Abstract
Developmental dyslexia is most commonly associated with phonological processing difficulties. However, children with dyslexia may experience poor speech-in-noise perception as well. Although there is an ongoing debate whether a speech perception deficit is inherent to dyslexia or acts as an aggravating risk factor compromising learning to read indirectly, improving speech perception might boost reading-related skills and reading acquisition. In the current study, we evaluated advanced speech technology as applied in auditory prostheses, to promote and eventually normalize speech perception of school-aged children with dyslexia, i.e., envelope enhancement (EE). The EE strategy automatically detects and emphasizes onset cues and consequently reinforces the temporal structure of the speech envelope. Our results confirmed speech-in-noise perception difficulties by children with dyslexia. However, we found that exaggerating temporal “landmarks” of the speech envelope (i.e., amplitude rise time and modulations)—by using EE—passively and instantaneously improved speech perception in noise for children with dyslexia. Moreover, the benefit derived from EE was large enough to completely bridge the initial gap between children with dyslexia and their typical reading peers. Taken together, the beneficial outcome of EE suggests an important contribution of the temporal structure of the envelope to speech perception in noise difficulties in dyslexia, providing an interesting foundation for future intervention studies based on auditory and speech rhythm training.
Keywords: dyslexia, speech perception, speech in noise, envelope enhancement, speech perception improvement, phonological awareness, phonological processing, intervention strategies
INTRODUCTION
Dyslexia is a developmental disorder affecting the ability to fluently read, spell, and decode words, despite adequate educational opportunities, and otherwise normal cognitive and intellectual abilities (Vellutino et al. 2004). Although it is highly unlikely that there is a “one size fits all” theoretical account describing the reading and spelling deficits in dyslexia (Pennington 2006), the prevailing view acknowledges the causal role of a phonological processing deficit showing impaired phonological skills in the majority of individuals with dyslexia (Snowling 2000; Ramus et al. 2003; Vellutino et al. 2004).
Recent research hypothesized that these phonological problems might stem from poor speech perception (Ziegler et al. 2009; Boets et al. 2011; Vanvooren et al. 2017; Calcus et al. 2018a). Over the last decades, many studies investigated the relation between speech perception, phonological processing, and reading development (Brady et al. 1983; Joanisse et al. 2000; Hazan et al. 2009; Robertson et al. 2009; Ziegler et al. 2009; Messaoud-Galusi et al. 2011; Calcus et al. 2016). Yet, the results are rather mixed, and some studies failed to provide clear evidence for a speech perception deficit altogether (e.g., Hazan et al., 2009; Robertson et al. 2009). One possible explanation for the fragility of speech perception difficulties might be that speech perception has typically been evaluated under optimal listening conditions (i.e., quiet). As a result, subtle deficits might remain unnoticed, due to the high redundancy inherent to speech. Conversely, numerous studies have demonstrated speech perception impairments in more challenging listening conditions (i.e., background noise; for a review see Calcus et al. 2018b). Therefore, it is hypothesized that individuals with dyslexia fail to use important acoustic cues in the speech stream in order to encode and acquire adequate phonological representations (e.g., Poelmans et al. 2011; Calcus et al. 2018a; Van Hirtum et al. 2019b).
Rhythmic, dynamic cues embedded in the overall envelope of speech signals such as amplitude changes (modulations) and transients acoustic cues (rise times) are particularly important for effortless speech processing. Recent evidence suggests that impaired processing of those dynamic cues might lead to poor encoding of stress patterns and syllables, and in turn hinder adequate phonological development (i.e., temporal sampling theory; Goswami 2011). Various studies have indeed shown poor sensitivity by individuals with dyslexia to amplitude modulations and rise time, supporting the temporal sampling theory at a perceptual level (Lorenzi et al. 2000; Goswami et al. 2002; Witton et al. 2002; Talcott et al. 2003; Richardson et al. 2004; Leong et al. 2011; Poelmans et al. 2011) as well as at a neural level (Hämäläinen et al. 2011; Stefanics et al. 2011; Power et al. 2013; Lallier et al. 2018; Van Hirtum et al. 2019a).
In summary, a growing literature demonstrates poor (neural) processing of amplitude modulation and rise time in particular, which could affect the development of speech perception (e.g., Lallier et al. 2018; Goswami 2019). However, individuals with dyslexia do seem to benefit from various types of acoustic cues to improve their perception. Speech perception of dyslexics benefits from cues such as spatial lateralization or the type of background noise (Dole et al. 2012; Calcus et al. 2015, 2016) and adaptations to the speaker’s intonation (Hazan et al. 2013). Additionally, the potential beneficial effect of musical training on phonological skills and reading abilities is intensively evaluated. Results from various training programs designed to enhance the perception of speech rhythm of persons with dyslexia, using for example music, drumming, or tapping to poetry, not only improved auditory processing skills, but also had the potential to transfer from basic auditory skills to more advanced literacy skills (Bhide et al. 2013; Thomson et al. 2013; Flaugnacco et al. 2015). A critical issue, however, concerning the efficacy of auditory training programs is the duration necessary to promote substantial changes in reading or spelling (e.g., Rosario Ortiz González et al. 2002; Thomson et al. 2013). Additionally, intensive application of such training programs might not be feasible and very laborious for pre-readers, for with targeted intervention might be the most effective (Ozernov-Palchik and Gaab 2016). Hornickel et al. (2012) made a first attempt to tackle these challenges by investigating the use of assistive listening devices as implemented in classroom FM systems to improve auditory processing. Their results showed indeed that children with dyslexia who used this system throughout the school day improved significantly on phonological awareness and reading abilities. However, their system improved clarity of the acoustic signal by enhancing the signal-to-noise ratio (SNR) of the teacher’s voice without altering any dynamic features.
In this study, we introduce a potentially more ecologically valid method to benefit speech perception: envelope enhancement (EE; Geurts and Wouters 1999; Koning and Wouters 2012; Van Hirtum et al. 2019b). Originally, the EE algorithm was developed for cochlear implant users, which means that real-time implementation would be possible and the complexity of the algorithm is low. In contrast to the earlier work by Hornickel et al. (2012), the EE strategy emphasizes specific parts of the envelope. That is, transient acoustic cues such as amplitude rise times are automatically detected and amplified. According to the temporal sampling theory, poor processing of these specific acoustic cues constrain effortless speech encoding the most in individuals with dyslexia. Therefore, it is hypothesized that emphasizing these important acoustic cues would facilitate temporal segmentation and increase envelope tracking in the brain, which in turn would improve speech intelligibility (Gross et al. 2013; Doelling et al. 2014).
Recently, this EE strategy was applied by Van Hirtum et al. (2019b) who tested 21 adults with dyslexia and 21 typical readers using a speech-in-noise perception task including two different types of signals: (1) natural, unprocessed speech and (2) vocoded speech. Using vocoded speech, temporal fine structure and spectral information is significantly degraded, whereas the amplitude envelope is preserved. Contrasting both types of speech signals with different levels of temporal fine structure and spectral information was aimed at disentangling the nature of the speech perception deficit in dyslexia. That is, in addition to the framework proposed by Goswami (2011), an alternative hypothesis attributes dyslexics’ speech-in-noise difficulties to a general noise exclusion deficit (e.g., Sperling et al. 2005; Ziegler et al. 2009). This hypothesis implies that poor speech perception in dyslexia results from poor processing of degraded speech signals more generally rather than to poor auditory processing of envelope cues. In accordance with the temporal sampling theory (Goswami 2011), it was expected that adults with dyslexia would have difficulties in processing the dynamic cues of the envelope and thus perform worse than typical readers when listening to natural as well as vocoded speech. However, adults with dyslexia would not be disproportionately hindered by the degradation of the vocoded signal. Our results showed that, relative to typical readers, adults with dyslexia had speech perception deficits, irrespective of spectral resolution and limited fine structure, suggesting that the auditory deficit is temporal-specific. Moreover, not only did the majority of adults with dyslexia benefit from the EE implementation, but their benefit was larger than typical readers. This finding suggested that EE had the potential to compensate speech perception deficits in individuals with dyslexia under adverse listening conditions (Van Hirtum et al. 2019b).
This adult study provided a first answer on the nature of the speech perception deficit and the feasibility of the EE strategy to potentiality improve these difficulties directly; however, the impact of sensory and cognitive deficits may change over the course of development (Goswami 2015; Vandermosten et al. 2019). Previous research has demonstrated reading-induced changes in the speech perception network and the neural representation of phonemes (Dehaene et al. 2010; Bonte et al. 2017). In addition, it has been demonstrated that varying levels of top-down compensation processes influence the results of auditory processing tasks in adults as well (e.g., Stoodley et al. 2006; Law et al. 2014). Therefore, the first goal of this study was to investigate whether the same pattern of speech-in-noise perception deficits is found in school-aged children who are less experienced in reading, to disentangle and explore the respective contributions of developmental aspects (i.e., ~ age) and compensational mechanisms (i.e., ~ amount of schooling and remedial experience) respectively. Therefore, we aimed to replicate the speech perception-in-noise experiment by Van Hirtum et al. (2019b) in school-aged children. We were interested in finding out whether children with dyslexia would exhibit similar speech-in-noise deficits when listening to both types of speech material, comparable with adults with dyslexia, and compare and integrate the findings of both age groups. Additionally, we explored the effect of the duration of specialized reading remediation as a compensational mechanism on the speech perception deficit.
Furthermore, it was of major interest to investigate whether children’s speech perception would benefit from EE. Hence, the second and main goal of this study was to evaluate children’s susceptibility to the EE strategy. Beyond group comparisons, it is important to consider the heterogeneity of individual profiles when it comes to speech perception deficits and dyslexia. Therefore, we included a deviance analysis method (Ramus et al. 2003) to investigate the proportion of individuals performing below the norm and to identify individuals susceptible to EE. Altogether, these investigations may provide an important step leading to new auditory-based interventions, not solely based on explicit learning strategies. Considering that it is crucial to the well-being of children who experience the lifelong implications of dyslexia to receive targeted intervention as early as possible (Ozernov-Palchik and Gaab 2016), it is of utmost importance to evaluate the beneficial effect of EE in a younger population, who are less experienced in reading.
METHODS
Subjects
Sixty-three children, recruited from mainstream elementary schools, participated in this study. To qualify for the study, children had to be between 9 and 12 years old, attain adequate nonverbal intelligence (IQ score ≥ 80; WISC-IV; Wechsler 1991), have normal peripheral hearing (hearing thresholds ≤ 25 dB HL for frequencies from 0.5 to 4 kHz), and have no other neurological or developmental disorders (e.g., specific language impairment, attention-deficit/hyperactivity disorder, dyscalculia). All children were native Dutch speakers. In line with current practice in Belgium, children classified as having dyslexia were required to have a formal clinical diagnosis or document a history of severe reading problems and show objective signs of persistent literacy impairments. Literacy impairments were defined as (1) severe reading problems, implemented as a score below the 10th percentile on a standardized word-reading (Brus and Voeten 1979) or pseudo-word reading test (Van den Bos et al. 1994) or (2) severe spelling problems, implemented as a score below the 10th percentile on a standardized spelling test (Deloof 2006). Children with severe spelling problems were additionally required to demonstrate reading scores below the 16th percentile because dyslexia is mostly defined as a reading impairment. Children selected to participate in the control group were required to have a reading score within the normal range. In four children originally selected for the control group, we found a mismatch between reading history reported by the parents and the measured reading scores. These children were excluded from the study. Based on our criteria, 30 children were identified as dyslexic readers (DR), and 29 children were identified as typical readers (TR). Table 2 displays a summary of the subject characteristics and the cognitive profile of the two groups.
Table 2.
Speech-in-noise perception depicted as average SRTs and their precision values for typical readers (TR) and children with dyslexia (DR). Standard deviations in (). Min/max values in []
| TR | DR | |||
|---|---|---|---|---|
| SRT | Precision | SRT | Precision | |
| Natural speech | ||||
| No EE (dB) | − 8.20 (0.86) [− 6.40 to − 10.80] | 0.24 [0.16 to 0.36] | − 7.64 (0.92) [− 5.70 to − 9.43] | 0.28 [0.15 to 0.48] |
| EE (dB) | − 8.79 (0.88) [− 7.23 to − 10.45] | 0.29 [0.16 to 0.46] | − 8.13 (0.91) [− 5.29 to − 10.12] | 0.29 [0.16 to 0.43] |
| Vocoded speech | ||||
| No EE (dB) | 0.26 (2.17) [4.77 to − 2.95] | 0.36 [0.19 to 0.59] | 1.37 (2.51) [5.99 to − 2.30] | 0.41 [0.19 to 0.98] |
| EE (dB) | − 0.12 (2.16) [5.14 to − 2.61] | 0.37 [0.20 to 0.53] | 0.96 (2.56) [6.64 to − 2.29] | 0.39 [0.23 to 0.85] |
The study protocol was approved by the Medical Ethics Committee of the University Hospital of Leuven and all parents provided written informed consent. All children received two movie tickets to thank them for participating.
Cognitive Tasks
Phonological Tasks
Various aspects of phonological processing were tested individually.
Phonological awareness was assessed with a phoneme deletion test (De Vos et al. 2017). Every trial consisted of a pseudo-word with a CCVC or CVCC structure and a target phoneme that had to be omitted. Depending on the trial, deletion of the first or second phoneme of a pseudo-word with onset consonant cluster (CCVC) or deletion of the penultimate or last phoneme of a pseudo-word with offset consonant cluster (CVCC) was required. Performance was scored as the number of correct responses (max 24).
Lexical retrieval speed was assessed through four different rapid automatic naming (RAN) tasks: objects, colors, digits, and letters (Boets et al. 2007; De Vos et al. 2017; Vanvooren et al. 2017). For all four tasks, children were instructed to name the 50 stimuli (10 times 5 different items randomly ordered) distributed over five columns from top to bottom as quickly and accurately as possible. Both speed and accuracy are taken into account in the outcome measure (# correct/second).
Verbal short-term memory (VSTM) was assessed by the digit span forward subtest from the Clinical Evaluation of Language Fundamentals 4th edition (CELF-4-NL; Kort et al. 2010). Children were instructed to recall orally presented sequences of digits, increasing in length. Performance is measured as the number of correctly recalled series of digits. In addition the digit span backward was administered to additionally explore working memory abilities. Here, the children were instructed to repeat a sequence of digits in reversed order. Note, however, that since digit span backward measures both verbal span, recruiting phonological abilities, and the additional demand of recruitment of executive processes (e.g., Baddeley 2012), it was included as a measure related to general cognitive functioning (i.e., working memory) rather than phonological processing.
Language
Children’s language abilities were screened by the Formulated Sentences subtest of the CELF-4-NL. Using given words, children are required to formulate complete, semantically and grammatically correct sentences that match a given illustration. This particular subtest was chosen based on the similarities with the speech perception task in terms of language complexity and memory load.
Performance on all cognitive tasks (phonology, working memory, and language) for TR versus DR groups is presented in Table 1. Differences between both groups are assessed using Welch’s t-tests. Effect sizes were calculated using Pearson’s r. Children with dyslexia showed strong deficits in phonological processing tasks, especially in phonological awareness (i.e., phoneme deletion) and lexical retrieval (i.e., RAN). Language (p = 0.008) and working memory outcomes (p = 0.006) were significantly lower for children with dyslexia, although all children performed well within age norms.
Table 1.
Participant characteristics and mean scores of the various literacy and cognitive tasks for typical reading children (TR) and children with dyslexia (DR). Standard deviations in ()
| TR | DR | |||
|---|---|---|---|---|
| M (SD) | M (SD) | p | r† | |
| Chronological age | 10.11 (0.11) | 10.6 (0.10) | 0.039 | 0.269 |
| Sex (M/F) | 13/16 | 13/17 | ||
| Non-verbal IQ‡ | 113.10 (14.11) | 107.33 (15.30) | 0.137 | 0.196 |
| Language | 31.62 (4.04) | 28.77 (3.89) | 0.008 | 0.344 |
| Literacy | ||||
| Word reading (# correct/min) | 77.10 (11.98) | 44.63 (8.68) | < 0.001 | 0.857 |
| Pseudo-word reading (# correct/2 min) | 75.76 (17.80) | 36.73 (9.59) | < 0.001 | 0.848 |
| Spelling (# correct) | 24.31 (2.89) | 14.79 (4.55) | < 0.001 | 0.810 |
| Phoneme deletion (# correct) | 22.00 (2.00) | 20.00 (3.00) | 0.001 | 0.420 |
| RAN (# correct/s) | ||||
| Objects | 1.37 (0.28) | 1.06 (0.28) | < 0.001 | 0.489 |
| Colors | 1.45 (0.29) | 1.12 (0.23) | < 0.001 | 0.543 |
| Digits | 2.25 (0.54) | 1.68 (0.35) | < 0.001 | 0.567 |
| Letters | 2.23 (0.58) | 1.62 (0.34) | < 0.001 | 0.591 |
| Digit span (# correct) | ||||
| Forward | 7.90 (1.37) | 7.30 (1.29) | 0.091 | 0.223 |
| Backward | 5.00 (2.00) | 4.00 (1.00) | 0.006 | 0.361 |
Chronological age is expressed in years.months. For phoneme deletion and digit span backward (i.e., non-normally distributed data), the median and interquartile ranges are presented and group comparisons were carried out using Mann-Whitney U-tests instead of Welch’s t-tests
Effect sizes based on non-parametric statistics were calculated using:
†Effect sizes (Pearson’s r) were calculated using:
‡Standardized IQ test (M = 100, SD = 15)
Speech-in-Noise Perception Task
Signal Processing
All signal processing was performed using Matlab R2013b (The MathWorks Inc 2013).
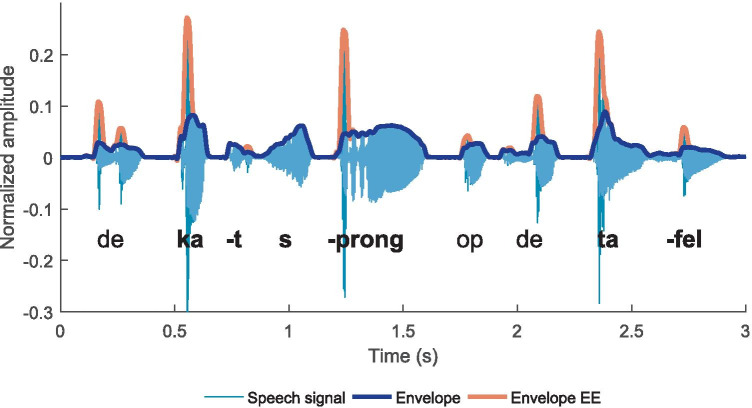
The experimental design, stimuli, and signal processing are identical to those reported in the adult study by Van Hirtum et al. (2019b). Children’s speech perception was evaluated in four experimental conditions: natural speech, vocoded speech, and their envelope-enhanced (EE) counterparts (see Fig. 1).
Fig. 1.
Waveform of one example sentence (i.e., the cat jumped on the table). The envelope of the speech signal without envelope enhancement (EE) is depicted in dark blue, whereas the envelope of the enhanced signal is shown in red. Keywords are highlighted in bold
In the vocoded condition, sentences were band-pass filtered using, fourth-order Butterworth filters into 8 frequency bands covering 187.5–7937.5 Hz. Technical details and cutoff frequencies can be found in Van Hirtum et al. (2019b). In each band, the envelope was extracted and used to modulate a broadband noise carrier with the same frequency characteristics as those of the initial band. The eight bands were then level matched to their original in-band input and summed over all frequency bands.
The EE strategy was identical to the strategy introduced in Van Hirtum et al. (2019b) and originally adapted from Koning and Wouters (2012) and Geurts and Wouters (1999). The input signal was samples with a rate of 16 kHz and split into frames of 128 samples and a frame advance of 32 samples. The signal was transformed into the frequency domain by computing the Fast Fourier Transform. The analysis window is a Hann analysis window. The envelope of each channel was extracted using a weighted summation of the power of its respective frequency bins. For each channel, the frequency limits were defined according to the critical bandwidths of Fastl and Zwicker (Fastl and Zwicker 2007) up to . Then, the slow envelope is obtained by filtering using a fourth order Butterworth filter with a cutoff frequency of 20 Hz. Due to this low cutoff frequency, F0 is removed. Additionally, this increases the time delay of when reacting to sudden increases in . was amplified by a factor = 8 to ensure that its level is higher than that of at quasi-stationary parts. At a sudden increases in energy (i.e., an onset), lies above and the peak signal was extracted by subtracting the amplified from . Then, half-wave rectification was required to ensure that no negative values (which occur in speech parts with stationary levels) are present. The half-wave rectified had values different from zero at onsets of . The peak signal was amplified by a factor = 18 to obtain the final signal . The enhanced envelope was obtained by adding and together. Finally, the time signals and were obtained by computing the inverse Fast Fourier Transform using a Hann synthesis window.
To enhance the signals in the vocoded conditions, was computed from the unprocessed signal (i.e., natural condition) and added to vocoded signals using broadband summation.
Test Materials and Procedure
Speech-in-noise perception was assessed by the Leuven Intelligibility Sentence Test (Van Wieringen and Wouters 2008). This test consists of 35 lists, each containing 10 semantically correct Dutch sentences spoken by a female speaker with 32 or 33 keywords. Keyword percent scores were counted to calculate the percentage-correct score. Therefore, children were instructed to repeat as many words of each sentence, even if it would result in an incorrect or incomplete sentence. All sentences were presented in a stationary speech-weighted (vocoded) noise at varying signal-to-noise ratios (SNR). The level of the noise was fixed at 65 dB SPL, and the speech levels was adapted to get the desired SNR in the same way as the speech material was standardized. Sentences were presented to the subjects at four fixed SNRs in 2 dB steps from − 3 to − 9 dB SNR for natural speech and + 5 to − 1 dB SNR for vocoded speech. All children received at least three training lists for both natural (silence, − 1 and − 3 dB SNR) and vocoded speech (silence, + 7 and + 5 dB SNR). In total, each child listened to at least 220 sentences (= 30 training sentences/condition + 10 sentences/condition × 4 SNR conditions). One list of 10 sentences was used per SNR. An additional list was presented (− 2 dB SNR) if no score below 50 % was obtained with the four fixed SNRs. Contrary, an additional list was presented (+ 2 dB SNR) if no score above 50 % was obtained as well in order to be able to reliably fit psychometric curves for each child individually. Note that, for three children (two controls and one with dyslexia), the vocoded speech condition resulted in a maximum score close to 50 % even after extended training. These results were excluded from further analyses. Natural and vocoded speech tests were administered in separate sessions of about 1 h each. The presentation order between sessions (natural versus vocoded) and within sessions (no EE versus EE) was randomized across children, and sentences were not repeated across conditions to limit learning effects.
Sentences were presented monaurally to the right ear over calibrated HDA-200 headphones using the software platform APEX (Francart et al. 2008). Right ear stimulation was chosen to allow for comparison with our previous work in children (Poelmans et al. 2011; Vanvooren et al. 2017) as well as adults (Law et al. 2014; Van Hirtum et al. 2019b). Additionally, previous research has shown that the postulated speech deficits emerge more clearly using a monaural configuration (Dole et al. 2012, 2014).
Statistical Analysis
All statistical analyses were carried out in R (version 3.5.3) (R Core Team 2015). The speech reception threshold (SRT; SNR corresponding to a 50 % percentage-correct score) was estimated for each child individually by fitting psychometric curves with a cumulative Gaussian function. Likewise, the precision on the SRT was calculated as the standard error along the x-axis (dB) (MASS package; Venables and Ripley 2002). After determining individual fits and precision values for each subject, the average SRTs were calculated as the arithmetic average of all individual SRTs. The overall precision was computed from the quadratic average of all error bars of the fit to the data of each individual.
For some children, it was not possible to get a reliable SRT estimate. Two children with dyslexia did not complete the vocoded speech condition. One child from the control group had a mild hearing loss during the natural speech condition. Consequently, N = 58 for analyses concerning natural speech and N = 54 for analyses concerning vocoded speech.
Our main analysis concerned the effect of reading group (TR vs DR) and the potential benefit of EE on the SRT. Linear mixed-effect models (LME) using maximum likelihood criteria were fitted with the lme4 package (Bates et al. 2015) to the SRT of each speech condition (natural and vocoded) separately. Group and EE were entered as fixed effects in the model, as well as by-subject random intercepts to account for dependencies between measures from the same child. In addition, children’s current grade was entered in the model to account for potential effects due to both age and school experience. Language and working memory scores were added as separate predictors to assess the relation between speech perception outcomes and these potential confounding factors. All interactions between predictors were investigated. Models with an interaction term between two or more fixed effects were fitted, however, were always insignificant (fitted using maximum likelihood). Insignificant interactions were therefore dropped from further analysis in the final models. Inspection of the assumptions of normality, homoscedasticity, and independence of the model’s residuals did not reveal any violations. Significant main effects are discussed in the results section by reporting the beta estimates for all fixed effects, with corresponding standard error (SE), degrees of freedom (df), corresponding test statistics (t-value), and p value.
Finally, we examined if the benefit that children with dyslexia obtained from the EE strategy was substantial to reach baseline performance of typical readers (i.e., SRTs without EE). Therefore, planned post hoc comparisons were performed using Welch’s t-tests (DR-EE versus TR-noEE).
RESULTS
Deficits in Speech Perception and the Effect of Envelope Enhancement
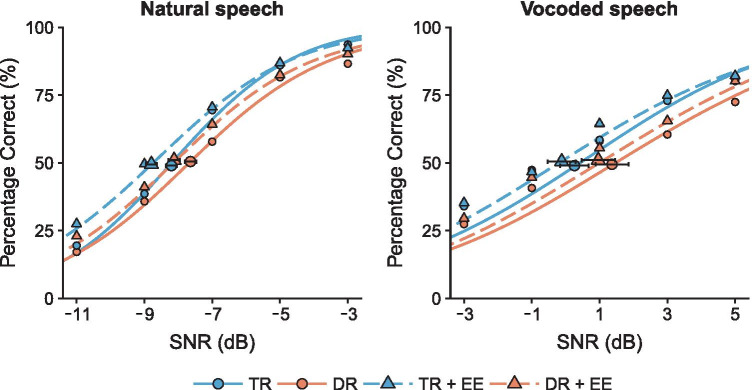
Table 2 shows speech perception performance as mean SRTs separately for each group and speech condition. Full psychometric functions of the group averages are illustrated in Fig. 2.
Fig. 2.
Psychometric functions fitted with a cumulative Gaussian distribution from the key word recognition scores as a function of SNR for each type of sentences. Blue circles illustrate the average key word recognition score for typical reading children (TR) and orange circles for children with dyslexia (DR) without EE, whereas blue and orange triangles indicate average performance with EE for TR and DR, respectively. Estimated speech reception thresholds are shown with their respective standard errors for each condition
For natural speech, SRT was significantly predicted by both grade (b = − 0.33, SE = 0.13, p = 0.014) and group (b = 0.55, SE = 0.21, p = 0.016; Table 3). There was no significant grade-by-group interaction (p > 0.05). This suggests that all children’s performance improved with age; however, children with dyslexia had more difficulty with speech-in-noise perception compared with typical reading peers, irrespective of their age. On average, SRTs were 0.55 dB worse for children with dyslexia. No significant effects of language (p = 0.486) or working memory (p = 0.583) were found. This indicates that although these skills are related to children’s reading outcomes, they do not influence speech perception outcomes found in this study.
Table 3.
Linear mixed-effect model: effect of language, digit span (backward), grade envelope enhancement (EE) and group on speech perception
| Fixed effect terms | Estimate | SE | df | t value | p |
|---|---|---|---|---|---|
| Natural speech | |||||
| (Intercept) | − 8.333 | 0.826 | 58 | − 10.093 | < 0.001 |
| Language | 0.018 | 0.026 | 58 | 0.701 | 0.486 |
| Backward | 0.042 | 0.077 | 58 | 0.551 | 0.584 |
| Grade | − 0.331 | 0.131 | 58 | − 2.528 | 0.014 |
| EE (with) | − 0.535 | 0.107 | 58 | − 4.982 | < 0.001 |
| Group (DR) | 0.545 | 0.219 | 58 | 2.481 | 0.016 |
| Vocoded speech | |||||
| (Intercept) | 2.023 | 2.326 | 54.10 | 0.869 | 0.388 |
| Language | 0.049 | 0.076 | 54 | 0.647 | 0.520 |
| Backward | − 0.099 | 0.218 | 54 | − 0.453 | 0.652 |
| Grade | − 1.248 | 0.373 | 54 | − 3.341 | 0.002 |
| EE (with) | − 0.389 | 0.143 | 54 | − 2.726 | 0.009 |
| Group (DR) | 0.427 | 0.634 | 54 | 0.673 | 0.504 |
Moreover, there was a main effect of EE (b = − 0.53, SE = 0.11, p < 0.001). This indicates that speech performance improved significantly after implementation of the EE algorithm (~ 0.54 dB) (Table 3). Moreover, post hoc comparisons using Welch’s t-tests showed that there was no significant difference (t(55.99) = − 0.33, p = 0.74, r = 0.045) between children with dyslexia provided with EE compared with typical readers (without EE), suggesting that the benefit derived from EE is large enough to bridge the initial gap between both groups.
Next, the model fitted on the outcomes of the vocoded speech condition revealed a significant main effect of grade (b = − 1.25, SE = 0.37, p < 0.01; Table 3). SRTs of children with dyslexia were on average 1.16 dB worse compared with typical readers; however, this effect did not reach significance (p > 0.05). Compared with the natural speech condition, there was a considerable amount of inter-subject variability (see Table 2). Additionally, a one-tailed Fisher’s F test confirmed that inter-subject variability observed in the vocoded condition was significantly higher than in the natural speech condition (F53,57 = 7.712, p < 0.001). However, implementation of the EE algorithms still provided a significant benefit to the SRT outcomes (b = − 0.39, SE = 0.14, p < 0.01), despite the increase in inter-subject variability. Finally, the model yielded no significant effect of language (p = 0.52) or working memory (p = 0.652).
In addition, the data of the present study were integrated with the previously obtained adult data (Van Hirtum et al. 2019b) to investigate child–adult differences for speech-in-noise perception abilities and construct a more comprehensive developmental framework. This integrated analysis consisted of an additional model with age band (children vs adults), group (DR vs TR), condition (natural vs vocoder), and EE (no EE vs EE) as fixed effects, as well as by-subject random intercepts. As expected, a significant difference in overall performance was found between children and adults (b = − 1.87, SE = 0.24, p < 0.001) showing that speech-in-noise perception abilities further improved after primary school. However, a significant age band-by-condition interaction was found (b = − 1.63, SE = 0.22, p < 0.001). Post hoc comparisons revealed a significant improvement from childhood to adulthood for both the natural (t(93.44) = 10.35, p < 0.001) and vocoded speech conditions (t(77.55) = 9.12, p < 0.001); however, this developmental effect was significantly larger for the vocoded one. No other interaction effects reached significance (all p > 0.05), indicating that subjects with dyslexia continuously perform worse compared with their typical reading peers, and EE improves speech perception abilities to a similar extent for both children and adults.
Deficits in Speech Perception and the Effect of Remedial Experience
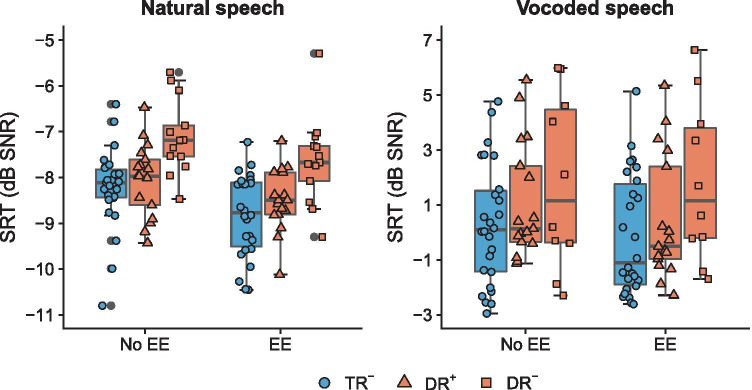
Children with dyslexia in Flanders (the Dutch speaking part of Belgium) are typically diagnosed between the ages of 9 and 11, since persistency of the reading deficits must be demonstrated first. Therefore, not all children with dyslexia in our sample received the same amount of remediation, i.e., specialized reading therapy, in addition to their mainstream educational trajectory. This specialized reading therapy comprises individual sessions with a speech and language pathologist, aimed at increasing reading ability (word recognition) and compensating for a low level of technical reading skills. Prior evidence (e.g., Horlyck et al. 2012) has shown that increased experience with the alphabetic principle can assist in the fine-tuning of phoneme categories and in turn boost speech perception. Therefore, increased experience with reading (i.e., specialized reading therapy) might act as a compensational mechanism effecting the speech perception deficits found in children with dyslexia and further exploratory analyses examined these therapy-related influences (see Fig. 3).
Fig. 3.
Speech-in-noise perception depicted as average SRTs for typical readers (TR−) and children with dyslexia with (DR+) and without (DR−) extensive amounts of specialized reading therapy. Individual data points in each boxplot represent individual participants, to show variation. Lines indicate the median value, and whiskers are 1.5× interquartile range
Firstly, we classified children with dyslexia into subgroups based on the amount of remedial experience: DR− (< 6 months of therapy, N = 13), DR+ (> 6 months of therapy, N = 17). Typical readers were labeled as TR−. Six months was set as the cutoff based on current practice in Belgium on the persistency of reading deficits, and there appeared to be no differences in speech perception abilities between the amounts of specialized reading therapy received after at least 6 months. Secondly, a model was constructed with SRT as the dependent variable and grade, group (including DR− and DR+), and EE as fixed effects. Language and working memory scores were again added as separate predictors. With regard to natural speech perception, results showed that DR− children had significantly worse SRTs compared with the TR− group (b = 1.02, SE = 0.27, p < 0.001; Table 4). Strikingly, children with dyslexia who had received at least 6 months of specialized therapy (DR+) only had slightly worse SRTs (i.e., 0.27 dB) compared with typical reading peers and their performance did not differ significantly (p > 0.05). This suggest that there is a significant effect of reading therapy, indicating increasingly better SRTs for children who had received extensive reading therapy. The main effect of EE (b = − 0.54, SE = 0.11, p < 0.001) remained unaltered indicating that EE implementation improved SRT outcomes significantly.
Table 4.
Linear mixed-effect model: effect of language, digit span (backward), grade envelope enhancement (EE), and remedial experience (group) on speech perception
| Fixed effect terms | Estimate | SE | df | t value | p |
|---|---|---|---|---|---|
| Natural speech | |||||
| (Intercept) | − 8.46 | 0.784 | 56.54 | − 10.785 | < 0.001 |
| Language | 0.019 | 0.025 | 56 | 0.739 | 0.463 |
| Backward | 0.024 | 0.074 | 56 | 0.328 | 0.744 |
| Grade | − 0.242 | 0.134 | 56 | − 1.801 | 0.077 |
| EE (with) | − 0.535 | 0.109 | 56 | − 4.903 | < 0.001 |
| Group (DR−) | 1.024 | 0.268 | 56 | 3.820 | < 0.001 |
| Group (DR+) | 0.267 | 0.238 | 56 | 1.124 | 0.266 |
| Vocoded speech | |||||
| (Intercept) | 1.981 | 2.349 | 53.10 | 0.843 | 0.403 |
| Language | 0.049 | 0.076 | 53 | 0.637 | 0.527 |
| Backward | − 0.104 | 0.220 | 53 | − 0.472 | 0.639 |
| Grade | − 1.213 | 0.395 | 53 | − 3.067 | 0.003 |
| EE (with) | − 0.402 | 0.145 | 53 | − 2.775 | 0.008 |
| Group (DR−) | 0.675 | 0.856 | 53 | 0.434 | 0.434 |
| Group (DR+) | 0.318 | 0.704 | 53 | 0.452 | 0.653 |
Again, the analyses were repeated for the vocoded speech data leading to similar outcomes. SRTs were on average 0.68 dB worse for DR− and 0.32 dB worse for children in the DR+ group compared with the TR− group; however, these effects did not reach significance (p > 0.05). Again, the EE effect (b = − 0.40, SE = 0.15, p < 0.001) remained significant.
This suggests that reading and speech perception may indeed be bi-directionally related (Horlyck et al. 2012), where extensive and specialized reading and spelling therapy seem to affect perception, over and above the aspect of school experience. It is, however, noteworthy that there are no differences in reading and spelling score or any literacy-related measures between both DR subgroups (see Table 5).
Table 5.
Participant characteristics and mean scores of the various literacy and cognitive tasks for typical reading children (TR−), children with dyslexia with more than 6 months of speech therapy (DR+) and children with dyslexia with less than 6 months of speech therapy (DR−). Standard deviations are shown in parentheses
| M (SD) | r† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TR− | DR+ | DR− | TR−-DR+ | TR−-DR− | DR+-DR− | |||||||
| Chronological age | 10.11 (0.11) | 10.71 (0.89) | 10.23 (0.89) | 0.246 | 0.48** | 0.276 | ||||||
| Years of remedial experience | 0 (0) | 1.82 (0.58) | 0.04 (0.14) | 0.955*** | 0.277 | 0.943*** | ||||||
| Sex (M/F) | 12/15 | 7/10 | 9/4 | |||||||||
| Non-verbal IQ‡ | 113.52 (14.53) | 109.71 (13.28) | 104.23 (17.66) | 0.146 | 0.345 | 0.197 | ||||||
| Language | 31.56 (4.16) | 29.00 (4.11) | 28.46 (3.73) | 0.322 | 0.418* | 0.072 | ||||||
| Literacy | ||||||||||||
| EMT (# correct/min) | 77.59 (12.19) | 45.76 (9.13) | 43.15 (8.16) | 0.840*** | 0.876*** | 0.156 | ||||||
| Klepel (# correct/2 min) | 76.59 (17.74) | 35.41 (9.87) | 38.46 (9.30) | 0.837*** | 0.824*** | 0.165 | ||||||
| Spelling (# correct) | 24.56 (2.79) | 15.41 (4.12) | 13.92 (5.14) | 0.848*** | 0.874*** | 0.182 | ||||||
| Phoneme deletion (# correct) | 22.00 (2.00) | 20.00 (3.00) | 19.50 (2.25) | − 0.495** | − 0.370* | − 0.135 | ||||||
| RAN (# correct/s) | ||||||||||||
| Objects | 1.37 (0.29) | 1.15 (0.31) | 0.94 (0.18) | 0.382* | 0.700*** | 0.398* | ||||||
| Colors | 1.45 (0.30) | 1.13 (0.24) | 1.12 (0.22) | 0.532*** | 0.587*** | 0.019 | ||||||
| Digits | 2.45 (0.55) | 1.73 (0.39) | 1.60 (0.30) | 0.492*** | 0.619*** | 0.188 | ||||||
| Letters | 2.67 (0.59) | 1.59 (0.34) | 1.66 (0.35) | 0.596*** | 0.571*** | 0.102 | ||||||
| Digit span (# correct) | ||||||||||||
| Forward | 7.93 (1.41) | 7.12 (1.05) | 7.54 (1.56) | 0.322* | 0.160 | 0.184 | ||||||
| Backward | 6.00 (2.00) | 4.00 (3.00) | 4.00 (1.00) | − 0.354* | − 0.378* | − 0.044 | ||||||
Chronological age is expressed in years.months. For phoneme deletion and digit span backward (i.e., non-normally distributed data) the median and interquartile ranges are presented and group comparisons were carried out using Mann-Whitney U-tests instead of independent t-tests
Effect sizes based on non-parametric statistics were calculated using:
*p < 0.05; ** p < 0.01; *** p < 0.001
†Effect sizes (Pearson’s r) were calculated using:
‡Standardized IQ test (M = 100, SD = 15)
Relation Between Speech Perception and Literacy-Related Measures
The relation between speech perception and literacy-related measures was assessed by calculating partial correlations between SRTs in each condition (natural and vocoded) and literacy and phonological abilities in children with dyslexia, while using age and language as controlling variables (see Table 6). All raw scores were converted to z-scores, and composite scores were calculated for reading (word and pseudo-word reading) and RAN (all 4 subtasks). There was a significant partial correlation between natural speech and RAN (r = 0.43, p = 0.001), indicating that children with worse lexical retrieval had worse SRTs. Furthermore, there was a significant negative partial correlation between the absolute benefit from EE (i.e., the difference between the SRT without and with EE) and RAN (r = − 0.37, p = 0.007) showing that children with dyslexia with lower lexical retrieval scores had larger improvements after EE. For vocoded speech, speech performance was significantly correlated with non-verbal IQ (r = 0.43, p = 0.001) and with spelling scores (r = 0.41, p = 0.003). However, non-verbal IQ and spelling abilities were strongly correlated with each other (r = 0.65, p < 0.001), and therefore, these relations seem to reflect the developmental delays observed for vocoded speech perception. Note that, all reported p-values remain significant after correcting for multiple comparisons using a Bonferroni correction.
Table 6.
Partial correlations between speech perception outcomes and literacy-related measures for children with dyslexia
| Literacy measures | SRT natural speech | SRT vocoded speech |
|---|---|---|
| Non-verbal IQ | 0.07 | 0.43** |
| Reading | − 0.09 | 0.06 |
| Spelling | − 0.21 | 0.41** |
| Phoneme deletion | − 0.12 | − 0.15 |
| RAN | 0.43** | 0.11 |
| VSTM | − 0.07 | − 0.27 |
All correlations are calculated using z-scores and partialled out for individual differences in age and language outcomes
*p < 0.05; **p < 0.01; ***p < 0.001
Analysis of Individual Deviance Profiles
Lastly, it is important to consider the proportion of individuals performing below the norm in each group because most studies showed that only a subset of persons with dyslexia are indeed consistently impaired across all auditory and phonological tasks (Ramus et al. 2003; Messaoud-Galusi et al. 2011; Calcus et al. 2018a; Van Hirtum et al. 2019b). In addition, investigating individual profiles would allow us to identify individuals with specific speech perception deficits with and without EE implementation and, thus, whether children with dyslexia would approach normal speech perception skills at the individual level as well as at group level.
Children demonstrating deviant performance were identified using a two-step procedure as described by Ramus et al. (2003). For each task, grade-corrected z-scores based on the mean and standard deviation of the TR group were analyzed using a threshold of − 1.65 SD. Individuals from the TR group performing below this threshold were identified, and the control mean and standard deviation were recomputed excluding these subjects. The number and proportion of children showing deviant performance (i.e., z-score below threshold of − 1.65 SD) in each group and each task is presented in Table 7. For all literacy-related measures, with the exception of VSTM, a significantly higher proportion of children with deviant performance were found in the DR group compared with the TR group. Overall, 57 % of the children with dyslexia did not perform within the norm on one of the speech conditions and were described as poor perceivers. For speech perception tasks, more than four times as many children with dyslexia than typical readers were identified as poor perceivers for the natural speech condition (46 % vs. 10.7 %). Twice as many children with dyslexia compared with typical readers had specific speech perception deficits when listening to vocoded speech (30 % vs. 15 %).
Table 7.
Number and proportion of children with deviant performance in each group
| Measure | TR | DR | χ² | ||
|---|---|---|---|---|---|
| n | % | n | % | TR vs DR | |
| Literacy | 2 | 6.90 | 30 | 100 | 51.50*** |
| PA | 3 | 10.34 | 14 | 48.28 | 10.07** |
| RAN | 1 | 3.45 | 13 | 44.83 | 13.56*** |
| VSTM | 0 | 0 | 3 | 10 | 3.06 |
| LIST | 3 | 10.71 | 14 | 46.67 | 11.18*** |
| LIST - EE | 9 | 30.00 | 4.33* | ||
| LIST vocoded | 4 | 14.81 | 8 | 29.63 | 2.05 |
| LIST vocoded - EE | 7 | 25.93 | 2.05 | ||
No individual deviances were calculated for TR with EE (gray boxes) since z-scores of TR without EE were used as the baseline to identify children with dyslexia with specific speech perception deficits before and after EE implementation
*p < 0.05; **p < 0.01; ***p < 0.001
Interestingly, 100 % of the poor perceivers with dyslexia were also poor in reading and spelling and around 65 % had poor phonological abilities (i.e., performed below the norm on at least one phonological processing task). Among the poor perceivers of the TR group, none were poor in reading and only one had poor phonological abilities. Nevertheless, it is noteworthy that three children from the DR group (10 %) performed within norms on all tasks requiring auditory and phonological abilities, whereas some of the typical reading children qualified as poor perceivers (24 %) and showed phonological deficits (10 %) without having reading impairments. This suggests that reading impairments seem entirely unaffected by any sensory or phonological disorders for some children, and pure auditory and phonological deficits are not sufficient to cause reading impairments either.
However, in those cases where specific speech perception deficits are apparent, EE does work as a valid tool to improve and even eliminates such speech perception deficits. That is, among the subgroup of poor perceivers, all but one child (94 %) had a clear and instantaneous benefit from the EE algorithm.
DISCUSSION
Dyslexia is typically characterized by impaired phonological processing skills. Recent studies hypothesized that these phonological difficulties might be a consequence of speech perception deficits given the impact of poor speech perception on the development of well-specified and readily accessible phonological representations and the process of learning grapheme-phoneme mappings (Calcus et al., 2018a; Ziegler et al. 2009). This learning process becomes even more stressed in noisy environments. The primary goal of this study was not only to examine the presence of speech-in-noise perception deficits but also to assess whether we can improve children’s inadequate speech perception. Therefore, we implemented an envelope enhancement (EE) strategy, a signal processing algorithm which automatically detects and exaggerates difficult parts of the speech signal (i.e., amplitude rise time).
In the present study, speech perception of natural speech was compared with vocoded speech in order to assess children’s abilities to specifically use the temporal envelope in their speech perception. Using vocoded speech, spectral information and temporal fine structure were restricted, which makes the signal inherently noisier. Hereby, we investigated the extent to which speech-in-noise perception deficits in dyslexia could be attributed to faulty amplitude envelope processing rather than poor noise exclusion in general. Additionally, working memory and language were evaluated to rule out an underlying, more basic contribution to the speech-in-noise perception deficit (Banai and Ahissar 2004). Our results showed robust speech perception deficits in children with dyslexia when listening to natural speech in noise, in line with previous studies (Brady et al. 1983; Bradlow et al. 2003; Ziegler et al. 2009; Poelmans et al. 2011; Dole et al. 2012; Calcus et al. 2018a). Yet, no significant speech perception deficit was obtained for the vocoded condition. However, previous findings in adults using an almost identical paradigm (Van Hirtum et al. 2019b) showed clear deficits in the perception of both natural and vocoded speech. Moreover, adults with dyslexia were not disproportionately hindered by the vocoded speech suggesting an underlying amplitude envelope processing deficit in favor of the temporal sampling theory (Goswami, 2011). The different findings, concerning vocoded speech between the current results in children compared with previous work in adults (Van Hirtum et al. 2019b), suggest that school-aged children might be more sensitive to sensory distortion in general, irrespective of reading difficulties. Although in many studies, on a wide range of speech-in-noise measures, mature performance has been reported by about 9–10 years of age (for a review, Leibold, 2017), speech-in-noise perception of vocoded sentences seems to mature at different rates, and higher-level processing (i.e., cognitive and linguistic factors) appears to have a much larger impact on perceptual outcomes. Our findings indeed support the idea of slower maturation of vocoded speech, showing a clear developmental effect, with younger children performing significantly worse and improving with age irrespective of reading level. However, this improvement was significantly larger for vocoded sentences (b = − 1.21) compared with natural sentences (b = − 0.30). Therefore, it seems unlikely that poor perception in the current study stems from general noise exclusion or even a spectral processing disorder. A finding which is in line with previous speech perception studies supporting preserved spectro-temporal processing in children with dyslexia by showing intact masking release from stationary to fluctuation background noise (e.g., Calcus et al. 2018a, b; Ziegler et al. 2009). In addition our results demonstrate that neither decreased language abilities nor impaired working memory alone can explain the emergence of speech perception deficits in children with dyslexia.
The ability to benefit from EE was investigated in children with dyslexia by presenting speech both with and without EE. Results showed that speech-in-noise perception improved significantly after implementation of the EE algorithm whether they were listening to natural or vocoded sentences. The current results are in line with previous work in adults (Van Hirtum et al. 2019b) and confirm the validity and robustness of the paradigm despite the increased inter-subject variability measured in the vocoded speech condition. Moreover, using EE, we were able to bring performance closer to normal functioning and for natural speech processing, the benefit derived from EE was large enough to completely bridge the initial gap between children with dyslexia and their typical reading peers.
To our knowledge, the present findings provide the first evidence of an ecological valid paradigm to improve perceptual abilities in school-aged children with dyslexia and which elevate performance to levels no longer different than typical reading peers. The fundamental importance of these findings lies in the fact that these results represent passive and instantaneous effects, meaning that no active or explicit learning is required. Children were only exposed to the EE processed sentences for a limited amount of time (i.e., one list of 10 sentences for each SNR), leading to the majority of children to benefit instantaneously from EE independent of the speech material (~ 76 %). Moreover, among the poor perceivers, 94 % had a clear improvement in speech perception after EE implementation, suggesting that for children with dyslexia with poor sensory sensitivity, amplifying rise times seems particularly beneficial (Goswami, 2011, 2015). Moreover, as EE might be incorporated as a training program to facilitate phonetic discrimination (Cheng et al. 2019), we hypothesize that repeated, longer-term exposure to EE speech might lead to stronger effects on individual’s perceptual abilities (Rosario Ortiz González et al. 2002; Thomson and Leong 2013; Flaugnacco et al. 2015).
Analyzing individual profiles revealed inconsistent deficits, not necessarily associated with reading impairments. Consequently, EE might not be effective for all individuals with dyslexia. Around 46 % of the children with dyslexia exhibited difficulties with natural speech performance, which is largely in line with previous studies investigating individual deviance profiles in children (Calcus et al. 2018a) as well as adults (Ramus et al. 2003; Hazan et al. 2009). Yet, among this subgroup of poor perceivers with dyslexia, most of them were also poor in reading and/or spelling and had poor phonological abilities, whereas this was not true for typical reading children who qualified as poor perceivers. Altogether, these findings seem to rule out a speech perception deficit that is inherently associated to dyslexia (Hazan et al. 2009; Robertson et al. 2009; Messaoud-Galusi et al. 2011; Calcus et al. 2016), however, do support the idea that sensory impairments and reading difficulties tend to co-occur in dyslexia.
Nevertheless, much debate remains concerning the direction of the respective influence of speech perception deficits. It is a well-established finding that reading acquisition itself drives phoneme awareness and impacts the quality of phonological representations, particularly in alphabetic orthographies (Bradley and Bryant 1983; Read et al. 1986; Kirtley et al. 1989; Horlyck et al. 2012). Therefore, reduced reading experience itself might cause perceptual impairments in dyslexia (Goswami, 2015). However, previous studies have found speech perception deficits when comparing dyslexics’ performance to younger reading level-matched children (Calcus et al. 2018a; Ziegler et al. 2009). In addition, sensory processing deficits such as rise time impairments have been reported in infants with (a family risk of) dyslexia at about 7 months of age (Kalashnikova et al. 2016). Altogether, this suggests that auditory processing and speech perception in noise deficits specifically are not simply the consequence of the reduced reading experience. Nevertheless, our exploratory analyses showed at least some evidence concerning a bidirectional connection between reading and speech perception (Horlyck et al. 2012). Interestingly, speech performance was influenced by remedial duration, over and above school experience. Consequently, speech perception deficits were only observable in children with dyslexia that received less than 6 months of specialized reading therapy. However, all children with dyslexia still obtained reading scores far below the normal age range, which raises questions concerning the strength of the bi-directionality. Another likely explanation is that training reading and spelling inherently recruits attentional control and/or memory (Temple et al. 2003). In line with this hypothesis, it might also be that reading practice increases semantic knowledge, and thus vocabulary size, which has been widely recognized as a mediating factor of speech perception abilities (Snowling 2000; Robertson et al. 2009; Kalashnikova 2019; Snowling et al. 2019). Considering that the nature of the remedial experience was not specifically controlled for in this exploratory analyses, and no speech perception data was available before the onset of the training program, future work that would serve to further clarify these results is warranted. In either case, poor speech perception in school-aged children can be expected to be influenced by remediation-related variables, which might alter its potential cascading effect for phonological processing and reading and potentially overshadow sensory processing deficits.
Given its signal processing nature, EE has some substantial strengths, such as the possibility of real-time implementation in assistive listening devices. The use of assistive listening devices as implemented in classroom FM systems to improve auditory processing in noisy classroom environments has already been proven to significantly benefit phonological awareness and reading abilities (Hornickel et al. 2012). However, improved clarity of the acoustic signal by enhancing the SNR of the teacher’s voice without altering any dynamic features might not necessarily boost tracking of the speech envelope nor might it lead to the development of robust phonological representations (Goswami, 2011). Therefore, it cannot be ruled out that literacy improvement found by Hornickel et al. (2012) might, at least partially, be the consequence of reducing the cognitive load of attending which allows for more resources to be allocated to the classroom-related activities (i.e., reading and spelling). Consequently, adjusting the EE algorithm for similar FM system use might lead to an additional benefit. Secondly, our findings show both instantaneous and passive effects of EE on perceptual outcomes; hence, age-specific training designs based on EE can be adapted easily taking the motivation and cognitive development of the individual child into consideration. Consequently, it has great potential to be used with very young (pre-reading) children who have been shown to be more susceptible for intervention (Butterworth and Kovas, 2013; Ozernov-Palchik and Gaab, 2016). Thereby, we believe it is feasible to support children to refine their phonologic representations when their literacy acquisition begins and learn grapheme-phoneme mappings in a less laborious way.
To conclude, our results confirm the presence of poor speech perception in noise in school-aged children with dyslexia. Moreover, the presence of these deficits in both school-aged children and adults indicate that speech perception difficulties persist over time. Yet, compensational mechanisms, such as extensive remedial experience, may alter the observed relation between speech perception and reading impairments without actually overcoming reading difficulties for children with dyslexia. More importantly, our results demonstrated that emphasizing amplitude rise times in speech using EE instantaneously improved speech perception in children with dyslexia. The same benefit was also found in adults with dyslexia (Van Hirtum et al. 2019b). Considering the beneficial outcomes of EE, we believe our findings provide a foundation for future intervention studies based on auditory and speech rhythm training, though the transfer to phonological and reading outcomes still needs verification.
Acknowledgements
The authors are grateful to all our subjects who made time to participate in this study. Our special thanks go to Arturo Moncada-Torres for his contributions to implementing and optimizing the signal processing, to Robin Gransier for his assistance and insightful comments with regard to data analysis, and to Astrid De Vos for her assistance in recruiting our participants.
Author Contribution
T.V.H. and J.W. designed the experiments. T.V.H. and P.G. designed the cognitive tasks and recruited the subjects. T.V.H. performed the research, analyzed the data and wrote the manuscript. P.G. and J.W. supervised the research. All authors edited the manuscript.
Funding
This work was supported by the Research Foundation—Flanders (FWO) (G.0A91.15N) and by the Research Council of KU Leuven (C14/17/046).
Data Availability
All data supporting the findings of this study are available from the corresponding author (tilde.vanhirtum@kuleuven.be) upon reasonable request
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baddeley A. Working memory: theories, models, and controversies. Annu Rev Psychol. 2012;63(1):29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Banai K, Ahissar M. Poor frequency discrimination probes. Audiol Neurootol. 2004;9:328–340. doi: 10.1159/000081282. [DOI] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. 10.18637/jss.v067.i01
- Bhide A, Power A, Goswami U. Intervention for poor readers: a comparison of efficacy with a letter-based intervention. Mind, Braind Educ. 2013;7:113–123. doi: 10.1111/mbe.12016. [DOI] [Google Scholar]
- Boets B, Vandermosten M, Poelmans H, et al. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Res Dev Disabil. 2011;32:560–570. doi: 10.1016/j.ridd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Boets B, Wouters J, van Wieringen A, Ghesquière P. Auditory processing, speech perception and phonological ability in pre-school children at high-risk for dyslexia: a longitudinal study of the auditory temporal processing theory. Neuropsychologia. 2007 doi: 10.1016/j.neuropsychologia.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Bonte M, Correia JM, Keetels M, et al. Reading-induced shifts of perceptual speech representations in auditory cortex. Sci Rep. 2017;7:1–11. doi: 10.1038/s41598-017-05356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley L, Bryant P (1983) Categorizing sounds and learning to read a causal connection. Nature 301:419–421. 10.1038/301419a0
- Bradlow AR, Kraus N, Hayes E. Speaking clearly for children with learning disabilities. J Speech Lang Hear Res. 2003;46:80. doi: 10.1044/1092-4388(2003/007). [DOI] [PubMed] [Google Scholar]
- Brady S, Shankweiler D, Mann V. Speech perception and memory coding in relation to reading ability. J Exp Child Psychol. 1983;35:345–367. doi: 10.1016/0022-0965(83)90087-5. [DOI] [PubMed] [Google Scholar]
- Brus BT, Voeten MJM (1979) Eén-Minuut-Test, vorm A en B. Verantwoording en Handleiding. Pearson, Nijmegen: Berkhout
- Butterworth B, Kovas Y (2013) Understanding neurocognitive developmental disorders can improve eduction for all. Science 340:300–305 [DOI] [PubMed]
- Calcus A, Colin C, Deltenre P, Kolinsky R. Informational masking of speech in dyslexic children. J Acoust Soc Am. 2015;137:496–502. doi: 10.1121/1.4922012. [DOI] [PubMed] [Google Scholar]
- Calcus A, Deltenre P, Colin C, Kolinsky R. Peripheral and central contribution to the difficulty of speech in noise perception in dyslexic children. Dev Sci. 2018;21:1–13. doi: 10.1111/desc.12558. [DOI] [PubMed] [Google Scholar]
- Calcus A, Hoonhorst I, Colin C, et al (2018b) The “rowdy classroom problem” in children with dyslexia: a review. In: Lachmann T, Weis T (eds) Reading and dyslexia. Springer, Cham
- Calcus A, Lorenzi C, Collet G, et al. Is there a relationship between speech identification in noise and categorical perception in children with dyslexia. Am J Speech-Language Pathol. 2016;25:1–15. doi: 10.1044/2016. [DOI] [PubMed] [Google Scholar]
- Cheng B, Zhang X, Fan S, Zhang Y (2019) The role of temporal acoustic exaggeration in high variability phonetic training: a behavioral and ERP study. Front Psychol 10:1178. 10.3389/fpsyg.2019.011782019.011782019.011782019.01178 [DOI] [PMC free article] [PubMed]
- De Vos A, Vanvooren S, Vanderauwera J, et al. A longitudinal study investigating neural processing of speech envelope modulation rates in children with (a family risk for) dyslexia. Cortex. 2017;93:206–219. doi: 10.1016/j.cortex.2017.05.007. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, et al (2010) How learning to read changes the cortical networks for vision and language. Sci 3;330(6009):1359-13564. 10.1126/science.1194140 [DOI] [PubMed]
- Deloof G (2006) Leerlingvolgsysteem VCLB. Spelling: Toetsen 1–6 - Basisboek. Garant, Antwerpen, Belgium
- Doelling KB, Arnal LH, Ghitza O, Poeppel D. Acoustic landmarks drive delta-theta oscillations to enable speech comprehension by facilitating perceptual parsing. Neuroimage. 2014;85:761–768. doi: 10.1016/j.neuroimage.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole M, Hoen M, Meunier F. Speech-in-noise perception deficit in adults with dyslexia: effects of background type and listening configuration. Neuropsychol. 2012;50:1543–1552. doi: 10.1016/j.neuropsychologia.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Dole M, Meunier F, Hoen M. Neuropsychologia Functional correlates of the speech-in-noise perception impairment in dyslexia: an MRI study. Neuropsychol. 2014;60:103–114. doi: 10.1016/j.neuropsychologia.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Fastl H, Zwicker E (2007) Psychoacoustics: fast and models, 3rd edn. Springer Science & Business Media
- Flaugnacco E, Lopez L, Terribili C, et al. Music training increases phonological awareness and reading skills in developmental dyslexia: a randomized control trial. PLoS One. 2015;10:e0138715. doi: 10.1371/journal.pone.0138715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francart T, van Wieringen A, Wouters J. APEX 3: a multi-purpose test platform for auditory psychophysical experiments. J Neurosci Methods. 2008;172:283–293. doi: 10.1016/j.jneumeth.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Geurts L, Wouters J. Enhancing the speech envelope of continuous interleaved sampling processors for cochlear implants. J Neurosci. 1999;105:2476–2484. doi: 10.1121/1.426851. [DOI] [PubMed] [Google Scholar]
- Goswami U. A temporal sampling framework for developmental dyslexia. Trends Cogn Sci. 2011;15:3–10. doi: 10.1016/j.tics.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Goswami U (2019) Speech rhythm and language acquisition an amplitude modulation phase hierarchy perspective. Ann N Y Acad Sci 1–12. 10.1111/nyas.14137 [DOI] [PubMed]
- Goswami U. Sensory theories of developmental dyslexia: three challenges for research. Nat Rev Neurosci. 2015;16:43–54. doi: 10.1038/nrn3836. [DOI] [PubMed] [Google Scholar]
- Goswami U, Thomson J, Richardson U, et al. Amplitude envelope onsets and developmental dyslexia: a new hypothesis. Proc Natl Acad Sci. 2002;99:10911–10916. doi: 10.1073/pnas.122368599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Hoogenboom N, Thut G, et al. Speech rhythms and multiplexed oscillatory sensory coding in the human brain. PLOS Biol. 2013;11:1–14. doi: 10.1371/journal.pbio.1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen JA, Fosker T, Szücs D, Goswami U. N1, P2 and T-complex of the auditory brain event-related potentials to tones with varying rise times in adults with and without dyslexia. Int J Psychophysiol. 2011;81:51–59. doi: 10.1016/j.ijpsycho.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Hazan V, Messaoud-galusi S, Rosen S. The Effect of talker and intonation variability on speech perception in noise in children with dyslexia. J Speech Lang Hear Res. 2013;56:44–62. doi: 10.1044/1092-4388(2012/10-0107)44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan V, Messaoud-Galusi S, Rosen S, et al. Speech perception abilities of adults with dyslexia: is there any evidence for a true deficit? J Speech, Lang Hear Res. 2009;52:1510–1529. doi: 10.1044/1092-4388(2009/08-0220). [DOI] [PubMed] [Google Scholar]
- Horlyck S, Reid A, Burnham D. The relationship between learning to read and language-specific speech perception: maturation versus experience. Sci Stud Read. 2012;16:218–239. doi: 10.1080/10888438.2010.546460. [DOI] [Google Scholar]
- Hornickel J, Zecker SG, Bradlow AR, Kraus N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc Natl Acad Sci. 2012;109:16731–16736. doi: 10.1073/pnas.1206628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse MF, Manis FR, Keating P, Seidenberg MS. Language deficits in dyslexic children: speech perception, phonology, and morphology. J Exp Child Psychol. 2000;77:30–60. doi: 10.1006/jecp.1999.2553. [DOI] [PubMed] [Google Scholar]
- Kalashnikova M, Goswami U, Burnham D. Mothers speak differently to infants at-risk for dyslexia. Dev Sci. 2016;21:1–15. doi: 10.1111/desc.12487. [DOI] [PubMed] [Google Scholar]
- Kalashnikova M, Goswami U, Burnham D (2019) Sensitivity to amplitude envelope rise time in infancy and vocabulary development at 3 years: A significant relationship. Dev Sci. 22:e12836. 10.1111/desc.12836 [DOI] [PubMed]
- Kirtley C, Bryant P, Maclean M. Rhyme, rime, and the onset of reading. J Exp Child Psychol. 1989;48:224–245. doi: 10.1016/0022-0965(89)90004-0. [DOI] [PubMed] [Google Scholar]
- Koning R, Wouters J. The potential of onset enhancement for increased speech intelligibility in auditory prostheses. J Acoust Soc Am. 2012;132:2569–2581. doi: 10.1121/1.4748965. [DOI] [PubMed] [Google Scholar]
- Kort W, Schittekatte M, Compaan E. CELF-4-NL: Test voor diagnose en evaluatie van taalproblemen. Amsterdam, The Netherlands: Pearson; 2010. [Google Scholar]
- Lallier M, Lizarazu M, Molinaro N, et al (2018) From auditory rhythm processing to grapheme-to-phoneme conversion: how neural oscillations can shed light on developmental dyslexia. In: Lachmann T, Weis T (eds) Reading and dyslexia. Springer, Cham
- Law JM, Vandermosten M, Ghesquiere P, et al (2014) The relationship of phonological ability, speech perception , and auditory perception in adults with dyslexia. 8:1–12. 10.3389/fnhum.2014.00482 [DOI] [PMC free article] [PubMed]
- Leibold LJ. Speech perception in complex acoustic environments: developmental effects. J Speech, Lang Hear Res. 2017;60:3001–3008. doi: 10.1044/2017_JSLHR-H-17-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong V, Hämäläinen J, Soltész F, Goswami U. Rise time perception and detection of syllable stress in adults with developmental dyslexia. J Mem Lang. 2011;64:59–73. doi: 10.1016/j.jml.2010.09.003. [DOI] [Google Scholar]
- Lorenzi C, Dumont A, Füllgrabe C. Use of temporal envelope cues by children with developmental dyslexia. J Speech Lang Hear Res. 2000;43:1367–1379. doi: 10.1044/jslhr.4306.1367. [DOI] [PubMed] [Google Scholar]
- Messaoud-Galusi S, Hazan V, Rosen S. Investigating speech perception in children with dyslexia: is there evidence of a consistent deficit in individuals? J Speech Lang Hear Res. 2011;54:1682–1701. doi: 10.1044/1092-4388(2011/09-0261). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O, Gaab N. Tackling the “dyslexia paradox”: reading brain and behavior for early markers of developmental dyslexiax. Wiley Interdiscip Rev Cogn Sci. 2016;7:156–176. doi: 10.1002/wcs.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF. From single to multiple deficit models of developmental disorders. Cognition. 2006;101:385–413. doi: 10.1016/j.cognition.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Poelmans H, Luts H, Vandermosten M, et al. Reduced sensitivity to slow-rate dynamic auditory information in children with dyslexia. Res Dev Disabil. 2011;32:2810–2819. doi: 10.1016/j.ridd.2011.05.025. [DOI] [PubMed] [Google Scholar]
- Power AJ, Mead N, Barnes L, Goswami U. Neural entrainment to rhythmic speech in children with developmental dyslexia. Front Hum Neurosci. 2013;7:1–19. doi: 10.3389/fnhum.2013.00777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015) R: a language and environment for statistical computing.
- Ramus F, Rosen S, Dakin SC, et al. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Read C, Yun-fei Z, Hong-yin NIE, Bao-qing D. The ability to manipulate speech sounds depends on knowing alphabetic writing. Cognition. 1986;24:31–44. doi: 10.1016/0010-0277(86)90003-X. [DOI] [PubMed] [Google Scholar]
- Richardson U, Thomson JM, Scott SK, Goswami U. Auditory processing skills and phonological representation in dyslexic children. Dyslexia. 2004;10:215–233. doi: 10.1002/dys.276. [DOI] [PubMed] [Google Scholar]
- Robertson EK, Joanisse MF, Desroches AS, Ng S. Categorical speech perception deficits distinguish language and reading impairments in children. Dev Sci. 2009;12:753–767. doi: 10.1111/j.1467-7687.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- Rosario Ortiz González M, García Espinel AI, Guzmán Rosquete R. Remedial interventions for children with reading disabilities: speech perception — an effective component in phonological training ? J Learn Disabil. 2002;35:334–342. doi: 10.1177/00222194020350040401. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. Dyslexia. 2. Oxford, UK: Blackwell; 2000. [Google Scholar]
- Sperling AJ, Lu ZL, Manis FR, Seidenberg MS. Deficits in perceptual noise exclusion in developmental dyslexia. Nat Neurosci. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Stefanics G, Fosker T, Huss M, et al (2011) Auditory sensory deficits in developmental dyslexia: a longitudinal ERP study. Neuroimage 57:723–732. 10.1016/j.neuroimage.2011.04.0052011.04.005 [DOI] [PubMed]
- Stoodley CJ, Hill PR, Stein JF, Bishop DVM. Auditory event-related potentials differ in dyslexics even when auditory psychophysical performance is normal. Brain Res. 2006;1121:190–199. doi: 10.1016/j.brainres.2006.08.095. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Gram A, Van Ingelghem M, et al (2003) Impaired sensitivity to dynamic stimuli in poor readers of a regular orthography. Brain Lang. 87:259-66. 10.1016/S0093-934X(03)00105-6 [DOI] [PubMed]
- Temple E, Deutsch GK, Poldrack RA, et al (2003) Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci 100:2860–2865. 10.1073/pnas.0030098100 [DOI] [PMC free article] [PubMed]
- The MathWorks Inc (2013) MATLAB and Statistics Toolbox Release 2013b
- Thomson JM, Leong V, Goswami U. Auditory processing interventions and developmental dyslexia: a comparison of phonemic and rhythmic approaches. Read Writ. 2013 doi: 10.1007/s11145-012-9359-6. [DOI] [Google Scholar]
- Van den Bos KP, Spelberg CLH, Scheepstra JMA, De Vries RJ (1994) De Klepel. Vorm A en B. Een test voor leesvaardigheid van pseudowoorden. Verantwoording, handleiding, diagnostiek en behandeling. Pearson, Nijmegen
- Vandermosten M, Correia J, Vanderauwera J, et al (2019) Brain activity patterns of phonemic representations are atypical in beginning readers with family risk for dyslexia. Dev Sci 23:e12857. 10.1111/desc.12857 [DOI] [PubMed]
- Van Hirtum T, Ghesquière P, Wouters J. Atypical neural processing of rise time by adults with dyslexia. Cortex. 2019;113:128–140. doi: 10.1016/j.cortex.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Van Hirtum T, Moncada-Torres A, Ghesquière P, Wouters J (2019b) Speech envelope enhancement instantaneously effaces atypical speech perception in dyslexia. Ear Hear 40:1242–1252. [DOI] [PubMed]
- Van Wieringen A, Wouters J. LIST and LINT: sentences and numbers for quantifying speech understanding in severely impaired listeners for Flanders and the Netherlands. Int J Audiol. 2008;47:348–355. doi: 10.1080/14992020801895144. [DOI] [PubMed] [Google Scholar]
- Vanvooren S, Poelmans H, De Vos A, et al. Do prereaders’ auditory processing and speech perception predict later literacy? Res Dev Disabil. 2017;70:138–151. doi: 10.1016/j.ridd.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Vellutino FR, Fletcher JM, Snowling MJ, Scanlon DM. Specific reading disability (dyslexia): what have we learned in the past four decades? J Child Psychol Psychiatry Allied Discip. 2004;45:2–40. doi: 10.1046/j.0021-9630.2003.00305.x. [DOI] [PubMed] [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S. 4. New York, USA: Springer; 2002. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 3. San Antonio, Texas: The Psychological Corporation; 1991. [Google Scholar]
- Witton C, Stein JF, Stoodley CJ, et al. Separate influences of acoustic AM and FM sensitivity on the phonological decoding skills of impaired and normal readers. J Cogn Neurosci. 2002;14:866–874. doi: 10.1162/089892902760191090. [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Pech-Georgel C, George F, Lorenzi C. Speech-perception-in-noise deficits in dyslexia. Dev Sci. 2009;12:732–745. doi: 10.1111/j.1467-7687.2009.00817.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data supporting the findings of this study are available from the corresponding author (tilde.vanhirtum@kuleuven.be) upon reasonable request