Abstract
Background
Understanding the impact of the tumor immune microenvironment and BRCA1/2-related DNA repair deficiencies on the clinical activity of immune checkpoint inhibitors may help optimize both patient and treatment selection in metastatic triple-negative breast cancer. In this substudy from the phase 3 IMpassion130 trial, immune biomarkers and BRCA1/2 alterations were evaluated for association with clinical benefit with atezolizumab and nab-paclitaxel (A+nP) vs placebo and nP in unresectable (P+nP) locally advanced or metastatic triple-negative breast cancer.
Methods
Patients were randomly assigned 1:1 to nab-paclitaxel 100 mg/m2 (days 1, 8, and 15 of a 28-day cycle) and atezolizumab 840 mg every 2 weeks or placebo until progression or toxicity. Progression-free survival and overall survival were evaluated based on programmed death-ligand 1 (PD-L1) expression on immune cells (IC) and tumor cells, intratumoral CD8, stromal tumor-infiltrating lymphocytes, and BRCA1/2 mutations.
Results
PD-L1 IC+ in either primary or metastatic tumor tissue was linked to progression-free survival and overall survival benefit with A+nP. PD-L1 IC+ low (26.9%; 243 of 902 patients) and high (13.9%; 125 of 902 patients) populations had improved outcomes that were comparable. Intratumoral CD8 and stromal tumor-infiltrating lymphocytes positivity (sTIL+) were associated with PD-L1 IC+ status; improved outcomes were observed with A+nP vs P+nP only in CD8+ and sTIL+ patients who were also PD-L1 IC+. BRCA1/2 mutations (occurring in 14.5% [89 of 612 patients]) were not associated with PD-L1 IC status, and PD-L1 IC+ patients benefited from A+nP regardless of BRCA1/2 mutation status.
Conclusions
Although A+nP was more efficacious in patients with richer tumor immune microenvironment, clinical benefit was only observed in patients whose tumors were PD-L1 IC+.
Patients with metastatic triple-negative breast cancer (mTNBC) have a median overall survival (OS) of less than 18 months with standard chemotherapy (1‐4). Targeted drugs, including bevacizumab plus chemotherapy (5) or poly(ADP-ribose) polymerase (PARP) inhibitors for BRCA1/2-mutant, HER2-negative metastatic breast cancer (6,7), are associated with progression-free survival (PFS) benefit. The randomized phase 3 study IMpassion130, evaluating atezolizumab and nab-paclitaxel (A+nP) vs placebo and nab-paclitaxel as first-line treatment for mTNBC, met its co-primary PFS endpoint in the intention-to-treat (ITT) population and in patients whose tumors had 1% or higher programmed death-ligand 1 (PD-L1)–expressing tumor-infiltrating immune cells (IC+). Improved activity with A+nP was only observed in PD-L1 IC+ patients (8,9). Although not testable for statistical significance because of the hierarchical study design, PD-L1 IC+ patients also had clinically meaningful OS improvement (hazard ratio = 0.71, 95% confidence interval [CI] = 0.54 to 0.93) with A+nP (9).
CD8+ T cells (10,11), stromal tumor-infiltrating lymphocytes (sTILs) (12‐15), and PD-L1 expression on tumor cells (TC) (16) are other biomarkers associated with improved clinical outcomes with PD-L1/PD-1 inhibition, and patients with mTNBC who have BRCA1/2 gene mutations derive clinical benefit with PARP inhibitors (6,7). It has been hypothesized that BRCA1/2-deficient tumors may be more responsive to immune checkpoint inhibitors because of DNA damage accumulation, but whether this occurs in breast cancer is not established. This exploratory analysis aimed to evaluate whether PD-L1 TC+, CD8+ T cells, sTILs, or BRCA1/2 mutation status, in addition to PD-L1 IC+, are suitable biomarkers for selecting patients likely to benefit from A+nP.
Methods
Study Design and Patients
IMpassion130 (ClinicalTrials.gov identifier: NCT02425891), a randomized, double-blind study of A+nP as first-line treatment in 902 patients with mTNBC, was previously described (8).
Eligible patients aged 18 years or older had metastatic or unresectable locally advanced, histologically documented TNBC. HER2 and progesterone receptor statuses per American Society of Clinical Oncology–College of American Pathologists guidelines were confirmed locally (17,18). Enrollment required measurable disease per Response Evaluation Criteria in Solid Tumors 1.1, Eastern Cooperative Oncology Group performance status 0-1, and TNBC tumor tissue from either an archival or fresh sample (formalin-fixed paraffin-embedded) for prospective central PD-L1 testing and exploratory biomarker analyses. Patients could not have received prior therapy for mTNBC, but prior therapy (including taxanes) in the curative setting was allowed if completed at least 12 months before randomization (8).
IMpassion130 was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki; the protocol was approved by independent ethics committees at each participating site (8). All participants provided written informed consent.
Biomarker Assessments
Biomarkers were centrally analyzed in pretreatment primary or metastatic tumor samples (hematoxylin and eosin immunohistochemistry [IHC]: HistoGeneX, Antwerp, Belgium; BRCA1/2 status: Foundation Medicine Inc, Cambridge, MA, USA). All primary tumor samples were from the breast. Study sites provided information on the stage, anatomical location, and collection date of tissue samples in the sample requisition forms. PD-L1 IC and TC status was assessed using the VENTANA SP142 PD-L1 IHC assay (Ventana Medical Systems, Oro Valley, AZ). Further details on PD-L1 evaluation are provided in the Supplementary Methods (available online). If multiple pretreatment biopsies were available, the highest PD-L1 score was used for classification. Scoring was based on PD-L1-expressing IC as a percentage of tumor area: IC negative (<1%) or positive (≥1%) (12,19). PD-L1 scoring on TC was based on the percentage of PD-L1–expressing TC: TC- (<1%) and TC+ (≥1%) (12,19). Intratumoral CD8+ T cells assessed by IHC (clone C8/144B; Dako North America, Carpinteria, CA) were digitally quantified. A cutoff of 0.5% of tumor center was used (12). Quantification of CD8 IHC was performed on digital scans following pathologist designation of the tumor area (see the Supplementary Methods, available online, for further details). sTILs were assessed by hematoxylin and eosin stain following International TILs Working Group evaluation guidelines, with a 10% cutoff used to distinguish low vs high levels (20,21). Pathologists from HistoGeneX were trained by representatives from the International Immuno-Oncology Biomarker Working Group. Tumor BRCA1/2 status (mutant: known or likely deleterious mutations; nonmutant: wild type or variants of unknown significance) was assessed using a FoundationOne DNA-based panel (Foundation Medicine), which can discriminate germline from somatic mutations.
Outcomes and Statistical Analyses
Investigator-assessed PFS and OS were evaluated in biomarker-evaluable populations (BEPs; Supplementary Figure 1, available online). Potential effects of biomarkers on prognosis for PFS and OS were tested in the placebo and nP in unresectable (P+nP) arm using Cox regression analysis based on binary biomarker categorization, adjusted for key baseline prognostic factors (prior taxane treatment, liver metastases). Hazard ratio estimates with associated 95% confidence intervals and P values were derived to compare time-to-event endpoints between patient groups using Cox regression, adjusted for prognostic factors mentioned above. All analyses performed were hypothesis generating, and descriptive P values are presented for exploratory purposes only. P values for comparing proportions between groups were based on the Fisher exact test. Kaplan-Meier estimates and corresponding medians for time-to-event distributions were determined. Correlation between biomarkers as continuous variables was performed with the Spearman rank-correlation index. The Mann-Whitney test was used for the comparison of continuum variables by biomarker subgroup. Tests of statistical significance were 2-sided. A P value of less than .05 was considered statistically significant.
Results
Patients
In IMpassion130, the prevalence of PD-L1 IC+ (≥1%) was 41.0% (185 of 451 patients) in the A+nP arm and 40.8% (184 of 451 patients) in the P+nP arm (8). The median follow-up duration for the presented efficacy analyses in biomarker-defined patient subgroups was 18.0 months (clinical cutoff: January 2, 2019). Study flow and baseline characteristics for each BEP are summarized in Supplementary Figure 1 and Supplementary Table 1 (available online). Baseline characteristics by PD-L1 status are shown in Supplementary Table 2 (available online) and were generally similar between subgroups.
PD-L1 IC Expression Levels and Clinical Outcomes
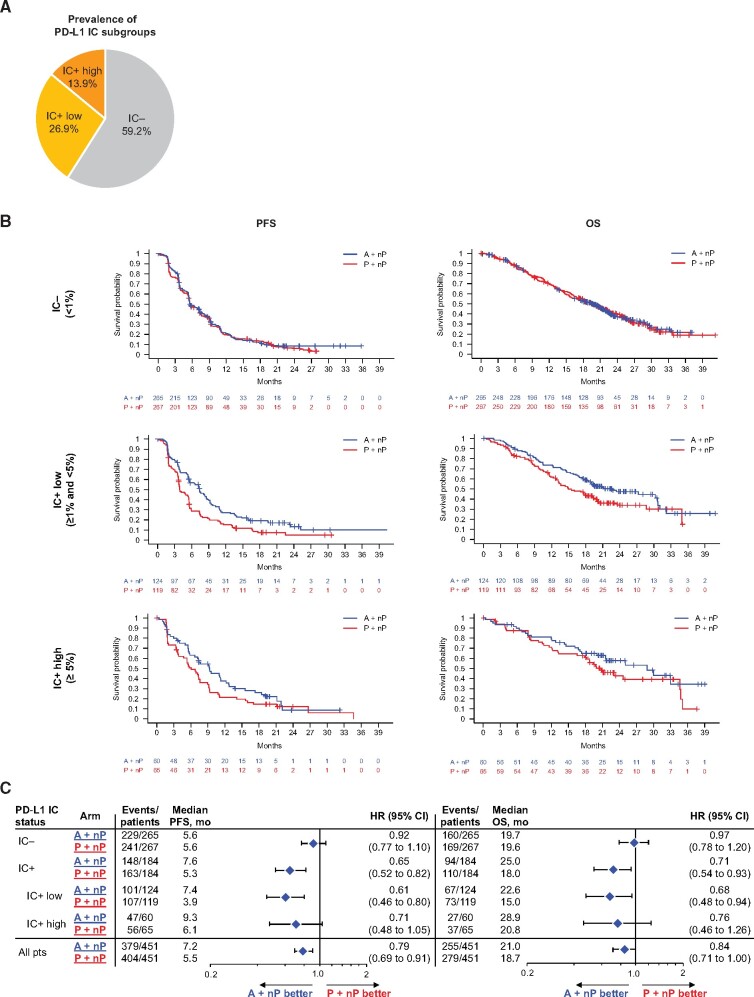
In IMpassion130, 40.8% (368 of 902) of patients in total were PD-L1 IC+ (8); 26.9% (243 of 902) of patients had low PD-L1 IC (PD-L1 IC ≥1% and <5%) and 13.9% (125 of 902) of patients had high PD-L1 IC (PD-L1 IC ≥5%; Figure 1, A). PFS hazard ratios for A+nP vs P+nP in the PD-L1 IC+ low and IC+ high subgroups were 0.61 (95% CI = 0.46 to 0.80, P < .005) and 0.71 (95% CI = 0.48 to 1.05, P = .09), respectively; corresponding OS hazard ratios were 0.68 (95% CI = 0.48 to 0.94, P = .02) and 0.76 (95% CI = 0.46 to 1.26, P = .29) (Figure 1, B and C). In the A+nP arm, the median PFS and OS were, respectively, 7.4 months and 22.6 months in PD-L1 IC+ low patients and 9.3 months and 28.9 months in PD-L1 IC+ high patients. Thus, although assessment of the PD-L1 IC+ high patient group is limited by small sample size and low event rates, we observed improved outcomes with A+nP in IMpassion130 patients whenever PD-L1 IC expression was 1% or more.
Figure 1.
PD-L1 expression on immune cells: sample disposition and efficacy outcomes by PD-L1 IC levels. A) Distribution of PD-L1 IC subgroups. B) Kaplan-Meier analysis of PFS and OS in the PD-L1 IC−, PD-L1 IC+ low, and PD-L1 IC+ high patient populations. C) Forest plots of PFS and OS in PD-L1 IC-defined patient populations. P values are descriptive, except for PD-L1 IC+ PFS. Analyses were adjusted for prior taxane treatment and liver metastases. A = atezolizumab; CI = confidence interval; HR = hazard ratio; IC = tumor-infiltrating immune cells; IC+ = PD-L1 ≥ 1% on IC; IC- = PD-L1 < 1% on IC; nP = nab-paclitaxel; OS = overall survival; P = placebo; PD-L1 = programmed death-ligand 1; PFS = progression-free survival; pts = patients.
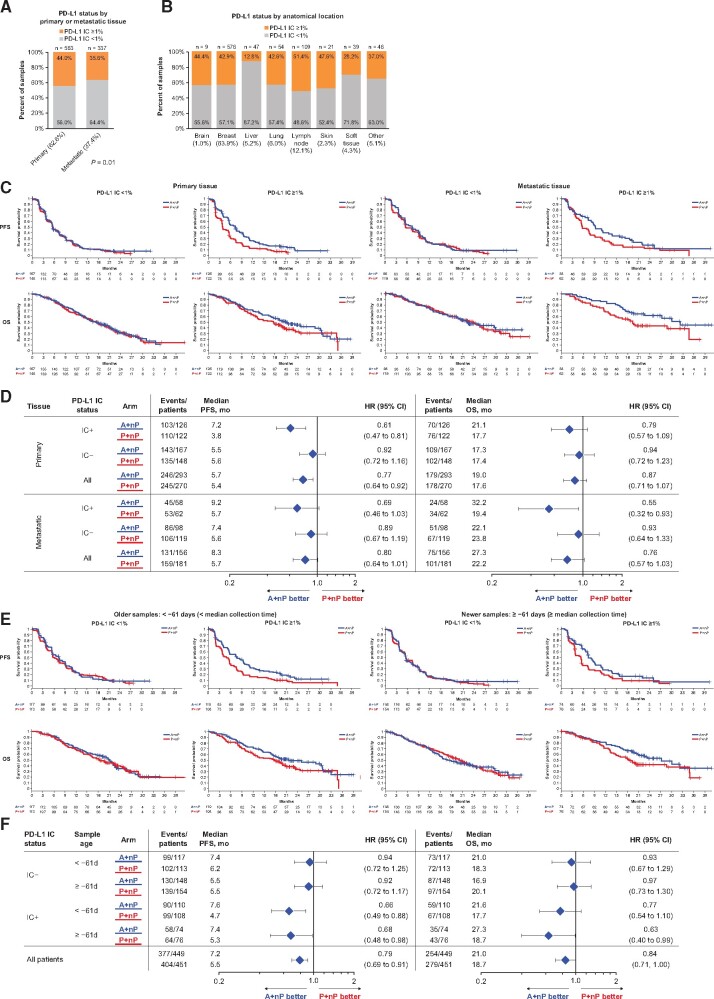
PD-L1 IC Status by Sample Location, Disease Stage, and Collection Time
Out of 900 annotated cases, 563 tissue samples (62.6%) were collected from primary tumors and 337 (37.4%) from metastases (Figure 2, A). The PD-L1 IC+ prevalence was higher in primary tumor samples (44.0% [248 of 563 patients]) than in metastatic cases (35.6% [120 of 337 patients]; P = .01). PD-L1 IC+ prevalence varied by anatomical location, with lymph nodes having the highest IC+ prevalence (51.3%) and liver lesions the lowest (12.8%; Figure 2, B). In matched sample pairs collected on the same day, PD-L1 IC+ status was concordant in 92.6% of cases (63 of 68; Supplementary Table 3, available online). In contrast, PD-L1 IC+ status was concordant in 54.1% (20 of 37) of matched primary and metastatic samples collected at different time points; no clear directionality of PD-L1 status change was observed over time in the asynchronous primary and metastatic pairs (Supplementary Table 4, available online). The median time from sample collection to randomization was 61 days (interquartile range [IQR] = 35-555 days; range = 7-3984 days; Supplementary Figure 2, available online). Improved PFS and OS with A+nP was observed in PD-L1 IC+ patients regardless of sample collection time and whether primary or metastatic tissue was PD-L1 IC+ (Figure 2, C−F).
Figure 2.
PFS and OS grouped by PD-L1 IC, tissue origin, and sample collection timing. A-B) Expression of PD-L1 on IC based on primary or metastatic source and anatomical location. Kaplan-Meier plots are shown based on PD-L1 IC and (C-D) biopsy origin (primary tumor vs metastases) or (E-F) sample collection timing. A = atezolizumab; CI = confidence interval; HR = hazard ratio; IC = tumor-infiltrating immune cells; IC+ = PD-L1 ≥ 1% on IC; IC− = PD-L1 < 1% on IC; nP = nab-paclitaxel; OS = overall survival; P = placebo; PD-L1 = programmed death-ligand 1; PFS = progression-free survival.
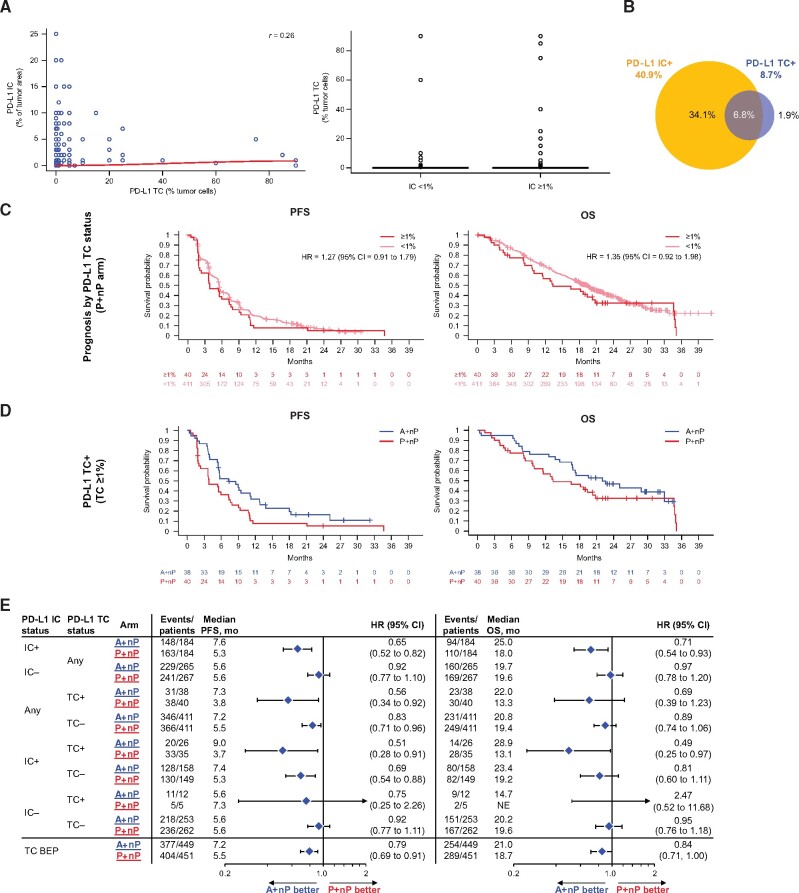
PD-L1 TC Expression and Clinical Outcomes
We also evaluated PD-L1 expression on TC, using the 1% cutoff value similar to IC, although PD-L1-expressing TC were scored by the proportion of tumor cells rather than a percentage of the tumor area. PD-L1 IC and TC were weakly correlated as continuous variables (r = 0.26; Figure 3, A). Consistent with previous reports (12,22), PD-L1 TC+ (≥1%) prevalence was low, with only 8.7% (78 of 900) of IMpassion130 TNBC samples classified as PD-L1 TC+ (Figure 3, B). Of the 78 PD-L1 TC+ samples, most were also PD-L1 IC+ (61 PD-L1 TC+/IC+ and 17 TC+/IC- samples). No difference in PFS and OS was observed between TC+ and TC- patients in the P+nP arm (Figure 3, C). We found that patients with PD-L1 TC+ tumors had improved PFS and OS outcomes with A+nP (Figure 3, D and E) and therefore assessed the potential link between PD-L1 TC+ and/or PD-L1 IC+ and clinical activity. PFS hazard ratios clearly favored A+nP vs P+nP in both TC+ and TC- patients who were IC+, with greatest benefit for IC+/TC+ patients. PFS benefit was not observed in IC- patients, although it should be noted that patient numbers were small in the IC-/TC+ subset. OS data showed similar patterns (Figure 3, E).
Figure 3.
Efficacy analyses in patient subgroups defined by PD-L1 expression on tumor cells. A) Correlation between PD-L1 IC+ as a percentage of tumor area and percentage of PD-L1 TC+ (left); distribution of PD-L1 IC as percentage of tumor area by PD-L1 TC status (right). B) Overlap of PD-L1 TC+ (≥1% TC) with PD-L1 IC+. C) PFS and OS Kaplan-Meyer survival curves by of PD-L1 TC status (<1% vs ≥1%) in P+nP arm. D) PFS and OS Kaplan-Meier curves for PD-L1 TC+ in A+nP vs P+nP arms. E) Forest plots of PFS and OS in PD-L1 IC/TC-defined patient subgroups. Analyses were adjusted for prior taxane treatment and liver metastases. All P values are for descriptive purposes only. A = atezolizumab; CI = confidence interval; HR = hazard ratio; IC = tumor-infiltrating immune cells; IC+ = PD-L1 ≥ 1% on IC; IC− = PD-L1 < 1% on IC; nP= nab-paclitaxel; OS = overall survival; P = placebo; PD-L1 = programmed death-ligand; PFS = progression-free survival; r = Spearman correlation index; TC = tumor cells; TC+ = PD-L1 ≥ 1% on TC; TC−= PD-L1 < 1% on TC.
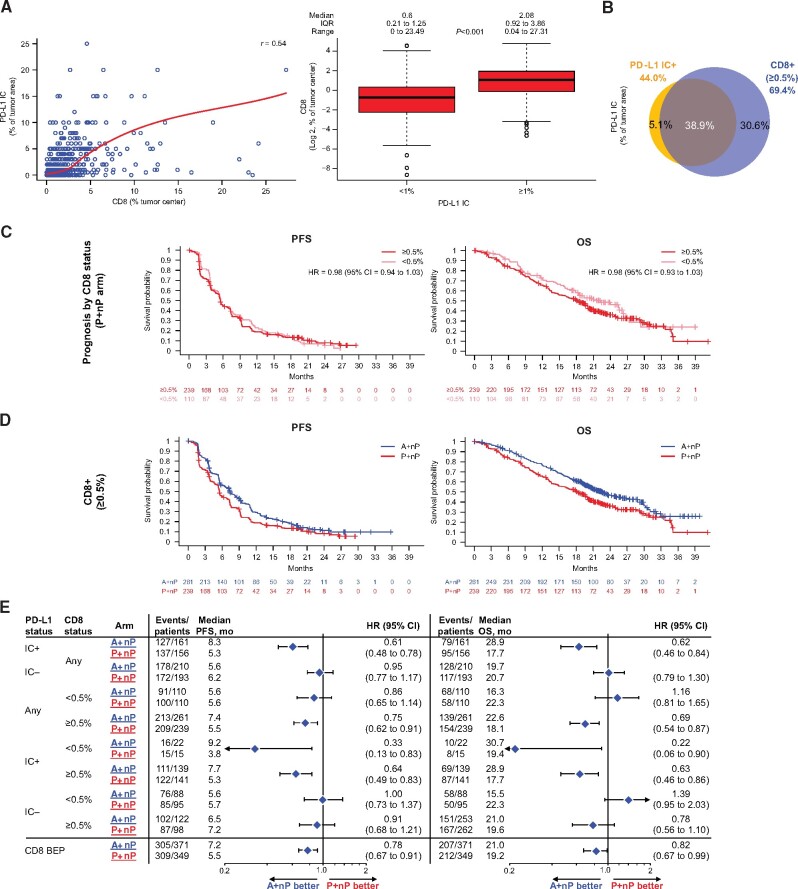
CD8+ T Cells and Clinical Outcomes
In a previous phase 1b study, CD8+ T cells at a 0.5% cutoff were associated with improved clinical activity with A+nP in mTNBC (22). In IMpassion130, CD8-BEP (79.8% [720 of 902] of ITT patients; Supplementary Figure 3, available online), the percentages of CD8+ T cells and IC expressing PD-L1 were moderately correlated when evaluated as continuous variables (r = 0.54; Figure 4, A). In IMpassion130, we used the 0.5% CD8+ T-cell cutoff suggesting differentiation in clinical activity in the phase 1b study (22) and found that 69.4% (500 of 720) of the IMpassion130 tumor samples were CD8+ (median CD8+ value = 1.0% [IQR = 0.4%-2.5%; range = 0%-27.3%]). In total, 38.9% (280 of 720) of tumors were both PD-L1IC+ and CD8+ (Fisher exact test P < .001; Figure 4, B). No difference in PFS and OS was observed between CD8+ and CD8- patients in the P+nP arm (Figure 4, C). Clinical activity favoring A+nP vs P+nP was observed in patients with PD-L1 IC+ tumors independent of CD8 status, and in CD8+ patients, improved outcomes were only seen when patients were also PD-L1 IC+ (Figure 4, D and E). Similar patterns were observed with OS. No PFS or OS improved outcome was observed in PD-L1 IC-/CD8- patients.
Figure 4.
Efficacy analyses in patient subgroups defined by tumor-infiltrating CD8+ T cells. A) Correlation between CD8 (as a percentage of tumor center) and PD-L1 IC as a percentage of tumor area (left); distribution of CD8 (log2) by PD-L1 IC status (right). B) Overlap of CD8+ (≥0.5%) with PD-L1 IC+. C) PFS and OS Kaplan-Meyer survival curves by CD8 status (<0.5% vs ≥0.5%) in P+nP arm. D) PFS and OS Kaplan-Meier curves for CD8+ in A+nP or P+nP arms. E) Forest plots of PFS and OS in CD8- and PD-L1 IC-defined patient subgroups. Analyses adjusted for prior taxane treatment and liver metastases. All P values are for descriptive purposes only. A = atezolizumab; BEP = biomarker-evaluable population; CI = confidence interval; HR = hazard ratio; IC = tumor-infiltrating immune cells; IC+ = PD-L1 ≥ 1% on IC; IC− = PD-L1 < 1% on IC; IQR= interquartile range; nP = nab-paclitaxel; OS = overall survival; P = placebo; PD-L1 = programmed death-ligand 1; PFS = progression-free survival; r = Spearman correlation index.
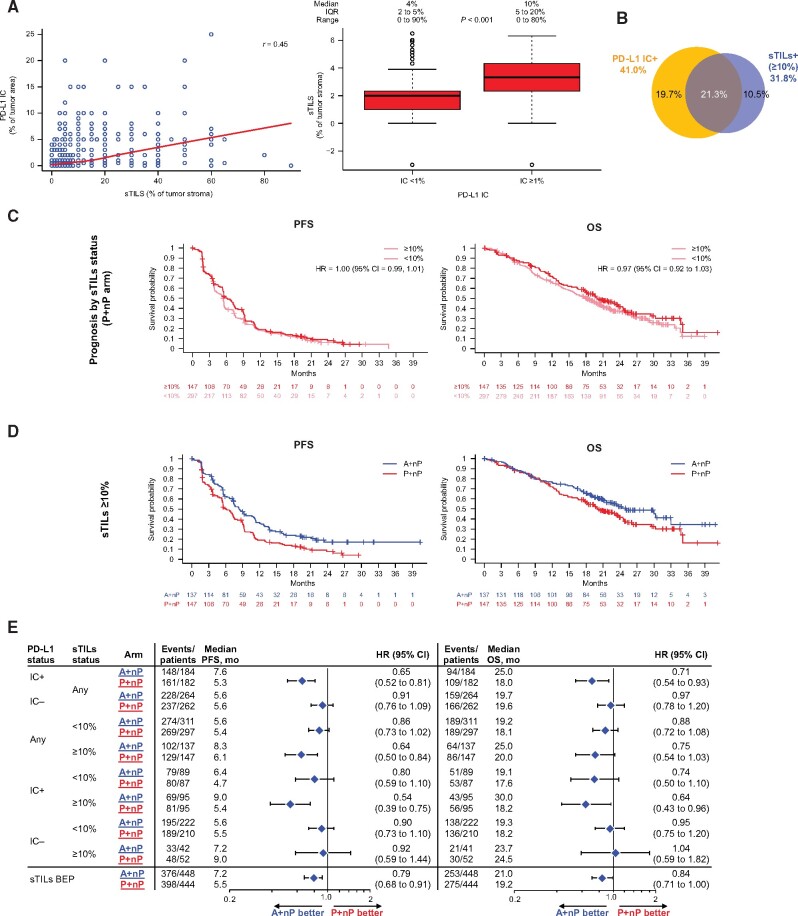
Stromal Tumor-Infiltrating Lymphocytes and Clinical Outcomes
sTILs have been associated with improved clinical outcomes with chemotherapy in early TNBC (eTNBC) and with PD-L1/PD-1 antagonists in mTNBC (15,22), including the phase 1 b study of A+nP in mTNBC. In the IMpassion130 sTILs-BEP (98.9% [892 of 902] of ITT patients), consistent with previous reports (23,24), sTIL counts were lower in metastatic than in matched primary tissue (data not shown). The median value of sTILs was 5% (IQR = 3%-10%; range = 0%-90%), sTILs and PD-L1 IC were moderately correlated as continuous values (Spearman correlation index r = 0.45), and PD-L1 IC+ tumors harbored more sTILs than IC- tumors (Figure 5, A). A prespecified threshold of 10% or more previously shown to be associated with improved clinical outcomes with chemotherapy in the neoadjuvant setting (21) was used to determine the role of sTILs in IMpassion130; 31.8% (284 of 892) of tumor samples were sTILs+. The sTIL levels were associated with PD-L1 IC+ status (Fisher exact test P < .001), and 66.9% (190 of 284) of sTIL+ cases were also PD-L1 IC+ (Figure 5, B).
Figure 5.
Efficacy analyses in patient subgroups defined by stromal TILs. A) Correlation between sTILs and PD-L1 IC (left); distribution of TILs (log2) by PD-L1 IC status (right). B) Overlap of sTILs+ (≥10%) with PD-L1 IC+. C) PFS and OS Kaplan-Meyer survival curves by sTILs status in P+nP arm. D) PFS and OS Kaplan-Meier curves by sTILs status in A+nP or P+nP arms. E) Forest plots of PFS and OS in sTIL- and PD-L1 IC-defined patient subgroups. Analyses were adjusted for prior taxane treatment and liver metastases. All P values are for descriptive purposes only. A = atezolizumab; BEP = biomarker-evaluable population; CI = confidence interval; HR = hazard ratio; IC = tumor-infiltrating immune cells; IC+ = PD-L1 ≥ 1% on IC; IC− = PD-L1 < 1% on IC; IQR = interquartile range; nP = nab-paclitaxel; OS = overall survival; P = placebo; PD-L1 = programmed death-ligand 1; PFS = progression-free survival; r = Spearman correlation index; sTIL = stromal tumor-infiltrating lymphocyte.
Although sTILs portend a good prognosis in eTNBC (21,25), sTILs did not appear to impact the PFS or OS in patients with mTNBC from the IMpassion130 comparator P+nP arm for PFS or OS (Figure 5, C). Compared with patients whose tumors lacked sTILs, sTIL+ patients had longer PFS and OS with A+nP (Figure 5, C and D); however, similar to observations with PD-L1 TC+ and CD8+ status, patients with sTILs+ tumors only profit from A+nP (Figure 5, E) if their tumors were also PD-L1 IC+. Improved PFS or OS outcomes with A+nP were not observed in patients negative for both biomarkers (48.4% [432 of 892 patients]). Collectively, these data suggest that although PD-L1 TC, CD8 T cells, and sTILs were correlated to PD-L1 IC, these immune biomarkers do not provide additional associative value beyond PD-L1 IC status in identifying patients who can potentially benefit from A+nP in mTNBC.
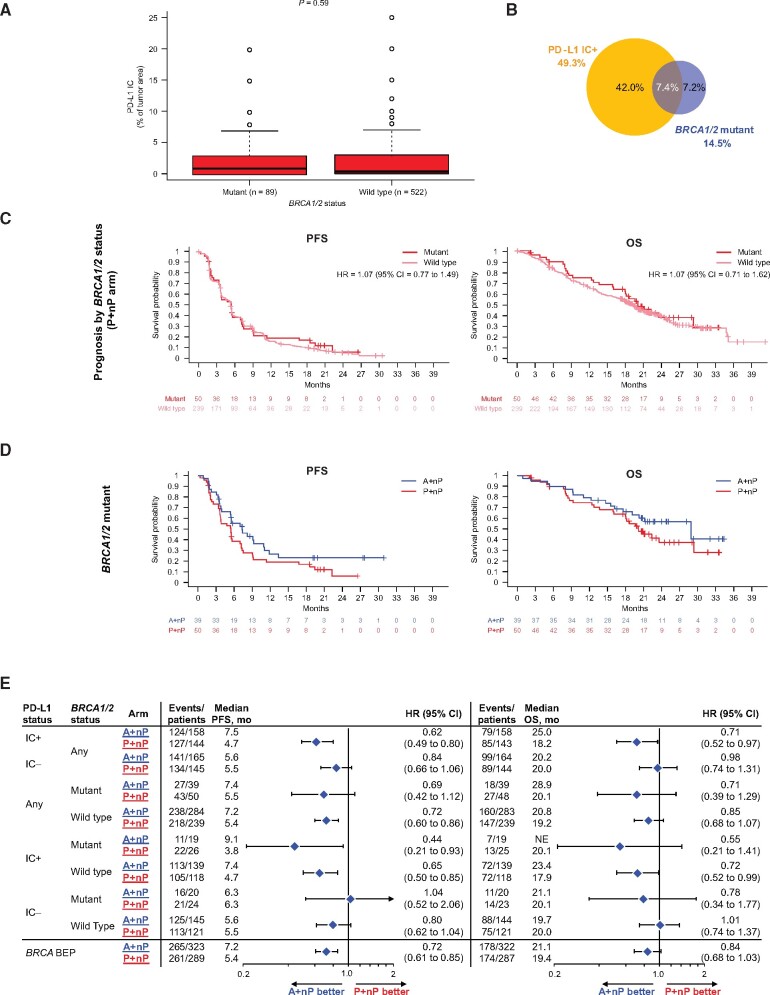
BRCA1/2 Mutation Status and Clinical Outcomes
BRCA1/2 mutation status was evaluable in 67.8% (612 of 902) of the ITT population. Deleterious BRCA1/2 mutations (BRCA1/2mut) were observed in 14.5% of patients (89 of 612), of whom 59 had a BRCA1 mutation alone, 28 had BRCA2 mutation alone, and 2 harbored mutations in both genes. The type of BRCA mutation (germline or somatic) was evaluable for 77.5% (69 of 89) of the BRCA1/2mut samples: 71.0% (49 of 69) were germline mutated, and 29.0% (20 of 69) had somatic alterations. BRCA1/2 deleterious mutations were not linked to PD-L1 IC status (P = .87; Figure 6, A and B), whereas PD-L1 IC status was equally distributed in the 89 BRCA1/2mut patients (50.6% and 49.4%, respectively) in this mTNBC sample dataset. BRCA1/2mut status was not associated with improved PFS or OS outcome in the P+nP arm (Figure 6, C), and hazard ratio point estimates in PD-L1 IC+ patients favored A+nP regardless of BRCA1/2mut status (Figure 6, D and E). Together, these data suggest that deleterious BRCA1/2 mutations may not be prognostic in mTNBC and that PD-L1 IC+ patients derived comparable clinical benefit with A+nP regardless of BRCA1/2 mutation status.
Figure 6.
Efficacy analyses in patient subgroups defined by PD-L1 on IC and deleterious BRCA1/2 mutations. A) Distribution of PD-L1 IC by BRCA mutation status. B) Prevalence of tumors with deleterious BRCA1/2 mutations and its overlap with PD-L1 IC+ cases. C) Kaplan-Meier curves for PFS and OS by BRCA1/2 mutation status in patients treated with P+nP. D) PFS and OS Kaplan-Meier curves for patients bearing tumors with BRCA1/2 mutations treated with A+nP or P+nP. E) Forest plots of PFS and OS by BRCA1/2 mutation status and PD-L1 IC-defined patient subgroups. Analyses were adjusted for prior taxane treatment and liver metastases. All P values are for descriptive purposes only. A = atezolizumab; BEP = biomarker-evaluable population; CI = confidence interval; HR = hazard ratio; IC = tumor-infiltrating immune cells; IC+ = PD-L1 ≥ 1% on IC; IC− = PD-L1 < 1% on IC; IQR = interquartile range; NE = not evaluable; nP = nab-paclitaxel; OS = overall survival; P = placebo; PD-L1 = programmed death-ligand 1; PFS = progression-free survival; r = Spearman correlation index.
Discussion
In the phase 3 IMpassion130 study, clinical benefit with A+nP was driven by the PD-L1 IC+ population (8,9). In the exploratory analyses presented here, we evaluated PD-L1 IC status along with other biomarkers potentially relevant to mTNBC. If multiple samples were available from a patient, the sample with the highest PD-L1 IC score was used for PD-L1 status designation, independent of disease stage or collection time. Although samples collected from the same lesion or on the same date were more likely to have the same PD-L1 IC status, PD-L1 IC expression might vary between primary and metastatic samples in our small substudy. Furthermore, PD-L1 expression varied by anatomical location, where we observed the lowest prevalence of PD-L1 IC+ status in liver metastases and the highest prevalence in lymph nodes; the variability in PD-L1 expression by metastatic site warrants further investigation. These analyses suggest that patients with PD-L1 IC+ status derived clinical benefit from A+nP regardless of sample collection time or type (primary vs metastatic tissue sample origin). Notably, whether the clinical benefit of PD-L1 IC status is definitively associated with any of these variables can be properly addressed only in the context of a prospective study collecting both primary archival and fresh metastatic samples.
The PD-L1 IC+ expression level (≥1%) did not appear to influence A+nP efficacy, as comparable improved PFS and OS hazard ratios were observed in patients with PD-L1 IC+ low (≥1% and <5%) or PD-L1 IC+ high (≥5%) status. The potential for clinical benefit with A+nP thus appears to be binary, with PD-L1 IC at 1% representing the threshold PD-L1 expression level associated with the clinical activity of A+nP. Consistent with previous observations in mTNBC (26,27), most PD-L1 TC+ tumors were also PD-L1 IC+. Despite this association, previous phase 1 data on single-agent atezolizumab in mTNBC (12) and our analyses of data from IMpassion130 patients show that PD-L1 on TC per se is not associated with the clinical activity of A+nP.
It was suggested based on descriptive signals that other immune biomarkers (sTILs, CD8+ T cells) were also associated with A+nP clinical activity, but patients whose tumors expressed these biomarkers in the context of PD-L1 IC positivity derived the greatest clinical improvements with A+nP. In contrast, atezolizumab and pembrolizumab mTNBC monotherapy studies showed that clinical activity was highest when CD8+ T cells and/or sTILs were present (12,15,28). Notably, the highest clinical activity with A+nP in IMpassion130 was observed in patients with tumors that were PD-L1 IC+ and either PD-L1 TC+ or with 10% or more sTILs. This observation raises interest in identifying the specific immune cells targeted by atezolizumab to trigger the antitumor immune response.
In eTNBC, the presence of sTILs and CD8+ T cells is associated with good prognosis (21,29,30). In contrast, sTILs and CD8+ T cells were not associated with longer PFS or OS in the IMpassion130 placebo plus nab-paclitaxel arm. It remains to be addressed whether the discrepancies in prognosis of these biomarkers between early and metastatic disease are because of the tumor and host immune biology, the type of therapies used in different disease settings (ie, limited cyclophosphamide and anthracycline use in mTNBC), the overall disease burden, or other host factors. Of note, intratumoral immune activation by ado-trastuzumab emtansine plus atezolizumab in HER2-positive metastatic breast cancer was subdued compared with early breast cancer (31), hinting at potential differences in immune biology between early and metastatic breast cancer.
We also evaluated BRCA1/2 mutation status in a subgroup of IMpassion130 patients, because patients harboring these alterations can benefit from PARP inhibitors, which are approved treatment options for BRCA1/2mut mTNBC (6,7). Although based on small patient numbers, our exploratory analyses suggest that improved PFS and OS with A+nP in PD-L1 IC+ patients was observed regardless of BRCA1/2 mutation status. Exploratory analyses of the OlympiAD trial in a small number of patients suggest a possible OS benefit if olaparib is used for first-line treatment of germline BRCAmut mTNBC (32). Our finding that the association of PD-L1 IC+ with clinical benefit from immunotherapy regardless of BRCA mutation status may guide the sequencing of A+nP and PARP inhibitors in patients who are both PD-L1 IC+ and germline BRCAmut.
Our exploratory analyses confirmed that although PD-L1 IC+ was the most informative biomarker associated with the clinical activity of A+nP in IMpassion130, patients with immune-rich tumor microenvironment, by also coexpressing other immune biomarkers, benefit the most from A+nP. Overall, patients with newly diagnosed mTNBC should be routinely tested for PD-L1 IC status with the clinically validated companion diagnostic (VENTANA PD-L1 SP142 assay) to determine whether they could benefit from first-line treatment with A+nP. Analyses and comparisons with other PD-L1 IHC assays are ongoing.
Funding
This work was supported by F. Hoffmann-La Roche Ltd /Genentech, Inc, a member of the Roche Group, which also funded medical writing support.
Notes
Role of the funder: F. Hoffmann-La Roche/Genentech provided atezolizumab and the matched placebo and collaborated with academic authors regarding the trial design, data collection, analysis, and interpretation. Celgene provided nab-paclitaxel but had no role in trial design, data collection, or analysis, although the company did review the manuscript.
Disclosures: All authors received grants and nonfinancial support from F. Hoffmann-La Roche during the conduct of the study. Editorial support, funded by the sponsor, was provided by an independent medical writer under the guidance of the authors. Leisha A. Emens is co-chair of the steering committee for the IMpassion130 study and chair of the KATE2 study steering committee; reports honoraria from AbbVie, Amgen, Celgene, Chugai, Gritstone, MedImmune, Peregrine, Shionogi, and Syndax; honoraria and travel support from AstraZeneca, Bayer, MacroGenics, Replimune, Vaccinex; travel support from Bristol Myers Squibb, Genentech/Roche, and Novartis; has potential future stock from Molecuvax; reports institutional support from Aduro Biotech, AstraZeneca, Bolt, the Breast Cancer Research Foundation, Bristol Myers Squibb, Corvus, the US Department of Defense, EMD Serono, Genentech, Maxcyte, Merck, the National Cancer Institute, the NSABP Foundation, Roche, Silverback, Tempest, the Translational Breast Cancer Research Consortium, and HeritX; and reports royalties from Aduro. Luciana Molinero is an employee of Roche/Genentech; reports stock ownership in Roche; and has a use patent to disclose with Roche/Genentech. Sherene Loi reports research funding to her institution from Novartis, Bristol Myers Squibb, Merck, Roche, Genentech, Puma, Pfizer, and Eli Lilly; has acted as an unpaid consultant to Seattle Genetics, Pfizer, Novartis, Bristol Myers Squibb, Merck, AstraZeneca, and Roche/Genentech; and acted as consultant to Aduro Biotech (fees paid to her institution). Hope S. Rugo reports research support for the IMpassion130 study and editorial support from F. Hoffmann-La Roche; institutional support from Pfizer, Novartis, Eli Lilly, Merck, OBI, Eisai, and Plexxikon; institutional and travel support from Genentech/Roche and MacroGenics; travel support from Puma, Mylan, Novartis, and Pfizer; research support from Immunomedics; research and travel support from Daiichi Sankyo; and honoraria from Celltrion. Andreas Schneeweiss reports research grants from Celgene, F. Hoffmann-La Roche, AbbVie, and Molecular Partners; consulting fees and travel expenses from F. Hoffmann-La Roche and AstraZeneca; honoraria from F. Hoffmann-La Roche, Celgene, AstraZeneca, Novartis, Merck Sharp and Dohme, Tesaro, and Eli Lilly; and honoraria and travel expenses from Pfizer. Véronique Diéras reports honoraria for serving on advisory boards for F. Hoffmann-La Roche, Genentech, Pfizer, Eli Lilly, Novartis, Daiichi Sankyo, AstraZeneca, AbbVie, and Odonate. Hiroji Iwata reports honoraria, consulting fees, and research support for the IMpassion130 study; editorial support from F. Hoffmann-La Roche and Chugai; serves as an uncompensated member of the steering committee for the IMpassion130 trial; and reports honoraria and consulting fees from Novartis, AstraZeneca, Pfizer, Eli Lilly, and Daiichi Sankyo. Carlos H. Barrios reports research grants from Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Roche/Genentech, Eli Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas, BioMarin, Bristol Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana BioSciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, and Millennium; and consulting fees from Roche/Genentech, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Eisai, Bayer, Merck Sharp and Dohme, and AstraZeneca. Marina Nechaeva has nothing else to disclose. Anh Nguyen Duc, Stephen Y. Chui, and Amreen Husain report employment at and received stock from Genentech/Roche during the conduct of the study. Eric P. Winer reports honoraria from Eli Lilly, Leap, Genentech, Infinite MD, Carrick Therapeutics, GlaxoSmithKline, Jounce, Genomic Health, Merck, and Seattle Genetics and is a scientific advisory board member for Leap. Sylvia Adams reports uncompensated consulting or advisory roles with Bristol Meyers Squibb, Genentech, and Merck and reports research funding from Amgen, Bristol Meyers Squibb, Celgene, Genentech, Merck, and Novartis. Peter Schmid reports research support (grants) to his institution from Genentech, F. Hoffmann-La Roche, Oncogenex, and Novartis; reports honoraria from AstraZeneca, F. Hoffmann-La Roche, Medscape, and GI Therapeutics; reports a consulting or advisory role with AstraZeneca, Novartis, F. Hoffmann-La Roche, Merck, Boehringer Ingelheim, Bayer, Eisai, Celgene, Pfizer, and Puma; is an uncompensated steering committee member for the IMpassion130 trial; and reports that his spouse has a consulting role for Genentech and F. Hoffmann-La Roche.
Prior presentations: Data from this manuscript were presented in part at the San Antonio Breast Cancer Symposium 2018; December 4-8, 2018; San Antonio, TX, and at the European Society for Medical Oncology Annual Meeting; September 27 to October 1, 2019; Barcelona, Spain.
Author contributions: Leisha A. Emens: Conceptualization, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision, Project Administration. Luciana Molinero: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision, Project Administration, Funding Acquisition. Sherene Loi: Conceptualization, Methodology, Investigation, Resources, Writing—Review and Editing, Supervision. Hope S. Rugo: Conceptualization, Investigation, Resources, Writing—Review and Editing, Visualization. Andreas Schneeweiss: Conceptualization, Methodology, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision. Véronique Diéras: Resources, Writing—Review and Editing. Hiroji Iwata: Investigation, Resources, Writing—Review and Editing. Carlos H. Barrios: Conceptualization, Methodology, Formal Analysis, Investigation, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision. Marina Nechaeva: Writing—Review and Editing. Anh Nguyen Duc: Methodology, Software, Validation, Formal Analysis, Writing—Review and Editing, Visualization. Stephen Y. Chui: Conceptualization, Methodology, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision, Project Administration, Funding Acquisition. Amreen Husain: Writing—Review and Editing. Eric P. Winer: Writing—Review and Editing. Sylvia Adams: Conceptualization, Formal Analysis, Investigation, Resources, Data Curation, Writing—Review and Editing. Peter Schmid: Conceptualization, Investigation, Resources, Writing—Original Draft, Writing—Review and Editing, Visualization, Supervision, Project Administration.
Acknowledgments: This study was sponsored by F. Hoffmann-La Roche Ltd/Genentech, Inc, a member of the Roche Group. We thank the patients participating in this trial and their families and the nurses, research coordinators, data managers, and clinical study site investigators. Medical writing assistance for this manuscript was provided by Steffen Biechele, PhD, and Ashley J. Pratt, PhD, of Health Interactions, Inc, and funded by F. Hoffmann-La Roche, Ltd.
Data Availability
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Supplementary Material
References
- 1. Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol. 2018;29(8):1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yardley DA, Coleman R, Conte P, et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol. 2018;29(8):1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer, V1. National Comprehensive Cancer Network; 2019.
- 4. Miles DW, Dieras V, Cortes J, et al. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24(11):2773–2780. [DOI] [PubMed] [Google Scholar]
- 5.Avastin (bevacizumab) [summary of product characteristics]. Grenzach-Wyhlen, Germany; Roche Registration GmbH; 2017. https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf. Accessed September 1, 2020.
- 6. Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. [DOI] [PubMed] [Google Scholar]
- 7. Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–2121. [DOI] [PubMed] [Google Scholar]
- 9. Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. [DOI] [PubMed] [Google Scholar]
- 10. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahmoud S, Lee A, Ellis I, et al. CD8(+) T lymphocytes infiltrating breast cancer: a promising new prognostic marker? Oncoimmunology. 2012;1(3):364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. [DOI] [PubMed] [Google Scholar]
- 14. Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loi S, Adams S, Schmid P, et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): results from KEYNOTE-086. Ann Oncol .2017;28(suppl 5):V608. [Google Scholar]
- 16. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. [DOI] [PubMed] [Google Scholar]
- 17. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 18. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27(2):92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26(2):259–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Denkert C, von Minckwitz G, Darb-Esfahani S, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40–50. [DOI] [PubMed] [Google Scholar]
- 22. Adams S, Diamond JR, Hamilton E, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 2019;5(3):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutchinson KE, Yost SE, Chang CW, et al. Comprehensive profiling of poor-risk paired primary and recurrent triple-negative breast cancers reveals immune phenotype shifts. Clin Cancer Res. 2020;26(3):657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29(11):2232–2239. [DOI] [PubMed] [Google Scholar]
- 25. Loi S, Drubay D, Adams S, et al. Tumor-infiltrating lymphocytes and prognosis: a pooled individual patient analysis of early-stage triple-negative breast cancers. J Clin Oncol. 2019;37(7):559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Chang CW, Tran D, et al. Prevalence of PDL1 and tumor infiltrating lymphocytes (TILs) in primary and metastatic TNBC. Cancer Res. 2018;78(suppl 4):PD6–01. [Google Scholar]
- 27. Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol. 2016;47(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–411. [DOI] [PubMed] [Google Scholar]
- 29. Ali HR, Provenzano E, Dawson SJ, et al. Association between CD8 T-cell infiltration and breast cancer survival in 12 439 patients. Ann Oncol. 2014;25(8):1536–1543. [DOI] [PubMed] [Google Scholar]
- 30. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25(8):1544–1550. [DOI] [PubMed] [Google Scholar]
- 31. Hamilton EP, Kaklamani V, Falkson C, et al. PD1-05. Atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer: safety and biomarker outcomes from a multi-cohort phase Ib study. In: Proceedings of the 2019 San Antonio Breast Cancer Symposium; December 10-14, 2019; San Antonio, TX. Cancer Res. 2020;80(suppl 4):PD1-05. [Google Scholar]
- 32. Robson ME, Tung N, Conte P, et al. OlympiAD final overall survival and tolerability results: olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019;30(4):558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).