Abstract
Digital images used in the field of ophthalmology are among the most important methods for automatic detection of certain eye diseases. These processes include image enhancement as a primary step to assist optometrists in identifying diseases. Therefore, many algorithms and methods have been developed for the enhancement of retinal fundus images, which may experience challenges that typically accompany enhancement processes, such as artificial borders and dim lighting that mask image details. To eliminate these problems, a new algorithm is proposed in this paper based on separating colour images into three channels (red, green, and blue). The green channel is passed through a Wiener filter and reinforced using the CLAHE technique before merging with the original red and blue channels. Reducing the green channel noise with this approach is proven effective over the other colour channels. Results from the Contrast Improvement Index (CII) and linear index of fuzziness (r) test indicate the success of the proposed algorithm compared with alternate algorithms in the application of improving blood vessel imagery and other details within ten test fundus images selected from the DRIVER database.
Keywords: Fundus images, Image enhancement, CLAHE, Wiener filter, CII, Linear index of fuzziness
Introduction
The retina is a thin tissue layer lining the back of the eye from the inside, located near the optic nerve [1]. The role of the retina is to convert light rays reflected onto it through the lens of the eye into electrical signals transmitted to the brain, which in turn interprets these signals as a visual image [2]. Through this process, we distinguish colours and light intensities of varying degrees, from regions of light and shadow [3, 4] enabling us to perform routine activities, such as reading and driving. Activating this vision mechanism requires cooperation between the retinas as it captures light from receiving cells and the optic nerve at the back of the eye as it transmits information to the brain [5]. If something interrupts this mechanism, such as physical damage to the retina, then decreased vision capacity or blindness may result [6]. With the vital role of the retina, any damage to it may even cause permanent blindness [5].
The photographic diagnostic test of retinal imaging obtains multiple images of the fundus and retina [7]. From an analysis of these images, various diseases affecting the retina can be detected, such as age-related macular degeneration, diabetic retinopathy, and pigmentary retinitis [8–10]. Initiating retinal imaging only requires the use of drops to dilate the pupil and is a simple, painless, safe, and diagnostically useful investigation for several diseases [7].
Images of the fundus suffer the same issues as other digital images due to contrast problems [11], where contrast is defined as the ratio between the illumination of an object and the background illumination [12]. Improving contrast depends on the spatial distribution of the bright and dark regions within an image [13]. The available intensity of brightness while capturing an image, measured by the amount of light reflected from the object, is an essential characteristic that impacts the image quality [14]. If an ophthalmologist lacks experience in adjusting the lighting, then the resulting picture of the back of the eye may be too dark or bright due to a poor distribution of the colour of the image caused by inadequate lighting. Good image contrast is considered when the lighting levels are available such that the image includes clear features. Low contrast occurs when the difference in the levels is too small, making the image faded such that its features cannot be distinguished, and a high contrast exists when the illumination difference is so significant that some areas of the image are too dark while others are too bright [15, 16].
Many methods and algorithms for enhancing fundus images have been proposed and are typically based on three principles of (1) enhancing the grey image; (2) separating the image colours into red, green, and blue channels and enhancing one of these channels; and (3) applying enhancements based on the colour image [11, 16]. These proposed algorithms depend on various existing contrast enhancement techniques. Histogram equalization (HE) is the simplest and most common contrast enhancement technique that features a fast implementation compared to other approaches but also leads to distortion in the image features [17]. Contrast stretching (CS) is another technique that can obscure features in the fundus image, so it is considered an unwanted approach for fundus image enhancement. Contrast limited adaptive histogram equalization (CLAHE) is considered one of the best enhancement methods but results in some artificial boundaries [18, 19], and other algorithms were designed to overcome these issues. Foracchia et al. suggested a model for correcting the luminosity, but it suffers from imaging fading as well as the disappearance of some image features [20]. Joshi et al. presented a method for colour fundus image enhancement that leverages mathematical equations to enhance the red, green, and blue channels, which yielded better results than those of Foracchia et al., although the improved image remained pale [21]. Setiawan et al. separated fundus colour images into red, green, and blue channels and applied the CLAHE technique to the green channel before combining it with the others. This approach proved useful but still resulted in artificial details [16]. Qureshi et al. applied an algorithm based on converting the red, green, and blue channel (RGB) image into a J module of the CIECAM02 colour model. Then, they applied a non-linear enhancement to the J module, resulting in a high degree of brightness that highlighted the blood clarity in the fundus image at the expense of other features [22].
From this previous literature, the methods applied did not fully solve the problems that accompany the process of image enhancement, as some results included faded images, hidden image details, and the appearance of artificial borders. In this paper, we suggest a hybrid algorithm to improve colour retinal fundus images and solve the issues experienced by the approaches described above.
Methodology
Contrast Enhancement Technique
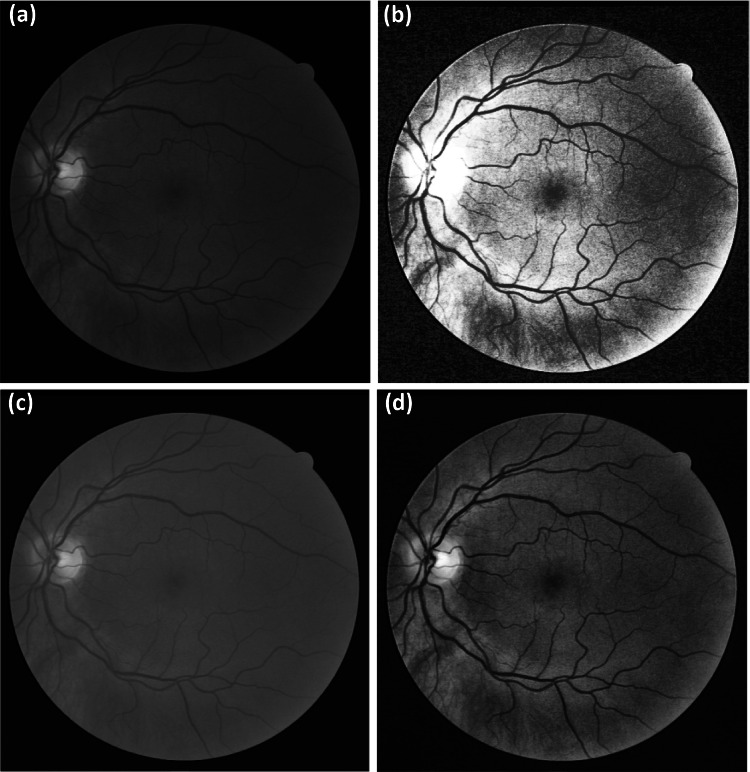
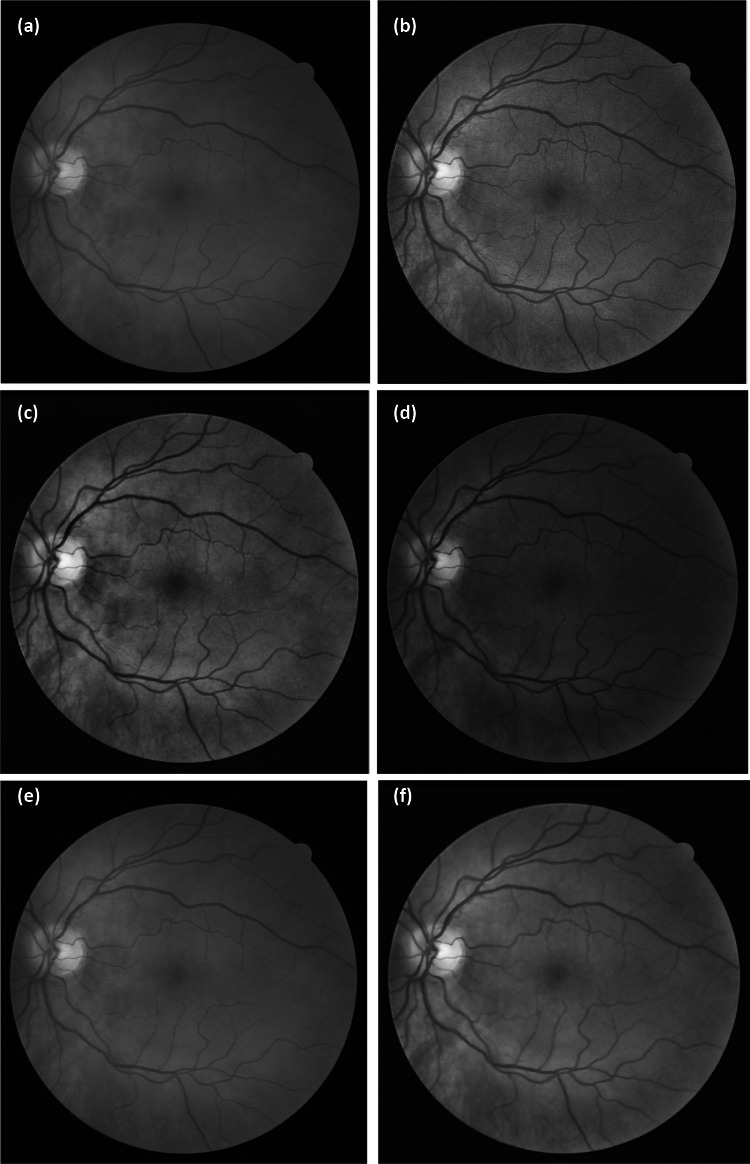
The CLAHE technique is useful for improving digital images, especially in medical imagery [11, 23], and provides better results compared to the HE and CS techniques [22, 24]. In a fundus image, the green channel responds to applied enhancements better than other channels [16], as can be seen in Fig. 1 that includes a comparison of the results from the CLAHE technique to other techniques applied to the green channel.
Fig. 1.
Different contrast enhancement techniques on the green channel of a random fundus image from the DRIVER database: (a) original green channel, (b) result from the HE technique, (c) result from the CS technique, and (d) result from the CLAHE technique
Figure 1a displays the green channel of the selected image obtained after separating the other two channels of red and blue. Figure 2b, c show the results from HE and CS, respectively. By a simple visual assessment, HE does not improve the green channel well, and it appears to distort the background illumination, which is essential for clarifying the details of the fundus image [18]. In the result from CS, the green channel is significantly obscured, such as in the vein details, optical disk, fovea, cup area, and blood vessels. The CLAHE technique result features an improvement in the details of the green channel, compared to the other techniques, although it still contains noise and unwanted boundaries [19]. An evaluation of the performance of these optimization techniques from Fig. 1 is presented in the image histograms shown in Fig. 2.
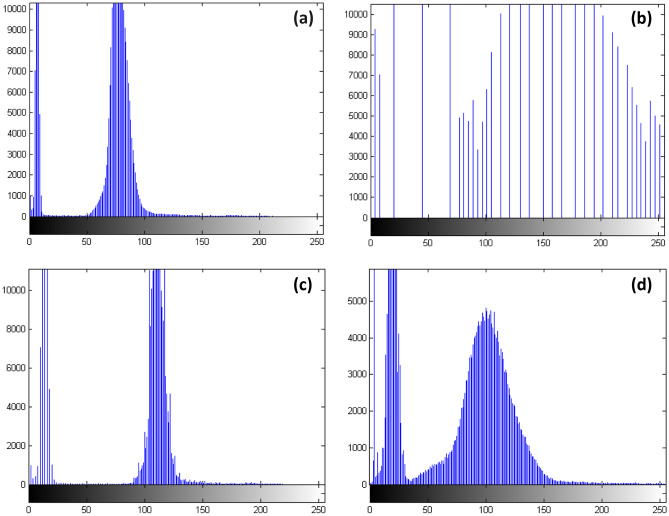
Fig. 2.
Histogram results of the corresponding images from Fig. 1a–d
The best iterative distribution of the image is considered to be the one that spans the most values from the grey levels ranging from 0 to 255. This type of iterative diagram offers good contrast, and the details of the image can be observed easily [25]. The normalization operations of the iterative pattern can be seen in Fig. 2, as the CLAHE technique applied to the green channel image extended (or withdrew) the grey levels better than the other techniques. Therefore, this approach resulted in a noticeable contrast within the resulting green channel.
Noise Reduction
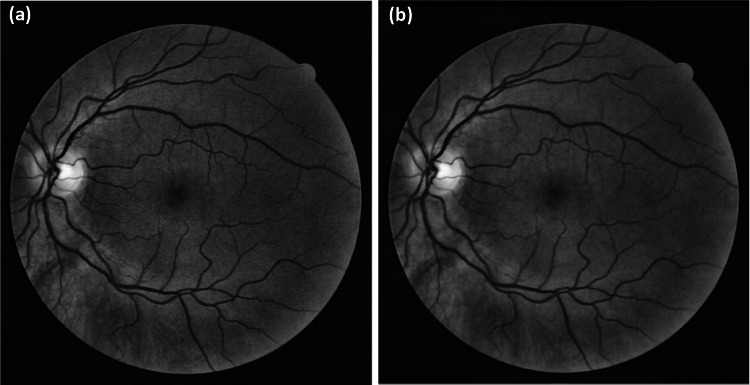
This section describes our methodology to reduce the noise present in the green channel before implementing CLAHE. Figure 3 shows a comparison between the CLAHE technique (Fig. 3a) and a Wiener filter pass before applying CLAHE (Fig. 3b), which results in significantly improved detail in the green channel as well as reducing noise and eliminating the artificial boundaries associated with the CLAHE algorithm.
Fig. 3.
The effect of applying a Wiener filter before the CLAHE technique on the green channel: (a) the result of the CLAHE technique alone and (b) application of the Wiener filter before the CLAHE technique
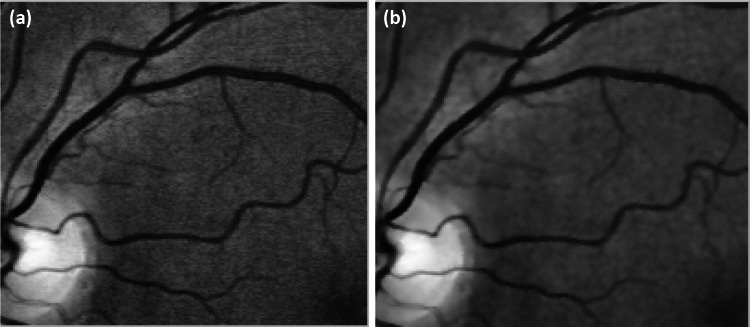
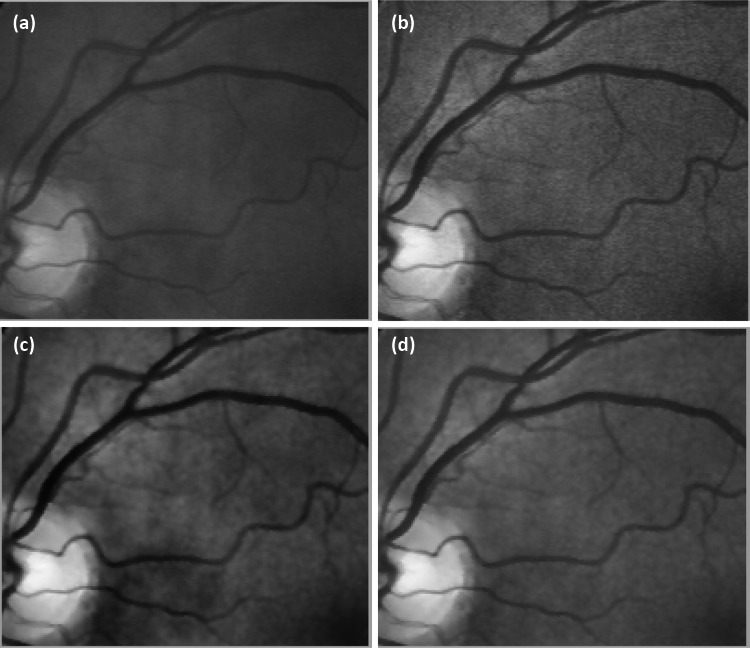
Figure 4 highlights a sub-region from the Fig. 3 comparison to further illustrate the improved quality of applying the Wiener filter to the green channel of the image before performing the CLAHE technique with the improved details and elimination of the artificial edge boundaries (Fig. 4b).
Fig. 4.
A selected sub-region from the Fig. 3 comparison: (a) the result of the CLAHE technique alone, and (b) application of the Wiener filter before the CLAHE technique
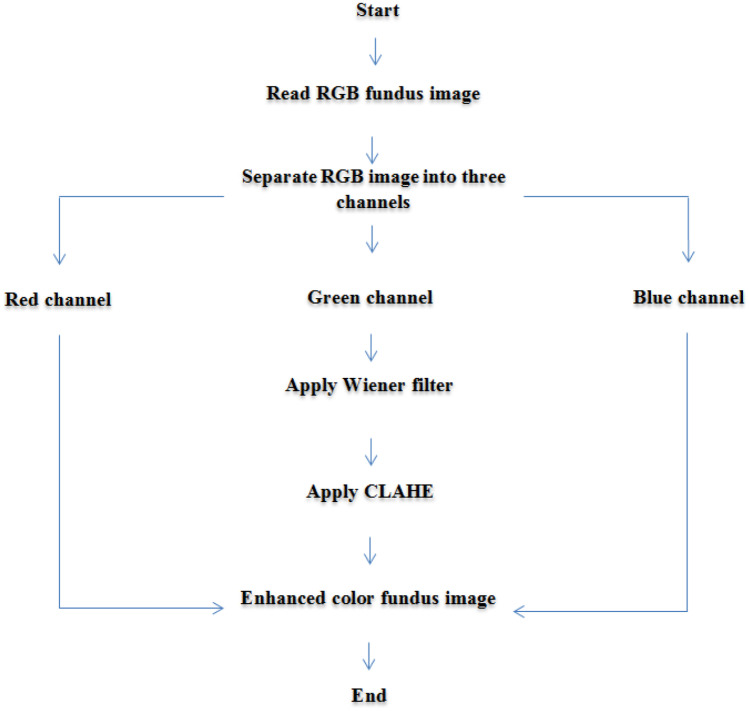
Proposed Algorithm for Enhancing a Colour Fundus Image
The algorithm relies on first removing noise in the green channel using a Wiener filter and enhancing the channel with CLAHE. The result of this preprocessing is combined with the red and blue channels obtaining an enhanced colour image of the base of the eye. Figure 5 presents an outline of the proposed algorithm, and the steps include the following:
Step one: read the colour fundus image.
Step two: separate the RGB colour channels into three distinct images.
Step three: apply a Wiener filter on the green channel.
Step four: apply CLAHE to the green channel enhanced by the Wiener filter from the previous step.
Step five: merge the green channel with the red and blue channels to create a final enhanced image.
Fig. 5.
The steps of the proposed algorithm
Results and Discussion
Ten images publicly available from the DRIVER database were tested using the proposed approach; each image has a FOV of 45° and pixel dimensions of 565 × 584. Figure 6 shows the result of the proposed algorithm of preprocessing the green channel and subsequent merge with the red and blue channels in comparison to the previous results from algorithms of Setiawan et al. and Qureshi et al. [16, 22] and is described in the following (Fig. 7).
Fig. 6.
The enhancement results of colour retinal fundus images from applying multiple algorithms: (a) the original colour image, and the results from (b) the algorithm of [16], (c) the algorithm of [22], (d) applying the Wiener filter and CLAHE to the red channel, (e) applying the Wiener filter with CLAHE on the blue channel, and (f) applying the proposed algorithm from this paper
Fig. 7.
A selected sub-region of the colour fundus image after being processed by multiple algorithms for comparison: (a) the original image, and the results from (b) the algorithm from [16], (c) the algorithm from [22], and (d) the proposed algorithm
Figure 6a is a randomly selected colour image from the DRIVER database with the results of applying the algorithm from [16] in Fig. 6b and from the algorithm from [22] in Fig. 6c. Figure 6d includes the result of applying the Wiener filter to first remove noise in the red channel followed by CLAHE and merging with green and blue channels, and Fig. 6e similarly applies the Wiener filter to the blue channel before CLAHE and merges with the red and green channels. Figure 6f is the result of the proposed algorithm.
From the result of applying the algorithm from [16] in Fig. 6b, the image can be seen to not be improved entirely, with the addition of artificial borders due to the effect of CLAHE [18, 19]. The result of applying the algorithm from [22] in Fig. 6c shows fading, and the key image details, such as blood vessels, the optic disc, and tissues, are obscured. However, these features do emerge from the blood exudation; so, this is a good result [22]. When first applying the Wiener filter before the CLAHE technique on the red channel, as seen in Fig. 6d, a visual evaluation suggests the appearance of noise as well as distortion of the colour image when combined with the green and blue channels with a lack of distinction between the blood vessels and blood exudation. Applying the Wiener filter before CLAHE on the blue channel and then merging with the red and green channels, as seen in Fig. 6e, also did not improve the image as it produced a blurry image.
With these visual reviews, each alternate method did not provide satisfactory performances due to hidden image details, contrast issues, and the emergence of artificial borders. The proposed algorithm successfully enhances the appearance of the blood vessels as well as improves all other features present in the image. This approach also demonstrates reducing the noise and eliminating the artificial boundaries caused by the CLAHE technique.
To quantitatively evaluate the performance of the proposed algorithm with another approach, we first consider the appearance of the blood vessels in the images by using the Contrast Improvement Index (CII), as defined by [26].
| 1 |
where Cen and Co are the contrast values of the blood vessels in the enhanced fundus image and the original image, respectively.
The contrast C of the blood vessel features in the fundus image is defined as [27].
| 2 |
where represents the mean grey values of the blood vessels feature, and is the mean grey values of the non-blood vessel features in the same region of the image.
The second method considered for quantitatively evaluating the proposed algorithm reviews the improvement of all details in the fundus image with the linear index of fuzziness measure, as defined by [28].
| 3 |
| 4 |
where is the maximum value of the grey values from the fundus image enhancement with size N × M.
A higher value of the CII represents an increased performance of the algorithm based on the enhancement of the blood vessel features (Fig. 8) [18]. A low value of r suggests that the features of the image are clear and include less noise [28]. Tables 1 and 2 list comparisons of the CII and r values between the proposed algorithm and the other methods.
Fig. 8.
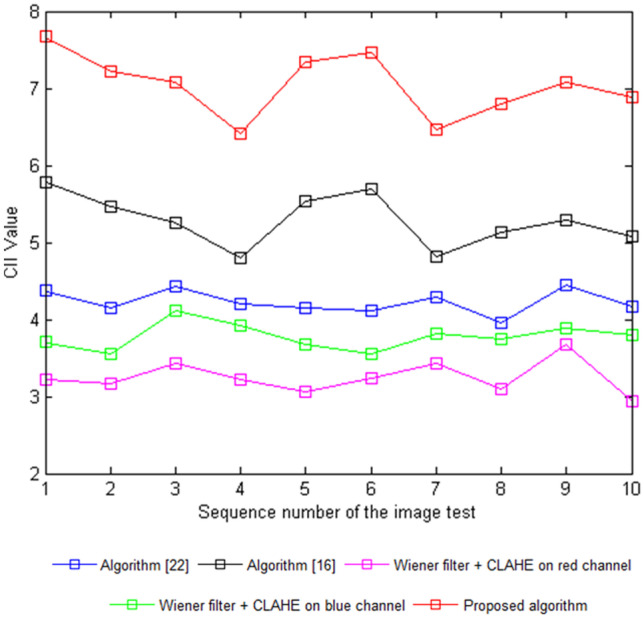
The CII values of different images
Table 1.
The CII values from the contrast enhancement techniques performed on the DRIVER images
| Image | Algorithm [22] | Algorithm [16] | Wiener filter + CLAHE on red channel | Wiener filter + CLAHE on blue channel | Proposed algorithm |
|---|---|---|---|---|---|
| 1 | 4.36951 | 5.78348 | 3.22034 | 3.70272 | 7.66875 |
| 2 | 4.14156 | 5.45886 | 3.17103 | 3.55682 | 7.21484 |
| 3 | 4.42766 | 5.26084 | 3.42192 | 4.10852 | 7.08141 |
| 4 | 4.19592 | 4.79228 | 3.21204 | 3.91452 | 6.41906 |
| 5 | 4.15508 | 5.53188 | 3.05438 | 3.68094 | 7.35056 |
| 6 | 4.12172 | 5.69776 | 3.23326 | 3.55356 | 7.46918 |
| 7 | 4.28783 | 4.81054 | 3.42694 | 3.81304 | 6.45889 |
| 8 | 3.95626 | 5.12478 | 3.10056 | 3.74408 | 6.79154 |
| 9 | 4.44706 | 5.29098 | 3.67122 | 3.88598 | 7.07041 |
| 10 | 4.17302 | 5.08358 | 2.93072 | 3.79712 | 6.88815 |
Table 2.
The r values from the contrast enhancement techniques performed on the DRIVER images
| Image | Algorithm [22] | Algorithm [16] | Wiener filter + CLAHE on red channel | Wiener filter + CLAHE on blue channel | Proposed algorithm |
|---|---|---|---|---|---|
| 1 | 0.62421 | 0.48095 | 0.78545 | 0.69862 | 0.33342 |
| 2 | 0.59165 | 0.45491 | 0.77342 | 0.67109 | 0.31368 |
| 3 | 0.63252 | 0.43843 | 0.83461 | 0.77519 | 0.30788 |
| 4 | 0.59941 | 0.39935 | 0.78342 | 0.73858 | 0.28008 |
| 5 | 0.59358 | 0.46099 | 0.76497 | 0.69451 | 0.31958 |
| 6 | 0.58881 | 0.47032 | 0.78863 | 0.67048 | 0.32474 |
| 7 | 0.61253 | 0.40088 | 0.83583 | 0.71944 | 0.28282 |
| 8 | 0.56518 | 0.42707 | 0.75623 | 0.70643 | 0.29528 |
| 9 | 0.63529 | 0.44092 | 0.84241 | 0.7332 | 0.3074 |
| 10 | 0.59614 | 0.42363 | 0.74483 | 0.71643 | 0.29948 |
The CII values resulting from the proposed algorithm over ten test fundus images were higher compared to other algorithms, indicating the success of the proposed algorithm in improving the blood vessel features, seen in Fig. 7. The r values resulted in lower values from the proposed algorithm in comparison to the others, also indicating its success in improving the details of the fundus images by reducing noise (Fig. 9).
Fig. 9.
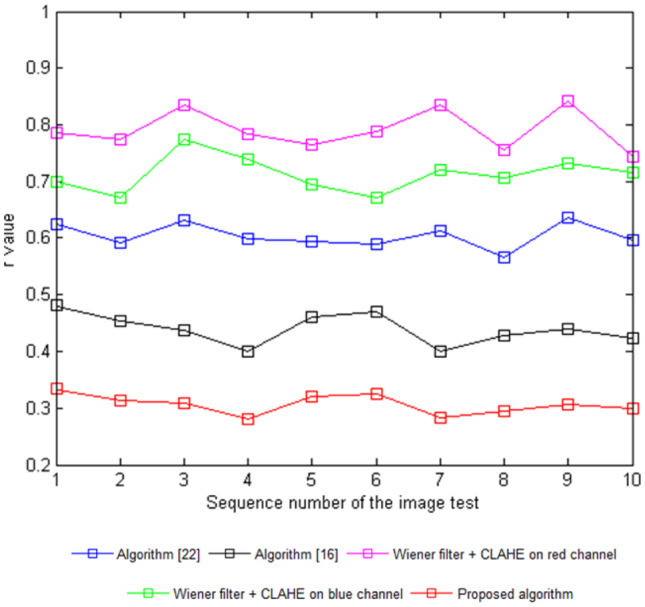
r values of different images
Despite the visual and quantitative success of applying the proposed algorithm, the approach requires a longer implementation time compared to that of the algorithm from [16], while taking less time than the algorithm from [22]. Table 3 lists the implementation times of these algorithms in a MATLAB R2016a environment on a 4.00 GB of RAM, Intel(R) Core TM i7 2.60-GHz CPU computer.
Table 3.
Comparison of the implementation times in seconds between the proposed algorithm and the algorithms from [16] and [22]
Conclusions
A hybrid algorithm was proposed for the enhancement of colour fundus images that followed a simple design based on separating the RGB channels of the image and applying a Wiener filter to reduce noise only in the green channel (Fig. 10). Then, this channel was further enhanced using the CLAHE technique before merging with the original red and blue channels. The experimental results of the CII and r calculations of ten test images obtained from the DRIVER database suggest the success of the proposed algorithm in improving the colour base eye images by eliminating the issues existing in previous methods. The proposed algorithm does suffer from a longer implementation time than other methods, which will be a focus for the future development of algorithms to reduce the time required to apply the processing.
Fig. 10.
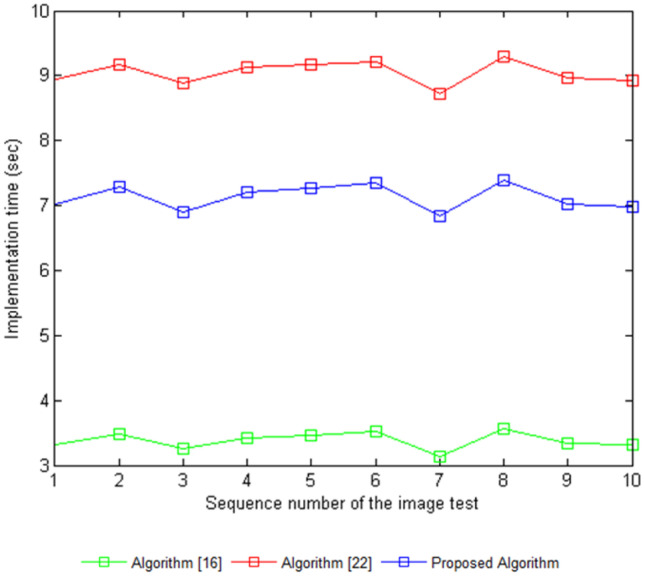
Implementation time in seconds of the proposed algorithm compared to the algorithms from [16] and [22]
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammed J. Alwazzan, Email: dr.mohammedjamal@huciraq.edu.iq
Mohammed A. Ismael, Email: mohammedismael520@gmail.com
Asmaa N. Ahmed, Email: anr.asoo@yahoo.com
References
- 1.Cense B, Chen TC, Park BH, Pierce MC, De Boer JF. In vivo birefringence and thickness measurements of the human retinal nerve fiber layer using polarization-sensitive optical coherence tomography. Journal of biomedical optics. 2004;9(1):121–126. doi: 10.1117/1.1627774. [DOI] [PubMed] [Google Scholar]
- 2.Gregory R: Eye and brain: the psychology of seeing (Vol. 80). Princeton university press.
- 3.Joesch M, Meister M. A neuronal circuit for colour vision based on rod–cone opponency. Nature. 2016;532(7598):236–239. doi: 10.1038/nature17158. [DOI] [PubMed] [Google Scholar]
- 4.Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Oertner TG. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141(4):618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Laha B, Stafford BK, Huberman AD. Regenerating optic pathways from the eye to the brain. Science. 2017;356(6342):1031–1034. doi: 10.1126/science.aal5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shepherd RK, Shivdasani MN, Nayagam DA, Williams CE, Blamey PJ. Visual prostheses for the blind. Trends in biotechnology. 2013;31(10):562–571. doi: 10.1016/j.tibtech.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Abràmoff MD, Garvin MK, Sonka M. Retinal imaging and image analysis. IEEE reviews in biomedical engineering. 2010;3:169–208. doi: 10.1109/RBME.2010.2084567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuerch K, Woods RL, Lee W, Duncker T, Delori FC, Allikmets R, Sparrow JR. Quantifying fundus autofluorescence in patients with retinitis pigmentosa. Investigative ophthalmology & visual science. 2017;58(3):1843–1855. doi: 10.1167/iovs.16-21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dysli C, Schuerch K, Escher P, Wolf S, Zinkernagel MS. Fundus autofluorescence lifetime patterns in retinitis pigmentosa. Investigative ophthalmology & visual science. 2018;59(5):1769–1778. doi: 10.1167/iovs.17-23336. [DOI] [PubMed] [Google Scholar]
- 10.Yun WL, Acharya UR, Venkatesh YV, Chee C, Min LC, Ng EYK. Identification of different stages of diabetic retinopathy using retinal optical images. Information sciences. 2008;178(1):106–121. doi: 10.1016/j.ins.2007.07.020. [DOI] [Google Scholar]
- 11.Sahu S, Singh AK, Ghrera SP, Elhoseny M. An approach for de-noising and contrast enhancement of retinal fundus image using CLAHE. Optics & Laser Technology. 2019;110:87–98. doi: 10.1016/j.optlastec.2018.06.061. [DOI] [Google Scholar]
- 12.Matkovic K, Neumann L, Neumann A, Psik T, Purgathofer W. Global contrast factor-a new approach to image contrast. Computational Aesthetics. 2005;2005:159–168. [Google Scholar]
- 13. Alwazzan J, Ismael MA, Hussain MK, et al: Brain tumour isolation in MRI images based on statistical properties and morphological process techniques. In Journal of Physics: Conference Series (Vol. 1279, No. 1, p. 012018). IOP Publishing,2019
- 14.Banić N, Lončarić S. Smart light random memory sprays retinex: a fast retinex implementation for high-quality brightness adjustment and color correction. JOSA A. 2015;32(11):2136–2147. doi: 10.1364/JOSAA.32.002136. [DOI] [PubMed] [Google Scholar]
- 15.Chiu K, Herf M, Shirley P, Swamy S, Wang C, Zimmerman K, et al: Spatially nonuniform scaling functions for high contrast images. In Graphics Interface (pp. 245–245). Canadian Information Processing Society,1993
- 16.Setiawan AW, Mengko TR, Santoso OS, Suksmono AB, et al: Color retinal image enhancement using CLAHE. In International Conference on ICT for Smart Society (pp. 1–3). IEEE,2013
- 17.Mitra A, Roy S, Roy S, Setua SK. Enhancement and restoration of non-uniform illuminated fundus image of retina obtained through thin layer of cataract. Computer methods and programs in biomedicine. 2018;156:169–178. doi: 10.1016/j.cmpb.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Dai P, Sheng H, Zhang J, Li L, Wu J, Fan M. 2016. International journal of biomedical imaging: Retinal fundus image enhancement using the normalized convolution and noise removing; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reza AM. Realization of the contrast limited adaptive histogram equalization (CLAHE) for real-time image enhancement. Journal of VLSI signal processing systems for signal, image and video technology. 2004;38(1):35–44. doi: 10.1023/B:VLSI.0000028532.53893.82. [DOI] [Google Scholar]
- 20.Foracchia M, Grisan E, Ruggeri A. Luminosity and contrast normalization in retinal images. Medical image analysis. 2005;9(3):179–190. doi: 10.1016/j.media.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Joshi GD, Sivaswamy J: Colour retinal image enhancement based on domain knowledge. In 2008 Sixth Indian Conference on Computer Vision, Graphics & Image Processing (pp. 591–598). IEEE,2008
- 22.Qureshi I, Ma J, Shaheed K. A hybrid proposed fundus image enhancement framework for diabetic retinopathy. Algorithms. 2019;12(1):14. doi: 10.3390/a12010014. [DOI] [Google Scholar]
- 23.Li L, Si Y, Jia Z. Medical image enhancement based on CLAHE and unsharp masking in NSCT domain. Journal of Medical Imaging and Health Informatics. 2018;8(3):431–438. doi: 10.1166/jmihi.2018.2328. [DOI] [Google Scholar]
- 24.Zhou M, Jin K, Wang S, Ye J, Qian D. Color retinal image enhancement based on luminosity and contrast adjustment. IEEE Transactions on Biomedical Engineering. 2017;65(3):521–527. doi: 10.1109/TBME.2017.2700627. [DOI] [PubMed] [Google Scholar]
- 25.Celik T, Tjahjadi T. Automatic image equalization and contrast enhancement using Gaussian mixture modeling. IEEE transactions on image processing. 2011;21(1):145–156. doi: 10.1109/TIP.2011.2162419. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Wang K, Yang F, Pan S, Han Y, Zhao X. Image enhancement for crop trait information acquisition system. Information Processing in Agriculture. 2018;5(4):433–442. doi: 10.1016/j.inpa.2018.07.002. [DOI] [Google Scholar]
- 27.Oh J, Hwang H. Feature enhancement of medical images using morphology-based homomorphic filter and differential evolution algorithm. International Journal of Control, Automation and Systems. 2010;8(4):857–861. doi: 10.1007/s12555-010-0418-y. [DOI] [Google Scholar]
- 28.Bai X, Zhou F, Xue B. Image enhancement using multi scale image features extracted by top-hat transform. Optics & Laser Technology. 2012;44(2):328–336. doi: 10.1016/j.optlastec.2011.07.009. [DOI] [Google Scholar]