Abstract
INTRODUCTION:
Medication-taking is a routine instrumental activity of daily living (IADL) affected by mild cognitive impairment (MCI) but difficult to measure with clinical tools. This prospective longitudinal study examined in-home medication-taking and transition from normative aging to MCI.
METHODS:
Daily, weekly, and monthly medication-taking metrics derived from an instrumented pillbox were examined in 64 healthy cognitively intact older adults (Mage = 85.5 years) followed for a mean of 2.3 years; nine transitioned to MCI during study follow-up.
RESULTS:
In the time up to and after MCI diagnosis, incident MCI participants opened their pillbox later in the day (by 19 minutes per month; β = 0.46, p <.001) and had increased day-to-day variability in the first pillbox opening over time (by four minutes per month) as compared to stable cognitively intact participants (β = 4.0, p = .003).
DISCUSSION:
Individuals who transitioned to MCI opened their pillboxes later in the day and were more variable in their medication-taking habits. These differences increased in the time up to and after diagnosis of MCI. Unobtrusive medication-taking monitoring is an ecologically valid approach for identifying early IADL changes that signal transition to MCI.
Keywords: Aging, Activities of Daily Living, Medication Adherence, In-Home Monitoring, Mild Cognitive Impairment, Digital Biomarkers
1. Introduction
As the world population ages1, a growing number of older adults are being diagnosed with mild cognitive impairment (MCI) and dementia. The number of older adults aged 65 and older who will be at increased risk for MCI and dementia is expected to skyrocket from 55 million in 2019 to 88 million by 20502. Identifying pre-symptomatic changes in healthy older adults that predict transition to MCI and Alzheimer’s Disease (AD) is critical to maximize benefits from currently available treatment, measure treatment response in clinical trials, and implement future disease-modifying treatments3,4. A delay in onset or progression to MCI and AD has the potential to significantly reduce the number of individuals affected, costs, caregiver burden, and staggering public health burden2.
Subtle changes in instrumental activities of daily living (IADLs) may be early behavioral markers of progression from normal aging to MCI5. Driving, computer use, and management of finances and medications are IADLs that decline in MCI and have important clinical implications6,7. Managing a medication regimen is a particularly important IADL for older adults, and proper management is critical to maintaining one’s health, safety, and independence at home. Medication non-adherence costs more than $100 billion annually8 and is associated with negative outcomes such as increased emergency visits, hospitalizations, and a greater probability of morbidities and deaths9.
Early declines in IADLs such as medication-taking are often insidious, slowly progressing, and difficult to detect due to inadequate tools and traditional clinic-based assessment paradigms. In clinical settings, assessment of cognition is brief and typically limited to short paper-and-pencil cognitive screeners (e.g., Mini Mental Status Exam (MMSE), Montreal Cognitive Assessment (MoCA), Mini-Cog) administered only once due to time and staffing restrictions. While a traditional neuropsychological evaluation comprised of a battery of cognitive tests may be a strong predictor of transition from normative aging to future dementia10, these evaluations are lengthy, costly, and not widely available to the growing number of older adults, particularly those who face socioeconomic, geographic, or other barriers to accessing specialty healthcare services. Clinical evaluations are also infrequent and do not always allow for repeated points of cognitive assessment. As such, they are not ideal for tracking subtle change or intra-individual variability over time, which may be a strong indicator of MCI11,12.
Cognitively intact older adults with relatively lower cognitive test scores13 and older adults with MCI14,15 have been shown to be at increased risk for medication non-adherence. Given the high executive and prospective memory demands of medication management, it is often one of the first IADLs to become compromised in older adults with MCI16. In fact, forgetting and being distracted from one’s routine are two commonly cited barriers to adherence among community-dwelling older adults and adults with a broad range of medical conditions14,17,18.
Assessment of medication-taking behaviors, like other IADLs, relies on accurate and sensitive measurement techniques. Routine clinical evaluations typically do not include any objective assessment of IADLs, and inferences about an older adult’s IADL abilities are often made based upon self-report, available cognitive data, and in rare cases, performance-based measures. Self-report measures are most frequently used to capture medication-taking behaviors; however, their accuracy is repeatedly questioned19. The validity of self-report is dependent on minimal social desirability bias, intact cognition and memory, and a psychometrically-sound instrument of medication-taking behaviors. Direct observation of medication management in a clinic setting is also limited in that it provides only a snapshot observation of functioning14. Pill count methods have the potential to both over- and under-estimate adherence when refills are obtained early or medications are disposed. In total, these indirect methods of measuring medication behaviors are limited as they are infrequently administered, assess the cognitive and physical abilities required to manage medications rather than real-world capacity, and are not sensitive to intra-individual changes19. While no single method is sufficiently reliable, electronic-monitoring is considered the gold standard for measuring medication-taking behaviors.
Advances in wireless sensing technology and pervasive computing have made it possible to unobtrusively and continuously monitor cognitively-demanding IADLs in the home environment, with the data analytics then applied to identify abnormal activity patterns and make predictions for detection of MCI20–22. Specifically, daily observation of cognitively challenging tasks such as use of everyday devices (e.g., medication pillbox, home computer) offers a practical, sensitive, and accurate approach to detecting intra-individual functioning changes that emerge before administration of traditional clinic-based cognitive assessments and for identifying the transition from normal cognition to MCI4,23. In fact, preliminary evidence suggests that factors beyond adherence, such as variability in medication-taking patterns, in healthy older adults reflects level of cognitive functioning24. Further research is needed to clarify the role of continuous passive sensor-based monitoring of medication-taking behaviors in early detection of cognitive decline thereby allowing for implementation of in-home interventions to support people with MCI and dementia and their caregivers.
The current study, embedded within the Oregon Center for Aging & Technology (ORCATECH), utilized in-home sensors and wireless technologies to continuously assess several routine medication behaviors (i.e., medication adherence, mean pill-taking time, and day-to-day variability in pill-taking time) in community-dwelling older adults. A longitudinal database with continuous measurement of medication-taking behaviors was collected with the volunteers undergoing annual cognitive batteries and weekly reporting of changes to health or medication. The first aim of the present study was to examine how routine medication behaviors discriminate between stable cognitively intact older adults and incident MCI older adults at baseline and longitudinally. Given the research suggesting that medication-taking behaviors can be a sensitive indicator of early cognitive decline13,15,24, we hypothesized that the incident MCI group would have poorer medication-taking behaviors (i.e., lower medication adherence, greater pill-taking time variability, and greater day-to-day variability in pill-taking time) at baseline as compared to the stable cognitively intact group. The second aim was to examine how medication-taking behaviors discriminate between groups longitudinally through group by time interactions. Over time, we hypothesized that subtle changes would emerge in incident MCI older adults’ medication-taking behaviors as compared with stable cognitively intact older adults.
2. Method
2.1. Participants
All participants were provided written informed consent and had been previously enrolled in ongoing longitudinal studies of aging and in-home monitoring (www.orcatech.com). Participants were recruited from the Portland, Oregon metropolitan area through advertisement and presentations at local retirement communities. The study protocols were approved by the Oregon Health & Science University Institutional Review Board (Life Laboratory IRB #2765; ISSAC IRB #2353). Additional details of the sensor systems and study protocols have been published elsewhere21,25. Inclusion criteria were 65 years and older, living independently (living with a companion/spouse was allowed, but not as caregiver), without poorly-controlled medical illnesses (e.g., hypertension, diabetes), and no evidence of dementia at study entry. Of the possible 480 homes with sensor technology, we report on 64 cognitively intact participants at the start of medication monitoring, as evidenced by a Clinical Dementia Rating Scale (CDR)26 score equal to 0, and without evidence of depression (Geriatric Depression Scale [GDS-15≤ 5])27 who met all listed inclusion criteria and had at least two weeks of data for the 7-day electronic pillbox.
2.2. Clinical Assessment Procedures
Participants received clinical assessments during annual visits in their homes using a standardized test battery including the CDR, MMSE, GDS-15, Functional Activities Questionnaire (FAQ)28, and well-validated neuropsychological measures used in prospective NIA-funded longitudinal aging cohort studies. The neuropsychological examination assessed the following cognitive domains: attention: Wechsler Adult Intelligence Scale-Revised Digit Span Forward (WAIS-R)29, WAIS-R Digit Symbol Test29, and Trail Making Test-Part A30; working memory: WAIS-R Digit Span Backward29 and WAIS-IV Digit Span Sequencing31; memory: Wechsler Memory Scale-Revised Logical Memory II (WMS-R)32, WMS-R Visual Reproduction II32, and CERAD Word-List Recall33; executive function: Animal and Vegetable Fluency34 and Trail Making Test-Part B30; and visual perception/construction: WAIS-R Block Design29 and WAIS-R Picture Completion29. Cognitive domain z-scores were calculated using group mean and standard deviations of the raw test scores from all cognitively intact subjects (CDR = 0) at study entry into the ORCATECH cohort (n = 180). Cognitive domain z-scores were then averaged to create a global cognition composite score. Data from the clinical evaluation closest to the start of medication-taking monitoring for each participant was used for the analyses. Classification of incident MCI or stable cognitively intact was made at each participant’s subsequent annual clinical evaluation based on CDR score.
2.3. Medication-Taking Metrics
Each participant received a MedTracker13,23,35 a 7-day electronic, wireless pillbox developed to continuously track adherence by detecting the opening and closing of each compartment door (see Figure 1). To be eligible for study participation, participants needed to take a medication at least once per day that was prescribed or recommended by their doctor. Participants were asked to use the pillbox for their daily medications in their typical manner. To capture their typical medication regimen, participants were asked to use the study pillbox only and not to use additional non-study pillboxes. Pillbox use was unsupervised (e.g., no camera or video), and there were no other guidelines about time of day or number of times per day they should use it. Three medication behavior metrics using the MedTracker were computed based on the first time any Medtracker door was opened each day: medication adherence, pill-taking clock time, and pill-taking clock time variability. Medication adherence was calculated by dividing the number of days with a door opening divided by the total number of days multiplied by 100. Pill-taking clock time metric was calculated as the average time of day the Medtracker was first opened (in minutes from midnight) across the participant’s monitoring period and presented as “clock time” in hours for ease of interpretation. Pill-taking clock time variability metric was calculated as the standard deviation (day-to-day variability) of the first time any Medtracker door was opened each day across the participant’s monitoring period. The metric is presented in hours. Participants were required to have medication adherence data for a period of at least two weeks between April 2014–October 2018.
FIGURE 1.
MedTracker electronic, wireless pillbox designed to track medication behavior metrics.
2.4. Statistical Approach
Cross-sectional group comparisons of demographic and clinical variables from the annual evaluation just prior to the medication monitoring period were made using Students t-test or Wilcoxon ranked sum test for continuous variables and the Pearson chi-square test for categorical variables (Table 1). To examine the medication-taking metrics cross-sectionally, we used the first month of available medication-taking data closest to the annual clinical evaluation for each participant. Group differences in medication behavior metrics were calculated using Students t-test or Wilcoxon ranked sum test, as appropriate. We used linear mixed effects models for repeated measures over time (SAS Proc Mixed) to analyze the impact of group (incident MCI vs. stable cognitively intact) on each of the medication-taking metrics with fixed effects of monitoring period time, group, and the interaction between monitoring period time and group adjusted for age and sex (Table 2). This procedure prevented listwise deletion due to missing data. The time scale for linear mixed effects model 1 is in days. Model 2 and 3 metrics were aggregated into monthly units. Analyses were performed using SAS software 9.4 (Cary, NC).
TABLE 1.
Demographics, baseline cognitive domain z-scores, and baseline period medication-taking metrics
| Variable | Total | Incident MCI | Stable cognitively intact | P-value | Cohen’s d |
|---|---|---|---|---|---|
| N | 64 | 9 | 55 | ||
| Age at baseline (yrs) | 85.5 (6.8) | 84.4 (7.1) | 85.7 (6.8) | .59 | 0.19 |
| Sex (% female) | 77% | 89% | 75% | .67 | -- |
| Race (% white) | 97% | 89% | 98% | -- | -- |
| Education (yrs) | 15.8 (2.5) | 16.3 (2.0) | 15.8 (2.5) | .52 | 0.22 |
| MMSE (raw score) | 28.8 (1.3) | 28.4 (1.3) | 28.9 (1.3) | .38 | 0.38 |
| WRAT (raw score) | 77.4 (8.8) | 77.4 (8.5) | 77.4 (8.9) | .84 | 0.0 |
| Global Cognition (z-score) | 0.2 (0.6) | −0.5 (0.6) | 0.3 (0.6) | <.001 | 1.33 |
| Executive function (z-score) | 0.4 (0.9) | −0.4 (0.8) | 0.5 (0.9) | <.01 | 1.06 |
| Working memory (z-score) | 0.0 (1.0) | −0.8 (1.2) | 0.1 (0.9) | <.01 | .85 |
| Attention (z-score) | 0.2 (0.7) | −0.4 (0.7) | 0.3 (0.6) | <.01 | 1.07 |
| Memory (z-score) | 0.2 (0.8) | −0.5 (0.8) | 0.4 (0.7) | <.01 | 1.20 |
| Visuospatial (z-score) | 0.4 (0.8) | −0.3 (0.8) | 0.5 (0.8) | <.01 | 1.00 |
| GDS-15 (raw score) | 0.8 (1.1) | 1.4 (1.6) | 0.7 (1.0) | .09 | 0.52 |
| FAQ (raw score) | 0.3 (0.7) | 0.3 (0.7) | 0.3 (0.7) | .97 | 0.0 |
| Mean number of medications | 9.4 (4.4) | 10.1 (6.1) | 9.3 (4.1) | .66 | 0.15 |
| Mean duration of medication monitoring (years) | 2.3 (1.2) | 1.7 (0.9) | 2.4 (1.3) | .12 | 0.63 |
| Baseline period adherence (% of days with a door opening) | 85 (25) | 83 (27) | 85 (24) | .61 | 0.08 |
| Baseline period mean pill-taking time of day | 9:36 AM (3.7 hrs) | 8:32 AM (0.8 hrs) | 9:47 AM (4.0 hrs) | .82 | 0.49 |
| Baseline period variability (SD) in pill-taking time (hours) | 2.4 (1.8) | 2.6 (1.9) | 2.4 (1.8) | .64 | 0.11 |
| Time between clinical evaluation and monitoring (days) | 125 (124) | 71 (70) | 134 (129) | 0.21 | 0.61 |
Baseline period is first month (30 days) of monitoring.
We calculated each volunteers’ within-person variability (SD) in pill-taking time during the first month. Then we calculated grand means and SD for the cohort and between group comparisons.
Abbreviations: FAQ, Functional Activities Questionnaire; GDS-15, Geriatric Depression Scale-15; MCI, Mild Cognitive Impairment; MMSE, Mini-mental Status Exam; SD, standard deviation; WRAT, Wide Range Achievement Test – Reading subtest.
TABLE 2.
Longitudinal mixed effects models of medication-taking by group
| Model 1 Pill-taking Time of Day | Model 2 Variability in pill-taking time (minutes) | Model 3 Number of days missed | ||||
|---|---|---|---|---|---|---|
| Covariate | β Coefficient | P-value | β Coefficient | P-value | β Coefficient | P-value |
| Incident MCI vs. Stable cognitively intact | −17.46 | .83 | −10.1 | .75 | 2.02 | .28 |
| Monitoring time | 0.07 | <.001 | 0.41 | .42 | 0.001 | .015 |
| Incident MCI * monitoring time | 0.46 | <.001 | 4.0 | .003 | 0.002 | .17 |
| Age (years) | 4.05 | .27 | −1.5 | .36 | −0.11 | .26 |
| Female vs. male | −45.7 | .61 | 35.1 | .19 | −0.15 | .92 |
Note. Model 1 metric is in daily units, Model 2 and 3 metrics are aggregated into monthly units.
Longitudinal models for pill-taking time of day are measured in minutes from 5am each day.
Stable cognitively intact (n = 55) vs. Incident MCI (n = 9)
3. Results
3.1. Participants’ Characteristics
Baseline demographic, clinical, cognitive domain composite z-scores, and medication-taking characteristics of the sample are presented in Table 1. The entire sample had a mean age of 85.5 (SD = 6.8) years; 77% were female (n = 49) and 97% (n = 62) were white. Of these 64 cognitively intact participants at the start of medication monitoring, nine (14%) transitioned to MCI (defined as CDR ≥ 0.5) at an annual clinical follow-up visit. On average the incident MCI group had 1.7 (SD = 0.9) years of medication monitoring (range = 236–1144 days) and the stable cognitively intact group had 2.4 (SD = 1.3) years of medication monitoring (range = 24–1621 days), p = .12, with the medication monitoring range related to rolling study enrollment. The incident MCI took an average of 10.1 (SD = 6.1) medications/day and the stable cognitively intact group took 9.3 (SD = 4.1) medications/day, p = .66. At baseline, there were no significant differences between stable cognitively intact and incident MCI groups in age, sex, education, Wide Range Achievement Test (WRAT) reading scores (a measure of premorbid IQ), self-reported mood (GDS-15), global cognition via a screening measure (MMSE), or informant-rated IADL (functional) level in complex activities (FAQ). The incipient MCI group had significantly lower performance on neuropsychological testing across all cognitive domains. There were no significant associations between neuropsychological test scores (global and domain-specific cognitive z-scores) or FAQ scores with the medication-taking metrics cross-sectionally by group or in the total sample (p >.05).
3.2. Cross-sectional differences in medication-taking metrics between stable cognitively intact and incident MCI groups
There were no significant group differences in the pill-taking clock time, pill-taking clock time variability (SD), or medication-taking adherence means for each group during the one-month cross-sectional baseline period, p >.05. During the cross-sectional baseline period (first month of available medication-taking data), the incident MCI group first opened their pillboxes at 8:32 AM on average while the stable cognitively intact group first opened their pillboxes at 9:47 AM. Medication-taking adherence during the baseline period was 83% for the incident MCI group and 85% for the stable cognitively intact group, p = .61. During the baseline period the incident MCI group had an average measure of day-to-day variability in pill-taking time of day of 2.6 hours and the stable cognitively intact group had an average variability of 2.4 hours, p = .64.
3.3. Longitudinal analysis of medication-taking metrics and transition to MCI
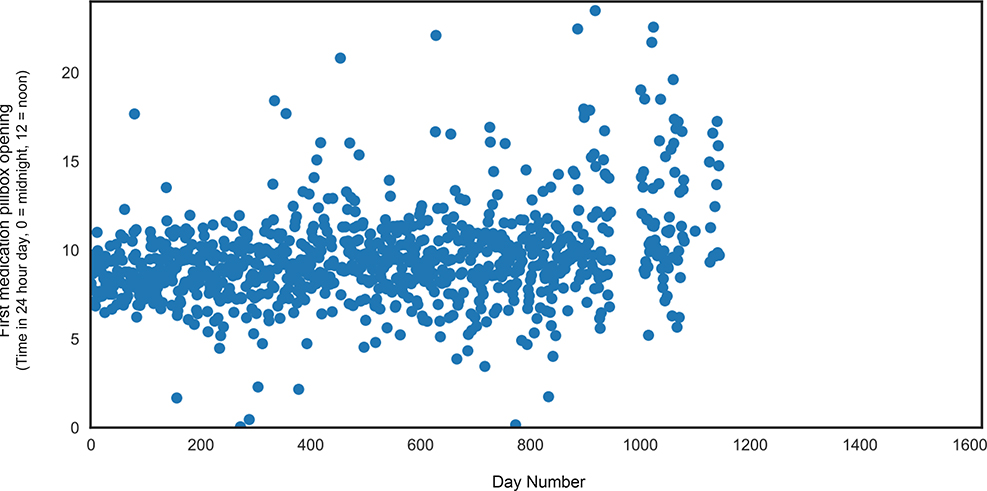
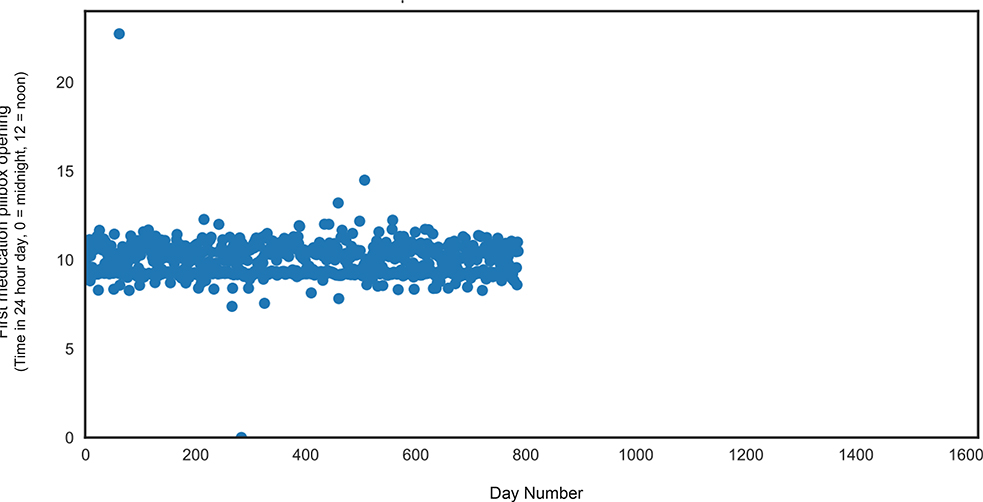
The linear mixed effects model for pill-taking time of day (in clock time) revealed no significant main effects of group or time, p’s >.05. The group x time interaction was statistically significant (β = 0.46, SE = 0.07, t = 6.2; p <.001); incident MCI individuals opened their pillboxes increasingly later in the day (by 19 minutes per month) as compared to stable cognitively intact older adults over the monitoring period (slope) (Table 2, Figure 2). The linear mixed effects model for pill-taking clock time variability aggregated into monthly intervals also revealed a significant group x time interaction; over the monitoring period incident MCI participants became significantly more variable in the time of their first pillbox opening each day (by four minutes per month) as compared to stable cognitively intact (slope) (β = 4.0, SE = 1.4, t = 2.9; p = .003). There was no main effect of group or time for pill-taking variability (in clock time), p’s >.05. The linear mixed effects model for adherence revealed a significant main effect of time; all participants, regardless of group, missed more days of medication over time (β = 0.001, SE = 0.0005, t =2.4; p = .015). There were no interaction effects for medication adherence, p >.05. Scatterplots of the raw data depict the variability in first pillbox opening over the monitoring period for a representative incident MCI participant (Figure 2) and a representative cognitively intact participant (Figure 3). Overall, individuals with incident MCI demonstrated greater variability over time as compared to cognitive intact individuals.
FIGURE 2.
Representative incident MCI participant. Figure 2 depicts the increased variability in first medication pillbox opening over an approximately three-year period for a participant with incident MCI.
MCI, Mild Cognitive Impairment.
FIGURE 3.
Representative Stable Cognitively Intact participant. Figure 3 depicts the variability in first medication pillbox opening over an approximately two-year period for a stable, cognitively intact participant.
4. Discussion
Overall, the present findings show that compared to older adults who remain cognitively intact, those who transition to MCI open their pillboxes increasingly later in the day and are more variable in their medication-taking habits, and these differences increase in the time up to and after diagnosis of MCI. There were no baseline differences in any of the medication-taking metrics between individuals who transitioned to MCI and those who remained cognitively intact, and there was no significant difference in medication-taking adherence over time between the two groups. This is notable given that medication adherence is typically used as the gold standard metric for assessing medication-taking behavior (and making inferences about cognitive and functional status) in aging and dementia research and clinical practice. Rather, the current evidence suggests that adherence itself may not be the most sensitive indicator of emerging MCI. Less overtly apparent medication-taking behaviors, such as time of day medication is taken and variability in that timing, even with intact adherence, are more sensitive early behavioral markers of underlying mild cognitive decline.
Prior research has showed that subtle variability in medication-taking patterns in healthy older adults was reflective of level of cognitive functioning in cognitively intact older adults23, and the current results further extend these findings by showing that these subtle aspects of medication-taking behavior are predictive of transition from normative aging to MCI. Another prior study from our lab demonstrated that individuals with MCI may engage in routine daily tasks such as going online to complete a weekly survey progressively later in the day compared to cognitively healthy older adults, independent of mood and/or time of morning awakening4. One possible explanation for these converging findings is that the efficiency by which older adults engage in cognitively complex IADLs like medication-taking is a more sensitive early indicator of MCI than traditional metrics of adherence, which likely only becomes affected after the person loses the ability to compensate for cognitive impairment. In the early stages of neurocognitive disorders, individuals begin to develop changes in variability (day-by-day performance fluctuations) in daily tasks that may reflect the brain’s ability to compensate for an underlying neuropathological process11. The ability to complete high level daily tasks typically does not become impaired outright with early cognitive decline, but growing evidence suggests there is a period of increased or decreased variability early on that could reflect diminishing compensatory mechanisms36. During this early period, the initiation and execution of complex daily tasks gradually becomes less efficient and effective until compensatory mechanisms are exhausted and functional impairment is detected. These hypotheses warrant further scientific examination incorporating assessment of underlying neural processes.
With advances in passive in-home activity monitoring, it is now possible to assess domains of everyday function that rely, to large extent, on those cognitive abilities and underlying brain structures and neural systems that are known to be affected in early MCI and AD (e.g., memory and executive functioning; medial temporal lobe structures)6,37. Managing a medication regimen is dependent on cognitive processes that are known to be affected by normative aging and MCI, such as memory and executive functioning38,39. Non-content memory processes such as prospective, temporal order, and source memory are independent predictors of IADL performance in older adults40. Further, divided attention, response selection and inhibition, cognitive flexibility, and conflict monitoring are a subset of executive functioning abilities that have been shown to be significantly associated with IADLs in older adults4,41. These select memory and executive processes rely on prefrontal cortical functioning and connectivity to parietal and striatal brain regions42,43 as well as medial temporal lobe functioning44. Current findings suggest that certain aspects of medication-taking may be more sensitive to early degradation processes, and future studies will explore links between passively monitored medication-taking and underlying neural correlates.
This study is not without limitations. The majority of participants were white, well-educated women living independently with few health comorbidities. Studies with larger and more diverse, medically-complex samples over longer periods of time are needed to replicate these findings. Additionally, there were baseline differences in neuropsychological scores. This difference is not unexpected given the sensitivity of neuropsychological testing, with all domain z-scores still falling in the average range. Given rolling study enrollment, medication monitoring periods varied, with more extended monitoring and larger study cohorts needed to confirm findings. Participants in the current study were asked to use the device for at least one daily medication, and conclusions regarding medication adherence, pill-taking clock time, and pill-taking clock time variability were based on the first medication taking behavior and not necessarily adherence to the overall regimen. The passive, unsupervised nature of the study allowed for high retention in the study, and future studies should consider monitoring individuals’ entire mediation regimen or individuals with more complex regimens for additional early indicators of cognitive decline. Future studies should also investigate the sensitivity and specificity of medication-taking metrics and consider potential moderators of medication-taking habits, such as medication knowledge and beliefs, given their impact on adherence in older adults45. As medication-taking habits rely on multiple cognitive abilities, future research should also examine the cognitive domains most strongly associated with medication habits to better target interventions that compensate for cognitive difficulties. In addition, examination of baseline predictors of medication-taking trajectories, such as health characteristics or neural markers, will allow for a more nuanced understanding of the relationship between medication-taking and cognitive decline.
This study has important implications. Clinicians may use this information to tailor questions about patient’s medication-taking habits, given the potential to infer a change in cognitive abilities. By tracking medication habits (i.e., time of day and variability in timing) with simple, non-invasive monitoring technologies, our hope is to reliably identify individuals at highest risk of cognitive decline, which will allow for earlier intervention. Encouraging the use of an electronically-monitored pillbox may allow for more objective data that signals the need for more comprehensive evaluation to providers and families. From a clinical trials perspective, using a sensitive and ecologically-valid functional outcome measure such as medication-taking timing variability may expedite the development and implementation of novel drug therapies by identifying and tracking IADL changes due to medication effects with greater accuracy and precision than current clinic-based measures. A multi-domain approach that allows for the integration of multiple data types from various in-home monitoring platforms, such as walking speed11, computer use6, online survey assessment4, and time outside the home46 in combination with medication-taking may offer digital behavioral signatures that are associated with increased risk of transition between clinical states and to higher care21.
Acknowledgements
The authors would like to thank the staff at the OHSU Layton Center for Aging & Alzheimer’s Research and at the Oregon Center for Aging & Technology for their assistance with data collection, data entry, data cleaning, and data processing. This work was supported in part by funding from the National Institutes of Health grants P30AG024978 and P30AG008017 and by the Minneapolis VA Health Care System. The contents do not represent the views of the US Department of Veteran Affairs or the US Government. The other authors have no conflicts of interest to disclose.
Conflicts of Interest and Source of Funding Statement: This work was supported in part by funding from the National Institutes of Health grants P30AG024978 and P30AG008017. There are no conflicts of interest to disclose.
References
- 1.United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing. http://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf Published 2015.Accessed July 15, 2020.
- 2.Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017;12:459–509. doi: 10.1016/j.jalz.2016.03.001 [DOI] [PubMed] [Google Scholar]
- 3.Rockwood K The measuring, meaning and importance of activities of daily living (ADLs) as an outcome. Int Psychogeriatrics. 2007;19:467–482. doi: 10.1017/S1041610207004966 [DOI] [PubMed] [Google Scholar]
- 4.Seelye A, Mattek N, Sharma N, et al. Weekly observations of online survey metadata obtained through home computer use allow for detection of changes in everyday cognition before transition to mild cognitive impairment. Alzheimer’s Dement. 2018;14:187–194. doi: 10.1016/j.jalz.2017.07.756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seelye A, Mattek N, Sharma N, et al. Passive assessment of routine driving with unobtrusive sensors: A new approach for identifying and monitoring functional level in normal aging and mild cognitive impairment. J Alzheimer’s Dis. 2017;59:1427–1437. doi: 10.3233/JAD-170116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaye J, Mattek N, Dodge HH, et al. Unobtrusive measurement of daily computer use to detect mild cognitive impairment. Alzheimers Dement. 2014;10:10–17. doi: 10.1016/j.jalz.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitter-Edgecombe M, Parsey CM. Assessment of functional change and cognitive correlates in the progression from healthy cognitive aging to dementia. Neuropsychology. 2014;28:881–893. doi: 10.1037/neu0000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54:57–60. doi: 10.1016/S0895-4356(01)00457-7 [DOI] [PubMed] [Google Scholar]
- 9.Chisholm-Burns MA, Spivey CA. The “cost” of medication nonadherence: Consequences we cannot afford to accept. J Am Pharm Assoc. 2012;52:823–826. doi: 10.1331/JAPhA.2012.11088 [DOI] [PubMed] [Google Scholar]
- 10.Bondi MW, Smith GE. Mild Cognitive Impairment: A Concept and Diagnostic Entity in Need of Input from Neuropsychology. J Int Neuropsychol Soc. 2014;20:129–134. doi: 10.1017/s1355617714000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodge HH, Mattek NC, Austin D, et al. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology. 2012;78:1946–1952. doi: 10.1212/WNL.0b013e318259e1de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodge HH, Zhu J, Mattek NC, et al. Use of high-frequency in-home monitoring data may reduce sample sizes needed in clinical trials. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0138095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes TL. Make a Big Difference. J Aging Heal. 2009;21:567–580. doi: 10.1177/0898264309332836.Medication [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodgers JE, Thudium EM, Beyhaghi H, et al. Predictors of Medication Adherence in the Elderly: The Role of Mental Health. Med Care Res Rev. 2018;75:746–761. doi: 10.1177/1077558717696992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stilley CS, Bender CM, Dunbar-Jacob J, et al. The impact of cognitive function on medication management: three studies. Health Psychol. 2010;29:50–55. doi: 10.1037/a0016940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jekel K, Damian M, Wattmo C, et al. Mild cognitive impairment and deficits in instrumental activities of daily living: A systematic review. Alzheimer’s Res Ther. 2015;7. doi: 10.1186/s13195-015-0099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer JA. Medication Compliance in Epilepsy. Arch Intern Med. 1991;151:1236–1237. doi: 10.1001/archinte.1991.00400060142033 [DOI] [PubMed] [Google Scholar]
- 18.Wagner GJ, Ryan GW. Relationship between routinization of daily behaviors and medication adherence in HIV-positive drug users. AIDS Patient Care STDS. 2004;18:385–393. doi: 10.1089/1087291041518238 [DOI] [PubMed] [Google Scholar]
- 19.Elliott RA, Marriott JL. Standardised assessment of patients’ capacity to manage medications: A systematic review of published instruments. BMC Geriatr. 2009;9:1–10. doi: 10.1186/1471-2318-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin M, Gong P, Yang T, et al. Big Data Analytical Approaches to the NACC Dataset: Aiding Preclinical Trial Enrichment. Alzheimer Dis Assoc Disord. 2018;32:18–27. doi: 10.1097/WAD.0000000000000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyons BE, Austin D, Seelye A, et al. Pervasive computing technologies to continuously assess Alzheimer’s disease progression and intervention efficacy. Front Aging Neurosci. 2015;7:1–14. doi: 10.3389/fnagi.2015.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peetoom KKB, Lexis MAS, Joore M, et al. Literature review on monitoring technologies and their outcomes in independently living elderly people. Disabil Rehabil Assist Technol. 2015;10:271–294. doi: 10.3109/17483107.2014.961179 [DOI] [PubMed] [Google Scholar]
- 23.Austin J, Klein K, Mattek N, et al. Variability in medication taking is associated with cognitive performance in nondemented older adults. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2017;6:210–213. doi: 10.1016/j.dadm.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salas M, In’t Veld BA, Van Der Linden PD, et al. Impaired cognitive function and compliance with antihypertensive drugs in elderly: The Rotterdam Study. Clin Pharmacol Ther. 2001;70:561–566. doi: 10.1067/mcp.2001.119812 [DOI] [PubMed] [Google Scholar]
- 25.Kaye JA, Maxwell SA, Mattek N, et al. Intelligent Systems for Assessing Aging Changes: Home-Based, Unobtrusive, and Continuous Assessment of Aging. Journals Gerontol Ser B. 2011;66B:i180–i190. doi: 10.1093/geronb/gbq095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- 27.Yesavage J a, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale. J Psychiatr Res. 1983;39:37–49. http://www.ncbi.nlm.nih.gov/pubmed/7183759. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer RI, Kurosaki TT, Harrah CH, et al. Measurement of functional activities in older adults in the community. Journals Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D Manual for the Wechsler Adult Intelligence Scale-Revised (WAIS-R). The Psychol Corp, New York. 1981. [Google Scholar]
- 30.Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychol Monogr. 1946; 60:1–48. doi: 10.1037/h0093567 [DOI] [Google Scholar]
- 31.Wechsler D Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV). San Antonio, TX: NCS Pearson. 2008; 22:1. doi: 10.1037/t5169-000 [DOI] [Google Scholar]
- 32.Wechsler D Manual for the Wechsler Memory Scale-Revised. San Antonio, TX, The Psychol Corp. 1987. [Google Scholar]
- 33.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984; 141:1356–1364. doi: 10.1176/ajp.141.11.1356 [DOI] [PubMed] [Google Scholar]
- 34.Chan AS, Butters N, Salmon DP, Johnson SA, Paulsen JS, Swenson MR. Comparison of the semantic networks in patients with dementia and amnesia. Neuropsychology. 1995; 9: 177–186. doi: 10.1037/0894-4105.9.2.177 [DOI] [Google Scholar]
- 35.Hayes TL, Hunt JM, Adami A, et al. An electronic pillbox for continuous monitoring of medication adherence. 2006 Int Conf IEEE Eng Med Biol Soc. 2006:6400–6403. doi: 10.1109/IEMBS.2006.260367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaye J, Dodge H, Hayes T, et al. Person-specific change in home computer use predicts the development of mild cognitive impairment (MCI). Alzheimer’s Dement. 2013;9:P445. doi: 10.1016/j.jalz.2013.05.889 [DOI] [Google Scholar]
- 37.Seelye A, Mattek N, Howieson DB, et al. Embedded Online Questionnaire Measures Are Sensitive to Identifying Mild Cognitive Impairment. Alzheimer Dis Assoc Disord. 2016;30:152–159. doi: 10.1097/WAD.0000000000000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insel K, Morrow D, Brewer B, et al. Executive function, working memory, and medication adherence among older adults. Journals Gerontol - Ser B Psychol Sci Soc Sci. 2006;61:102–107. doi: 10.1093/geronb/61.2.P102 [DOI] [PubMed] [Google Scholar]
- 39.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: A quantitative review. Neuropsychology. 2000;14:224–232. doi: 10.1037/0894-4105.14.2.224 [DOI] [PubMed] [Google Scholar]
- 40.Schmitter-Edgecombe M, Woo E, Greeley DR. Characterizing Multiple Memory Deficits and Their Relation to Everyday Functioning in Individuals With Mild Cognitive Impairment. Neuropsychology. 2009;23:168–177. doi: 10.1037/a0014186 [DOI] [PubMed] [Google Scholar]
- 41.Dobbs BM, Shergill SS. How effective is the trail making test (parts a and b) in identifying cognitively impaired drivers? Age Ageing. 2013;42:577–581. doi: 10.1093/ageing/aft073 [DOI] [PubMed] [Google Scholar]
- 42.Reynolds JR, West R, Braver T. Distinct neural circuits support transient and sustained processes in prospective memory and working memory. Cereb Cortex. 2009;19:1208–1221. doi: 10.1093/cercor/bhn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowe DA, Goodwin SJ, Blackman RK, et al. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci. 2013;16:1484–1491. doi: 10.1038/mm.3509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon BA, Shelton JT, Bugg JM, et al. Structural correlates of prospective memory. Neuropsychologia. 2011;49:3795–3800. doi: 10.1016/j.neuropsychologia.2011.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuz B, Marx C, Wurm S, et al. Medication beliefs predict medication adherence in older adults with multiple illnesses. J Psychosom Res. 2011;70:179–187. doi: 10.1016/j.jpsychores.2010.07.014 [DOI] [PubMed] [Google Scholar]
- 46.Petersen J, Austin D, Mattek N, et al. Time out-of-home and cognitive, physical, and emotional wellbeing of older adults: A longitudinal mixed effects model. PLoS One. 2015;10:e0139643. doi: 10.1371/journal.pone.0139643 [DOI] [PMC free article] [PubMed] [Google Scholar]