Abstract
The aim of the study was to investigate differences in metabolic profiles between patients with major depressive disorder (MDD) with full remission (FR) and healthy controls (HCs). A total of 119 age-matched MDD patients with FR (n = 47) and HCs (n = 72) were enrolled and randomly split into training and testing sets. A 1H-nuclear magnetic resonance (NMR) spectroscopy-based metabolomics approach was used to identify differences in expressions of plasma metabolite biomarkers. Eight metabolites, including histidine, succinic acid, proline, acetic acid, creatine, glutamine, glycine, and pyruvic acid, were significantly differentially-expressed in the MDD patients with FR in comparison with the HCs. Metabolic pathway analysis revealed that pyruvate metabolism via the tricarboxylic acid cycle linked to amino acid metabolism was significantly associated with the MDD patients with FR. An algorithm based on these metabolites employing a linear support vector machine differentiated the MDD patients with FR from the HCs with a predictive accuracy, sensitivity, and specificity of nearly 0.85. A metabolomics-based approach could effectively differentiate MDD patients with FR from HCs. Metabolomic signatures might exist long-term in MDD patients, with metabolic impacts on physical health even in patients with FR.
Subject terms: Neuroscience, Biomarkers, Diseases, Medical research
Introduction
Major depressive disorder (MDD) is a common mental disorder. However, no robust objective laboratory test is available for the diagnosis of MDD or evaluation of the severity of depression. A metabolomics-based approach can be employed to identify products of a given biochemical system and metabolic substrates, and therefore this approach has emerged as a method by which to increase our understanding of diseases and biological systems in a large-scale manner1. The technology of metabolomics offers significant potential as a tool to investigate the diagnosis of diseases and responses to medications. Metabolomics has been used in MDD-related research, such as to evaluate the severity of depression2, identify biomarkers of MDD3–9, for predictive diagnosis of MDD10–13, identify metabolic profiles post-antidepressant treatment14–17, pinpoint biomarkers of metabolites for drug response phenotypes18,19, and differentiate MDD from bipolar disorder12,20,21.
Full remission (FR) of depression is a treatment goal for patients with MDD. One of the commonly-used definitions of FR is a 17-item Hamilton Depression Rating Scale (HAMD) score ≤ 722. However, MDD with FR does not equate to achieving health23,24. For example, cognitive dysfunction, which may hinder functional recovery, is one of the common residual symptoms of depression, and may persist during the remission phase25. This raises two interesting questions: (1) are there any differences in the metabolic profiles between MDD patients with FR and healthy controls (HCs), and (2) is it possible to establish an algorithm based on metabolites as biomarkers to differentiate MDD patients with FR from HCs?
The majority of studies of metabolomics in MDD patients, as described above, have been concerned with identifying biomarkers or obtaining a predictive diagnosis of MDD, or predicting the response to antidepressants. Few studies have focused on investigating differences in metabolite expressions between MDD patients with FR and HCs using targeted metabolomics analysis26,27, and to our knowledge, no study has comprehensively investigated differences in metabolite levels in peripheral plasma between MDD patients with FR and HCs. An algorithm based on metabolomics analysis to differentiate MDD patients with FR from HCs is still lacking. However, investigation of the above two issues is important, because MDD has negative impacts on multiple physical systems28–30. Abnormalities in metabolites among MDD patients with FR might be associated with long-term negative impacts on physical health. Furthermore, recurrence is common in MDD, and investigation of these issues may provide clues as to the recurrence of depression and subsequent prevention of depression.
Therefore, this study aimed to comprehensively investigate the differences in metabolomic profiles in peripheral plasma between patients with MDD with FR and HCs, and to then establish an algorithm based on metabolomics analysis to differentiate MDD patients with FR from HCs. We hypothesized that an algorithm based on metabolomics analysis could be effective in differentiating MDD patients with FR from HCs.
Methods
Subjects
The subjects included in this study were nested within a project that examined MDD patients and were recruited at the 10-year follow-up point from August 2014 to December 201629,31,32. At baseline (from January 2004 to August 2007), patients diagnosed with MDD in that project were enrolled from outpatient clinics of the Psychiatric Department of Chang Gung Memorial Hospital at Linkou, a medical center in northern Taiwan. The outpatients fulfilled the criteria for MDD, and were diagnosed using the Structured Clinical Interview for DSM-IV-text revision (TR) Axis I Disorders33.
At baseline, 229 participants with MDD were enrolled, then were treated by antidepressants. At the 10-year follow-up point, 137 (47.2%) subjects attended follow-up. The severity of depression was evaluated using the 17-item Hamilton Depression Rating Scale (HAMD)34 administered by a psychiatrist, and among the 137 subjects, a total of 47 MDD patients were in FR, which was defined as a HAMD score ≤ 722, and had been medication-free for at least 6 months and had no history of substance abuse or dependence.
Sixty-seven healthy persons were simultaneously enrolled as controls. The exclusion criteria for the HCs were as follows: (1) any current or previous lifetime history of neurological or DSM-IV-TR axis I/II diagnoses; (2) systemic medical diseases, such as hypertension, diabetes mellitus, and others; and (3) any family history of psychiatric disorders. The project was approved by the Institutional Review Board of Chang Gung Memorial Hospital (No. 105-5895C). Based on the guidelines regulated in the Declaration of Helsinki, written informed consent was acquired from all subjects.
The enrolled subjects, including 47 MDD patients with FR and 67 HCs, were then randomly split (3/5 for training, 2/5 for testing) into training (30 MDD patients with FR and 42 HCs) and testing sets (17 MDD patients with FR and 30 HCs) for algorithm development. Twelve-hour fasting plasma samples of the 47 MDD patients with FR and 67 HCs were collected and analyzed at the 10-year follow-up point. Fasting plasma parameters including glucose, cholesterol, or triglyceride, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) analyzed by completely automated methods at clinical laboratories were also analyzed.
Plasma sample preparation
Fasting blood samples for plasma collection were obtained at 9–10 a.m., aliquoted and stored immediately at − 80 °C until analysis. Thawed plasma samples were centrifuged at 12,000×g at 4 °C for 30 min. 500 μL of plasma supernatant were mixed with 500 μL of 0.075 M phosphate buffer (pH 7.40) in 20% deuterium water containing 0.08% 3-(trimethylsilyl)-propionic-2,2,3,3-d4 acid sodium salt (TSP) as an internal chemical shift reference standard. The mixed samples were vortexed for 20 s and centrifuged at 12,000×g at 4 °C for 30 min, following which 600 μL of the supernatant were loaded into a standard 5-mm NMR tube (Bruker BioSpin, Billerica, MA, USA) for further analysis.
Nuclear magnetic resonance (NMR) spectrum acquisition
NMR experiments were performed at Chang Gung Healthy Aging Research Center, Taiwan. 1H-NMR spectra were acquired on a Bruker Avance 600-MHz spectrometer (Bruker-Biospin GmbH, Karlsruhe, Germany) equipped with a 5-mm CPTCI 1H cryoprobe. Temperature was controlled at 300 K throughout the experiments. Relaxation-edited spectra were acquired using Carr–Purcell–Meiboom–Gill (CPMG)-presat pulse sequence. In CPMG method, a series of 180° pulse was applied with the radio-frequency (RF) pulses in 27.24 μs and the water presaturation bandwidth 25 Hz. Low-power water pre-saturation pulse sequence was used for water signal suppression during the relaxation time of 4 s. For each spectrum, 64 transients were collected into 64 K data points using a spectral window of 20 ppm during a relaxation time of 4 s. The temperature-controlled Bruker SampleJet automation unit was installed for sample handling and laboratory automation. Prior to Fourier transformation, all 1H-NMR spectra were processed with zero-filling and exponential line-broadening of 0.3 Hz. The acquired spectra were manually phased, baseline corrected, and the internal TSP signal calibrated to δ 0.0 ppm using TopSpin 3.2 software (Bruker BioSpin, Rheinstetten, Germany).
NMR data processing and analysis
NMRProcFlow (https://www.nmrprocflow.org), an open-source software, provides comprehensive tools for the processing and visualization of 1D NMR data. The raw 1H-NMR spectra were imported into NMRProcFlow 1.3 for ppm calibration, baseline correction, alignment, spectra bucketing and data normalization35. Spectra bucketing was performed using the method of intelligent bucketing and variable size bucketing with the full range of 10.0–0.00 ppm36. Metabolite identification was performed using Chenomx NMR Suite 8.0 professional software (Chenomx Inc., Edmonton, AB, Canada). The compounds were identified by comparing spectra to database Chenomx 600 MHz Version 9 (Chenomx Inc., Edmonton, Canada) with 332 metabolites in this particular database. A standard two-dimensional (2D) NMR experiment (1H and 13C NMR spectrum) was conducted on a pooled plasma sample and metabolites were further assigned by comparison with reference spectra from the Human Metabolome Database (HMDB). The area of individual resonances of glucose metabolite was significantly correlated with biochemical glucose concentration (Supplementary Fig. S1). The exported bucketing data of the 1H-NMR spectra were uploaded to MetaboAnalyst 4.0 (http://www.metaboanalyst.ca) with mean-centered, generalized log transformation and scaled by Pareto scaling. To identify metabolites that may be used to distinguish MDD patients with FR from healthy controls, partial least squares-discriminant analysis (PLS-DA) was applied with the variable importance in projection (VIP) score and fold-change values. Pathway analysis of the potential metabolites selected owing to a p-value lower than 0.05 was carried out to identify the implicated pathways. The potential metabolites were selected from the training samples, and Receiver Operating Characteristic (ROC) analysis was performed to investigate the accuracy of the training and testing models using four well-established algorithms, including PLS-DA, Random Forest, Support Vector Machine (SVM) and Logistic Regression Models. One-hundred cross-validations were performed to obtain a more reliable prediction model and the permutation test was used 1000 times to evaluate the performance of the model.
Results
Subjects
Table 1 shows the differences in demographic variables and biochemical indices between the MDD patients with FR and the HCs in the training and testing groups. There were no significant differences in age, gender, BMI, fasting plasma glucose, cholesterol, or triglyceride levels between groups. A significant difference was noted in the HAMD score between the MDD patients with FR and the HCs in the training group; however, both scores were within the range of FR (HAMD score ≤ 7).
Table 1.
Demographic variables and biochemical indices in the MDD patients with full remission and the healthy controls.
| Training group | Testing group | |||
|---|---|---|---|---|
| MDD with remission | Healthy controls | MDD with remission | Healthy controls | |
| Number | 30 | 42 | 17 | 30 |
| Age (years) | 42.1 ± 9.2 | 41.2 ± 7.3 | 38.2 ± 5.2 | 40.6 ± 8.7 |
| Female (%) | 70.0 | 71.4 | 58.8 | 66.7 |
| HAMD score | 3.7 ± 2.0 | 1.5 ± 2.4* | 2.9 ± 1.9 | 2.1 ± 4.2 |
| BMI | 24.2 ± 4.8 | 22.8 ± 3.7 | 22.8 ± 4.3 | 23.8 ± 3.5 |
| Fasting plasma glucose (mg/dL) | 92.6 ± 19.7 | 89.5 ± 10.2 | 104.9 ± 62.2 | 86.7 ± 5.7 |
| Cholesterol | 190.8 ± 29.2 | 185.5 ± 25.1 | 193.5 ± 24.2 | 191.5 ± 30.8 |
| Triglycerides (mg/dL) | 104.9 ± 61.1 | 94.4 ± 61.1 | 97.4 ± 60.8 | 94.5 ± 51.3 |
| AST | 23.4 ± 9.7 | 22.6 ± 5.4 | 22.9 ± 4.9 | 25.0 ± 7.4 |
| ALT | 21.8 ± 15.8 | 19.1 ± 12.1 | 19.2 ± 9.7 | 23.7 ± 16.4 |
Full remission was defined as a HAMD score ≤ 7.
MDD major depressive disorder, HAMD Hamilton Depression Rating Scale, BMI body mass index, ALT alanine aminotransferase, AST aspartate aminotransferase.
*p < 0.05.
Metabolites significantly differentially-expressed between the MDD patients with FR and the HCs in the training group
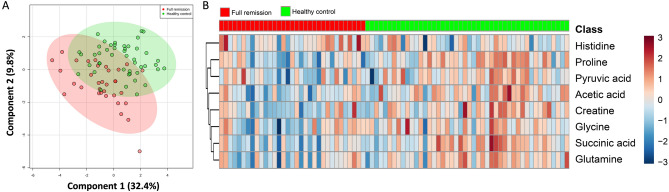
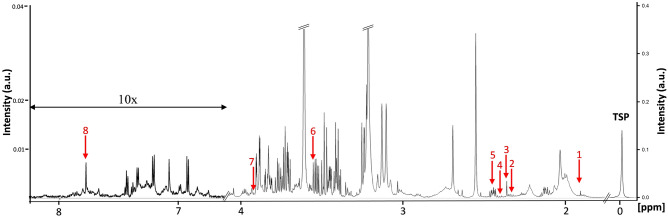
1H-NMR spectra obtained from plasma corresponded to 27 known metabolites (Supplementary Table S1). Metabolites that contributed to discrimination between the groups were identified using supervised PLS-DA (Fig. 1A, score plots). Table 2 shows the metabolites significantly differentially-expressed between the MDD patients with FR and the HCs in the training group. Compared with the HCs, eight metabolites were found to be significantly associated with the MDD patients with FR (p < 0.05), among which seven metabolites, including succinic acid, proline, acetic acid, creatine, glutamine, glycine, and pyruvic acid, had significantly lower expressions in the MDD patients with FR, while in contrast histidine had a significantly higher expression in the MDD patients with FR than in the HCs. Figure 1B shows a heatmap of these eight metabolites clustered using Hierarchical Clustering. A representative 600 MHz 1H-NMR spectra of selected eight metabolite signals are shown in Fig. 2.
Figure 1.
PLS-DA score plots from the analysis of 1H-NMR spectra using plasma samples and a heat map of eight metabolites significantly differentially-expressed between the major depressive disorder (MDD) patients with full remission (FR) and healthy controls (HCs). (A) Two-dimensional scatter plot showing the model’s degree of separation between the two groups: x axis, component 1 (% of total variance); y axis, component 2 (% of total variance). (B) Each column represents a plasma sample and each row represents the expression profile of a metabolite. The fold changes from the overall mean concentration are shown in a color-coded manner, with blue representing a decrease and red an increase.
Table 2.
Significantly differentially-expressed metabolites between the MDD patients with full remission and the healthy controls.
| Metabolite | Chemical shift (ppm) | VIP score | Fold change | p |
|---|---|---|---|---|
| Succinic acid | 2.394–2.397 (s) | 1.32 | 0.85 | < 0.001 |
| Proline | 2.322–2.357 (m) | 1.79 | 0.75 | < 0.001 |
| Acetic acid | 1.907–1.914 (s) | 1.37 | 0.83 | < 0.001 |
| Creatine | 3.918–3.926 (s) | 1.03 | 0.89 | 0.001 |
| Glutamine | 2.403–2.409 (m) | 0.79 | 0.93 | 0.005 |
| Glycine | 3.548–3.565 (s) | 0.63 | 0.94 | 0.020 |
| Pyruvic acid | 2.357–2.369 (s) | 0.77 | 0.91 | 0.032 |
| Histidine | 7.760–7.783 (s) | 1.39 | 1.06 | 0.039 |
MDD major depressive disorder, VIP variable importance in the projection, s singlet, m multiplet.
Figure 2.
Representative 600 MHz 1H-NMR spectra of plasma showing the selected eight metabolite signals (δ1–9). x axis, parts per million (ppm); y axis, intensity (a.u.). 1, Acetic acid; 2, Proline; 3, Pyruvic acid; 4, Succinic acid; 5, Glutamine; 6, Glycine; 7, Creatine; 8, Histidine.
Metabolic pathway associated with MDD patients with FR
Table 3 shows functional pathways of the metabolic network associated with the MDD patients with FR. Pyruvate metabolism via the tricarboxylic acid (TCA) cycle linked to amino acid metabolism, including alanine, aspartate and glutamate; arginine and proline; and glycine, serine and threonine metabolisms, was significantly associated with the MDD patients with FR (p < 0.01).
Table 3.
Functional pathway analysis of metabolites associated with MDD with full remission.
| Pathway name | Match status | Metabolitesa | P | FDR | Impact |
|---|---|---|---|---|---|
| Alanine, aspartate and glutamate metabolism | 3/24 | Pyruvic acid, glutamine, succinic acid | < 0.001 | 0.002 | 0.207 |
| Aminoacyl-tRNA biosynthesis | 4/75 | Histidine, glutamine, glycine, proline | < 0.001 | 0.002 | 0.000 |
| Arginine and proline metabolism | 4/77 | Glutamine, proline, creatine, pyruvic acid | < 0.001 | 0.002 | 0.134 |
| Nitrogen metabolism | 3/39 | Glutamine, histidine, glycine | < 0.001 | 0.004 | 0.000 |
| Glycine, serine and threonine metabolism | 3/48 | Glycine, creatine, pyruvic acid | < 0.001 | 0.006 | 0.188 |
| Taurine and hypotaurine metabolism | 2/20 | Pyruvic acid, acetic acid | 0.002 | 0.020 | 0.022 |
| Citrate cycle (TCA cycle) | 2/20 | Succinic acid, pyruvic acid | 0.002 | 0.020 | 0.105 |
| Glycolysis or Gluconeogenesis | 2/31 | Pyruvic acid, acetic acid | 0.004 | 0.041 | 0.096 |
| Pyruvate metabolism | 2/32 | Pyruvic acid, acetic acid | 0.005 | 0.041 | 0.282 |
MDD major depressive disorder, FDR false discovery rate, TCA tricarboxylic acid.
aMetabolites for which p < 0.05 were selected.
Model of metabolites in MDD patients with FR
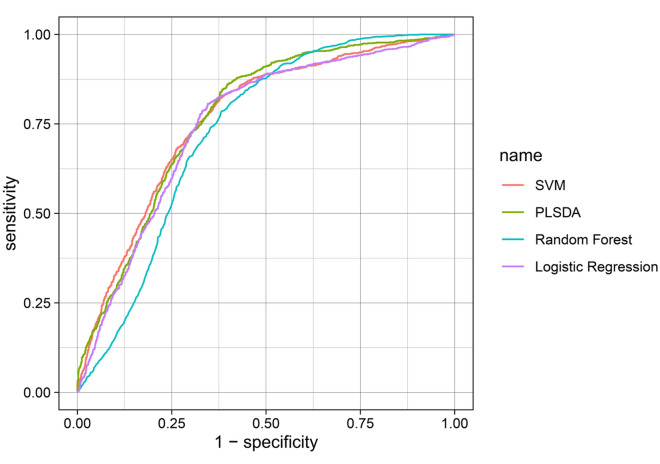
Table 4 shows the performance of the model of metabolites in terms of discriminating the MDD patients with FR from the HCs using four types of machine learning algorithm. Figure 3 shows the ROC curves for the SVM, PLS-DA, random forest, and logistic regression models. The model included the eight metabolites that had been identified as being significantly associated with the MDD patients with FR, with a highest AUC value of 0.784 and a highest predictive accuracy of 0.715 in the traing group (Ppermutation test < 0.05). Using linear SVM classification in the testing group, the predictive accuracy, sensitivity, and specificity were 0.846, with a positive predictive value of 0.733 and a negative predictive value of 0.917.
Table 4.
Model of metabolites in MDD with full remission using different types of machine learning algorithm.
| Model metabolitea | Machine learning model | Training model | Testing model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | Ppermutation testb | Predictive accuracy | Ppermutation test | Predictive accuracy | Sensitivity | Specificity | Positive predictive value | Negative predictive value | ||
|
Succinic acid Proline Acetic acid Creatine Glutamine Glycine Pyruvic acid Histidine |
Linear SVM | 0.784 | 0.007 | 0.707 | 0.011 | 0.846 | 0.846 | 0.846 | 0.733 | 0.917 |
| PLS-DA | 0.779 | 0.003 | 0.705 | 0.011 | 0.846 | 0.923 | 0.808 | 0.706 | 0.955 | |
| Random FOREST | 0.738 | 0.007 | 0.677 | 0.029 | 0.821 | 0.769 | 0.846 | 0.714 | 0.880 | |
| Logistic regression | 0.772 | 0.004 | 0.715 | 0.005 | 0.821 | 0.769 | 0.846 | 0.714 | 0.880 | |
MDD major depressive disorder, AUC area under the receiver operating characteristic curve, SVM support vector machine, PLS-DA partial least squares-discriminant analysis.
aMetabolites for which p < 0.05 were selected.
b1000 random permutations were performed for validation testing.
Figure 3.
Receiver operating characteristics (ROC) curves for supportive vector machine (SVM), PLS-DA, random forest, and logistic regression models.
Discussion
This study demonstrated the potential of metabolic profiling in MDD patients with FR. A model based on metabolomics analysis using machine learning could effectively differentiate MDD patients with FR from HCs. Several studies have reported differences in metabolomes between MDD patients in a depressive episode and HCs5,12,37. Our findings implied the long-term existence of biological characteristics of MDD, even in patients with FR. Despite the fact that MDD with FR does not equate to achieving health in terms of clinical symptoms, such as cognitive dysfunction23,25, our results further demonstrated that FR of depression might not be equivalent to biological health based on the aspect of metabolomics.
In this study, eight metabolites were identified as being significantly differentially-expressed between the MDD patients with FR and the HCs. One review article identified several differentially-expressed metabolites between patients with MDD and controls from 46 studies37. Different studies of MDD patients might present controversial results in terms of elevated or decreased levels of metabolites as compared with HCs37–39. However, all eight metabolites identified in this study had been previously reported to be associated with MDD37, with the exception of acetic acid. Among these metabolites, significantly lower levels of l-glutamine and pyruvic acid were identified in the MDD patients with FR as compared with the HCs, which was compatible with the findings of an integrated meta-analysis of metabolites in MDD patients37. As was the case in this study, lower levels of succinic acid and glutamine have been reported to be significant in the diagnosis of MDD using metabolomics analysis12. However, in contrast to the decreased level of proline and elevated level of histidine observed in this study, antidepressant-free MDD patients have previously been reported to have a conversely increased level of proline and decreased level of l-histidine37.
A recent study reported that serum levels of methionine, phenylalanine, tryptophan, and tyrosine were significantly decreased in MDD patients compared to HCs40. Three of these four metabolites including methionine, tryptophan, and tyrosine related to aminoacyl-tRNA biosynthesis, glycine, serine and threonine metabolism, and citrate cycle were associated with MDD as in this study. Higher serum serine levels have reported to be significantly higher in patients with depression41. In addition, plasma levels of glutamate, glutamine, glycine, and taurine were found to be significantly increased in the depressed patients, particularly reflecting the severity of depression42. Despite the differences between studies, the available evidence suggests the importance of amino acid metabolites in patients with depressive disorder.
Amino acids, in particular glycine, glutamate, and glutamine, have been reported to significantly affect macrophage atherogenicity through modulation of the cellular triglyceride metabolism43. Most importantly, the anti-atherogenic properties of glycine have been further confirmed in vivo43. In this study, the MDD patients with FR appeared to have steady, low glycine levels, which may imply a risk of atherosclerosis in MDD patients, even with FR. Indeed, depression is clinically associated with an increased risk of cardiovascular diseases28,30. The findings of this study indicated that MDD patients might suffer persistent metabolic impacts on physical health, despite FR of the disease.
Nine functional metabolic pathways associated with MDD with FR were identified in this study, most of which have been reported previously37. Amino acid metabolism in the peripheral blood, such as nitrogen metabolism and aminoacyl-tRNA biosynthesis, appeared to be prominently associated with MDD patients44,45. The majority of the differentially-expressed metabolites identified in this study were significantly lower in the MDD patients with FR. In fact, previous studies have also reported some reductions in amino acid bioavailability in MDD patients37,38.
Several points are worthy of note. (1) Ali-Sisto et al. identified a significant difference between MDD patients and HCs in purine metabolism by analysis of fasting serum samples; however, there were no significant differences in metabolite levels between remitted and non-remitted MDD patients26. Most of the metabolites identified in this study had been reported in previous studies that investigated differences in metabolites between MDD patients in a depressive episode and HCs37. These results demonstrated that metabolomic signatures of MDD might not disappear, even with FR. (2) Kaddurah-Daouk et al. reported significant differences in tryptophan and tyrosine metabolism in cerebrospinal fluid in MDD patients with FR in comparison with HCs27. Our study ascertained that differences in metabolomics between MDD patients with FR and HCs were also present in peripheral plasma. (3) There is a possibility that people in the community who have similar metabolomic characteristics to MDD patients with FR may be at greater risk of the onset of depression; however, this hypothesis requires more evidence for confirmation.
There were some limitations and bias in this study. (1) The course of depression fluctuates, and it was difficult to clarify how long the patients with MDD had been in FR at the time point of the investigation. It was also unknown whether the duration of FR might affect the results of metabolomics analysis. (2) The HAMD score in the MDD patients with FR was still significantly higher than that in the HCs in the training group. It was unknown whether this difference in the HAMD score was a factor associated with metabolomic differences between the MDD patients with FR and the HCs. (3) The study did not control the phase of menstrual cycle, which might affect metabolomic profiles46, in female subjects. This might cause bias.
Conclusion
There were significant differences in the expressions of eight metabolites between the MDD patients with FR and the HCs. Pyruvate metabolism via the TCA cycle linked to amino acid metabolism may play a biological role in the potential depression status. Using machine learning Linear SVM, a model containing the eight metabolites related to MDD with FR was developed, which provided a predictive accuracy, sensitivity, and specificity of nearly 0.85 for discrimination of MDD patients with FR from HCs. Metabolomic signatures might exist long-term in MDD patients and could have a persisting impact on physical health, despite FR of the disease.
Supplementary Information
Acknowledgements
This study was supported in part by Chang Gung Memorial Hospital Research Programs (CMRPG 3E2141, CMRPG 3G0251 and CMRPG 3H1781).
Author contributions
C.-I.H. and C.-Y.C. designed the study and wrote the protocol. C.-I.H., G.L. and M.-H.C. collected the data. G.L. and M.-H.C. performed NMR experiments. C.-I.H. and C.-Y.C. undertook the statistical analysis and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95221-1.
References
- 1.Bilello JA. Seeking an objective diagnosis of depression. Biomark. Med. 2016;10:861–875. doi: 10.2217/bmm-2016-0076. [DOI] [PubMed] [Google Scholar]
- 2.Chen JJ, et al. Differential urinary metabolites related with the severity of major depressive disorder. Behav. Brain Res. 2017;332:280–287. doi: 10.1016/j.bbr.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 3.Gadad BS, et al. Peripheral biomarkers of major depression and antidepressant treatment response: Current knowledge and future outlooks. J. Affect. Disord. 2018;233:3–14. doi: 10.1016/j.jad.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, et al. Metabolomic biosignature differentiates melancholic depressive patients from healthy controls. BMC Genom. 2016;17:669. doi: 10.1186/s12864-016-2953-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, et al. Discovery and validation of plasma biomarkers for major depressive disorder classification based on liquid chromatography–mass spectrometry. J. Proteome. Res. 2015;14:2322–2330. doi: 10.1021/acs.jproteome.5b00144. [DOI] [PubMed] [Google Scholar]
- 6.Ding X, et al. The potential biomarker panels for identification of Major Depressive Disorder (MDD) patients with and without early life stress (ELS) by metabonomic analysis. PLoS ONE. 2014;9:e97479. doi: 10.1371/journal.pone.0097479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng P, et al. Identification and validation of urinary metabolite biomarkers for major depressive disorder. Mol. Cell Proteom. 2013;12:207–214. doi: 10.1074/mcp.M112.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, et al. Polyunsaturated fatty acids metabolism, purine metabolism and inosine as potential independent diagnostic biomarkers for major depressive disorder in children and adolescents. Mol. Psychiatry. 2019;24:1478–1488. doi: 10.1038/s41380-018-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald K, et al. Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am. J. Med. Genet. B Neuropsychiat. Genet. 2019;180:122–137. doi: 10.1002/ajmg.b.32680. [DOI] [PubMed] [Google Scholar]
- 10.Zheng H, et al. Predictive diagnosis of major depression using NMR-based metabolomics and least-squares support vector machine. Clin. Chim. Acta. 2017;464:223–227. doi: 10.1016/j.cca.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 11.Zheng P, et al. Metabolite signature for diagnosing major depressive disorder in peripheral blood mononuclear cells. J. Affect. Disord. 2016;195:75–81. doi: 10.1016/j.jad.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Pan JX, et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiatry. 2018;8:130. doi: 10.1038/s41398-018-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, et al. The metabolic factor kynurenic acid of kynurenine pathway predicts major depressive disorder. Front. Psych. 2018;9:552. doi: 10.3389/fpsyt.2018.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandu R, et al. Liquid chromatography/mass spectrometry-based plasma metabolic profiling study of escitalopram in subjects with major depressive disorder. J. Mass Spectrom. 2018;53:385–399. doi: 10.1002/jms.4070. [DOI] [PubMed] [Google Scholar]
- 15.Woo HI, et al. Plasma amino acid profiling in major depressive disorder treated with selective serotonin reuptake inhibitors. CNS Neurosci. Ther. 2015;21:417–424. doi: 10.1111/cns.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhattacharyya S, et al. Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients. Transl. Psychiatry. 2019;9:173. doi: 10.1038/s41398-019-0507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moaddel R, et al. Plasma metabolomic profiling of a ketamine and placebo crossover trial of major depressive disorder and healthy control subjects. Psychopharmacology. 2018;235:3017–3030. doi: 10.1007/s00213-018-4992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rotroff DM, et al. Metabolomic signatures of drug response phenotypes for ketamine and esketamine in subjects with refractory major depressive disorder: new mechanistic insights for rapid acting antidepressants. Transl. Psychiatry. 2016;6:e894. doi: 10.1038/tp.2016.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu H, et al. Pharmacometabolomics of response to sertraline and to placebo in major depressive disorder—Possible role for methoxyindole pathway. PLoS ONE. 2013;8:e68283. doi: 10.1371/journal.pone.0068283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JJ, et al. Divergent urinary metabolic phenotypes between major depressive disorder and bipolar disorder identified by a combined GC-MS and NMR spectroscopic metabonomic approach. J. Proteome Res. 2015;14:3382–3389. doi: 10.1021/acs.jproteome.5b00434. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K. Metabolomics of major depressive disorder and bipolar disorder: Overview and future perspective. Adv. Clin. Chem. 2018;84:81–99. doi: 10.1016/bs.acc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Moller HJ. Outcomes in major depressive disorder: The evolving concept of remission and its implications for treatment. World J. Biol. Psychiatry. 2008;9:102–114. doi: 10.1080/15622970801981606. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre RS, Lee Y, Mansur RB. Treating to target in major depressive disorder: Response to remission to functional recovery. CNS Spectr. 2015;20(Suppl 1):20–30. doi: 10.1017/S1092852915000826. [DOI] [PubMed] [Google Scholar]
- 24.Sawamura J, Ishigooka J, Nishimura K. Re-evaluation of the definition of remission on the 17-item Hamilton Depression Rating Scale based on recovery in health-related quality of life in an observational post-marketing study. Health Qual. Life Outcomes. 2018;16:14. doi: 10.1186/s12955-018-0838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bortolato B, et al. Cognitive remission: A novel objective for the treatment of major depression? BMC Med. 2016;14:9. doi: 10.1186/s12916-016-0560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali-Sisto T, et al. Purine metabolism is dysregulated in patients with major depressive disorder. Psychoneuroendocrinology. 2016;70:25–32. doi: 10.1016/j.psyneuen.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Kaddurah-Daouk R, et al. Cerebrospinal fluid metabolome in mood disorders-remission state has a unique metabolic profile. Sci. Rep. 2012;2:667. doi: 10.1038/srep00667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodwin GM. Depression and associated physical diseases and symptoms. Dialogues Clin. Neurosci. 2006;8:259–265. doi: 10.31887/DCNS.2006.8.2/mgoodwin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung CI, Liu CY, Yang CH, Wang SJ. Migraine and greater pain symptoms at 10-year follow-up among patients with major depressive disorder. J. Headache Pain. 2018;19:56. doi: 10.1186/s10194-018-0884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copeland VC, et al. Major depressive disorder and cardiovascular disease in African–American women. J. Soc. Serv. Res. 2017;43:624–634. doi: 10.1080/01488376.2017.1370682. [DOI] [Google Scholar]
- 31.Hung CI, Liu CY, Yang CH, Gan ST. Comorbidity with more anxiety disorders associated with a poorer prognosis persisting at the 10-year follow-up among patients with major depressive disorder. J. Affect. Disord. 2020;260:97–104. doi: 10.1016/j.jad.2019.08.085. [DOI] [PubMed] [Google Scholar]
- 32.Hung CI, Liu CY, Yang CH. Persistent depressive disorder has long-term negative impacts on depression, anxiety, and somatic symptoms at 10-year follow-up among patients with major depressive disorder. J. Affect. Disord. 2019;243:255–261. doi: 10.1016/j.jad.2018.09.068. [DOI] [PubMed] [Google Scholar]
- 33.First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). Biometrics Research. New York State Psychiatric Institute, New York (2002).
- 34.Hamilton M. Development of a rating scale for primary depressive illness. Br. J. Soc. Clin. Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacob D, Deborde C, Lefebvre M, Maucourt M, Moing A. NMRProcFlow: A graphical and interactive tool dedicated to 1D spectra processing for NMR-based metabolomics. Metabolomics. 2017;13:36. doi: 10.1007/s11306-017-1178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Meyer T, et al. NMR-based characterization of metabolic alterations in hypertension using an adaptive, intelligent binning algorithm. Anal. Chem. 2008;80:3783–3790. doi: 10.1021/ac7025964. [DOI] [PubMed] [Google Scholar]
- 37.Pu J, et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry. 2020 doi: 10.1038/s41380-020-0645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogawa S, et al. Plasma amino acid profile in major depressive disorder: Analyses in two independent case–control sample sets. J. Psychiatr. Res. 2018;96:23–32. doi: 10.1016/j.jpsychires.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Inoshita M, et al. Elevated peripheral blood glutamate levels in major depressive disorder. Neuropsychiatr. Dis. Treat. 2018;14:945–953. doi: 10.2147/NDT.S159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Islam MR, et al. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naïve first-episode major depressive disorder. BMC Psychiatry. 2020;20(1):333. doi: 10.1186/s12888-020-02738-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashimoto K, et al. Increased serum levels of serine enantiomers in patients with depression. Acta Neuropsychiatr. 2016;28(3):173–178. doi: 10.1017/neu.2015.59. [DOI] [PubMed] [Google Scholar]
- 42.Mitani H, et al. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(6):1155–1158. doi: 10.1016/j.pnpbp.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Grajeda-Iglesias C, Aviram M. Specific amino acids affect cardiovascular diseases and atherogenesis via protection against macrophage foam cell formation: review article. Rambam Maimonides Med. J. 2018;9:e0022. doi: 10.5041/RMMJ.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu G. Amino acids: Metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 45.Francklyn CS, Mullen P. Progress and challenges in aminoacyl-tRNA synthetase-based therapeutics. J. Biol. Chem. 2019;294:5365–5385. doi: 10.1074/jbc.REV118.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace M, et al. Effects of menstrual cycle phase on metabolomic profiles in premenopausal women. Hum. Reprod. 2010;25(4):949–956. doi: 10.1093/humrep/deq011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.