Abstract
Background
Nonadherence to aromatase inhibitors (AIs) is common and increases risk of breast cancer (BC) recurrence. We analyzed factors associated with nonadherence among patients enrolled in S1105, a randomized trial of text messaging.
Methods
At enrollment, patients were required to have been on an adjuvant AI for at least 30 days and were asked about financial, medication, and demographic factors. They completed patient-reported outcomes (PROs) representing pain (Brief Pain Inventory), endocrine symptoms (Functional Assessment of Cancer Therapy–Endocrine Symptoms), and beliefs about medications (Treatment Satisfaction Questionnaire for Medicine; Brief Medication Questionnaire). Our primary endpoint was AI nonadherence at 36 months, defined as urine AI metabolite assay of less than 10 ng/mL or no submitted specimen. We evaluated the association between individual baseline characteristics and nonadherence with logistic regression. A composite risk score reflecting the number of statistically significant baseline characteristics was examined.
Results
We analyzed data from 702 patients; median age was 60.9 years. Overall, 35.9% patients were nonadherent at 36 months. Younger patients (younger than age 65 years) were more nonadherent (38.8% vs 28.6%, odds ratio [OR] = 1.51, 95% confidence interval [CI] = 1.05 to 2.16; P = .02). Fourteen baseline PRO scales were each statistically significantly associated with nonadherence. In a composite risk model categorized into quartile levels, each increase in risk level was associated with a 46.5% increase in the odds of nonadherence (OR = 1.47, 95% CI =1.26 to 1.70; P < .001). The highest-risk patients were more than 3 times more likely to be nonadherent than the lowest-risk patients (OR = 3.14, 95% CI = 1.97 to 5.02; P < .001).
Conclusions
The presence of multiple baseline PRO-specified risk factors was statistically significantly associated with AI nonadherence. The use of these assessments can help identify patients for targeted interventions to improve adherence.
Despite the proven efficacy of aromatase inhibitors (AIs) for the treatment and prevention of hormone-sensitive breast cancer (BC) (1,2), full adherence to therapy for the duration of 5 years is only 50% (3). Nonadherence to endocrine therapy is associated with worsened disease-free survival (4). Numerous observational studies have evaluated the reasons for nonadherence to hormonal therapy (5-8). Barriers include patient, physician, medication, and system-related variables. Poor adherence is usually associated with a combination of these factors, although adverse effects from the medication appear to be the primary reason for discontinuation (6–8). It remains unclear how to identify patients early who are at the highest risk of nonadherence.
Patient-reported outcomes (PROs) are standardized measures used to obtain the patient’s perspective. Use of PROs improves patient-provider communication and patient satisfaction (9). In advanced cancer, use of a web-based tool to actively monitor symptoms between clinic visits improved health-related quality of life (HRQOL) (10) and increased overall survival (11,12). Serial symptom monitoring using electronic PROs has also resulted in greater attention to and better management of symptoms (13). Less is known about whether PROs can be used to predict other outcomes, such as therapy adherence, especially among patients with nonmetastatic cancer.
SWOG S1105 was a prospective, multicenter, randomized trial conducted to determine if a text message educational intervention improved AI adherence (14). No effect of the intervention was observed on the rates of adherence, because a one-size-fits-all approach to interventions will likely not suffice (15). A strength of the study was detailed prospective assessment of adherence with a urinary biomarker, a measure of adherence thought to be less subject to false-positive findings than self-report. The study collected PROs as well as other factors potentially associated with adherence; using this large study cohort, we examined the association between PROs and nonadherence at 3 years from enrollment.
Methods
Study Design
Patients were randomly assigned to receive either text messaging or no text messaging. Patients were assessed at enrollment and every 3 months for 36 months. The study was conducted after approval by individual institutional review boards (Columbia University AAAJ8301). Patients were aware they were being monitored for medication adherence. The study was registered with ClinicalTrials.gov (NCT01515800).
Patient Characteristics
Subjects were women with stage I-III BC. Patients were required to be taking a third-generation AI for at least 30 days prior to registration and have at least 3 years of AI therapy remaining.
Covariates and Patient-Reported Outcomes
Covariates and PROs were selected based on factors potentially related to adherence to AIs (5–8). Medication and socioeconomic information was collected at enrollment by patient self-report, including sociodemographic characteristics (age, race and ethnicity, education, annual household income, and insurance status), number of medications per day, number of pills per refill (30 vs 90 pills), use of mail-order pharmacy, and out-of-pocket cost. Prior treatment variables included the protocol-specified study stratification factors AI duration (coded <12 months vs 12-24 months) and AI type, as well as prior chemotherapy and prior radiation therapy.
The Brief Pain Inventory Short Form (BPI-SF) was used to assess arthralgias, the most common adverse effect from AIs (8). The BPI is a measure used to assess cancer pain (16). The BPI-SF is a 14-item questionnaire that asks subjects to rate joint pain over the prior week and the degree to which it interferes with activities on a 0 to 10 scale, where higher scores indicate more pain or interference. This form was modified to include stiffness. For this analysis, we examined individual items reflecting worst pain, least pain, average pain, and pain interference within the past 7 days as well as pain right now.
The Functional Assessment of Cancer Therapy—Endocrine Symptoms (FACT-ES) measures physical, social, family, emotional, and functional well-being; a subscale reflecting the total of these domains (the FACT-general [G]); and endocrine symptoms (17). The FACT scales have 5 response levels (“not at all” to “very much”), where higher scores reflect better well-being and fewer symptoms. This scale provides a broader measure of HRQOL that may be impacted by endocrine symptoms, especially joint pain and stiffness. We examined each individual well-being domain, the FACT-G total score, and the FACT-ES total score (the HRQOL domains plus endocrine symptoms).
The Beliefs about Medication Questionnaire (BMQ) is a 5-item scale assessing patients’ beliefs about the necessity of prescribed medication for controlling their disease and their concerns about potential adverse consequences of not taking it. This was adapted to specifically ask about the AI therapy. Respondents indicated their degree of agreement with each statement on a 5-point Likert scale (“strongly disagree” to “strongly agree”). Scores obtained for individual items within all scales are summed (18).
The Treatment Satisfaction Questionnaire for Medication (TSQM) (19) is a 14-item instrument (with items scored 0-100) to assess patients’ satisfaction with AI medication, providing scores for 4 scales (side effects, effectiveness, convenience, and global satisfaction). Higher scores indicate greater treatment satisfaction.
Urine Assay and Patient-Reported Adherence Measures
Assessments were collected at enrollment and every 3 months for 36 months. The scheduled time window for each 3-month follow-up assessment was +/-21 days. Urine assays to detect the presence of aromatase inhibitor and metabolites were used for the primary endpoint (20). The assays are highly sensitive and were conducted by Sports Medicine Research and Testing Laboratory by either liquid chromatography or tandem mass spectrometry for anastrozole and exemestane and its metabolites or gas chromatography or mass spectrometry for letrozole and its metabolites (14).
Statistical Analysis
The endpoint of interest for this analysis was nonadherence to AI, defined as a negative urine test (assay < 10 ng/mL) at 36 months ± 6 months or no urine test during this time frame. Associations of factors at enrollment with 36-month nonadherence were analyzed using multivariable logistic regression, adjusting for the study stratification factors (AI duration and type) as covariates and stratifying by treatment arm (text messaging vs no text messaging). The association of AI duration and AI type with nonadherence was examined separately; however, because these factors were included a priori as covariates in the regression analyses (because they were specified as stratification factors), they were not included in the risk model. PRO scale scores at enrollment were converted to binary indicator variables, split at the observed median. Nonoverlapping individual baseline PRO scales (that is, scales that contained distinct questions, none of which were repeated in different scales) that were statistically significantly associated with nonadherence (P < .05) in these models were summed to create a composite risk score, with patients receiving 1 point for each adverse risk factor they had at baseline (Figure 1). Analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC) and R-version-4.0.2 (R-Project for Statistical Computing). Statistical tests in logistic regression were conducted using a Wald test. Differences in baseline characteristics between patients based on prior AI duration were tested using Wilcoxon tests (for continuous variables) and χ2 tests (or Fisher exact tests for cell counts <5) for categorical variables. All statistical tests were 2-sided. P values less than .05 were considered statistically significant.
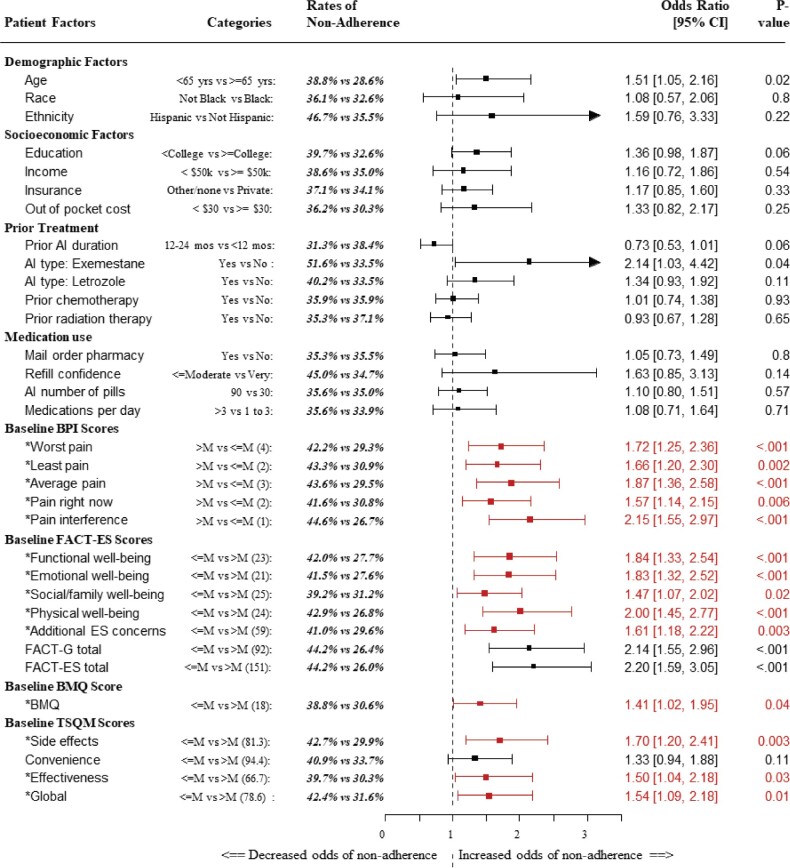
Figure 1.
Results of logistic regression models with nonadherence at 36 months (±6 months) coded as a binary outcome and the protocol-specified stratification variables AI duration and AI type as covariates. AI type (exemestane vs letrozole vs anastrozole) was coded using indicator variables, with anastrozole as the reference category. Each model was stratified by treatment arm. PRO scores were split at the median value, which is shown in parentheses. Baseline factors included in the PRO-based composite baseline risk score are represented with an asterisk. P values were derived from Wald tests in logistic regression analyses. AI = aromatase inhibitor; BMQ = Brief Medication Questionnaire; BPI = Brief Pain Inventory; CI = confidence interval; ES = endocrine symptoms; FACT-ES = Functional Assessment of Cancer Therapy–Endocrine Symptoms; FACT-G = Functional Assessment of Cancer Therapy–General; M = median; PRO = patients-reported outcomes; TSQM = Treatment Satisfaction Questionnaire for Medicine.
Additional Analyses
As a sensitivity analysis, any sociodemographic variables associated with nonadherence were also included in the composite score, with the adverse risk level counted as a risk factor. The association between level of the composite risk score and 36-month nonadherence was analyzed as stated above, treating risk group as a numeric factor, adjusting for AI duration and type, and stratifying by treatment arm. Additionally, to account for the potential that adherence may fluctuate over time, we evaluated the risk model at other time points throughout follow-up (at 1.0, 1.5, 2.0, 2.5 years), using the same approach as used for the 3-year assessment time. Finally, we examined whether risk of nonadherence differed between patients who received prior AI therapy in less than 12 months vs 12-24 months.
Results
Patient Characteristics
A total of 724 patients were registered from 40 institutions between May 2012 and September 2013, thus the study met its primary enrollment goal (14). Of the patients, 22 were ineligible; therefore, 702 eligible patients are included in these analyses. Baseline characteristics of the cohort are displayed in Table 1. The median age was 60.9 years. Thirty patients (4.3%) were of Hispanic origin, and 46 (6.6%) were Black. The majority of patients (64.5%) had received AI therapy for less than 12 months prior to randomization. The predominant AI type was anastrozole (71.5%).
Table 1.
Baseline patient characteristics (n = 702)
| Characteristic | No. (%) |
|---|---|
| Median age (range), y | 60.9 (30.7-82.4) |
| SD | 7.3 |
| Hispanic | |
| Yes | 30 (4.3) |
| No | 668 (95.2) |
| Unknown | 4 (0.6) |
| Race | |
| White | 625 (89.0) |
| Black | 46 (6.6) |
| Asian | 16 (2.3) |
| Other | 15 (2.1) |
| Aromatase inhibitor duration | |
| 12-24 months | 249 (35.5) |
| <12 months | 453 (64.5) |
| Aromatase inhibitor type | |
| Anastrozole | 502 (71.5) |
| Exemestane | 31 (4.4) |
| Letrozole | 169 (24.1) |
| Stage | |
| Stage I | 444 (63.2) |
| Stage II-III | 258 (36.8) |
| No. of comorbiditiesa | |
| 0 | 121 (17.2) |
| 1 | 300 (42.7) |
| 2 | 187 (26.6) |
| >2 | 94 (13.4) |
| Performance statusb | |
| 0 | 617 (87.9) |
| 1 | 80 (11.4) |
| 2 | 5 (0.7) |
| Prior chemotherapy | |
| No | 396 (56.4) |
| Yes | 306 (43.6) |
| Prior radiation | |
| No | 240 (34.2) |
| Yes | 462 (65.8) |
| Prior tamoxifen | |
| No | 659 (93.9) |
| Yes | 43 (6.1) |
Baseline comorbidities included renal insufficiency; abnormal pulmonary function; hypertension; heart disease; hearing impairment; visual impairment; deformity or orthopedic impairment; diminished mental capacity, neurologic impairment, and/or dementia; arthritis; HIV or AIDS; and other, as assessed by the treating site.
Performance status was graded according to the Zubrod performance status scale.
Clinical Factors and Nonadherence
At baseline, 3.7% of the patients were nonadherent to AIs. At 36 months, 35.9% of patients were nonadherent. Younger age (younger than 65 years) was associated with greater nonadherence compared with those 65 years or older (38.8% vs 28.6%, odds ratio [OR] = 1.51, 95% confidence interval [CI] = 1.05 to 2.16; P = .02). With respect to the model stratification variables, patients with longer prior AI duration were marginally less likely to be nonadherent (12-24 months, 31.3%; <12 months, 38.4%; OR = 0.73, 95% CI = 0.53 to 1.01; P =.06). Further, nonadherence was statistically significantly higher for patients receiving exemestane compared with anastrazole (51.6% vs 33.5%, OR = 2.14, 95% CI = 1.03 to 4.42; P =.04). Neither race, ethnicity, socioeconomic factors, nor other prior treatment variables (chemotherapy or radiation therapy) were associated with nonadherence. Similarly, medication-related factors were not associated with nonadherence (Figure 1).
Patient-Reported Outcomes and Nonadherence
AI nonadherence was greater at 36 months among those with more baseline endocrine symptoms, higher pain, and worse HRQOL. Similarly, nonadherence was greater among those with less baseline satisfaction with medications and lower scores on beliefs about medication (Figure 1). With the exception of the TSQM convenience score, for every baseline PRO, worse scores were statistically significantly associated with greater nonadherence. With respect to pain scores, the BPI item demonstrating the greatest association with nonadherence was the pain interference score (>median 44.6% vs ≤median 26.7%; OR = 2.15, 95% CI = 1.55 to 2.97; P < .001). Patients with worse HRQOL according to the FACT-ES total score had much greater risk of nonadherence (≤median 44.2% vs >median 26.0%; OR = 2.20, 95% CI = 1.59 to 3.05; P < .001). Among the FACT-G domains, worse physical well-being demonstrated the greatest association with nonadherence (≤median 42.9% vs >median 26.8%; OR = 2.00, 95% CI = 1.45 to 2.77; P < .001). Among domains of treatment satisfaction, the baseline TSQM side effects score was most associated with nonadherence (≤median 42.7% vs >median 29.9%; OR = 1.70, 95% CI = 1.20 to 2.41; P = .003).
Composite Risk Score and Nonadherence
The nonoverlapping PRO domains statistically significantly associated with nonadherence included the BPI worst, least, and average pain scores; pain right now and pain interference scores; the FACT-ES functional, emotional, social, family, and physical well-being scores and the additional ES concerns scale; the BMQ score; and the TSQM side effects, effectiveness, and global scores. Patients were categorized according to the number of adverse categories of the 14 domains they reported. The 4 categories of the composite risk score, distributed into quartiles, were 1 (0-3, n = 186), 2 (4-7, n = 206), 3 (8-10, n = 163), and 4 (11-14, n = 147) (Figure 2). For each increase in category level, the odds of nonadherence increased by 46.5% (OR = 1.47, 95% CI = 1.26 to 1.70; P < .001). The findings were similar when the endpoint assessment time was allowed to vary from 1.0 to 2.5 years in 6-month intervals (Table 2). Results were similar when age was included in the risk model (OR = 1.45, 95% CI = 1.25 to 1.69; P < .001). The highest-risk patients (group 4, 11-14 risk factors) were more than 3 times as likely to be nonadherent at 36 months than the lowest-risk patients (52.4% vs 25.3%; OR = 3.14, 95% CI = 1.97 to 5.02; P < .001; Figure 3). Results were very similar when age was included (data not shown).
Figure 2.
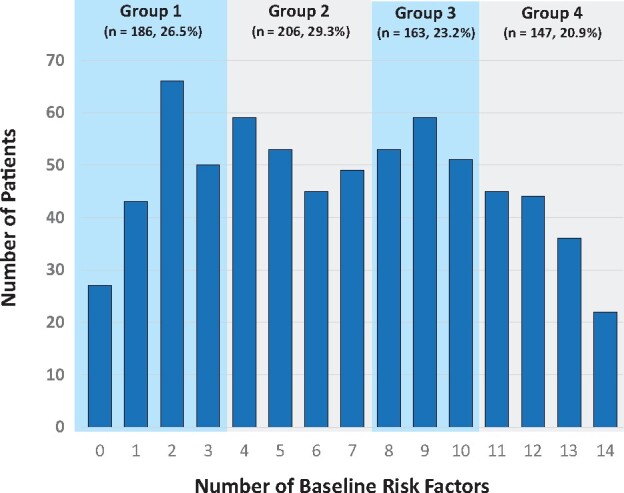
Distribution of number of baseline risk factors. Patients are categorized into groups based on their number of risk factors at baseline: 0-3, 4-7, 8-10, or 11-14. Risk factors included the 14 nonoverlapping patients-reported outcomes domain scores that were statistically significantly associated with nonadherence, including BPI-SF worst, least, average, and right now pain, and pain interference; FACT functional, emotional, social, and physical well-being and additional ES concerns; BMQ; and TSQM side effects, effectiveness, and global scales. BMQ = Beliefs about Medication Questionnaire; BPI-SF = Brief Pain Inventory Short Form; FACT-ES = Functional Assessment of Cancer Therapy–Endocrine Symptoms; TSQM = Treatment Satisfaction Questionnaire for Medicine.
Table 2.
Evaluation of the derived risk model at different assessment times
| Nonadherence time | Model | Model resultsa |
||
|---|---|---|---|---|
| Level (%) | OR (95% CI) | P b | ||
| 1.0 years | 4 independent groups | 0-3 (10.2) | 1.00 (referent) | — |
| 4-7 (10.2) | 0.97 (0.5 to 1.87) | .93 | ||
| 8-10 (14.1) | 1.38 (0.72 to 2.66) | .34 | ||
| 11-14 (19.7) | 1.98 (1.05 to 3.74) | .04 | ||
| Ordinal categorical variable | 1 unit increase | 1.28 (1.04 to 1.58) | .02 | |
| 1.5 years | 4 independent groups | 0-3 (12.9) | 1.00 (referent) | — |
| 4-7 (15.5) | 1.22 (0.69 to 2.17) | .49 | ||
| 8-10 (19.0) | 1.55 (0.87 to 2.79) | .14 | ||
| 11-14 (32.7) | 3.13 (1.79 to 5.46) | <.001 | ||
| Ordinal categorical variable | 1 unit increase | 1.47 (1.23 to 1.75) | <.001 | |
| 2.0 years | 4 independent groups | 0-3 (15.6) | 1.00 (referent) | — |
| 4-7 (18.4) | 1.20 (0.70 to 2.05) | .50 | ||
| 8-10 (27.0) | 1.97 (1.16 to 3.35) | .01 | ||
| 11-14 (37.4) | 3.07 (1.82 to 5.20) | <.001 | ||
| Ordinal categorical variable | 1 unit increase | 1.48 (1.26 to 1.75) | <.001 | |
| 2.5 years | 4 independent groups | 0-3 (19.4) | 1.00 (referent) | — |
| 4-7 (24.3) | 1.32 (0.81 to 2.15) | .26 | ||
| 8-10 (32.5) | 1.97 (1.20 to 3.23) | .01 | ||
| 11-14 (44.9) | 3.24 (1.98 to 5.30) | <.001 | ||
| Ordinal categorical variable | 1 unit increase | 1.49 (1.27 to 1.74) | <.001 | |
| 3.0 years | 4 independent groups | 0-3 (25.3) | 1.00 (referent) | — |
| 4-7 (31.1) | 1.31 (0.84 to 2.04) | .24 | ||
| 8-10 (39.3) | 1.89 (1.19 to 2.99) | .01 | ||
| 11-14 (52.4) | 3.14 (1.97 to 5.02) | <.001 | ||
| Ordinal categorical variable | 1 unit increase | 1.47 (1.26 to 1.7) | <.001 | |
Results of logistic regression models with nonadherence at 1.0 years up to 3.0 years, by 6-month intervals. For each assessment time, a window of ±6 months was allowed, consistent with the primary analysis at 3.0 years. Model covariates included the protocol-specified stratification variables AI duration and AI type. AI type (exemestane vs letrozole vs anastrozole) was coded using indicator variables, with anastrozole as the reference category. Each model was stratified by treatment arm. AI = aromatase inhibitor; CI = confidence interval; OR = odds ratio.
Comparisons were tested using a Wald test in logistic regression. All tests were 2-sided.
Figure 3.
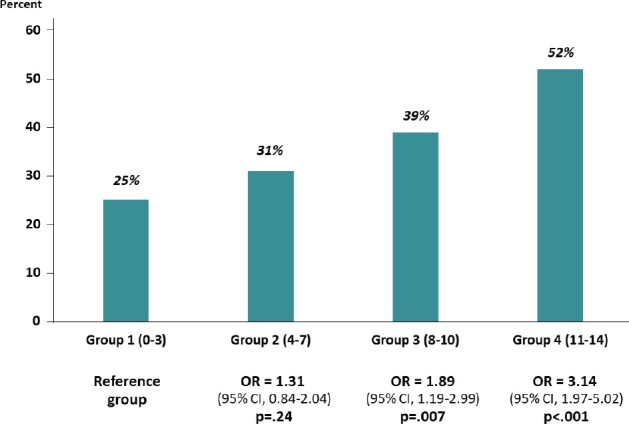
Observed rates of nonadherence according to level of risk. Risk factors included the nonoverlapping patient-report outcome domain scores that were statistically significantly associated with nonadherence, including BPI-SF worst, least, average, and right now pain, and pain interference; FACT functional, emotional, social, and physical well-being and additional ES concerns; BMQ; and TSQM side effects, effectiveness, and global scales. P values were derived from Wald tests in logistic regression analyses. BMQ = Beliefs about Medication Questionnaire; BPI-SF = Brief Pain Inventory Short Form; CI = confidence interval; ES = endocrine symptoms; OR = odds ratio; FACT-ES = Functional Assessment of Cancer Therapy–Endocrine Symptoms; TSQM = Treatment Satisfaction Questionnaire for Medicine.
The baseline PRO measures were also evaluated as continuous variables rather than as binary indicator variables, split at the median. Results were similar (Supplementary Table 1, available online), with 1 additional PRO (the TSQM convenience baseline score) identified as statistically significantly associated with nonadherence. In a revised risk model, for each increase in risk level from 0-3 risk factors (24.9% nonadherence) to 4-7 risk factors (30.6% nonadherence) to 8-10 risk factors (37.5% nonadherence) to 11-15 risk factors (52.1%), the odds of nonadherence increased by 46.4% (OR = 1.46, 95% CI = 1.27 to 1.69; P <.001), very similar to our base case model, which showed an increased odds of nonadherence of 46.5% for each increase in risk level. Additionally, the highest-risk patients (11-15 factors) were more than 3 times as likely to be nonadherent at 36 months than the lowest-risk patients (OR = 3.16, 95% CI = 2.00 to 5.01; P <.001).
Further, there were no statistically significant differences in baseline characteristics between patients based on prior AI duration, with the exception of age (Supplementary Table 2, available online). With risk categorized as an ordinal categorical variable, we found no evidence of an interaction between the risk score and prior AI duration (P = .59). In the subgroup of patients with prior AI duration less than 12 months prior, there was a 52.9% increase in risk for each increase in risk level (OR = 1.53, 95% CI = 1.27 to 1.84; P <.001), and in the subgroup of patients with AI duration 12-24 months, there was a 37.6% increase in risk for each increase in risk level (OR = 1.38, 95% CI = 1.07 to 1.77; P = .01).
Discussion
In this large, prospective, multicenter trial, among patients on AIs for at least 30 days, we found that the 3-year nonadherence rates to AIs, based on urinary biomarkers, was high at 35.9%. Long-term AI nonadherence was greater among those with more baseline endocrine symptoms, higher pain, and worse HRQOL, and AI nonadherence was also greater among those with less satisfaction with medications and lower scores on beliefs about medication. Furthermore, when baseline PRO scores were combined into a composite risk score and grouped into 4 distinct risk groups, each increase in level of risk group increased the odds of nonadherence by 46.5%. The findings were similar at multiple assessment times, suggesting that the association of risk and adherence was reasonably constant over time. Because PROs are increasingly used in clinical practice, patients at high risk for nonadherence can be identified early after therapy initiation for monitoring and interventions.
Prior studies using PROs to evaluate AIs have shown that women who report symptoms prior to the initiation of AIs are at greater risk of treatment discontinuation (21). In a prospective substudy of 686 women enrolled in MA-27, 32% of patients discontinued therapy prior to completion at 5 years. Patients who reported moderate or severe symptoms prior to initiation had nearly a twofold higher rate of discontinuation (21). Similar results were reported in a cohort study of about 400 patients, showing that women with multiple menopausal symptoms prior to AI initiation had an 89% increase in the odds of treatment discontinuation (22).
Other studies have shown that early treatment emergent symptoms on AIs also predict nonadherence; however, the degree of nonadherence varies substantially from study to study, and the majority of these studies evaluated adherence at 12 months. In a study of approximately 3800 women, self-reported discontinuation at 12 months was 14.6%; among those who discontinued, 63% discontinued because of early side effects. The most common side effects reported were sleep disorders, musculoskeletal syndrome, or menopausal disorders; each was associated with approximately a twofold increased risk of discontinuation (23). Similarly, in a prospective study of 500 patients, 32% of patients discontinued therapy over a 2-year period because adverse effects, and the median time to discontinuation was 6 months (8). Patients enrolled on S1105 had been on AIs for at least 30 days, and more than 30% had received AIs between 12 and 24 months at the time of enrollment. Patients with early treatment emergent toxicities may have discontinued AI therapy quickly and would therefore be ineligible for this trial.
Symptom management is a cornerstone of clinical care. However, symptoms, subjective toxicities, and impaired functioning can go undetected by clinicians (24). Several studies have supported the routine collection of PROs with feedback in clinical practice to overcome these barriers. A systematic review of studies using PROs showed a benefit of enhancing patient-provider communication, improving patient satisfaction, and detecting unrecognized problems (9). A more recent randomized controlled trial of electronic patient-reported symptom monitoring vs usual care in advanced cancer patients showed a survival improvement in the PRO group (12). Our data supports routine use of PROs to identify patients at highest risk of AI discontinuation.
A strength of our study was the use of a biomarker to assess endocrine therapy adherence. Determining adherence to adjuvant hormonal therapy and assessing treatment discontinuation can be methodologically challenging because many measures result in bias, which can make it difficult to compare results across studies (6,20). In addition, patients were followed prospectively over 3 years. Prior studies have assessed short-term discontinuation.
The study also has potential limitations. Because patients enrolled had already initiated AI therapy, the reported nonadherence rate for this study may not reflect nonadherence for individuals first initiating AI therapy. Moreover, we recognize that patients may have been more likely to remain adherent because they were aware they were being monitored and enrolled in a randomized clinical trial. This may limit generalizability. Nonetheless, the nonadherence rate was high and generally reflected other studies in the literature (25). We evaluated PROs related to symptoms and HRQOL as well as satisfaction and beliefs about medications. Although we did not find an association between economic- and insurance-related factors and nonadherence, patients with economic barriers may have been less likely to enroll in a randomized trial of a text messaging intervention (26). Additionally, although the PRO items in the risk model asked distinct, nonoverlapping questions, they represented conceptually overlapping domains.
A key consideration for risk models is understanding the extent to which each variable contributes independent information sufficient to justify its inclusion in the model. In this study, we built a simplified model that included all statistically significant variables; moreover, we did not attempt to establish cutpoints that optimized differences between patients with respect to observed adherence, and further, some power may have been lost by dichotomizing continuous variables in variable selection. Even in the context of this limited, agnostic approach, we observed a pronounced dose-response pattern, suggesting sufficient independent information among the PRO scores. This affirms the utility of our approach and establishes the principle that risk of nonadherence to AI therapy can be predicted based on patient reports. A future analysis that independently verifies the predictive capacity of PRO domains in this setting is required before any clinical decision tool can be reliably established. Further, our study cannot rule out the possibility that some reverse causation exists, whereby greater adherence leads to more symptoms. Finally, the study was limited to postmenopausal women.
In conclusion, in a prospective, multicenter study that included women on AIs for less than 24 months, worse PRO scores were associated with higher rates of nonadherence. Moreover, the impact was cumulative, with patients with the highest number of composite PRO-based risk factors having more than 3 times the odds of nonadherence as patients with the fewest such risk factors. These observations suggest that PROs identifying individuals with greater baseline symptoms, worse HRQOL, more limited satisfaction, and belief in medication use—and the accumulation of these factors—can be used to guide care for BC survivors by establishing which patients may be at highest risk of AI discontinuation. Such a strategy may be especially advantageous for interventions such as duloxetine or acupuncture that address symptoms such as arthralgia (27,28). A priority will be to determine if early symptom management will reduce symptoms and improve adherence to AI therapy.
Funding
NIH/NCI/DCP grant UG1CA189974 and legacy grant U10CA37429. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funding also provided by the Conquer Cancer Foundation and the Breast Cancer Research Foundation.
Notes
Role of the funder: The funder had no role in the conduct of the trial, the analysis, or the writing of the manuscript.
Disclosures: The authors have no disclosures to report
Author contributions: Conceptualization—Hershman, Unger. Data curation—Hershman, Moseley, Unger. Formal analysis—Unger, Moseley. Funding acquisition—Hershman, Unger, Neugut. Investigation—All. Methodology—Unger, Moseley, Hershman. Project administration—Hershman, Unger. Resources—All. Software—Moseley, Unger. Supervision—Hershman, Unger, Neugut. Validation—Moseley, Unger. Visualization—Unger, Moseley, Hershman. Writing—original draft—Hershman, Unger. Writing—review & editing—All.
Prior presentations: The results were presented at a poster discussion session at the virtual ASCO 2020 meeting.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28(23):3784–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–1048. [DOI] [PubMed] [Google Scholar]
- 3. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chirgwin JH, Giobbie-Hurder A, Coates AS, et al. Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 trial of tamoxifen and letrozole, alone and in sequence. J Clin Oncol. 2016;34(21):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hershman DL. Sticking to it: improving outcomes by increasing adherence. J Clin Oncol. 2016;34(21):2440–2442. [DOI] [PubMed] [Google Scholar]
- 6. Accordino MK, Hershman DL.. Disparities and challenges in adherence to oral antineoplastic agents. Am Soc Clin Oncol Educ Book. 2013;(33):271–276. [DOI] [PubMed] [Google Scholar]
- 7. Chim K, Xie SX, Stricker CT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer survivors. BMC Cancer. 2013;13(1):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30(9):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Ou L, Hollis SJ.. A systematic review of the impact of routine collection of patient reported outcome measures on patients: providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Basch E, Deal AM, Kris MG, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol. 2016;34(6):557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denis F, Lethrosne C, ourel N, et al. Randomized trial comparing a web-mediated follow-up with routine surveillance in lung cancer patients. J Natl Cancer Inst. 2017;109(9):436. [DOI] [PubMed] [Google Scholar]
- 12. Basch E, Deal AM, Dueck AC, et al. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berry DL, Hong F, Halpenny B, et al. Electronic self-report assessment for cancer and self-care support: results of a multicenter randomized trial. J Clin Oncol. 2014;32(3):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hershman DL, Unger JM, Hillyer GC, et al. Randomized trial of text messaging to reduce early discontinuation of adjuvant aromatase inhibitor therapy in women with early-stage breast cancer: SWOG S1105. J Clin Oncol. 2020;38(19):2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Easthall C, Barnett N.. Using theory to explore the determinants of medication adherence: moving away from a one-size-fits-all approach. Pharmacy (Basel). 2017;5(4):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen MP, Turner JA, Romano JM, Fisher LD.. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83(2):157–162. [DOI] [PubMed] [Google Scholar]
- 17. Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. [DOI] [PubMed] [Google Scholar]
- 18. Horne R, Weinman J.. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47(6):555–567. [DOI] [PubMed] [Google Scholar]
- 19. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2(12):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clarke Hillyer G, Neugut AI, Crew KD, et al. Use of a urine anastrozole assay to determine treatment discontinuation among women with hormone-sensitive breast cancer: a pilot study. J Oncol Pract. 2012;8(5):e100-104–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner LI, Zhao F, Goss PE, et al. Patient-reported predictors of early treatment discontinuation: treatment-related symptoms and health-related quality of life among postmenopausal women with primary breast cancer randomized to anastrozole or exemestane on NCIC Clinical Trials Group (CCTG) MA.27 (E1Z03). Breast Cancer Res Treat. 2018;169(3):537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kidwell KM, Harte SE, Hayes DF, et al. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer. 2014;120(16):2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nabieva N, Fehm T, Haberle L, et al. Influence of side-effects on early therapy persistence with letrozole in post-menopausal patients with early breast cancer: results of the prospective EvAluate-TM study. Eur J Cancer. 2018;96:82–90. [DOI] [PubMed] [Google Scholar]
- 24. Di Maio M, Gallo C, Leighl NB, et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J Clin Oncol. 2015;33(8):910–915. [DOI] [PubMed] [Google Scholar]
- 25. Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A.. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26(4):556–562. [DOI] [PubMed] [Google Scholar]
- 26. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henry NL, Unger JM, Schott AF, et al. Randomized, multicenter, placebo-controlled clinical trial of duloxetine versus placebo for aromatase inhibitor-associated arthralgias in early-stage breast cancer: SWOG S1202. J Clin Oncol. 2018;36(4):326-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hershman DL, Unger JM, Greenlee H, et al. Effect of acupuncture vs sham acupuncture or waitlist control on joint pain related to aromatase inhibitors among women with early-stage breast cancer: a randomized clinical trial. JAMA. 2018;320(2):167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.