Abstract
Background
Traditional count-based measures of comorbidity are unlikely to capture the complexity of multiple chronic conditions (multimorbidity) in older adults with cancer. We aimed to define patterns of multimorbidity and their impact in older United States veterans with multiple myeloma (MM).
Methods
We measured 66 chronic conditions in 5076 veterans aged 65 years and older newly treated for MM in the national Veterans Affairs health-care system from 2004 to 2017. Latent class analysis was used to identify patterns of multimorbidity among these conditions. These patterns were then assessed for their association with overall survival, our primary outcome. Secondary outcomes included emergency department visits and hospitalizations.
Results
Five patterns of multimorbidity emerged from the latent class analysis, and survival varied across these patterns (log-rank 2-sided P < .001). Older veterans with cardiovascular and metabolic disease (30.9%, hazard ratio [HR] = 1.33, 95% confidence interval [CI] = 1.21 to 1.45), psychiatric and substance use disorders (9.7%, HR = 1.58, 95% CI = 1.39 to 1.79), chronic lung disease (15.9%, HR = 1.69, 95% CI = 1.53 to 1.87), and multisystem impairment (13.8%, HR = 2.25, 95% CI = 2.03 to 2.50) had higher mortality compared with veterans with minimal comorbidity (29.7%, reference). Associations with mortality were maintained after adjustment for sociodemographic variables, measures of disease risk, and the count-based Charlson Comorbidity Index. Multimorbidity patterns were also associated with emergency department visits and hospitalizations.
Conclusions
Our findings demonstrate the need to move beyond count-based measures of comorbidity and consider cancer in the context of multiple chronic conditions.
Older adults make up the growing majority of patients newly diagnosed with cancer, expected to comprise 75% of all cancer survivors by the year 2040 (1). This is especially true for multiple myeloma (MM), where the median age at diagnosis is currently 69 years and increasing (2). Comorbidities have been traditionally measured in oncology as either a simple function of the number of comorbid conditions or a count-based index (3-6). These count-based comorbidity measures have then been used as a covariate for case-mix adjustment in trials and observational studies primarily focused on the independent effects of a particular cancer therapy or exposure (7). However, for older patients, comorbidities such as advanced cardiovascular disease, neuropsychiatric disorders, and multi-system diagnoses can each by themselves or collectively pose a threat equal to their cancer (8-11).
Accordingly, MM and its treatment must be considered in the context of multiple other chronic conditions, where disease–disease, disease–drug, and drug–drug interactions can be prevalent and hazardous (9). Count-based comorbidity measures do not capture how the particular combination or pattern of chronic conditions may complicate one’s care (12-14). Novel methods of defining and analyzing the complexity of multimorbidity in older adults with MM would allow for more personalized treatment decision making and supportive care interventions.
Older US veterans with MM are a prime example of a population needing more relevant measures of multimorbidity. Older veterans on average carry more chronic conditions and are even more underrepresented in clinical trials than the general US population (15,16). The objective of this study was to identify and define patterns of multimorbidity in veterans aged 65 years and older newly treated for MM in the national Veterans Affairs (VA) health-care system from 2003 to 2017. Moreover, we wished to assess the impact of these multimorbidity patterns on survival, unplanned hospitalizations, and emergency department (ED) visits. We hypothesized that older veterans have a number of distinct patterns of multimorbidity that affect outcomes. We further hypothesized that these multimorbidity patterns predict mortality beyond the Charlson Comorbidity Index, a widely used count-based comorbidity measure (17).
Methods
Data Source and Population
We designed a retrospective cohort study analyzing data collected from the VA Corporate Data Warehouse (CDW), which collects clinical, billing, and electronic health record information from veterans treated at VA facilities nationwide (18). To capture chronic conditions managed outside the VA, we linked the VA CDW with data from Centers for Medicare and Medicaid Services (CMS) (19). We selected for veterans newly treated for MM throughout the VA (see the Supplementary Methods, available online for our selection criteria). Treatments included hematopoietic stem cell transplant or medical therapy (any class of medical therapy for MM, including proteasome inhibitors, immune-modifying drugs, and chemotherapy).
This study was approved by the VA Boston Healthcare System Institutional Review Board.
Measurement of Chronic Conditions and Covariates
To measure chronic conditions in our population, we used the CMS Chronic Conditions Data Warehouse, which includes 66 chronic conditions that are tracked in administrative claims data with International Classification of Diseases (ICD)-9 and ICD-10 diagnostic and procedural codes (Supplementary Table 1, available online) (20). We used the lists of conditions that were present in the February 2, 2019, revision, with a few modifications (Supplementary Methods; Supplementary Table 2, available online). As a comparative comorbidity measure based on comorbidity count, we calculated the Charlson Comorbidity Index using the R comorbidity package based on ICD codes in the 3 years before index date (17,21).
Covariates were extracted from the VA CDW and included the sociodemographic variables age at initiation of treatment, sex, race, and income. Laboratory data related to myeloma stage and prognosis were measured in a time period starting 90 days before the index date, with the latest value being used. Myeloma stage was measured using the Myeloma International Staging System (22) based on serum albumin and beta-2 microglobulin. Calcium, creatinine, hemoglobin, and platelet levels were also measured using prespecified cutoffs validated in the literature (23). Specific myeloma therapies were measured at time of treatment initiation, defined as the first 90 days after the index date, and over the patient’s lifetime. Among these therapies, we classified novel therapy as any proteasome inhibitor (bortezomib, carfilzomib, or ixazomib) or immunomodulatory agent (thalidomide, lenalidomide, or pomalidomide).
Outcomes
Our primary outcome was overall survival, measured using death data in the VA CDW. Our secondary outcomes were ED visits and unplanned hospitalizations (admissions not prescheduled, eg, for elective surgery) within the VA health-care system, measured from the CDW. Veterans were followed until their last record in CDW or end of the study period (after which they were censored).
Statistical Analysis
Defining Patterns of Multimorbidity
To define patterns of multimorbidity present at the initiation of myeloma treatment, latent class analysis (LCA) was performed using the chronic conditions from the CCW measured in the entire population consisting of both transplant-eligible and -ineligible veterans. In brief, LCA is a data-driven method that identifies latent classes, in our case multimorbidity patterns, that best explain observed data, in our case chronic conditions (24,25). Similar to a prior analysis using LCA to define multimorbidity patterns in a general population of older adults (10), we examined 4 to 7 classes, choosing the final number of classes based on model fit (Bayesian information criterion) and clinical meaningfulness as assessed jointly by licensed geriatricians C.D. and D.K. We used the R poLCA package to fit LCA model (26). After obtaining a final model fit, each participant was assigned to a multimorbidity pattern based on the highest probability of class membership (Supplementary Methods, available online).
Analyzing the Impact of Multimorbidity on Mortality and Care Utilization
To minimize unmeasured confounding across patients who are eligible and ineligible for transplants, we restricted analyses of our outcomes to veterans who received medical myeloma therapy only. Kaplan-Meier analyses and log-rank tests were used to determine whether time to death, time to ED visit, and time to hospitalization varied across multimorbidity patterns. To assess for an independent association between multimorbidity patterns and outcomes, Cox proportional hazards regression models were used to estimate hazard ratios (HRs) for mortality, ED visits, and hospitalizations adjusting for age and other sociodemographic, myeloma stage, and laboratory covariates. The proportional hazards assumption was assessed graphically and with Schoenfeld residuals. There was no evidence that the assumption was violated. Multiple imputation using chained equations as implemented in the R MICE package was used to impute any baseline missingness in covariates from available baseline data (27,28). Analyses of outcomes were run on the imputed dataset, followed by a complete case analysis as a sensitivity analysis. As a secondary analysis to assess for the ability of multimorbidity patterns to predict our outcomes beyond a count-based comorbidity measure, separate fully adjusted models using the imputed dataset were additionally adjusted for the Charlson Index. In other fully adjusted models, we further tested for interactions between each pattern and age. All statistical tests were 2-sided, and a P value less than .05 was considered statistically significant. All analyses were performed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). In reporting this study, we followed the guidelines put forth by the Strengthening the Reporting of Observational Studies in Epidemiology Statement (29).
Results
Study Population
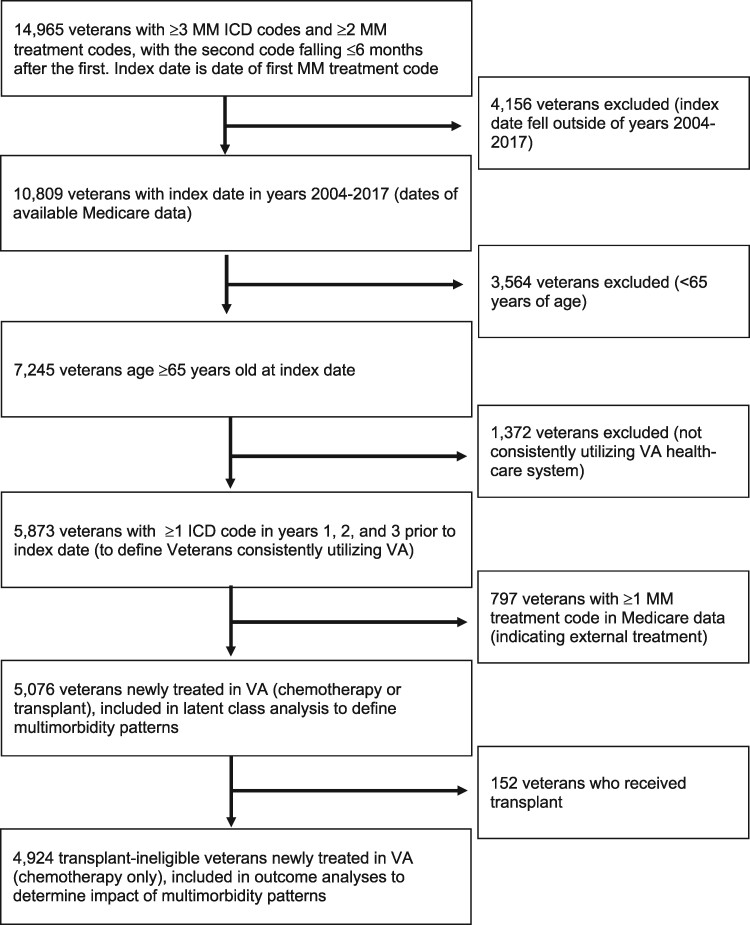
Figure 1 displays the selection of our study population within the VA CDW. We conducted the LCA to define patterns of multimorbidity in 5076 veterans newly treated in VA with either chemotherapy or transplant. After excluding transplant, we analyzed the impact of multimorbidity patterns on outcomes in 4924 veterans treated with medical therapies.
Figure 1.
Flow diagram showing inclusion of study population of veterans aged 65 years and older, with multiple myeloma (MM) newly treated in Veterans Affairs (VA). ICD = International Classification of Diseases.
Multimorbidity Patterns at Treatment Initiation
From the LCA, 5 multimorbidity patterns yielded the optimal balance between model fit and clinical meaningfulness. Increases in model fit were negligible with increasing the number of classes beyond 5 (Supplementary Figure 1, available online). The final model classified the majority of individuals in their respective patterns with a probability greater than or equal to 75% (Supplementary Table 3, available online). Supplementary Figure 2 (available online) is a heat map displaying the probabilities of each condition being present conditional on a veteran being assigned to each multimorbidity pattern, and Table 1 displays the 5 multimorbidity patterns and their defining and prevalent conditions.
Table 1.
Multimorbidity patterns and their defining and prevalent conditions among 5076 veterans with MM newly treated in Veterans Affairs
| Characteristic | Minimal comorbidity (n = 1507) | Cardiovascular and metabolic (n = 1568) | Psychiatric and substance use (n = 494) | Chronic lung disease (n = 805) | Multisystem impairment (n = 702) |
|---|---|---|---|---|---|
| Median No. of chronic conditions (IQR) | 6.00 (4.00-7.00) | 10.00 (8.00-11.00) | 11.00 (10.00-13.00) | 10.00 (9.00-12.00) | 15.00 (14.00-17.00) |
| Defining and prevalent conditions in each pattern |
Hypertension (71.5%) Hyperlipidemia (59.1%) Arthritis (39.8%) |
Diabetes (59.4%) Hyperlipidemia (87.7%) Ischemic heart disease (54.0%) Chronic kidney disease (61.7%) Cataracts (74.0%) |
Mood disordersa (93.5%) Bipolar disorder (24.3%) Fibromyalgia, chronic pain, and fatigue (37.9%) Alcohol use disorder (43.3%) Drug use disordersb (25.5%) Dementia (18.6%) |
COPD and bronchiectasis (100.0%) Asthma (17.1%) Tobacco (28.1%) HIV (42.1%) |
Ischemic heart disease (86.6%) Heart failure (73.8%) Atrial fibrillation (44.3%) Mood disorders (56.7%) COPD and bronchiectasis (71.2%) Chronic kidney disease (81.8%) |
Mood disorders include Centers for Medicare and Medicaid Chronic Condition Data Warehouse categories anxiety disorders, depression, depressive disorders, and posttraumatic stress disorder. COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency virus; IQR = interquartile range; MM = multiple myeloma.
Drug use disorders includes drug use disorders and opioid use disorder.
The first pattern (n = 1507, 29.7%) consisted of veterans who carried minimal comorbidity beyond their myeloma diagnosis, including hypertension, hyperlipidemia, arthritis, and cataracts. The second pattern (n = 1568, 30.9%) consisted of veterans with cardiovascular and metabolic diseases, including diabetes, ischemic heart disease, and chronic kidney disease. The third pattern (n = 494, 9.7%) consisted of veterans with psychiatric and substance use disorders, including mood disorders, chronic pain, drug (eg, opioid) use disorders, and alcohol use disorder. The fourth pattern (n = 805, 15.9%) consisted of veterans with chronic lung disease, including chronic obstructive pulmonary disease (COPD) and tobacco use disorder. Finally, the fifth pattern (n = 702, 13.8%) consisted of veterans with multisystem impairment, including cardiovascular disease (ischemic heart disease, heart failure, atrial fibrillation), lung disease (COPD), psychiatric disorders, and sensory impairments. All patterns had high prevalence of anemia (55.1%-91.2%). The complete distribution of chronic conditions by multimorbidity pattern is shown in Supplementary Table 4 (available online).
Table 2 displays the baseline characteristics of our population by multimorbidity pattern (with additional characteristics in Supplementary Table 5, available online). The majority of the population was male (98.6%). Compared with veterans with minimal comorbidity, veterans with multisystem impairment tended to be older (median age of 76.5 years vs 74.8 years) and carry a greater median number of comorbidities (15 vs 9). Mean income was lowest among veterans with psychiatric and substance use disorders ($33 529.48). Although there were minimal differences in the receipt of novel myeloma therapy across multimorbidity patterns (92.9% of all veterans received some form of novel therapy in their lifetime), veterans with multisystem impairment were the least likely to receive both lenalidomide and bortezomib during their induction treatment (8.3% vs 15.5%, respectively, in veterans with minimal comorbidity).
Table 2.
Baseline characteristics of older veterans newly treated for MM in the VA, according to 5 multimorbidity patterns that emerged from LCA
| Characteristic | Overall | Minimal comorbidity | Cardiovascular and metabolic | Psychiatric and substance use disorders | Chronic lung disease | Multisystem impairment |
|---|---|---|---|---|---|---|
| Total No. | 5076 | 1507 | 1568 | 494 | 805 | 702 |
| Median age at diagnosis (IQR), y | 74.8 (69.6-80.6) | 73.2 (68.6-78.8) | 76.0 (70.4-81.5) | 71.0 (67.7-76.7) | 76.5 (70.6-81.2) | 76.5 (71.3-82.3) |
| Male, No. (%) | 5006 (98.6) | 1488 (98.7) | 1544 (98.5) | 487 (98.6) | 797 (99.0) | 690 (98.3) |
| Race, No. (%) | ||||||
| White | 3388 (66.7) | 1034 (68.6) | 1002 (63.9) | 301 (60.9) | 572 (71.1) | 479 (68.2) |
| Black | 1136 (22.4) | 317 (21.0) | 387 (24.7) | 150 (30.4) | 138 (17.1) | 144 (20.5) |
| Other | 66 (1.3) | 20 (1.3) | 19 (1.2) | 8 (1.6) | 9 (1.1) | 10 (1.4) |
| Missing | 486 (9.6) | 136 (9.0) | 160 (10.2) | 35 (7.1) | 86 (10.7) | 69 (9.8) |
| Mean income (SD), $ | 43325.27 (74 118.31) | 47192.74 (78 126.27) | 45062.77 (81 577.57) | 33529.48 (53 647.44) | 38853.29 (57 720.40) | 43580.08 (76 743.92) |
| ISS stage, No. (%) | ||||||
| ISS 1 | 523 (10.3) | 223 (14.8) | 131 (8.4) | 64 (13.0) | 76 (9.4) | 29 (4.1) |
| ISS 2 | 958 (18.9) | 315 (20.9) | 273 (17.4) | 101 (20.4) | 162 (20.1) | 107 (15.2) |
| ISS 3 | 641 (12.6) | 164 (10.9) | 228 (14.5) | 68 (13.8) | 86 (10.7) | 95 (13.5) |
| Missing | 2954 (58.2) | 805 (53.4) | 936 (59.7) | 261 (52.8) | 481 (59.8) | 471 (67.1) |
| Calcium ≥11 mg/dL, No. (%) | 161 (3.2) | 50 (3.3) | 59 (3.8) | 15 (3.0) | 18 (2.2) | 19 (2.7) |
| Missing | 425 (8.4) | 148 (9.8) | 132 (8.4) | 20 (4.0) | 66 (8.2) | 59 (8.4) |
| Creatinine >2 mg/dL, No. (%) | 1009 (19.9) | 156 (10.4) | 392 (25.0) | 109 (22.1) | 138 (17.1) | 214 (30.5) |
| Missing | 350 (6.9) | 125 (8.3) | 106 (6.8) | 18 (3.6) | 50 (6.2) | 51 (7.3) |
| Hemoglobin <10 g/dL, No. (%) | 1770 (34.9) | 423 (28.1) | 615 (39.2) | 176 (35.6) | 262 (32.5) | 294 (41.9) |
| Missing | 349 (6.9) | 122 (8.1) | 98 (6.2) | 18 (3.6) | 57 (7.1) | 54 (7.7) |
| Platelet <150 000/microL, No. (%) | 1152 (22.7) | 306 (20.3) | 368 (23.5) | 116 (23.5) | 177 (22.0) | 185 (26.4) |
| Missing | 636 (12.5) | 209 (13.9) | 181 (11.5) | 55 (11.1) | 97 (12.0) | 94 (13.4) |
| Transplantation, No. (%) | 152 (3.0) | 85 (5.6) | 37 (2.4) | 17 (3.4) | 12 (1.5) | 1 (0.1) |
| Novel therapy at induction,a No. (%) | 4376 (86.2) | 1312 (87.1) | 1358 (86.6) | 434 (87.9) | 674 (83.7) | 598 (85.2) |
| Thalidomide | 1119 (22.0) | 319 (21.2) | 338 (21.6) | 77 (15.6) | 219 (27.2) | 166 (23.6) |
| Lenalidomide | 1950 (38.4) | 662 (43.9) | 591 (37.7) | 196 (39.7) | 286 (35.5) | 215 (30.6) |
| Bortezomib | 2069 (40.8) | 590 (39.2) | 667 (42.5) | 250 (50.6) | 275 (34.2) | 287 (40.9) |
| Thalidomide and bortezomib | 75 (1.5) | 23 (1.5) | 22 (1.4) | 10 (2.0) | 11 (1.4) | 9 (1.3) |
| Lenalidomide and bortezomib | 671 (13.2) | 234 (15.5) | 207 (13.2) | 81 (16.4) | 91 (11.3) | 58 (8.3) |
Therapy at induction is defined as therapy received in the first 90 days after the index date. Novel therapy is defined to include any proteasome inhibitor (bortezomib, carfilzomib, or ixazomib) or immunomodulatory agent (thalidomide, lenalidomide, or pomalidomide). IQR = interquartile range; ISS = International Staging System; LCA = latent class analysis; MM = multiple myeloma; VA = Veterans Affairs.
Multimorbidity Patterns and Mortality
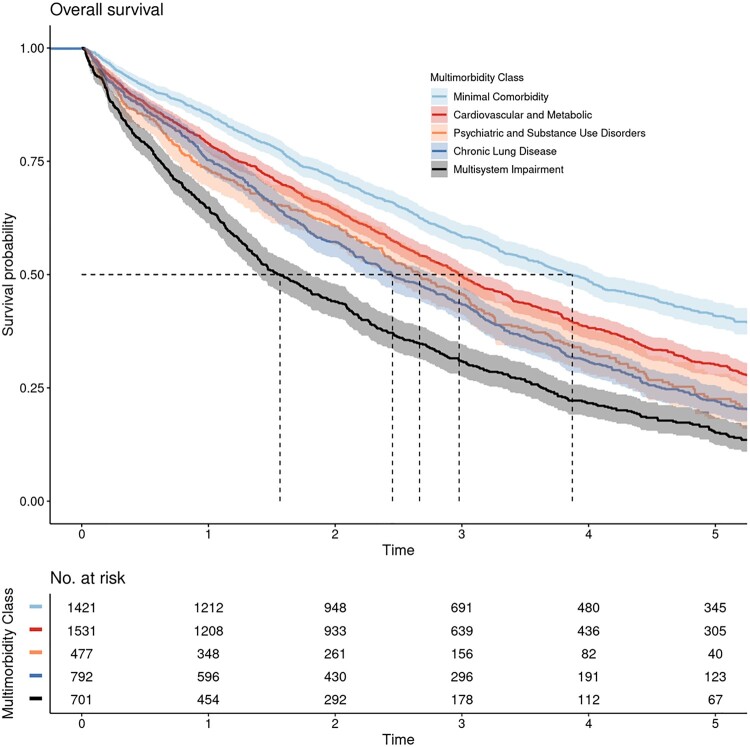
Median follow-up was 2.46 years (interquartile range = 1.18-4.29 years), and 3614 veterans (73.4%) died during the study period. Survival varied across multimorbidity patterns (log-rank test P < .001; Figure 2), with veterans with minimal comorbidity demonstrating the best survival (median survival = 3.87 years, 95% confidence interval [CI] = 3.61 to 4.11 years) and veterans with multisystem impairment demonstrating the worse survival (median survival = 1.56 years, 95% CI = 1.39 to 1.81 years). Veterans with cardiovascular and metabolic diseases (median survival = 2.98 years, 95% CI = 2.82 to 3.15 years), psychiatric and substance use disorders (median survival = 2.67 years, 95% CI = 2.39 to 3.03 years), and chronic lung disease (median survival = 2.45 years, 95% CI = 2.22 to 2.78 years) also demonstrated worse survival than veterans with minimal comorbidity.
Figure 2.
Kaplan-Meier curves demonstrating overall survival by multimorbidity pattern in older transplant-ineligible veterans newly treated for multiple myeloma in Veterans Affairs. Survival varied across patterns (log-rank 2-sided P < .001). Shaded areas around solid curves represent 95% confidence intervals. Dashed lines represent median survival for each multimorbidity class, as follows: minimal comorbidity = 3.87 years, diabetes and complications = 2.98 years, psychiatric and substance use disorders = 2.67 years, chronic lung disease = 2.45 years, and multisystem impairment = 1.56 years.
In univariate Cox regression models, veterans with cardiovascular and metabolic diseases (HR = 1.33, 95% CI = 1.21 to 1.45), psychiatric and substance use disorders (HR = 1.58, 95% CI = 1.39 to 1.79), chronic lung disease (HR = 1.69, 95% CI = 1.53 to 1.87), and multisystem impairment (HR = 2.25, 95% CI = 2.03 to 2.50) all had a higher hazard of death compared with veterans with minimal comorbidity. In multivariable Cox regression, associations with mortality were maintained after adjustment for baseline sociodemographic variables, Myeloma International Staging System, and prognostic laboratory studies (Table 3). The complete case analysis was similar (Supplementary Table 6, available online). In our secondary analysis, associations between multimorbidity patterns and mortality were maintained after further adjustment for the Charlson Index (Table 3). In our interaction analyses, the chronic lung disease pattern interacted with age in its effect on mortality (chronic lung disease × age HR = 0.98, 95% CI = 0.96 to 0.99), suggesting that increasing age had slightly less impact on hazard of death in veterans with chronic lung disease compared with veterans with minimal comorbidity. No other patterns interacted with age.
Table 3.
Multivariable Cox proportional hazards regression models estimating effect of multimorbidity patterns on overall mortality, hospitalizations, and ED visitsa
| Multimorbidity pattern | Mortality HR (95% CI) | ED visit HR (95% CI) | Hospitalization HR (95% CI) |
|---|---|---|---|
| Primary analysis | |||
| Minimal comorbidity | Reference | Reference | Reference |
| Cardiovascular and metabolic | 1.16 (1.06 to 1.26) | 1.22 (1.11 to 1.34) | 1.17 (1.07 to 1.29) |
| Psychiatric and substance use disorders | 1.62 (1.42 to 1.84) | 1.67 (1.47 to 1.90) | 1.52 (1.34 to 1.73) |
| Chronic lung disease | 1.52 (1.37 to 1.68) | 1.28 (1.15 to 1.44) | 1.44 (1.29 to 1.61) |
| Multisystem impairment | 1.89 (1.70 to 2.11) | 1.62 (1.44 to 1.82) | 1.55 (1.38 to 1.74) |
| Secondary analysis, further adjusting for Charlson Index | |||
| Minimal comorbidity | Reference | Reference | Reference |
| Cardiovascular and metabolic | 1.02 (0.93 to 1.13) | 0.94 (0.85 to 1.04) | 0.92 (0.84 to 1.02) |
| Psychiatric and substance use disorders | 1.42 (1.24 to 1.63) | 1.26 (1.10 to 1.45) | 1.17 (1.03 to 1.34) |
| Chronic lung disease | 1.32 (1.18 to 1.47) | 0.95 (0.84 to 1.08) | 1.11 (0.99 to 1.25) |
| Multisystem impairment | 1.39 (1.21 to 1.59) | 0.87 (0.76 to 1.02) | 0.88 (0.76 to 1.01) |
All analyses were on imputed data. For our primary analysis, models were adjusted for all covariates, including age at MM diagnosis, sex, race, income, ISS stage, calcium greater than or equal to 11 mg/dL, creatinine greater than 2 mg/dL, hemoglobin less than 10 g/dL, and platelet less than 150 000/microL. For our secondary analysis, models were further adjusted for the Charlson Index. CI = confidence interval; ED = emergency department; HR = hazard ratio; International Staging System; MM = multiple myeloma.
Multimorbidity Patterns and Care Utilization
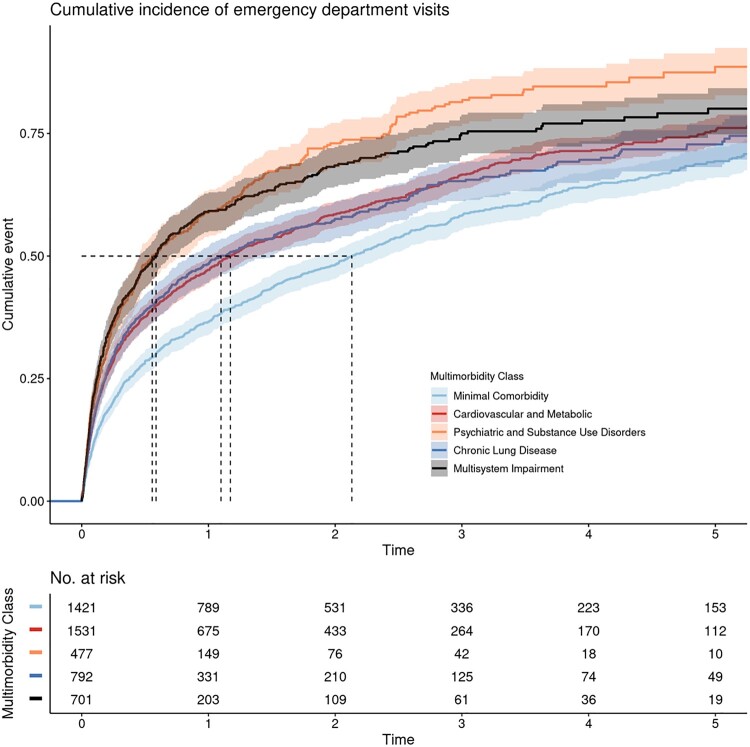
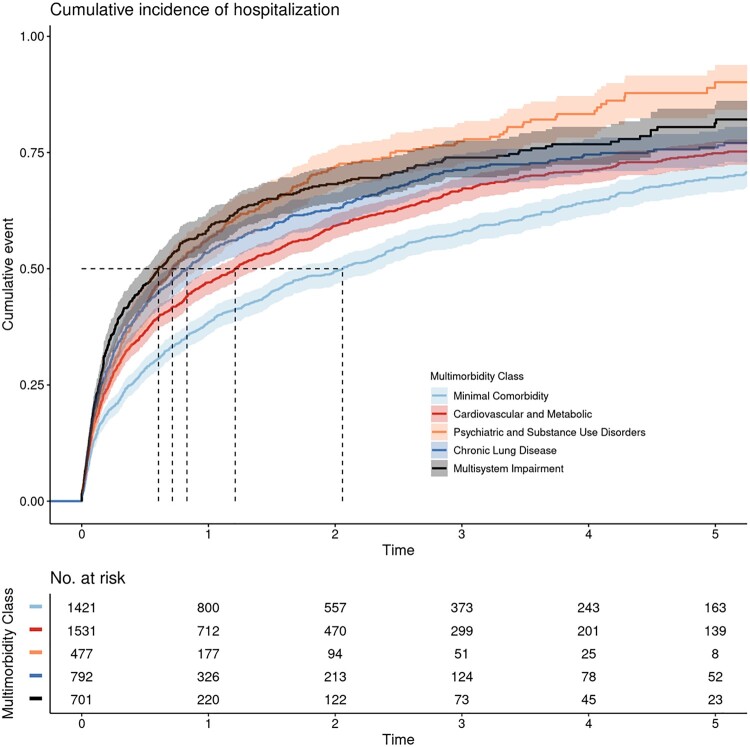
ED visits and hospitalizations varied across multimorbidity patterns (log-rank P value for both outcomes < .001; Figures 3 and 4). Compared with veterans with minimal comorbidity, veterans with cardiovascular and metabolic diseases, psychiatric and substance use disorders, chronic lung disease, and multisystem impairment all had a higher hazard of ED visits and hospitalizations; these associations held after adjustment for all covariates (Table 3). Complete case analyses were similar (Supplementary Table 6, available online). In our secondary analysis, only the psychiatric and substance use pattern maintained its association with ED visits and hospitalizations after further adjustment for the Charlson Index (Table 3).
Figure 3.
Kaplan-Meier curves demonstrating time to first emergency department (ED) visit by multimorbidity pattern in older transplant-ineligible veterans newly treated for multiple myeloma in Veterans Affairs. Time to first ED visit varied across patterns (log-rank 2-sided P < .001). Shaded areas around solid curves represent 95% confidence intervals. Dashed lines represent median time to first ED visit for each multimorbidity class, as follows: minimal comorbidity = 2.13 years, diabetes and complications = 1.17 years, chronic lung disease = 1.10 years, multisystem impairment = 0.59 years, and psychiatric and substance use disorders = 0.56 years.
Figure 4.
Kaplan-Meier curves demonstrating time to first unplanned hospitalization by multimorbidity pattern in older transplant-ineligible veterans newly treated for multiple myeloma in Veterans Affairs. Time to first hospitalization varied across patterns (log-rank 2-sided P < .001). Shaded areas around solid curves represent 95% confidence intervals. Dashed lines represent median time to first hospitalization for each multimorbidity class, as follows: minimal comorbidity = 2.06 years, diabetes and complications = 1.21 years, chronic lung disease = 0.83 years, psychiatric and substance use disorders = 0.72 years, and multisystem impairment = 0.61 years.
Discussion
We used a novel method to define multimorbidity in older adults with cancer, identifying 5 distinct patterns of multimorbidity among US veterans age 65 years and older newly treated for MM. Older veterans with cardiovascular and metabolic diseases, psychiatric and substance use disorders, chronic lung disease, and multisystem impairment demonstrated higher mortality and care use compared with older veterans with minimal comorbidity. Associations with mortality were independent of sociodemographic variables and measures of disease risk, and were observed even after adjustment for the traditional count-based measure of comorbidity in the Charlson Index. Our findings highlight that in many older patients with MM, prognosis and treatment of cancer must not be considered in isolation but rather in the context of their multiple chronic conditions. Considering the nature or pattern of these chronic conditions rather than merely their number better captures the complexity of multimorbidity.
Health policymakers, national research organizations such as the National Cancer Institute, and the World Health Organization have prioritized research into novel methods of analyzing multimorbidity that fully harness the multitude of data available in electronic health record systems (30-33). Our study aligned with this goal, applying a data-driven approach to administrative and electronic health record data in the nationally integrated VA health-care system. Other approaches to defining and analyzing multimorbidity exist, each with strengths and limitations (34). A widely accepted definition identifies it as the presence of 2 or more chronic conditions (34), but this definition is nonspecific and classifies 70% or greater of older adults as multimorbid in certain populations (35). Other definitions focus on measuring prespecified clusters or patterns of 2 or more chronic conditions, such as dyads or triads of hyperlipidemia, hypertension, and cardiovascular disease (15). Finally, empirical data-driven approaches such as the LCA used in our study allow for examination of how patterns of chronic conditions arise nonrandomly, due to either a shared underlying mechanism or ability to predict outcomes such as survival, function, and quality of life (9,10,15,36-39). For these reasons, LCA has been similarly applied to patient-reported measures and other data, for example, to reveal distinct clusters of symptoms in patients with cancer (40,41).
In our cohort of veterans with MM, we found multimorbidity patterns similar to those found in other populations of older adults (9,10). In the cardiovascular and metabolic pattern, for example, the clustering of diabetes, microvascular complications such as nephropathy and neuropathy, and macrovascular complications such as ischemic heart disease likely reflects the common pathophysiology of the metabolic syndrome prevalent in the United States and other high-income countries (42,43). The pattern of psychiatric and substance use disorders likely reflects in veterans the high rates of traumatic brain injury, posttraumatic stress disorder, and depression, all of which contribute to higher risk of comorbid mood disorders, alcoholism, and opioid use disorders (44-46). The increasing prevalence of Vietnam veterans aged 65 years and older, who are particularly at risk of these conditions, makes recognition of this pattern especially important (47).
The higher rates of death, ED use, and hospitalizations in older veterans with MM who had cardiovascular and metabolic diseases, psychiatric and substance use disorders, chronic lung disease, and multisystem impairment not only demonstrate the predictive validity of these distinct multimorbidity patterns but also call for further investigation into their distinct mechanisms of adverse outcomes. It is known that immunomodulatory MM agents elevate the risk of thrombosis and that proteasome inhibitors confer a higher risk of nerve damage; these drugs are used with caution in patients with preexisting cardiovascular disease and neuropathy (48). However, little is known how MM and its treatments interact with multiple psychiatric conditions, chronic lung disease, or the presence of multisystem diagnoses.
Veterans with depression, posttraumatic stress disorder, and substance use disorders could have any one of these conditions exacerbated by the stress of their MM and its treatment, potentially leading to treatment discontinuations (and thus disease progression) and reduced physical and social quality of life (49). Moreover, veterans with COPD have a higher risk of pulmonary toxicity related to their myeloma treatments (50). Lastly, veterans with multisystem impairment not only carry multiple life-limiting conditions that present competing risks in and of themselves but also present with potential disease–disease, disease–drug, and drug–drug interactions with MM that endanger their care (51). Further investigation is warranted to determine the specific mediators of adverse outcomes within each pattern of multimorbidity (48). As prior evidence suggests, interventions targeting mediators or functional difficulties associated with specific conditions hold the most promise in improving outcomes in older adults with multimorbidity (52).
There are limitations to our study. Our criteria used to include veterans newly treated for MM within the VA introduce immortal time bias by excluding veterans who are diagnosed with MM but not treated. However, most patients with newly diagnosed MM are indicated for immediate treatment (48). Moreover, many veterans receive prior treatment or part of their current treatment outside VA (53), risking misclassification if they are selected based on diagnostic codes alone. Our algorithm, which combines diagnostic and treatment codes and uses CMS data to exclude external treatments, minimizes this greater threat to our study’s internal validity. Second, our results are potentially subject to residual confounding by the absence of MM cytogenetics. Third, we did not measure frailty, a construct related to but distinct from multimorbidity (54). Finally, although LCA can assign an individual to a particular pattern of multimorbidity, it does not identify all of the comorbidities present within that individual.
In conclusion, we identified patterns of multimorbidity in older veterans with MM that affected outcomes beyond a count-based comorbidity index. Future research should elucidate the mechanisms by which these multimorbidity patterns lead to worse outcomes. Moreover, future work should compare patterns of multimorbidity that arise in other malignancies as well as patterns that arise in other, nonveteran populations. If this approach is validated, then tools should be developed to assist oncologists at the point of care with identifying patterns of multimorbidity and the specific comorbidities within each patient. Such efforts will advance our understanding of how cancer and its treatment interact with an older adult’s multiple chronic conditions, moving beyond the concept of count-based comorbidity toward more individualized prognosis and decision making.
Funding
This work was supported by the VA Office of Research and Development, Cooperative Studies Program (NRF, NVD, MTB); HCSRN-OAICs AGING Initiative Pilot (1R33AG057806) (NRF, CD, ME), Harvard Translational Research in Aging Training Program (National Institute on Aging of the National Institutes of Health: T32AG023480) (CD); VA Merit Review Award 1I01BX001584 (NCM), and NIH grants P01-155258–07 and P50-100707 (NCM). Support for VA/CMS data is provided by the Department of Veterans Affairs, VA Health Services Research and Development Service, VA Information Resource Center (Project Numbers SDR 02–237 and 98–004).
Notes
Role of the funders: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures: NCM is consultant for BMS, Janssen, OncoPep, Amgen, Abbvie and Takeda and on the board of directors for OncoPep.
Prior presentation: Preliminary findings of this work were presented at the American Society of Clinical Oncology’s Annual Meeting in 2019.
Disclaimer: The views expressed are those of the authors and do not represent the views of VA or the United States Government.
Author contributions: Conceptualization (NRF, CD, DHK, NCM, JAD); Methodology (NRF, CD, CY, JL, ME, DC, DHK, NCM, JAD); Software (NRF, CY, JL); Data Curation (NRF, CD, CY, JL); Formal analysis (NRF, CD, CY, JL); Validation (NRF, CD, CY, JL, DHK); Interpretation of Data/Analyses (all authors); Writing—original draft preparation (NRF, CD); Writing—review and editing (all authors); Supervision (NVD, MTB, DHK, NCM, and JD); Funding acquisition (NRF, CD, ME, NVD, MTB, NCM).
Data Availability
The data underlying this article were accessed from the VA Corporate Data Warehouse. The derived data generated in this research may be shared on reasonable request to the corresponding author as permitted by VA policy. We have also uploaded key portions of our code related to the latent class analysis here: https://github.com/bostoninformatics/mm_multimorbidity_jnci.
Supplementary Material
References
- 1. Shapiro CL. Cancer survivorship. N Engl J Med. 2018;379(25):2438–2450. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) (2013-2017). Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 01, 2020.
- 3. Kleber M, Ihorst G, Terhorst M, et al. Comorbidity as a prognostic variable in multiple myeloma: comparative evaluation of common comorbidity scores and use of a novel MM-comorbidity score. Blood Cancer J. 2011;1(9):e35–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engelhardt M, Domm A-S, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102(5):910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gregersen H, Vangsted AJ, Abildgaard N, et al. The impact of comorbidity on mortality in multiple myeloma: a Danish nationwide population-based study. Cancer Med. 2017;6(7):1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol. 2012;65(9):924–933. [DOI] [PubMed] [Google Scholar]
- 7. Søgaard M, Thomsen RW, Bossen KS, et al. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl 1):3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stirland LE, González-Saavedra L, Mullin DS, et al. Measuring multimorbidity beyond counting diseases: systematic review of community and population studies and guide to index choice. BMJ. 2020;368:m160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prados-Torres A, Calderon-Larranaga A, Hancco-Saavedra J, et al. Multimorbidity patterns: a systematic review. J Clin Epidemiol. 2014;67(3):254–266. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen QD, Wu C, Odden MC, et al. Multimorbidity patterns, frailty, and survival in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2018;74(8):165–1270. 10.1093/gerona/gly205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American Geriatrics Society: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. J Am Geriatr Soc. 2012;60(10):1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wallace E, McDowell R, Bennett K, et al. Comparison of count-based multimorbidity measures in predicting emergency admission and functional decline in older community-dwelling adults: a prospective cohort study. BMJ Open. 2016;6(9):e013089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nardi R, Scanelli G, Corrao S, et al. Co-morbidity does not reflect complexity in internal medicine patients. Eur J Intern Med. 2007;18(5):359–368. [DOI] [PubMed] [Google Scholar]
- 14. Pearson-Stuttard J, Ezzati M, Gregg EW.. Multimorbidity—a defining challenge for health systems. Lancet Public Health. 2019;4(12):e599–e600. [DOI] [PubMed] [Google Scholar]
- 15. Steinman MA, Lee SJ, John Boscardin W, et al. Patterns of multimorbidity in elderly veterans. J Am Geriatr Soc. 2012;60(10):1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs Medical Centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. [DOI] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 18. Price LE, Shea K, Gephart S.. The Veterans Affairs's corporate data warehouse: uses and implications for nursing research and practice. Nurs Adm Q. 2015;39(4):311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee on Future Information Architectures Processes and Strategies for the Centers for Medicare and Medicaid Services, Shortliffe EH, Millet LI, et al. Strategies and Priorities for Information Technology at the Centers for Medicare and Medicaid Services. Washington, DC: National Academies Press; 2012:xiv177. [Google Scholar]
- 20.Centers for Medicare and Medicaid Services Chronic Conditions Data Warehouse: condition categories. https://www2.ccwdata.org/web/guest/about-ccw. Accessed October 01, 2020.
- 21. Gasparini A. Comorbidity: an R package for computing comorbidity scores. J Open Source Softw. 2018;3(23):648. [Google Scholar]
- 22. Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. [DOI] [PubMed] [Google Scholar]
- 23. Kyle RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78(1):21–33. [DOI] [PubMed] [Google Scholar]
- 24. Mori M, Krumholz HM, Allore HG.. Using latent class analysis to identify hidden clinical phenotypes. JAMA. 2020;324(7):700–701. [DOI] [PubMed] [Google Scholar]
- 25. Agresti A. Categorical Data Analysis. 2nd ed. New York: Wiley-Interscience; 2002. [Google Scholar]
- 26. Linzer DA, Lewis JB.. poLCA: An R Package for Polytomous Variable Latent Class Analysis. J Stat Soft. 2011;42(10):29. [Google Scholar]
- 27. van Buuren S, Groothuis-Oudshoorn K.. Mice: Multivariate Imputation by Chained Equations in R. J Stat Soft. 2011;45(3):67. [Google Scholar]
- 28. Buuren S. Flexible imputation of missing data. In: Morgan B, Heijden P, Wikle C, eds. Chapman and Hall/CRC Interdisciplinary Statistics. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 29. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. [DOI] [PubMed] [Google Scholar]
- 30. Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ. 2020;368:l6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Lancet. Making more of multimorbidity: an emerging priority. Lancet. 2018;391(10131):1637. [DOI] [PubMed] [Google Scholar]
- 32. Pearson-Stuttard J, Ezzati M, Gregg EW.. Multimorbidity-a defining challenge for health systems. Lancet Public Health. 2019;4(12):e599–e600. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Multimorbidity. Geneva: World Health Organization; 2016. [Google Scholar]
- 34. Johnston MC, Crilly M, Black C, et al. Defining and measuring multimorbidity: a systematic review of systematic reviews. Eur J Public Health. 2019;29(1):182–189. [DOI] [PubMed] [Google Scholar]
- 35. Maciejewski ML, Hammill BG.. Measuring the burden of multimorbidity among Medicare beneficiaries via condition counts and cumulative duration. Health Serv Res. 2019;54(2):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei MY, Mukamal KJ.. Multimorbidity, mortality, and long-term physical functioning in 3 prospective cohorts of community-dwelling adults. Am J Epidemiol. 2018;187(1):103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei MY, Ratz D, Mukamal KJ.. Multimorbidity in Medicare beneficiaries: performance of an ICD-coded multimorbidity-weighted index. J Am Geriatr Soc. 2020;68(5):999–1006. 10.1111/jgs.16310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hidalgo CA, Blumm N, Barabási A-L, et al. A dynamic network approach for the study of human phenotypes. PLOS Comput Biol. 2009;5(4):e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Olaya B, Moneta MV, Caballero FF, et al. Latent class analysis of multimorbidity patterns and associated outcomes in Spanish older adults: a prospective cohort study. BMC Geriatr. 2017;17(1):186–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miaskowski C, Barsevick A, Berger A, et al. Advancing symptom science through symptom cluster research: expert panel proceedings and recommendations. J Natl Cancer Inst. 2017;109(4):djw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miaskowski C, Dunn L, Ritchie C, et al. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. 2015;50(1):28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sattar N, Gill JMR, Alazawi W.. Improving prevention strategies for cardiometabolic disease. Nat Med. 2020;26(3):320–325. [DOI] [PubMed] [Google Scholar]
- 44. Hoge CW, McGurk D, Thomas JL, et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358(5):453–463. [DOI] [PubMed] [Google Scholar]
- 45. Lwi SJ, Barnes DE, Xia F, et al. Ten-year prevalence of cognitive impairment diagnoses and associated medical and psychiatric conditions in a national cohort of older female veterans. Am J Geriatr Psychiatry. 2019;27(4):417–425. [DOI] [PubMed] [Google Scholar]
- 46. Hassan AN, Le Foll B, Imtiaz S, et al. The effect of post-traumatic stress disorder on the risk of developing prescription opioid use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Drug Alcohol Depend. 2017;179:260–266. [DOI] [PubMed] [Google Scholar]
- 47. Marini CM, Fiori KL, Wilmoth JM, et al. Psychological adjustment of aging Vietnam veterans: the role of social network ties in reengaging with wartime memories. Gerontology. 2020;66(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548–567. [DOI] [PubMed] [Google Scholar]
- 49. Wachen JS, Patidar SM, Mulligan EA, et al. Cancer-related PTSD symptoms in a veteran sample: association with age, combat PTSD, and quality of life. Psycho-Oncology. 2014;23(8):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fakhri B, Fiala MA, Shah N, et al. Measuring cardiopulmonary complications of carfilzomib treatment and associated risk factors using the SEER-Medicare database. Cancer. 2020;126(4):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sarfati D, Koczwara B, Jackson C.. The impact of comorbidity on cancer and its treatment. CA: A Cancer J Clin. 2016;66(4):337–350. [DOI] [PubMed] [Google Scholar]
- 52. Smith SM, Soubhi H, Fortin M, et al. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ. 2012;345(sep03 1):e5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aggarwal NK. Ramifications of the VA MISSION Act of 2018 on mental health: potential Implementation challenges and solutions. JAMA Psychiatry. 2020;77(4):337–338. [DOI] [PubMed] [Google Scholar]
- 54. Vetrano DL, Palmer K, Marengoni A, et al. ; Joint Action ADVANTAGE WP4 Group. Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74(5):659–666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were accessed from the VA Corporate Data Warehouse. The derived data generated in this research may be shared on reasonable request to the corresponding author as permitted by VA policy. We have also uploaded key portions of our code related to the latent class analysis here: https://github.com/bostoninformatics/mm_multimorbidity_jnci.