Abstract
Continuous glucose monitoring (CGM) use is associated with improved A1C outcomes and quality of life in adolescents and young adults with diabetes; however, CGM uptake is low. This article reports on a quality improvement (QI) initiative of the T1D Exchange Quality Improvement Collaborative to increase CGM use among patients in this age-group. Ten centers participated in developing a key driver diagram and center-specific interventions that resulted in an increase in CGM use from 34 to 55% in adolescents and young adults over 19–22 months. Sites that performed QI tests of change and documented their interventions had the highest increases in CGM uptake, demonstrating that QI methodology and sharing of learnings can increase CGM uptake.
Consistent glycemic management is necessary to prevent long-term microvascular and macrovascular complications in individuals with type 1 diabetes (1,2). In the American Diabetes Association’s (ADA’s) most recent Standards of Medical Care in Diabetes (3), an A1C target of <7% is recommended for most children and adults with type 1 diabetes but should be personalized. However, data from the T1D Exchange have shown that a majority of children, adolescents, and young adults in the United States did not meet an earlier A1C target of <7.5% (4). In fact, patients in the 2016–2018 T1D Exchange cohort had worse A1C outcomes than those in the 2010–2012 cohort (mean A1C of 7.8% in the 2010–2012 cohort and 8.4% in the 2016–2018 cohort) (4,5).
Data from the T1D Exchange collected before the widespread use of continuous glucose monitoring (CGM) technology suggests that increased frequency of self-monitoring of blood glucose (i.e., with a glucose meter) is strongly associated with lower A1C (6). CGM provides more data (up to 288 glucose readings per day), as well as glycemic trends and alerts to improve clinical outcomes. Newer-generation CGM systems are more accurate, factory-calibrated, and have nonadjunctive use indications. In contrast, earlier CGM systems required fingerstick glucose checks for calibration, making these devices less attractive for use, particularly by adolescents and young adults (7).
Uptake of CGM is increasing worldwide, including in the United States (8). In 2017 data from the T1D Exchange, ∼20% of youth and young adults between the ages of 13 and 26 years used CGM. Recent data from a randomized control trial demonstrated that adolescents and young adults started on CGM sustained CGM use and had a mean A1C reduction of 0.37% after 6 months of CGM use (9). Several studies have demonstrated that initiating CGM early in the course of type 1 diabetes can improve clinical outcomes (10,11).
However, there are barriers to technology adoption at the structural, patient, and provider levels (12,13). Structural barriers to CGM adoption include financial challenges related to insurance coverage and associated out-of-pocket costs. Patient-level barriers may include reluctance to wear a diabetes device, exacerbation of diabetes distress (potentially resulting from CGM alarms [12]), or simply inadequate information or perceptions about CGM. Examples of provider-level barriers include lack of time to learn about, promote, and complete paperwork for insurance approval of CGM.
The T1D Exchange Quality Improvement Collaborative (T1DX-QI) is a learning collaborative established to improve care delivery for people with type 1 diabetes (14). Between May 2018 and March 2020, the 10 sites initially participating in the collaborative worked on improving CGM use among adolescents and young adults (12–26 years of age) from an aggregated baseline of 34% to a collaborative target of 50% over a 2-year time frame. Each center adapted preexisting barriers questionnaires (15) to assess barriers to CGM adoption in their populations and developed targeted interventions to address structural-, patient-, and provider-level barriers to CGM use.
Research Design and Methods
Interventions to Improve CGM Use
This project was deemed nonhuman subject research by the Western Institutional Review Board. All participating centers also received local institutional review board approval to share aggregate data and participate in this quality improvement (QI) project. No identifiable patient information was collected for this project.
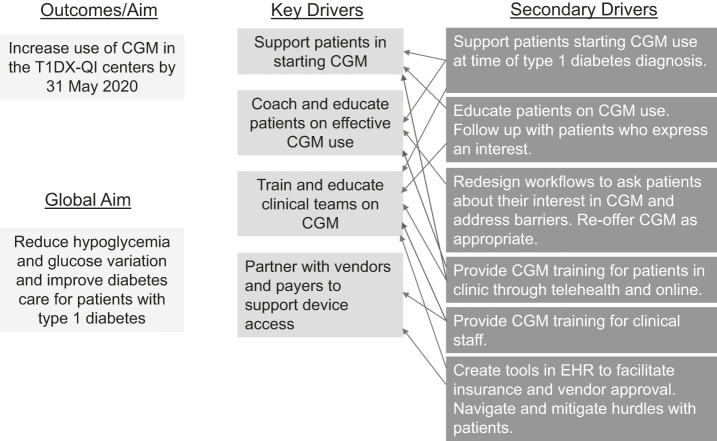
Ten diabetes centers in the T1DX-QI (seven pediatric clinics [Barbara Davis Center, Children’s Mercy Hospital, Cincinnati Children’s Medical Center, C.S. Mott Children’s Hospital, Nationwide Children’s Hospital, Stanford Children’s Hospital, and Texas Children’s Hospital] and three adult clinics [Barbara Davis Center, SUNY Upstate, and University of Pennsylvania) used QI methodology, including the development of a theoretical model or key driver diagram (Figure 1). The global aim of the T1DX-QI is to improve clinical outcomes and patient experience by increasing patient achievement of the ADA-recommended A1C target, reducing hypoglycemia and glucose variation, improving diabetes care delivery to individuals with type 1 diabetes, and advancing quality of life. One of the outcomes of focus to promote this aim is increased use of CGM within the collaborative clinics. The clinical leadership team reviewed the literature and clinic operations and identified four key drivers to achieve this outcome: 1) support patients starting CGM, 2) coach and educate patients on effective CGM use, 3) train and educate clinical teams on CGM, and 4) partner with vendors and payers to support device access.
FIGURE 1.
Key driver diagram for increasing CGM uptake across the T1DX-QI collaborative. The secondary drivers are interventions to support the key drivers.
Interventions were center-specific, and each site used iterative Plan-Do-Study-Act cycles to test and expand various interventions to increase CGM use among their patients aged 12–26 years. Outcomes were shared on an ongoing basis via a common website platform (Life QI) and during monthly collaborative calls during which participating centers presented updates. Resources and artifacts from successful testing were shared on an online sharing platform accessible to all members of the T1DX-QI (Trello). The sites began testing improvement ideas in May 2018; data were collected and analyzed for 22 months in pediatric sites (May 2018 to February 2020) and 19 months in adult sites (May 2018 to December 2019). All work was completed before the onset of the coronavirus disease 2019 pandemic.
Each center in the collaborative was responsible for designing and implementing its own interventions. Sites identified barriers to CGM uptake at their sites and designed interventions to target those barriers. The interventions were grouped into 12 categories that mapped to one of the four key drivers (Table 1).
TABLE 1.
Interventions to Improve CGM Uptake in the T1DX-QI
| Site | Support Patients Starting CGM | Coach and Educate Patients on Effective CGM Use | Train and Educate Clinical Teams on CGM | Partner With Vendors and Payers to Support Device Access | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tip Sheets for New CGM Users (n = 1) | “30-60-90 Rule” Education Tool (n = 4) | Insurance Navigator to Support Families (n = 1) | Promotion of CGM Use in New-Onset Patients (n = 7) | Barriers Assessment Survey (n = 5) | Educational Programs and Tools to Promote CGM Use (n = 5) | Trial CGM (n = 1) | Staff Training (n = 2) | Tools to Help Providers Track Whether CGM Was Offered (n = 3) | Incorporation of CGM Data into the EHR (n = 2) | Tools to Facilitate Prescribing by Clinic Staff (n = 3) | Advocacy Efforts to Promote CGM Coverage by State Medicaid Program (n = 10) | |
| P 1 | x | x | x | x | x | |||||||
| P 2 | x | x | ||||||||||
| P 3 | x | x | x | x | ||||||||
| P 4 | x | x | x | x | x | x | x | x | x | |||
| P 5 | x | x | x | x | x | |||||||
| P 6 | x | x | x | x | ||||||||
| P 7 | x | x | x | x | x | x | ||||||
| A 1 | x | x | x | |||||||||
| A 2 | x | x | x | x | x | x | ||||||
| A 3 | x | |||||||||||
A, adult; P, pediatric.
Support Patients Starting CGM
To help support patients new to CGM, one of the sites prepared material for common issues such as skin reactions to CGM sensors, devices falling off, and alarms and provided guidance on troubleshooting. One of the sites developed a class for helping patients and families interpret CGM data. To guide data interpretation for real-time decision-making, one of the sites created the “30-60-90 Rule” to encourage dynamic diabetes management, and this intervention was adopted by three other sites (pediatric sites 1, 4, 6, and 7). This rule incorporated the rate of change arrows provided by CGM systems to optimize bolus dosing (16). Pediatric center 4 created the role of a patient navigator to help patients and families through the process of obtaining payer approval for CGM.
Coach and Educate Patients on Effective CGM Use
All pediatric sites focused on promoting CGM to patients with new-onset type 1 diabetes. Each site developed interventions that were specific to its own needs. However, through collaborative calls and learning sessions, tools were frequently shared and later adopted by other sites. For patients with established diabetes, several sites (pediatric sites 1, 4, and 5 and adult sites 1 and 2) used tools to assess barriers to glucose monitoring and/or device adoption. Providers used questionnaire responses as a starting point to discuss CGM and its benefits with patients and their families.
Five of the sites (pediatric sites 1, 4, 5, and 7; adult center 2) developed programs and tools to educate patients and families on the benefits of and appropriate expectations of CGM. One site developed a tool comparing the different options for glucose monitoring to allow for shared decision-making, and this tool was adopted by two other sites (Supplementary Figure S1). Adult center 2 created a program that allowed patients to have a trial of CGM.
Train and Educate Clinical Teams on CGM
Pediatric sites 3, 4, and 7 developed site-specific provider education for physicians, nurse practitioners, and certified diabetes educators, so these providers could better inform patients of the benefits of CGM. The contents of these education sessions were shared among collaborative members. Pediatric center 4 and adult sites 1 and 2 developed flow sheets in the electronic health record (EHR) to track whether patients were using or had been offered information about CGM. These tools helped to ensure that CGM was reviewed with all patients. Two of the sites (pediatric sites 5 and 6) incorporated CGM data into their EHR system to facilitate data review.
Partner With Vendors and Payers to Support Device Access
Three of the sites (pediatric sites 3, 4, and 7) developed tools to facilitate prescribing by clinic staff. One clinic created letters of medical necessity within its EHR, one facilitated ordering through its EHR, and a third streamlined its internal workflows for ordering CGM. Although these tools were developed locally, they were shared across the collaborative so other sites could adapt and implement them.
All sites engaged in advocacy efforts to promote CGM coverage by their state Medicaid program. During this time frame, some states expanded coverage of CGM for publicly insured individuals. The group of 10 centers represented eight states (Table 2). Only three of the states had coverage during the entire study period; four did not have CGM coverage for individuals with public insurance for at least a part of the study period; and one had intermittent coverage during this period. In four states, individuals with public insurance had to meet certain criteria such as performing a fixed number of glucose checks per day to receive CGM approval.
TABLE 2.
Medicaid Coverage of CGM in States Where T1DX-QI Sites Were Located
| State | State Medicaid CGM Coverage |
|---|---|
| California | Covered with criteria for approval; gaps in coverage during the study period |
| Colorado | Covered with criteria for approval |
| Michigan | Coverage started April 2019 |
| Missouri | Coverage started April 2020 |
| New York | Covered |
| Ohio | Coverage started April 2019 with criteria for approval |
| Pennsylvania | Covered with criteria for approval |
| Texas | Coverage started April 2020 |
Data Collection
The participating diabetes centers shared monthly aggregate data using a secure data collection tool (www.smartsheet.com) with the T1DX-QI central coordinating office. The denominator was the number of patients with type 1 diabetes who had two A1C values in the preceding 12 months, of which the last value was from the reporting month. The numerator was the number of patients in the denominator who used CGM at the time of their last visit. A1C changes are beyond the scope of this analysis.
Data Analysis
CGM use over time was plotted on a control chart, a type of process-behavior chart, to assess changes/shifts in CGM use and evaluate project effectiveness (17). Eight data points above the mean or four out of five consecutive data points outside the first σ control limits, a threshold set at 3 SDs above the mean, were used to determine the shifts. Data were further used for ongoing QI coaching and for promoting sharing of best practices. A t test was used to evaluate statistical significance between the pre- and post-intervention means.
Control charts were created using the SPC for Excel plug-in software (https://www.spcforexcel.com), and the t test was completed using R software (https://www.r-project.org).
Results
CGM Use Increased Across the Collaborative in Patients 12–26 Years of Age
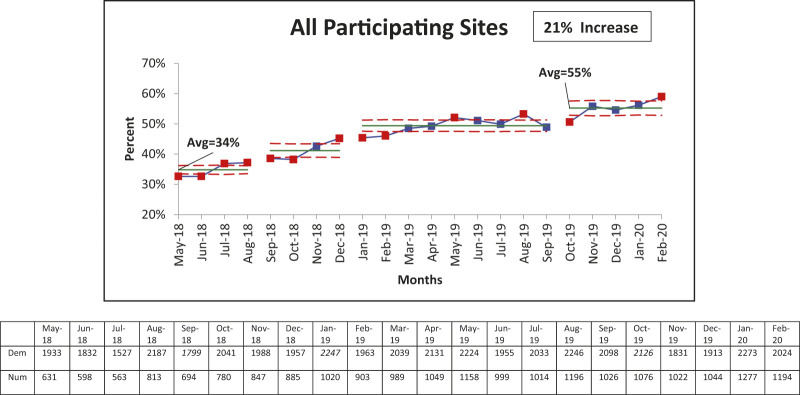
The median CGM use across the 10 sites was 34% in May 2018. Through targeted interventions specific to each center, this use increased to 55% by February 2020, an increase of 21% by March 2020 (Figure 2). This increase in CGM use was statistically significant (P <0.0001). Seven of 10 sites had increased CGM use during the intervention period.
FIGURE 2.
Control chart of CGM use among 12- to 26-year-old youths from all 10 participating sites in the T1DX-QI collaborative. CGM use increased from 34% baseline to 55% in 20 months after testing various interventions. Dashed lines are control lines, the solid line represents the mean. Dem, denominator; Num, numerator.
CGM Use Increased in Five of Seven Pediatric Sites
CGM use data from the seven pediatric sites are shown in Supplementary Figure S2. At the start of the intervention period in May 2018, CGM use among pediatric clinic sites varied from 21 to 68%. Except for pediatric clinics 3 and 7, which already had high CGM uptake (40 and 68%, respectively), all other sites experienced increases of 10–25% in CGM use. No pediatric center used one single intervention approach to increase CGM use (Table 1). Sites 4 and 5 experienced an increase in CGM use of ∼25% by implementing processes for initiating CGM in patients with new-onset type 1 diabetes, developing a tool to assess barriers to CGM adoption, and creating educational material. Center 4 also improved the process for CGM prescriptions, increased staff training, and created the role of an insurance navigator. Center 5 incorporated CGM data into its EHR to facilitate use by the staff. Center 1, which had an increase of 19% in CGM uptake, developed processes for identifying barriers to CGM use, improving CGM education, and using devices in the hospitalized setting. Center 2, which promoted CGM uptake in patients with new-onset type 1 diabetes, also increased CGM use by 19%. Center 6, which promoted CGM use in patients with new-onset type 1 diabetes, implemented the 30–60–90 education tool and incorporated CGM data into its EHR, had a 9.3% increase in CGM use. Sites 3 and 7, which did not see increases in CGM use, each had multiple interventions (Table 1).
CGM Use Increased in Two of Three Adult Sites
As was the case for pediatric centers, use of CGM was variable among adult clinics. At the start of the intervention period, CGM use ranged from 29 to 64% (Supplementary Figure S3). During the intervention period from May 2018 to December 2019, adult clinic 1 had no change in CGM use, whereas the other two adult clinics did. Adult center 2 developed tip sheets to support new CGM users, implemented a barriers assessment survey with an associated educational program, tracked CGM discussions in its EHR, and developed a program for trialing CGM. Adult center 3 advocated for CGM coverage by its state’s Medicaid program. Adult center 1, which did not see an increase in CGM use, developed a barriers assessment survey and tools to track CGM discussions with patients.
Discussion
During the study period, CGM use in the T1DX-QI collaborative centers overall increased from 34 to 55% (a 21% increase) in adolescents and young adults, which surpassed the target of 50%. Seven of the 10 participating centers experienced an increase in CGM use. All 10 sites performed assessments to identify barriers to CGM use in their clinics and designed interventions to address these barriers (15) and increase CGM use. Broadly, these interventions promoted CGM use to all patients, included discussion of CGM in the new-onset period, increased education to patients before and after CGM initiation, educated staff to promote CGM, and incorporated CGM prescribing and data review into clinical workflows. During collaborative calls and learning sessions, sites took turns presenting their work and sharing slides, templates, and tools. The learnings and tools from these calls could be adapted for use at other sites.
Two of the pediatric sites did not experience an increase in CGM use. Center 3 had implemented several interventions to increase CGM uptake, but their state did not have Medicaid coverage for CGM until April 2020, which was after the study period. Pediatric clinic 7 had very high CGM uptake at baseline (68%) and developed multiple interventions but did not experience further growth. Adult center 1 also experienced no change in CGM uptake; however, it is unclear whether its steady CGM use data were the result of no change in uptake or incomplete documentation of CGM use.
As CGM technology improves, its importance in diabetes care continues to increase. Since 2020, the ADA’s Standards of Care has recommended CGM as the standard of care for individuals with diabetes who are on insulin therapy (3). CGM use is associated with improved clinical outcomes, as measured by A1C, and a lower incidence of hypoglycemia (7,9,18). In addition, CGM is a key component of automated insulin delivery systems, which have the potential to improve diabetes management while easing the burden of type 1 diabetes (19).
Lack of access is a universal barrier to increasing CGM use. Although most private insurance plans cover CGM, the copayments prevent some individuals from using it. In addition, public insurance coverage for CGM varies by state, further limiting uptake. Half of the states represented in this initiative had prerequisites such as a certain number of glucose checks or boluses per day as requirements for CGM approval. These requirements may limit access to CGM for some patients, especially those with public insurance. Of eight states in which collaborative sites were located, only one had consistent Medicaid coverage of CGM throughout the initiative time period, whereas the others expanded Medicaid coverage or had intermittent Medicaid coverage of CGM (Table 2). Historically, state Medicaid programs limited access to CGM based on older data showing that youths and young adults had poor adherence to early CGM systems (7). However, recent randomized controlled trials and real-world studies have shown that publicly insured youths and young adults who are provided access show sustained use of CGM and improved A1C (9,20–22). Data from multistate networks such as the T1DX-QI can advocate for policy changes to allow for universal CGM coverage and decrease the work burden on clinics to increase CGM uptake (23). Data from the T1D Exchange clinic registry indicate a widening disparity in CGM use in youths with type 1 diabetes of lower compared with higher socioeconomic status, in contrast to data from the German DPV registry (24).
This project had limitations that should be noted. Many of the sites relied on clinical documentation of CGM use, which may have led to underreporting of CGM use. Additionally, this was an observational study, and many sites implemented multiple interventions; therefore, we are unable to assess the success of a single intervention in increasing CGM use. However, the overall trend is consistent with other reports that CGM use is increasing across the collaborative (8,23).
The use of CGM increased from 34 to 55% in adolescents and young adults across the T1DX-QI during an interval of active improvement initiatives. Identification of barriers and targeted approaches by diabetes care teams can increase CGM uptake in clinical practice. Further analyses are planned to determine whether these interventions to increase CGM use also improve clinical outcomes. Data from the T1DX-QI will support advocacy for changes to public policy and insurance coverage to promote CGM use as a standard of care for individuals with type 1 diabetes.
Article Information
Acknowledgments. The authors thank the Leona M. and Harry B. Helmsley Charitable Trust for funding the T1DX-QI. The authors acknowledge the contributions of patients, families, diabetes care teams, and collaborators within the T1DX-QI, who continually seek to improve care and outcomes for people with diabetes.
Duality of Interest
O.E. is a compensated Health Equity Advisory Board member for Medtronic Diabetes and serves as principal investigator for investigator-led projects sponsored by Abbott, Eli Lilly, Insulet, and Medtronic. M.C. has consulting arrangements with Eli Lilly and Medtronic, is an employee (chief medical officer) of Glooko, and has received research support from Abbott Diabetes Care and Dexcom and travel support from Intrexon and Provention Bio. J.M.L. is on a medical advisory board for GoodRx. D.M.M. has received research support from the Helmsley Charitable Trust, JDRF, the National Institutes of Health, and the National Science Foundation; his institution has received research support from Bigfoot Biomedical, Dexcom, Insulet, Medtronic, Roche, and Tandem; and he has been a consultant for Abbott, Aditxt, Dompe, Eli Lilly, the Helmsley Charitable Trust, Insulet, Medtronic, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
P.P. wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and contributed to the implementation of interventions and data collection. O.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation
A portion of the data included in this article was presented as a poster at the American Diabetes Association’s virtual 80th Scientific Sessions, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14403269.
This article is part of a special article collection available at https://clinical.diabetesjournals.org/collection/quality-improvement-initiatives-in-type-1-diabetes.
References
- 1. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2. Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . 13. Children and adolescents: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S163–S182 [DOI] [PubMed] [Google Scholar]
- 4. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 6. Miller KM, Beck RW, Bergenstal RM, et al.; T1D Exchange Clinic Network . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013;36:2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 8. Miller KM, Hermann J, Foster N, et al.; T1D Exchange and DPV Registries . Longitudinal changes in continuous glucose monitoring use among individuals with type 1 diabetes: international comparison in the German and Austrian DPV and U.S. T1D Exchange registries. Diabetes Care 2020;43:e1–e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laffel LM, Kanapka LG, Beck RW, et al.; CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group; CDE10 . Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulinacci G, Alonso GT, Snell-Bergeon JK, Shah VN. Glycemic outcomes with early initiation of continuous glucose monitoring system in recently diagnosed patients with type 1 diabetes. Diabetes Technol Ther 2019;21:6–10 [DOI] [PubMed] [Google Scholar]
- 11. Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care 2020;43:e3–e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes technology: uptake, outcomes, barriers, and the intersection with distress. J Diabetes Sci Technol 2016;10:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care 2021;44:14–16 [DOI] [PubMed] [Google Scholar]
- 14. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes 2020;38:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Divan V, Greenfield M, Morley CP, Weinstock RS. Perceived burdens and benefits associated with continuous glucose monitor use in type 1 diabetes across the lifespan. J Diabetes Sci Technol. Online ahead of print on 24 December 2020 (doi: 10.1177/1932296820978769) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corathers SD, DeSalvo DJ. Therapeutic inertia in pediatric diabetes: challenges to and strategies for overcoming acceptance of the status quo. Diabetes Spectr 2020;33:22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf 2011;20:46–51 [DOI] [PubMed] [Google Scholar]
- 18. Polonsky WH, Hessler D, Ruedy KJ; DIAMOND Study Group . The impact of continuous glucose monitoring on markers of quality of life in adults with type 1 diabetes: further findings from the DIAMOND randomized clinical trial. Diabetes Care 2017;40:736–741 [DOI] [PubMed] [Google Scholar]
- 19. Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prahalad P, Addala A, Buckingham BA, Wilson DM, Maahs DM. Sustained continuous glucose monitor use in low-income youth with type 1 diabetes following insurance coverage supports expansion of continuous glucose monitor coverage for all. Diabetes Technol Ther 2018;20:632–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ravi SJ, Coakley A, Vigers T, Pyle L, Forlenza GP, Alonso T. Pediatric Medicaid patients with type 1 diabetes benefit from continuous glucose monitor technology. J Diabetes Sci Technol 2021;15:630–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes 2020;21:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller KM, Beck RW, Foster NC, Maahs DM. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socioeconomic status on HbA1c in the T1D Exchange clinic registry findings. Diabetes Technol Ther 2020;22:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care 2021;44:133–140 [DOI] [PMC free article] [PubMed] [Google Scholar]