Abstract
Breast cancer (BC) incidence is increasing around the globe, including in Taiwan, though the cause of the increasing incidence is less clear. We followed up 11,296 Taiwanese females who did not have BC at baseline, and ascertained new invasive BC (N = 351) through data linkage to the National Cancer Registry from 1991 to 2018 to examine whether reproductive, lifestyle and environmental risk factors including polycyclic aromatic hydrocarbons (PAH) were associated with BC risk. We conducted a nested case–control study using baseline blood available from a total of 305 women with BC and 598 women without BC matched on time in cohort. We examined the association of PAH-albumin adducts and BC risk using conditional logistic regression models. Age at menarche (HR 0.6 (95% CI 0.5–0.9) for ≥ 15 vs. < 13 years) and multiparity were associated with BC risk (HR 2.0 (95% CI 1.4–2.8), 2.8 (1.9–4.2), and 2.4 (1.0–5.0) for 3–4, 1–2 and 0 live birth, compared with women ≥ 5 births). PAH-albumin adducts were not associated with BC risk. Given the increasing BC incidence in Taiwan, there is a need to identify environmental factors that are important to this population.
Subject terms: Breast cancer, Biomarkers
Introduction
Breast cancer (BC) is the most common non-skin cancer malignancy in women worldwide, including Asia1. Although, historically the incidence of female BC in Asian countries is lower than that in western countries, the incidence is rising among Asian countries2. In Taiwan, the annual percent change (APC) for women under 50 is 4.71 (4.20, 5.22). There was a 2.7-fold difference in age-standardized incidence rates from 1990 to 20173. This compares to an APC of 0.53 (0.29–0.78) in women under 50 in the U.S.4. Moreover, the peak age of onset in Western countries is 60–70 years, while in Asia it is 40–505. Using a cancer registry, Shen et al. reported that changes in the reproductive pattern might be associated with the increase in the younger generation of Taiwanese women6. Environmental exposure such as air pollution is also suggested as a risk factor in Asian women7. Polycyclic aromatic hydrocarbons (PAHs), possible human carcinogens, are ubiquitous pollutants caused by incomplete combustion of various materials, including diesel fuel and tobacco8. Recently, we conducted a prospective study of women from the New York site of the BC Family Registry and reported that detectable levels of PAH-albumin adducts in blood was associated with a twofold increased BC risk compared with women with non–detectable levels9. The association was even stronger among women with higher absolute BC risk9.
In the present population-based long-term prospective study, we followed up a total of 11,296 Taiwanese females who did not have BC at study entry. The goal of this study was to understand the epidemiology of BC and examine the association of reproductive risk factors in BC. We also used a prospective nested case–control study design to examine the association of PAH-albumin adducts in baseline bloods and BC risk.
Results
Table 1 presents the baseline characteristics for the total population at risk and for cases. Higher level of education was associated with a twofold increased risk (HR 2.2, 95% CI 1.1–4.3 for high school degree vs. never/elementary school). The prevalences of smoking and alcohol drinking were low in the cohort female participants. However, alcohol drinking was associated with an elevated, but not statistically significant, risk (HR 2.4, 95% CI 0.9–5.7). The prevalence of women whose mother had BC is less than 1%. The majority of women had menarche at age above 15 years and had 5 or more pregnancies. The HR for women with menarche at age of 15 and above was 0.6 (95% CI 0.5–0.9). Compared to women who had 5 or more pregnancies, the HR for women who had 3–4 pregnancies was 1.4 (95% CI 1.1–1.7). The HRs were 1.8 (95% CI 1.3–2.6) and 1.8 (95% CI 0.9–3.5) for women with 1–2 or 0 pregnancies. Compared to women who had 5 or more live births, the HR for women who had 3–4 live births was 2.0 (95% CI 1.4–2.8). The HRs were 2.8 (95% CI 1.9–4.2) and 2.4 (95% CI 1.1–5.0) for women with 1–2 or 0 live births. Birth control was not associated with BC. The associations were consistent across different birth cohorts (data not shown).
Table 1.
The hazard ratios (HR) of selected variables for breast cancer, the cancer screening program cohort.
| Population at risk/cases N = 11,296/351 N (%) |
Person-years (278,301) | Age adjusted HR | |
|---|---|---|---|
| Birth cohort | |||
| 1918–1940 | 4138/97 | 95,253 | 1.0 |
| 1941–1950 | 3106/105 | 78,434 | 1.3 (0.9–1.7) |
| 1951–1962 | 4052/149 | 104,613 | 1.4 (1.1–1.8) |
| Age at recruitment (years) | |||
| < 40 | 3458/132 | 89,452 | 1.0 |
| 40–50 | 3207/100 | 81,347 | 0.8 (0.6–1.1) |
| 50–60 | 3379/96 | 80,750 | 0.8 (0.6–1.1) |
| 60–70 | 1252/23 | 26,751 | 0.6 (0.4–0.9) |
| Education | |||
| Never/elementary | 8105/198 | 196,564 | 1.0 |
| High school | 3032/144 | 77,662 | 1.9 (1.4–2.4) |
| Colleague and above | 159/9 | 4,074 | 2.2 (1.1–4.3) |
| Cigarette smoking | |||
| No | 11,171/348 | 275,367 | 1.0 |
| Yes | 125/3 | 2,933 | 0.8 (0.3–2.5) |
| Alcohol consumption | |||
| No | 11,226/346 | 276,638 | 1.0 |
| Yes | 70/5 | 1,662 | 2.4 (0.9–5.7) |
| BMI (kg/m2) | |||
| < 24 | 6046/189 | 151,213 | 1.0 |
| 24–27 | 3194/105 | 78,025 | 1.1 (0.9–1.5) |
| ≥ 27 | 2056/57 | 49,062 | 1.0 (0.7–1.4) |
| Mother had breast cancer | |||
| No | 11,253/349 | 277,241 | 1.0 |
| Yes | 43/2 | 1,059 | 1.5 (0.4–5.8) |
| Age at regular menarche (year) | |||
| < 13 | 1436/61 | 35,914 | 1.0 |
| 13–15 | 4101/149 | 102,454 | 0.9 (0.6–1.2) |
| ≥ 15 | 5759/141 | 139,931 | 0.6 (0.5–0.9) |
| No. of pregnancy | |||
| ≥ 5 | 6156/151 | 148,980 | 1.0 |
| 3–4 | 4008/146 | 100,840 | 1.4 (1.1–1.7) |
| 1–2 | 935/45 | 23,552 | 1.8 (1.3–2.6) |
| 0 | 197/9 | 4,929 | 1.8 (0.9–3.5) |
| No. of live birth | |||
| ≥ 5 | 3193/51 | 74,759 | 1.0 |
| 3–4 | 5892/200 | 147,465 | 2.0 (1.4–2.8) |
| 1–2 | 1978/91 | 50,265 | 2.8 (1.9–4.2) |
| 0 | 233/9 | 5,809 | 2.4 (1.1–5.0) |
| Menopausal status at enrolment | |||
| Premenopausal | 7343/264 | 187,712 | 1.0 |
| Postmenopausal | 3953/87 | 90,588 | 0.8 (0.6–1.1) |
| Oral contraceptives use | |||
| Never | 7866/233 | 192,518 | 1.0 |
| Ever | 3430/118 | 85,782 | 1.1 (0.9–1.5) |
| Intrauterine devices (IUDs) use | |||
| Never | 4675/140 | 114,673 | 1.0 |
| Ever | 6621/211 | 163,627 | 1.1 (0.9–1.3) |
BMI body mass index, HR hazard ratio.
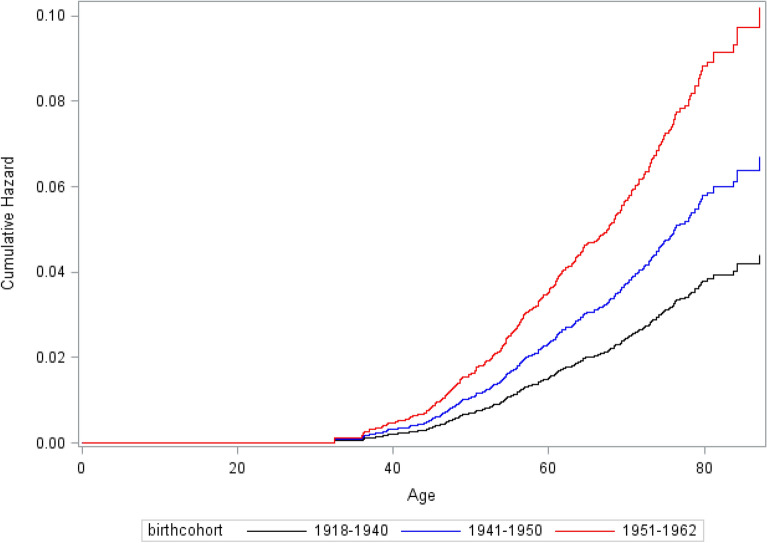
Cumulative hazard risk
The cumulative hazard was 0.04 (95% CI 0.03–0.05) for the birth cohort of 1918–1940 (Fig. 1). The cumulative hazards were 0.07 (95% CI 0.05–0.09) for birth cohort of 1941–1950 and 0.10 (95% CI 0.08–0.13) for birth cohort of 1951–1962.
Figure 1.
The cumulative hazard of breast cancer in Taiwanese women by different birth cohort.
Table 2 presents the association of PAH-albumin adducts and BC risk. We did not see any significant difference in the PAH-albumin adducts and BC risk overall, or by selected risk factors.
Table 2.
The association of PAH-albumin adducts and breast cancer risk by selected risk factors, the cancer screening program cohort.
| Cases | Matched controls | Age adjusted OR (95% CI) | |
|---|---|---|---|
| N = 305 | N = 598 | ||
| No | No | ||
| PAH-Alb | |||
| Above versus below the median | 135/170 | 299/299 | 0.8 (0.6–1.0) |
| BMI < 25 | |||
| PAH-Alb above versus below the median | 58/87 | 140/173 | 0.8 (0.5–1.4) |
| BMI ≥ 25 | |||
| PAH-Alb above versus below the median | 66/73 | 130/103 | 0.8 (0.4–1.3) |
| Age at baseline < 45 | |||
| PAH-Alb above versus below the median | 58/98 | 130/171 | 0.8 (0.5–1.2) |
| Age at baseline ≥ 45 | |||
| PAH-Alb above versus below the median | 66/62 | 140/105 | 0.8 (0.5–1.3) |
| Premenopausal status at recruitment | |||
| PAH-Alb above versus below the median | 84/127 | 169/210 | 0.8 (0.6–1.2) |
| Postmenopausal status at recruitment | |||
| PAH-Alb above versus below the median | 40/33 | 101/66 | 0.7 (0.4–1.4) |
Alb albumin, OR odds ratio, PAH polycyclic aromatic hydrocarbons.
Discussion
Overall our findings in the cohort analysis of Taiwanese women found only a small number of BC cases had a mother with BC. We also did not observe any association of ever oral contraceptive use with BC risk. Consistent with those from Western countries10 and other East Asian countries such as China and Korean11–14, we observed that selected reproductive factors were associated with BC in Taiwanese women: older age onset of menarche and multiparity were negatively associated with BC. The lack of an association with BC family history in this Taiwanese population and the large difference in APC across the countries, suggests that other risk factors such as lifestyle, reproductive factors or environmental exposures might be important15.
Although we did not observe a birth cohort difference in the association of reproductive factors and BC risk, the patterns of reproductive factors have changed in Taiwanese women. Similar to other countries, there has been a decrease in the age at menarche in the younger generation of Taiwan16. Among 214 adolescent girls, there was a 1.5 year difference in age at menarche between mothers and daughters, and a 2.1 year difference between grandmothers–granddaughters16. The fertility rate in Taiwan also decreased from 1.76 in 1991 to 1.15 in 2018 and the fertility rate in 2021 is only 1.07 which is the lowest fertility rate ever observed in Taiwan17. It is important to understand how much of the increase in the incidence of BC in Taiwan can be explained by the change in the reproductive factors. More studies are also needed to quantify the impact of changes in these reproductive factors on future BC incidence rate in Taiwan and compare the finding with other Asian countries. For example, the incidence of breast cancer is increasing in China18. Potential reasons for this increase may be related to substantially decline in the age of menarche and fertility rate19,20. According to a national survey21, the prevalence of both smoking and alcohol use are very low in Taiwanese women. Although the prevalences of smoking and alcohol use were low in our study participants, we observed an elevated increase in BC from alcohol consumption although this risk was not statistically significant. A recent survey of adolescents showed the prevalence of peer drinking in females was 49%22. Thus, the number of BC attributed to alcohol use would be expected to increase in the future.
Measuring urinary PAH metabolites, a biomarker of short-term PAH exposure, a nested case–control study in the Shanghai Women’s Health Study also reported no association between PAH exposure and BC risk in Chinese women23. We also did not observe an association between PAH-albumin adducts and BC risk. However, both our and the Shanghai Women’s Health Study were unable to time the exposure measurements to a window of susceptibility and were also unable to control for underlying genetic susceptibility23. The influence of environmental exposure on BC risk may be greater during several windows of susceptibility such as puberty24. Moreover, the effect of PAH exposure on cancer risk may vary with an individual’s ability to detoxify or eliminate the contaminant as well as DNA repair capacity. For example, we previously reported that polymorphisms in DNA repair genes modulate individual BC susceptibility related to exposure to PAHs25,26. In addition, we also found individuals with greater BC risk score based on a risk model that uses pedigree information were more vulnerable to PAH exposure9. As the prevalence of women whose mother had BC was low in our population, we were not able to examine the association of PAH exposure on BC risk stratified by family history of BC.
Although we did not observe an increased risk of BC associated with PAH-albumin adducts, other environmental exposures such as DDT and perfluoroalkly substances have been reported to be associated with BC in Taiwan27,28. In our systematic review of epidemiological literature on environmental exposure and BC risk, we concluded that studies enriched for women at higher BC risk through family history, younger age of onset and/or genetic susceptibility consistently support an association between an environment exposure and BC risk29.
A limitation of our study includes the lack of information on breast cancer molecular subtype. A hospital study examining the distribution of molecular subtypes of breast cancer in Taiwan reported that luminal A was the major subtype contributing to 61% of cases, following by basal-like (13%), HER-2+/ER−-(12%), luminal B (11%)30. It is unclear if the associations of reproductive factors differ by different subtypes in Taiwanese females. A case–control study conducted in China reported that late menopause, and lack of breastfeeding appear to increase risk of both luminal and ER-PR-tumors, while early menarche and nulliparity mainly impacted luminal tumor risk31. In addition, these associations were not impacted by menopausal status. Another limitation of our cohort analysis is that the epidemiologic information was collected at recruitment and not updated during the follow-up. Some of the reproductive information such as number of pregnancies and family history of breast cancer might change over time. In addition, PAH-albumin adduct level is a single measurement, and may not represent long-term PAH exposure from all exposure routes. The major strength of our study is that PAH-albumin adducts were measured in blood samples collected prior to cancer diagnosis. This rules out the possibility that the biomarker levels were due to metabolic changes associated with an already existing cancer.
In summary, we found no statistically significant association between PAH-albumin adducts and BC risk in Taiwanese women but similar reproductive risk factors as found in Western women. There is a need to understand what are the clinical drivers of the increase in BC risk in Taiwan and identify environmental factors that are important to this population.
Materials and methods
Study subjects
We used female participants from the community-based Cancer Screening Program cohort recruited in Taiwan. The cohort characteristics and methods of screening and follow-up have been described in detail previously32. Briefly, individuals who were between 30 and 65 years old and lived in seven townships in Taiwan with no history of cancer were recruited between 1991 and 1992 with a total of 11,973 males and 11,847 females. Participants were personally interviewed based on a structured questionnaire regarding epidemiological information and donated a 10 mL fasting blood sample at recruitment. Biospecimens were transported on dry ice to a central laboratory at the National Taiwan University and stored at − 80 °C until transport to Columbia University for analysis. Epidemiological information included socio-demographic characteristics, reproductive factors (female participants only), habits of alcohol intake and cigarette smoking, and mother’s BC status. Habitual cigarette smoking was defined as having smoked > 4 days/week for at least 6 months. Information about duration and intensity was also obtained. Habitual alcohol intake was defined as drinking alcohol containing products > 4 days/week for at least 6 months. Women who had no menstrual flow for 12 months were considered as postmenopausal and the rest were considered as premenopausal. Anthropometric measurements including height, weight, and hip and waist circumference were recorded using standardized protocols during the interview.
We ascertained newly developed female breast cancer (n = 365) among a total of 11,847 female participants by computerized data linkage with the National Cancer Registry from January 1, 1991, through December 31, 2018. For the cohort analysis, we used information from 11,296 female participants including 351 cases who completed questionnaire information. Among those 365 incident breast cancer cases, there were three cases were diagnosed within 1 year after recruitment. However, there three cases were not included in the cohort analysis as they did not completed questionnaire. All 351 cases were all diagnosed with breast cancer at least 1 year after baseline.
For the nested case–control study design, we used pre-diagnostic blood available from 305 cases for PAH-albumin adduct measurement. For controls, we randomly selected 598 women from prospective cohort subjects who were not affected with BC through the follow-up period by matching to each case by age (± 5 years), residential township, date of recruitment (± 6 months) and follow up time.
This study was approved by Columbia University’s Institutional Review Board (AAAB7226) as well as the Research Ethics Committee of the College of Public Health, National Taiwan University (AS-IRB01-18065), which conforms to the STROBE GUIDELINE for observation studies. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all subjects and strict quality controls and safeguards were used to protect confidentiality33.
PAH-albumin adducts
We measured plasma PAH-albumin adducts using a competitive enzyme linked immunosorbent assay with monoclonal antibody 8E11 which we generated34. This antibody recognizes benzo(a)pyrene diolepoxide tetrols and related PAH metabolites32. All other reagents including secondary goat anti-mouse antiserum and p-nitrophenylphosphate were from Sigma-Aldrich. Four 96 well plates were run each day with one pooled quality control sample assayed in duplicate on each plate. The intra and inter-batch CV were 17.1% and 23.4%. Values are expressed as fmol of PAH per mg albumin.
Statistical methods
Cohort analysis
Follow-up (in years) was considered as the time interval between the study entry and the earliest of these endpoints: date of BC diagnosis, date of death, or end of follow-up in the absence of BC diagnosis (December 31, 2018), whichever came first. To estimate the effect of various variables on the hazard of BC, we used Cox proportional hazards regression models using PROC PHREG procedure of SAS adjusting for age to calculate hazard ratios (HRs) and their 95% confidence intervals (CIs). We used follow-up time as the time scale and Schoenfeld’s global test to test the assumption of proportional hazards. For the figure, we used age as time scale to present the cumulative hazard by different birth cohort.
Nested case–control study analysis
We considered PAH-albumin adduct levels as a continuous variable and dichotomized into a single binary variable (above the median level vs. below the median level of controls). PAH-albumin adduct levels were natural log-transformed to normalize the distribution. We used the χ2 test for categorical variables and ANOVA for continuous variables to assess the difference of selected characteristics between cases and controls. After descriptive analyses, we used conditional logistic regression based on the matched sets (1:2 age matched) to estimate BC risk. All analyses were performed with SAS software 9.2 (SAS Institute, Cary, NC, USA). All statistical tests were based on two-tailed probability.
Abbreviations
- APC
Annual percent change
- BC
Breast cancer
- CSP
Cancer Screening Program
- CI
Confidence interval
- PAH
Polycyclic aromatic hydrocarbons
Author contributions
Study design: HC.W, R.S. MB.T, HI.Y, and CJ.C; organized and performed the analysis: HC.W and R.S; Data analysis: HC.W, R.S and MB.T; Paper writing: HC.W, R.S, MB.T, HI.Y, PH, L and CJ.C. HC. W, R.S. MB.T wrote the main manuscript. HC.W prepared figures and tables. All authors review the manuscript and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol. Biomark. Prev. 2016;25:16–27. doi: 10.1158/1055-9965.epi-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin.68, 394–424. 10.3322/caac.21492 (2018). [DOI] [PubMed]
- 3.(IHME), T. I. f. H. M. a. E. Institute of Health Metrics and Evaluation. GBD Results Tool, 2017. Global Health Data Exchange. Available at http://ghdx.healthdata.org/gbd-results-tool (2017).
- 4.Kehm RD, Yang W, Tehranifar P, Terry MB. 40 years of change in age- and stage-specific cancer incidence rates in US women and men. JNCI Cancer Spectrum. 2019 doi: 10.1093/jncics/pkz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 2006;24:2137–2150. doi: 10.1200/jco.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y-C, et al. Significant difference in the trends of female breast cancer incidence between Taiwanese and Caucasian Americans: Implications from age-period-cohort analysis. Cancer Epidemiol. Biomark. Prev. 2005;14:1986–1990. doi: 10.1158/1055-9965.Epi-04-0932. [DOI] [PubMed] [Google Scholar]
- 7.Su S-Y, et al. Associations between ambient air pollution and cancer incidence in Taiwan: An ecological study of geographical variations. BMC Public Health. 2019;19:1496. doi: 10.1186/s12889-019-7849-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC. in International Agency for Research on Cancer Monographs on the Carcinogenic Risk of Chemicals to Humans (1984).
- 9.Shen J, et al. Dependence of cancer risk from environmental exposures on underlying genetic susceptibility: An illustration with polycyclic aromatic hydrocarbons and breast cancer. Br. J. Cancer. 2017;116:1229–1233. doi: 10.1038/bjc.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. The Lancet. Oncology13, 1141–1151. 10.1016/S1470-2045(12)70425-4 (2012). [DOI] [PMC free article] [PubMed]
- 11.Liu YT, et al. Physiological, reproductive factors and breast cancer risk in Jiangsu province of China. Asian Pac. J. Cancer Prev. 2011;12:787–790. [PubMed] [Google Scholar]
- 12.Sung H, et al. Breast cancer risk factors and mammographic density among high-risk women in urban China. NPJ Breast Cancer. 2018;4:3. doi: 10.1038/s41523-018-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry CS, Otero JC, Palmer JL, Gross AS. Risk factors for breast cancer in East Asian women relative to women in the West. Asia Pac. J. Clin. Oncol. 2009;5:219–231. doi: 10.1111/j.1743-7563.2009.01242.x. [DOI] [Google Scholar]
- 14.Park B, et al. Reproductive factors as risk modifiers of breast cancer in BRCA mutation carriers and high-risk non-carriers. Oncotarget. 2017;8:102110–102118. doi: 10.18632/oncotarget.22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiatt RA, Brody JG. Environmental determinants of breast cancer. Annu. Rev. Public Health. 2018;39:113–133. doi: 10.1146/annurev-publhealth-040617-014101. [DOI] [PubMed] [Google Scholar]
- 16.Chang SR, Chen KH. Age at menarche of three-generation families in Taiwan. Ann. Hum. Biol. 2008;35:394–405. doi: 10.1080/03014460802154777. [DOI] [PubMed] [Google Scholar]
- 17.Total fertility rate. The world factbook (2021).
- 18.Cao W, Chen H-D, Yu Y-W, Li N, Chen W-Q. Changing profiles of cancer burden worldwide and in China: A secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021;134:783. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng X, Li S, Duan W, Sun Y, Jia C. Secular trend of age at menarche in Chinese adolescents born from 1973 to 2004. Pediatrics. 2017;140:e20170085. doi: 10.1542/peds.2017-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewington S, et al. Temporal trends of main reproductive characteristics in ten urban and rural regions of China: The China Kadoorie Biobank study of 300,000 women. Int. J. Epidemiol. 2014;43:1252–1262. doi: 10.1093/ije/dyu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng W-J, Cheng Y, Huang M-C, Chen C-J. Alcohol dependence, consumption of alcoholic energy drinks and associated work characteristics in the Taiwan working population. Alcohol Alcohol. 2012;47:372–379. doi: 10.1093/alcalc/ags034. [DOI] [PubMed] [Google Scholar]
- 22.Cui Y, et al. Gender differences in cigarette smoking and alcohol drinking among adolescents and young adults in Hanoi, Shanghai, and Taipei. J. Int. Med. Res. 2018;46:5257–5268. doi: 10.1177/0300060518807292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K-H, et al. Breast cancer and urinary biomarkers of polycyclic aromatic hydrocarbon and oxidative stress in the Shanghai Women's Health Study. Cancer Epidemiol. Biomarkers Prev. 2010;19:877–883. doi: 10.1158/1055-9965.EPI-09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terry MB, et al. Environmental exposures during windows of susceptibility for breast cancer: A framework for prevention research. Breast Cancer Res. 2019;21:96. doi: 10.1186/s13058-019-1168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crew KD, et al. Polymorphisms in nucleotide excision repair genes, polycyclic aromatic hydrocarbon-DNA adducts, and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2007;16:2033–2041. doi: 10.1158/1055-9965.Epi-07-0096. [DOI] [PubMed] [Google Scholar]
- 26.Terry MB, et al. Polymorphism in the DNA repair gene XPD, polycyclic aromatic hydrocarbon-DNA adducts, cigarette smoking, and breast cancer risk. Cancer Epidemiol. Biomark. Prev. 2004;13:2053–2058. [PubMed] [Google Scholar]
- 27.Chang S, El-Zaemey S, Heyworth J, Tang M-C. DDT exposure in early childhood and female breast cancer: Evidence from an ecological study in Taiwan. Environ. Int. 2018;121:1106–1112. doi: 10.1016/j.envint.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Tsai M-S, et al. A case–control study of perfluoroalkyl substances and the risk of breast cancer in Taiwanese women. Environ. Int. 2020;142:105850. doi: 10.1016/j.envint.2020.105850. [DOI] [PubMed] [Google Scholar]
- 29.Zeinomar N, Oskar S, Kehm RD, Sahebzeda S, Terry MB. Environmental exposures and breast cancer risk in the context of underlying susceptibility: A systematic review of the epidemiological literature. Environ. Res. 2020;187:109346. doi: 10.1016/j.envres.2020.109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CH, et al. Molecular subtypes of breast cancer emerging in young women in Taiwan: evidence for more than just westernization as a reason for the disease in Asia. Cancer Epidemiol. Biomarkers Prev. 2009;18:1807–1814. doi: 10.1158/1055-9965.Epi-09-0096. [DOI] [PubMed] [Google Scholar]
- 31.Li H, et al. BMI, reproductive factors, and breast cancer molecular subtypes: A case–control study and meta-analysis. J. Epidemiol. 2017;27:143–151. doi: 10.1016/j.je.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu H-C, et al. Polycyclic aromatic hydrocarbon- and Aflatoxin-albumin adducts, and hepatitis B virus infection and hepatocellular carcinoma in Taiwan. Cancer Lett. 2007;252:104–114. doi: 10.1016/j.canlet.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Elm E, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Santella R, Lin C, Cleveland W, Weinstein I. Monoclonal antibodies to DNA modified by a benzo[a]pyrene diol epoxide. Carcinogenesis. 1984;5:373–377. doi: 10.1093/carcin/5.3.373. [DOI] [PubMed] [Google Scholar]