Abstract
With the increasing number of older adults around the world, the overall number of dementia cases is expected to rise dramatically in the next 40 years. In 2020, nearly 6 million individuals in the USA were living with Alzheimer’s disease, the most common type of dementia, with anticipated growth to nearly 14 million by year 2050. This increasing prevalence, coupled with high societal burden, makes prevention and intervention of dementia a medical and public health priority. As clinicians and researchers, we will continue to see more individuals with hearing loss with other comorbidities including dementia. Epidemiologic evidence suggests an association between hearing loss and increased risk of dementia, presenting opportunity for targeted intervention for hearing loss to play a fundamental role in dementia prevention. In this discussion, we summarize current research on the association between hearing loss and dementia and review potential casual mechanisms behind the association (e.g., sensory-deprivation hypothesis, information-degradation hypothesis, common cause). We emphasize key areas of research which might best inform our investigation of this potential casual association. These selected research priorities include examination of the causal mechanism, measurement of co-existing hearing loss and cognitive impairment, and potential of aural rehabilitation. Addressing these research gaps and how results are then translated for clinical use is paramount for dementia prevention and overall health of older adults.
Keywords: hearing loss, cognitive decline, dementia, prevention
INTRODUCTION
In both the initial 2017 and updated 2020 Lancet Commission reports on dementia prevention (Livingston 2017; Livingston 2020), hearing loss was identified as the leading potentially modifiable risk factor for dementia. A sharp increase in research on the association between hearing loss and dementia has developed over the past decade, building upon initial research from decades ago. As early as 1968, in an experimental condition designed to mimic hearing loss and decreased speech intelligibility, participants demonstrated increased difficulty of performance on word-recall cognitive tasks compared to normal listening conditions (Rabbitt 1968). Some 20 years later, a landmark case-control study (Uhlmann et al. 1989) reported higher odds of dementia in those with hearing loss compared to normal hearing.
Given the rapidly aging population, the global pace of research has accelerated with regards to potentially modifiable risk factors of dementia, including hearing loss. While there is a growing body of knowledge on the association of hearing loss with dementia, significant gaps remain in our understanding. In this article, we aim to (1) provide a foundational understanding of dementia epidemiology, presentation, and diagnosis in the USA; (2) contextualize research on the association between hearing loss and dementia to guide dementia prevention and intervention; (3) review mechanistic theories about the hearing-dementia association; (4) review priorities for future research; and (5) provide a perspective on how we can utilize current and future evidence to improve patient care. We conclude by offering context as we look toward the coming decades and posit that targeted collaboration between the auditory science and cognitive science communities may present a unique opportunity to alter the landscape of cognitive aging and dementia care.
EPIDEMIOLOGY AND CLINICAL DIAGNOSIS OF DEMENTIA
In its 2020 updated report, the Lancet Commission estimated up to 40 % of dementia cases could, in theory, be prevented through intervention on modifiable dementia risk factors (Livingston 2020). These risk factors include lower educational attainment, hypertension, smoking, obesity, depression, physical inactivity, diabetes, infrequent social contact, excessive alcohol consumption, head injury, air pollution, and, notably, hearing impairment. Among these modifiable risk factors, it was estimated that intervention on hearing impairment could prevent or delay up to 8 % of dementia cases (Livingston 2020). The disproportionate weight attributed to hearing loss is due, in part, to the high prevalence of hearing loss in the older adult population. It is estimated that two-thirds of adults over the age of 70 years have bilateral hearing loss, with significant growth in the population prevalence of hearing loss expected in the coming decades as the population ages (Fig.1) (Lin et al. 2011; Goman and Lin 2016; Alzheimer’s Disease Facts and Figures 2020). In addition to its high prevalence, hearing loss has a strong relationship with dementia. Hearing impairment is associated with an estimated 94 % increase in risk of dementia over time (Livingston 2017) compared to non-hearing impaired. We provide a population-based perspective and review of the evidence leading to this high level of risk and discussion of the potential mechanism behind this risk in the coming sections. To understand where auditory science may fit into the broader picture of dementia prevention, we must first understand the role and presentation of dementia within the US population.
Fig. 1.
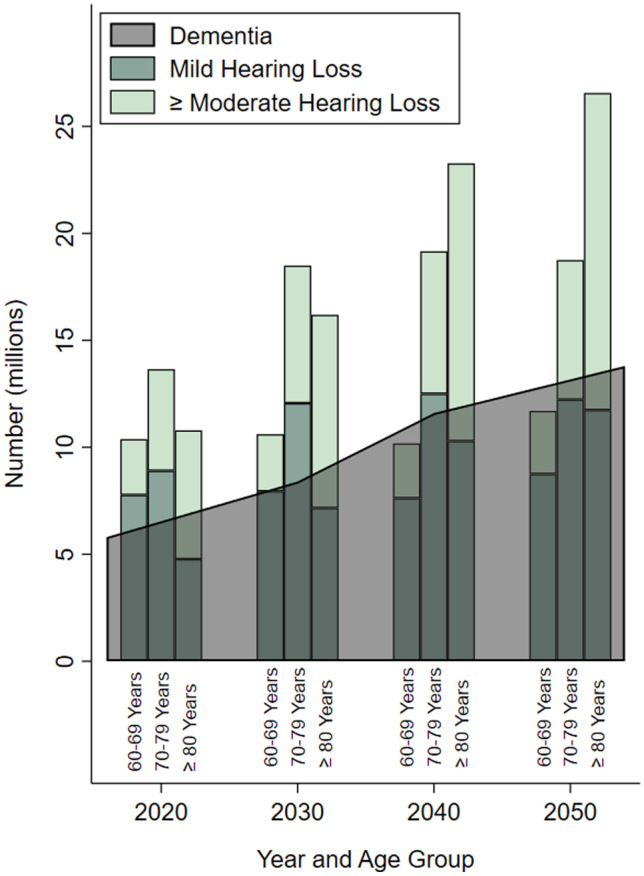
Estimated and projected trends in prevalence of hearing loss (mild hearing loss and moderate or greater hearing loss) and dementia in older adults by age categories in the USA from 2020 to 2050. Data compiled from Goman et al. 2016 and the Alzheimer’s Disease Facts and Figures 2020 Report
The Prevalence and Cost of Dementia
By 2034, the population over the age of 65 years in the USA is projected to outnumber those under the age of 19 years for the first time in US history (Vespa et al. 2020). Moreover, by 2060, the number of individuals over the age of 65 will almost double from 49 million in 2016 to over 94 million (Vespa et al. 2020). In the USA, an estimated 5.8 million individuals were living with Alzheimer’s disease in 2020, which is expected to increase to nearly 14 million by 2050 (Alzheimer’s Disease Facts and Figures 2020). The growing prevalence of dementia translates to increased financial, social, and emotional burdens on both communities and families. The total cost of care for those over 65 years living with dementia in the USA is estimated at $305 billion in the year 2020 alone (Alzheimer’s Disease Facts and Figures 2020). Family and social care account for up to 85 % of the cost of care for those with dementia (Livingston 2017). In 2019, 16 million family members in the USA provided over 18.6 billion hours of care to individuals with Alzheimer’s disease, a labor cost valued at $244 billion (Alzheimer’s Disease Facts and Figures 2020)—cost that often goes unrecognized. These numbers do little to express the social and emotional strain on individuals and families who are providing or requiring care. The astronomical financial and emotional costs of dementia are among the many drivers behind the research for prevention or intervention at all stages of cognitive aging.
Identifying Dementia
Cognitive decline may be thought of as a trajectory along a continuum spanning normal cognition, expected cognitive changes observed with aging, mild cognitive impairment (MCI), and dementia (Fig. 2). Cognitive decline then represents a change in cognitive functioning from an individual’s prior ability (Knopman et al. 2014). While a general decrease in cognitive performance is anticipated with increasing age for many cognitive processes, particularly processing speed and memory (Salthouse 1996), departures from prior ability greater than that expected for an individual’s age and education suggest possible transition along the clinical spectrum.
Fig. 2.
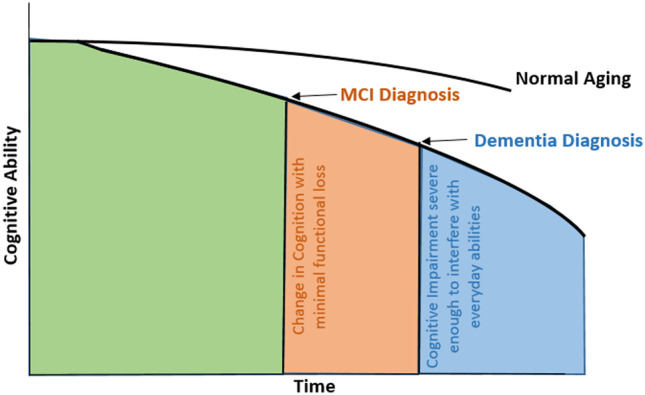
Conceptual model of cognitive trajectory along the cognitive decline dementia continuum. This continuum represents a departure from normal aging with decreased cognitive ability greater than expected with normal aging (green region). Mild cognitive impairment (MCI) diagnosis may be provided with a change in cognition with minimal functional loss (orange region). For those who do progress from MCI to dementia, a dementia diagnosis may be provided with further progression with cognitive impairment severe enough to interfere with every ability (blue region)
Broadly, mild cognitive impairment refers to a symptomatic predementia phase in which the cognitive impairment demonstrated is beyond that expected based on age, education history, or other individual characteristics (Albert et al. 2011). A formal diagnosis of MCI is made via clinical, cognitive, and functional observed criteria (Albert et al. 2011). MCI is considered if concern over a change in cognition from previous ability is expressed from the individual, informant, or clinician, and if cognitive performance is poorer in one or more cognitive domains (e.g., memory, executive function, attention, language, and visuospatial skills) compared to that expected based on age and educational background. With MCI, while difficulty may exist performing complex tasks, the individual can maintain independence with everyday functional activities or requires minimal assistance for social or occupational functioning (Albert et al. 2011; Knopman et al. 2014). Not all individuals will continue to progress from MCI to dementia. However, a diagnosis progresses to dementia when the symptoms of cognitive impairment begin to interfere with the individual’s independence in everyday activities (McKhann et al. 2011). Of paramount importance to understanding research challenges of dementia is the generally insidious progression of disease and clinical presentation. For example, Alzheimer’s disease and related dementias (ADRD) are generally slow and progressive disorders without a definitive onset or clear transition points between an asymptomatic phase, symptomatic predementia phase, and the transition to dementia (McKhann et al. 2011). Importantly, defined cut-points between age-expected cognitive ability, MCI, and dementia currently do not exist. Therefore, diagnoses are made based on clinical expertise and judgment and may be supported by pathophysiology (e.g., labs, imaging) if available (Jack et al. 2011, 2018). Readers may seek additional information elsewhere for the distinctions between clinical based dementia diagnosis and diagnosis in research studies which follow a different framework for diagnosis recommendations (Jack et al. 2011; Albert et al. 2011; McKhann et al. 2011; Sperling et al. 2011; Jack et al. 2018).
Clinical diagnosis of dementia is made by a careful history and physical exam, cognitive testing, laboratory, and imaging studies. Neurocognitive evaluations are instrumental components in a provider’s clinical examination of a patient’s cognitive status. As such, an abundance of neurocognitive tests exist to inform clinical judgment. The assessments evaluate global cognitive performance or cognitive ability within five/six recognized cognitive domains: memory, attention, executive function, language, and visuospatial (and social) as recognized by the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM-V) (Hugo and Ganguli 2014). This variety of available cognitive tests is completed across visual and auditory modalities, including some designed to be administered over the phone.
In contrast to the criteria for clinical diagnosis described above, recommendations for criteria for diagnosis in a research setting allow for the additional incorporation of biomarkers. Biomarkers are loosely categorized as those reflecting amyloid-beta deposition, tau deposition, or signs of neuronal injury (Albert et al. 2011; Jack et al. 2011; Sperling et al. 2011; Jack et al. 2018). Importantly, the biomarkers within each category are not necessarily specific to Alzheimer’s disease (AD) and may be indicative of AD as well as other dementia etiology. In research regarding preclinical dementia, biomarkers are used to identify participants with AD pathophysiology with emphasis placed on the hypothesized ordering to biomarker presentation (Sperling et al. 2011). However, in MCI- or AD-related research, usage of biomarkers for diagnosis is more conservative due to an expressed need for additional outcomes research (Jack et al. 2011). While biomarkers suggesting amyloid pathology are thought to precede neuronal injury in research settings, much remains to determine how biomarkers may be used to determine dementia etiology and prognostication, or their utility in short- vs. long-term dementia trajectories before widespread use in clinical diagnosis (Albert et al. 2011). A more recent research framework from the National Institute on Aging and Alzheimer’s Association (Jack et al. 2018) proposed a biological definition of Alzheimer’s disease. The new framework proposes an AT(N) classification system based on the presence/absence of β amyloid deposition, pathologic tau, and neurodegeneration and may be expanded to included future biomarkers. Recent research has placed blood biomarkers at the forefront as the next potential biomarkers (Molinuevo et al. 2018). P-tau181 has been proposed as an easily accessible biomarker as an AD-specific biomarker for predicting and evaluating neurodegeneration (Moscoso et al. 2021). P-tau231 has been found as a biomarker of early tau pathology and has demonstrated similar ability to identify clinical stages of AD as p-tau181 earlier than other means of identification (Ashton et al. 2021). The plasma concentration of the neurofilament light chain (NfL) has also been suggested as a plasma biomarker for AD (Lewczuk et al. 2018) and in combination with amyloid-β42 may help in evaluating risk of developing dementia in those dementia-free (de Wolf et al. 2020). The variety of diagnostic criteria across phase of dementia progression within clinical vs. research setting presents challenges to summarizations and inference of this research.
Alzheimer’s disease is recognized as the most common type of dementia (Alzheimer’s Disease Facts and Figures 2020), but increasing evidence recognizes that many older adults demonstrating cognitive impairment have mixed pathology (Boyle et al. 2019). Other types of dementia such as vascular dementia, dementia with Lewy bodies, and frontotemporal dementia should also be recognized and represent different etiology (Hugo and Ganguli 2014). While biomarker testing for AD (e.g., amyloid-PET, CSF amyloid-beta and tau) is not required, if available, it increases the certainty that the dementia is due to the AD pathophysiological process vs. other dementias and could aid in disease and symptom management (McKhann et al. 2011). At this time, amyloid-PET is approved by the FDA, and there are on-going studies to examine its utility (Rabinovici JAMA 2019). Some biomarkers that are thought to be characteristic of AD may also be seen in other dementias. The last few decades have informed our understanding of progression of dementia pathophysiology and the connection to disease stage and the pathophysiologic process. However, as noted, a biomarker-based diagnosis is currently only supported for research-based diagnosis (Jack et al. 2018) and remains to be clarified for clinical use (McKhann et al. 2011).
As discussed, current evidence suggests a pre-clinical asymptomatic phase may be years if not decades for some individuals (Braak and Braak 1991; Jack et al. 2011). A long pre-clinical phase of disease presents challenges for epidemiologic studies and interventions or trials for dementia, yet provides opportunity for early detection and potential prevention of clinical symptom progression. Further clouding the diagnostic picture, not all individuals who demonstrate the neuropathological hallmarks of AD with amyloid beta accumulation and tau protein will develop clinical symptoms (i.e., cognitive decline or functional impairment) during their lifetime (Sperling et al. 2011; Jack et al. 2013). Alternatively, some individuals may demonstrate significant cognitive impairment early with fewer pathologic changes (Sperling et al. 2011; Jack et al. 2013). The imperfect relationship between pathological changes and the presentation of symptoms opens questions of what contributes to this varied presentation of disease and makes clinical prediction for the progression of disease challenging.
Dementia Prevention
What remains clear is that the expected growth in dementia cases worldwide requires action on dementia prevention and intervention as currently there is no FDA approved disease modifying treatment for ADRD. Current treatment considerations for most forms of dementia have the goal of delaying or minimizing the impact of clinical symptoms for a period of time (Livingston 2020). In the absence of a cure, the primary objective of epidemiologic research is to identify dementia risk factors associated with pathologic progression or dementia diagnosis or identify factors associated with the progression of dementia symptoms. Subsequent goals of this research are to then identify opportunities to prevent or postpone dementia along the continuum. With identification of such risk factors, we may then emphasize research on the implementation and delaying of dementia onset, which could have great implications at the population level. An intervention which could delay the onset of dementia by 5 years could lead to a 57 % reduction in the number of patients with dementia (Sperling et al. 2011) and 40 % lower cost by 2050 (Zissimopoulos 2014). With foundational evidence of the association between hearing impairment and dementia from epidemiologic research, the expertise and potential collaboration between auditory scientists and disciplines involved with cognitive aging may enrich our evidence base and contribute towards prevention and intervention efforts.
THE LINK BETWEEN HEARING AND DEMENTIA—WHAT WE KNOW NOW
In this review, we will primarily highlight evidence of the association and potential causal effect within studies with completed objective measures of auditory function. Self-reported hearing not only is important but also incorporates an individual’s perception of their hearing and communication ability (i.e., communication demands, regular listening environments, mental health).
Significant heterogeneity in study methodology and study design exists, making comparisons and data synthesis across studies challenging. Prior work has varied in audiometric parameters to define hearing loss, auditory frequencies used, sample size, study population, consideration of potential confounders, and how dementia was measured and operationalized, to name a few. Differing ways of measuring cognitive impairment or dementia are particularly challenging as different criteria may be used across studies. Dementia ascertainment in studies through medical chart history compared to clinical, research-based settings, or in-person dementia diagnosis may introduce variability in dementia diagnosis across studies. Therefore, cognitive function may be assessed in a variety of formats depending on the clinical or research environment. A wide variety of additional neurocognitive tests and test batteries across cognitive domains exist (Belleville et al. 2017), in addition to global cognitive screeners (i.e., Mini-Mental State Exam ([MMSE], Folstein et al. 1975) or the Montreal Cognitive Assessment ([MoCA], Nasreddine et al. 2005), to aid in evaluation of cognitive function. These test batteries are often comprehensive, requiring additional time considerations as well as implementation by trained personnel experienced in the delivery of neurocognitive assessments in both clinical and research settings.
Peripheral Hearing Loss
A handful of previous reviews have provided summaries of current evidence (Albers et al. 2015; Loughrey 2018; Thomson et al. 2017; Yuan et al. 2018; Fortunato et al. 2016). In perhaps the most rigorous review to date, The Lancet Commission (Livingston 2017) reported a pooled relative risk from three longitudinal studies (Lin et al. 2011; Deal et al. 2016; Gallacher et al. 2012) which indicated 1.9 times greater risk of incident dementia among hearing impaired individuals 55 years of age and older compared to those with normal hearing. The Commission found only these three studies on the association between peripheral hearing impairment and incident dementia met the specified inclusion criteria of audiometrically (objective) measured hearing, longitudinal evaluation (at least 5 years), and covariate adjustment. Each of these criteria is a fundamental component toward investigation of causality. Objectively measured hearing is important when attempting to isolate the independent effect of hearing loss due to cochlear damage on dementia. Cross-sectional evaluations, where hearing and cognition are measured at the same time point, present challenges for causal inference of cognitive impairment. With cross-sectional studies, we are not able to assess which comes first—hearing loss or dementia. Additionally, cognition is particularly susceptible to confounding by psychosocial factors (i.e., episodic depression, anxiety, stress, exhaustion, comorbid conditions, or medications) when measured at one time point but is less susceptible with longitudinal measures. Therefore, comparing group differences in cognitive test scores cross-sectionally may not accurately depict changes in underlying cognitive function but may instead include the effect of these other psychosocial factors. Additionally, many prior studies failed to account for known additional factors (i.e., confounders) which could contribute toward, and therefore cloud, the estimated association found between hearing and cognition.
Other reviews have also reported associations between hearing and dementia. A meta-analysis (Loughrey et al. 2018) of prospective studies found 1.22 (95 % CI 1.09, 1.36) or 1.28 (95 % CI 1.02, 1.59) greater odds of cognitive impairment or dementia, respectively, for those with age-related hearing loss. In addition, hearing loss has been specifically associated with declines in global cognitive function, executive function, processing speed, and memory (Lin et al. 2011; Loughrey et al. 2018; Alattar et al. 2020; Brewster et al. 2021; Gao et al. 2020). However, what constitutes hearing ability or impairment often differs across studies. Some studies have included hearing ability continuously per decibels in hearing level (dB HL) (Armstrong et al. 2019), while others have categorized hearing ability (i.e., mild or greater loss (Lin et al. 2011; Lin et al. 2013), moderate or greater loss (Gates et al. 1996) or clinically recognized categories (WHO Grades of hearing 2020; Lin et al. 2011; Deal et al. 2016) or only considered specific frequency regions (Uhlman et al. 1989; Gates et al. 1996) rather than the more traditional range of 500–4000 Hz or 500–8000 Hz. This heterogeneity of definitions presents challenges for pooling or summarizing results across studies. The degree of hearing difficulty varies by categorization used and represents different levels of impairment and functional ability such as in categorization of those with any measured hearing impairment (i.e., pure tone average [PTA] ≥ 25 dB HL) vs. those with a moderate or greater hearing impairment (PTA > 40 dB HL) (WHO Grades of hearing 2020).
As mentioned, many studies summarize peripheral hearing acuity as a four-frequency pure tone average (PTA, 500–4000 Hz). While this is informative and sufficient for many hypothesis considering functional performance of hearing acuity, when reviewing the potential biological or physiological causal association between hearing and dementia, it may also be worth considering specific frequency ranges depending on the research hypothesis. Additional heterogeneity in study definitions of hearing impairment might include “better” vs. “worse ear PTA” or might fail to define which ear is referenced. Studies examining the association of hearing loss and its possible consequences (e.g., hearing loss is the exposure) may wish to conservatively use “better ear PTA,” as many of the proposed mechanistic pathways underlying these relationships have to do with an individual’s overall hearing ability. In comparison, for studies examining biological mechanism and etiology of hearing loss (i.e., hearing loss is the outcome of interest), it may be more informative to consider worse ear PTA.
Central Auditory Function
While an association between age-related peripheral hearing loss and dementia is well established (Livingston 2020), the relationship between cognitive decline/dementia and central auditory function remains much more abstract. Termed cognitive hearing science by some (Dryden et al. 2017; Tune et al. 2012), the interdependence between central auditory function and cognitive processing blurs the distinction between the two processing abilities. Not only does the integrity of an auditory stimulus rely on an accurately encoded signal by a functioning peripheral system, but the stimulus must further be decoded by the central auditory system. This decoding requires the involvement of higher-level cognitive processes. Distinguishing the boundaries between central auditory dysfunction and cognitive impairment has been a subject of research for decades (CHABA 1988; Humes et al. 2013b). However, as with peripheral hearing loss, studies have used a diverse grouping of definitions, tests, cognitive domains, and sample populations (e.g., both young and old (Humes 2013, b)) or hearing impaired and non-impaired populations (Nuesse 2018). This heterogeneous operationalization of both processing abilities creates significant barriers to pooled evidence and causal inference.
Dryden et al. systematically reviewed evidence in 2017. While the quality and potential bias in the studies included were mixed, among studies involving adult unaided listeners with normal to moderate hearing loss, a correlation between cognitive performance and speech perception was overall weak depending on the speech-in-noise test used and cognitive domain of interest, with the greatest correlation seen with processing speed. The degree un-aided hearing loss plays in moderating the relationship between central auditory functioning (e.g., decoding of speech stimuli or speech perception ability in noise) and cognitive performance remains to be determined. However, results continue to highlight the interdependence of cortical resources utilized during both central auditory processing and cognitive processes (Humes et al. 2013a, b; Humes and Young 2016; Sardone et al. 2019).
The interdependence between central auditory function and cognitive processing presents not only challenges to inference but also unique opportunities in identifying targets for prevention. Prior work has hypothesized that central auditory dysfunction (CAD) may be a prodromal symptom and therefore an early marker of cognitive decline in older adults (Gates et al. 1996). AD pathology is known to reach the primary auditory cortex. Work conducted nearly 30 years ago demonstrated AD pathology of plaques and neurofibrillary tangles within the medial geniculate body and central nucleus of the inferior colliculus as well as the primary auditory and auditory association cortices of patients diagnosed with AD, but not in non-AD elderly patients (Sinha et al. 1993). Braak staging (Braak and Braak 1997) indicates that auditory association cortices are one of the last brain regions affected by AD pathology, supporting central auditory dysfunction as a marker of AD, but with unclear indication of CAD as an early marker (Sardone et al. 2020). In a volunteer sample from a dementia surveillance cohort (Gates et al. 2011), the hazard ratio for incident dementia was 9.9 (95 % CI 3.6, 26.7) among those with severe central auditory dysfunction, as measured using the Dichotic Sentence Identification test, compared to those demonstrating normal central auditory function. Further work suggested that the Dichotic Sentence Identification test in free report mode may be the most sensitive test for the presence of memory impairment among those demonstrating mild memory impairment without dementia (Gates et al. 2008). Investigation of the association between central auditory processing and biomarkers for AD neurodegeneration including CSF tau, cortical thickness, and volumetric measures of AD-related brain regions was consistent across measures of central auditory processing (Tuwaig et al. 2017). Whether CAD has utility for predicting dementia in addition to predictions possible with current ADRD biomarkers has yet to be determined.
The nature of the association between central auditory function, as measured via speech-in-noise ability, and cognition may differ by aided vs. unaided hearing. Additional work has demonstrated that for aided listening with background noise, higher level cognitive processing capacity was the most important factor in the differences observed in speech understanding performance. In contrast, for unaided listening, peripheral hearing was the predominant factor driving performance. For select individuals, high-frequency hearing played a predominant role regardless of aided status or degree of loss (Humes et al. 2013a). Investigation of various measures of central hearing ability suggests an association of central auditory processing dysfunction with subjective memory complaints/impairment (Fausto et al. 2018; Idrizbegovic et al. 2011; Armstrong et al. 2019) potentially further supporting the idea of speech-in-noise difficulty as a marker of cognitive function.
Some researchers have described an idea of central presbycusis meant to represent “age-related auditory processing disorder underlying poor speech understanding in noise, competing speech, or distorted speech” (Gates 2012). The existing research on this construct is heterogeneous, incorporating measures of both speech and non-speech stimuli (Humes et al. 2012). A review of past research (Humes et al. 2012) concluded insufficient evidence existed to claim central presbycusis as an independent construct, but proposed that it may instead represent a disease manifestation from multiple conditions involving age-related or disease-related changes in both the auditory system and the brain.
Management of Hearing Loss for Dementia Prevention
As mentioned, the treatment of hearing loss makes it a worthy target of interest among dementia risk factors due to the readily available management strategies for hearing loss (e.g., hearing aids). The data on the use of hearing aids to decrease risk of cognitive decline in observational or cross-sectional studies are mixed (Ray et al. 2018; Amieva et al. 2015; Dawes et al. 2019; Maharani et al. 2018, Amieva et al. 2020). In observational studies, we are often not able to control factors which influence if someone becomes a hearing aid user (e.g., higher education, higher socioeconomic status, greater access to healthcare), many of which are also factors which are protective against dementia. Therefore, it is challenging to disentangle the potential effects of hearing aids on cognition vs. the effect of these other factors, possibly influencing the effect seen and therefore introducing bias into our study. However, the question of whether hearing aid use or other treatments for hearing impairment may alter the subsequent risk of dementia has begun to receive attention in well-designed pilot studies and clinical trials. These clinical trials aid in minimizing this potential bias through study design tools such as randomization and masking (Celentano and Szklo 2020). The Aging and Cognitive Health Evaluation in Elders (ACHIEVE) pilot study conducted in 40 older adults demonstrated clear efficacy in hearing handicap and social outcomes and a suggestion of efficacy in improving or stabilizing memory scores (although not powered to detect a difference) for those randomized to the use of a hearing aid compared to a successful aging education control (Deal et al. 2017). A full-scale (N = 977) randomized clinical trial is currently being conducted (Deal et al. 2018). A pilot study of hearing aid use by older adults with depression, another possible downstream risk of hearing impairment which may mediate or moderate the hearing-dementia association, observed small to moderate improvement in depressive symptoms, memory, and general cognitive functioning (Brewster et al. 2020). Further consideration of the age and rapidity of hearing loss onset and its proximity to hearing aid uptake and the influence of other rehabilitation strategies on dementia risk is vastly understudied.
Although encompassing a significantly smaller portion of the hearing impaired population compared to hearing aid users, the influence of cochlear implantation on cognition has also received study (Claes et al. 2018). Work in a pilot sample among 23 cochlear implant candidates and 16 implant recipients demonstrated better performance on measures of reaction time, cognitive flexibility, working memory, and strategy use compared to implant candidates (Jayakody et al. 2017). Prospective evaluation of cognitive performance among individuals pre- and post-cochlear implant surgery has suggested improved cognitive performance on global cognition or executive function post implantation (Sarant et al. 2019; Mosnier 2015). However, many studies have focused on pre/post performance on speech perception, perceived function, and quality of life for older adults rather than neurocognitive outcomes specifically. While these outcomes are absolutely important, investigation of the impact on cognitive performance following cochlear implant surgery using neurocognitive assessments in multivariate models with appropriately considered control groups is limited (Miller et al. 2015; Moberly et al. 2019). With expanding cochlear implant candidacy and our aging population, well-designed longitudinal studies could vastly improve our understanding and counseling considerations for older adult cochlear implant candidates.
MECHANISTIC THEORIES
As described above, the association between central auditory function and dementia is complex and its interdependence with dementia presents challenges for causal inference. Therefore, current mechanistic theories predominantly consider peripheral hearing impairment as a potential cause of dementia and central auditory function as a marker of cognitive decline. While there are gaps in the understanding of mechanism(s) behind the relationship between age-related hearing loss and dementia, elucidating these mechanisms is vital for the design, implementation, and evaluation of preventive strategies, interventions, or recommendations for patient care or health policy. Hypotheses on the causal mechanism of the association have eloquently been described in prior works (Griffiths et al. 2020; Wayne and Johnsrude 2015; Lin et al. 2014b). While limited evidence supports the notion that dementia leads to hearing loss (CHABA, 1988; Baltes and Lindenberger 1997), the direction of the association between age-related hearing loss and dementia remains to be clarified. A greater body of evidence supports how age-related hearing loss may contribute to cognitive decline directly (“sensory-deprivation” hypothesis) or indirectly (“information-degradation” hypothesis) or how both may be the result of an external variable all together (“common-cause” hypothesis) (Wayne and Johnsrude 2015). We will briefly review evidence for each of these mechanistic theories as depicted in our modified mechanistic framework (Fig. 3). Readers may seek additional evidence for each proposed hypothesis in Wayne and Johnsrude (2015) or Lin et al. (2014b).
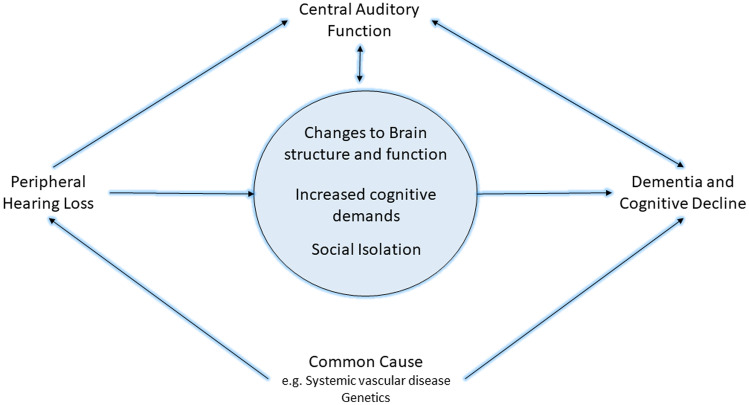
Fig. 3.
Hypothesized framework for the mechanism of the hearing and dementia association. The center blue circle includes potential causal paths between peripheral hearing loss and cognitive decline or dementia, including changes to brain structure and function (i.e., sensory deprivation hypothesis), increased cognitive demands (i.e., information degradation hypothesis), and other effects such as social isolation. Additionally, a common cause such as systemic vascular disease or genetic factors may lead to both peripheral hearing loss and cognitive decline and dementia. Further inclusion of central auditory function as a result of direct and indirect effects from this causal pathway and may serve as a marker of cognitive decline or dementia. It is likely more than one of the pathways depicted may explain the link between hearing and dementia
Sensory Deprivation Hypothesis
Evidence suggests that prolonged sensory deprivation due to age-related hearing loss has a lasting adverse effect on brain structure and function. Age-related hearing loss could lead to cortical re-allocation, deafferentation, or atrophy to support speech perception processing. This reorganization can be to the detriment of general cognitive performance, adding to existing brain pathology (e.g., amyloid burden, neuronal loss) by altering critical brain regions which could otherwise be utilized for higher-level cognitive processing. Prolonged sensory deprivation from peripheral hearing loss may lead to changes and decreases in cortical brain volume similar to that seen with cognitive decline (Lin 2014a; Eckert et al. 2019). In particular, changes to temporal lobe volume and reduced gray matter density have been noted in those with peripheral hearing loss (Armstrong et al. 2019; Lin et al. 2014b; Wingfield and Peelle 2015). These are brain regions important for semantic memory and involved in progression along the dementia continuum. Brain regions affected include the superior temporal gyrus and Heschel’s gyrus as well as frontal and pre-frontal brain regions (Husain et al. 2011). Reduced whole-brain gray matter volume is significantly associated with brain regions recruited for speech understanding, including the frontal cortex and hippocampus (Rudner et al. 2019). Evidence indicates that hearing loss is associated with lower white matter microstructural integrity in brain regions critical for cognitive processing, even in older adults considered dementia-free (Croll et al. 2020; Rigters et al. 2017; Alfandari et al. 2018; Armstrong et al. 2019). Critical ages or length of sensory deprivation necessary to evoke these structural changes remains to be characterized. Even in early stages of cognitive impairment, cortical reorganization due to age-related hearing loss is apparent (Campbell and Sharma 2014; Sharma and Glick 2017); however, the degree of reorganization necessary to evoke brain atrophy and changes to cognitive performance has not been determined. This research suggests prolonged sensory deprivation may lead to a reduction in cortical volume beyond that seen from dementia pathology alone or may necessitate reorganization due to prolonged deprivation. This additional structural and functional decline results in further restriction of cortical capacity available for cognitive processing.
Information Degradation Hypothesis
In contrast to the physical nature of the association between age-related hearing loss and cognitive decline posed by the sensory deprivation hypothesis, the information degradation hypothesis proposes that the association is due to the increased cognitive processing required to compensate for an impoverished sensory input. This increased processing for degraded perception draws on the same resources needed for other higher-level cognitive processing and semantic encoding (Peelle 2018). The subsequent increased cognitive demands required to accurately encode information takes extensive listening effort with particular demands on attention, memory, and executive function (Wingfield et al. 2005; Tun et al. 2009; Peelle 2018; Pichora-Fuller et al. 2016). The cognitive capacity of an individual and ability to compensate for decreased cognitive performance varies and may partially explain some of the variance between existing brain pathology and presentation of clinical symptoms as previously described. This listening effort required with hearing impairment may increase the cognitive load and the full demands placed on the brain at any time (Whitson et al. 2018). These demands then draw on the compensatory buffering ability of the individual, potentially resulting in the earlier presentation of clinical symptoms and dementia.
Additionally, differences between compensatory mechanisms activated for cognitive performance between older and younger adults with sensory deficits are evident and may highlight increased fatigue in older adults for the dual tasks of listening and understanding (Schneider et al. 2002). Thus, this hypothesis implies temporary cognitive impairment. Through the amelioration of auditory input, cognitive performance on higher-level tasks might be restored.
Common Cause
The association between hearing and cognition in older adults could also simply stem from the same underlying mechanism, resulting in both impairments (Baltes and Lindenberger 1997; Wayne and Johnsrude 2015). Investigation of a common cause associated with both hearing and cognitive impairment has been primarily evaluated through PTA due to the presumed independent effect of peripheral hearing. General neural degeneration commonly seen with aging could potentially result in decreased cognitive function and auditory performance (Wayne and Johnsrude 2015; Surprenant and Neath 2006). Age-associated slowing of processing speed may result in slower overall cognitive functioning as well as slower processing for sensory integration and perception (CHABA 1988; Salthouse 1996). Concurrent changes in both cognitive and sensory modalities in older adults could also stem from conditions such as cerebrovascular disease (Livingston 2017; Eckert et al. 2013; Laughlin et al. 2013). Systemic vascular pathology impacting the spiral ganglion or stria vascularis as well as the vasculature of the brain could potentially alter both auditory and neural functioning. Investigation into an underlying common genetic risk between AD and hearing loss suggests that genetic risk for AD (as represented by a weighted sum of identified polymorphisms for AD and the inflammatory pathway) is associated with poorer speech-in-noise performance and self-reported difficulty hearing with background noise (Brenowitz et al. 2020). The apolipoprotein ε4 (APOE ε4) allele is the strongest known genetic risk factor for Alzheimer’s disease, but is not associated with hearing impairment (Morita et al. 2019), nor does it modify the association between hearing loss and cognitive decline (Alattar et al. 2020), suggesting that APOE ε4 may not be a driving factor that may explain the relationship between hearing loss and dementia in older adults. Alternatively, other works suggest that APOE ε4 status may be marginally associated with better hearing thresholds (Mener et al. 2016). Consideration of identified genetic risk factors for hearing loss may provide further into insight avenues for investigation of a common cause between hearing and dementia. The genetic determinants of age-related hearing loss remain largely unknown but have become a focus of recent work (Wells et al. 2019). However, question of causality in this association between shared genes remains (Mitchell et al. 2020) as the number of studies investigating genetic risk is limited, warranting further investigation.
Other Potential Factors in the Association Between Hearing and Cognition
The association of hearing and cognition may also be mediated through other potential risk factors for dementia, including social isolation, depression, or decreased physical activity. Social isolation and loneliness as a consequence of hearing loss is not a new concept, nor is the idea that decreased social interaction increases risk of dementia (Rafnsson 2020; Rutherford et al. 2018; Shukla et al. 2020; Maharani et al. 2019). While evidence is mixed, hearing loss may lead to increased risk of depression in older adults, which has been considered either a prodromal symptom or an independent risk factor for cognitive decline (see also for review Rutherford et al 2018). Further, hearing loss may lead to reduced physical activity due to anxiety from decreased auditory awareness (Choi et al. 2016; Chen et al. 2015). Additional evidence suggests that hearing loss is associated with increased prevalence of frailty which has been suggested to increase risk for dementia or may serve as an early marker of dementia (Panza et al. 2015; Liljas 2017). How these potential mediators fit within the larger context of the above hypothesis remains to be clarified, yet each offers opportunity for targeted intervention.
Summary of Proposed Hypothesis and Mechanism
It is likely that one or more of the proposed mechanisms explain and/or mediate the association between hearing loss and dementia. The specific contribution of each proposed mechanism toward risk of dementia may vary individually. While common risk factors (i.e., age, education, vascular disease) are thought to contribute towards the association (Humes 2013a; Whalley et al. 2004; Wayne and Johnsrude 2015), these factors likely do not explain the full story. In epidemiologic studies which have attempted to control for these confounding factors as best as able, the association persists, potentially indicating that other mechanisms are likely involved.
PRIORITIES FOR FUTURE RESEARCH
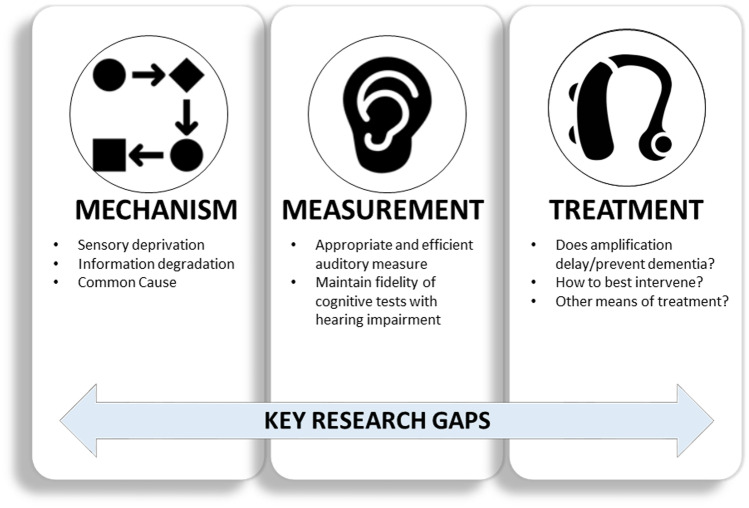
Given the wide multi-disciplinary scope of research on hearing and dementia, identification of research priorities can help propel the field of auditory science toward meaningful advances in both hearing and dementia care. A foundational objective is identifying subgroups of individuals who may be at a greater risk of cognitive decline or may benefit most from intervention. Many crucial research questions and additional perspectives exist (Wayne & Johnsrude 2015; Whitson et al. 2018). Here we highlight three areas of research priorities which we believe will provide the greatest advancements in understanding the association and possible causal role of hearing impairment along the dementia continuum. These include (1) the driving mechanism behind the association, (2) standardized use of validated measurement tools for the assessment of hearing and cognition in older adults, and (3) evaluating the efficacy/effectiveness of interventions to treat hearing impairment to delay the onset of dementia (Fig. 4).
Fig. 4.
Key research gaps in hearing impairment and cognition. Targeted research on the mechanism behind the association between hearing and cognition will guide intervention and prevention strategies. Additional research gaps include careful study and identification of the most appropriate and efficient auditory measure for association and causal effect studies of hearing and cognition, as well as ensuring fidelity of cognitive tests with hearing impairment. Further understanding of if treating hearing loss via amplification or another means influences dementia risk is vital for public health
What is the Driving Mechanism Behind the Hearing-cognition Association?
If we can determine the link(s) or driver(s) behind the association of hearing loss with cognition, we may better plan for and provide appropriate intervention options to delay the onset or alter the trajectory of the clinical course of dementia. How best to intervene, and ultimately whether treatment for hearing loss will be effective in decreasing or delaying dementia risk, largely depends on the underlying mechanism. Research has yet to determine if there is an independent effect of hearing loss on cognitive impairment for older adults. As described above, the relationship between AD pathology and the presentation of symptoms is heterogeneous. We do not yet know by what means hearing impairment may influence a potential buffer which delays/prevents the presentation of symptoms in some individuals yet leads to earlier symptomatology in others.
If the association between hearing impairment and dementia was only due to a common cause of both hearing loss and dementia, such as vascular disease or genetics, the opportunity for directly preventing or changing the progression of dementia through treating hearing loss would be limited. In turn, research focus would shift to intervention on the underlying biological or neurological mechanism at hand which could have significant downstream effects on hearing and cognition for older adults.
Both the sensory-deprivation and information degradation hypotheses imply a more direct effect of hearing impairment on dementia risk. While the long-term vs. short-term timeline of this effect varies by hypothesis, both suggest that hearing impairment leads to downstream effects on available cognitive resources. However, the nature of the effect from either hypothesis has implications for approaches to intervention. With structural and functional changes in the brain due to hearing impairment via the sensory deprivation hypothesis, early identification of hearing impairment and options to potentially delay or prevent deafferentation or atrophy within the auditory or sensory regions of the brain are essential to prevent lasting structural damage. This hypothesis may further demonstrate treatment benefit at later stages of hearing impairment, for example, by delaying additional neural reorganization or atrophy. In contrast, the information degradation hypothesis would provide a strong argument for new and existing hearing intervention options at any stage of the disease process—with improved integrity of auditory input via rehabilitation or hearing treatment, the strain on cognitive resources for environmental awareness or communication would be reduced.
Disentangling central auditory dysfunction and cognitive decline will be essential for targeting prevention efforts or early identification of subgroups at greater risk for cognitive decline. The temporality of the association and whether central auditory dysfunction is an early marker of dementia or represents an independent process, and how to differentiate the two, remain a challenge in understanding the link between hearing loss and dementia. The dependence of both peripheral and central hearing on higher-level, auditory-based cognitive functioning leaves significant questions of how to interpret the influence of each component along the auditory pathway. If central auditory dysfunction represents an early marker of dementia, how measures of CAD may fit within the context of existing early biomarkers of dementia or neuropsychiatric test batteries remains to be determined.
In all likelihood, multiple mechanisms are involved in the hearing-dementia association; thus, further investigation should determine if one mechanism serves as a primary driver and how each might interact to alter risk. It is possible the primary driver is unique to the individual, allowing for a person-centered approach to intervention that alters the progression to dementia.
How Do We Best Measure Hearing and Dementia in this Process?
Data are only as good as the measurement tools used to collect them. If we are not able to effectively measure our construct of interest, then our data may not appropriately answer the question of interest. The majority of studies on hearing impairment and dementia or cognitive decline have focused on utilizing audiometry or self-reported hearing—yet these measures reflect two differing constructs for hearing impairment. While both measures reflect aspects of hearing function, understanding the primary research objective will determine the most effective measure. We need to identify what hearing measures (i.e., self-reported hearing, pure tone average, specific configuration of impairment, central auditory function measures) provide the best representation of the mechanisms driving the association. We should also identify the most practical and useful measure to estimate a causal effect or for detection of cognitive change in a clinical environment or population-based research study. Synthesis and harmonization of data are most effective and informative if less heterogeneous and streamlined types of measures are collected across a wide range of studies and represented populations. Investigation of the agreement of the various objective measures utilized (i.e., four-frequency PTA in the better hearing ear vs. 500–8000 Hz in the better hearing ear) and how self-reported hearing ability or electrophysiologic measures may supplement knowledge gained from pure tone averages may foster consistency across studies. Because the auditory pathway encompasses both peripheral and central hearing components, effectively and efficiently discerning and utilizing these respective measures for study of cognition in older adults are imperative to progress in this research.
There is a need to disentangle the potential for sensory bias in cognitive testing and the dependence on cognitive functioning for auditory measures, particularly speech-in-noise measures. Cognitive tests and screening tools rely on an individual’s auditory or visual ability for completion. This dependence has led some to question if auditory or visual impairments may lead to potential sensory bias with cognitive tests (Füllgrabe 2020; Guerreiro and Van Gerven 2017). Confirming the validity of the test within the cognitive domain and construct of interest is essential for use in the growing number of older adults with sensory impairments (Pye et al. 2017). Among important questions to consider is whether accommodations during testing for individuals with hearing loss are appropriate. Cognitive tests which maintain integrity of the assessment but are simple and efficient for hearing impaired older adults, researchers, and clinicians will have the greatest utility. Addressing hearing and vision needs prior to cognitive assessments will be important for confidence and synthesis of study results.
Prior work suggests challenges with completion of standard audiometry for those with more advanced dementia, particularly those living in managed care settings. A systematic review of only 3 studies suggested that only 56–59 % of dementia participants were not able to appropriately complete audiometric testing (Bott et al. 2019). However, limitations of this review, including the small number of studies included, small sample size, and variability in methodology and study design, leave significant clinical and methodologic questions. Recent work suggests test-retest reliability for a wide scope of standard clinical audiology measures in those with mild dementia is comparable to cognitively normal adults (McClannahan et al. 2021). More than 30 years ago, it was recommended that auditory-evoked potential testing be used as an alternative for those with dementia (Hedner et al. 1987) and more recent study (Villeneuve et al. 2017; Bott 2020) suggests feasibility. However, given the variability in performance ability demonstrated for those across the trajectory of cognitive impairment, more information is needed for clinical providers to determine the most appropriate means of hearing evaluation for their patients with more severe cognitive impairment.
Does Treating Hearing Loss Alter Dementia Risk?
Evidence for the impact treating hearing impairment has on cognitive decline or dementia is accumulating. However, until the results of the current longer-term clinical trials are available, the evidence of these effects on cognitive decline and dementia risk largely stem from observational studies. There is great power in our insights gained from observational studies; however, evidence of causality for decreased or delayed cognitive decline due to hearing aid use is more challenging. Challenges include selective effects of who chooses to pursue hearing aids as well as heterogeneity in device, fitting, use, temporality, or unmeasured confounding. Not only must we still determine if treating hearing loss using hearing aids attenuates dementia risk or delays presentation, we also have a limited understanding of how and when to best intervene.
Public health prevention strategies recognize the potential for targeted intervention at each stage of the disease process—known as primary, secondary, and tertiary prevention (Fig. 5) (Celentano and Szklo 2020). Evidence for hearing intervention at these levels of prevention remains limited, and an understanding of how hearing loss treatment throughout the process might influence upstream higher-level cognitive processing is virtually non-existent. Determining the best choices for prevention within a population is dependent on evidence of efficacy, effectiveness, and cost efficiency—all areas with extensive gaps in evidence for hearing loss and dementia.
Fig. 5.
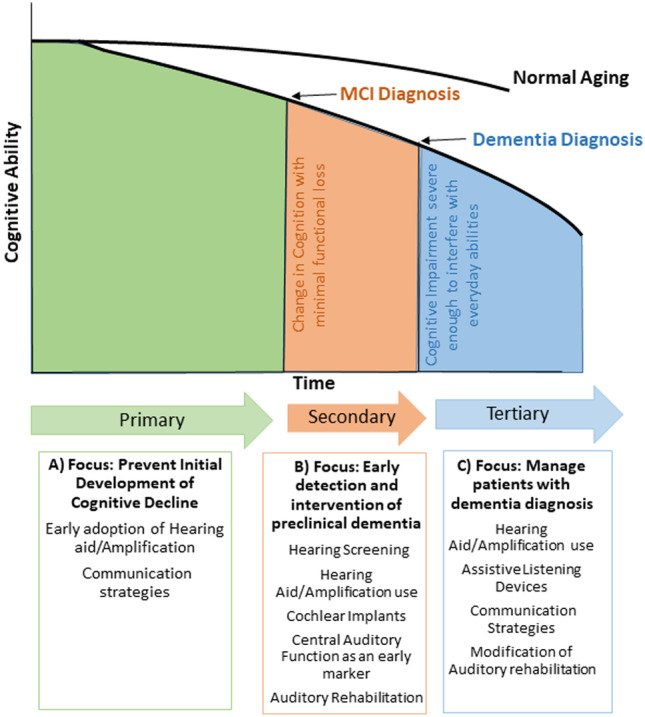
Schematic of levels for dementia prevention through identification of and management of hearing loss in combination with the cognitive decline continuum. (A) Primary prevention focusing on the prevention of initial development of cognitive decline in normal cognitive aging. (B) Secondary prevention focusing on early detection and intervention of preclinical dementia. (C) Tertiary prevention with a focus on management of patients with a dementia diagnosis
Primary prevention strategies involve the reduction of risk factors before the disease process begins (Celentano and Szklo 2020). In this association, primary prevention focuses on reducing the onset of cognitive decline among those with cognitive abilities with that expected for age and background. Early identification of hearing loss and adoption of hearing treatment may prevent structural changes in the brain (sensory deprivation hypothesis), thereby reducing the additional “hit” to cortical structures and demands on neural allocation. Key objectives of hearing aid clinical trials are to determine if adoption of hearing aids for hearing impairment (at least peripheral hearing impairment) may delay progression of cognitive decline and how the use of amplification may therefore influence the outcome.
Secondary prevention involves the early detection and prevention of disease through screening and early identification and encompasses intervention upon those at clear risk of progression of cognitive impairment. Identification of hearing impaired older adults with concurrent mild cognitive impairment, subjective cognitive complaints, or significant biomarkers of dementia is the focus of these efforts. Delay of further cognitive decline and transition to dementia through amplification use, aural rehabilitation, and communication strategies would make a lasting contribution to dementia prevention. Attention should be paid to following best practices for device fitting if possible to ensure patient hearing needs are met as best as able, including the use of real ear measures for hearing aid programming and objective measures for cochlear implant mapping such as neural response telemetry (NRT) and electrical stapedial reflex threshold (ESRT).
The last stage of prevention, tertiary prevention, in this case follows the onset of dementia and focuses on supportive and rehabilitative services to minimize morbidity and mortality or maximize quality of life. The use of hearing aids may decrease risk presented by mediators within the hearing and dementia pathway by reducing social isolation or depression risk for older adults, which can worsen dementia symptom trajectories. Importantly, neuropsychiatric (i.e., depression, agitation, anxiety, delusions, aggression, apathy, etc.) symptoms are fairly common at all stages of dementia and are associated with poor outcomes for both patients and caregivers (Kales et al. 2014). However, hearing aid use has been associated with fewer and lower severity neuropsychiatric symptoms and less severe depressive symptoms in older adults with cognitive impairment (Kim et al. 2020). Therefore, not only does hearing aid use among ADRD patients demonstrate improved communication, decreased hearing handicap, and improved quality of life (Mamo et al. 2018; Dawes et al. 2015, 2019), but it may also reduce common dementia-related behaviors detrimental for individuals and challenging for caregivers and providers.
Within tertiary prevention, we must further determine how to provide auditory rehabilitation for adults with cognitive impairment (Hubbard et al. 2018). While current evidence suggests treating hearing loss in cognitively impaired older adults yields benefits as described above, the strength of the evidence is limited due to its case-report and subjective nature. Determining alternative accessible and effective means of hearing rehabilitation for cognitively impaired and aging adults is imperative to meeting future population needs. Consideration for hearing rehabilitation might include alternative strategies such as hearable devices if appropriate or simple assistive listening devices. Even with changes in health policy improving access to hearing care and rehabilitation, hearing aids may not be feasible for some older adults and likely do not meet the listening needs of many adults when used as the only form of rehabilitation. Alternative strategies which present non-technology driven proposals (Nieman et al. 2017) should be evaluated to determine if these approaches better meet the auditory and cognitive needs for some older adults.
UTILIZING CURRENT AND FUTURE EVIDENCE TO IMPROVE PATIENT CARE
How auditory scientists, cognitive scientists, epidemiologists, and other investigators present the research on hearing and cognitive impairment can have significant implications for how clinical providers educate patients and their families. Epidemiologic studies comprise much of the research on hearing and cognitive impairment in older adults. These studies are primarily observational, conducted using population-level data. This design allows for the observation of health-related trends within a selected population of individuals. Thus, on average, observation of a significant positive association in epidemiologic data suggests that those with hearing impairment demonstrate an increased risk of cognitive impairment compared to those with normal hearing. This type of data does not imply that a given individual with hearing impairment will go on to develop dementia but helps identify groups of individuals whom primary care providers may wish to monitor for cognitive change.
In comparison, some of the work by cognitive and auditory researchers has been conducted within selective and controlled clinical or research environments. These environments allow for ease of participant recruitment and control of variables, yet the use of clinic-based populations does not necessarily ensure that the complex relationship between hearing ability and cognitive impairment seen within the broader real world is captured. Many clinic- or research-based participants may have underlying concerns which have led them to participate in studies, and participants often possess characteristics which may not be generalizable or representative of the general population or under-represented groups. Thus, while epidemiologic cohorts also have their limitations, understanding trends within a population helps identify areas of further research. These population trends inform health program planning, health policies, and identification of areas for targeted research. Each research environment presents differing angles or perspectives with which scientists may address research needs, enriching collaborations and our comprehensive approach to the public health issue.
Given the varied disciplines involved in research on hearing and cognitive impairment, understanding how to synthesize the current evidence, translate findings, and guide future research for scientific progression is vital. However, current research has primarily remained siloed within disciplines. Auditory science and cognitive science researchers have the opportunity and expertise to address aspects of the primary research gaps described above. Transparency of and careful consideration of study design, measurement tools implemented, and discussion of results’ applicability for clinical or community populations are essential to characterize the role of hearing impairment within the dementia timeline and meet the needs of our current and future older adults.
CONCLUSION
While research on the contribution of age-related hearing loss to dementia risk has been around for decades, growing evidence of the adverse consequences of hearing loss for older adults has brought conversations about hearing health and care into the clinical exam room and to the attention of older adults and policy makers. The human and economic expense of caring for the coming wave of adults reaching or past retirement age demands interdisciplinary collaboration. Auditory science has the opportunity to contribute towards efforts to reduce this impact. Identification of the mechanism(s) driving the association between hearing and dementia, directed hearing-dementia research for the greatest public health impact and societal needs, and thoughtful translation of this research for clinicians and patients can have a monumental impact on prevention and intervention strategies for older adults. The interdependent and synergistic processes of hearing and cognition require careful approach. Optimizing our strategies to treat hearing loss could diminish the risk of adverse outcomes and enhance health and quality of life for older adults.
Funding
Dr. Deal was supported by NIH/NIA grant K01AG054693. Dr. Oh was supported by NIH grant R01AG057725.
DECLARATIONS
Conflict of Interest
Dr. Frank Lin reports being a consultant to Frequency Therapeutics, speaker honoraria from Caption Call, and being the director of a public health research center funded in part by a philanthropic gift from Cochlear Ltd to the Johns Hopkins Bloomberg School of Public Health. Dr. Danielle Powell, Dr. Jennifer Deal, and Dr. Esther Oh declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alattar A, Bergstrom J, Laughlin G, Kritz-Silberstein D, Richard E, et al. Hearing impairment and cognitive decline in older, community dwelling adults. J Gerontol A Biol Sci Med Sci. 2020;75(3):567–573. doi: 10.1093/gerona/glz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers MW, Gilmore GC, Kaye J, Murphy C, Wingfield A, et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimer’s & Dementia. 2015;11(1):70–98. doi: 10.1016/j.jalz.2014.04.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guideline for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfandari D, Criend C, Heslenfeld DJ, Versfeld NJ, Kramer SE, Zekveld AD. Brain volume differences associated with hearing impairment in adults. Trends in Hearing. 2018;22:1–8. doi: 10.1177/2331216518763689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Disease Facts and Figures (2020) Alzheimers Dement 16: 391-460
- Amieva H, Ouvrard C, Giulioli C, Meillon C, Rullier L, et al. Self-reported hearing loss, hearing aids, and cognitive decline in elderly adults: a 25-year study. Journal of the American Geriatrics Society. 2015;63:2099–2104. doi: 10.1111/jgs.13649. [DOI] [PubMed] [Google Scholar]
- Amieva H, Ouvrard C (2020) Does treating hearing loss in older adults improve cognitive outcomes? Rev J Clin Med 9(3):805 [DOI] [PMC free article] [PubMed]
- Armstrong NM, An Y, Doshi J, Erus G, Ferrucci L, et al. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngology-Head & Neck Surgery. 2019;145(9):794–802. doi: 10.1001/jamaoto.2019.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N, Pascoal T, Karikari T, Benedet A, Lantero-Rodriguez J et al (2021) Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. Published Online 14/2/2021. 10.1007/s00401-021-02275-6 [DOI] [PMC free article] [PubMed]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive function across the adult lifespan: a new window to the study of cognitive aging? Psychology and Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Belleville S, Fouquet C, Hudon C, Tchala Vignon Zomahoun H, Croteau J for the Consortium for the Early Identification of Alzheimer’s disease-Quebec Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer’s type dementia in older adults: a systematic review and meta-analysis. Neuropsychol Rev. 2017;27:328–353. doi: 10.1007/s11065-017-9361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott A, Meyer C, Hickson L, Pachana, Can adults living with dementia complete pure-tone audiometry. International Journal of Audiology. 2019;58(4):185–192. doi: 10.1080/14992027.2018.1550687. [DOI] [PubMed] [Google Scholar]
- Bott A, Hickson L, Meyer C, Bardy F, Van dun B et al (2020) Is cortical automatic threshold estimation a feasible alternative for hearing threshold estimation with adults with dementia living in aged care? Int J Audiol 59(10):745-752 [DOI] [PubMed]
- Boyle PA, Yu L, Leurgans SE, Wilson RS, Broomeyer R, et al. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Annals of Neurology. 2019;85(1):114–124. doi: 10.1002/ana.25380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiology of Aging. 1997;18(4):551–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- Brenowitz WD, Filshtein TJ, Yaffe K, Walter S, Ackley SF, et al. Association of genetic risk score for Alzheimer disease and hearing impairment. Neurology. 2020;95(16):e2225–e2234. doi: 10.1212/WNL.0000000000010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster KK, Pavlicova M, Stein A, Chen M, Chen C, et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. International Journal of Geriatric Psychiatry. 2020;35(8):842–850. doi: 10.1002/gps.5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster KK, Hu M, Wall M, Brown P, Zilcha-Mano S et al (2021) Age-related hearing loss, neuropsychological performance, and incident dementia in older adults. J Alzheimers Dis 80(2):855-864. 10.3233/JAD-200908 [DOI] [PMC free article] [PubMed]
- Campbell J, Sharma A. Cross-modal re-organization in adults with early stage hearing loss. PLOS One. 2014;9(2):1–8. doi: 10.1371/journal.pone.0090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano DD, Szklo M. Gordis Epidemiology. 6. Philadelphia: Elsevier; 2020. [Google Scholar]
- CHABA Speech understanding and aging. Working group on speech understanding and aging. Committee on Hearing, Bioacoustics, and Biomechanics, Commission on Behavioral and Social Sciences and Education, National Research Council. Journal of the Acoustical Society of America. 1988;83:859–895. [PubMed] [Google Scholar]
- Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. Journals of Gerontology: Series A Biological Sciences and Medical Sciences. 2015;70(5):654–661. doi: 10.1093/gerona/glu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Betz J, Deal J, Contrera KJ, Genther DJ. A comparison of self-report and audiometric measures of hearing and their associations with functional outcomes in older adults. Journal of Aging and Health. 2016;28(5):890–910. doi: 10.1177/0898264315614006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes A, Van de Heyning P, Gilles A, Van Rompaey V, Mertens G. Cognitive performance of severely hearing-impaired older adults before and after cochlear implantation: preliminary results of a prospective, longitudinal cohort study using the RBANS-H. Otology & Neurotology. 2018;39:e765–773. doi: 10.1097/MAO.0000000000001936. [DOI] [PubMed] [Google Scholar]
- Croll PH, Vernooij MW, Reid RI, Goedegebure A, Power MC, et al. Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimer’s & Dementia. 2020;16(11):1515–1523. doi: 10.1002/alz.12151. [DOI] [PubMed] [Google Scholar]
- De Wolf F, Ghanbari M, Licher S, McRae-McKee K, Gras L, et al. Brain. 2020;143:1220–1232. doi: 10.1093/brain/awaa054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes P, Emsley R, Cruickshanks KJ, Moore DR, Fortnum H et al (2015) Hearing loss and cognition: the role of hearing aids, social isolation and depression. PLoS One 10(3):e0119616 [DOI] [PMC free article] [PubMed]
- Dawes P, Wolski L, Himmelsbach I, Regan J, Leroi I. Interventions for hearing and vision impairment to improve outcomes for people with dementia: a scoping review. International Psychogeriatrics. 2019;31(2):203–221. doi: 10.1017/S1041610218000728. [DOI] [PubMed] [Google Scholar]
- Deal JA, Beta J, Yaffe K, Harris T, Purchase-Helzner E, et al. Hearing Impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2016;72(5):703–709. doi: 10.1093/gerona/glw069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal JA, Albert MS, Arnold M, Bangdiwala SI, Chisolm T, Davis S, et al. A randomized feasibility pilot trial of hearing treatment for reducing cognitive decline: results from the Aging and Cognitive Health Evaluation in Elders Pilot Study. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2017;3:410–415. doi: 10.1016/j.trci.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal JA, Goman AM, Albert MS, Arnold ML, Burgard S, et al. Hearing treatment for reducing cognitive decline: design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4:499–507. doi: 10.1016/j.trci.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden A, Allen HA, Henshaw H, Heinrich A. The Association between cognitive performance and speech-in-noise perception for adult listeners: a systematic literature review and meta-analysis. Trends in Hearing. 2017;21:1–21. doi: 10.1177/2331216517744675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Kuchinsky SE, Vaden KI, Cute SL, Spampinato MV, et al. White matter hyperintensities predict low frequency hearing in older adults. Journal of the Association of Research in Otolaryngology. 2013;14:425–433. doi: 10.1007/s10162-013-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Vaden KI, Jr, Dubno JR. Age-related hearing loss associations with changes in brain morphology. Trends in Hearing. 2019;23:1–14. doi: 10.1177/2331216519857267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto B, Badana A, Arnold M, Lister J, Edwards J. Comparison of subjective and objective measures of hearing, auditory processing, and cognition among older adults with and without mild cognitive impairment. Journal of Speech, Language, and Hearing Research. 2018;61(4):945–956. doi: 10.1044/2017_JSLHR-H-17-0263. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fortunato S, Forli F, Guglielmi V, De Corso E, Paludetti G, et al. A review of new insights on the association between hearing loss and cognitive decline in ageing. Acta Otorhinolaryngologica Italica. 2016;36:155–166. doi: 10.14639/0392-100X-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füllgrabe C. On the possible overestimation of cognitive decline: the impact of age-related hearing loss on cognitive-test performance. Frontiers in Neuroscience. 2020;14:454. doi: 10.3389/fnins.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher J, Ilubaera V, Ben-Shlomo Y, Bayer A, Fish M, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. 2012;79:1583–1590. doi: 10.1212/WNL.0b013e31826e263d. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cogg JL, Linn RT, Rees T, Wolf PA, D’Agostino RB. Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Archives of Otolaryngology- Head & Neck Surgery. 1996;122:161–167. doi: 10.1001/archotol.1996.01890140047010. [DOI] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction in older people with memory impairment or Alzheimer’s dementia. Archives of Otolaryngology- Head & Neck Surgery. 2008;134(7):771–777. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer’s dementia. Archives of Otolaryngology- Head & Neck Surgery. 2011;137(4):390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA. Central presbycusis: an emerging view. Otolaryngology-Head & Neck Surgery. 2012;141(1):1–2. doi: 10.1177/0194599812446282. [DOI] [PubMed] [Google Scholar]
- Gao J, Armstrong N, Deal J, He Lin F, P, Hearing loss and cognitive function among Chinese older adults: the role of participation in leisure activities. BMC Geriatrics. 2020;20:215. doi: 10.1186/s12877-020-01615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goman A, Lin FR. Prevalence of hearing loss by severity in the United States. American Journal of Public Health. 2016;106(10):1820–1822. doi: 10.2105/AJPH.2016.303299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths TD, Lad M, Kumar S, Holmes E, McMurrary B et al (2020) How can hearing loss cause dementia? Neuron 108(3):401-412 [DOI] [PMC free article] [PubMed]
- Guerreiro MJS, Van Gerven PWM. Disregarding hearing loss leads to overestimation of age-related cognitive decline. Neurobiology of Aging. 2017;56:180–189. doi: 10.1016/j.neurobiolaging.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Hedner K, Broms P, Harris S, Steen B (1987) Hearing in geriatric long-stay patients. Comprehensive gerontology. Sec A Clin Lab Sci 1(2):69-71 [PubMed]
- Hubbard HI, Mamo SK, Hopper T. Dementia and hearing loss: interrelationships and treatment considerations. Seminars in Speech and Language. 2018;39(3):197–210. doi: 10.1055/s-0038-1660779. [DOI] [PubMed] [Google Scholar]
- Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis and treatment. Clinics in Geriatric Medicine. 2014;30:421–442. doi: 10.1016/j.cger.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Dubno J, Gordon-Salant S, Lister JL, Cacae AT, et al. Central presbycusis: a review and evaluation of the evidence. Journal of the American Academy of Audiology. 2012;23:635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE. Understanding the speech-understanding problems of older adults. American Journal of Audiology. 2013;22:303–305. doi: 10.1044/1059-0889(2013/12-0066). [DOI] [PubMed] [Google Scholar]
- Humes LE, Busey RA, Craig J, Kewley-Port D. Are age-related changes in cognitive function driven by age-related changes in sensory processing? Atten Percept Psychophys. 2013;75:508–524. doi: 10.3758/s13414-012-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Kidd GR, Lentz JL (2013b) Auditory and cognitive factors underlying individuals differences in aided-speech-understanding among older adults. Front Syst Neurosci 7:55 [DOI] [PMC free article] [PubMed]
- Humes LE, Young LA. Sensory-cognitive interactions in older adults. Ear & Hearing. 2016;37(Suppl 1):52S–61S. doi: 10.1097/AUD.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain FT, Medina RE, Davis CW, Szymko-Bennett Y, Simonyan K, et al. Neuoanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Research. 2011;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizbegovic E, Hederstierna C, Dahlquist M, Kämpfe Nordström C, Jelic V, et al. Central auditory function in early Alzheimer’s disease and in mild cognitive impairment. Age Ageing. 2011;40(2):249–254. doi: 10.1093/ageing/afq168. [DOI] [PubMed] [Google Scholar]
- Jack CR, Albert M, Knopman DS, McKhann GM, Sperling RA, et al. Introduction to revised criteria for the diagnosis of Alzheimer’s disease: National Institute on Aging and the Alzheimer Association workgroups. Alzheimer’s & Dementia. 2011;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Peterson RC, Weiner MW. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. The Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakody D, Friedland P, Nel E, Martins R, Atlas M, et al. Impact of cochlear implantation on cognitive functions of older adults: pilot test results. Otology and Neurotology. 2017;38(8):e289–e295. doi: 10.1097/MAO.0000000000001502. [DOI] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, Lyketsos CG for the Detroit Expert Panel on the Assessment and Management of the Neuropsychiatric Symptoms of Dementia Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multisensory expert panel. Journal of the American Geriatrics Society. 2014;62(4):762–769. doi: 10.1111/jgs.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AS, Garcia Morales EE, Amjad H, Cotter VT, Lin FR, et al. Association of hearing loss with neuropsychiatric symptoms in older adults with cognitive impairment. American Journal of Psychiatry. 2020;S1064–7481(20):30510–8. doi: 10.1016/j.jagp.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Roberts RO, Pankratz S, Cha RH, Rocca WA, et al. Incidence of dementia among participants and nonparticipants in a longitudinal study of cognitive aging. American Journal of Epidemiology. 2014;180(4):414–423. doi: 10.1093/aje/kwu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin GA, McEvoy LK, Barrett-Connor E, Daniels LB et al (2013) Fetuin-A, a new vascular biomarker of cognitive decline in older adults. Clin Endocrinol 81(1):134-40. [DOI] [PMC free article] [PubMed]
- Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s Research and Therapy. 2018;10(1):1–10. doi: 10.1186/s13195-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljas A, Carvalho L, Papachristou E, De Oliveira C, Wannamethee G et al (2017) Self-reported hearing impairment and incident frailty in English community-dwelling older adults: a 4-year follow-up study. J Am Geriatr Soc 65(5): 958-965 [DOI] [PMC free article] [PubMed]
- Lin FL, Metter J, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Archives of Neurology. 2011;68(2):214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, et al. Association of hearing impairment with brain volume changes in older adults. Neuorimage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Albert M. Hearing loss and dementia- who’s listening? Aging and Mental Health. 2014;18(6):671–673. doi: 10.1080/13607863.2014.915924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Yaffe K, Xia J, Xue Q, Harris T et al. (2013) Hearing Loss and Cognitive Decline Among Older Adults. JAMA Internal Medicine 173(4):293-299 [DOI] [PMC free article] [PubMed]
- Livingston G et al (2017) Dementia prevention, intervention, and care. The Lancet 390(10113):2673-2734 [DOI] [PubMed]
- Livingston G et al (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet 396(10248):413-446 [DOI] [PMC free article] [PubMed]
- Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of Age-related hearing loss with cognitive function, cognitive impairment and dementia: a systematic review and meta-analysis. JAMA Otolaryngology-Head & Neck Surgery. 2018;144(2):115–126. doi: 10.1001/jamaoto.2017.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Longitudinal relationship between hearing aid use and cognitive function in older Americans. Journal of the American Geriatrics Society. 2018;66:1130–1136. doi: 10.1111/jgs.15363. [DOI] [PubMed] [Google Scholar]
- Maharani A, Pendleton N, Leroi I. Hearing impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English Longitudinal Study on Ageing. American Journal of Geriatric Psychiatry. 2019;27:1348–1356. doi: 10.1016/j.jagp.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Mamo SK, Reed NS, Price C, Occhipinti D, Pletnikova A. Hearing loss treatment in older adults with cognitive impairment: a systematic review. Journal of Speech Language and Hearing Research. 2018;61(10):2589–2603. doi: 10.1044/2018_JSLHR-H-18-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClannahan K, Chiu YF, Somers M, Peelle J. Test-retest reliability of audiometric assessment in individuals with mild dementia. JAMA Otolaryngology Head & Neck Surgery. 2021 doi: 10.1001/jamaoto.2021.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Cherkow H, Hyman BT, Jack CR, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging- Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mener DJ, Betz J, Yaffe K, Harris TB, Helzner EP, et al. Apolipoprotein E allele and hearing thresholds in older adults. American Journal of Alzheimer’s Disease & Other Dementias. 2016;31(1):34–39. doi: 10.1177/1533317514537549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Miler C, Marrone N, Howe C, Fain M, et al. The impact of cochlear implantation on cognition in older adults: a systematic review of clinical evidence. BMC Geriatrics. 2015;15(1):16. doi: 10.1186/s12877-015-0014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BL, Thorp JG, Evans DM, Nyholt DR, Martin NG. Exploring the genetic relationship between hearing impairment and Alzheimer’s disease. Alzheimer’s & Dementia. 2020;12:e12108. doi: 10.1002/dad2.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly A, Doerfer K, Harris M (2019) Does cochlear implantation improve cognitive performance? Laryngoscope 129:2208-2209. 10.1002/lary.28140 [DOI] [PubMed]
- Molinuevo J, Ayton S, Batrla R, Bednar M, Bittner T, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neruopathol. 2018;136(6):821–853. doi: 10.1007/s00401-018-1932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Sasaki T, Takahashi K, Kitazawa M, Nonomura Y, et al. Age-related hearing loss is strongly associated with cognitive decline regardless of APOE4 polymorphism. Otology & Neurotology. 2019;40:1263–1267. doi: 10.1097/MAO.0000000000002415. [DOI] [PubMed] [Google Scholar]
- Moscoso A, Grothe M, Ashton N, Karikari T, Lantero-Rodriguez, , et al. Longitudinal associations of blood phosphorylated Tau181 and neurofilament light chain with neurodegeneration in alzheimer disease. JAMA Neurology. 2021 doi: 10.1001/jamaneurol.2020.4986.publishedonlineJanuary11,2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnier I, Bebear J, Marx M, Fraysse B, Truy E et al (2015) Improvement of cognitive function after cochlear implantation in elderly patients. JAMA Otolarynogol Head Neck Surg 141(5): 442-450 [DOI] [PubMed]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nieman CL, Marrone N, Mamo SK, Betz J, Choi JS, et al. The Baltimore HEARS pilot study: an affordable, accessible community-delivered hearing care model. Gerontologist. 2017;57(6):1173–1186. doi: 10.1093/geront/gnw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuesse T, Steenken R, Neher T, Holube I (2018) Exploring the link between cognitive abilities and speech recognition in the elderly under different listening conditions. Front Psychol 9: 678. 10.3389/fpsyg.2018.00678 [DOI] [PMC free article] [PubMed]
- Panza F, Solfrizzi V, Seripa D, Imbimbo B, Capozzo R et al (2015) Age-related hearing impairment and frailty in Alzheimer’s disease: interconnected associations and mechanisms. Front Aging Neurosci 7:113. 10.3389/fnagi.2015.00113 [DOI] [PMC free article] [PubMed]
- Peelle J. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear and Hearing. 2018;39(2):204–214. doi: 10.1097/AUD.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BWY, et al. Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL) Ear & Hearing. 2016;37:5S–27S. doi: 10.1097/AUD.0000000000000312. [DOI] [PubMed] [Google Scholar]
- Pye A, Charalambous AP, Leroi I, Thodi C, Dawes P. Screening tools for the identification of dementia for adults with age-related acquired hearing or vision impairment: a scoping review. International Psychogeriatrics. 2017;29(11):1771–1784. doi: 10.1017/S104161021700120X. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Channel-capacity, intelligibility and immediate memory. Quarterly Journal of Experimental Psychology. 1968;20:241–248. doi: 10.1080/14640746808400158. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission topography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. Journal of the American Medical Association. 2019;321(13):1286–1294. doi: 10.1001/jama.2019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnsson SB, Orrell M, d’Orsi E, Hogervorst E, Steptoe A. Loneliness, social integration, and incident dementia over 6 years: prospective findings from the English Longitudinal Study of Ageing. Journals of Gerontology- Series B Psychological Sciences and Social Sciences. 2020;75(1):114–124. doi: 10.1093/geronb/gbx087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Popli G, Gell G. Association of cognition and age-related hearing impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngology-Head & Neck Surgery. 2018;144(10):876–882. doi: 10.1001/jamaoto.2018.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigters SC, Bos D, Metselaar M, Roshchupkin GV, Baatengurg de Jong RJ, et al. Hearing impairment is associated with smaller brain volume in aging. Frontiers in Aging Neuroscience. 2017;9:2. doi: 10.3389/fnagi.2017.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner M, Seeto M, Keidser G, Johnson B, Rönnberg J. Poorer speech reception threshold in noise is associated with lower brain volume in auditory and cognitive processing regions. Journal of Speech Language and Hearing Research. 2019;62:117–1130. doi: 10.1044/2018_JSLHR-H-ASCC7-18-0142. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. American Journal of Psychiatry. 2018;175(3):215–224. doi: 10.1176/appi.ajp.2017.17040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T (1996) The processing-speed theory of adult age differences in cognition. Psychol Rev 403-428 [DOI] [PubMed]
- Sarant J, Harris D, Busby P, Maruff P, Schembri A et al (2019) The effect of cochlear implants on cognitive function in older adults: initial baseline and 18-month follow up results for a prospective international longitudinal study. Front Aging Neurosci 13 10.3389/fnins.2019.00789 [DOI] [PMC free article] [PubMed]
- Sardone R, Battista P, Lozupone Panza F, M, Griseta C, , et al. The age-related central auditory processing disorder: silent impairment of the cognitive ear. Frontiers in Neuroscience. 2019;13:619. doi: 10.3389/fnins.2019.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardone R, Donghia R, Tortelli R, Grasso A, Castellana F, et al. Age-related central auditory processing disorder, MCI, and dementia in an older population in southern Italy. Otolaryngology- Head and Neck Surgery. 2020;163(2):348–355. doi: 10.1177/0194599820913635. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Daneman M, Pichora-Fuller MK. Listening in aging adults: from discourse comprehension to psychoacoustics. Can J Exp Pscyhol. 2002;56:139–152. doi: 10.1037/h0087392. [DOI] [PubMed] [Google Scholar]
- Sharma A, Glick H. Cross-modal plasticity in developmental and age-related hearing loss: clinical implications. Hear Res. 2017;343:191–201. doi: 10.1016/j.heares.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla A, Harper M, Pedersen E, Goman A, Suen JJ, et al. Hearing loss, loneliness, and social isolation: a systematic review. Otolaryngology-Head and Neck Surgery. 2020;5:622–633. doi: 10.1177/0194599820910377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer’s disease. Neurology. 1993;43:779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, et al. toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging and the Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant AM, Neath I. Cognitive Aging. In: Wilmoth JA, Ferraro K, editors. Gerontology: perspectives and issues. New York NY: Springer; 2006. pp. 89–109. [Google Scholar]
- Thomson RS, Auduong P, Miller AT, Gurgel RK. Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investigative Otolaryngology. 2017;2(2):69–79. doi: 10.1002/lio2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, McCoy A, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychology and Aging. 2009;24(3):761–766. doi: 10.1037/a0014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, Willaims VA, Small BJ, Hafter ER. The effects of aging on auditory processing and cognition. American Journal of Audiology. 2012;21:344–350. doi: 10.1044/1059-0889(2012/12-0030). [DOI] [PubMed] [Google Scholar]
- Tuwaig M, Savard M, Jutras B, Poirier J, Louis Collins D, et al. Deficit in central auditory processing as a biomarker of pre-clinical Alzheimer’s disease. Journal of Alzheimer’s Disease. 2017;60:1589–1600. doi: 10.3233/JAD-170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann RF, Larson EB, Rees RS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. Journal of the American Medical Association. 1989;261(13):1916–1919. [PubMed] [Google Scholar]
- Vespa J, Medina L, Armstrong DM (2020) Demographic turning points for the United States: population projections for 2020 to 2060. U.S. Department of Commerce, U.S. Census Bureau. https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1144.pdf
- Villeneuve A, Hommet C, Aussedat C, Lescanne E, Reffet K, et al. Audiometric evaluation in patients with Alzheimer’s disease. Eur Arch Otorhinolaryngol. 2017;274:151–157. doi: 10.1007/s00405-016-4257-1. [DOI] [PubMed] [Google Scholar]
- Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Research Reviews. 2015;23:154–166. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Wells H, Freidin M, Zainul Abidin F, Payton A, Dawes P, et al. GWAS identifies 44 independent associated genomic loci for self-reported adult hearing difficulty in UK Biobank. American Journal of Human Genetics. 2019;105:788–802. doi: 10.1016/j.ajhg.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Wingfield A, Tun PA, McCoy SL. Hearing loss in older adulthood-what it is and how it interacts with cognitive performance. Curr Directions Psychol. Sci. 2005;14(3):144–148. [Google Scholar]
- Wingfield A, Peelle JE (2015) The effects of hearing loss on neural plasticity and processing. Front Syst Neurosci 9:35 [DOI] [PMC free article] [PubMed]
- Whitson HE, Cronin-Golomb A, Cruickshanks KJ, Gilmore GC, Owsley C. American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: sensory impairment and cognitive decline in older adults. Journal of the American Geriatrics Society. 2018;00:1–7. doi: 10.1111/jgs.15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2020) Grades of hearing impairment. https://www.who.int/pbd/deafness/hearing_impairment_grades/en/. Accessed 20 December 2020
- Yuan J, Sun Y, Sang S, Huynh Pham J, Kon W. The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Scientific Reports. 2018;8(1):1–10. doi: 10.1038/s41598-018-20496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissimopoulos J. The value of delaying Alzheimer’s disease onset. Forum for Health Economics and Policy. 2014;18(10):25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]