Abstract
Uncontrolled type 2 diabetes can lead to a multitude of health complications. Insulin therapy is recommended when patients are unable to reach their A1C goal with oral or noninsulin injectable diabetes medications. This study evaluated the clinical benefits of switching from multiple daily insulin injections to a wearable insulin delivery device (V-Go). A retrospective chart review was conducted on 44 patients who received prescriptions for the V-Go at two family medicine offices. Investigators found a significant reduction in A1C and daily insulin requirements with no impact on weight or BMI.
The Centers for Disease Control and Prevention estimated that, in 2018, 34.1 million American adults had diabetes, of whom 90–95% were diagnosed with type 2 diabetes (1). Despite significant risk of complications from uncontrolled diabetes, only 36% of patients met individual A1C treatment goals from 2013 to 2016 (2). Many patients with type 2 diabetes will eventually require basal and bolus insulin therapy to reach their glycemic goals (3). Although the American Association of Clinical Endocrinologists recognizes a full basal-bolus insulin regimen as the most effective insulin option for those who are unable to achieve glycemic control with other medications, its use can be limited by concerns of both health care professionals (HCPs) and patients (4).
Even when insulin is initiated, data show that many patients do not reach their A1C goal. One market research study (5) found that 51% of patients with type 2 diabetes had not reached their A1C goal despite treatment with basal insulin for at least 6 months. Database reviews of insurance claims have reflected similar results (6–8). Approximately 75% of insulin users had an A1C >7%, and 20–30% had an A1C >9% 6–12 months after starting basal insulin (6–8). Insulin adherence and persistence play a role, as studies have noted that approximately one-fourth of patients discontinue insulin after 1 year (5,7). Studies have also shown that insulin regimens that require more frequent injections are associated with lower adherence rates than the use of premixed or basal insulin alone, which require fewer injections (9,10).
Nonadherence may be influenced by patient-reported concerns such as fear of hypoglycemia, weight gain, or painful injections; belief that insulin indicates worsening diabetes or a personal failure; concerns about how insulin may affect daily life and reduce flexibility; and frustration about taking too much time to reach goal (5,11,12). Patients who admitted stopping bolus insulin in the previous year were most likely to do so because of difficulties calculating doses, regulating food intake, and keeping up with two types of insulin (13). Interestingly, studies have found that HCPs identify fear of hypoglycemia, patients’ belief that a lack of symptoms means there is no need to increase their doses, limited patient motivation and involvement, and cost concerns as the most common barriers to basal insulin initiation and titration (5,6). These beliefs of HCPs can lead to clinical inertia, which further contributes to poor glycemic control.
Although a multipronged approach must be considered to overcome barriers that lead to clinical inertia and nonadherence, the use of technology can play a role. The V-Go insulin delivery device may offer a solution for individuals who express concerns about the complexity of a multiple daily injection (MDI) treatment regimen, injection discomfort, or perceived negative effects on quality of life.
The V-Go was approved by the U.S. Food and Drug Administration (FDA) in 2012 for adults with type 2 diabetes who require insulin. Patients fill the device with fast-acting insulin and apply it like a patch to their abdomen or the back of their arm. A small needle inserts into the subcutaneous tissue to continuously release a small amount of fast-acting insulin for basal dosing. A push-button release allows additional units of insulin to be released for bolus dosing. After 24 hours, patients remove their V-Go and replace it with a new device (14). Three device options are available, providing 20, 30, or 40 units of basal insulin with up to 36 additional units of bolus insulin. Bolus doses are delivered in increments of 2 units per click of a button on the device. Initial dosing is based on patients’ weight (15).
The device is recognized by the American Diabetes Association (ADA) Standards of Medical Care in Diabetes as an option for insulin delivery (16). Commercial insurance coverage varies, but a cost savings card is available to decrease the 30-day supply cost to $0 for the first fill and a maximum of $75 for each subsequent fill, with a maximum benefit of $472 and $397 for the first and subsequent fills, respectively. However, uninsured patients are not eligible to use this cost savings card, and there is no manufacturer assistance program. Medicare Part D provides coverage for disposable insulin delivery devices, so most individuals with Part D insurance will have access to the device; however, the cost can vary significantly based on deductibles, required copayments, and coinsurance (17). For some patients, buying V-Go plus rapid-acting insulin is more cost-effective than purchasing basal insulin, bolus insulin, and needles or syringes (18,19).
Previous studies have shown that patients initiated on the V-Go experienced significant reductions in A1C ranging from ∼1 to 2%, and either stable or significantly decreased total daily doses (TDDs) of insulin (18–25). However, to date, almost all V-Go studies have been completed in endocrinology or other diabetes specialty settings. This study evaluates use of the V-Go device in a primary care setting.
Research Design and Methods
This descriptive study involved retrospective electronic medical record (EMR) review of all patients prescribed a V-Go device from April 2017 to May 2019. The target population was from two rural, hospital-owned family medicine clinics in the southeastern United States. The clinics included four physicians and three nurse practitioners and are part of a network of 26 outpatient clinics. Adults ≥21 years of age with type 2 diabetes who had at least one A1C result 3 months after starting the V-Go were included. Exclusion criteria included pregnancy, A1C levels that did not correlate with home blood glucose readings, or any condition associated with an altered relationship between A1C and glycemic control (e.g., sickle cell disease, dialysis, postpartum status, HIV, recent blood loss or transfusion, and anemia).
Data collection included A1C levels, BMI, body weight, other medications for diabetes, baseline basal insulin dose, TDD, and starting dose and titration for the V-Go. The TDD for the V-Go was considered to be the basal insulin dose plus the 36 units allowed for bolus doses because specific directions were not always documented in the clinic notes. The study was approved by the hospital’s institutional review board.
The primary outcome was change in A1C after using the V-Go for 3–6 months. Baseline A1C was assessed at the visit when the V-Go was started. Secondary outcomes included change in weight, BMI, and TDD, as well as starting dose and titration for the V-Go.
Statistical Analysis
Differences in A1C, body weight, BMI, and insulin TDD from baseline to follow-up at 3–6 months were evaluated using two-tailed, paired t tests and reported as mean ± SD. A paired t test was also used to compare the baseline basal insulin dose to the V-Go starting dose, and a Student t test was used to compare final A1C between baseline MDI and basal insulin–only groups. Statistical significance was determined to be P ≤0.05. Descriptive statistics included frequency, range, and percentage. All analyses were performed using SYSTAT 13 (Systat Software, Inc., San Jose, CA).
Results
Review of the EMR revealed 65 potential subjects. Twenty-one were excluded for the following reasons: failure to use the V-Go for at least 3 months (n = 9), prescribed but not using the V-Go (n = 10), and lack of follow-up after starting the V-Go (n = 2). Table 1 depicts baseline characteristics of the eligible study population (N = 44). Subjects’ mean age was 59.2 ± 11.7 years, and most were females (61.4%, 27/44). The duration of type 2 diabetes ranged from 2 to 30 years, with almost half the patients having diabetes for >10 years (45.4%, 20/44). The majority of patients were managed by a physician (79.5%, 35/44), and the remainder by a nurse practitioner. Patients were trained on V-Go by a representative from the company.
TABLE 1.
Baseline Characteristics of the Study Population
| Study Population (N = 44) | |
|---|---|
| Age, years | 59.2 ± 11.7 (37–81) |
| Female sex | 27 (61.4) |
| Caucasian race | 36 (81.8) |
| Baseline A1C, % | 9.7 ± 1.6 |
| Weight, lb | 222.2 ± 17.1 |
| BMI, kg/m2 | 36.3 ± 9.5 |
| Insulin TDD, units | 86 ± 46.4 |
| Patients on oral medications | 31 (70.5) |
| Patients on GLP-1 receptor agonist therapy | 4 (9) |
Data are mean ± SD or n (%) except for age, which is mean ± SD (range).
Before starting insulin delivery using the V-Go device, the majority of patients (65.9%, 29/44) were on a basal-bolus regimen. Other insulin regimens included basal insulin only (22.7%, 10/44) and premixed insulin (11.4%, 5/44). Basal insulin regimens at baseline included NPH insulin (n = 1) and long-acting analog insulin (n = 9). Long-acting insulins included glargine U-300 (n = 3), detemir (n = 3), glargine U-100 (n = 2), and degludec (n = 1). Additional therapies used with the V-Go included oral medications (70%, 31/44) and glucagon-like peptide-1 (GLP-1) receptor agonists with or without other medications (9%, 4/44). Medications prescribed along with the V-Go are shown in Table 2.
TABLE 2.
Medications Used With the V-Go
| n | |
|---|---|
| Metformin | 12 |
| Metformin and SGLT2 inhibitor | 9 |
| Metformin and GLP-1 receptor agonist | 1 |
| Metformin, SGLT2 inhibitor, and GLP-1 receptor agonist | 2 |
| SGLT2 inhibitor | 3 |
| DPP-4 inhibitor | 1 |
| TZD | 1 |
| SU | 1 |
| GLP-1 receptor agonist | 1 |
DPP-4, dipeptidyl peptidase 4; SGLT2, sodium–glucose cotransporter 2; SU, sulfonylurea; TZD, thiazolidinedione.
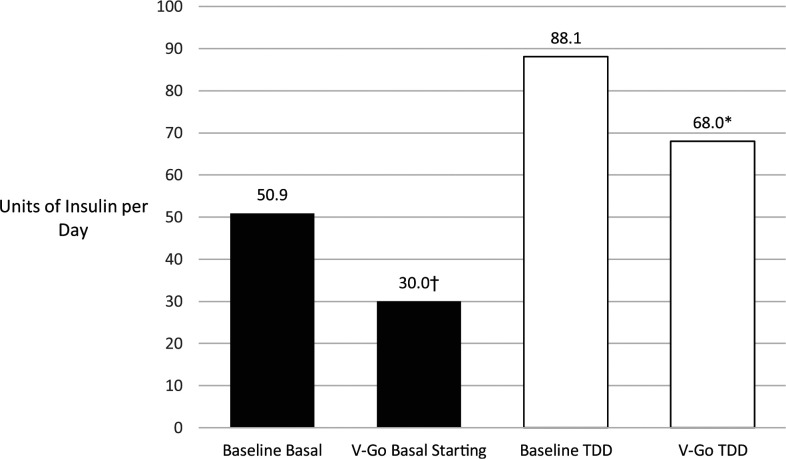
Baseline glycemic control was poor (A1C >9%) in 61% of patients. The mean A1C after an average of 5.5 months of V-Go use decreased from 9.62 to 8.25%, a change of −1.37% (P <0.001). There was no difference in A1C lowering based on baseline insulin regimens (basal/bolus vs. basal only). The TTD before V-Go initiation ranged from 14 to 210 units, with a mean of 88.07 units. After starting the V-Go, the mean TDD decreased by 20.02 units, which was statistically significant (P = 0.005) (Figure 1).
FIGURE 1.
Comparison of baseline and V-Go starting basal insulin and insulin TDDs. Data are means. *V-Go TDD compared with baseline P = 0.005. †V-Go starting basal insulin compared with baseline P <0.001.
Further analysis revealed an average reduction of 65.12 units in patients with a baseline TDD of 88–210 units (n = 18) after V-Go initiation. There were decreases and increases in TDD after starting V-Go among patients with a baseline TDD between 14 and 87. For those who experienced a reduction in TDD (n = 9), a mean decrease of 9.89 units was observed, compared with an average increase of 21.12 units in the remaining patients (n = 17) with a baseline TDD <88 units.
Although the V-Go manufacturer recommends individualizing initial dosing based on prior insulin requirements, there is also guidance to select the starting dose based on body weight: for <200 lb, the V-Go 20; 200–250 lb, the V-Go 30; and >250 lb, the V-Go 40 (17). In this retrospective study, starting doses with the V-Go device included 20 (n = 14), 30 (n = 16), and 40 (n = 14) units. Only 15 patients were started on the V-Go dose that corresponded to the weight-based recommendations.
The V-Go mean starting dose was 21 units less than the mean baseline basal dose, which was a statistically significant difference (P <0.001) (Figure 1). The majority of patients (72.7%, 32/44) were started on a lower V-Go dose when compared with basal doses at baseline. However, after starting the V-Go, the dose was increased in nine patients (20.5%), and there were no changes to lower doses. Three patients were increased from V-Go 20 to V-Go 30, and six were increased from V-Go 30 to V-Go 40.
A nonsignificant increase in weight, from 222.2 to 224.7 lb, was observed after 3–6 months using the V-Go (P = 0.340). Likewise, a 1-point increase in mean BMI after 3–6 months was not statistically significant (P = 0.420). There was no clinic standard for documenting hypoglycemia, but the review of clinic notes found no evidence of severe hypoglycemia. Five patients (11%) eventually stopped using the V-Go device; two stopped after 3 months, and the remainder used the product for 6–12 months. The reasons for discontinuation included cost/insurance coverage (n = 3) and adverse effects (n = 2).
Discussion
Primary care is on the front lines of type 2 diabetes management. Interestingly, many of the studies using the V-Go have been conducted in endocrinology offices or clinics specializing in the management of diabetes (20–23,26). This study demonstrates the successful use of the V-Go wearable insulin delivery device in primary care clinics. In this real-world setting, patients using the V-Go experienced statistically significant reductions in A1C and insulin TDD with no substantial change in weight or BMI.
Numerous retrospective studies have compared use of the V-Go to MDI insulin therapy. All studies documented the superiority of the V-Go, with significantly lower A1C and significant reductions in TDD compared with MDI insulin regimens. Lajara et al. (22) reported a 1.98% decrease in A1C after 27 weeks and lower daily insulin use with the V-Go. This retrospective study involving 116 participants compared the V-Go to MDI for therapy intensification and documented similar weight gain in both groups (22). A retrospective study (N = 103) by Sutton et al. (20) documented a 1.67% reduction in A1C, as well as a decrease in TDD after 14 months. Another retrospective study reported a 0.9% reduction in A1C after 2–4 months in 60 patients who were started on the V-Go (21). A larger, retrospective study (N = 204) documented A1C reductions of 1.34 and 1.58% at 14 and 27 weeks, respectively (23). Participants used significantly less insulin with the V-Go, and there was no change in the frequency of hypoglycemia compared with baseline. In a follow-on study the next year, the investigators reported an A1C decrease of 2% in a subset of patients (N = 97) with A1C levels >9% (average A1C at baseline 10.5%) (26). A prospective study of six patients compared V-Go to MDI regimens and showed a 1.5% reduction in A1C compared with a 0.2% increase in the MDI group (24). More recently, a pragmatic, community-based trial (N = 415) demonstrated a 1% reduction in A1C after 4 months of V-Go use compared with patients receiving standard treatment (18). There was a statistically significant reduction in TDD, and the V-Go was more cost-effective. Lastly, a claims-based study (N = 236) documented significant improvement in glycemic control in both the V-Go and MDI cohorts, but V-Go users had lower diabetes medication costs and a lower TDD (19). In the current study, a statistically significant A1C reduction of 1.37% was observed, which is comparable to the decreases highlighted in the literature mentioned above. Among these published studies, A1C reductions ranged from 0.9 to 2.0% among variable subject populations (6–415 patients).
A similar study in terms of design, subjects, and evaluation time points reported a 0.9% A1C decrease in patients from an endocrinology office using the V-Go (21). However, its setting and those of the other studies stand in contrast to the current study. It is notable that this research was conducted in the primary care setting, where the majority of patients with type 2 diabetes receive their diabetes care.
When compared with MDI insulin therapy, the V-Go provides a more physiologic form of insulin delivery, which can lead to lower TDDs (27). The Opt2mise trial (28) documented significantly lower insulin use with insulin pump therapy (not the V-Go) compared with MDI in patients with type 2 diabetes (28). The current study showed a statistically significant decrease in the mean TDD of 20 units with improved glycemic control. Other studies using the V-Go have documented improved glycemic control with decreases in mean TDD of 17–39 units (18–20,22,24,26).
Some barriers to starting insulin or intensifying treatment include fear of weight gain, concern about hypoglycemia, embarrassment, complicated and costly regimens, and misconceptions that insulin causes complications or even death (26,27). Weight gain is a known side effect of insulin therapy and intensification; therefore, it is notable that the current study observed a nonsignificant weight increase when using the V-Go device. With regard to treatment complexity, many patients struggle to incorporate mealtime insulin into their daily routine. In another V-Go study, patients reported only missing one dose of mealtime insulin during the past 30 days (25). Patients in the study reported the V-Go device was discreet, easy to use, and comfortable to wear. Interestingly, A1C levels decreased by 1.2% after 12 weeks of using the V-Go but increased by 0.6% when patients stopped using the V-Go. Greater patient satisfaction has been documented with the V-Go compared with standard therapies, and this greater satisfaction may increase adherence and ultimately lead to better glycemic control (18,25,29).
Cost can be a barrier to adherence, and numerous studies have documented lower costs with the V-Go compared with MDI therapy (18–20,22). In the current study, the primary reason for discontinuation was affordability issues, followed by adverse reactions. Lajara et al. (22) reported significantly lower costs with the V-Go when compared with MDI for insulin intensification. Despite the reported lower cost, cost was the main reason for discontinuation of V-Go use in two other studies (20,22). As with other prescription medications, telephone encounter and clinical message documentation revealed problems with cost and insurance coverage for the V-Go among our study subjects.
Primary care clinicians care for ∼90% of the patients with type 2 diabetes (30,31). In addition, the complexity of diabetes management is increasing with the availability of more medications and devices. Glycemic goals for nonpregnant adults include A1C levels <7% without a significant increase in episodes of hypoglycemia. Less stringent glycemic goals are recommended for patients ≥65 years of age and those with a history of severe hypoglycemia, limited life expectancy or complications, or comorbid conditions. The ADA highlights the importance of dose titration after initiating a basal-bolus regimen and specifically recommends increasing prandial insulin by 1–2 units twice weekly (3).
Effective insulin therapy requires four components: initiation, adherence, persistence, and intensification (32). HCPs and patients play crucial roles in all four components. HCPs must overcome time and resource constraints and be overly cautious in prescribing to avoid side effects. There are also barriers related to health care resources, including poor data flow between patients and HCPs (lack of glucose monitoring data), insufficient support for technologies (i.e., meters and continuous glucose monitoring systems), and lack of a team approach for diabetes education and management. Patients can be hesitant to start insulin or intensify therapy because of fear of needles or concerns about pain, weight gain, and hypoglycemia. Some patients are not able to manage complicated basal-bolus regimens or remember to take mealtime insulin. Any of these barriers can lead to poor glycemic control and increased risk for complications (31,33,34).
Time constraints in primary care practices and the lack of glucose monitoring by patients can prevent the titration of insulin, which may explain why the V-Go dose was increased in only nine patients in this study. The same HCP performed most of the titrations, highlighting the need for HCP education about starting doses and titration for the V-Go. Education is available for HCPs and patients (in written, video, and face-to-face formats).
Weight-based dosing can make initiation of the V-Go easy for HCPs. The majority of patients in this study were started on a V-Go dose that was lower than their baseline basal insulin dose, yet still experienced an improvement in A1C. Moreover, the product is dispensed at pharmacies, where additional education can occur. Studies have documented improved adherence with mealtime dosing resulting from the ability to deliver prandial doses by simply pressing two buttons. The V-Go is applied every 24 hours, negating the need for multiple injections, and patients also do not see the needle. The V-Go uses one type of insulin to simplify regimens; thus, patients do not have to remember to carry a separate pen or syringe for mealtime dosing. With pharmacy dispensing, patients can retrieve the V-Go with their other prescriptions. All of these factors can positively affect the adherence and persistence components of effective insulin therapy. Finally, the V-Go provides two methods of insulin intensification: the number of clicks for mealtime dosing can be increased and patients can be switched to a higher dose of basal insulin, if needed.
There were several limitations to this study, including its retrospective design, small sample size, and lack of a control group. The baseline data of the study group served as a comparison. Also, there was not consistent documentation in the EMR with regard to hypoglycemia, so these data could not be examined and extrapolated. Nonetheless, the results document that real-world use of V-Go in the primary care setting can lower A1C. Despite the V-Go’s FDA approval in 2010, large prospective studies of the device are still needed.
Conclusion
Patients with type 2 diabetes and poor glycemic control often need insulin intensification. In this real-world, primary care study, the use of V-Go significantly lowered A1C without causing significant changes in weight or BMI. These findings are consistent with evidence from previous trials in endocrinology and specialty practices. The V-Go device offers primary care clinicians and patients a treatment option that addresses all four components of effective insulin therapy, with the potential for enhanced compliance and improved glycemic control compared with MDI therapy.
Article Information
Acknowledgments
The authors thank Rebecca Creech Tart, PhD, contractor at Catawba Valley Medical Center, for editorial assistance and Susan Bates, PharmD, and Molly Casey, PharmD, for their assistance with data collection.
Duality of Interest
No potential conflicts of interest relevant to this article were reported.
Author Contributions
L.T.M. researched data and wrote, reviewed, and edited the manuscript. D.B. wrote, reviewed, and edited the manuscript. L.T.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report, 2020: Estimates of Diabetes and Its Burden in the United States. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 22 September 2020
- 2. American Diabetes Association . 1. Improving care and promoting health in populations: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S7–S13 [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association . 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S98–S110 [DOI] [PubMed] [Google Scholar]
- 4. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2020 executive summary. Endocr Pract 2020;26:107–139 [DOI] [PubMed] [Google Scholar]
- 5. Berard L, Bonnemaire M, Mical M, Edelman S. Insights into optimal basal insulin titration in type 2 diabetes: results of a quantitative survey. Diabetes Obes Metab 2018;20:301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalal MR, Grabner M, Bonine N, Stephenson JJ, DiGenio A, Bieszk N. Are patients on basal insulin attaining glycemic targets? Characteristics and goal achievement of patients with type 2 diabetes mellitus treated with basal insulin and physician-perceived barriers to achieving glycemic targets. Diabetes Res Clin Pract 2016;121:17–26 [DOI] [PubMed] [Google Scholar]
- 7. Mocarski M, Yeaw J, Divino V, et al. Slow titration and delayed intensification of basal insulin among patients with type 2 diabetes. J Manag Care Spec Pharm 2018;24:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blak BT, Smith HT, Hards M, Maguire A, Gimeno V. A retrospective database study of insulin initiation in patients with type 2 diabetes in UK primary care. Diabet Med 2012;29:e191–e198 [DOI] [PubMed] [Google Scholar]
- 9. Yavuz DG, Ozcan S, Deyneli O. Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens. Patient Prefer Adherence 2015;9:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnelly LA, Morris AD; DARTS/MEMO collaboration . Adherence to insulin and its association with glycaemic control in patients with type 2 diabetes. QJM 2007;100:345–350 [DOI] [PubMed] [Google Scholar]
- 11. Larkin ME, Capasso VA, Chen CL, et al. Measuring psychological insulin resistance: barriers to insulin use. Diabetes Educ 2008;34:511–517 [DOI] [PubMed] [Google Scholar]
- 12. Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: the translating research into action for diabetes insulin starts project. Diabetes Care 2010;33:733–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfeiffer KM, Basse A, Lee XY, Waldman LT. Diabetes management and healthcare resource use when intensifying from basal insulin to basal-bolus: a survey of type 2 diabetes patients. Diabetes Ther 2018;9:1931–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valeritas . What is V-Go? Available from https://www.go-vgo.com/hcp/about-v-go/what-is-v-go. Accessed 22 September 2020
- 15. Valeritas . V-Go dosing guide. Available from https://www.go-vgo.com/hcp/prescribing-dosing/overview. Accessed 22 September 2020
- 16. American Diabetes Association . 7. Diabetes technology: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S77–S88 [DOI] [PubMed] [Google Scholar]
- 17. Valeritas . V-Go coverage and savings. Available from https://www.go-vgo.com/hcp/coverage-savings/overview. Accessed 22 September 2020
- 18. Cziraky MJ, Abbott S, Nguyen M, et al. A pragmatic clinical trial to compare the real-world effectiveness of V-Go versus standard delivery of insulin in patients with advanced type 2 diabetes. J Health Econ Outcomes Res 2019;6:70–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raval AD, Nguyen MH, Zhou S, Grabner M, Barron J, Quimbo R. Effect of V-Go versus multiple daily injections on glycemic control, insulin use, and diabetes medication costs among individuals with type 2 diabetes mellitus. J Manag Care Spec Pharm 2019;25:1111–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sutton D, Higdon CD, Nikkel C, Hilsinger KA. Clinical benefits over time associated with use of V-Go wearable insulin delivery device in adult patients with diabetes: a retrospective analysis. Adv Ther 2018;35:631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johns BR, Jones TC, Sink JH 2nd, Cooke CE. Real-world assessment of glycemic control after V-Go® initiation in an endocrine practice in the southeastern United States. J Diabetes Sci Technol 2014;8:1060–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lajara R, Davidson JA, Nikkel CC, Morris TL. Clinical and cost-effectiveness of insulin delivery with V-Go® disposable insulin delivery device versus multiple daily insulin injections in patients with type 2 diabetes inadequately controlled on basal insulin. Endocr Pract 2016;22:726–735 [DOI] [PubMed] [Google Scholar]
- 23. Lajara R, Fetchick DA, Morris TL, Nikkel C. Use of V-Go® insulin delivery device in patients with sub-optimally controlled diabetes mellitus: a retrospective analysis from a large specialized diabetes system. Diabetes Ther 2015;6:531–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Winter A, Lintner M, Knezevich E. V-Go insulin delivery system versus multiple daily insulin injections for patients with uncontrolled type 2 diabetes mellitus. J Diabetes Sci Technol 2015;9:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosenfeld CR, Bohannon NJ, Bode B, et al. TheV-Go insulin delivery device used in clinical practice: patient perception and retrospective analysis of glycemic control. Endocr Pract 2012;18:660–667 [DOI] [PubMed] [Google Scholar]
- 26. Lajara R, Nikkel C, Abbott S. The clinical and economic impact of the V-Go® disposable insulin delivery device for insulin delivery in patients with poorly controlled diabetes at high risk. Drugs Real World Outcomes 2016;3:191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapitza C, Fein S, Heinemann L, Schleusener D, Levesque S, Strange P. Basal-prandial insulin delivery in type 2 diabetes mellitus via the V-Go: a novel continuous subcutaneous infusion device. J Diabetes Sci Technol 2008;2:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reznik Y, Cohen O, Aronson R, et al.; OpT2mise Study Group . Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet 2014;384:1265–1272 [DOI] [PubMed] [Google Scholar]
- 29. Reznik Y, Cohen O. Insulin pump for type 2 diabetes: use and misuse of continuous subcutaneous insulin infusion in type 2 diabetes. Diabetes Care 2013;36(Suppl. 2):S219–S225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson JA. The increasing role of primary care physicians in caring for patients with type 2 diabetes mellitus. Mayo Clin Proc 2010;85(Suppl.):S3–S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Perreault L, Vincent L, Neumiller JJ, Santos-Cavaiola T. Initiation and titration of basal insulin in primary care: barriers and practical solutions. J Am Board Fam Med 2019;32:431–447 [DOI] [PubMed] [Google Scholar]
- 32. Peyrot M, Barnett AH, Meneghini LF, Schumm-Draeger PM. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med 2012;29:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med 2013;126(Suppl. 1):S38–S48 [DOI] [PubMed] [Google Scholar]
- 34. LaSalle JR, Berria R. Insulin therapy in type 2 diabetes mellitus: a practical approach for primary care physicians and other health care professionals. J Am Osteopath Assoc 2013;113:152–162 [PubMed] [Google Scholar]