapy of chronic pain syndromes including osteoarthritis.
Highlights.
Cortisol awakening response (CAR) was positively correlated with pain threshold
Relatively blunted CAR was associated with higher anxiety and lower pain threshold
Relatively reduced post awakening cortisol was associated with higher pain interference
Women had lower pain threshold and lower CAR
Pain threshold mediated the influence of sex on post-awakening cortisol levels
Acknowledgments:
We thank Marise Cornelius for coordinating the data collection.
Funding:
This work was supported by National Institutes of Health grant R01 AG034982 to R.R. Edwards. The funding body had no direct role in study design, data analysis, or the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
References
- Adam Emma K. and Kumari Meena (2009), ‘Assessing salivary cortisol in large-scale, epidemiological research’, Psychoneuroendocrinology, 34 (10), 1423–36. [DOI] [PubMed] [Google Scholar]
- Almeida David M., Piazza Jennifer R., and Stawski Robert S. (2009), ‘Interindividual differences and intraindividual variability in the cortisol awakening response: an examination of age and gender’, Psychology and aging, 24 (4), 819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi M, et al. (2006), ‘Changes in salivary cortisol and corticosteroid receptor-alpha mRNA expression following a 3-week multidisciplinary treatment program in patients with fibromyalgia’, Psychoneuroendocrinology, 31 (9), 1076–86. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, et al. (1989), ‘The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research’, Psychiatry Res, 28 (2), 193–213. [DOI] [PubMed] [Google Scholar]
- Carlesso LC, Sturgeon JA, and Zautra AJ (2016), ‘Exploring the relationship between disease-related pain and cortisol levels in women with osteoarthritis’, Osteoarthritis and Cartilage, 24 (12), 2048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, et al. (2010), ‘The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008’, J Clin Epidemiol, 63 (11), 1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS and Ryan KM (1994), ‘Pain assessment: global use of the Brief Pain Inventory’, Ann Acad Med Singapore, 23 (2), 129–38. [PubMed] [Google Scholar]
- Crofford LJ, et al. (2004), ‘Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome’, Brain Behav Immun, 18 (4), 314–25. [DOI] [PubMed] [Google Scholar]
- Cross M, et al. (2014), ‘The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study’, Ann Rheum Dis, 73 (7), 1323–30. [DOI] [PubMed] [Google Scholar]
- Dedovic K, et al. (2010), ‘Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder?’, Biol Psychiatry, 68 (9), 847–53. [DOI] [PubMed] [Google Scholar]
- Edwards S, et al. (2001), ‘Exploration of the awakening cortisol response in relation to diurnal cortisol secretory activity’, Life Sci, 68 (18), 2093–103. [DOI] [PubMed] [Google Scholar]
- Fabian LA, et al. (2009), ‘The association of the cortisol awakening response with experimental pain ratings’, Psychoneuroendocrinology, 34 (8), 1247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries Eva, Dettenborn Lucia, and Kirschbaum Clemens (2009), ‘The cortisol awakening response (CAR): Facts and future directions’, International Journal of Psychophysiology, 72 (1), 67–73. [DOI] [PubMed] [Google Scholar]
- Gaab J, et al. (2005), ‘Reduced reactivity and enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic whiplash-associated disorder’, Pain, 119 (1–3), 219–24. [DOI] [PubMed] [Google Scholar]
- Griep EN, et al. (1998), ‘Function of the hypothalamic-pituitary-adrenal axis in patients with fibromyalgia and low back pain’, J Rheumatol, 25 (7), 1374–81. [PubMed] [Google Scholar]
- Heim C, et al. (1999), ‘Psychological and endocrine correlates of chronic pelvic pain associated with adhesions’, J Psychosom Obstet Gynaecol, 20 (1), 11–20. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C and Hellhammer DH (1994), ‘Salivary cortisol in psychoneuroendocrine research: recent developments and applications’, Psychoneuroendocrinology, 19 (4), 313–33. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, and Kirschbaum C (2003), ‘Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects’, Psychosom Med, 65 (2), 313–9. [DOI] [PubMed] [Google Scholar]
- Kuehl LK, et al. (2010), ‘Increased basal mechanical pain sensitivity but decreased perceptual wind-up in a human model of relative hypocortisolism’, Pain, 149 (3), 539–46. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N, et al. (2008), ‘Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: associations with sleep quality and metabolic parameters’, Psychoneuroendocrinology, 33 (2), 143–51. [DOI] [PubMed] [Google Scholar]
- Lauche R, et al. (2013), ‘A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome’, J Psychosom Res, 75 (6), 500–10. [DOI] [PubMed] [Google Scholar]
- Lentjes EG, et al. (1997), ‘Glucocorticoid receptors, fibromyalgia and low back pain’, Psychoneuroendocrinology, 22 (8), 603–14. [DOI] [PubMed] [Google Scholar]
- Losina Elena, et al. (2015), ‘Lifetime medical costs of knee osteoarthritis management in the United States: impact of extending indications for total knee arthroplasty’, Arthritis care & research, 67 (2), 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, et al. (2002), ‘Diurnal cortisol variations and symptoms in patients with interstitial cystitis’, J Urol, 167 (3), 1338–43. [PubMed] [Google Scholar]
- McBeth J, et al. (2005), ‘Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents’, Arthritis Res Ther, 7 (5), R992–r1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nees F, et al. (2019), ‘Hypothalamic-pituitary-adrenal axis feedback sensitivity in different states of back pain’, Psychoneuroendocrinology, 101, 60–66. [DOI] [PubMed] [Google Scholar]
- Osman A, et al. (1997), ‘Factor structure, reliability, and validity of the Pain Catastrophizing Scale’, J Behav Med, 20 (6), 589–605. [DOI] [PubMed] [Google Scholar]
- Pilkonis PA, et al. (2013), ‘Assessment of self-reported negative affect in the NIH Toolbox’, Psychiatry Res, 206 (1), 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, and Kirschbaum C (1999), ‘Burnout, perceived stress, and cortisol responses to awakening’, Psychosom Med, 61 (2), 197–204. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. (2003), ‘Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change’, Psychoneuroendocrinology, 28 (7), 916–31. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. (1997), ‘Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity’, Life Sci, 61 (26), 2539–49. [DOI] [PubMed] [Google Scholar]
- Riva R, et al. (2012), ‘Comparison of the cortisol awakening response in women with shoulder and neck pain and women with fibromyalgia’, Psychoneuroendocrinology, 37 (2), 299–306. [DOI] [PubMed] [Google Scholar]
- Sapse AT (1997), ‘Cortisol, high cortisol diseases and anti-cortisol therapy’, Psychoneuroendocrinology, 22 Suppl 1, S3–10. [DOI] [PubMed] [Google Scholar]
- Smith E, et al. (2014), ‘The global burden of other musculoskeletal disorders: estimates from the Global Burden of Disease 2010 study’, Ann Rheum Dis, 73 (8), 1462–9. [DOI] [PubMed] [Google Scholar]
- Steptoe A and Serwinski B (2016), ‘Chapter 34 - Cortisol Awakening Response’, in Fink George (ed.), Stress: Concepts, Cognition, Emotion, and Behavior (San Diego: Academic Press; ), 277–83. [Google Scholar]
- Sudhaus S, et al. (2007), ‘Die Cortisol-Aufwachreaktion bei Patienten mit akuten und chronischen Rückenschmerzen’, Der Schmerz, 21 (3), 202–11. [DOI] [PubMed] [Google Scholar]
- Therrien F, et al. (2008), ‘Awakening cortisol response in relation to psychosocial profiles and eating behaviors’, Physiol Behav, 93 (1–2), 282–8. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM, et al. (2010), ‘Sex differences in hypothalamic-pituitary-adrenal axis function in patients with chronic pain syndrome’, Stress, 13 (4), 292–300. [DOI] [PubMed] [Google Scholar]
- van Eck M, et al. (1996), ‘The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol’, Psychosom Med, 58 (5), 447–58. [DOI] [PubMed] [Google Scholar]
- Vargas I, Mayer S, and Lopez-Duran N (2017), ‘The Cortisol Awakening Response and Depressive Symptomatology: The Moderating Role of Sleep and Gender’, Stress Health, 33 (3), 199–210. [DOI] [PubMed] [Google Scholar]
- Villafañe JH, et al. (2020), ‘Exploring the relationship between chronic pain and cortisol levels in subjects with osteoarthritis: results from a systematic review of the literature’, Osteoarthritis Cartilage, 28 (5), 572–80. [DOI] [PubMed] [Google Scholar]


