Abstract
Background
Glioblastoma is the deadliest brain tumor in adults, and the standard of care consists of surgery followed by radiation and treatment with temozolomide. Overall survival times for patients suffering from glioblastoma are unacceptably low indicating an unmet need for novel treatment options.
Methods
Using patient-derived HK-157, HK-308, HK-374, and HK-382 glioblastoma lines, the GL261 orthotopic mouse models of glioblastoma, and HK-374 patient-derived orthotopic xenografts, we tested the effect of radiation and the dopamine receptor antagonist quetiapine on glioblastoma self-renewal in vitro and survival in vivo. A possible resistance mechanism was investigated using RNA-sequencing. The blood-brain-barrier–penetrating statin atorvastatin was used to overcome this resistance mechanism. All statistical tests were 2-sided.
Results
Treatment of glioma cells with the dopamine receptor antagonist quetiapine reduced glioma cell self-renewal in vitro, and combined treatment of mice with quetiapine and radiation prolonged the survival of glioma-bearing mice. The combined treatment induced the expression of genes involved in cholesterol biosynthesis. This rendered GL261 and HK-374 orthotopic tumors vulnerable to simultaneous treatment with atorvastatin and further statistically significantly prolonged the survival of C57BL/6 (n = 10 to 16 mice per group; median survival not reached; log-rank test, P < .001) and NOD Scid gamma mice (n = 8 to 21 mice per group; hazard ratio = 3.96, 95% confidence interval = 0.29 to 12.40; log-rank test, P < .001), respectively.
Conclusions
Our results indicate promising therapeutic efficacy with the triple combination of quetiapine, atorvastatin, and radiation treatment against glioblastoma without increasing the toxicity of radiation. With both drugs readily available for clinical use, our study could be rapidly translated into a clinical trial.
Despite decades of drug development and technical improvement in radiotherapy, glioblastoma is still the deadliest brain cancer in adults with almost all patients ultimately dying from the disease (1). Attempts to add chemotherapeutic drugs that serve as radiosensitizers have largely failed because of either lack of blood-brain-barrier (BBB) penetration or lack of a proper therapeutic window with temozolomide being the only radiosensitizer that has so far been included into the standard of care (2). However, median survival time under the current standard of care with gross tumor resection, temozolomide, and radiotherapy is only 15-18 months, thus indicating an urgent need to develop novel strategies against glioblastoma. Glioblastomas are thought to be organized hierarchically with a small number of glioma-initiating cells and able to produce more differentiated progeny and to repopulate a tumor after sublethal treatment. The ability to identify glioma-initiating cells prospectively (3,4) and the recognition of their intrinsic radioresistance (5) have sparked research aiming to target glioma-initiating cells specifically (6).
In a high-throughput screen of 83 000 compounds (7), we identified the first-generation dopamine receptor antagonist trifluoperazine as a Food and Drug Administration (FDA)–approved drug with known BBB penetration that interferes with the self-renewal of glioma cells alone and prevents the induction of a glioma-initiating cell phenotype in combination with radiation (11). Recently, dopamine receptor antagonists have generated considerable interest in their repurpose as anticancer agents with well-established pharmacological properties and demonstrated some single-agent anticancer activity (8-14). Considering the unfavorable clinical side effect profile of trifluoperazine, we have extended our studies to include second-generation dopamine receptor antagonists with milder side effects.
In this study, we hypothesized that a combination of quetiapine and radiation would have equal or better efficacy against self-renewal of patient-derived glioma-initiating cells in vitro than first-generation dopamine receptor antagonists and radiation and it would prolong survival in mouse models of glioblastoma in vivo. Furthermore, RNA-sequencing (RNA-Seq) uncover novel resistance mechanisms against this combination treatment that could be exploited to improve survival of glioblastoma-bearing mice.
Methods
An extended description of the materials and methods is provided in the Supplementary Methods (available online).
Cell Culture
Primary human glioblastoma cell lines were established at the University of California, Los Angeles (UCLA) as described previously (3). Characteristics of specific gliomasphere lines can be found in Laks et al. (15). The GL261 murine glioma cell line was obtained from Charles River Laboratories, Inc (Frederick, MD). Cells were grown as previously described (11).
Drug Treatment
After confirming tumor grafting via bioluminescence imaging, mice implanted with the HK-374 or GL261 specimen were injected subcutaneously (quetiapine 30 mg/kg) or intraperitoneally (atorvastatin 30 mg/kg) on a 5-days on, 2-days off schedule with quetiapine, combined quetiapine and atorvastatin, or saline until they reached euthanasia endpoints. Quetiapine was dissolved in acidified phosphate buffered saline (0.4% glacial acetic acid) at a concentration of 5 mg/ml. Atorvastatin was dissolved in corn oil containing 2.5% dimethyl sulfoxide at a concentration of 5 mg/mL.
Irradiation
Cells were irradiated as previously described (11). Corresponding controls were sham irradiated. Mice were irradiated as previously described (16). For the assessment of the effect of quetiapine and quetiapine plus atorvastatin in combination with irradiation in vivo, mice were treated with corresponding drugs 1 hour prior to irradiation. Animals received a single dose of 10 Gy on day 3 or day 7 after tumor implantation.
In Vitro Sphere Formation Assay
For the assessment of self-renewal in vitro, patient-derived glioblastoma cells (HK-374, HK-382, HK-308, and HK-157) were irradiated with (0, 2, 4, 6, or 8 Gy) and treated with daily doses of quetiapine (0, 5, or 10 µM). Glioblastoma cells were treated with a single dose of quetiapine (0, 5, 10 µM) and atorvastatin (0, 50, 100 250, 500, 1000 nM) with or without irradiation at 0, 2, 4, 6, or 8 Gy. The number of spheres formed at each dose point was normalized against the control. The resulting data points were fitted using a linear-quadratic model.
For the tertiary sphere formation assay ith quetiapine, the spheres from patient-derived specimens were seeded in 10-cm dishes and treated with daily doses of either solvent or quetiapine (5 or 10 µM) for 5 days. The surviving spheres after the initial treatment were collected, dissociated, and subjected to additional 2 rounds of identical treatment to attain the tertiary sphere cells. These spheres were plated in a 96-well plate and treated with a single dose of control or quetiapine. Ten days after treatment, the number of spheres formed in each condition were counted, and the percentage of clonal cells forming spheres was calculated.
RNA-Seq
RNA was extracted from HK-374 cells using Trizol 48 hours after 0 or 4 Gy. RNA-Seq analysis was performed by Novogene (Chula Vista, CA) as previously described (11).
Shotgun Lipidomics Analysis
Forty-eight hours after irradiation with 0 or 4 Gy in the presence or absence of quetiapine, HK-374 cells were trypsinized and resuspended in 0.2 mL of 1X phosphate buffered saline. A small aliquot of cell suspension from each sample was saved for cell counting, and the remaining cell suspension was immediately stored at -80°C and subjected to shotgun lipidomics analysis at the UCLA Lipidomics Core.
Statistical Analysis
All data shown are represented as mean (95% confidence interval [CI]) and result from at least 3 biologically independent experiments. A P value of no more than .05 in an unpaired 2-sided Student t test or 2-way ANOVA test indicated a statistically significant difference. For sphere-forming assays, data points were fitted using linear-quadratic model (surviving fraction = exp(-alpha*dose-beta*dose2). For Kaplan-Meier estimates, a P value of .05 in a log-rank test indicated a statistically significant difference. Hazard ratios (HRs) and 95% confidence intervals for the survival curves were calculated using the Mantel-Haenszel model. All statistical analyses were performed using the GraphPad Prism Software package (GraphPad Software, San Diego, CA). All tests were 2-sided.
Results
Quetiapine and Radiation-Induced Phenotype Conversion In Vitro
We had previously reported that radiation treatment induced a phenotype conversion of glioma cells into glioma-initiating cells through reexpression of Yamanaka factors. Furthermore, we reported that treatment with the first-generation dopamine receptor antagonist trifluoperazine prevented this phenotype conversion and prolonged survival in a syngeneic GL261 model of glioblastoma and patient-derived orthotopic glioma xenografts (11).
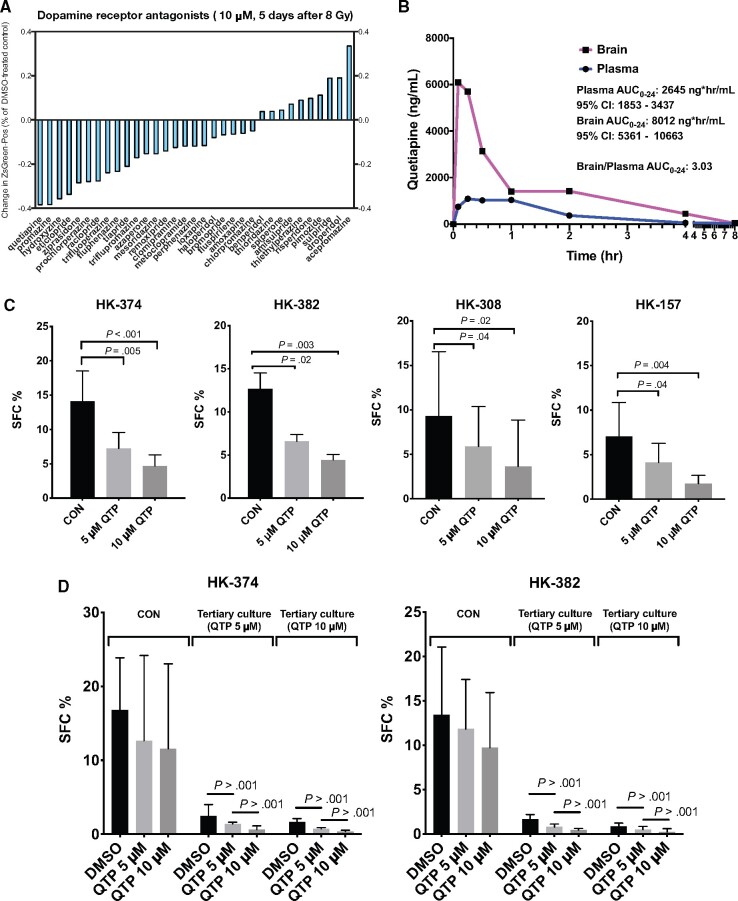
Given the unfavorable side effect profile of trifluoperazine, we screened a panel of additional 32 dopamine receptor antagonists for their ability to interfere with the process of radiation-induced phenotype conversion. Compared with trifluoperazine, the second-generation dopamine receptor antagonist quetiapine, known for a milder side effect profile (17), showed higher efficacy against radiation-induced phenotype conversion than trifluoperazine in this screening assay (Figure 1, A).
Figure 1.
Quetiapine reduces self-renewal in patient-derived glioblastoma lines. (A) Inhibition of radiation-induced phenotype conversion by a panel of dopamine receptor antagonists. (B) Brain and plasma levels of quetiapine (QTP) in mice after a single injection (30 mg/kg, intraperitoneal) represented by Area Under Curve from 0-24 hours (AUC0-24). (C) Sphere-forming capacity of patient-derived glioblastoma lines in quetiapine vs. control (CON) treated cells. (D) Tertiary sphere-forming assays with glioblastoma cells treated with quetiapine or control (DMSO). (E) Surviving fraction of spheres treated with radiation in the presence or absence of quetiapine. All experiments in this figure have been performed with at least 3 independent biological repeats. P values were calculated using 2-way ANOVA. CI = confidence interval.
Even though quetiapine is a psychotropic drug, we next ensured that quetiapine would penetrate into the central nervous system at relevant levels. Animals were treated with the mouse equivalent of 25% of the human maximum tolerated dose via intraperitoneal injection (30 mg/kg). Blood and brains were harvested at various time points after injection and subjected to mass spectrometry. Quetiapine rapidly crossed the BBB with a threefold higher Area Under Curve from 0-24 hours (AUC0-24) compared with plasma levels (Figure 1, B).
To test the effects of quetiapine on the self-renewal of patient-derived glioblastoma specimens, we treated gliomaspheres of the HK-374, HK-382, HK-308, or HK-157 lines with 5 daily doses of quetiapine at 0, 5, or 10 µM. Quetiapine caused a statistically significant dose-dependent reduction in sphere formation in all 4 cell lines (all P < .05) (Figure 1, C). To further evaluate the efficacy of quetiapine in inhibiting the self-renewal capacity of gliomaspheres, we performed tertiary sphere-forming assays and observed a statistically significant exhaustion in sphere-forming capacity in glioblastoma cells treated with quetiapine (all P < .001) (Figure 1, D). Next, we combined a single dose of radiation with 5 daily doses of quetiapine to assess if quetiapine would act as a radiosensitizer in gliomaspheres. Quetiapine did not sensitize glioblastoma cells to radiation in the 4 patient-derived lines tested, suggesting that quetiapine treatment would not increase the toxicity of radiation (Figure 1, E; Supplementary Table 1, available online).
Effects of Quetiapine on Survival in Mouse Models of Glioblastoma
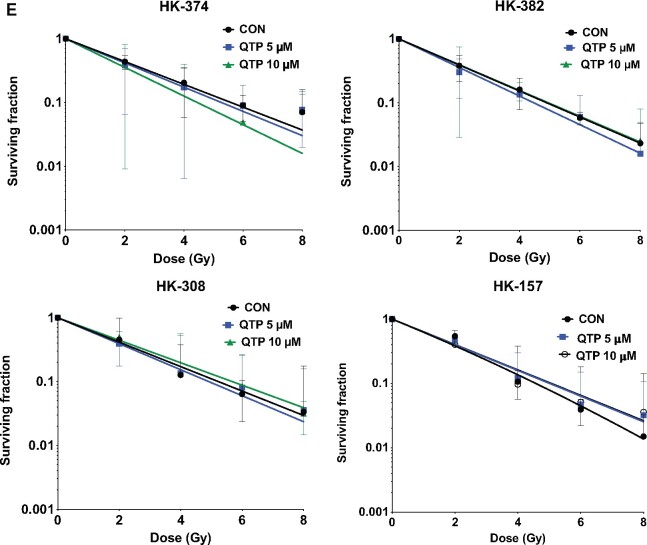
We next grafted GL261 cells into the striatum of C57BL/6 mice. Finding a statistically significant gain in the median survival in response to quetiapine but no clear dose dependency (Figure 2, A), we performed all subsequent in vivo experiments with quetiapine at 30 mg/kg (median survival: saline 23 days, quetiapine 31 days; HR = 1.34, 95% CI = 0.53 to 3.39; P = .02, log-rank test). Treatment of mice bearing GL261-StrawberryRed tumors with 5 daily injections of quetiapine for 1 or 2 weeks statistically significantly reduced the sphere-forming capacity of the surviving tumor cells in vitro, thus indicating the efficacy of quetiapine against glioma-initiating cells in vivo (Figure 2, B).
Figure 2.
A combination of quetiapine and radiation prolongs survival in mouse models of glioblastoma. (A) Dose escalation of quetiapine (QTP) in GL261 tumor-bearing C57Bl/6 mice. Seven days after injection of the tumor cells the animals were treated with escalating doses of quetiapine. Five daily doses of quetiapine per week for 3 weeks led to a statistically significant gain in the median survival (hazard ratio [HR] of control vs quetiapine = 2.64, 95% confidence interval [CI] = 0.93 to 7.44) with no clear dose dependency. (B) Treatment of GL261 tumor-bearing C57BL/6 mice with quetiapine reduces the sphere-forming capacity of surviving tumor cells. This experiment has been performed with at least 2 independent biological repeats (unpaired Student t test). (C and D) A single dose of 10 Gy or daily injection of quetiapine alone had small effects on medical survival (HR of control vs 10 Gy = 2.66, 95% CI = 0.94 to 7.49; HR control vs quetiapine = 3.96, 95% CI = 0.29 to 12.40). A combination of radiation (10 Gy) and daily injections of quetiapine statistically significantly prolongs survival in the GL261 glioma mouse model (HR of control vs quetiapine and 10 Gy = 5.46, 95% CI = 1.54 to 19.32) (C). Likewise, in mice carrying patient-derived orthotopic xenografts, radiation or quetiapine alone led to only small increases in median survival (HR of control vs quetiapine = 1.95; control vs 10 Gy = 1.77), whereas the combined treatment statistically significantly prolonged median survival from 27.5 to 52.5 days (log-rank HR = 4.70, 95% CI = 1.90 to 11.62) (D). All statistical tests were 2-sided. i.p. = intraperitoneal; tx = treatment.
Considering that quetiapine did not act as a radiosensitizer, we decided to treat all animals with a single, sublethal dose of radiation, reflecting the inability of radiation to locally control glioblastoma in patients instead of using fractionated radiotherapy, which would normally be used to explore a therapeutic window for a radiosensitizer (18). A single radiation dose of 10 Gy (equivalent to 18 Gy in 2 Gy fractions in glioblastoma considering an alpha to beta ratio of 8 Gy), 7 days after orthotopic implantation of GL261 cells in mice, led to a small increase in median survival (23 days saline, 31 days 10 Gy, HR of control vs 10 Gy = 2.66, 95% CI = 0.94 to 7.49), comparable to the effect of quetiapine-alone treatment (23 days saline, 31 days quetiapine, HR control vs quetiapine = 3.96, 95% CI = 0.29 to 12.40). However, irradiation with 10 Gy rendered the tumors vulnerable to 5 daily injections of quetiapine per week, leading to a statistically significant increase in median survival (23 days saline, 71 days quetiapine and 10 Gy, HR of control vs quetiapine and 10 Gy = 5.46, 95% CI = 1.54 to 19.32; P < .001, log-rank test; Figure 2, C).
Next, we verified these findings using patient-derived orthotopic xenografts. Three days after orthotopic implantation of HK-374 glioblastoma cells in mice, animals were treated with a single dose of 0 or 10 Gy followed by daily injection of quetiapine (30 mg/kg) or saline (5 days on, 2 days off schedule). Although NOD-scid IL2Rgammanull mice are deficient in DNA repair via nonhomologous end joining, normal brain tissue toxicity from radiation does not differ from that in immune-competent mice (19). Again, quetiapine treatment or irradiation alone led to only small increases in median survival (29 days saline, 34.5 days quetiapine, HR of control vs quetiapine = 1.95, 95% CI = 0.78 to 4.88; 29 days saline, 32 days 10 Gy HR of control vs 10 Gy = 1.77, 95% CI = 0.82 to 5.57). However, the combination of a single radiation dose of 10 Gy followed by treatment with quetiapine statistically significantly increased the median survival (29 days saline, 52.5 days quetiapine and 10 Gy, HR = 4.70, 95% CI = 1.90 to 11.62; P < .001, log-rank test; Figure 2, D).
Dopamine Receptor Inhibition, Radiation, and Cholesterol Biosynthesis
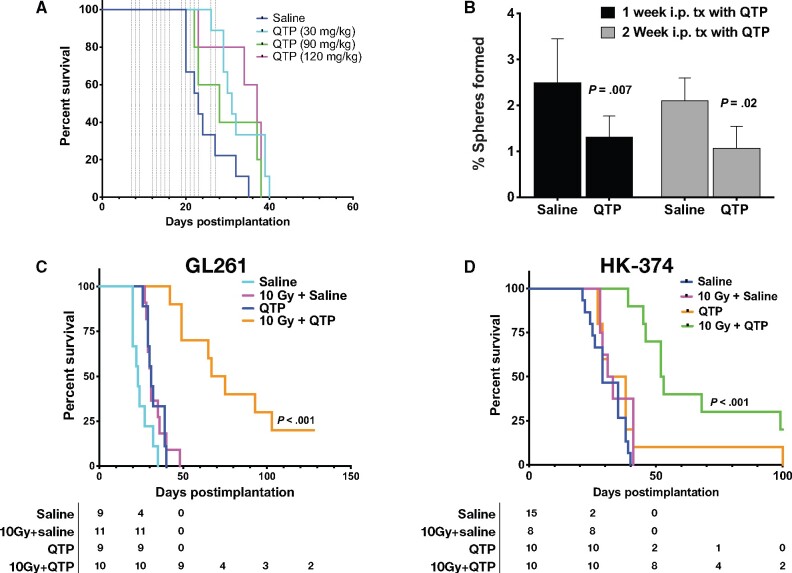
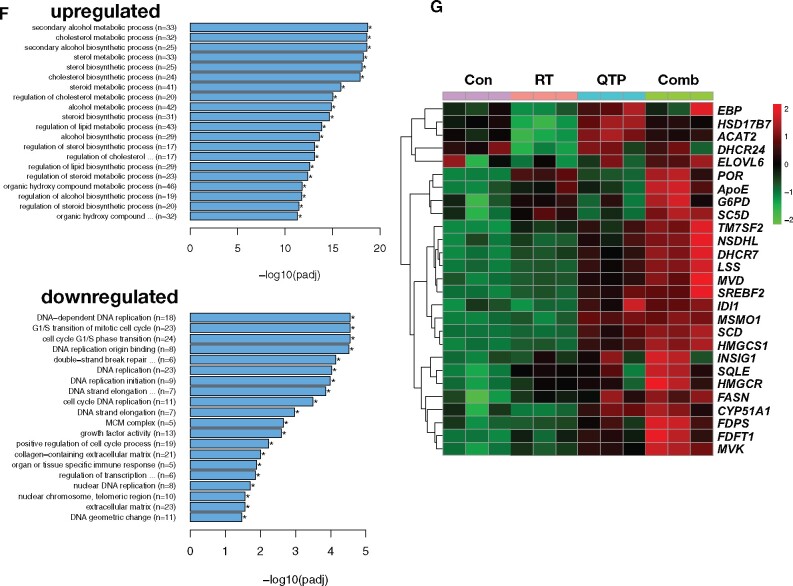
Although the combined treatment with radiation and quetiapine statistically significantly improved the median survival of the animals, almost all animals eventually succumbed to the implanted tumor, thus suggesting that the tumor cells initiated a response to the treatment that led to resistance to quetiapine. To study this possibility in more detail, we next performed RNA-Seq on HK-374 cells at 48 hours after irradiation and/or quetiapine treatment to assess changes in the expression of genes in response to combined treatment. When compared with samples irradiated with a single dose of 4 Gy, the combined treatment with radiation and quetiapine led to the differential upregulation of 516 and the downregulation of 468 genes (Figure 3, A, last panel). The combination of quetiapine and radiation led to the differential expression of 122 unique genes (Figure 3, B), and irradiated samples showed a distinct genes expression profile (Figure 3, C). The top 10 up- and downregulated genes are presented as a heatmap (Figure 3, D) and were validated using qRT-PCR (Figure 3, E). Gene ontology enrichment analysis revealed overlap of the differentially downregulated genes in the samples treated with radiation and quetiapine with gene sets involved in DNA-dependent DNA replication, G1/S transition of the mitotic cell cycle, double-strand break repair, and cell cycle DNA replication (Figure 3, F).
Figure 3.
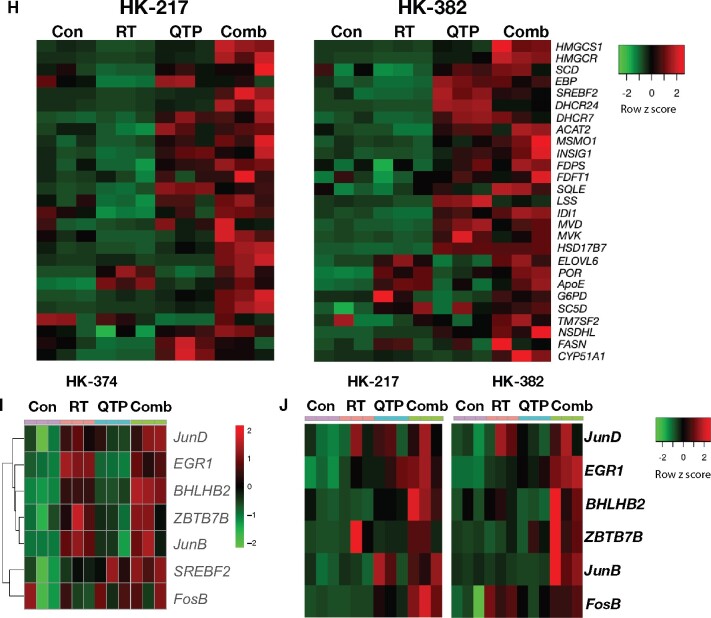
RNA-Seq analysis of glioma cells treated with quetiapine and radiation. (A) Volcano diagrams of differentially expressed genes (DEG) in HK-374 cells, 48 hours after treatment with radiation (IR, 4 Gy, RT), quetiapine (QTP), or quetiapine and radiation (Comb) compared with untreated control (Cnt) cells. (B) Venn diagrams of differentially expressed genes in HK-374 cells, 48 hours after treatment with radiation, quetiapine, or quetiapine and radiation. (C) Heatmap of differential expression genes in HK-374 cells, 48 hours after treatment with radiation, quetiapine, or quetiapine and radiation. (D) Top 10 up- and downregulated genes and their validation by qRT-PCR (E). Top 20 gene ontology gene set overlapping with genes differentially up- or downregulated in HK-374 cells, 48 hours after treatment with radiation and quetiapine (F). Genes involved in the biosynthesis of cholesterol are upregulated in HK-374 (G), HK-217, and HK-382 (H) cells, 48 hours after treatment with radiation (4 Gy) and quetiapine or radiation. Genes of the first-level regulator network of SREBF2, the master regulator of cholesterol biosynthesis, are upregulated in HK-374 (I), HK-217, and HK-382 (J) cells, 48 hours after treatment with radiation (4 Gy) and quetiapine or radiation.
The most prominent genes set that overlapped with genes differentially upregulated after combined treatment was involved in cholesterol, sterol, and lipid biosynthesis (Figure 3, F). The majority of genes involved in the cholesterol biosynthesis pathway were upregulated by quetiapine treatment, but this effect was enhanced when quetiapine treatment was combined with radiation (Figure 3, G). Analysis of the same set of genes in a second RNA-Seq data set of HK-374 cells treated with radiation, trifluoperazine, or a combination of trifluoperazine and radiation at the 48-hour timepoint showed a similar upregulation of genes involved in cholesterol biosynthesis after combined treatment and trifluoperazine treatment alone, thus indicating that this effect was not restricted to quetiapine alone (Supplementary Figure 1, A and B, available online).
Most of the genes in this pathway are under the control of sterol regulatory element binding transcription factor 2 (SREBF2) (20), and our analysis found SREBF2 as well as several genes of the SREBF2 first-level regulatory network upregulated in cells treated with quetiapine (Figure 3, I). To demonstrate that these effects of radiation and dopamine receptor antagonist were not cell-line specific, we performed qRT-PCR using specific primers for all genes in this pathway on 2 additional patient-derived glioblastoma lines—HK-217 and HK-382—which confirmed the upregulation of the expression of key enzymes in the cholesterol biosynthesis pathway and the first-level regulatory network of SREBF2 (Figure 3, H and J).
Inhibition of Cholesterol Biosynthesis in Combination With Quetiapine and Radiation in Glioblastoma
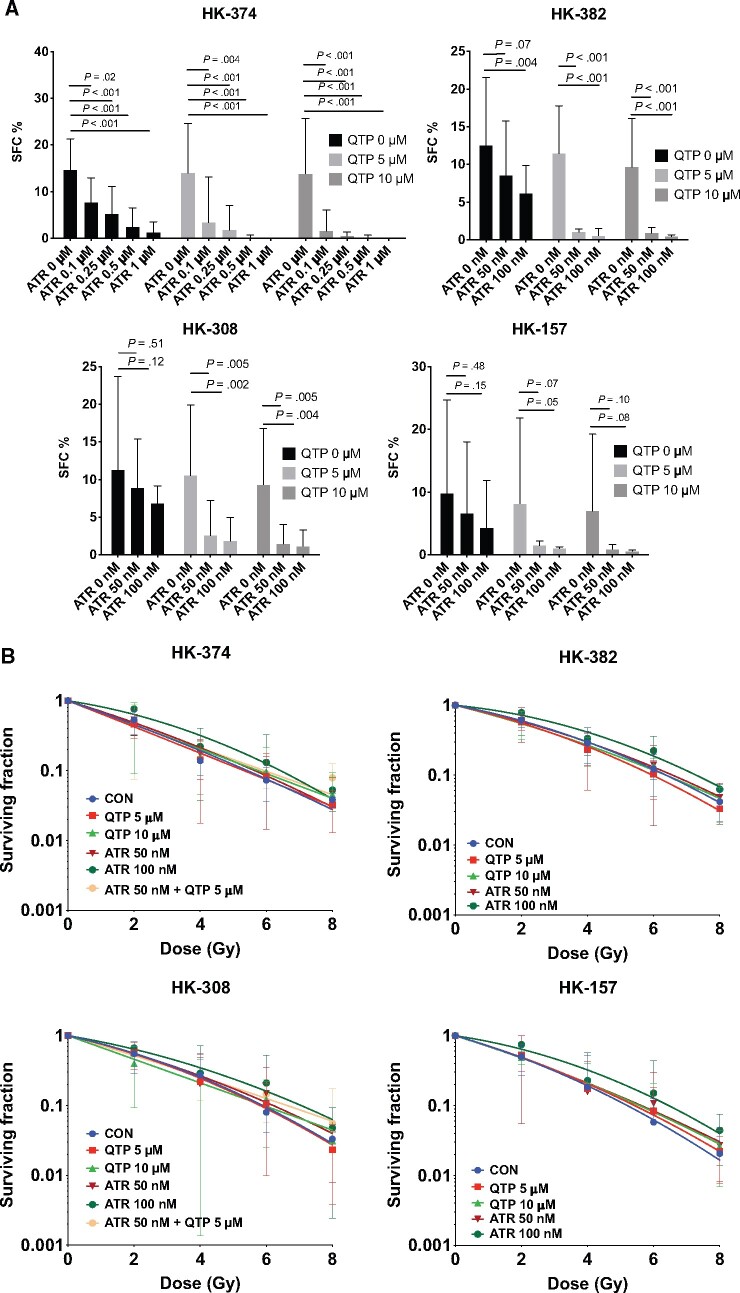
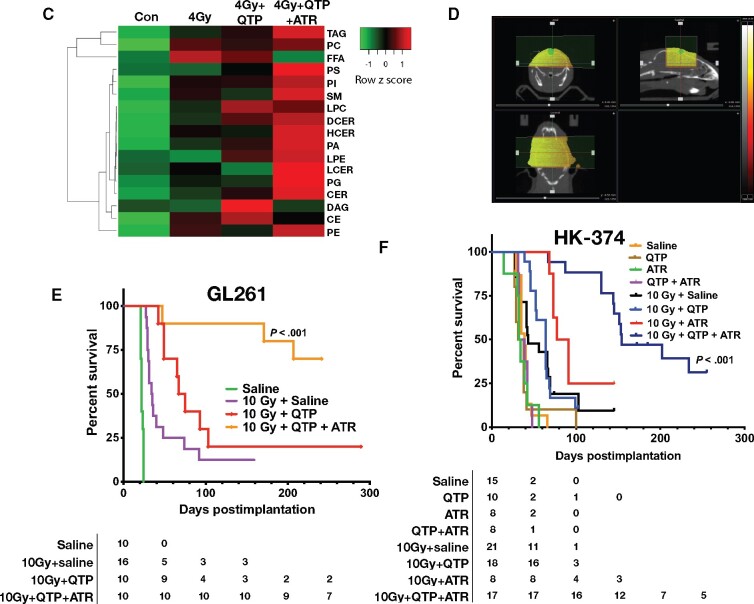
Results from our RNA-Seq study led us to hypothesize that the upregulation of cholesterol biosynthesis is part of a defense mechanism of glioblastoma cells against radiation combined with dopamine receptor inhibition. We therefore tested if treatment with quetiapine and radiation would render glioblastoma cells vulnerable to treatment with the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin, a well-established and FDA-approved statin. Using the same 4 patient-derived glioblastoma specimens in sphere-formation assays, we reduced the very effective 5 daily treatments with quetiapine to only 1 treatment and combined it with atorvastatin at 0.1, 0.25, 0.5, or 1 µM concentrations. Whereas a single dose of quetiapine had limited inhibitory effect on the self-renewal of gliomaspheres, the addition of atorvastatin to quetiapine treatment led to a statistically significant, dose-dependent reduction in sphere formation (all P < .02) (Figure 4, A). When combined with radiation, the addition of atorvastatin did not radiosensitize gliomaspheres in the presence or absence of quetiapine (Figure 4, B; Supplementary Table 1, available online). Performing shotgun lipidomics on HK-374 cells after irradiation, quetiapine, or combined treatment, we found the lipid species of free fatty acids, diglycerides or diacylglycerols, and cholesterol esters upregulated, which agreed with our RNA-Seq findings. Addition of atorvastatin reversed the effects of the combined radiation and quetiapine treatment on those 3 lipid species while globally upregulating all other lipids (Figure 4, C). In agreement with our in vitro findings, daily treatment with quetiapine and atorvastatin (5 days on, 2 days off schedule) after a single dose of 10 Gy (Figure 4, D) statistically significantly increased the survival of C57Bl/6 mice implanted with GL261 cells with 90% of the animals surviving 157 days (median survival = 22 days saline, 31 days quetiapine, 34.5 10 Gy, 71 days quetiapine and 10 Gy, median survival not reached for quetiapine and atorvastatin and 10 Gy; HR of control vs 10 Gy and quetiapine and atorvastatin = 8.33, 95% CI = 2.50 to 27.77; P < .001, log-rank test; Figure 4, E). In mice-bearing HK-374 patient-derived orthotopic xenografts, the same combination treatment statistically significantly increased the median survival to 154 days (median survival = 39 days saline, 34.5 quetiapine, 33 days atorvastatin, 35.5 days quetiapine and atorvastatin, 43 days 10 Gy and saline, 64 days quetiapine and 10 Gy, 84 days atorvastatin and 10 Gy, 154 days quetiapine and atorvastatin and 10 Gy; HR of control vs 10 Gy and quetiapine and atorvastatin = 5.94, 95% CI = 2.21 to 15.95; P < .001, log-rank test; Figure 4, F).
Figure 4.
A combination of quetiapine and radiation with atorvastatin improves survival in mouse modelsof glioblastoma. (A) Quetiapine (QTP) and atorvastatin (ATR) synergistically decreases sphere-forming capacities (SFC) in patient-derived glioblastoma lines. All experiments in this figure have been performed with at least 3 independent biological repeats. (Two-way ANOVA; P values are indicated in the figure). (B) QTP, ATR, or a combination of both does not alter the radiation sensitivity of patient-derived glioblastoma lines. (C) Shotgun lipidomics analysis was performed with HK-374 cells treated with either control (CON), 4 Gy, 4 Gy + QTP, or 4 Gy + QTP + ATR that included at least 3 independent biological repeats. Radiation and quetiapine upregulated fatty acid, diglyceride or diacylglycerol, and cholesterol ester synthesis, and this effect was reversed by the addition of atorvastatin. (D) Treatment plan for patient-derived orthotopic xenografts using an image-guided small animal irradiator. A combination of QTP and ATR (both 30 mg/kg) with a single dose of radiation (10 Gy) statistically significantly prolongs survival in the GL261 glioma mouse model (hazard ratio [HR] of control vs 10 Gy and quetiapine and atorvastatin = 8.33, 95% confidence interval [CI] = 2.50 to 27.77) (E) and HK-374 patient-derived orthotopic xenografts (F) (log-rank HR of control vs 10 Gy and quetiapine and atorvastatin = 5.94, 95% CI = 2.21 to 15.95). All statistical tests were 2-sided.
Discussion
Radiotherapy is one of the main pillars in the standard of care for patients suffering from glioblastoma and one of the few modalities that robustly prolongs the survival of the patients over surgery alone (21). Out of many attempts that added conventional chemotherapy or targeted therapies to the standard of care, so far only temozolomide met the threshold to be included in the treatment regimen (22). However, despite decades of effort, the overall survival of patients with glioblastoma remains unacceptably low, thus indicating an unmet need for novel treatment options against glioblastoma.
Recent reports in the literature have suggested an antitumor activity of dopamine receptor type 2 antagonists against glioblastoma (9,23-25), and dopamine receptors have been found to be expressed in various tumor types including glioblastoma (8). However, the antitumor efficacy of dopamine receptor antagonists as single agents was found to be limited in preclinical (26) and clinical studies (14).
We have recently reported that a combination of a single high dose of radiation and continuous application of the first-generation dopamine receptor antagonist trifluoperazine improved survival in mouse models of glioblastoma (11). Here, we report that quetiapine, a second-generation dopamine receptor antagonist with a more favorable side effect profile than trifluoperazine, combined with radiation also shows efficacy against glioma-initiating cells in vitro and prolongs survival in mouse models of glioblastoma without affecting the radiation sensitivity of glioma cells. Although the effect on survival was highly statistically significant and meaningful, most animals eventually showed tumor progression and ultimately succumbed to the disease. Our search for a possible resistance mechanism analyzing data obtained by RNA-Seq revealed an upregulation of key components of the cholesterol biosynthesis pathway in the response of glioblastoma cells to the combination of quetiapine and radiation.
Antipsychotics like quetiapine, commonly used for the treatment of mental disorders, have been associated with dyslipidemia (27,28) and are known to cause an upregulation in serum lipid levels (29,30). However, even high concentrations of quetiapine alone are not able to induce the cholesterol and fatty acid synthesis pathway in in vitro (31). The microenvironmental and molecular changes that drive this dyslipidemic effect of quetiapine are incompletely understood but have recently been linked to the activation of the Pregnane X Receptor signaling in the intestines (27).
Survival of Glioblastoma cells is known to depend on cholesterol (32). The potential beneficial impact of statin use in glioblastoma patients is discussed controversially (33-36), but a recent meta-analysis did not indicate a beneficial effect of statins in glioblastoma under the current standard of care (37). We report here that quetiapine treatment of glioblastoma cells but not irradiation alone led to a moderate upregulation of the key components of the cholesterol biosynthesis. This effect was statistically significantly amplified when quetiapine and radiation were combined and led to an upregulation of the intracellular levels of fatty acids and cholesterol esters species. Additionally, although the normal brain contains cholesterol, only its free form, sterol esters, has long been known to be specific for glioblastomas (38). In agreement with the literature, inhibition of the cholesterol biosynthesis using atorvastatin by itself had some effect on the self-renewal capacity of glioma-initiating cells in vitro, and this was enhanced by the addition of quetiapine but failed to radiosensitize glioma-initiating cells. However, the combined treatment with radiation and quetiapine rendered the glioma-initiating cells and tumors sensitive to the addition of atorvastatin, resulting in statistically significantly reduced self-renewal capacity of glioma-initiating cells and increased median survival in mouse models of glioblastoma. Our study falls short of identifying the specific cholesterol esters that facilitate the survival in glioblastoma after combined treatment and the pathways they engage. Future studies will potentially identify novel targets in this pathway that interfere with this resistance mechanism more specifically.
In summary, we conclude that dopamine receptor antagonists are readily available, FDA-approved drugs with well-known toxicity profiles that enhance the efficacy of radiotherapy. Furthermore, the addition of atorvastatin can further improve survival, a treatment combination that can be easily tested in future clinical trials.
Funding
FP was supported by a grant from the National Cancer Institute (R01CA200234). DN, PLN, TC, LL, HK, and FP were supported by a grant from the National Cancer Institute (P50CA211015).
Notes
Role of the funder: The National Cancer Institute played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: TFC is cofounder, major stock holder and board member of Katmai Pharmaceuticals, Member of the board for the 501c3 Global Coalition for Adaptive Research, holds stock option of Notable Labs, has provided consulting services to QED, Roche, Trizel, Medscape, Bayer, Amgen, Odonate Therapeutics, Pascal Biosciences, Bayer, Del Mar Pharmaceuticals, Tocagen, Karyopharm, GW Pharma, Kiyatec, Abbvie, Boehinger Ingelheim, VBI, Deciphera, VBL, Agios, Merck, Roche, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable labs, Medqia and has contracts with UCLA for the Brain Tumor Program with Amgen, Abbvie, DNAtrix, Beigene, BMS, AstraZeneca, Kazia, Agios, Boston Biomedical, Deciphera, Tocagen, Orbus, AstraZeneca, Karyopharm. No potential conflicts of interests are disclosed by the other authors.
Author contributions: FP, PLN, TFC conceived of the study. KB, MS, FC, LH, LZ, AI, DN, JT, SJB performed the experiments, KB, MS and FP analyzed the data, LML and HIK provided materials, FP and KB drafted the manuscript. All authors contributed to the writing of the manuscript
Data Availability
All data generated and analyzed in this study are available from the corresponding author on reasonable request. The RNA-Seq data are accessible through GSE165624 (quetiapine) and GSE146358 (trifluoperazine).
Supplementary Material
References
- 1. van Linde ME, Brahm CG, de Witt Hamer PC, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135(1):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen RJ, Mehta MP.. Role of radiosensitizers in radiation treatment of gliomas. Prog Neurol Surg. 2018;31:102–115. [DOI] [PubMed] [Google Scholar]
- 3. Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100(25):15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 5. Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. [DOI] [PubMed] [Google Scholar]
- 6. Spencer DA, Auffinger BM, Murphy JP, et al. Hitting a moving target: glioma stem cells demand new approaches in glioblastoma therapy. Curr Cancer Drug Targets. 2017;17(3):236–254. [DOI] [PubMed] [Google Scholar]
- 7. Zhang L, Bochkur Dratver M, Yazal T, et al. Mebendazole potentiates radiation therapy in triple-negative breast cancer. Int J Radiat Oncol Biol Phys. 2019;103(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caragher SP, Shireman JM, Huang M, et al. Activation of dopamine receptor 2 prompts transcriptomic and metabolic plasticity in glioblastoma. J Neurosci. 2019;39(11):1982–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinheiro T, Otrocka M, Seashore-Ludlow B, et al. A chemical screen identifies trifluoperazine as an inhibitor of glioblastoma growth. Biochem Biophys Res Commun. 2017;494(3-4):477–483. [DOI] [PubMed] [Google Scholar]
- 10. Brami-Cherrier K, Valjent E, Garcia M, et al. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22(20):8911–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhat K, Saki M, Vlashi E, et al. The dopamine receptor antagonist trifluoperazine prevents phenotype conversion and improves survival in mouse models of glioblastoma. Proc Natl Acad Sci USA. 2020;117(20):11085–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall MD, Odia Y, Allen JE, et al. First clinical experience with DRD2/3 antagonist ONC201 in H3 K27M-mutant pediatric diffuse intrinsic pontine glioma: a case report. J Neurosurg Pediatr. 2019;5:1–7. [DOI] [PubMed] [Google Scholar]
- 13. Prabhu VV, Morrow S, Rahman Kawakibi A, et al. ONC201 and imipridones: anti-cancer compounds with clinical efficacy. Neoplasia. 2020;22(12):725–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chi AS, Tarapore RS, Hall MD, et al. Pediatric and adult H3 K27M-mutant diffuse midline glioma treated with the selective DRD2 antagonist ONC201. J Neurooncol. 2019;145(1):97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laks DR, Crisman TJ, Shih MY, et al. Large-scale assessment of the gliomasphere model system. Neuro Oncol. 2016;18(10):1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saki M, Bhat K, Sodhi SS, et al. Effects of brain irradiation in immune-competent and immune-compromised mouse models. Radiat Res. 2019;193(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang SY, Kao Yang YH, Chong MY, et al. Risk of extrapyramidal syndrome in schizophrenic patients treated with antipsychotics: a population-based study. Clin Pharmacol Ther. 2007;81(4):586–594. [DOI] [PubMed] [Google Scholar]
- 18. Holthusen H. Erfahrungen über die Verträglichkeitsgrenze für Röntgenstrahlen und deren Nutzanwendung zur Verhütung von Schäden. Strahlentherapie. 1936;(57):254–269. [Google Scholar]
- 19. Saki M, Bhat K, Sodhi SS, et al. Effects of brain irradiation in immune-competent and immune-compromised mouse models. Radiat Res. 2020;193(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eberle D, Hegarty B, Bossard P, et al. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–848. [DOI] [PubMed] [Google Scholar]
- 21. Walker MD, Strike TA, Sheline GE.. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–1731. [DOI] [PubMed] [Google Scholar]
- 22. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 23. Liu Z, Jiang X, Gao L, et al. Synergistic suppression of glioblastoma cell growth by combined application of temozolomide and dopamine D2 receptor antagonists. World Neurosurg. 2019;128:e468–e477. [DOI] [PubMed] [Google Scholar]
- 24. Chen X, Luo X, Cheng Y.. Trifluoperazine prevents FOXO1 nuclear excretion and reverses doxorubicin-resistance in the SHG44/DOX drug-resistant glioma cell line. Int J Mol Med. 2018;42(6):3300–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kang S, Lee JM, Jeon B, et al. Repositioning of the antipsychotic trifluoperazine: synthesis, biological evaluation and in silico study of trifluoperazine analogs as anti-glioblastoma agents. Eur J Med Chem. 2018;151:186–198. [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Xu R, Zhang C, et al. Trifluoperazine, a novel autophagy inhibitor, increases radiosensitivity in glioblastoma by impairing homologous recombination. J Exp Clin Cancer Res. 2017;36(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng Z, Gwag T, Sui Y, et al. The atypical antipsychotic quetiapine induces hyperlipidemia by activating intestinal PXR signaling. JCI Insight. 2019;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olfson M, Marcus SC, Corey-Lisle P, et al. Hyperlipidemia following treatment with antipsychotic medications. Am J Psyhiatry. 2006;163(10):1821–1825. [DOI] [PubMed] [Google Scholar]
- 29. Meyer JM, Koro CE.. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70(1):1–17. [DOI] [PubMed] [Google Scholar]
- 30. de Leon J, Susce MT, Johnson M, et al. A clinical study of the association of antipsychotics with hyperlipidemia. Schizophr Res. 2007;92(1-3):95–102. [DOI] [PubMed] [Google Scholar]
- 31. Lauressergues E, Staels B, Valeille K, et al. Antipsychotic drug action on SREBPs-related lipogenesis and cholesterogenesis in primary rat hepatocytes. Naunyn-Schmied Arch Pharmacol. 2010;381(5):427–439. [DOI] [PubMed] [Google Scholar]
- 32. Villa GR, Hulce JJ, Zanca C, et al. An LXR-cholesterol axis creates a metabolic co-dependency for brain cancers. Cancer Cell. 2016;30(5):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seliger C, Schaertl J, Gerken M, et al. Use of statins or NSAIDs and survival of patients with high-grade glioma. PLoS One. 2018;13(12):e0207858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shojaei S, Alizadeh J, Thliveris J, et al. Statins: a new approach to combat temozolomide chemoresistance in glioblastoma. J Investig Med. 2018;66(8):1083–1087. [DOI] [PubMed] [Google Scholar]
- 35. Bhavsar S, Hagan K, Arunkumar R, et al. Preoperative statin use is not associated with improvement in survival after glioblastoma surgery. J Clin Neurosci. 2016;31:176–180. [DOI] [PubMed] [Google Scholar]
- 36. Gaist D, Hallas J, Friis S, et al. Statin use and survival following glioblastoma multiforme. Cancer Epidemiol. 2014;38(6):722–727. [DOI] [PubMed] [Google Scholar]
- 37. Happold C, Gorlia T, Nabors LB, et al. ; for the EORTC Brain Tumor Group and on behalf of the CENTRIC and CORE Clinical Trial Groups. Do statins, ACE inhibitors or sartans improve outcome in primary glioblastoma? J Neurooncol. 2018;138(1):163–171. [DOI] [PubMed] [Google Scholar]
- 38. White HB Jr, Smith RR.. Cholesteryl esters of the glioblastoma. J Neurochem. 1968;15(4):293–299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed in this study are available from the corresponding author on reasonable request. The RNA-Seq data are accessible through GSE165624 (quetiapine) and GSE146358 (trifluoperazine).