Abstract
Background
The prevalence of persistent peripheral neuropathy (PN) in early-stage breast cancer (ESBC) survivors is largely unknown. We explored the occurrence and risk factors of PN among long-term ESBC survivors treated with taxane chemotherapy.
Methods
A population-based cohort of 884 recurrence-free ESBC survivors diagnosed 2010–2015 in the South East Health Care region, Sweden and 1768 control women without prior cancer received a postal questionnaire that included the European Organisation for Research and Treatment of Cancer chemotherapy-induced peripheral neuropathy (CIPN20) items. Prevalence, relative risks (RRs) (Poisson regression) and risk factors (binomial regression) were calculated. Adjustments were made for confounding factors (e.g. age, body mass index, comorbidities).
Results
The response rate was 79% for survivors and 59% for controls. The median time post taxane was 3.6 years (1.5–7.3 years). The adjusted RR was highest (RR 1.8) for “tingling/numbness of toes/feet”. Individual sensory symptoms occurred in 8.9–48.4% and motor symptoms in 7.2–61.3% of survivors; the most prevalent symptoms were “difficulty opening jar” and “cramps in feet”. Paclitaxel, older age, overweight, diabetes mellitus, vibrating hand tools, autoimmune disease and smoking were independent risk factors.
Conclusions
PN was more common among ESBC survivors than control women and many symptoms persisted over time. Risk factors should be considered when treatment decisions are made.
Subject terms: Breast cancer, Chemotherapy
Background
Worldwide, breast cancer is the most common cancer among women. The prognosis for early-stage breast cancer (ESBC) patients is excellent, but the risk of long-term adverse events must be considered. Treatment for ESBC includes taxane chemotherapy,1 a treatment strongly associated with peripheral neuropathy (PN). The latter may have a detrimental effect on the quality of life.2–4 PN is well described during the first few years following taxane chemotherapy, but little is known about the risk for and prevalence of persistent PN in ESBC survivors.
The incidence and severity of taxane-induced peripheral neuropathy (TIPN) differ with the type of taxane, cumulative dose and treatment schedule.5–7 In addition, pre-existing PN has been associated with the risk for TIPN.8 TIPN can start within days of the first treatment and is often dose-limiting.9 Symptoms often improve with time, but not always, and >80% of affected women experience neuropathic symptoms 1–3 years post-taxane treatment.10,11 Few studies provide data longer than 3 years post treatment.11,12
Risk factors for PN, in addition to previous use of chemotherapeutic drugs, include increasing age, female gender, diabetes mellitus, body mass index (BMI),13,14 alcohol overconsumption,15 cardiovascular disease, nutritional deficiencies, autoimmune diseases, hereditary factors16 and vibrating tools.17,18 Normative data could help to interpret TIPN in ESBC survivors.19
We performed a population-based cohort study on recurrence-free ESBC survivors from the South East Health Care region in Sweden treated 2–6 years earlier with (neo)adjuvant taxane chemotherapy regimens. Here, we present the primary objective of the study, the prevalence and severity of self-reported symptoms of PN and compare their occurrence with that of women, without prior cancer, randomly sampled from the Swedish Population Registry. In addition, we report on risk factors for PN among the ESBC survivors.
Methods
Study population
A cohort of women diagnosed with breast cancer between 1 January 2010 and 30 June 2015 was identified from the Swedish Cancer Register (SCR). Reporting to the SCR is mandatory, and the registry achieves >95% coverage for all malignant tumours, of which 99% have been histologically verified.20
The inclusion criteria were women older than 18 years, diagnosed with early-stage (T1-3, N0-2) invasive breast cancer in the South East Health Care Region and treated with taxane chemotherapy. Women with advanced stage of disease, other malignancies (except cervical carcinoma in situ and basal cell carcinoma) and recurrent disease were excluded. The file from SCR was linked with the population register to exclude deceased or emigrated women, and to the CSAM Cytodos software system to identify those treated with taxanes. The CSAM Cytodos software is a chemotherapy prescription system for both prescription and administration that documents the doses delivered. Medical records were screened for stage and recurrence. The latest date of follow-up for vital status was 25 August 2017.
Each eligible breast cancer survivor was matched for birth year and residency with up to four individuals from the Swedish Population Registry and controlled against the SCR to exclude those with prior or current malignant disease. An introductory letter and a questionnaire were sent to eligible survivors and two matched cancer-free women, since we anticipated a lower response rate among controls. The study was approved by the Regional Ethics Committee in Linköping (Ref. no. 2016/548-31).
Questionnaire
A study-specific questionnaire was constructed consisting of 134 questions. These included the validated European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire, QLQ-C30,21 the HADS instrument for anxiety and depression22 and the EORTC QLQ chemotherapy-induced peripheral neuropathy, CIPN20, questionnaire.23,24 Additional questions concerned demographics, confounding factors (e.g. lifestyle factors, BMI, comorbidities, exogenous oestrogens, menopause status, the use of vibrating tools) and questions regarding other chemotherapy-induced side effects. The additional questions were tested for face validity. A total of ten individuals (survivors and experienced healthcare professionals) were asked to read and comment on the content and phrasing to ensure the relevance and the intelligibility of the questions. Adjustments of the questions were made successively until no further comments were made.
The CIPN20 was chosen as the primary outcome measure due to its reported strong psychometric properties supporting validity and reliability.23–27 The CIPN20 consists of a sensory scale of nine items, a motor scale of seven items and an autonomic scale of two items. The item “difficulty driving” is conditioned.24 The item on erectile disorder was omitted since it is applicable only to males. Each item is measured on a four-point Likert scale ranging from “not at all” to “very much”. Physical activity and alcohol consumption were assessed as specified by Olsson et al.28 and Bush et al.29 The questionnaire for control women consisted of 120 questions. Health-related quality of life in relation to PN will be reported later.
All eligible women received a study-specific ID number. The questionnaires were pre-labelled with this study-specific ID number to maintain confidentiality. For ESBC survivors an encrypted code key linking the study-specific ID number to the personal identity was constructed, in accordance with regulations from the authorities. Up to two postal reminders were sent out to non-responders within a total time frame of 5 weeks. Eligible women gave informed consent by sending back a completed questionnaire.
A pilot study of 100 breast cancer survivors and 100 controls was performed in September to October 2017 to explore if a response rate exceeding 60% for survivors and 50% for control women was achievable. The results showed a response rate of 77% and 60%, respectively. Hence, the questionnaires for the remaining eligible women were sent out (October 2017 to January 2018).
Medical records
Medical records were scrutinised, and data on tumour and treatment characteristics were recorded in a case report form. Data on chemotherapy regimens, treatment dates and received doses were obtained from the CSAM Cytodos software system. Topographical codes C50.0-C50.9 from the International Classification of Diseases (ICD-10) and the TNM staging classification (7th Edition) for breast cancer were used. Grading was in accordance with the Nottingham Histologic Score system. The limit for positive immunohistochemical staining for oestrogen (ER) and progesterone receptor (PgR) was set at 10% positive tumour cells.
Taxane regimens of docetaxel and paclitaxel were considered interchangeable in the guidelines in use and the choice made depended on local preferences. The predominant regimens were three courses of docetaxel 100 mg/m2 every 3 weeks and 12 courses of weekly paclitaxel 80 mg/m2. The most widely used anthracycline regimens consisted of either three cycles of fluorouracil, epirubicin (100 mg/m2) and cyclophosphamide (FEC100) every 3 weeks, or three cycles of epirubicin (90 mg/m2) and cyclophosphamide every 3 weeks (EC90). Lower dosing of epirubicin or docetaxel was prescribed for fragile (high age or comorbidity) patients.
Study size
The required sample size was calculated on the assumption that the prevalence of neuropathy was 7% among the unexposed group of cancer-free women16 and 15% among long-term breast cancer survivors.4 The calculation used the following parameters: two-sided log-rank test, 80% power and 5% significance level, and showed that 540 survivors would be needed.
Statistical analysis
All pages from the questionnaire were scanned and a computer software program was used to transform this data to Excel. Survivors and control women were compared using Student’s t test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Different cut-off levels were used. For the primary analysis, a symptom was dichotomised into either having the symptom—“a little”, “quite a bit” or “very much”—or not having had the symptom in the past 6 months. For exploring moderate–severe symptoms, we combined the response categories “quite a bit” and “very much”. The absolute difference in symptom occurrence between survivors, classified by time elapsed since completed taxane treatment, and control women was evaluated with logistic regression and was presented as a p value. Unadjusted and adjusted relative risk (RR) with 95% confidence intervals (CIs) for persistent TIPN were calculated with Poisson regression.30
A comprehensive literature search was performed when planning the study to explore potential confounders for neuropathy. The data were collected with the rationale that extensive information on comorbidities and other factors should be available and adjusted for to more clearly be able to see differences in RRs. Since our study was large, we considered the data to be sufficiently robust for numerous covariate analyses. Covariates were age, BMI, alcohol consumption, folic acid deficiency, vitamin B12 deficiency, joint pain, osteoporosis, thrombosis, diabetes mellitus, autoimmune disease, cardiovascular disease, menopausal status, exogenous oestrogens and the use of vibrating hand tools using the categorisation shown in Table 1. Recent psychometric testing of the EORTC QLQ-CIPN20 have indicated an unstable factor structure wherefore we chose to present mean scores of individual items alone.27 The item mean scores and standard deviations of the EORTC CIPN20 module were calculated.24 We explored whether time since completing taxane therapy had an effect among patients, using median time since treatment 3.6 years to form categories. The results are also adjusted for confounding factors (linear regression). Plausible risk factors for sensory and motor symptoms (rated as “a little”, “quite a bit” or “very much”) of PN among ESBC survivors treated exclusively with either docetaxel or paclitaxel were explored, and RRs (95% CI) were calculated using binominal regression (univariable or multivariable analysis). The following variables were entered into the univariable analysis: type of taxane (docetaxel vs paclitaxel), age at diagnosis (<65 vs ≥65 years), BMI at diagnosis (<25 vs ≥25), receiving treatment for diabetes mellitus (no vs yes), use of vibrating hand tools at work (no vs yes), autoimmune disease (no vs yes), alcohol risk consumption (none vs risk consumption), cardiovascular disease (no vs yes), current smoking (no vs current smoker), mastectomy (no vs yes) and lymph node metastases (N0 vs N1, N0 vs N2). Only predictive factors with a statistically significant association (p < 0.05) with an individual symptom were entered into the multivariable model. Individuals with missing data were excluded from the calculations of each respective outcome. Statistical analyses were performed using IBM SPSS version 25 and Stata SE version 16.1 for Poisson and binomial regressions. Tests were two-sided and p values were regarded significant if p value was <0.05.
Table 1.
Characteristics of early-stage breast cancer survivors compared with the general female population cohort.
| Characteristics | Breast cancer survivors n = 646 No. (%) |
General female population n = 1040 No. (%) |
P value |
|---|---|---|---|
| Age at survey, years | |||
| Mean (SD) | 60.7 (11.2) | 61.6 (11.2) | 0.092a |
| Median (min–max) | 62.0 (31–86) | 64.0 (25–86) | |
| Age at diagnosis, years | |||
| Mean (SD) | 56.6 (11.2) | NA | |
| Median (min–max) | 58.0 (27–82) | ||
| Body mass index, BMI at survey, no. | 637 | 1013 | |
| <18.5 (underweight) | 11 (1.7) | 6 (0.6) | |
| 18.5–24.9 (normal) | 241 (37.9) | 468 (46.2) | |
| 25–29.9 (overweight) | 259 (40.7) | 359 (35.4) | |
| 30–34.9 (obese) | 91 (14.3) | 138 (13.6) | |
| >35 (severely obese) | 39 (6.1) | 42 (4.1) | |
| Mean (SD) | 26.8 (4.8) | 26.1 (4.4) | 0.004a |
| Not stated | 9 | 27 | |
| BMI at diagnosis, no. | 646 | NA | |
| Mean (SD) | 26.7 (5.0) | ||
| Alcohol, no. | 646 | 1020 | 0.582b |
| Risk consumption | 68 (10.5) | 96 (9.4) | |
| Consumers | 479 (74.1) | 752 (73.7) | |
| No consumption | 99 (15.3) | 172 (16.9) | |
| Not stated | 0 | 20 | |
| Smoking, no. | 641 | 1019 | 0.327b |
| Current smoker | 64 (10) | 115 (11) | |
| Former smoker | 244 (38) | 353 (35) | |
| Never smoked | 333 (52) | 551 (54) | |
| Not stated | 5 | 21 | |
| Comorbidities, self-reported, no. | |||
| Painful joints | 291/629 (46.3) | 396/986 (40.2) | 0.016b |
| Cardiovascular diseasec | 197/611 (32.2) | 340/939 (36.2) | 0.109b |
| Psychiatric disorder | 101/629 (16.1) | 151/974 (15.5) | 0.766b |
| Carpal tunnel syndrome | 77/626 (12.3) | 140/977 (14.3) | 0.247b |
| Osteoporosis | 76/613 (12.4) | 74/961 (7.7) | 0.002b |
| Thromboembolic event | 75/621 (12.1) | 68/962 (7.1) | 0.001b |
| Hypothyroidism | 72/627 (11.5) | 105/972 (10.8) | 0.672b |
| Herniated disc | 56/625 (9.0) | 110/976 (11.3) | 0.139b |
| Autoimmune diseased | 54/621 (8.7) | 96/960 (10.0) | 0.387b |
| Vitamin B12 deficiency | 45/611 (7.4) | 67/953 (7.0) | 0.802b |
| Lung disease | 39/628 (6.2) | 81/976 (8.3) | 0.121b |
| Diabetes mellitus | 38/629 (6.0) | 72/999 (7.2) | 0.400b |
| Folic acid deficiency | 25/625 (4.0) | 41/970 (4.2) | 0.824b |
| Neurological diseasee | 21/610 (3.4) | 46/948 (4.9) | 0.181b |
| Kidney disease | 12/619 (1.9) | 33/969 (3.4) | 0.086b |
| Liver disease | 6/620 (1.0) | 10/966 (1.0) | 0.896b |
| Hereditary polyneuropathy | 2/626 (0.3) | 2/956 (0.2) | 0.650f |
| Operating vibrating tools, no. | 639 | 1015 | 0.006b |
| Yes | 38 (5.9) | 99 (9.8) | |
| No | 601 (94.1) | 916 (90.2) | |
| Not stated | 7 | 25 | |
| Menopausal status at survey, no. | 628 | 995 | <0.001b |
| Premenopausal | 47 (7.5) | 214 (21.5) | |
| Postmenopausal | 581 (92.5) | 781 (78.5) | |
| Not stated | 18 | 45 | |
| Oestrogen, exogenous (systemic or local), no. | 622 | 1008 | <0.001b |
| Yes | 59 (9.5) | 199 (19.7) | |
| No | 563 (90.5) | 809 (80.3) | |
| Not stated | 24 | 32 | |
| TNM classification (7th edition) | NA | ||
| Tumour size, no. | 643 | ||
| T1 | 293 (45.4) | ||
| T2 | 311 (48.1) | ||
| T3 | 39 (6.0) | ||
| Not stated | 3 (0.5) | ||
| Nodal status | |||
| N0 | 258 (40.0) | ||
| N1 | 301 (46.6) | ||
| N2 | 87 (13.5) | ||
| Surgery | NA | ||
| Breast-conserving + SN dissection | 138 (21.3) | ||
| Breast-conserving + axillary dissection | 126 (19.5) | ||
| Mastectomy + SN dissection | 121 (18.7) | ||
| Mastectomy + axillary dissection | 260 (40.2) | ||
| Only axillary dissection | 1 (0.2) | ||
| Anthracycline-based regimens, no. | 644 | NA | |
| FEC100 | 311 (48.3) | ||
| FEC75 | 233 (36.2) | ||
| FEC60 | 26 (4.0) | ||
| EC90 | 63 (9.8) | ||
| Other | 11 (1.7) | ||
| Taxane-based regimens, no. | 646 | NA | |
| Docetaxel, dose intensity | 345 (53.4) | ||
| 75–80 mg/m2 every 3 weeks | 77 (11.9) | ||
| 100 mg/m2 every 3 weeks | 275 (42.6) | ||
| 35 mg/m2 per week | 9 (1.4) | ||
| Cumulative doses, mean mg/m2 (SD) | 273 (65) | ||
| Paclitaxel, dose intensity | |||
| 80 mg/m2 per week | 283 (43.8) | ||
| Cumulative dose, mean mg/m2 (SD) | 866 (159) | ||
| Alternating between docetaxel and paclitaxel | 18 (2.7) | ||
| Additional chemotherapy | |||
| Metotrexate | 2 | ||
| Carboplatin | 1 | ||
| Current endocrine antitumoural treatmentg | NA | ||
| Tamoxifen | 255 (39.5) | ||
| Aromatase inhibitor | 114 (17.7) | ||
| GnRH analogues | 14 (2.2) | ||
NA not applicable, SN sentinel node, FEC fluorouracil, epirubicin and cyclophosphamide, EC epirubicin and cyclophosphamide, GnRH gonadotropin-releasing hormone.
Denominator is dependent on number of respondents answering a specific item and may differ from the maximum sum.
aStudent’s t test.
bPearson’s χ2 test.
cHypertension, heart failure, angina pectoris and myocardial infarction.
dRheumatism, scleroderma, systemic lupus erythematosus and Sjogren’s syndrome.
eParkinson’s disease, multiple sclerosis and epilepsy, stroke.
fFisher’s exact test.
gSelf-reported data.
Results
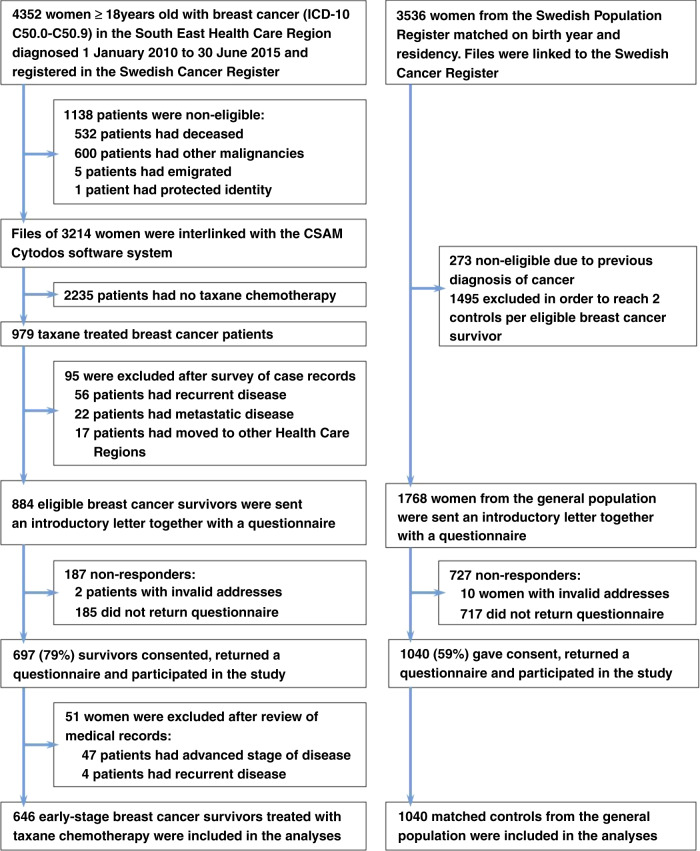
A total of 4352 breast cancer survivors and 3536 women without cancer were examined for eligibility (Fig. 1). Of these, 884 survivors and 1768 controls met the eligibility criteria. In total, 697 of 884 (78.8%) cancer survivors and 1040 (58.8%) control women returned a completed questionnaire and participated in the study. After a review of medical records, 51 survivors were excluded from the analyses.
Fig. 1.
Flow diagram of the study population.
Table 1 presents the characteristics of the participants. Survivors (n = 646) were more obese (61.1 vs 53.2% with BMI ≥ 25) and reported more painful joints (46.3 vs 40.2%), osteoporosis (12.4 vs 7.7%) and thromboembolic events (12.1 vs 7.1%) than control women. Control women used exogenous oestrogens (19.7 vs 9.5%), operated vibrating tools (9.8 vs 5.9%) and were more often premenopausal (21.5 vs 7.5%) than ESBC survivors. The median time from the end of taxane therapy to completing the questionnaire was 3.6 years (range 1.5–7.3 years). The median time since diagnosis was 4.1 years (range 2.2–7.8 years). Supplementary Tables 1 and 2 gives additional information on demographic, clinical, tumour and treatment characteristics of the participants.
Table 2 shows the prevalence and estimates of RR of neuropathic symptoms in ESBC survivors classified by time since completing taxane chemotherapy, compared with women without prior cancer. The occurrence of sensory symptoms among survivors ranged between 8.9 (difficulty distinguishing hot/cold water) and 48.4% (tingling hands), motor symptoms between 7.2 (foot drop) and 61.3% (difficulty opening a jar), and autonomic symptoms between 29.3 (blurred vision) and 45.5% (dizziness). The most prevalent neuropathic symptoms among survivors were “difficulty opening a jar” followed by “cramps in feet” with adjusted RR of 1.4 (95% CI 1.1–1.6) and 1.5 (95% CI 1.2–1.7) when compared with controls (Table 2 and Fig. 2a). The adjusted RR was highest for “tingling of toes/feet” (1.8, 95% CI 1.5–2.1) and “numbness of toes/feet” (1.8, 95% CI 1.5–2.2) (see Table 2 and Fig. 2b). There was no difference in the rate of “shooting/burning fingers/hands”, “hearing difficulties”, “problems holding a pen” and “difficulty using pedals when driving” between survivors and control women. The mean scores for individual items of the EORTC CIPN20 module are shown in Table 3.
Table 2.
Unadjusted and adjusted RR (95% CI) for symptoms of neuropathy in early-stage breast cancer survivors classified by median time since completing taxane chemotherapy, and in women without cancer (controls).
| EORTC QLQ-CIPN20 | Survivors n = 646 No. (%) |
Controls n = 1040 No. (%) |
Survivors vs. controls Unadjusted RR Adjusteda RR (95% CI) | Survivors <3.6 years n = 321 No. (%) |
Survivors <3.6 years vs controls Adjusteda RR (95% CI) |
Survivors ≥3.6 years n = 325 No. (%) |
Survivors ≥3.6 years vs controls Adjusteda RR (95% CI) |
|---|---|---|---|---|---|---|---|
| Sensory symptoms | |||||||
| Tingling of fingers or hands | 311/642 (48.4) | 333/1025 (32.5) |
1.5 (1.3–1.7) 1.4 (1.2–1.7) |
168/320 (52.5) | 1.7 (1.4–2.2) | 143/322 (44.4) | 1.3 (1.1–1.7) |
| Not at all | 331 (51.6) | 692 (67.6) | 152 (47.5) | 179 (55.6) | |||
| A little | 190 (29.6) | 232 (22.6) | 98 (30.6) | 92 (28.6) | |||
| Quite a bit | 84 (13.1) | 70 (6.8) | 45 (14.1) | 39 (12.1) | |||
| Very much | 37 (5.8) | 31 (3.0) | 25 (7.8) | 12 (3.7) | |||
| Tingling of toes or feet | 308/642 (48.0) | 236/1025 (23.0) |
1.9 (1.6–2.2) 1.8 (1.5–2.1) |
169/319 (53.0) | 2.5 (1.9–3.2) | 139/323 (43.0) | 1.8 (1.4–2.3) |
| Not at all | 334 (52.0) | 789 (77.0) | 150 (47.0) | 184 (57.0) | |||
| A little | 159 (24.8) | 168 (16.4) | 79 (24.8) | 80 (24.8) | |||
| Quite a bit | 98 (15.3) | 52 (5.1) | 54 (17.0) | 44 (13.6) | |||
| Very much | 51 (7.9) | 16 (1.6) | 36 (11.3) | 15 (4.6) | |||
| Numbness of fingers or hands | 309/640 (48.3) | 365/1021 (35.8) |
1.4 (1.2–1.6) 1.3 (1.1–1.6) |
150/319 (47.0) | 1.6 (1.2–2.0) | 143/321 (44.6) | 1.3 (0.9–1.6) |
| Not at all | 331 (51.7) | 656 (64.3) | 153 (48.0) | 178 (55.6) | |||
| A little | 188 (29.4) | 262 (25.7) | 96 (30.1) | 92 (28.7) | |||
| Quite a bit | 90 (14.1) | 68 (6.7) | 50 (15.7) | 40 (12.5) | |||
| Very much | 31 (4.8) | 35 (3.4) | 20 (6.3) | 11 (3.4) | |||
| Numbness of toes or feet | 308/641 (48.1) | 243/1025 (23.7) |
1.9 (1.6–2.2) 1.8 (1.5–2.2) |
175/320 (54.7) | 2.6 (2.0–3.3) | 133/321 (41.4) | 1.8 (1.4–2.3) |
| Not at all | 333 (52.0) | 782 (76.3) | 145 (45.3) | 188 (58.6) | |||
| A little | 156 (24.3) | 170 (16.6) | 85 (26.6) | 71 (22.1) | |||
| Quite a bit | 105 (16.4) | 53 (5.2) | 60 (18.8) | 45 (14.0) | |||
| Very much | 47 (7.3) | 20 (2.0) | 30 (9.4) | 17 (5.3) | |||
| Shooting or burning of fingers or hands | 142/637 (22.3) | 176/1024 (17.2) |
1.2 (1.1–1.5) 1.2 (0.9–1.4) |
78/316 (24.7) | 1.2 (0.9–1.6) | 64/321 (19.9) | 1.1 (0.8–1.5) |
| Not at all | 495 (77.7) | 848 (82.8) | 238 (75.3) | 257 (80.1) | |||
| A little | 90 (14.1) | 114 (11.1) | 45 (14.2) | 45 (14.0) | |||
| Quite a bit | 36 (5.7) | 44 (4.3) | 23 (7.3) | 13 (4.1) | |||
| Very much | 16 (2.5) | 18 (1.8) | 10 (3.2) | 6 (1.9) | |||
| Shooting or burning of toes or feet | 191/641 (29.8) | 177/1024 (17.3) |
1.5 (1.3–1.8) 1.5 (1.2–1.7) |
104/320 (32.5) | 1.7 (1.3–2.2) | 87/321 (27.1) | 1.6 (1.2–2.0) |
| Not at all | 450 (70.2) | 847 (82.7) | 216 (67.5) | 234 (72.9) | |||
| A little | 102 (15.9) | 108 (10.6) | 51 (15.9) | 51 (15.9) | |||
| Quite a bit | 64 (10.0) | 47 (4.6) | 34 (10.6) | 30 (9.3) | |||
| Very much | 25 (3.9) | 22 (2.1) | 19 (5.9) | 6 (1.9) | |||
| Problems in standing or walking because of difficulty feeling the ground under feet | 157/638 (24.6) | 107/1025 (10.4) |
1.7 (1.4–2.1) 1.7 (1.4–2.0) |
88/319 (27.6) | 2.1 (1.6–2.8) | 69/319 (21.6) | 1.8 (1.3–2.4) |
| Not at all | 481 (75.4) | 918 (89.6) | 231 (72.4) | 250 (78.4) | |||
| A little | 93 (14.6) | 66 (6.4) | 46 (14.4) | 47 (14.7) | |||
| Quite a bit | 45 (7.1) | 22 (2.1) | 31 (9.7) | 14 (4.4) | |||
| Very much | 19 (3.0) | 19 (1.9) | 11 (3.4) | 8 (2.5) | |||
| Difficulty distinguishing between hot and cold water | 57/640 (8.9) | 31/1025 (3.0) |
1.8 (1.3–2.3) 1.7 (1.3–2.2) |
30/319 (9.4) | 2.0 (1.3–2.9) | 27/321 (8.4) | 2.0 (1.3–3.0) |
| Not at all | 583 (91.1) | 994 (97.0) | 289 (91.0) | 294 (91.6) | |||
| A little | 42 (6.6) | 20 (2.0) | 23 (7.2) | 19 (5.9) | |||
| Quite a bit | 9 (1.4) | 5 (0.5) | 4 (1.3) | 5 (1.6) | |||
| Very much | 6 (0.9) | 6 (0.6) | 3 (0.9) | 3 (0.9) | |||
| Difficulty hearing | 237/643 (36.9) | 393/1018 (38.6) |
1.0 (0.8–1.1) 0.9 (0.8–1.1) |
126/319 (39.5) | 1.0 (0.8–1.3) | 111/324 (34.3) | 0.9 (0.7–1.1) |
| Not at all | 406 (63.1) | 625 (61.4) | 193 (60.5) | 213 (65.7) | |||
| A little | 167 (26.0) | 301 (29.6) | 83 (26.0) | 84 (25.9) | |||
| Quite a bit | 51 (7.9) | 70 (6.9) | 30 (9.4) | 21 (6.5) | |||
| Very much | 19 (3.0) | 22 (2.2) | 13 (4.1) | 6 (1.9) | |||
| Motor symptoms | |||||||
| Cramps in hands | 143/638 (22.4) | 200/1024 (19.5) |
1.3 (1.1–1.5) 1.3 (1.1–1.6) |
90/318 (28.3) | 1.5 (1.1–1.9) | 83/320 (25.9) | 1.4 (1.1–1.8) |
| Not at all | 465 (72.9) | 824 (80.5) | 228 (71.7) | 237 (74.1) | |||
| A little | 120 (18.8) | 144 (14.1) | 64 (20.1) | 56 (17.5) | |||
| Quite a bit | 39 (6.1) | 40 (3.9) | 20 (6.3) | 19 (5.9) | |||
| Very much | 14 (2.2) | 16 (1.6) | 6 (1.9) | 8 (2.5) | |||
| Cramps in feet | 362/642 (56.4) | 390/1025 (38.1) |
1.6 (1.3–1.8) 1.5 (1.2–1.7) |
176/320 (55.0) | 1.5 (1.2–1.9) | 185/322 (57.5) | 1.7 (1.4–2.2) |
| Not at all | 281 (43.8) | 635 (62.0) | 144 (45.0) | 137 (42.6) | |||
| A little | 196 (30.5) | 285 (27.8) | 95 (29.7) | 100 (31.1) | |||
| Quite a bit | 119 (18.5) | 85 (8.3) | 59 (18.4) | 60 (18.6) | |||
| Very much | 47 (7.3) | 20 (2.0) | 22 (6.9) | 25 (7.8) | |||
| Problem holding a pen, which made writing difficult | 91/639 (14.2) | 110/1026 (10.7) |
1.2 (0.9–1.5) 1.2 (0.9–1.5) |
49/319 (15.4) | 1.4 (0.9–1.9) | 42/320 (13.1) | 1.1 (0.8–1.6) |
| Not at all | 548 (85.8) | 916 (89.3) | 270 (84.6) | 278 (86.9) | |||
| A little | 66 (10.3) | 79 (7.7) | 35 (11.0) | 31 (9.7) | |||
| Quite a bit | 18 (2.8) | 17 (1.7) | 9 (2.8) | 9 (2.8) | |||
| Very much | 7 (1.1) | 14 (1.4) | 5 (1.6) | 2 (0.6) | |||
| Difficulty manipulating small objects with fingers | 259/642 (40.3) | 255/1021 (25.0) |
1.5 (1.3–1.8) 1.5 (1.3–1.8) |
137/318 (43.1) | 1.8 (1.4–2.3) | 122/324 (37.7) | 1.6 (1.2–2.0) |
| Not at all | 383 (59.7) | 766 (75.0) | 181 (56.9) | 202 (62.3) | |||
| A little | 176 (27.4) | 173 (16.9) | 94 (29.6) | 82 (25.3) | |||
| Quite a bit | 56 (8.7) | 60 (5.9) | 27 (8.5) | 29 (9.0) | |||
| Very much | 27 (4.2) | 22 (2.2) | 16 (5.0) | 11 (3.4) | |||
| Difficult opening a jar or bottle because of weakness in hands | 394/643 (61.3) | 491/1022 (48.0) |
1.4 (1.2–1.6) 1.4 (1.1–1.6) |
198/320 (61.9) | 1.4 (1.1–1.8) | 196/323 (61.0) | 1.5 (1.2–1.9) |
| Not at all | 249 (38.7) | 531 (52.0) | 122 (38.1) | 127 (39.3) | |||
| A little | 239 (37.2) | 322 (31.5) | 103 (32.2) | 136 (42.1) | |||
| Quite a bit | 109 (17.0) | 115 (11.3) | 68 (21.3) | 41 (12.7) | |||
| Very much | 46 (7.2) | 54 (5.3) | 27 (8.4) | 19 (5.9) | |||
| Difficulty walking because of foot drop | 46/639 (7.2) | 35/1019 (3.4) |
1.5 (1.1–2.1) 1.5 (1.1–2.1)` |
24/316 (7.6) | 1.8 (1.1–2.8) | 22/323 (6.8) | 1.6 (0.9–2.5) |
| Not at all | 593 (92.8) | 984 (96.6) | 292 (92.4) | 301 (93.2) | |||
| A little | 32 (5.0) | 23 (2.3) | 17 (5.4) | 15 (4.6) | |||
| Quite a bit | 7 (1.1) | 9 (0.9) | 4 (1.3) | 3 (0.9) | |||
| Very much | 7 (1.1) | 3 (0.3) | 3 (1.0) | 4 (1.2) | |||
| Difficulty climbing stairs or getting up out of a chair because of weakness in legs | 254/644 (39.4) | 255/1023 (24.9) |
1.5 (1.3–1.7) 1.4 (1.2–1.7) |
139/320 (43.4) | 1.7 (1.3–2.2) | 115/324 (35.5) | 1.5 (1.1–1.9) |
| Not at all | 390 (60.6) | 768 (75.1) | 181 (56.6) | 209 (64.5) | |||
| A little | 161 (25.0) | 164 (16.0) | 89 (27.8) | 72 (22.2) | |||
| Quite a bit | 68 (10.6) | 62 (6.1) | 39 (12.2) | 29 (9.0) | |||
| Very much | 25 (3.9) | 29 (2.8) | 11 (3.4) | 14 (4.3) | |||
| Autonomic symptoms | |||||||
| Dizziness when standing up from a sitting or lying position | 291/640 (45.5) | 378/1023 (37.0) |
1.2 (1.1–1.5) 1.3 (1.1–1.5) |
140/316 (44.3) | 1.3 (1.1–1.6) | 151/324 (46.6) | 1.4 (1.1–1.8) |
| Not at all | 349 (54.5) | 645 (63.1) | 176 (55.7) | 173 (53.4) | |||
| A little | 223 (34.8) | 313 (30.6) | 109 (34.5) | 114 (35.2) | |||
| Quite a bit | 56 (8.8) | 54 (5.3) | 26 (8.2) | 30 (9.3) | |||
| Very much | 12 (1.9) | 11 (1.2) | 5 (1.6) | 7 (2.2) | |||
| Blurred vision | 188/642 (29.3) | 186/1019 (18.3) |
1.4 (1.2–1.7) 1.3 (1.1–1.5) |
100/320 (31.3) | 1.4 (1.1–1.8) | 88/322 (27.3) | 1.3 (1.1–1.7) |
| Not at all | 454 (70.7) | 833 (81.7) | 220 (68.8) | 234 (72.7) | |||
| A little | 145 (22.6) | 147 (14.4) | 78 (24.4) | 67 (20.8) | |||
| Quite a bit | 32 (5.0) | 36 (3.5) | 15 (4.7) | 17 (5.3) | |||
| Very much | 11 (1.7) | 3 (0.3) | 7 (2.2) | 4 (1.2) | |||
| Only if you drive a car | |||||||
| Difficulty using pedals when driving a car | 24/530 (4.5) | 22/852 (2.6) |
1.4 (0.9–2.1) 1.1 (0.7–1.8) |
13/260 (5.0) | 1.2 (0.6–2.3) | 11/270 (4.1) | 1.2 (0.6–2.4) |
| Not at all | 506 (95.5) | 830 (97.4) | 247 (95.0) | 259 (96.0) | |||
| A little | 16 (3.0) | 18 (2.1) | 8 (3.1) | 8 (3.0) | |||
| Quite a bit | 7 (1.3) | 3 (0.4) | 5 (1.9) | 2 (0.7) | |||
| Very much | 1 (0.2) | 1 (0.1) | 0 | 1 (0.4) | |||
RR relative risk, CI confidence interval, EORTC QLQ-CIPN20 European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy Module.
Denominator is dependent on the number of respondents answering a specific item and may differ from the maximum sum.
a Adjusted for age, body mass index, alcohol consumption, folic acid deficiency, B12 deficiency, joint pain, osteoporosis, thrombosis, diabetes mellitus, autoimmune disease, cardiovascular disease, menopausal status, exogenous oestrogen and use of vibrating hand tools.
Fig. 2. Self-reported symptoms of peripheral neuropathy (EORTC QLQ-CIPN20) among early-stage breast cancer survivors and control women.
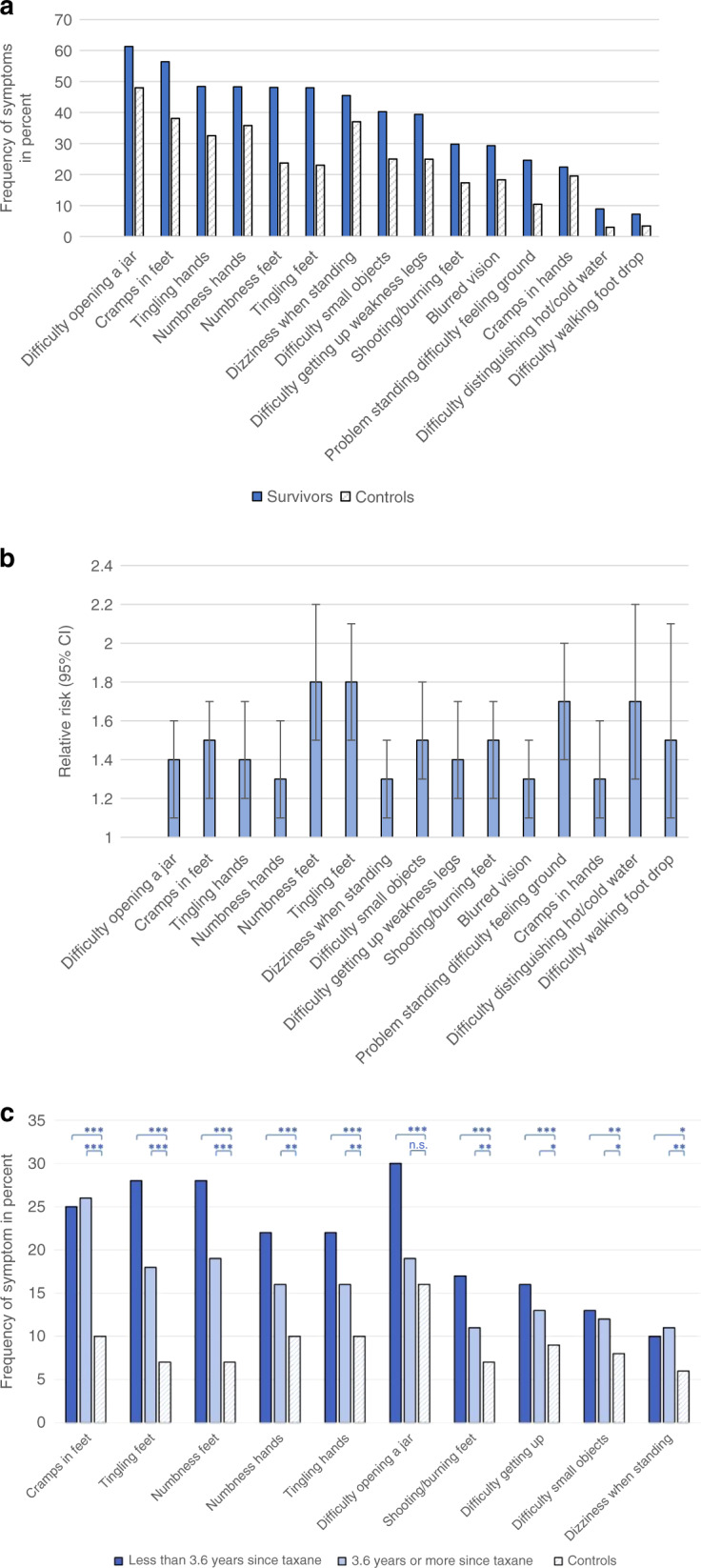
a The most prevalent neuropathic symptoms in descending order among survivors compared with control women. b The corresponding adjusted1 relative risk of neuropathic symptoms when comparing survivors with control women. Error bars show 95% confidence intervals of relative risk estimates. 1Adjusted for age, body mass index, alcohol consumption, folic acid deficiency, B12 deficiency, joint pain, osteoporosis, thrombosis, diabetes mellitus, autoimmune disease, cardiovascular disease, menopausal status, exogenous oestrogen and use of vibrating hand tools. c The ten symptoms of neuropathy, self-reported as moderate–severe, with a largest absolute difference in prevalence between early-stage breast cancer survivors, classified by time elapsed since completed taxane treatment, and control women. Analysis was performed by logistic regression. Significance is indicated as ***P < 0.001, **P < 0.01, *P < 0.05 or n.s. non-significant.
Table 3.
Mean scores (SD) of EORTC QLQ-CIPN20 individual items among cancer survivors and control women.
| Individual items | Mean (SD)a |
Pb Pc |
Mean (SD)a |
Pa Pc |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breast cancer survivors n = 646 |
Not stated | General female population n = 1040 |
Not stated | Breast cancer survivors <3.6 years n = 321 |
Not stated | Breast cancer survivors ≥3.6 years n = 325 |
Not stated | |||
| Sensory symptoms | ||||||||||
| Tingling of fingers or hands | 1.73 (0.90) | 4 | 1.45 (0.75) | 15 |
<0.001 <0.001 |
1.82 (0.95) | 1 | 1.64 (0.84) | 3 |
0.010 0.001 |
| Tingling of toes or feet | 1.79 (0.97) | 4 | 1.31 (0.64) | 15 |
<0.001 <0.001 |
1.92 (1.04) | 2 | 1.66 (0.88) | 2 |
0.001 <0.001 |
| Numbness of fingers or hands | 1.72 (0.88) | 6 | 1.49 (0.77) | 19 |
<0.001 <0.001 |
1.80 (0.92) | 2 | 1.64 (0.83) | 4 |
0.018 0.006 |
| Numbness of toes or feet | 1.79 (0.97) | 5 | 1.33 (0.66) | 15 |
<0.001 <0.001 |
1.92 (1.00) | 1 | 1.66 (0.91) | 4 |
0.001 <0.001 |
| Shooting or burning of fingers or hands | 1.33 (0.70) | 9 | 1.25 (0.62) | 16 |
0.018 0.037 |
1.38 (0.76) | 5 | 1.28 (0.63) | 4 |
0.056 0.029 |
| Shooting or burning of toes or feet | 1.48 (0.83) | 5 | 1.26 (0.64) | 16 |
<0.001 <0.001 |
1.55 (0.91) | 1 | 1.40 (0.74) | 4 |
0.023 0.038 |
| Problems in standing or walking because of difficulty feeling the ground under feet | 1.38 (0.75) | 8 | 1.16 (0.54) | 15 |
<0.001 <0.001 |
1.44 (0.81) | 2 | 1.31 (0.67) | 6 |
0.026 0.033 |
| Difficulty distinguishing between hot and cold water | 1.12 (0.38) | 6 | 1.05 (0.30) | 15 |
<0.001 <0.001 |
1.13 (0.44) | 2 | 1.12 (0.44) | 4 |
0.840 0.672 |
| Difficulty hearing | 1.51 (0.77) | 3 | 1.50 (0.72) | 22 |
0.809 0.828 |
1.57 (0.82) | 2 | 1.44 (0.70) | 1 |
0.037 0.009 |
| Motor symptoms | ||||||||||
| Cramps in hands | 1.38 (0.70) | 8 | 1.27 (0.61) | 16 |
0.001 <0.001 |
1.38 (0.69) | 3 | 1.37 (0.71) | 5 |
0.788 0.181 |
| Cramps in feet | 1.89 (0.95) | 4 | 1.50 (0.73) | 15 |
<0.001 <0.001 |
1.87 (0.95) | 1 | 1.92 (0.96) | 3 |
0.556 0.890 |
| Problem holding a pen, which made writing difficult | 1.19 (0.53) | 7 | 1.15 (0.49) | 14 |
0.105 0.038 |
1.44 (0.81) | 2 | 1.17 (0.49) | 5 |
0.323 0.503 |
| Difficulty manipulating small objects with fingers (e.g. fastening small buttons) | 1.57 (0.82) | 4 | 1.35 (0.69) | 19 |
<0.001 <0.001 |
1.62 (0.84) | 3 | 1.53 (0.8) | 1 |
0.203 0.088 |
| Difficult opening a jar or bottle because of weakness in hands | 1.93 (0.92) | 3 | 1.70 (0.87) | 18 |
<0.001 <0.001 |
2.00 (0.97) | 2 | 1.85 (0.86) | 2 |
0.040 0.091 |
| Difficulty walking because of foot drop | 1.10 (0.43) | 7 | 1.05 (0.29) | 21 |
0.004 0.003 |
1.11 (0.42) | 5 | 1.10 (0.43) | 2 |
0.872 0.179 |
| Difficulty climbing stairs or getting up out of a chair because of weakness in legs | 1.58 (0.83) | 2 | 1.37 (0.72) | 17 |
<0.001 <0.001 |
1.63 (0.83) | 1 | 1.53 (0.83) | 1 |
0.150 0.325 |
| Autonomic symptoms | ||||||||||
| Dizziness when standing up from a sitting or lying position | 1.58 (0.73) | 6 | 1.44 (0.65) | 17 |
<0.001 <0.001 |
1.56 (0.71) | 5 | 1.60 (0.75) | 1 |
0.437 0.524 |
| Blurred vision | 1.38 (0.66) | 4 | 1.21 (0.51) | 21 |
<0.001 <0.001 |
1.40 (0.68) | 1 | 1.35 (0.64) | 3 |
0.318 0.599 |
| Only if you drive a car | ||||||||||
| Difficulty using pedals when driving a car | 1.06 (0.31) | 116 | 1.03 (0.21) | 188 |
0.046 0.098 |
1.07 (0.32) | 61 | 1.06 (0.30) | 55 |
0.613 0.537 |
SD standard deviation, EORTC QLQ-CIPN20 European Organization for Research and Treatment of Cancer Quality of-Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy Module.
aA higher mean score indicates a worse symptom.
bStudent’s t test.
cLinear regression, adjusted for age, body mass index, alcohol consumption, folic acid deficiency, B12 deficiency, joint pain, osteoporosis, thrombosis, diabetes mellitus, autoimmune disease, cardiovascular disease, menopausal status, exogenous oestrogen and use of vibrating hand tools.
Moderate–severe symptoms
The prevalence of moderate–severe PN among survivors was highest for “cramps in feet” (25.9%) followed by “numbness of toes/feet” (23.7%) and “tingling of toes/feet” (23.2%) (Supplementary Table 3). The corresponding adjusted RR for survivors, when compared with control women, was 1.7 (95% CI 1.4–2.0), 2.0 (95% CI 1.6–2.4) and 1.9 (95% CI 1.5–2.3), respectively. The largest absolute difference between survivors and controls was for “cramps in feet” followed by “tingling of toes/feet” and “numbness of toes/feet” (Fig. 2c).
Symptoms over time among survivors
Except for “cramps in feet”, the prevalence of neuropathic symptoms among survivors decreased over time. However, the adjusted RR for these symptoms remained significantly higher among survivors at least 3.6 years post-taxane treatment compared with control women, except for “difficulty walking because of foot drop” (Table 2). The observed decrease in the mean score, when comparing survivors <3.6 years with those at least 3.6 years post taxane, was statistically significant for all but one sensory symptom but did not significantly decrease for any motor or autonomic symptom (Table 3). Symptoms of PN reported as moderate–severe among survivors decreased over time. Only moderate–severe “tingling of toes/feet” (RR 2.1, 95% CI 1.5–2.9), “numbness of toes/feet” (RR 2.3, 95% CI 1.7–3.1) and “cramps in feet” (RR 2.0, 95% CI 1.5–2.6) had a higher risk among survivors at least 3.6 years post-taxane treatment compared with controls (Supplementary Table 3).
Risk factors for PN among survivors
Risk factors for individual symptoms of sensory and motor PN among ESBC survivors previously treated with a taxane were explored in univariable analysis (Supplementary Table 4) and those who were statistically significantly associated with an individual symptom were entered into the multivariable model (Table 4). Paclitaxel treatment was associated with a higher risk for six individual symptoms of PN compared with docetaxel, of which the highest risk (RR 1.9, 95% CI 1.1–3.2) was found for “difficulty distinguishing hot/cold water”. Older age at diagnosis (at least 65 years) was the only risk factor for “cramps in feet” (RR 1.2, 95% CI 1.1–1.4) but was also associated with “tingling/numbness in feet”, “hearing difficulty”, “cramps in hands”, “difficulty manipulating small objects”, “difficulty opening a jar” and “difficulty climbing stairs”. Overweight was associated with seven symptoms of PN; the highest risk was for “difficulty walking because of foot drop” (RR 2.3, 95% CI 1.1–4.6). The risk for “distinguishing hot/cold water” was four times as high (RR 3.8, 95% CI 2.0–7.4) among ESBC survivors treated for diabetes mellitus compared with survivors who were not. Diabetes was also associated with six other individual symptoms. The use of vibrating hand tools was associated with “tingling/numbness in hands” and “difficulty manipulating small objects”. Autoimmune disease was associated with six symptoms, of which the highest risk was for “difficulty walking” (RR 2.5, 95% CI 1.2–5.3). Alcohol risk consumption was only associated with “cramps in hands”, for which the risk was lower (RR 0.5, 95% CI 0.3–0.8) compared with no consumption. Smoking was associated with “difficulty manipulating small objects” and “difficulty climbing stairs”. Cardiovascular disease was not a risk factor for any symptom of PN. Mastectomy and a greater number of lymph node metastases were not associated with PN in the univariable analysis (Supplementary Table 4).
Table 4.
Multivariable analysesa of risk factors for sensory and motor symptoms of PN (EORTC QLQ-CIPN20) among ESBC survivors treated with a taxaneb. Estimates of relative risk and 95% confidence intervals are shown.
| Item# | Paclitaxel | Diabetes mellitusc | Age | BMI | Vibrating hand toolsc | Autoimmune diseasec,d | Cardiovascular diseasec,e | Alcohol risk consumptionc,f | Current smokingc |
|---|---|---|---|---|---|---|---|---|---|
| Sensory | |||||||||
| Tingling fingers/hands | 1.2 (1.1–1.4) | 1.3 (0.9–1.6) | NE | NE | 1.3 (1.1–1.7) | NE | NE | NE | NE |
| Tingling toes/feet | 1.3 (1.1–1.5) | 1.3 (1.1–1.7) | 1.2 (1.1–1.5) | 1.2 (1.1–1.5) | NE | NE | NE | NE | NE |
| Numbness fingers/hands | NE | 1.5 (1.2–1.8) | NE | NE | 1.3 (1.1–1.7) | NE | NE | NE | NE |
| Numbness toes/feet | 1.3 (1.1–1.5) | 1.3 (0.9–1.6) | 1.3 (1.1–1.6) | 1.2 (1.1–1.5) | NE | NE | NE | NE | NE |
| Shooting/burning fingers/hands | NE | 2.3 (1.6–3.3) | NE | 1.4 (1.1–1.9) | 1.5 (0.9–2.4) | NE | NE | NE | NE |
| Shooting/burning toes/feet | NE | 1.8 (1.3–2.5) | NE | 1.5 (1.2–2.0) | NE | NE | NE | NE | NE |
| Problems standing | 1.5 (1.1–2.0) | 1.5 (0.9–2.4) | NE | 1.6 (1.1–2.2) | NE | NE | 1.2 (0.9–1.6) | 0.9 (0.5–1.5) | NE |
| Difficulty hot/cold | 1.9 (1.1–3.2) | 3.8 (2.0–7.4) | NE | NE | NE | NE | 1.3 (0.8–2.4) | NE | NE |
| Hearing difficulty | NE | NE | 1.3 (1.1–1.6) | NE | NE | 1.4 (1.1–1.8) | NE | NE | NE |
| Motor | |||||||||
| Cramps in hands | NE | NE | 1.9 (1.5–2.4) | NE | NE | 1.9 (1.5–2.4) | NE | 0.5 (0.3–0.8) | NE |
| Cramps in feet | NE | NE | 1.2 (1.1–1.4) | NE | NE | NE | NE | NE | NE |
| Problem holding pen | NE | 2.4 (1.3–4.3) | NE | NE | NE | 1.9 (1.1–3.3) | NE | NE | NE |
| Difficulty manipulating small objects | 1.3 (1.1–1.5) | 1.3 (0.9–1.8) | 1.5 (1.2–1.8) | NE | 1.4 (1.1–1.9) | 1.3 (0.9–1.7) | NE | NE | 1.4 (1.1–1.8) |
| Difficulty opening a jar | NE | 1.4 (1.2–1.5) | 1.2 (1.1–1.3) | NE | NE | 1.3 (1.1–1.4) | NE | NE | NE |
| Difficulty walking/foot drop | NE | 2.3 (0.9–5.6) | NE | 2.3 (1.1–4.6) | NE | 2.5 (1.2–5.3) | NE | 0.8 (0.3–2.0) | NE |
| Difficulty climbing stairs | NE | 1.0 (0.7–1.5) | 1.6 (1.3–2.0) | 1.6 (1.2–2.0) | NE | NE | 1.0 (0.8–1.3) | 0.8 (0.6–1.2) | 1.6 (1.2–2.2) |
PN peripheral neuropathy, ESBC early-stage breast cancer, BMI body mass index, NE variable not entered in multivariable analyses.
Bold values shows significant relative risks.
aThe following variables were entered into the univariable analysis: type of taxane (docetaxel vs paclitaxel), age at diagnosis (<65 vs ≥65 years), BMI at diagnosis (<25 vs ≥25), receiving treatment for diabetes mellitus (no vs yes), use of vibrating hand tools at work (no vs yes), autoimmune disease (no vs yes), alcohol risk consumption (no vs yes), cardiovascular disease (no vs yes), current smoking (no vs yes), mastectomy (no vs yes) and lymph node metastases (no vs yes). Only predictive factors with a statistically significant association (p < 0.05) in the univariable analyses with an individual symptom were entered into the multivariable model.
bN = 628 treated with either docetaxel or paclitaxel. The 18 survivors treated with both docetaxel and paclitaxel were excluded from the analysis.
cSelf-reported at the time of completing the questionnaire.
dRheumatism, scleroderma, systemic lupus erythematosus and Sjogren’s syndrome.
eHypertension, heart failure, angina pectoris and myocardial infarction.
fAlcohol risk consumption measured by AUDIT-C.
Discussion
In this large population-based study, the risk for symptoms of PN was higher among ESBC survivors, treated up to 7 years earlier with a taxane, than among age- and residency-matched control women without prior cancer from the general population. Survivors had the highest risk for “tingling of toes/feet” and “numbness of toes/feet” compared with controls. The highest prevalence was for “difficulties opening a jar” and “cramps in feet”, which affected more than half of the survivors. Moderate–severe neuropathy affected at least every fourth survivor. The prevalence of most neuropathic symptoms decreased with time but remained higher compared with controls. The use of paclitaxel, age >65 years, overweight, treatment for diabetes mellitus, vibrating hand tools, autoimmune disease and smoking were independent risk factors for individual symptoms of PN among ESBC survivors.
In a recent systematic review of CIPN among ESBC survivors, only five publications (from four studies) provided data beyond 1 year post treatment.11 The frequency of CIPN ranged from 11% to >80% of participants, up to 3 years following treatment. Differences in sample size, study design, chemotherapy exposure and outcome measures contributed to the wide range in prevalence. Eckhoff et al.4 found an overall PN of 52% 1–3 years after docetaxel treatment for ESBC when assessed by CIPN20. As shown in our study, the prevalence of individual symptoms, within and between categories, differed. Hence, reporting of an overall prevalence that combines all categories of PN may not be so clinically meaningful. We suggest that studies also should investigate and report results on specific symptoms of PN as we have done here. Furthermore, psychometric testing of the EORTC QLQ-CIPN20 instrument indicates an unstable factor structure questioning reporting of subscale scores.27
Long-term symptoms of sensory neuropathy among ESBC survivors have also been reported by others. Bandos et al.8 reported the results from a quality of life sub-study of a randomised phase III trial evaluating different docetaxel regimens. Two years after treatment initiation, 42% of 1512 patients reported that they were bothered by “numbness and tingling in hands and feet”, with 10% reporting “quite a bit” to” very much” “bother” level. Six years after diagnosis, the reported prevalence of sensory symptoms of the lower extremities was 47% in a study including cancer survivors from exercise-intervention trials31 and between 31% (docetaxel) and 44% (paclitaxel) in a cross-sectional study of 254 breast cancer survivors.32 Bao et al.14 reported a much higher prevalence (58%; 27% was rated as moderate–severe) of tingling/numbness in hands and/or feet, in a study of 296 hormone-receptor-positive ESBC survivors treated with taxane-based chemotherapy 6 years earlier.14 In comparison, 48% of ESBC survivors in our study reported “tingling/numbness in hands and/or feet”. In contrast to others, our results are from a large population-based cohort. The ESBC survivors had the highest risk for tingling/numbness in the lower extremities, which may lead to functional deficits and higher fall risk, which potentially may be life-threatening.3,14,31 It is, therefore, important to increase the awareness of the risk of this long-term adverse event so that measures can be taken early during taxane treatment.
Apart from our study, individual symptoms of motor and autonomic symptoms of PN have scarcely been studied among long-term ESBC survivors. The most prevalent symptom of all categories was “difficulties opening a jar” and “cramps in feet”, which persisted over time. Although many symptoms categorised as motor in the CIPN20 module may be due to weakness in upper and/or lower extremities, it is more likely that at least some symptoms (e.g. “problem holding a pen”) are due to a mixed motor–sensory dysfunction rather than a pure motor dysfunction33,34, which further emphasises the importance of reporting and monitoring individual symptoms. Our results regarding the autonomic items “dizziness” and “blurred vision” should be interpreted with caution since recent psychometric testing of the EORTC QLQ-CIPN20 indicate that these items are not reliable indicators of PN.26
The risk of “hearing difficulties”, “shooting/burning fingers/hands”, “problems holding a pen” and “difficulty using pedals when driving” was not higher among survivors compared with controls. “Hearing difficulties” are rather associated with age, noise exposure and cisplatin treatment than taxane chemotherapy.27,35,36 Although we found that the mean scores for “shooting/burning of fingers/hands” or “problems holding a pen” were higher among survivors than controls, survivors did not have a higher risk for these symptoms. It may be that other conditions such as diabetes mellitus constitute a higher risk than taxane chemotherapy. Few participants reported “difficulty using pedals when driving”, which could have influenced the risk ratio between survivors and control women. This conditioned item has also been reported to have low instrument validity due to respondents concerns about their driving license.33
The prevalence of polyneuropathy reported in the general population ranges between 1 and 7%.16 Recently, the prevalence of “definitive” polyneuropathy (diagnosed by a consensus panel using combined data from questionnaires, neurologic examinations and nerve conduction studies) was reported to be 5.5% in an unselected community-based population of 1310 participants (mean age 70 years, 55% female). The combined prevalence of “definitive” and “probable” neuropathy was 9.4%, range 7.9–11.1%.37 These normative data are consistent with our findings from the Swedish female general population. Our normative data are also compatible with those reported in a Dutch population without cancer using the EORTC QLQ-CIPN20 questionnaire.19 Fewer control women in our study reported the absence of PN symptoms compared with the Dutch normative data, which may be explained by the high mean age, and the exclusion of the male gender.
At the time of the survey, survivors were more often postmenopausal, osteoporotic and used less often exogenous oestrogens than the age- and residence-matched control women without prior cancer. These differences are likely a consequence of cancer treatment since chemotherapy may induce earlier menopause6,38 and aromatase inhibitors are associated with osteoporosis and bone mineral loss.39 The survivors were more often overweight than control women, but the mean weight at diagnosis did not differ from when completing the questionnaire. Since overweight is associated with an increased risk for breast cancer in postmenopausal women, the difference in BMI between survivors and control women probably mirror the breast cancer population.40 Breast cancer survivors are advised against the use of exogenous oestrogens at diagnosis. We have no obvious explanation for the difference in the use of vibrating tools among survivors and control women.
Several risk factors for TIPN have previously been reported, e.g. cumulative dose of taxane, paclitaxel, increasing age, higher BMI, diabetes mellitus,41 mastectomy, >3 positive lymph nodes and pre-existing PN.8 Due to the cross-sectional study design of our study, we did not have access to all baseline factors of relevance, e.g. pre-existing PN. We chose not to ask the survivors about this issue due to the risk of recall bias. We did not have access to comorbidities at baseline, but we assumed that severe comorbidities (such as diabetes requiring treatment, autoimmune disease and cardiovascular disease) reported at the time of the survey also may, at least to some extent, have existed when initiating chemotherapy. Consistent with other reports of PN during and up to 2 years after taxane therapy, we found older age, higher BMI, diabetes mellitus and treatment with paclitaxel to be predictive for several symptoms of persistent TIPN, especially of “tingling of toes/feet”.
The use of vibrating hand tools has to our knowledge not been studied in relation to CIPN before, but it is a well-known occupational hazard. Workers exposed to hand–arm vibration has been reported to experience tingling and numbness in their fingers and hands.17,18 Our findings underscore the relevance of including questions also about the use of vibrating tools when exploring risk factors for PN among newly diagnosed ESBC patients.
We found that a history of autoimmune disease was associated with symptoms of TIPN among ESBC survivors. Neuropathy has been reported among patients with various autoimmune diseases (e.g. systemic lupus erythematosus, rheumatoid arthritis and Sjögren’s syndrome) and can consist of pure sensory or sensorimotor modalities.42 We found no increased risk for pure sensory symptoms among survivors with autoimmune disease, but several of the associated motor symptoms are probably sensorimotor, e.g. “difficulty holding a pen”. In contrast to our results, Hershman et al.41 found a borderline, significant, protective effect of a history of autoimmune disease with the development of CIPN, which intrigued the authors. The protective effect of alcohol risk consumption of “cramps in hands” should be interpreted with caution. We asked about alcohol consumption at the time of the survey and alcohol habits may change over time. Smoking was associated with the development of two symptoms, “difficulty manipulating small objects” and “difficulty climbing stairs”, of TIPN and has previously been implicated as a risk factor of CIPN.12,43 CIPN is considered dose-dependent44, wherefore we included a cumulative dose of docetaxel and paclitaxel, respectively, in the first regression analysis. We found, however, a protective effect with a higher cumulative dose (data not shown), which probably reflects dose reductions due to toxicity as have been reported by others.9 We, therefore, chose not to include these variables in the final regression analyses.
This is the first study to compare self-reported PN among long-term ESBC survivors in a large population-based cohort with women without prior cancer from the general population. The use of unique personal identity numbers and public registers, together with the fact that all breast cancer patients in Sweden belong to one of six geographical catchment areas, minimises the risk for selection-induced problems. The use of a validated questionnaire is a strength and enables comparison with other studies. Interviewer-induced bias was avoided by using a postal questionnaire. Access to all medical records has ensured accurate information regarding clinical characteristics and administered chemotherapy. Another strength of our study is that only one patient had received platinum chemotherapy, which is also associated with PN. Our data were based on early-stage, recurrent-free breast cancer survivors, and it may not be possible to generalise the results to populations with more advanced stage or recurrent disease. We have no information about non-responders, and our assessment was limited to self-reported CIPN. However, studies comparing objective and subjective assessments of CIPN suggest that patient-related outcome measures are reliable.45
Conclusions
We have shown that symptoms of PN were common among long-term ESBC survivors compared with control women and that many symptoms persisted over time. The highest risk ratios among survivors were for tingling and/or numbness in the lower extremities. We found that in addition to receiving paclitaxel, age >65 years, overweight, a history of treatment for diabetes mellitus, autoimmune disease, use of vibrating hand tools and smoking were independent risk factors for persistent TIPN. The prevalence of symptoms varied within and between categories of PN and some symptoms were not more common among survivors than controls, wherefore reporting individual symptoms of PN may be more clinically meaningful than subscales. ESBC patients’ needs to be informed about the risk of long-term adverse events, particularly conditions associated with functional deficits and socioeconomic burdens. The treatment regimens received by the patients in our study are in accordance with currently prescribed regimens in Europe and the United States. It is, therefore, important to take our results into consideration when making shared treatment decisions for ESBC patients. Patients with risk factors such as older age, overweight and diabetes mellitus may choose to avoid taxane chemotherapy, especially paclitaxel, if other options exist. Monitoring individual symptoms of PN early during taxane chemotherapy is important so that appropriate interventions can be done (dose modification or treatment alteration) to decrease the risk of persistent PN.
Supplementary information
Acknowledgements
We wish to thank all the study participants, Marika Wenemark for assistance in developing the questionnaire and Rasmus Mikiver for cancer register assistance.
Author contributions
Conceptualisation; data curation; formal analysis; methodology; resources; software; supervision; validation; visualisation; roles/writing—original draft: K.E., M.F., H.G. and E.Å.-L. Funding acquisition; investigation; project administration; writing—review and editing: K.E., H.G. and E.Å.L.
Ethics approval and consent to participate
The Regional Ethics Committee in Linköping approved the study (Ref. no. 2016/548-31). All women who participated in the study gave informed consent by returning the questionnaire. The study was performed in accordance with the Declaration of Helsinki.
Consent to publish
This study contained no individual person’s data.
Data availability
The dataset analysed during the current study is available from the corresponding author on reasonable request.
Competing interests
The funding sources played no role in the study. The authors declare no competing interests.
Funding information
This work was supported by the Swedish Cancer Society (190224); the Medical Research Council of Southeast Sweden (FORSS-932359); Futurum—The Academy for Health and Care, Jönköping County Council (575361); Forsknings-ALF (LIO-901261).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01429-3.
References
- 1.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard PL, Di Leo A, Piccart-Gebhart MJ. Taxanes: optimizing adjuvant chemotherapy for early-stage breast cancer. Nat. Rev. Clin. Oncol. 2010;7:22–36. doi: 10.1038/nrclinonc.2009.186. [DOI] [PubMed] [Google Scholar]
- 3.Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support. Care Cancer. 2014;22:2261–2269. doi: 10.1007/s00520-014-2255-7. [DOI] [PubMed] [Google Scholar]
- 4.Eckhoff L, Knoop A, Jensen MB, Ewertz M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur. J. Cancer. 2015;51:292–300. doi: 10.1016/j.ejca.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Tanabe Y, Hashimoto K, Shimizu C, Hirakawa A, Harano K, Yunokawa M, et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int. J. Clin. Oncol. 2013;18:132–138. doi: 10.1007/s10147-011-0352-x. [DOI] [PubMed] [Google Scholar]
- 6.Nitz U, Gluz O, Huober J, Kreipe HH, Kates RE, Hartmann A, et al. Final analysis of the prospective WSG-AGO EC-Doc versus FEC phase III trial in intermediate-risk (pN1) early breast cancer: efficacy and predictive value of Ki67 expression. Ann. Oncol. 2014;25:1551–1557. doi: 10.1093/annonc/mdu186. [DOI] [PubMed] [Google Scholar]
- 7.Ewertz M, Qvortrup C, Eckhoff L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015;54:587–591. doi: 10.3109/0284186X.2014.995775. [DOI] [PubMed] [Google Scholar]
- 8.Bandos, H., Melnikow, J., Rivera, D. R., Swain, S. M., Sturtz, K., Fehrenbacher, L. et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG Oncology/NSABP B-30. J. Natl Cancer Inst. 110, 10.1093/jnci/djx162 (2018). [DOI] [PMC free article] [PubMed]
- 9.Speck RM, Sammel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, et al. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 2013;9:e234–e240. doi: 10.1200/JOP.2012.000863. [DOI] [PubMed] [Google Scholar]
- 10.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J. Clin. Oncol. 2012;30:3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 11.Rivera, D. R., Ganz, P. A., Weyrich, M. S., Bandos, H. & Melnikow, J. Chemotherapy-Associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J. Natl Cancer Inst.110, 10.1093/jnci/djx140 (2018). [DOI] [PMC free article] [PubMed]
- 12.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Greenlee, H., Hershman, D. L., Shi, Z., Kwan, M. L., Ergas, I. J., Roh, J. M. et al. BMI, lifestyle factors and taxane-induced neuropathy in breast cancer patients: the Pathways Study. J. Natl Cancer Inst. 109, 10.1093/jnci/djw206 (2017). [DOI] [PMC free article] [PubMed]
- 14.Bao T, Basal C, Seluzicki C, Li SQ, Seidman AD, Mao JJ. Long-term chemotherapy-induced peripheral neuropathy among breast cancer survivors: prevalence, risk factors, and fall risk. Breast Cancer Res. Treat. 2016;159:327–333. doi: 10.1007/s10549-016-3939-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julian T, Glascow N, Syeed R, Zis P. Alcohol-related peripheral neuropathy: a systematic review and meta-analysis. J. Neurol. 2019;266:2907–2919. doi: 10.1007/s00415-018-9123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 2016;31:5–20. doi: 10.1007/s10654-015-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolke R, Rolke S, Vogt T, Birklein F, Geber C, Treede RD, et al. Hand-arm vibration syndrome: clinical characteristics, conventional electrophysiology and quantitative sensory testing. Clin. Neurophysiol. 2013;124:1680–1688. doi: 10.1016/j.clinph.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 18.Heaver C, Goonetilleke KS, Ferguson H, Shiralkar S. Hand-arm vibration syndrome: a common occupational hazard in industrialized countries. J. Hand Surg. Eur. Vol. 2011;36:354–363. doi: 10.1177/1753193410396636. [DOI] [PubMed] [Google Scholar]
- 19.Mols F, van de Poll-Franse LV, Vreugdenhil G, Beijers AJ, Kieffer JM, Aaronson NK, et al. Reference data of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CIPN20 Questionnaire in the general Dutch population. Eur. J. Cancer. 2016;69:28–38. doi: 10.1016/j.ejca.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48:27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J. Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 22.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 23.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur. J. Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Kieffer JM, Postma TJ, van de Poll-Franse L, Mols F, Heimans JJ, Cavaletti G, et al. Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20) Qual. Life Res. 2017;26:2999–3010. doi: 10.1007/s11136-017-1626-1. [DOI] [PubMed] [Google Scholar]
- 25.Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann. Oncol. 2013;24:454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Qual. Life Res. 2013;22:2787–2799. doi: 10.1007/s11136-013-0379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith EML, Banerjee T, Yang JJ, Bridges CM, Alberti P, Sloan JA, et al. Psychometric Testing of the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Chemotherapy-Induced Peripheral Neuropathy 20-Item Scale Using Pooled Chemotherapy-Induced Peripheral Neuropathy Outcome Measures Standardization and Alliance for Clinical Trials in Oncology A151408 Study Data. Cancer Nurs. 2019;42:179–189. doi: 10.1097/NCC.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsson SJ, Ekblom Ö, Andersson E, Börjesson M, Kallings LV. Categorical answer modes provide superior validity to open answers when asking for level of physical activity: a cross-sectional study. Scand. J. Public Health. 2016;44:70–76. doi: 10.1177/1403494815602830. [DOI] [PubMed] [Google Scholar]
- 29.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 30.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 31.Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J. Clin. Oncol. 2017;35:2604–2612. doi: 10.1200/JCO.2016.71.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustafa Ali, M., Moeller, M., Rybicki, L. & Moore, H. C. F. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res. Treat.10.1007/s10549-017-4437-8 (2017). [DOI] [PubMed]
- 33.Lavoie Smith EM, Haupt R, Kelly JP, Lee D, Kanzawa-Lee G, Knoerl R, et al. The content validity of a chemotherapy-induced peripheral neuropathy patient-reported outcome measure. Oncol. Nurs. Forum. 2017;44:580–588. doi: 10.1188/17.ONF.580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M, Cheng HL, Lopez V, Sundar R, Yorke J, Molassiotis A. Redefining chemotherapy-induced peripheral neuropathy through symptom cluster analysis and patient-reported outcome data over time. BMC Cancer. 2019;19:1151. doi: 10.1186/s12885-019-6352-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molassiotis A, Cheng HL, Lopez V, Au JSK, Chan A, Bandla A, et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer. 2019;19:132. doi: 10.1186/s12885-019-5302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J. Clin. Oncol. 2016;34:2712–2720. doi: 10.1200/JCO.2016.66.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanewinckel R, Drenthen J, van Oijen M, Hofman A, van Doorn PA, Ikram MA. Prevalence of polyneuropathy in the general middle-aged and elderly population. Neurology. 2016;87:1892–1898. doi: 10.1212/WNL.0000000000003293. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Fidalgo JA, Roselló S, García-Garré E, Jordá E, Martín-Martorell P, Bermejo B, et al. Incidence of chemotherapy-induced amenorrhea in hormone-sensitive breast cancer patients: the impact of addition of taxanes to anthracycline-based regimens. Breast Cancer Res. Treat. 2010;120:245–251. doi: 10.1007/s10549-009-0426-x. [DOI] [PubMed] [Google Scholar]
- 39.Gonnelli S, Petrioli R. Aromatase inhibitors, efficacy and metabolic risk in the treatment of postmenopausal women with early breast cancer. Clin. Interv. Aging. 2008;3:647–657. doi: 10.2147/CIA.S3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015;1:611–621. doi: 10.1001/jamaoncol.2015.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in southwest oncology group clinical trials. J. Clin. Oncol. 2016;34:3014–3022. doi: 10.1200/JCO.2015.66.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattacharyya S, Helfgott SM. Neurologic complications of systemic lupus erythematosus, Sjögren syndrome, and rheumatoid arthritis. Semin. Neurol. 2014;34:425–436. doi: 10.1055/s-0034-1390391. [DOI] [PubMed] [Google Scholar]
- 43.Brydøy M, Oldenburg J, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J. Natl Cancer Inst. 2009;101:1682–1695. doi: 10.1093/jnci/djp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan B, Margulies A, Cardoso F, Cavaletti G, Haugnes HS, Jahn P, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020;31:1306–1319. doi: 10.1016/j.annonc.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 45.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res. Treat. 2011;125:767–774. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed during the current study is available from the corresponding author on reasonable request.