Abstract
The translucent appearance of the conjunctiva allows for immediate visualization of changes in the circulation of the conjunctival microvasculature consisting of extensive branching of superficial and deep arterial systems and corresponding drainage pathways, and the translucent appearance of the conjunctiva allows for immediate visualization of changes in the circulation. Conjunctival hyperemia is caused by a pathological vasodilatory response of the microvasculature in response to inflammation due to a myriad of infectious and non-infectious etiologies. It is one of the most common contributors in ocular complaints that prompts visits to medical centers. Our understanding of these neurogenic and immune-mediated pathways has progressed over time and played a critical role in developing targeted novel therapies. Due to a multitude of underlying etiologies, patients must be accurately diagnosed for the efficacious management of conjunctival hyperemia. The diagnostic techniques used for the grading of conjunctival hyperemia have also evolved from descriptive and subjective grading scales to more reliable computer-based objective grading scales.
Keywords: conjunctiva, conjunctival vasculature, microcirculation, conjunctivitis
1. INTRODUCTION
Ocular complaints are responsible for approximately 2–3% of patient visits to primary care physicians and emergency facilities, of which the majority are for the management of conjunctival hyperemia.[1–3] However, the current literature reports limited evidence about the prevalence of conjunctival hyperemia due to its self-limiting nature. Conjunctival hyperemia is caused by a wide range of etiologies that prompts a pathological vasodilatory response of the microvasculature in the conjunctival tissue and is the most common non-refractive ocular complaint requiring medical care.[4]
The localized inflammatory changes due to infectious and non-infectious etiologies cause vasodilation of conjunctival microvessels, and subsequently, hyperemia and edema. Although most cases of conjunctivitis are benign and self-limiting, some may forebode vision-threatening disorders or even act as an indicator of underlying systemic disease; therefore, it is necessary to discern the underlying causes of conjunctival hyperemia.[5–7] Ocular redness with variable degrees of conjunctival erythema is also associated with inflammation of other ocular tissues such as uveitis, episcleritis, scleritis, glaucoma, keratitis, and subconjunctival hemorrhage.[8] Due to its high prevalence, conjunctival hyperemia can impinge on the loss of time from work and social activities, as well as creating a recurrent burden of the cost of treatment and care for the patients.[1,4,9]
Herein, we provide an overview of the microanatomy of the conjunctival vasculature, followed by a detailed analysis of the different etiologies that cause conjunctival hyperemia. We outline the immune response and associated pathophysiological mechanisms prompting the disease to provide a comprehensive summary. Furthermore, we briefly discuss available diagnostic modalities and novel platforms under development for the clinical diagnosis of conjunctival hyperemia.
2. ANATOMY OF CONJUNCTIVAL VASCULATURE
2.1. ARTERIAL SUPPLY OF THE CONJUNCTIVA
The arterial circulation of the conjunctiva consists of marginal and peripheral tarsal arcades, anterior and deep ciliary systems. (Figure 1) The peripheral tarsal arcade runs along the upper border of the tarsus muscle between the two parts of the levator palpebrae superioris, in the tarsal plate, fornix, and proximal bulbar conjunctiva. [10] The perforating branches of the peripheral tarsal arcade either pass over the tarsal plate or through the palpebral muscle, further bifurcating into ascending and descending conjunctival arteries. [11] The ascending conjunctival arteries pass above the fornix towards the orbit, further dividing into the posterior conjunctival arteries. By contrast, the descending branches provide blood supply to the proximal two-thirds of the tarsal conjunctiva, anastomosing with the shorter branches of the marginal artery, which have pierced the tarsal plate at the subtarsal fold. [12] The primary arterial supply for the bulbar conjunctiva consists of anastomoses between the anterior conjunctival arteries and ascending branches of the conjunctival arteries, close to the limbus. The peripheral tarsal arcade in the lower lid arises from the lacrimal, transverse facial, or superficial temporal arteries and occurs anteriorly to the inferior palpebral muscle of Muller with a similar distribution to that of the upper lid. [13] However, when it is not formed, the inferior tarsal plate, fornix, or bulbar conjunctiva are then supplied by the marginal arcade or muscular arteries to the inferior rectus.
Figure 1:
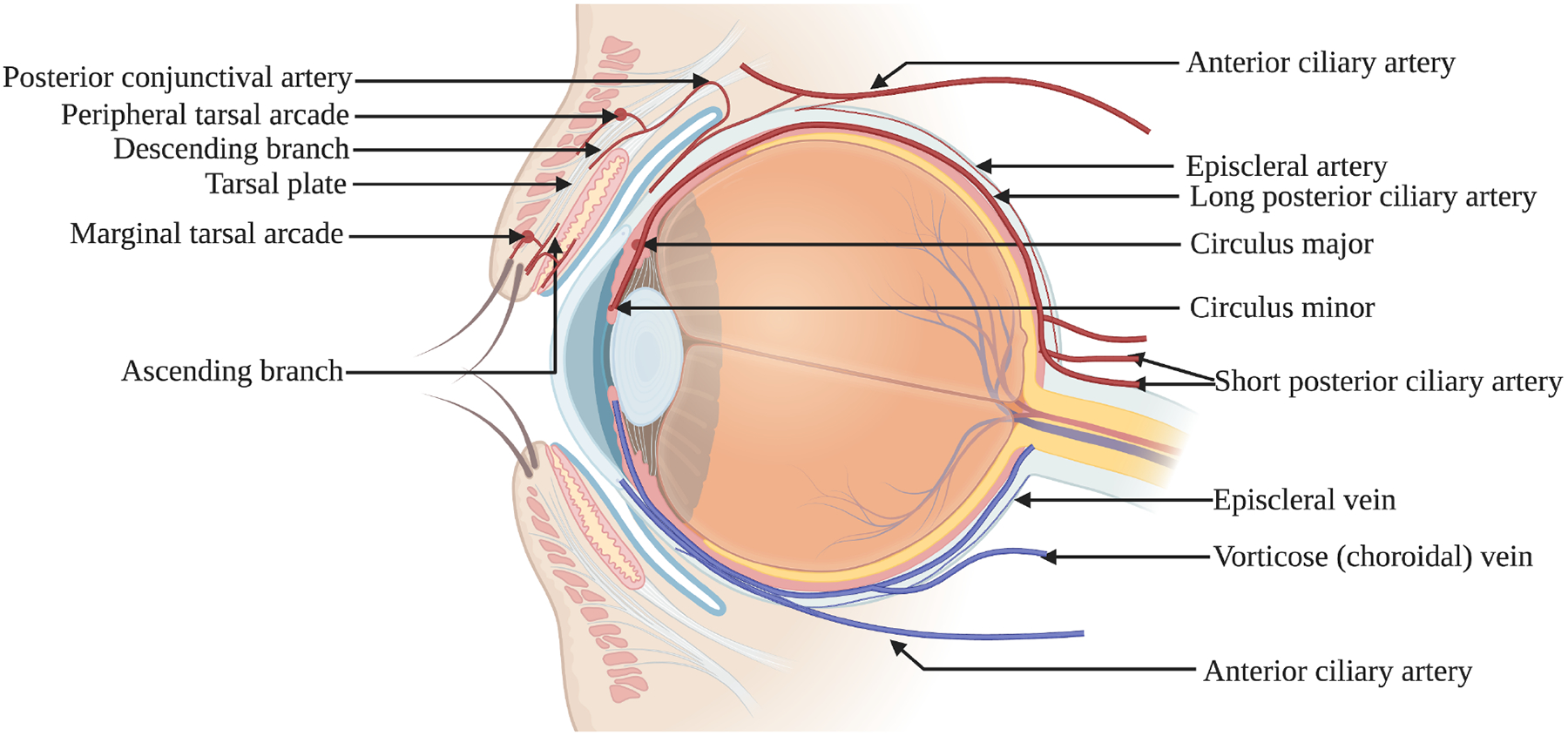
The arterial supply (red) and venous draining (blue) of the conjunctival tissue.
The episcleral branches of the anterior ciliary artery communicate to form the deep episcleral capillary net of the pericorneal plexus. At the limbus, the episcleral branches enter the bulbar conjunctiva to form the anterior conjunctival arteries, which form an anastomosis with branches of the posterior conjunctival artery in proximity to the limbus. The perilimbal branches of the anterior ciliary artery anastomose to form the superficial or conjunctival part of the pericorneal plexus. At the limbus, the episcleral arteries give rise to a marginal pericorneal arcade extending to the peripheral edge of Bowman’s layer of the cornea as well as extending to the palisades of Vogt at the upper and lower limbus. [14]
Patterns of redness due to various underlying etiologies are attributed to the distinct vascular anatomy of the different parts of the conjunctiva. Therefore, in cases of conjunctivitis affecting the bulbar conjunctiva, the redness extends to the fornix and tarsal plate, sparing the episcleral circulation. The bulbar conjunctiva in the perilimbal area, episcleral and limbal circulation consists of the deep ciliary arterial circle formed by the scleral perforating and the anterior ciliary arteries.
The deep red color of the tarsal conjunctiva is attributed to its extensive circulation. By contrast, the bulbar conjunctiva is colorless unless congested. Additionally, the congested bulbar vessels’ arc is mobile with the conjunctiva and blanches poorly upon pressure, whereas the palpebral vasculature is fixed, and therefore blanches on direct application of pressure.
2.2. VENOUS DRAINAGE OF THE CONJUNCTIVA
The venous drainage of the conjunctiva consists of one or more veins corresponding to their respective arteries. (Figure 1) Palpebral veins drain the fornix, tarsal, and posterior bulbar conjunctiva. [15] The venous return from the upper lid occurs via venous plexus formed in the tendons of the levator palpebrae superioris, which drains into the veins of the levator and superior rectus and eventually into the ophthalmic vein. [16]
The perilimbal venous circle consists of three communicating veins occurring posteriorly to the limbal venous arcades and anteriorly to the episcleral arterial circle. [17] The venous return from the limbus, marginal arcades, and anterior conjunctival veins is drained by communicating veins into radial episcleral collecting veins, which eventually reach the venous drainage of rectus muscles. [18] Additionally, these veins receive venous blood from the episcleral veins as well as veins draining the deeper parts of the sclera. These veins leave the globe surface above the rectus muscles.
3. PHYSIOLOGY OF CONJUNCTIVAL CIRCULATION
The conjunctival and retinal microvascular systems both originate from the internal carotid artery and consist of extensive anatomical networks of branching capillaries, arterioles, and venules, with multiple anastomoses described in the previous section. Numerous studies have placed the vasculature diameter between 5 and 70 μm with flow velocities in venules and arterioles ranging from 0.52 to 3.26 mm/s. [19–21] Moreover, the conjunctival circulation has an anatomical resemblance to areas of the brain supplied by branches of the internal carotid artery, mainly due to comparative vessel caliber of the vasculature and equal distance from the feeding vessel in the branching tree. Therefore, these two circulations have corresponding hydraulic fluid dynamics [20]. The bulbar conjunctival microvascular bed is encased in a semi-transparent membrane above the white sclera, thereby rendering real-time, in-vivo , and non-invasive measurements of conjunctival hyperemia possible, thus helping in the diagnosis of microvascular dysfunction and vasculopathies associated with the cerebral cortex and kidneys [19,22,23]. Duench and colleagues have used a spectrophotometer to report cyclical patterns of conjunctival hyperemia [24]. Multiple novel instruments such as digital imaging analysis and measurement of circulating blood flow velocity based on red blood cell displacement in successive image frames of the conjunctival vasculature have also been previously reported in normal subjects, as well as in pathological changes associated with diseases such as dry eye disease (DED), sickle cell anemia, diabetes, and Alzheimer’s [25–33]
4. ETIOLOGIES OF CONJUNCTIVAL HYPEREMIA
Conjunctival hyperemia is associated with a multitude of etiologies that cause dilation of the microvasculature, resulting in a reddish appearance of the conjunctival tissue. Clinically, it is primarily a nonspecific sign associated with seasonal allergens, ocular surface infections, fatigue, and even underlying systemic diseases in some cases. The etiologies causing conjunctivitis can be distinguished into infectious or non-infectious.
A detailed ocular and systemic history along with a thorough assessment of the hyperemic patterns, laterality, duration, associated pain, intraocular pressure (IOP) are relevant for an accurate diagnosis. [2]. Accurate assessment of the underlying causes of conjunctival hyperemia is vital in differentiating systemic causes from a localized inflammatory response to tailor the treatment plan accordingly. Upon initial analysis of the eye, if causes of conjunctival hyperemia are not associated with corneal or conjunctival disorders, assessment of commonly associated factors such as photophobia, duration, ocular pain, and IOP provides insight into internal structures such as the sclera, uvea, or iris that may be affected, requiring a comprehensive ophthalmological examination [34] Other clinical presentations, such as changes in the skin of the eyelids, presence, color, and consistency of ocular discharge, contact lens wear, and ocular medications, should also be considered. Timing and seasonality of onset of symptoms help in differentiating types of allergic conjunctivitis. By contrast, discerning immediate history before the presentation can be beneficial in elucidating the traumatic causes with suspicion of foreign body involvement. These determinations are essential for distinguishing self-limiting causes of conjunctival hyperemia and those that require observation, further medical management, and referral to a tertiary care center in cases that constitute ocular emergencies and require immediate intervention.
4.1. Non-infectious etiologies
Allergic conjunctivitis is one of the leading causes of conjunctival hyperemia. [1] In Europe, allergic conjunctivitis is the most common cause of red eyes (35%), with recent studies estimating up to 40% of the population affected. [35,36] The most observed form of allergic conjunctivitis is due to seasonal and perennial allergic conjunctivitis (SAC, PAC), representing approximately 50% of ocular allergic disease. [37] Other atopic disorders include atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC), and giant papillary conjunctivitis (GPC) [38]. Symptoms of allergic conjunctivitis arise due to the exposure of the ocular surface to environmental allergens that can cause conjunctival inflammation and microvascular dilation in the conjunctival tissue resulting in hyperemia. SAC is more common, presenting at higher rates in the spring or fall season, varying with the geographical location, primarily attributed to the higher presence of allergens such as ragweed and grass pollens. [39] PAC is noted year-long in patients and is triggered by common allergens such as dust and animal dander. Both types of allergic conjunctivitis present with increased itching, chemosis, and tearing, leading to hyperemia of the conjunctiva and edema [40,41]. PAC and SAC rarely have corneal involvement and are self-limiting, and do not pose threats to vision [38]. AKC and VKC may hold more serious pathological implications and can threaten vision if there is corneal involvement.
While most cases of allergic conjunctivitis led to acute conjunctival hyperemia, dry eye disease (DED) can also be associated with chronic conjunctival hyperemia. DED is used as an overarching term to describe the sign and symptom complex of various conditions that result in decreased tear quality, tear film instability, hyperosmolarity, and ocular surface inflammation and damage. [42,43] The pathogenesis of DED has been associated with environmental conditions such as wind and climate that can affect tear evaporation and autoimmune disorders. [44] DED is broadly categorized into aqueous deficient dry eye and evaporative dry eye, and patients typically present with ocular surface inflammation, and it is not uncommon for patients to present with both forms of DED. [45] Characteristics of aqueous deficient dry eye are a marked decrease in tear production while evaporative dry eye is frequently due to Meibomian gland dysfunction (MGD) that normally supplies the lipid layer of the tear film to reduce tear evaporation. Both conditions create an ocular environment that triggers a myriad of proinflammatory responses resulting in inflammation and subsequent conjunctival hyperemia. [46]
The use of ocular medications can also induce conjunctival hyperemia. Drug pH, formulation, mechanism of action, among other factors, can lead to hyperemia and other issues with drug tolerability. Ocular hyperemia is the most commonly reported adverse effect in up to 50% of patients who are prescribed prostaglandin analog based IOP reducing eyedrops for the management of glaucoma [47,48]. Furthermore, ocular drug preparations typically include preservatives that allow for a longer shelf life by preventing drug decomposition and decreasing chances of microbial contamination [49]. However, cytotoxic effects have been observed with long-term use, such as with the common antimicrobial preservatives benzalkonium chloride (BAK), resulting in altered homeostasis of the conjunctival surface that may worsen conjunctival hyperemia. [50] A comparative analysis showed that patients treated with BAK-free anti-ocular hypertension medications had fewer reports of hyperemia due to topical eye drop associated conjunctival hyperemia [51]. Other non-infectious mediated causes of conjunctival hyperemia can stem from irritant-based sources that induce an inflammatory response, such as chlorinated water, cosmetics, chemicals, smoke, and pollutants [52].
4.2. Infectious etiologies
Acute conjunctivitis refers to cases with a duration of three weeks or less [52]. Cases of acute conjunctivitis are more common, affecting an estimated six million people in the United States [1]. Overlap of symptoms among bacterial and viral cases can make accurate diagnosis of the etiology of infection challenging, resulting in high rates of misdiagnosis of viral conjunctivitis as bacterial [53]. Cases of infectious conjunctivitis often self-resolve without the aid of any therapeutic intervention and do not affect ocular health or vision after clearing of symptoms[1].
4.2.1. Viral conjunctivitis
Viral conjunctivitis accounts for up to 80% of acute conjunctivitis cases [1], with Adenoviruses causing an estimated 90% of cases. Geographical considerations can help in elucidating specific serotypes of adenovirus that infect populations in the area [52,54,55]. Less common origins of viral conjunctivitis are attributed to herpes zoster virus (HSV), Varicella-zoster virus (VZV), and Molluscum contagiosum [1,56].
Viral infection of the conjunctiva induces conjunctival hyperemia that typically presents unilaterally with subsequent infection of the other eye following shortly thereafter, giving the typical appearance of pink-hued conjunctiva because of a diffuse fine microvascular pattern [57]. Viral conjunctivitis is highly contagious typically spreading due to eye-to-hand contact as ocular itching is a common symptom; therefore, emphasis is laid on vigorous hand washing to reduce transmission. Follicles on the palpebral conjunctiva can also accompany adenovirus-induced conjunctival hyperemia.
Conjunctivitis due to viruses such as HSV and VZV do not transmit as easily as adenoviruses and are almost always unilateral in immunocompetent hosts [58]. Patients with conjunctival hyperemia secondary to HSV infection have a similar appearance as observed in cases with adenoviral etiologies, along with unilateral foreign body sensation, pain, and burning sensation. Patients with conjunctivitis caused by VZV present with severe pain along with conjunctival hyperemia in a smaller proportion of re-activated cases of herpes zoster ophthalmicus (HZO) [59].
4.2.2. Bacterial conjunctivitis
Bacteria are commonly transmitted through contaminated fingertips introducing microbes to the ocular surface and transmission from contaminated eye drop applicators, contact lenses, and improper hand hygiene causing conjunctivitis [61]. Acute bacterial conjunctivitis presents with burning, irritation, excessive tearing, and usually mucopurulent or purulent discharge [60]. Furthermore, conjunctival inflammation, hyperemia, and mild eyelid edema are often noted. Higher incidences of bacterial infection are observed in school-aged children and elderly patients [52]. As seen in DED, patients with abnormal lacrimation obstructions to the nasolacrimal duct, ectropion or entropion, and injuries to the conjunctiva and cornea predispose the ocular surface to bacterial infections.
A wide range of Gram-positive and Gram-negative bacteria have been known to induce infectious conjunctivitis, however, Gram-positive bacteria have been linked to the majority of the recorded cases. In adults, the most common bacteria are Staphylococcus species, Streptococcus pneumoniae, and Haemophilus influenzae [60]. Patients belonging to sexually active age groups presenting with conjunctival hyperemia should be suspected of Chlamydia trachomatis infection. [62] The pediatric patients with conjunctival symptoms, including hyperemia, are typically infected by Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis. [9,61] The most indicative sign of bacterial conjunctivitis is a yellow-white purulent discharge collected at the base of the eyelashes and matting of the eyelids, particularly upon waking in the morning. High clinical suspicion for timely identification of the causative organism is critical as bacteria, such as Neisseria gonorrhoeae, can produce proteolytic enzymes that can result in irreversible damage to conjunctival layers. [63]
Contact lens wearers are also prone to certain forms of conjunctivitis that can progress to keratitis due to microbial organisms accumulate on the contact lenses and in contact lens fluid [64]. Contact lens wearers are at risk of development of keratoconjunctivitis from opportunistic pathogens following corneal trauma associated with common ocular infections caused by gram-negative bacteria such as Pseudomonas aeruginosa, that pose an increased risk of vision loss, with severe conjunctival hyperemia also reported due to Acanthamoeba infections. [61,65].
5. PATHOGENESIS OF CONJUNCTIVAL HYPEREMIA
The vasodilatation of conjunctival microvessels is clinically reported as hyperemia (or redness), plays a critical role in the efferent component of the immune system, delivering both humoral and cellular immune components to the site of inflammation [66]. The inflammatory process in the conjunctival tissue is mediated by physiologically active molecules such as histamine, cytokines, and associated neuropeptides.
5.1. Histamine-induced vasodilation
Histamine is an endogenous organic nitrogenous compound that plays a critical role in localized immune responses and is released predominantly from mast cell granules and in lesser quantities by basophils and neutrophils. [67] In normal conjunctiva, approximately 5,000–6,000 mast cells/mm3 are found below the basement membrane in the substantia propria and contain approximately 4.6 pg/cell of histamine. [39] The proteases released by the allergens activate protease-activated receptor-2 in the conjunctival tissue which leads to the breakdown of the tight junctions between conjunctival epithelial cells and enter the subepithelial layer [68]. The dendritic cells (DCs) in the conjunctiva process the allergens to undergo maturation, with the expression of peptides that form a complex with major histocompatibility complex class II (MHC-II) molecules (Figure 2). Subsequently, the mature conjunctival dendritic cells (D.C.) migrate to the regional secondary lymphoid organs to interact with naïve T cells through the upregulated MHC-II and costimulatory molecules – Cluster of differentiation (CD) 80 and CD86, leading to activation, proliferation, and differentiation of CD4 T cells primarily into allergen-specific Th2 cells. Thereafter, the interaction between the CD40 on B cells and CD40 ligand on allergen-specific Th2 cells leads to the release of interleukin 4 (IL-4), which induces the proliferation and differentiation of allergen-specific B cells into plasma cells that produce allergen-specific IgE. [69]. Conjunctival mast cells are primed when allergen-specific IgE binds via its Fc region to the Ig-like domain of the alpha chain of Fc Epsilon Receptor I (FcεRI), leading to degranulation and histamine release in the early phase allergic reaction. However, in the late phase, histamine is released by both mast cells and basophils when stimulated by non-IgE stimulating factors such as the C-C chemokine family, including monocyte chemoattractant protein-1, Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted (RANTES) and eotaxin, cytokines such as IL-1, IL-3, IL-5, IL-6 and other molecules such as neurotrophic growth factor and c-kit ligand in the late phase allergic reaction, releasing approximately 23 ng/mm3 of histamine [70,71].
Figure 2:
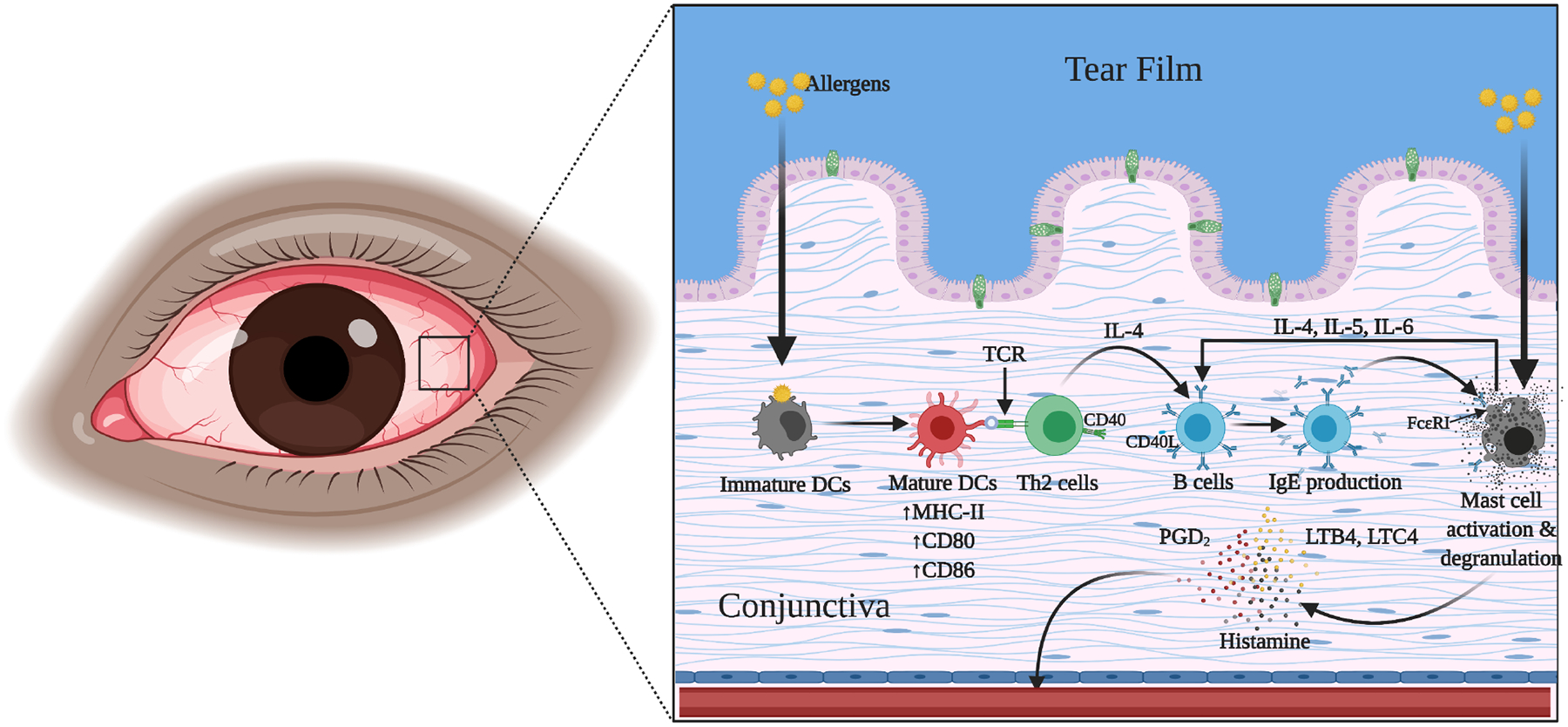
The immunopathological mechanisms triggered on allergen exposure in the conjunctiva.
Researchers previously believed that the action of histamine released by mast cells occurred through Histamine 1 (H1) and H2 receptors only. (Figure 3) [72]. However, more recently, Leonardi and colleagues have also reported the presence of H4 receptors in normal conjunctiva. [73]. In allergic conjunctivitis, histamine binds to H1 receptors expressed on the endothelial cells of the conjunctival microvessels upon stimulation, leading to the formation of the local vasodilator substance nitric oxide (NO). [74]. NO increases cyclic guanosine monophosphate (cGMP) production by activation of guanylate cyclase. Elevation of cGMP levels causes arterial smooth muscle cell relaxation leading to vasodilatation. [75]. It has been reported that NO plays an essential role as a mediator in the process of both vasodilation and edema formation in the acute phase of allergic conjunctivitis. [76–79] Additionally, evidence suggests that higher doses of histamine also lead to H1 mediated vasopermeability in the conjunctival vasculature, causing localized edema. [80]
Figure 3:
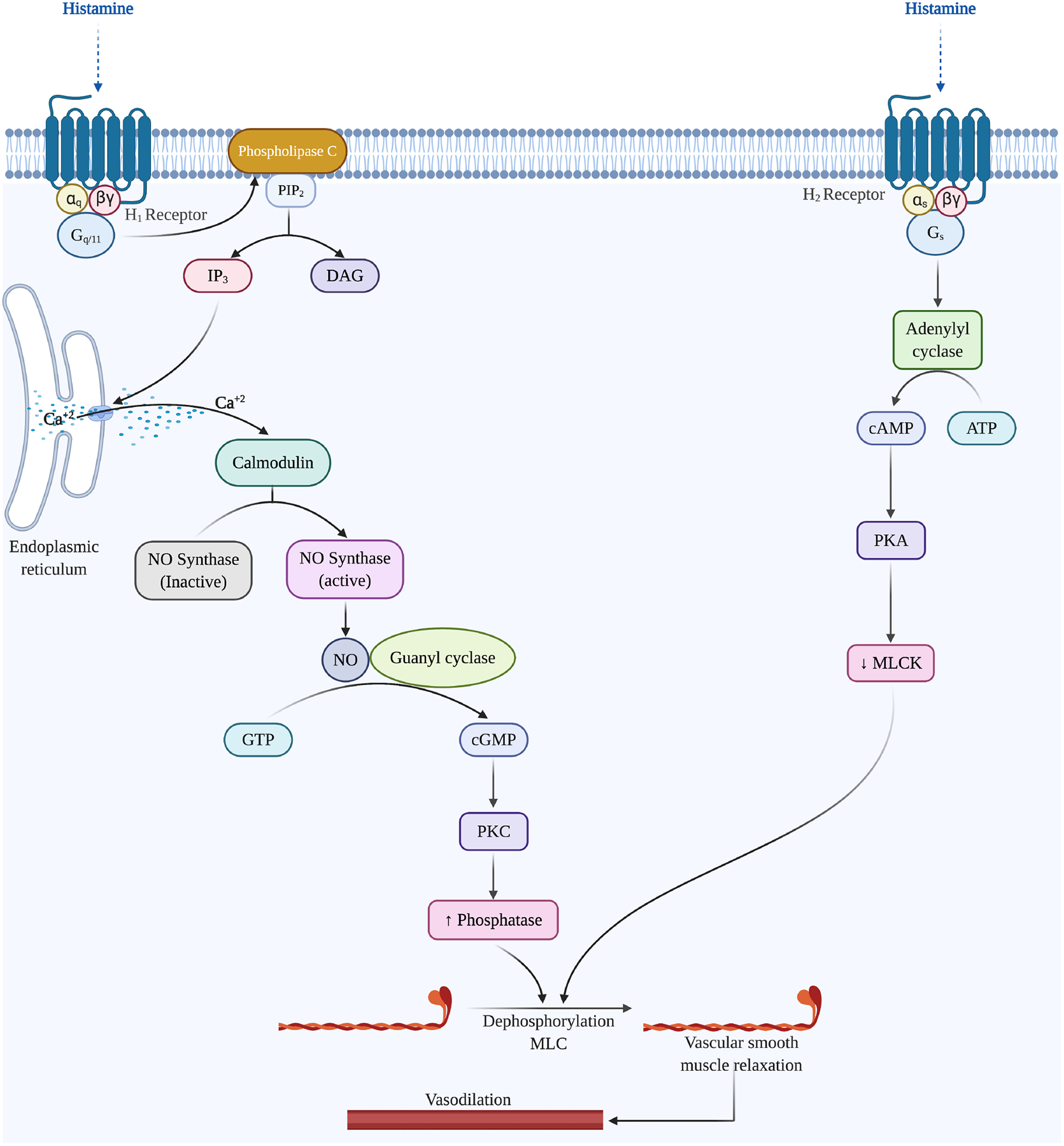
The signaling pathway activated by histamine through H1 and H2 receptors activated in the vascular smooth muscle cells, leading to vasodilation of microvessels in the conjunctiva.
Histamine stimulation of H2 receptors in the conjunctiva causes vasodilation [81]. H2 receptors are coupled to adenylate cyclase via Gs, which upon activation, stimulates the production of cyclic AMP (cAMP), subsequently activating protein kinase A (PKA) [82]. The increase in PKA activity causes phosphorylation and subsequent suppression of the myosin light chain kinase resulting in myosin light chain dephosphorylation by myosin light chain phosphorylase, inhibiting contraction. [83] The relaxation of vascular smooth muscles in the conjunctiva leads to vasodilation. [72]
Furthermore, upon degranulation, mast cells also release prostaglandin (PGD2) and leukotrienes (LTB4, LTC4). [84] PGD2 acts on the vascular smooth muscles adding to the vasodilatory effect of histamine on the vasodilation of conjunctival blood vessels as well as aggravates ocular pruritus. [85] Leukotrienes also induce vasodilation and increased permeability of conjunctival blood vessels, which manifest as conjunctival hyperemia and chemosis, respectively. [86]
5.2. Cytokine-induced vasodilation
The conjunctival vasculature generates a robust response against the pathogenic invasion of the tissue despite the biological defense responses mounted by the components of the tear fluid. The resultant inflammation leads to the dilation and permeabilization of conjunctival microvessels, leading to exacerbation of inflammation and hyperemia. Pathogen-associated lipopolysaccharide (LPS) generates tumor necrosis factor-α(TNFα), IL-1β, and IL-6 under the regulation of nuclear factor-κB (NF-κB). [87] The vascular endothelial and smooth muscle cells have been shown to have TNF-α and IL-1β receptors. [88,89] These cytokines induce the expression of several vasodilatory mediators, including PGI2, PGE2, and nitric oxide (NO). Brian Jr. and Faraci have shown the progressive vasodilatory action of TNF-α, which could be blocked by aminoguanidine and dexamethasone, suggesting the critical role of NO in vasodilation [90]. Additionally, several studies have shown that TNF-α activates NAD(P)H oxidase, resulting in an increase in intracellular H2O2 that stimulates Ca2+ sparks and transient K + −Ca2+ currents, leading to vascular smooth muscle cell hyperpolarization, reduction in Ca2+, and vasodilation. [91–93]
In response to local inflammatory changes, macrophages and T cells enter the stromal layer of the conjunctiva from the vascular system to mount a second defense against the invading pathogens. Both T cells and macrophages may secrete IL-1, interferon-gamma (IFNγ), and TNF-α; TNF-α, in particular causes vasodilation by inducing smooth muscle cell synthesis of NO, which itself, in turn, contributes to hyperemia and inflammation [94,95].
5.3. Neurogenic vasodilation
The inflammatory response observed in DED and allergic diseases can mediate vasodilation [96,97] via the nervous system due to the dense innervation of the ocular surface by the trigeminal sensory system [98]. The conjunctiva consists of a dense network of sensory and sympathetic/parasympathetic innervation within the stroma and the surrounding epithelium [99]. Conjunctival insults detected by afferent sensory neurons are relayed to the central nervous system and lead to an efferent sympathetic/parasympathetic response at the ocular surface, causing local release of neuromediators, including Substance P SP) and calcitonin gene-related peptide (CGRP).
5.3.1. Substance P
SP is an undecapeptide tachykinin expressed in the central and peripheral nervous systems. [100] S.P. from the peripheral nerves in the conjunctiva mediates immunological action via high-affinity neurokinin one receptor (NK-1R). SP causes vasodilation in the conjunctiva either through direct action on the microvasculature or through enhanced mast cell degranulation and release of TNF-α. [101]
Initially, the mast cell degranulation by S.P. was considered to be direct G-protein mediated activation-dependent, instead of NK-1R mediated response. [102,103] However, recent studies provide evidence of a high expression of NK-1R by mast cells. Also, studies show a bidirectional signal between mast cells and S.P. expressing nerves. [104,105] S.P. released from sensory nerve endings also acts on mast cells to synthesize TNF-α, which plays a role in vasodilation. [106]
5.3.2. Calcitonin gene-related peptide
Calcitonin gene-related peptide (CGRP) is reported to be a potent microvascular vasodilator in most vascular beds, in the heart, and in trigeminal circulations. [107–109] CGRP has an approximately10-fold potency as a microvascular vasodilator compared to most prostaglandins and significantly greater than other vasodilators such as acetylcholine (ACh) and SP. [107] CGRP is produced in abundance in trigeminal ganglion neurons and is released from the peripheral nerves and central nerve terminals as well as being secreted within the trigeminal ganglion, and causes vasodilation through both NO-dependent and -independent dilatory pathways in the peripheral vessels. [110]
The primary mode of action of the CGRP occurs by an increase in intracellular cAMP as a result of direct stimulation of adenylate cyclase by Gαs in smooth muscle cells. (Figure 4) The subsequent activation of PKA by cAMP can lead to the phosphorylation and opening of ATP-sensitive K+ channels, resulting in relaxation [111].
Figure 4:
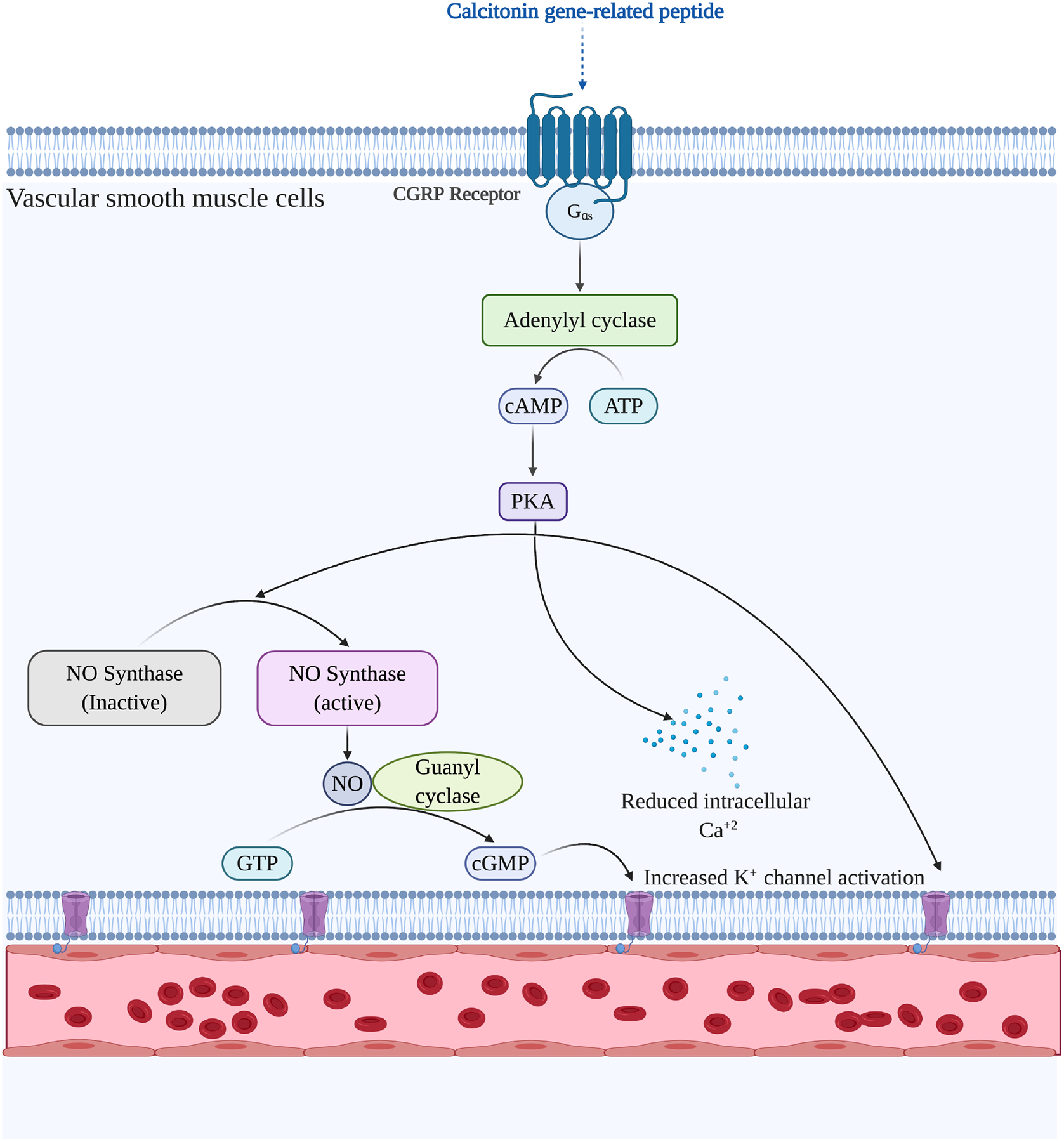
Neurogenic vasodilation trigger by calcitonin gene related peptide (CGRP) in the vascular smooth muscle cells, causing vasodilation of conjunctival microvessels.
6. GRADING OF CONJUNCTIVAL HYPEREMIA
The unique anatomic location of the conjunctival microvasculature makes it the only circulatory system that is readily visible to the external observer (e.g.greater visibility than the nailbed), allowing for the detection of physiological and pathological changes using noninvasive modalities. For accurate diagnosis and efficacious management, it is critical on the part of the ophthalmologists to accurately detect conjunctival hyperemia at initial presentation and closely follow its progression. Before the first grading scale was established by C.W. McMonnies and A. Chapman Davis in 1987, the assessment of conjunctival hyperemia was performed using descriptive terminology which was highly variable depending upon the examining observer [112]. (Table 1) Although clinical presentation of conjunctival hyperemia continues to be recorded using descriptive terminology in-patient records, novel and more standardized scales for assessment of conjunctival hyperemia are being adopted into clinical practice such methods allow for comparisons during follow-up, thereby facilitating detection of changes in the conjunctival microvasculature, allowing appropriate alterations in the treatment plan.
Table 1:
Subjective Quantification Methods of Ocular Redness
| Scale | Description | Region of conjunctiva | Characteristics | Limitations |
|---|---|---|---|---|
| Mcmonnies/Chapman-Davies (MC-D) scale | 6 photographs from 0 to 5 entitled conjunctival redness | Inferior bulbar | First grading scale | Low sensitivity and narrow range of ocular redness |
| Institute for Eye Research (IER, previously known as CCLRU) scale | 4 photographs from 1 to 4 entitled bulbar redness | Temporal bulbar | Widely used in clinic | Not sensitive at low levels of redness, need a threshold beyond which grades of bulbar redness are discriminated |
| Efron scale | 5 images from 0 to 4 entitled conjunctival redness | Temporal bulbar | Widely used in clinic | Developed from artist rendered paintings, low sensitivity and high variability |
| Validated bulbar redness (VBR) scale | 5 photographs from 10 to 90 entitled ocular redness (20 point increments) | Temporal bulbar | Finer categorization and linear scale | Highest hyperemia images is the same image modified using Adobe Photoshop to artificially create more severe grades of bulbar redness |
6.1. Descriptive grading scales
6.1.1. Slit-lamp findings classification scale
In an attempt to standardize the description of conjunctival hyperemia observed in patients during slit-lamp biomicroscopy, Food and Drug Administration established the first descriptive slit lamp grading scale in 1984. [112] The conjunctival hyperemia was graded numerically along with a descriptive term which was further defined, i.e., 0 (None - No injection present),1 (Trace-Slight limbal, bulbar and/or palpebral injection), 2 (Mild- Mild limbal, bulbar, and/or palpebral injection), 3 (Moderate- Significant, segmented limbal, diffuse bulbar or palpebral injection) and 4 (Severe- Severe limbal injection in the circumcorneal region, diffuse bulbar involving episcleral or scleral vessels, or palpebral injection.
6.1.2. Mandell scale for conjunctival injection
In 1987, Robert Mandell prepared an expanded descriptive scale for conjunctival injection based on the FDA classification scale. [113]. The whole number grading from 0 through 4 was divided into a decimal grading scale to differentiate the different anatomical locations of the conjunctiva. (Table 2)
Table 2:
Mandell slit lamp classification system
| Classification | Grade | |
|---|---|---|
| A | None | 0 |
| B | Mild conjunctival hyperaemia | |
| a) palpebral | 1.1 | |
| b) palpebral and/or bulbar | 1.2 | |
| C | Mild circumcorneal injection | 1.3 |
| D | Moderate conjunctival hyperaemia | |
| a) palpebral | 2.1 | |
| b) palpebral and/or bulbar | 2.2 | |
| E | Moderate circumcorneal injection | 2.3 |
| F | Severe conjunctival hyperaemia | |
| a) palpebral | 3.1 | |
| b) palpebral and/or bulbar | 3.2 | |
| G | Severe circumcorneal injection | 3.3 |
| H | Other. (Grade by severity as either 1.9, 2.9, 3.9, 4.9) | |
6.2. Reference image-based subjective grading scales
6.2.1. McMonnies and Chapman-Davies scale
In 1987, C.W. McMonnies and A. Chapman Davis developed the first scale to detect conjunctival hyperemia in non-, hard and soft contact lens wearers. [114] The authors induced vasodilation using hypertonic (20%) saline solution to induce hyperemia, and the photographs of the lower conjunctival quadrant were taken at standardized intervals and compared to a pre-determined photographic reference scale to grade microvascular dilation. Although the authors reported statistically significant differences in conjunctival hyperemia responses between hard, soft, and non-contact lens wearing patients and high inter-and intra-observer reliability, the subjective interpretations of the reference images reduced the reliability of the scale. Moreover, the scoring was exclusive to the lower quadrant of the conjunctiva only.
6.2.2. Brian Holden Vision Institute scale
The Cornea and Contact Lens Research Unit in Australia developed a subjective grading scale focused on the complications associated with contact lens usage, including bulbar redness, limbal redness, lid redness, lid roughness, corneal staining type, depth and extent, and conjunctival staining [115] This scale was later renamed as the Institute for Eye Research (IER) or the Brian Holden Vision Institute grading scale. Each of the ten different complications was graded into four different severity categories ranging from grade 1 (very slight) to grade 4 (severe).
6.2.3. Efron scale
In 1997, Nathan Efron utilized reference artist-rendered illustrations for preparing a grading scale instead of photographs as done previously [116]. The illustrations were used as a reference for corneal epithelial staining and microcysts, stromal neovascularization and edema, variation endothelial cell morphology and blebs, conjunctival hyperemia, and papillary conjunctivitis, and were denoted with a traffic-light color scheme with an increase in severity grading from normal (Grade 0, green framing) to severe (Grade 4, red framing).
6.2.4. Validated bulbar redness scale
Schulz et al. developed the validated bulbar redness (VBR) scale, which consists of a comprehensive 100-point photographic scale for grading conjunctival hyperemia. [117]. Conjunctival hyperemia was induced in patients by instilling hypertonic saline (5%) to the patient’s eyes. The observer took photographs of the nasal conjunctiva of the recovering eye at standardized magnification, illumination, and gaze settings. The photographs were taken with a digital camera attached to a zoom photo slit lamp that was interfaced to a personal computer for comparative analysis with the standardized photographic scale.
6.2.5. Shortcomings of subjective grading scales
The clinical application of subjective scales for grading conjunctival hyperemia is relatively easy; however, these scales have several important limitations. Multiple studies have shown that these scales demonstrate limited inter-and intra-observer repeatability. [118–120] Additionally, despite assessing the same ocular sign, i.e., conjunctival hyperemia, the different scales vary in the number of images used for establishing the reference scale, the range of hyperemia severity, the linearity of the scores, and the conjunctival region included in the reference as well as examination images. Unsurprisingly, this heterogeneity of metrics and the inherent subjectivity of these assessment tools renders comparisons between each scale unreliable.
6.3. Computer-assisted image grading systems
The limitations noted above have led to the development of novel models of conjunctival hyperemia grading objectively by the application of computer-based photograph-analysis techniques, which has now progressed to automated image analysis through the application of artificial intelligence. (Table 3) These systems grade the images of conjunctiva based on multiple variable parameters that determine the severity of conjunctival hyperemia, such as blood vessel area, overall redness intensity, vessel diameter, and tortuosity. [121]
Table 3:
Objective Quantification Methods of Ocular Redness
| Group | Description | Region of Interest (ROI) | Characteristics | Limitations |
|---|---|---|---|---|
| Willingham FF et al | Mean relative redness (RR) Blood vessel area ratio (VA) | Temporal and nasal bulbar (circle region) | Reduce the impact of operator in consistency during image acquisition | Need specific camera and image system |
| Owen CG et al | Proportion of vasculature | Temporal bulbar (square region) | Distinguish vasculature between conjunctivae and sclera, increase sensitivity | ROI was not the same area among the subjects. |
| Guillon M et al | Individual vessel width Percentage vessel coverage |
All four quadrants of limbal and bulbar | Provide a precise and sufficient sensitive measure of the level of conjunctival redness | Not relate closely to the subjective measurements. |
| Sorbara L et al | CIEu* chromaticity values (0–107 (deep red)) | Temporal and nasal bulbar (circle region) | Offer accurate and more sensitive measurements of redness | Not variable at low levels of ocular redness |
| Yoneda T et al | Proportion of blood vessels in the conjunctiva | Temporal bulbar (square region) | Simple analysis and images can be analyzed promptly | All photographs were taken by a single photographer and not reliable |
| Amparo F et al | Ocular redness index (ORI): a continuous centesimal (0–100) scale | Whole temporal bulbar(freehand selection) | Intuitive, efficient and user friendly | The way of white balance affects final scores a lot |
| Fieguth P et al | Edge-Redness Assessed Grade (0–100) | Whole temporal and nasal bulbar | Minimum amount of operator intervention, more accuracy and less variability | Need more time and too complicated |
| Ferrari G et al | Relative redness of image (RRI) Edge feature (EF) scales |
Freehand selection around the exposed conjunctiva | A semiautomatic method to objectively quantify ocular surface inflammation in a simple and low cost way | Long time required to process images |
| Wolffsohn JS et al | Edge detection (ED) Relative color extraction (RCE) |
Temporal bulbar (rectangular region) | More sensitive and reliable than subjective grading. | Non-linear scale |
| Park IK et al | Occupied area by blood vessels | Whole temporal bulbar(freehand selection) | Cover the whole range of conditions that may cause conjunctival injection with various severity levels, not limited to specific conditions | Complicate algorithms need to be applied |
| Rodriguez JD et al | Total average redness intensity (Com-Red) Proportion of horizontal conjunctival vessels (Com-Hor) |
Temporal bulbar (rectangular region) | Objective measurement of the geometry of vessels | Only proved in Dry eye Disease induced ocular redness |
| Wu et al | Redness score (RS):Area percentage ratio between the vessels and the remainder of analyzed area | Nasal limbal, temporal limbal, nasal bulbar, temporal bulbar and global | A reliable and objective commercial product that can be used easily | Machine restricts the visible ocular area, overestimated the scores obtained using the subjective scale |
| Romano V et al | Pixel densitometry index (PDI): The mean intensity of the pixels in the ROI | Nasal, temporal, superior, and inferior conjunctival quadrant. | Avoid the need for white balance standardization of a photograph | Invasive measurement and PDI cannot be as amenable to interpatient comparison |
Villumesen and colleagues developed the computer-assisted, conjunctival hyperemia quantification system. [122] The authors analyzed the monochrome images generated from the captured video and quantified the vessel area by pixel edge detection analysis after smoothening. Conjunctival hyperemia was estimated by the number of pixels with vessels in a 3×3 area of analysis. Willingham et al. quantified temporal and nasal conjunctiva using pixelated images of the external eye using two parameters - mean relative redness (the percentage of red color in the image) and the blood vessel area ratio (the fraction of pixels which are blood vessels). [123] The acquired images were validated by a C++ programming-based software using a pre-established photographic reference scale of ocular hyperemia, established by instillation of 0.5% dapiprazole hydrochloride to increase hyperemia and 2.5% phenylephrine hydrochloride to reduce hyperemia. Gullion and Shah quantified the severity and geographic distribution of hyperemia and evaluated the diurnal variation in soft contact lens wearers. [124] The images were analyzed after electronic sectioning with differential intensity color analysis at fixed intervals away from the limbus and quantified the number and size of the vessels present. In the past two decades, evolution in software production, the advent of portable computers and cameras have promoted the clinical application of objective analyzers. Wollfsohn and Purslow quantified bulbar hyperemia, tarsal redness, corneal staining, and tarsal staining using thresholding, color extraction, and edge detection techniques. [125] The authors reported high repeatability on the assessment of bulbar hyperemia and close correlation with the subjective IER scale as well. The accuracy of the automated analysis heavily relies on the characterization of a region of interest (ROI) in the images of the conjunctiva, and the outcomes may vary depending upon a multitude of factors such as illumination conditions, the location of the eye in the image, the devices used to take the pictures or videos, the distance from the eye to the camera, or the presence of eyelashes. [126] Yoneda and colleagues reported high repeatability as well as reliability on accurate detection of ROI features. [127] They reported the optimal ROI was 400 vertical pixels by 300 horizontal pixels with high reproducibility of the analysis. The automated analysis of the ROI showed significantly higher blood vessel coverage in subjects with bimatoprost-induced hyperemia and hyperemia in allergic conjunctival diseases compared to images from control subjects.
Rodriguez et al. developed a new automated computer grading system tailored exclusively to dry eye disease-associated conjunctival hyperemia. [128] This system focused on the geometry of the vessels of the most clinically relevant region in dry eye, the interpalpebral fissure, and showed promising reliability and repeatability compared to other computer-based grading scales. The growing interest in the use of noninvasive imaging techniques for the evaluation of the ocular surface, the reliability of quantifying conjunctival hyperemia by using the Oculus Keratograph 5M Topographer was reported by Wu et al. [129] As the only commercially available device that can objectively evaluate conjunctival hyperemia, and this system is based on the area percentage ratio between the vessels and the rest of the analyzed area. However, it has been reported that redness scores provided by the keratograph 5M overestimated the scores obtained using the Efron and MC-D scales, showing poor agreement and moderate correlation with the subjective scales. [130]
To address some of these relevant challenges, we have established a novel objective assessment system, the ocular redness index (ORI), to overcome the shortcomings of previously established grading systems [131]. ORI has certain advantages over some of the previously established computer-assisted grading systems due to its high consistency, inferable scoring system, and easy application in clinics due to its portability and user interface that can perform analysis of images from different devices and file formats without loss of reproducibility [132]. The software uses a universal grading system that allows for intra- and inter-institutional coordination of patient data. Moreover, a non-clinician with minimal training can perform the grading by selecting the conjunctival area of interest using the provided seven-point region of interest (ROI) selection tool, including all the conjunctival areas exposed in the photographs except the caruncle. (Figure 5) After the selection of ROI, the software computes the redness score on a continuous centesimal (0–100) scale in approximately 30 seconds. The software algorithm consists of two components – white balance correction of images and redness quantification of images. The white-balance correction removes atypical color cast due to variable lighting conditions during image acquisition. After white-balance correction, the observer selected the conjunctival area to score. The generated ORI scores showed a significant correlation to analyses performed by experienced clinicians using the more subjective Efron and VBR scales on the same images.
Figure 5:
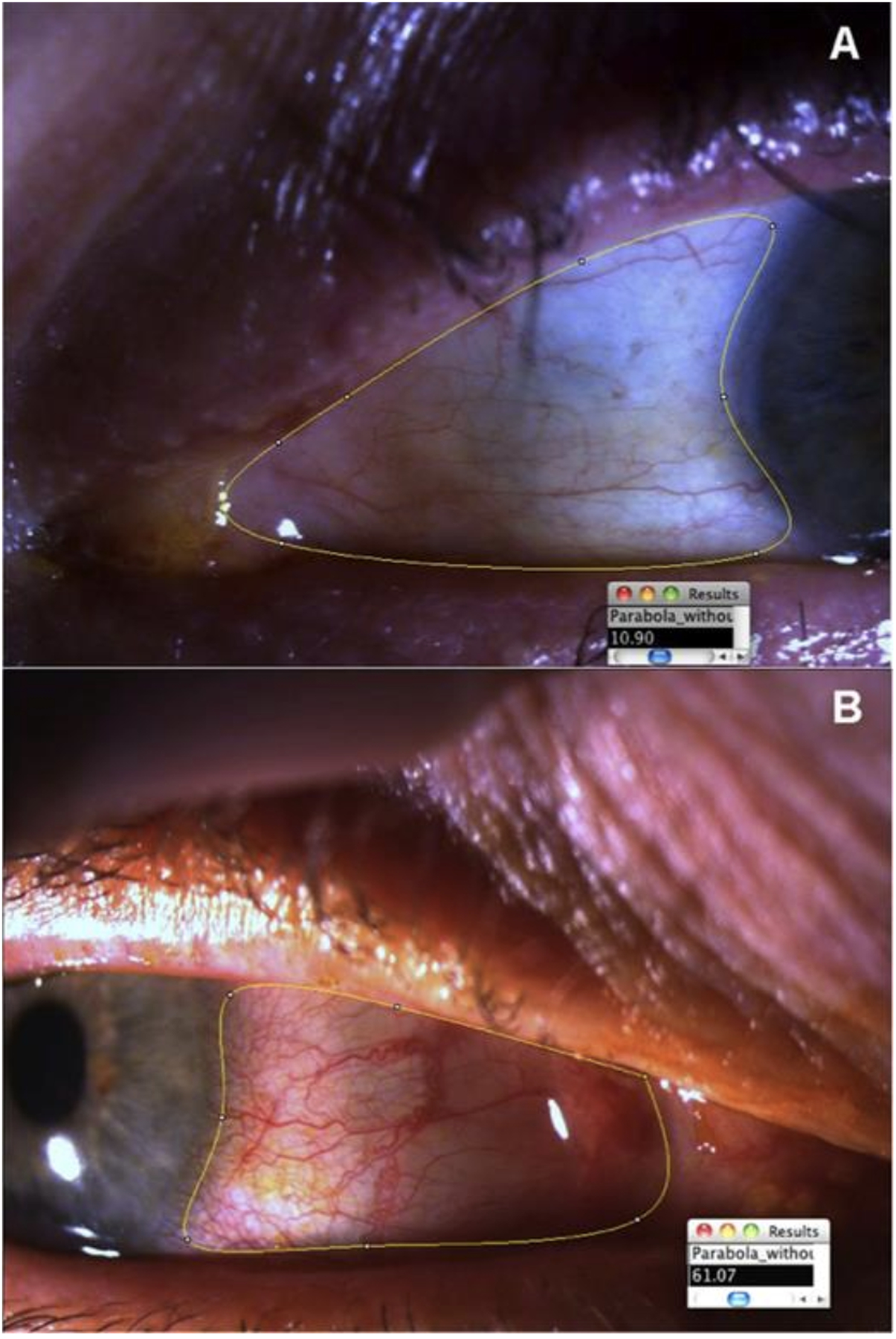
The region of interest (ROI) marked by the observer before analysis of the Ocular Redness Index by the software using a preset formula. (From Amparo et al., 2013)
7. CONCLUSION
The unique anatomy of the conjunctival microvasculature allows for easy and noninvasive examination and detection of physiological and pathological changes. The development of novel imaging modalities has immensely contributed to our understanding of the microcirculatory physiology of the conjunctiva. Moreover, the recent insights into the immunological mechanisms that cause vascular changes have helped to elucidate the pathophysiology of conjunctival hyperemia. Inflammation due to the vasodilatory response of the conjunctiva microvasculature can be prompted in response to a myriad of infectious and non-infectious etiologies that can be differentiated from detailed patient history and assessment of conjunctivitis characteristics such as redness patterns, duration of symptoms, and ocular pain. Accurate diagnosis of the source instigating conjunctival hyperemia allows physicians to effectively treat patients to ensure the greatest treatment outcomes in terms of ocular health and vision. Despite our understanding of the neurogenic and immune-mediated pathways that regulate the microvasculature of the ocular surface has significantly increased in recent years, large gaps in knowledge continue to persist.
9. ACKNOWLEDGEMENTS
We would like to thank Shudan Wang, M.D. and Mojgan Pishyareh, M.S. for their critical review of the manuscript.
All the figures in this manuscript were prepared by Rohan Bir Singh, using Biorender.com
Funding:
This review article was supported by the National Eye Institute/National Institutes of Health (K12-EY016335 to J.Y.; R01-EY12963 and R01-EY20889 to R.D.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Rohan Bir Singh: None, Lingjia Liu: None, Sonia Anchouche: None, Ann Yung: None, Sharad K. Mittal: None, Tomas Blanco: None, Jia Yin: None, Reza Dana: None
8. REFERENCES:
- [1].Azari AA, Barney NP. Conjunctivitis: A systematic review of diagnosis and treatment. JAMA - J Am Med Assoc 2013;310:1721–9. doi: 10.1001/jama.2013.280318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Frings A, Geerling G, Schargus M. Rotes Auge - Leitfaden für den Nicht-Ophthalmologen. Dtsch Arztebl Int 2017;114:302–12. doi: 10.3238/arztebl.2017.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Pflipsen M, Massaquoi M, Wolf S. Evaluation of the painful eye. Am Fam Physician 2016. [PubMed] [Google Scholar]
- [4].Dunlop AL, Wells JR. Approach to Red Eye for Primary Care Practitioners. Prim Care - Clin Off Pract 2015. doi: 10.1016/j.pop.2015.05.002. [DOI] [PubMed] [Google Scholar]
- [5].Du Toit N, Van Zyl L. The red eye. South African Fam Pract 2013;55:33–40. doi: 10.1080/20786204.2013.10874299. [DOI] [Google Scholar]
- [6].Leibowitz HM. Primary care: The red eye. N Engl J Med 2000;343:345–51. doi: 10.1056/NEJM200008033430507. [DOI] [PubMed] [Google Scholar]
- [7].Roy FH, Fraunfelder FW (Frederick W., Fraunfelder FT, Ralph Erskine Conrad Memorial Fund. Roy and Fraunfelder’s current ocular therapy. Elsevier Saunders; 2008. [Google Scholar]
- [8].Torkildsen GL, Sanfilippo CM, DeCory HH, Gomes PJ. Evaluation of Efficacy and Safety of Brimonidine Tartrate Ophthalmic Solution, 0.025% for Treatment of Ocular Redness. Curr Eye Res 2018;43:43–51. doi: 10.1080/02713683.2017.1381269. [DOI] [PubMed] [Google Scholar]
- [9].Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician 2010;81:137–44. [PubMed] [Google Scholar]
- [10].Lopez R, Lauwers F, Paoli JR, Boutault F, Guitard J. The vascular system of the upper eyelid. Anatomical study and clinical interest. Surg Radiol Anat 2008;30:265–9. doi: 10.1007/s00276-008-0323-8. [DOI] [PubMed] [Google Scholar]
- [11].Gupta N, Motlagh M, Singh G. Anatomy, Head and Neck, Eye Arteries. 2020. [PubMed]
- [12].Meighan SS. Blood vessels of the bulbar conjunctiva in man. Br J Ophthalmol 1956;40:513–26. doi: 10.1136/bjo.40.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Erdogmus S, Govsa F. The arterial anatomy of the eyelid: importance for reconstructive and aesthetic surgery. J Plast Reconstr Aesthetic Surg 2007;60:241–5. doi: 10.1016/j.bjps.2006.01.056. [DOI] [PubMed] [Google Scholar]
- [14].Goldberg MF, Bron AJ. Limbal palisades of Vogt. Trans Am Ophthalmol Soc 1982;Vol. 80:155–71. [PMC free article] [PubMed] [Google Scholar]
- [15].Lee SH, Lee HJ, Kim YS, Tansatit T, Kim HJ. Novel anatomic description of the course of the inferior palpebral vein for minimally invasive aesthetic treatments. Dermatologic Surg 2016. doi: 10.1097/DSS.0000000000000700. [DOI] [PubMed] [Google Scholar]
- [16].Azzam D, Cypen S, Tao J. Anatomy, Head and Neck, Eye Ophthalmic Vein. StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- [17].Meyer PAR. The Circulation of the Human Limbus. vol. 3. 1989. [DOI] [PubMed] [Google Scholar]
- [18].Kiel J. Anatomy - The Ocular Circulation 2010. [PubMed]
- [19].Cheung ATW, Perez RV., Chen PCY. Improvements in diabetic microangiopathy after successful simultaneous pancreas-kidney transplantation: A computer-assisted intravital microscopy study on the conjunctival microcirculation. Transplantation 1999;68:927–32. doi: 10.1097/00007890-199910150-00005. [DOI] [PubMed] [Google Scholar]
- [20].Shahidi M, Wanek J, Gaynes B, Wu T. Quantitative assessment of conjunctival microvascular circulation of the human eye. Microvasc Res 2010;79:109–13. doi: 10.1016/j.mvr.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Koutsiaris AG, Tachmitzi SV., Batis N. Wall shear stress quantification in the human conjunctival pre-capillary arterioles in vivo. Microvasc Res 2013;85:34–9. doi: 10.1016/j.mvr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- [22].Chew SH, Meighan Tomic M, Cheung ATW. Alzheimer’s disease: More than amyloid. Clin Hemorheol Microcirc 2010;46:69–73. doi: 10.3233/CH-2010-1356. [DOI] [PubMed] [Google Scholar]
- [23].Smith MM, Chen PCY, Li CS, Ramanujam S, Cheung ATW. Whole blood viscosity and microvascular abnormalities in alzheimer’s disease. Clin Hemorheol Microcirc 2009;41:229–39. doi: 10.3233/CH-2009-1174. [DOI] [PubMed] [Google Scholar]
- [24].Duench S, Simpson T, Jones LW, Flanagan JG, Fonn D. Assessment of variation in bulbar conjunctival redness, temperature, and blood flow. Optom Vis Sci 2007;84:511–6. doi: 10.1097/OPX.0b013e318073c304. [DOI] [PubMed] [Google Scholar]
- [25].Cheung ATW, Harmatz P, Wun T, Chen PCY, Larkin EC, Adams RJ, et al. Correlation of abnormal intracranial vessel velocity, measured by transcranial Doppler ultrasonography, with abnormal conjunctival vessel velocity, measured by computer-assisted intravital microscopy, in sickle cell disease. Blood 2001;97:3401–4. doi: 10.1182/blood.V97.11.3401. [DOI] [PubMed] [Google Scholar]
- [26].Schaser KD, Settmacher U, Puhl G, Zhang L, Mittlmeier T, Stover JF, et al. Noninvasive analysis of conjunctival microcirculation during carotid artery surgery reveals microvascular evidence of collateral compensation and stenosis-dependent adaptation. J Vasc Surg 2003;37:789–97. doi: 10.1067/mva.2003.139. [DOI] [PubMed] [Google Scholar]
- [27].Koutsiaris AG, Tachmitzi SV., Batis N, Kotoula MG, Karabatsas CH, Tsironi E, et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology 2007;44:375–86. [PubMed] [Google Scholar]
- [28].Cheung ATW, Miller JW, Craig SM, To PL, Lin X, Samarron SL, et al. Comparison of real-time microvascular abnormalities in pediatric and adult sickle cell anemia patients. Am J Hematol 2010;85:899–901. doi: 10.1002/ajh.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cheung ATW, Tomic MM, Chen PCY, Miguelino E, Li CS, Devaraj S. Correlation of microvascular abnormalities and endothelial dysfunction in Type-1 diabetes mellitus (T1DM): A real-time intravital microscopy study. Clin Hemorheol Microcirc 2009;42:285–95. doi: 10.3233/CH-2009-1199. [DOI] [PubMed] [Google Scholar]
- [30].Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, et al. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 2007;56:2790–6. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cheung ATW, Hu BS, Wong SA, Chow J, Chan MS, To WJ, et al. Microvascular abnormalities in the bulbar conjunctiva of contact lens users. Clin Hemorheol Microcirc 2012;51:77–86. doi: 10.3233/CH-2011-1513. [DOI] [PubMed] [Google Scholar]
- [32].Wanek J, Gaynes B, Lim JI, Molokie R, Shahidi M. Human bulbar conjunctival hemodynamics in hemoglobin S.S. and S.C. disease. Am J Hematol 2013;88:661–4. doi: 10.1002/ajh.23475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rodriguez JD, Johnston PR, Ousler GW, Smith LM, Abelson MB. Automated grading system for evaluation of ocular redness associated with dry eye. Clin Ophthalmol 2013;7:1197–204. doi: 10.2147/OPTH.S39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Roy FH. The red eye. Ann Ophthalmol 2006;38:35–8. doi: 10.1385/AO:38:1:35. [DOI] [PubMed] [Google Scholar]
- [35].Rosario N, Bielory L. Epidemiology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol 2011;11:471–6. doi: 10.1097/ACI.0b013e32834a9676. [DOI] [PubMed] [Google Scholar]
- [36].Petricek I, Prost M, Popova A. The differential diagnosis of red eye: A survey of medical practitioners from Eastern Europe and the Middle East. Ophthalmologica 2006;220:229–37. doi: 10.1159/000093076. [DOI] [PubMed] [Google Scholar]
- [37].Chigbu DGI. The pathophysiology of ocular allergy: a review. Cont Lens Anterior Eye 2009;32:3–4. doi: 10.1016/j.clae.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [38].Stahl JL, Cook EB, Barney NP, Graziano FM. Pathophysiology of ocular allergy: the roles of conjunctival mast cells and epithelial cells. Curr Allergy Asthma Rep 2002;2:332–9. doi: 10.1007/s11882-002-0062-6. [DOI] [PubMed] [Google Scholar]
- [39].Bielory L. Allergic and immunologic disorders of the eye. Part II: Ocular allergy. J Allergy Clin Immunol 2000;106:1019–32. doi: 10.1067/mai.2000.111238. [DOI] [PubMed] [Google Scholar]
- [40].Manzouri B, Flynn TH, Larkin F, Ono SJ, Wyse R. Pharmacotherapy of allergic eye disease. Expert Opin Pharmacother 2006;7:1191–200. doi: 10.1517/14656566.7.9.1191. [DOI] [PubMed] [Google Scholar]
- [41].Mishra GP, Tamboli V, Jwala J, Mitra AK Recent Patents and Emerging Therapeutics in the Treatment of Allergic Conjunctivitis. Recent Pat Inflamm Allergy Drug Discov 2011;5:26–36. doi: 10.2174/187221311794474883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nelson JD, Craig JP, Akpek EK, Azar DT, Belmonte C, Bron AJ, et al. TFOS DEWS II Introduction. Ocul Surf 2017;15:269–75. doi: 10.1016/j.jtos.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [43].Clayton JA. Dry eye. N Engl J Med 2018;378:2212–23. doi: 10.1056/NEJMra1407936. [DOI] [PubMed] [Google Scholar]
- [44].Stevenson W, Chauhan SK, Dana R. Dry Eye Disease. Arch Ophthalmol 2012;130:90. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: A retrospective study. Cornea 2012;31:472–8. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- [46].Stevenson W, Chauhan SK, Dana R. Dry eye disease: An immune-mediated ocular surface disorder. Arch Ophthalmol 2012. doi: 10.1001/archophthalmol.2011.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Aronson JK. Chapter 21 - Diuretics. In: Aronson JKBT-SE of DA, editor. vol. 31, Elsevier; 2009, p. 371–81. doi: 10.1016/S0378-6080(09)03121-3. [DOI] [Google Scholar]
- [48].Feldman RM. Conjunctival Hyperemia and the Use of Topical Prostaglandins in Glaucoma and Ocular Hypertension. J Ocul Pharmacol Ther 2003;19:23–35. doi: 10.1089/108076803762718088. [DOI] [PubMed] [Google Scholar]
- [49].Noecker R. Effects of common ophthalmic preservatives on ocular health. Adv Ther 2001;18:205–15. doi: 10.1007/BF02853166. [DOI] [PubMed] [Google Scholar]
- [50].Arıcı MK, Arıcı DS, Topalkara A, Güler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clin Experiment Ophthalmol 2000;28:113–7. doi:doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- [51].Henry JC, Peace JH, Stewart JA, Stewart WC. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol 2008;2:613–21. doi: 10.2147/opth.s3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Haq A, Wardak H, Kraski N. Infective Conjunctivitis – Its Pathogenesis, Management and Complications. Common Eye Infect., InTech; 2013. doi: 10.5772/52462. [DOI] [Google Scholar]
- [53].Jackson CL. Misdiagnosis of acute eye diseases by primary health care providers: Incidence and implications. Med J Aust 2009;190:343–4. doi: 10.5694/j.1326-5377.2009.tb02439.x. [DOI] [PubMed] [Google Scholar]
- [54].Jonas RA, Ung L, Rajaiya J, Chodosh J. Mystery eye: Human adenovirus and the enigma of epidemic keratoconjunctivitis. Prog Retin Eye Res 2020. doi: 10.1016/j.preteyeres.2019.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ghebremedhin B. Human adenovirus: Viral pathogen with increasing importance. Eur J Microbiol Immunol 2014;4:26–33. doi: 10.1556/eujmi.4.2014.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yeu E, Hauswirth S. A review of the differential diagnosis of acute infectious conjunctivitis: Implications for treatment and management. Clin Ophthalmol 2020;14:805–13. doi: 10.2147/OPTH.S236571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jhanji V, Chan TCY, Li EYM, Agarwal K, Vajpayee RB. Adenoviral keratoconjunctivitis. Surv Ophthalmol 2015;60:435–43. doi: 10.1016/j.survophthal.2015.04.001. [DOI] [PubMed] [Google Scholar]
- [58].ShinYoshi K. Viral conjunctivitis. Japanese J Clin Ophthalmol 2016. doi: 10.1016/b978-0-7506-4063-3.50014-8. [DOI] [Google Scholar]
- [59].Meduri A, Grenga PL, Kaufman SC. Herpes zoster ophthalmicus. Expert Rev Ophthalmol 2009. doi: 10.1586/eop.09.45. [DOI] [Google Scholar]
- [60].Hutnik C, Mohammad H, Mohammad-Shahi M. Bacterial conjunctivitis. Clin Ophthalmol 2010. doi: 10.2147/OPTH.S10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Epling J. Bacterial conjunctivitis. BMJ Clin Evid 2010;2010. [PMC free article] [PubMed] [Google Scholar]
- [62].Kreisel K, Weston E, Braxton J, Llata E, Torrone E. Keeping an eye on chlamydia and gonorrhea conjunctivitis in infants in the United States, 2010–2015. Sex Transm Dis 2017. doi: 10.1097/OLQ.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mayor MT, Roett MA, Uduhiri KA. Diagnosis and management of gonococcal infections. Am Fam Physician 2012. [PubMed] [Google Scholar]
- [64].Frings A, Geerling G, Schargus M. Rotes Auge - Leitfaden für den Nicht-Ophthalmologen. Dtsch Arztebl Int 2017;114:302–12. doi: 10.3238/arztebl.2017.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Thomas PA, Geraldine P. Infectious keratitis. Curr Opin Infect Dis 2007;20:129–41. doi: 10.1097/QCO.0b013e328017f878. [DOI] [PubMed] [Google Scholar]
- [66].Bron AJ, Mengher LS, Davey CC. The normal conjunctiva and its responses to inflammation. Trans Ophthalmol Soc U K 1985;104:424–35. [PubMed] [Google Scholar]
- [67].Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, et al. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med 2006;203:2907–17. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Irkec MT, Bozkurt B. Molecular immunology of allergic conjunctivitis. Curr Opin Allergy Clin Immunol 2012;12:534–9. doi: 10.1097/ACI.0b013e328357a21b. [DOI] [PubMed] [Google Scholar]
- [69].Butrus S, Portela R. Ocular allergy: Diagnosis and treatment. Ophthalmol Clin North Am 2005;18:485–92. doi: 10.1016/j.ohc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- [70].Rees PH, Hillier K, Church MK The secretory characteristics of mast cells isolated from the human large intestinal mucosa and muscle. Immunology 1988;65:437–42. [PMC free article] [PubMed] [Google Scholar]
- [71].Leonardi A. Role of histamine in allergic conjunctivitis. Acta Ophthalmol Scand Suppl 2000;78:18–21. doi: 10.1034/j.1600-0420.2000.078s230018.x. [DOI] [PubMed] [Google Scholar]
- [72].Bielory L, Ghafoor S. Histamine receptors and the conjunctiva. Curr Opin Allergy Clin Immunol 2005;5:437–40. doi: 10.1097/01.all.0000183113.63311.11. [DOI] [PubMed] [Google Scholar]
- [73].Leonardi A, Di Stefano A, Vicari C, Motterle L, Brun P. Histamine H4 receptors in normal conjunctiva and in vernal keratoconjunctivitis. Allergy Eur J Allergy Clin Immunol 2011;66:1360–6. doi: 10.1111/j.1398-9995.2011.02612.x. [DOI] [PubMed] [Google Scholar]
- [74].Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- [75].Moncada S, Palmer RMJ, Higgs EA. Biosynthesis of nitric oxide from l-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol 1989;38:1709–15. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- [76].Meijer F, Van Delft JL, Garrelds IM, Van Haeringen NJ, Kijlstra A. Nitric oxide plays a role as a mediator of conjunctival edema in experimental allergic conjunctivitis. Exp Eye Res 1996;62:359–66. doi: 10.1006/exer.1996.0041. [DOI] [PubMed] [Google Scholar]
- [77].Ko SM, Kim MK, Kim JC. The role of nitric oxide in experimental allergic conjunctivitis. Cornea 2000;19:84–91. doi: 10.1097/00003226-200001000-00017. [DOI] [PubMed] [Google Scholar]
- [78].Meijer F, Ruijter JM, Van Delft JL, Van Haeringen NJ. Nitric oxide induces vascular permeability changes in the guinea pig conjunctiva. Eur J Pharmacol 1995;284:61–7. doi: 10.1016/0014-2999(95)00376-V. [DOI] [PubMed] [Google Scholar]
- [79].Meijer F, Tak C, Van Haeringen NJ, Kijlstra A. Interaction between nitric oxide and prostaglandin synthesis in the acute phase of allergic conjunctivitis. Prostaglandins 1996;52:431–46. doi: 10.1016/S0090-6980(96)00123-2. [DOI] [PubMed] [Google Scholar]
- [80].Umemoto M, Tanaka H, Miichi H, Hayashi S. Histamine receptors on rat ocular surface. Ophthalmic Res 1987;19:200–4. doi: 10.1159/000265494. [DOI] [PubMed] [Google Scholar]
- [81].Abelson MB, Udell IJ. H2-receptors in the human ocular surface. Arch Ophthalmol 1981;99:302–4. doi: 10.1001/archopht.1981.03930010304018. [DOI] [PubMed] [Google Scholar]
- [82].Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, et al. International union of pharmacology. XIII. Classification of histamine receptors. Pharmacol Rev 1997;49:253–78. [PubMed] [Google Scholar]
- [83].Thangam EB, Jemima EA, Singh H, Baig MS, Khan M, Mathias CB, et al. The role of histamine and histamine receptors in mast cell-mediated allergy and inflammation: The hunt for new therapeutic targets. Front Immunol 2018;9:1873. doi: 10.3389/fimmu.2018.01873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Krystel-Whittemore M, Dileepan KN, Wood JG Mast cell: A multi-functional master cell. Front Immunol 2016;6:620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Stewart WC, Kolker AE, Stewart JA, Leech J, Jackson AL. Conjunctival hyperemia in healthy subjects after short-term dosing with latanoprost, bimatoprost, and travoprost. Am J Ophthalmol 2003;135:314–20. doi: 10.1016/S0002-9394(02)01980-3. [DOI] [PubMed] [Google Scholar]
- [86].Spada CS, Woodward DF, Hawley SB, Nieves AL. Leukotrienes cause eosinophil emigration into conjunction tissue. Prostaglandins 1986;31:795–809. doi: 10.1016/0090-6980(86)90181-4. [DOI] [PubMed] [Google Scholar]
- [87].Peters K, Unger RE, Brunner J, Kirkpatrick CJ. Molecular basis of endothelial dysfunction in sepsis. Cardiovasc Res 2003;60:49–57. doi: 10.1016/S0008-6363(03)00397-3. [DOI] [PubMed] [Google Scholar]
- [88].Iversen PO, Nicolaysen A, Kvernebo K, Benestad HB, Nicolaysen G. Human cytokines modulate arterial vascular tone via endothelial receptors. Pflügers Arch - Eur J Physiol 1999;439:93–100. doi: 10.1007/s004249900149. [DOI] [PubMed] [Google Scholar]
- [89].van der Poll T, Lowry SF. Tumor necrosis factor in sepsis: Mediator of multiple organ failure or essential part of host defense? Shock 1995;3:1–12. doi: 10.1097/00024382-199501000-00001. [DOI] [PubMed] [Google Scholar]
- [90].Brian JE, Faraci FM. Tumor necrosis factor-α-induced dilatation of cerebral arterioles. Stroke 1998;29:509–15. doi: 10.1161/01.STR.29.2.509. [DOI] [PubMed] [Google Scholar]
- [91].Wilkinson MF, Earle ML, Tricole CR, Barnes S. Interleukin‐lβ, tumor necrosis factor‐α, and LPS enhance calcium channel current in isolated vascidar smooth muscle cells of rat tail artery. FASEB J 1996;10:785–91. doi: 10.1096/fasebj.10.7.8635696. [DOI] [PubMed] [Google Scholar]
- [92].Cheranov SY, Jaggar JH. TNF-α dilates cerebral arteries via NAD(P)H oxidase-dependent Ca 2+ spark activation. Am J Physiol - Cell Physiol 2006;290. doi: 10.1152/ajpcell.00499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Orshal JM, Khalil RA. Interleukin-6 impairs endothelium-dependent NO-cGMP-mediated relaxation and enhances contraction in systemic vessels of pregnant rats. Am J Physiol - Regul Integr Comp Physiol 2004;286. doi: 10.1152/ajpregu.00729.2003. [DOI] [PubMed] [Google Scholar]
- [94].Barath P, Fishbein MC, Cao J, Berenson J, Helfant RH, Forrester JS. Detection and localization of tumor necrosis factor in human atheroma. Am J Cardiol 1990;65:297–302. doi: 10.1016/0002-9149(90)90291-8. [DOI] [PubMed] [Google Scholar]
- [95].Geng YJ, Hansson GK, Holme E. Interferon-γ and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res 1992;71:1268–76. doi: 10.1161/01.RES.71.5.1268. [DOI] [PubMed] [Google Scholar]
- [96].Sheng Y, Zhu L. The crosstalk between autonomic nervous system and blood vessels. Int J Physiol Pathophysiol Pharmacol 2018;10:17–28. [PMC free article] [PubMed] [Google Scholar]
- [97].McDougal DH, Gamlin PD. Autonomic control of the eye. Compr Physiol 2015;5:439–73. doi: 10.1002/cphy.c140014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Belmonte C, Aracil A, Acosta MC, Luna C, Gallar J. Nerves and sensations from the eye surface. Ocul Surf 2004;2:248–53. doi: 10.1016/S1542-0124(12)70112-X. [DOI] [PubMed] [Google Scholar]
- [99].Sousa-Valente J, Brain SD. A historical perspective on the role of sensory nerves in neurogenic inflammation. Semin Immunopathol 2018;40:229–36. doi: 10.1007/s00281-018-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Taketani Y, Marmalidou A, Dohlman TH, Singh RB, Amouzegar A, Chauhan SK, et al. Restoration of Regulatory T-Cell Function in Dry Eye Disease by Antagonizing Substance P/Neurokinin-1 Receptor. Am J Pathol 2020;190:1859–66. doi: 10.1016/j.ajpath.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Suvas S. Role of substance p neuropeptide in inflammation, wound healing, and tissue homeostasis. J Immunol 2017;199. doi: 10.4049/jimmunol.1601751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Columbo M, Horowitz EM, Kagey-Sobotka A, Lichtenstein LM. Substance P activates the release of histamine from human skin mast cells through a pertussis toxin-sensitive and protein kinase C-dependent mechanism. Clin Immunol Immunopathol 1996;81:68–73. doi: 10.1006/clin.1996.0159. [DOI] [PubMed] [Google Scholar]
- [103].Repke H, Bienert M. Structural requirements for mast cell triggering by substance P-like peptides. Agents Actions 1988;23:207–10. doi: 10.1007/BF02142542. [DOI] [PubMed] [Google Scholar]
- [104].Okada T, Hirayama Y, Kishi S, Miyayasu K, Hiroi J, Fujii T. Functional neurokinin NK-1 receptor expression in rat peritoneal mast cells. Inflamm Res 1999;48:274–9. doi: 10.1007/s000110050459. [DOI] [PubMed] [Google Scholar]
- [105].Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol 1993;150:4478–85. [PubMed] [Google Scholar]
- [107].Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature 1985;313:54–6. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- [108].McCulloch J, Uddman R, Kingman TA, Edvinsson L. Calcitonin gene-related peptide: Functional role in cerebrovascular regulation. Proc Natl Acad Sci U S A 1986;83:5731–5. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 2014;94:1099–142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Iyengar S, Johnson KW, Ossipov MH, Aurora SK. CGRP and the Trigeminal System in Migraine. Headache 2019;59:659–81. doi: 10.1111/head.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Crossman DC, Dashwood MR, Brain SD, McEwan J, Pearson JD. Action of calcitonin gene-related peptide upon bovine vascular endothelial and smooth muscle cells grown in isolation and co-culture. Br J Pharmacol 1990;99:71–6. doi: 10.1111/j.1476-5381.1990.tb14656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Woods R. Quantitative slit lamp observations in contact lens practice. J Br Contact Lens Assoc 1989;1989:42–5. doi: 10.1016/S0141-7037(89)80008-4. [DOI] [Google Scholar]
- [113].Mandell RB. Slit lamp classification system. J Am Optom Assoc 1987;58:198–201. https://pubmed.ncbi.nlm.nih.gov/3571821/ (accessed September 1, 2020). [PubMed] [Google Scholar]
- [114].McMonnies CW, Chapman-Davies A. Assessment of conjunctival hyperemia in contact lens wearers. part I. Optom Vis Sci 1987;64:246–50. doi: 10.1097/00006324-198704000-00003. [DOI] [PubMed] [Google Scholar]
- [115].Phillips AJ and Speedwell L. CCLRU grading scales (Appendix D). Contact Lenses 1997:863–7. [Google Scholar]
- [116].Efron N, Morgan PB, Katsara SS. Validation of grading scales for contact lens complications. Ophthalmic Physiol Opt 2001;21:17–29. doi: 10.1046/j.1475-1313.2001.00575.x. [DOI] [PubMed] [Google Scholar]
- [117].Schulze MM, Jones DA, Simpson TL. The development of validated bulbar redness grading scales. Optom Vis Sci 2007;84:976–83. doi: 10.1097/OPX.0b013e318157ac9e. [DOI] [PubMed] [Google Scholar]
- [118].Peterson RC, Wolffsohn JS. Sensitivity and reliability of objective image analysis compared to subjective grading of bulbar hyperaemia. Br J Ophthalmol 2007;91:1464–6. doi: 10.1136/bjo.2006.112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Sorbara L, Simpson T, Duench S, Schulze M, Fonn D. Comparison of an objective method of measuring bulbar redness to the use of traditional grading scales. Contact Lens Anterior Eye 2007;30:53–9. doi: 10.1016/j.clae.2006.12.003. [DOI] [PubMed] [Google Scholar]
- [120].Schulze MM, Hutchings N, Simpson TL. Grading bulbar redness using cross-calibrated clinical grading scales. Investig Ophthalmol Vis Sci 2011;52:5812–7. doi: 10.1167/iovs.10-7006. [DOI] [PubMed] [Google Scholar]
- [121].Papas EB Key factors in the subjective and objective assessment of conjunctival erythema. Investig Ophthalmol Vis Sci 2000;41:687–91. doi:doi: [PubMed] [Google Scholar]
- [122].Villumsen J, Ringquist J, Alm A. Image analysis of conjunctival hyperemia: A personal computer based system. Acta Ophthalmol 1991;69:536–9. doi: 10.1111/j.1755-3768.1991.tb02036.x. [DOI] [PubMed] [Google Scholar]
- [123].Willingham FF, Cohen KL, Coggins JM, Tripoli NK, Ogle JW, Goldstein GM. Automatic quantitative measurement of ocular hyperemia. Curr Eye Res 1995. doi: 10.3109/02713689508995816. [DOI] [PubMed] [Google Scholar]
- [124].Guillon M, Shah D. Objective measurement of contact lens-induced conjunctival redness. Optom Vis Sci 1996;73:595–605. doi: 10.1097/00006324-199609000-00006. [DOI] [PubMed] [Google Scholar]
- [125].Wolffsohn JS, Purslow C. Clinical monitoring of ocular physiology using digital image analysis. Contact Lens Anterior Eye 2003;26:27–35. doi: 10.1016/S1367-0484(02)00062-0. [DOI] [PubMed] [Google Scholar]
- [126].Brea MLS, Rodríguez NB, González AM, Evans K, Pena-Verdeal H. Defining the optimal region of interest for hyperemia grading in the bulbar conjunctiva. Comput Math Methods Med 2016;2016. doi: 10.1155/2016/3695014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Yoneda T, Sumi T, Takahashi A, Hoshikawa Y, Kobayashi M, Fukushima A. Automated hyperemia analysis software: Reliability and reproducibility in healthy subjects. Jpn J Ophthalmol 2012;56:1–7. doi: 10.1007/s10384-011-0107-2. [DOI] [PubMed] [Google Scholar]
- [128].Rodriguez JD, Johnston PR, Ousler GW, Smith LM, Abelson MB. Automated grading system for evaluation of ocular redness associated with dry eye. Clin Ophthalmol 2013;7:1197–204. doi: 10.2147/OPTH.S39703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Wu S, Hong J, Tian L, Cui X, Sun X, Xu J. Assessment of bulbar redness with a newly developed Keratograph. Optom Vis Sci 2015;92:892–9. doi: 10.1097/OPX.0000000000000643. [DOI] [PubMed] [Google Scholar]
- [130].Pérez-Bartolomé F, Sanz-Pozo C, Martínez-de la Casa JM, Arriola-Villalobos P, Fernández-Pérez C, García-Feijoó J. Assessment of ocular redness measurements obtained with keratograph 5M and correlation with subjective grading scales. J Fr Ophtalmol 2018;41:836–46. doi: 10.1016/j.jfo.2018.03.007. [DOI] [PubMed] [Google Scholar]
- [131].Amparo F, Wang H, Emami-Naeini P, Karimian P, Dana R. The ocular redness index: A novel automated method for measuring ocular injection. Investig Ophthalmol Vis Sci 2013;54:4821–6. doi: 10.1167/iovs.13-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Amparo F, Wang H, Yin J, Marmalidou A, Dana R. Evaluating Corneal Fluorescein Staining Using a Novel Automated Method. Invest Ophthalmol Vis Sci 2017;58:BIO168–73. doi: 10.1167/iovs.17-21831. [DOI] [PubMed] [Google Scholar]


