Abstract
A functional vascular system is indispensable for drug delivery and fundamental for responsiveness of the tumour microenvironment to such medication. At the same time, the progression of a tumour is defined by the interactions of the cancer cells with their surrounding environment, including neovessels, and the vascular network continues to be the major route for the dissemination of tumour cells in cancer, facilitating metastasis. So how can this apparent conflict be reconciled? Vessel normalisation—in which redundant structures are pruned and the abnormal vasculature is stabilised and remodelled—is generally considered to be beneficial in the course of anti-cancer treatments. A causality between normalised vasculature and improved response to medication and treatment is observed. For this reason, it is important to discern the consequence of vessel normalisation on the tumour microenvironment and to modulate the vasculature advantageously. This article will highlight the challenges of controlled neovascular remodelling and outline how vascular normalisation can shape disease management.
Subject terms: Tumour angiogenesis, Cancer microenvironment
Background
The vascular system spans about 100,000 miles within the human body, distributing oxygen and essential nutrients to organs and cells and taking away the by-products of metabolism.1 Ever since William Harvey first described this system in 1628, much has been written about this amazing network of arteries, veins and capillaries.2 Furthermore, one of the first scientific reports that documented a systematic approach observing the architectural changes in the vascular system after external stimuli date back as early as the 1850s.3–6
Vessels are generated by two distinct mechanisms. In early development, vasculogenesis involves the de novo formation of blood vessels by endothelial precursor cells (EPCs), which follow cues from growth factors and cytokines to create a primitive vascular tree; this vascular tree then gets remodelled and expanded by another mechanism, angiogenesis.7–9 However, EPCs from the bone marrow are known to participate in normal and pathological vessel formation in an adult.10,11
In healthy adults, endothelial cells tend to be quiescent and their turnover is very low compared with cells from other organs, for example, the gut. With the exception of the female reproductive cycle, angiogenesis is almost always pathological and the result of a trauma, surgery or an illness, such as cancer. Cancer cells are able to grow into solid tumours only by procuring a supply of nutrients and oxygen to meet their increasing demands for energy. They achieve this by creating a blood supply, either by exploiting the existing vasculature through co-option or vascular mimicry and/or by coaxing the existing vessels to expand through angiogenesis. As mentioned before, bone marrow-derived EPCs are also able to contribute to new vessel formation in an adult, although it is difficult to determine the exact extent of their contribution towards the tumour vasculature;12–14 some indication can be gained by indirectly assessing the number of cells that circulate in the blood.15
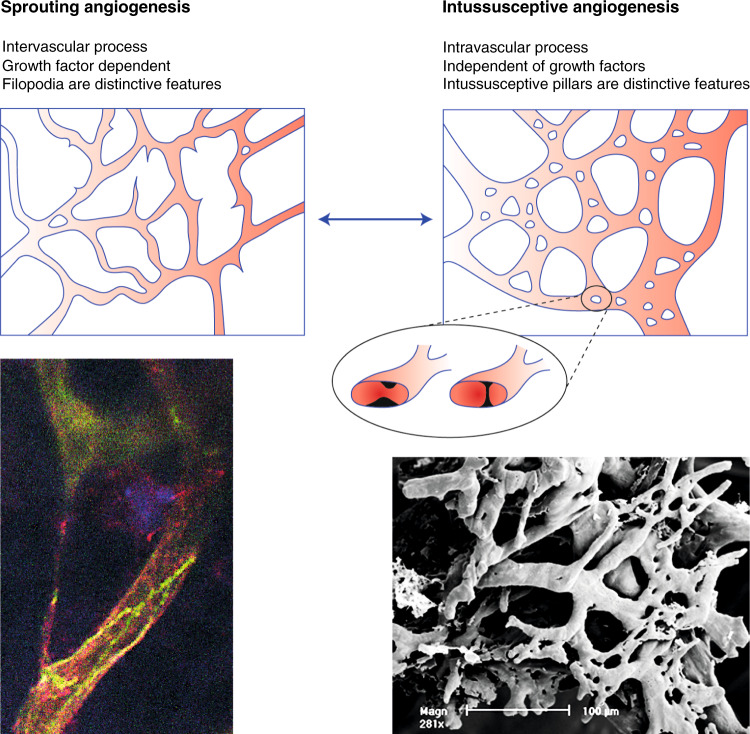
The majority of solid tumours are sustained by sprouting angiogenesis, in which endothelial cells can become tip cells or stalk cells (Fig. 1). Not all tumour growth necessarily depends on this mechanism. Intussusceptive angiogenesis is a less frequent, non-sprouting mechanism to increase vessel density that splits the existing vessel longitudinally, in principle dividing the lumen. Intussusceptive pillar formation is the defining feature (Fig. 1). There is little endothelial cell proliferation in the early stages of intussusceptive angiogenesis and pillar formation appears independent of angiogenic factors. There is no distinct growth factors gradient that guides the pillar forming endothelial cells in the way growth factors guide filopodial protrusions of a tip cell.16 This independence of growth factors can be a bypass around typical modes of anti-angiogenic targeting before sprouting angiogenesis prevails again.17,18
Fig. 1. Schematic illustration of the vascular network undergoing sprouting or intussusceptive angiogenesis.
The two mechanism of angiogenesis are not always exclusive, and a tumour can present characteristics of both, especially after anti-angiogenic therapy. On the left the cartoon illustrates how endothelial filaments called sprouts extending into the extravascular space. The accompanying confocal microscope image shows a filamentous bridge between two capillaries across the extracellular space. The endothelial cells and filaments are positive for PECAM-1 (green) and α5β1 integrin (red) in the Rip-Tag2 mouse model of pancreatic cancer. The cartoon on the right magnifies the cylindrical microstructures spanning the lumen of a capillary that are the distinctive feature of intussusception. For reasons not well understood endothelial cells on opposite sides on the lumen start to bulge until they meet to create the intussusceptive pillar. In the scanning electron micrograph on the right, those pillars appear as tiny holes on the outer surface on the vessel. The vascular casts of a colon tumour xenograft model reveal those very characteristic tiny holes of intussusceptive angiogenesis in the larger capillaries. The small diameter of ≈3–5 µm exclude them as mesh-structure in the vasculature. At this point, intussusceptive angiogenesis seems favoured over endothelial cell sprouting in those capillaries.
The fact that the circulation is required for tumour growth was known before the expression ‘tumour angiogenesis’ was coined.19,20 Evidence that existing host vessels are indeed seeding new vasculature following an activation signal and begin to sprout emerged with Judah Folkman’s ground breaking discovery in 1971 of a ‘tumour angiogenesis factor’.21 The theory had been that if a cancer could be stopped from growing its own blood supply it would wither and die. We now know that a ‘tumour angiogenesis factor’ does not exist in this simplistic form. Rather, a plethora of growth factors, together with other cytokines and biomolecules of various shapes and sizes, are involved in the process of angiogenesis (even different mechanisms of angiogenesis itself),22–25 such that targeting only one of the factors or receptors often spontaneously leads to the upregulation or activation of another factor to compensate for the loss.26 We also know that an intact, functional vascular system constitutes a key systemic route for the delivery of drugs and in particular is required for the responsiveness of the tissue microenvironment to these, and other, therapeutic agents, especially in the case of the tumour microenvironment (TME). Biologically and mechanistically treatment resistance is best understood in the context of tumour hypoxia. In the absence of a functional vascular network oxygenation of the TME is insufficient hence rendering chemotherapy, radiation and photodynamic therapy less efficient and the success of the latter depends on the generation of reactive oxygen species (ROS).27,28
Thus, targeting the blood supply of a tumour presents a challenge (Box 1). What has emerged from the study of the vasculature in response to anti-angiogenic drugs is that these agents can induce the phenomenon of vascular normalisation. This phenomenon can be exploited to confer improved efficacy in the course of anti-cancer treatments. In this article, we review the factors that regulate the vasculature and approaches to distinguishing between normalised and impaired vascular function. We conclude by proposing that temporal and spatial monitoring of vascular function will be of critical importance to maximising treatment impact and suggest ways in which this could be achieved in the future .
Box 1.
Endothelial cell heterogeneity
The vascular system can be classified according to size, function, cell morphology, gene and protein expression, depending on the organs that are supplied—in other words, on the nature of the vascular bed. Arteries, veins, arterioles and venules are larger vessels that narrow into a network of capillaries. The larger vessels consist of a continuous endothelium, held together by tight junctions, with three strong outer support layers. Capillaries are fine and narrow vessels that can be continuous, fenestrated or discontinuous, and are stabilised by the basal lamina and pericytes. The phenotypes of endothelial cells vary with their function and vascular bed. Extreme examples would be the highly fenestrated or discontinuous endothelium of capillaries in filtrating organs, like the kidneys and liver, and the tight endothelium of capillaries in the brain that constitutes the blood–brain barrier.146 Endothelial cells are capable of sensing and responding to their environment, which accounts for their phenotypic heterogeneity. Separated from their vascular bed and cultured in situ, they undergo a phenotypic drift. DNA microarrays reveal that about 50% of their site-specific genes are lost after passage. Concurrently, some specific properties are retained under cell culture conditions, which means that their gene expression is mediated by both their immediate environment and by epigenetics.147
Endothelial cell heterogeneity in tumour vessels
The phenotype of tumour endothelial cells (TEC) differs greatly from the normal endothelial cells in the host organ. The epigenetic footprint of endothelial cells in the tumour vasculature, however, is conserved, meaning the specific properties linked to their original vascular bed are kept.
For a long time, it was accepted that TEC cells within tumours were genotypically equal to their normal counterparts and that any changes in phenotype were a mere reaction to the cancerous environment. Today it is appreciated that TECs can display cytogenetic abnormalities. TECs derived from malignant melanoma and liposarcoma are not normal diploid cells but contain an abnormal number of chromosomes in the nucleus (aneuploidy). The chromosomal instabilities are not clonal but heterogeneic within a TEC population. However, the mechanisms that cause TEC heterogeneity are still pinned on the tumour environment.62,148
Tumours contain abnormal blood vessels
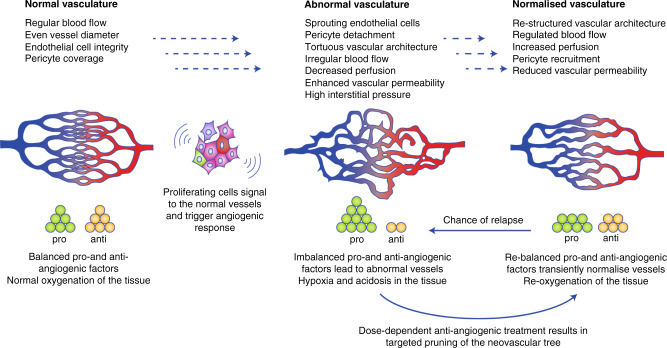
Whichever of the mechanisms that a solid tumour uses for increasing the blood supply (and, in many cases, a combination to various degrees of all seems the reality), the newly formed vessels (neovessels) are abnormal in the majority of cases.29,30 A relentless exposure to angiogenic factors released from the hypoxic tumour environment renders the endothelial cells in a state of constant activation, resulting in an uneven and dilated lumen and a tortuous vessel architecture (Fig. 2). Despite a seeming abundance of blood vessels, many of which retain blood flow, large areas within the tissues remain hypoxic. Chronic hypoxia stems from the shortened radial oxygen diffusion distance in tumour blood vessels. Moreover, irregular blood flow and fluctuation in perfusion adds acute or ‘cycling hypoxia’ to the overall chronic hypoxic state of the tumour.31 Mural cells called pericytes on capillaries are no longer adherent, and thereby lose their regulatory influence on the vessel stability.32 The basement membrane becomes thickened in some places and is absent in others.33 Weak endothelial cell–cell junctions in the tumour vasculature enable rogue cancer cells to enter the circulation—the first step of potential metastasis34—as well as creating gaps and leaky vessels. Although vessel leakiness might improve drug delivery by facilitating the passage of molecules through the endothelial lining,35 this benefit is offset by restricted blood flow and high interstitial pressure in the tumour. The lack of a pressure gradient shifts the distribution mechanism (of particles in general) from being directional (movement from a high-pressure region to a low-pressure region) to being non-directional (‘diffusion’). Diffusion through the dense extracellular matrix (ECM) is extremely slow so that some macromolecules can be re-absorbed into the circulation. This has been exemplified experimentally for globular IgG, albumin and dextrans using fluorescence correlation spectroscopy.36,37
Fig. 2. Schematic illustration of the vascular network in its normal, abnormal and normalised state.
Angiogenic and anti-angiogenic factors are finely tuned in healthy tissues to create an organised vessel structure and to maintain vascular function. In tumours, the angiogenic switch has taken place and the balance has tipped in favour of angiogenic factors. As a result, the structure of neovessels is abnormal on all levels, with highly impaired vascular function. In the normalised state, angiogenic and anti-angiogenic factors are nearly balanced and vascular function is transiently re-established.
Angiogenesis inhibitors and vascular normalisation
The rationale behind anti-angiogenic treatment is that blocking blood vessel formation in tumours or its regression will deprive cancer cells of nutrients and oxygen and finally starve tumours to death or induce tumour dormancy. However, anti-angiogenic treatment fails to bring about permanent de-vascularisation; rather, normalised tumour vessels emerge from the neovascular tree. Drug-induced vessel normalisation was observed and documented in 1972 by Le Serve and Hellman, when treatment with ICRF-159 (razoxane) was seen to cause the chaotic tumour neovasculature of Lewis Lung Cancer (LLC) in mice to appear normal.38 The drug was discovered through random screening and selected for its cytotoxic properties. At the time, it was largely unknown how ICRF-159 changed the architecture of the tumour vessels. Today we know that ICRF-159 is an inhibitor of the platelet-derived growth factor-β (PDGF-β) receptor, and its anti-angiogenic properties and potential as a vessel normalisation drug have been established.39 The response of the pathological neovasculature to anti-angiogenic treatment had not been anticipated and the effect that normalisation has on malignant and non-malignant cells opened new avenues for cancer combination therapy.40–42
Vessel normalisation almost always follows the same recognisable pattern in terms of vascular structure plasticity and changes to the TME.43 The immature parts of the neovasculature seem to be more susceptible to anti-angiogenic treatment than more mature vessels are, indicating that mature vessels are evidently less dependent on angiogenic factors. Tumour vessels that do not regress after anti-angiogenic treatment appear normalised, as illustrated in Fig. 2. The reduced cellular demand for blood supply re-establishes the equilibrium between angiogenic and anti-angiogenic factors. During the following vessel stabilisation effect, the endothelial cells in the immature vessels secrete basement membrane proteins and send signals that recruit mural cells. Cell junction integrity is resumed and permeability is again tightly regulated.44 However, the most important biological aspect of normalised vessels is that a reliable circulation replaces the previously flawed system, such that restored perfusion reverses almost all the abnormalities.
Assessing normalised vessels
In animal models, vessel normalisation is mostly observed by microscope image analysis, either at fixed time points or in real time (via a dorsal window chamber).45,46 Contemporary image analysis software allows for the quantitative analysis of vessel branching, number of sprouting endothelial cells, vessel length and diameter. Spatial image analysis of distance, for example, between pericytes and the endothelium is an indicator of vessel maturity. Blood flow and perfusion can be monitored by systemic optical tracers or non-invasively by functional magnetic resonance imaging (fMRI) and photoacoustic imaging.47,48 Visual and functional assessment of vascular normalisation is usually supported by complementary analysis at the molecular level, such as the analysis of the expression of angiogenic factors.
We can define normalised vessels according to several prominent characteristics. A significant drop in angiogenic factors results in fewer sprouting endothelial cells. Mural cells, such as pericytes, adhere again to the vessel walls and pericyte coverage increases. Through remodelling and reduced branching, leaner and less tortuous vessels with an even luminal diameter emerge, reducing the blood back flow that is typical for abnormal vessels and restoring a uniform blood pressure. Pruning of truncated vessels and endothelial structures that lack a lumen improves the perfusion. In the surrounding tissue, oxygen levels rise and hypoxia decreases, which, in turn, reduces hypoxia-induced activation of angiogenesis. In short, blood vessel maturation takes place.49 In reality, a combination of these restored normal blood vessel characteristics usually appears, with degrees of intra and inter tumour variation, which might depend on the tumour type and treatment.
Current strategies and potential targets for tumour vessel normalisation
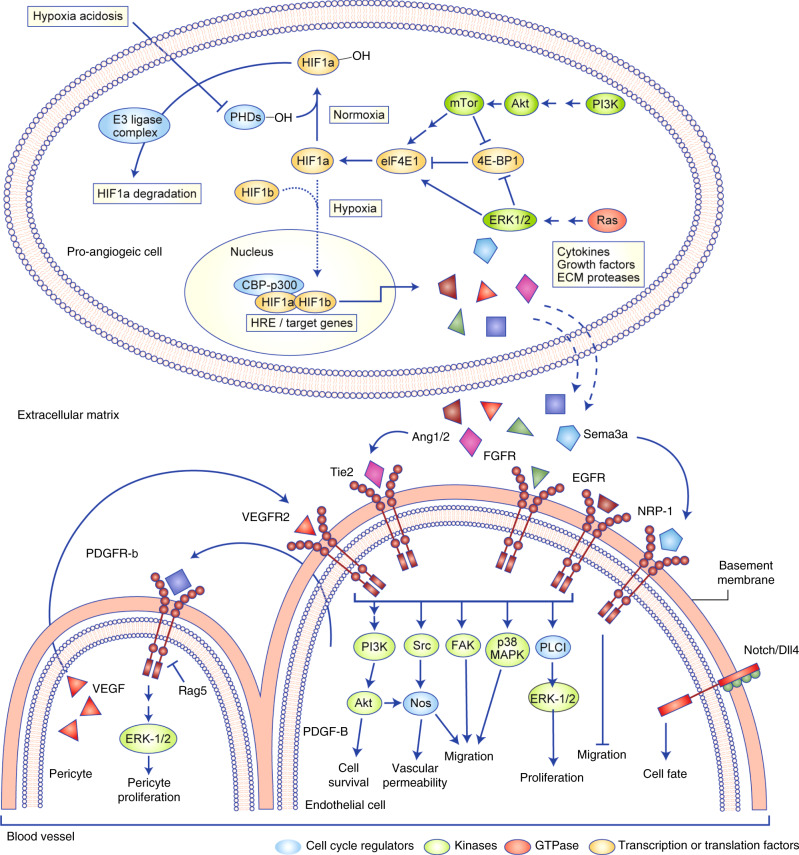
The signalling pathways that lead to sustained angiogenesis are illustrated in Fig. 3, and include several different types of molecule, such as kinases and GTPases. Targeting any of these molecules is likely to bring about changes in the vasculature that might influence tumour vessel normalisation. Similarly, promoting factors that initiate vascular maturation and sustain maintenance can induce vascular normalisation.
Fig. 3. Illustration of the major signalling pathways that either control angiogenesis and therefore represent potential targets for anti-angiogenic intervention.
In normal tissues, prolyl hydroxylases (PHDs) hydroxylate hypoxia-inducible factor (HIF)-1α; the level of HIF-1α is low or it gets degraded. Hypoxia, however, prevents the hydroxylation and HIF-1α levels rise. The HIF-1α/β complex binds to the hypoxia-response element (HRE) of the gene promoter for transactivation and expression of angiogenic factors, prominent among which are VEGF/VEGFR-2 and other activated tyrosine kinase receptors that determine cell survival, proliferation and cell motility. Tie2 and Ang-1 are vital for maturation and maintenance of the neovessels. Ang-2 in combination with VEGF promotes neovascularisation, but in the absence of VEGF triggers vessel regression. Neuropilin-1 is the specific receptor for class 3 Semaphorin (Sema) proteins. The anti-angiogenic Sema3/neuropilin complex controls endothelial cell motility by negatively regulating cytoskeletal events via small GTPase signalling. VEGF-dependent Notch-1/DLL4 receptor activation specifies endothelial cell fate. In cells with high Notch-1 activation the angiogenic potential is lowered, those cells remain quiescent, in cells with low Notch-1 activation the angiogenic potential is raised and they become sprouting cells. The pathways that regulate angiogenesis are interlinked.
Inhibiting proangiogenic signalling
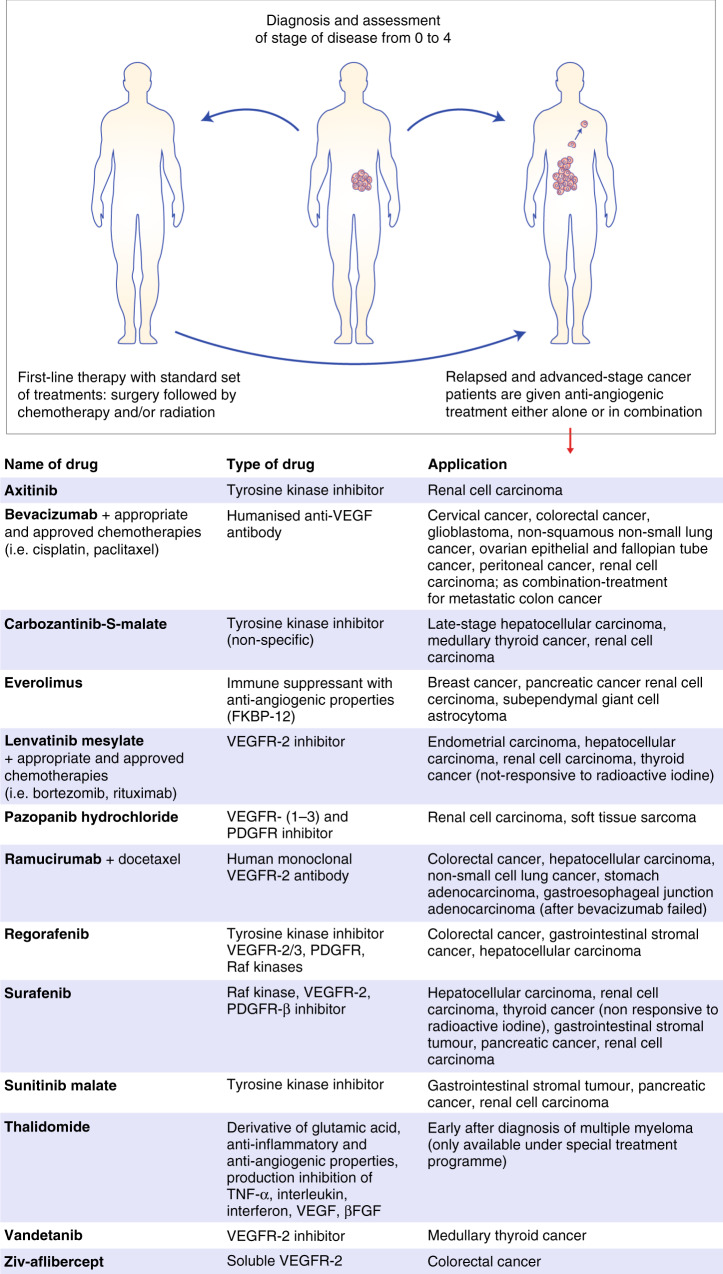
As represented in the table in Fig. 4, targeting VEGF-A with blocking antibodies or using soluble VEGF receptors, or directly inhibiting the VEGF-receptor 2 (or, less specifically, tyrosine kinase receptors in general) was, and still is, the first choice to downregulate neovascularisation.39 Early research to prove the hypothesis of vessel normalisation used DC101, a monoclonal antibody against VEGFR-2. A single intravenous dose of DC101 administered to tumour-bearing mice could reduce the interstitial fluid pressure by 50% without evidence of lymphatic vessel restoration.46 Extensive vascular pruning of vessels in the tumour was observed through a dorsal window chamber. Vascular density dropped and vessel diameter shrunk. In addition, metalloproteinases that degraded layers of the basement membrane to a normal thickness were activated, and levels of angiopoietin-1 (Ang-1), which supports the recruitment of new pericytes, increased temporarily. Another important finding from these investigations was that the vascular normalisation effect was transient and occurred 3–5 days post treatment, and that, during this time, the response to radiation therapy was greatly improved.50,51 These findings gave rise to the idea that, rather than inhibiting angiogenesis altogether, controlling angiogenesis would be a preferable approach.
Fig. 4. Anti-angiogenic treatment is approved by the FDA for the treatment of advanced cancers after conventional chemotherapy has failed or if cancer has metastasised (last reviewed 2019 source NIH, cancer.gov).
Only three anti-angiogenic dugs are applied in combination with traditional chemotherapy (bevacizumab, lenvatinib mesylate, ramucirumab). Thalimodide and lenvatinib mesylate are the only two treatments that are used in the early stages following diagnosis or as a first-line treatment and if surgery is not an option. The vast majority of anti-angiogenic drugs are tyrosine kinase inhibitors with some of them acting specifically on the VEGFR-2 receptor.
VEGF is highly expressed in hypoxic areas. The ectopic expression of a recombinant soluble form of VEGFR-2 in melanoma cells under hypoxic conditions did indeed bind murine and human VEGF sufficiently to slow down endothelial cell growth in vitro and reduce the tumour mass in vivo,52 and increased oxygen levels and reduced hypoxia suggested normalisation of the vessels. Despite controversy about the biological properties and function of VEGFxxx isoforms they were reported to promote angiogenesis while VEGFxxxb isoforms (generated by alternative splicing) have been shown to inhibit angiogenesis.53 Re-establishing the natural equilibrium of angiogenic and anti-angiogenic VEGF-A splice variants might offer an alternative means of tuning the angiogenic switch instead of blocking it. Although this method of regulation was achieved by locally adding the anti-angiogenic variant, modulation on a transcriptional level by inhibiting SRPK-1, the key factor in the spliceosome that regulates VEGF-165/VEGF-165b expression might be a more promising approach in the future.54–56 The regulatory effect of the VEGF-165b variant on the neovasculature is considered particularly advantageous in diabetic retinopathy, where a normalised vascular network is preferable over a significant de-vascularisation.57
Angiogenesis is regulated by a complex network of direct and indirect factors. In many ways, these factors are not entirely independent of VEGF/VEGFR-2 signalling. Some of them bear, for example, a resemblance to VEGFR because they are also tyrosine kinase receptors and signal via the same pathways, and/or trigger VEGF upregulation or otherwise influence the VEGF/VEGFR-2 pathway further downstream. Although it mediates downstream signalling by inducing serine/threonine kinase activity, rather than by activating a tyrosine kinase receptor, transforming growth factor β (TGF-β) plays a role in tumour progression.58,59 Blocking TGF-β and its receptors decreased the tumour size and prevented metastasis in breast cancer and glioblastoma models and significantly improved the intratumoural penetration of low-molecular weight chemotherapy drugs and nanoparticles in breast cancer models. The effect was attributed to tumour vessel normalisation, as a higher number of perfused vessels was counted and pericyte recruitment to these vessels was observed.
Epidermal growth factor receptor (EGFR), a tyrosine kinase receptor that, among several other functions, activates H-Ras to stimulate angiogenesis, and phosphoinositide 3-kinase (PI3K) and protein kinase B (PKB/Akt), which both regulate angiogenesis downstream of VEGFR-2, are additional targets that could be considered to achieve vascular normalisation. Selectively blocking one or more of these molecules resulted in persistent vascular changes for up to 2 weeks post treatment in human xenograft-bearing mice.60,61
Rgs5: a master gene for abnormal vascular morphology in the tumour
Endothelial cells in abnormal tumour vessels are not homogeneous, and differ in phenotype from their non-tumour counterparts.30,62 Although transcriptomic profiles can vary between tumour vessels and normal vessels, few genes directly associated with the abnormal neovasculature have been identified.59 The regulator of G-protein signalling 5 (Rgs5) has been shown to be a master gene for abnormal vascular morphology in the tumour.63 Rgs5 is expressed in a variety of organs and upregulated on blood vessels in Rip1-Tag5 tumours. Pericytes around the vessels of these tumours were predominantly PDGFR-β positive. Crossing Rip1-Tag5 with Rgs5−/− mice resulted in vascular remodelling and pericyte maturation, which was assessed by α-smooth muscle actin (αSMA) and neural/glial antigen 2 (NG2) expression. Additional observations such as reduced vascular leakage, increased perfusion and higher oxygen levels are consistent with vascular normalisation. Other tumour models crossed with the Rgs5−/− mice also generated similar findings. A gene whose expression directly relates to abnormal vessel formation could theoretically become a target for therapeutic genome editing.64
Promoting factors that support vessel normalisation
As well as blocking factors that are actively proangiogenic, promoting factors that initiate vascular maturation and sustain maintenance were found to normalise the vessels in a tumour. Unlike oncogenic H-Ras GTPases, R-Ras has been identified as an inhibitor of endothelial cell proliferation and functions instead as a regulator of vessel integrity and maturation during vascular remodelling to promote normalisation of tumour vasculature.61
Members of the family of angiopoietins have contrasting effects on the vessels. Ang-1 promotes vessel maturation and pericyte recruitment, whereas Ang-2 promotes endothelial cell death and vascular disruption and also works as an antagonist to Ang-1 via the Tie-2 receptor. Inhibiting Ang-2 while simultaneously activating the Tie-2 receptor leads to vascular normalisation in glioma and LLC in mouse models.65,66
Semaphorins comprise a large and diverse family of secreted, membrane-associated or membrane-bound signalling proteins that are essential for the development and maintenance of many tissues.67 The class 3 semaphorins Sema3A and Sema3F are assigned to the vascular system and counterbalance the actions of VEGF via the Sema3A–neuropilin receptor (NRP-1) complex. In tumour angiogenesis this balance falls in favour of VEGF, but in studies that followed the targeted delivery of Sema3A, tumour angiogenesis was inhibited and vessels regained a functional morphology.68,69
Nucleolin is predominantly localised in the nucleolus but is also found on the surface of proliferating endothelial cells. Its inhibition using a blocking antibody reduced tube formation and led to vessel normalisation in vivo, possibly via endothelial cell apoptosis as the levels of the anti-apoptotic molecule Bcl-2 were also reduced.70
Notch/Delta like ligand (DLL)1 signalling is an important determinant of cell fate in early development.71 In sprouting angiogenesis, Notch-1/DLL4 signalling shapes the vascular network by downregulating the VEGF-induced activation required for an endothelial cell to become a tip cell, which leads the way for proliferating stalk cells that eventually form the tubular network. Notch-1-deleted endothelial cells therefore preferentially become tip cells, which promotes an abnormal vasculature.72,73 The anti-malaria drug chloroquine was found to induce vessel normalisation by stimulating the Notch-1/DLL4 signalling pathway.74,75 In vivo, chloroquine reduced the tumour mass and improved the tumour milieu by increasing tissue perfusion, thereby lowering hypoxia and reducing tumour cell invasion while enhancing tissue sensitivity to chemotherapy.
The TME in solid cancers is acidic as a consequence of hypoxia and increased glucose metabolism. The metabolism of tumour endothelial cells (TECs), which are also hyperglycolytic, has gained attention over past years,76 as the normalisation of TEC metabolism is a major contributor to tumour vessel normalisation. Notably, pharmacological inhibition of tumour-cell-specific cyclooxygenase (COX)-2 leads to reduced expression of VEGF and, consequently, of PFKFB3, the gene that encodes 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3, in the TECs. Treatment with a COX-2 inhibitor normalises glucose metabolism in TECs and inhibits PFKFB3-mediated endothelial cell motility, leading to a reduction in the formation of tip cells and filopodia and decreased branching in the neovessels.77 An earlier extensive study that used either a blocking agent against PFKFB3 or transgenic mice +/− knockout mice, with the aim of slowing glucose metabolism, resulted in a similar normalisation effect on the neovessels.78
External factors that promote blood vessel normalisation
Tumour blood vessel normalisation can also occur without specifically targeting blocking or activating molecular angiogenic signalling factors. Cell death in response to radiation is largely dependent on the dosing and time frame of the therapy. A single high dose of radiation (30–60 Gy) is known to destroy most cells in the targeted tissue, causing a lasting decrease in vascularity.79 However, fractionated radiation, in which the dose is split and given over several intervals allows time for the normal cells to recover and undergo repair as shown in an orthotopic prostate xenograft mouse model of PC3-luc cells; here, the mice were treated with fractionated radiation of 2 Gy on each of 5 consecutive days over a total time of 2 weeks. Examination of the tissues at given time points (days 0, 1, 3, 7 and 14) revealed a remodelled vascular network with increased pericyte coverage and positive normalisation effects, such as increased perfusion and low hypoxia.80
Compelling observational evidence indicates that regular exercise synergises with anti-cancer therapy and rehabilitation. Data from preclinical studies suggest that physical activities/exercise promote tumour vessel maturity, followed by positive changes in tissue and oxygen perfusion and drug delivery and a boost to the immune response, all of which combine to improve the outcome of chemotherapy and radiotherapy in individual patients.81,82 When the underlying mechanisms of the influence of moderate exercise on tumour vessel normalisation were studied in detail in vivo and in vitro,83 the increased blood flow after exercise was seen to confer mechanical force on the endothelial cells. The resulting fluid shear stress on the vessel walls stimulates endothelial cells, triggering the production of nitric oxide and vascular remodelling mechanisms.84 The neovasculature of exercised tumour-bearing mice demonstrated all the signs of vessel normalisation, except for pericyte coverage. When the authors further investigated the effect of shear stress on endothelial cells, they observed that serum from exercised mice inhibited the formation of typical vascular structures by endothelial cells cultured on matrigel, suggesting that soluble angiogenesis inhibitory factors were secreted in the mouse model.85 Experiments on endothelial cells cultured under shear stress confirmed this observation. Subsequently, the authors discovered that the activation of nuclear factor of activated T cells (NFAT), via calcineurin, in endothelial cells is central to vascular remodelling in response to shear stress and leads to the upregulation of thrombospondin-1 (TSP-1), an inhibitor of angiogenesis. TSP-1 is essential for exercise-induced vessel normalisation.85
Vascular normalisation, anti-cancer treatment and the TME
Even before systematic research into vessel normalisation began, the benefit of anti-angiogenic treatment in combination with chemotherapy and/or radiation became obvious. Patients who received bevacizumab, a humanised blocking antibody to VEGF-A, in combination with chemotherapy in clinical trials fared much better than patients who were given either treatment alone.86 It was originally thought that this outcome occurred because tumour cells, once starved and weakened, were more susceptible to treatment. This turned out to be only a partial explanation, because less perfused tissue tends to be hypoxic, and hypoxic tissue is ultimately more resistant to radiotherapy and chemotherapy.27 The main explanation for the benefit on patients was that anti-angiogenic treatment promoted vessel normalisation. Improved perfusion is overcoming physiological barriers to tissue oxygenation and makes radiation more effective owing to a decrease in hypoxia-induced radiation resistance.28 Reducing vessel leakiness decreases the interstitial hypertension and restoring a distinct pressure gradient leads to the deeper penetration of macromolecules (chemo-therapeutics) in the tissue and facilitates the migration of immune cells.
A normalised vasculature boosts the tumour immune response
The immune response to cancer could be considered as a specialised case of immunity.87 In its early stages, a tumour engages in crosstalk with the innate immune system, and innate immune cells, such as macrophages, monocytes and dendritic cells that patrol the blood, accumulate at the neoplastic site. So why can the immune system not prevent tumour growth? In cancer, pathogen recognition triggers an acute inflammatory response, which recruits cytotoxic immune cells. These cells recognise and eliminate the more immunogenic cells, but this process selects for less immunogenic, often more aggressive, cells. As part of the TME, endothelial cells actively participate in the attraction of immunoregulatory factors, and can enhance or suppress the immune response, depending on their interactions and their expression of inflammatory cytokines.88 Lymphocyte trafficking, for example, is highly orchestrated: many molecular and physical factors must align to facilitate the initial and crucial step—lymphocyte rolling—in the process that enables T cells to exit the endothelium and make contact with the antigen-presenting cells (APCs).89 Although endothelial cells are not professional APCs, they do possess the capacity to express MHC-I and MHC-II molecules and can act like APCs.90,91 However, the presence of an aberrant tumour vasculature means that none of these factors can be co-ordinated.92–94 Consequently, the inflammatory response in a tumour no longer has the positive effect of an acute reaction to harmful stimuli but becomes a chronic inflammatory site; to quote Harold Dvorak, “tumours are wounds that never heal”.95,96 That may explain why a majority of patients do not benefit from anti-cancer immunotherapy in the long term. Vessel normalisation, linked to increased tissue perfusion and reduction of stromal components such as cancer-associated fibroblasts (CAFs) and collagen, to mention a few, directly promotes immune cell infiltration and functionality and as a result enhances the response to immune therapy.97
As indicated above, blood vessel normalisation leads to an improved immune response, involving T-lymphocyte recruitment from the secondary lymphoid organs to the tumour tissue and the accumulation of functional immune cells in the TEM,93 and unrestricted blood flow and restored perfusion of the tissue reduce hypoxia. Hypoxia is known to contribute to immune resistance and immune suppression in the TEM,28,98 as hypoxic regions are highly infiltrated by immune-suppressive cells such as myeloid-derived suppressor cells, tumour-associated macrophages and T-regulatory (Treg) cells, which are immune-suppressive.99,100 Vessel normalisation also restores the functionality of the luminal lining, or glycocalyx, in the neovessels, which is essential for leukocyte rolling.101 High levels of VEGF suppress T-cell infiltration into tumours by decreasing the expression of the T-cell-attracting chemokines CXCL10 and CXCL11; consequently, this effect is reversed upon vessel normalisation.102
The success of treatment using immune checkpoint inhibitors is increased in patients with a higher number of pre-existing tumour-infiltrating immune cells that express PD-L1, the ligand for programmed death-1 (PD-1).103,104 PD-1, alongside cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and lymphocyte activation gene 3 (LAG-3), is a well-studied inhibitory immune checkpoint that negatively regulates T-cell effector function and, by themselves synthesising these checkpoint proteins, cancer cells can avoid being attacked.105–107 Vascular normalisation mediated by low-dose anti-angiogenic treatment can convert cancers that are unresponsive to immunotherapy into responders, as demonstrated in a small group of patients with glioma in whom the beneficial effects of the immune checkpoint inhibitor ipilimumab were observed only in combination with bevacizumab.108 A combination of bevacizumab and CTLA-4 immune checkpoint inhibitors has proven favourable for the treatment of melanoma patients, possibly by evoking humoral immunity to galectin-1, an angiogenic and pro-tumour factor.109,110
An improved immune response supports vessel normalisation
It also transpires that an improved immune response is not only a consequence of tumour vessel normalisation but that it also contributes to vessel normalisation. Patients with tumours that were eosinophil-rich had better overall prognoses than those with fewer eosinophils.111 Eosinophils contribute to tumour immunity by secreting chemoattractants for CD8+ T cells. The intravenous transfer of activated eosinophils into MO4 (mouse melanoma) bearing mice (in combination with activated T cells) induced a remarkable normalisation effect on the neovasculature, including enhanced pericyte recruitment and downregulation of the Rgs5 gene.112 Activated eosinophils altered the TME by shifting the balance of TAMs from the invasion- and metastasis-promoting phenotype, M2, to the classically activated phenotype, M1.113,114 A similar effect on macrophage polarisation could be achieved by inhibition of the Ang/Tie2 and the pyruvate dehydrogenase/hypoxia-inducible factor 1-α (PDH/HIF-1-α) pathways.115,116 CD4+ and CD8+ T cells adoptively transferred into Rgs5-negative Rip1-Tag5 mice prolonged their survival because the T cells were able to significantly infiltrate the ‘normalised’ tumours of the knockout mice but not of wild type mice.63 Experimentally disrupting the vessel normalisation process using an NG2-knockout mouse model (which hampers pericyte recruitment) resulted in reduced CD4+ TH1 cell infiltration. In turn, the depletion or deactivation of CD4+ TH1 cells decreases tumour vessel normalisation.117 T cells are known to secrete interferon γ, which is known to regulate angiogenesis.118,119 The normalised tumour vasculature and CD4+ TH1 cell immune response create some sort of positive feedback loop that generates a controlled angiogenic response.120,121
Lessons from glioblastoma
In a clinical study in which glioblastoma patients were given a single dose of AZD2171 (cediranib, a VEGFR inhibitor) signs of vessel normalisation—based on the calculation of a ‘vascular normalisation index’—lasted for as long as 28 days, with some features of the normalised network persisting for up to 4 months. Vascular permeability/flow and microvessel volume assessed by fMRI correlated positively with the clinical outcome of patients with glioblastoma. For most patients who showed a high degree of vascular normalisation after a single dose of cediranib only, the clinical outcome improved.122,123 In a subset of patients with newly diagnosed glioblastoma within a Phase 2 trial that had received a regime of cediranib and chemoradiation, better perfusion and, consequently, higher oxygenation of the tumour tissue were associated with improved overall survival. Oedema in the brain was reduced and responses to radiotherapy were enhanced.124
The dose-dependent response to bevacizumab in the orthotopic glioma mouse model U87 showed that vessel regression and vessel normalisation occurs in animal treated with low (subclinical) and medium to high doses. Tumour regression and prolonged survival of the animals, however, was only observed in the medium to high dose group. The authors did not find bevacizumab triggered invasive behaviour of the glioma cells within their treatment time frame of 12–25 days.125 Although the anti-angiogenic treatment of brain tumours and accompanying vessel normalisation are associated with an overall benefit in some studies, data from other studies have revealed conflicting results. Keunen et al. reported that treatment of human glioblastoma xenografts in rats with bevacizumab decreased the number of larger sized vessels and rendered the tumour tissue more homogeneous but also increased tumour cell migration into healthy parts of the rat brain.126 The authors suggested that a drop in tumour oxygenation caused by fewer blood vessels triggered a metabolic change in the tumour cells that shifted them towards an invasive phenotype. Whether or not these findings translate to end-stage glioblastoma patients treated with bevacizumab is unknown.127
Normalisation of brain tumour vessels might be expected to help to restore the blood–brain barrier (BBB), which could be considered as an adverse effect on therapy, as this reduces the efficiency by which subsequent chemotherapy agents pass through the endothelium.128,129 Furthermore, gliomas are amongst a special group of cancers that are known to use vascular co-option for the growth of primary tumour sites and for metastasis. The cancer cell cytoskeleton and cell motility are fundamental factors in the molecular mechanisms of vascular co-option and mediated by Cdc42, a small Rho GTPase that regulates filopodia formation of the actin cytoskeleton and integrins.130–132 Integrin adhesion via the β1 chain seems essential for cancer cells to adhere to endothelial cells to both invade and escape from the vascular system through intercalation.133,104 In the brain, glioblastoma cells with cytoskeletal actin extensions, called flectopodia, adhere to the blood vessels by connecting in an astrocyte-like manner to the supporting pericytes suggesting that vessel normalisation and stabilisation by pericytes could potentially support vascular co-option and hence metastasis.134 However, a strong link between vascular co-option and vessel normalisation after anti-angiogenic treatment needs to be determined and the potential for therapeutically targeting vascular co-option evaluated.
How can we seize the moment?
Most FDA-approved anti-angiogenic drugs are primarily given when standard first-line cancer treatment has failed or when a patient is diagnosed with an advanced stage of the disease (see Fig. 4). We also know that, in principle, it is possible to use these agents to create optimised conditions in the TME to push the therapeutic impact of other approaches further. We thus need to be able to identify this ‘window of opportunity' by scrutinising the temporal changes in the TME as cancers evolve so that we define or at least predict the providential moment to for treatment.
Predicting a response to vessel normalisation treatment
Clinical trials involving breast cancer patients who received a single dose of bevacizumab followed by chemotherapy showed that the microvessel density prior to bevacizumab treatment could be used as a marker to predict treatment outcome—only those women whose tumours were highly vascularised before the treatment responded to bevacizumab-induced vessel normalisation.135
The genetic composition of tumour cells can determine whether or not a patient will respond to treatment by undergoing tumour vessel normalisation. So-called ‘Good Prognosis Angiogenesis Genes’ (GPAGs), discovered by screening breast cancer gene databases, are predominantly associated with cell–cell adhesion and smooth-muscle cell proliferation, and such gene expression programs are common in pericytes and pericyte recruitment in vessel normalisation GPAGs are also associated with immune response pathways, especially T-cell receptor signalling.117 This association underscores the potential to use gene expression signatures associated with pericyte function to predict not merely vessel normalisation but perhaps also the efficacy of an associated immune response. As such it exemplifies the intimate relationship between vascular function and the immunological state of cancers.
Determining the window of normalisation
In humans, non-invasive monitoring down to the resolution of microvessels still remains a challenge. MRI, computed tomography (CT) and positron emission tomography (PET) scans are imaging methods that are routinely used in the clinic to measure the size and location of tumours and, although large vessel such as arteries and veins can be seen using a contrast agent (magnetic resonance angiogram or ‘MRA’), the spatial resolution is too low for microvessels, and the movement of fluids, essentially blood, cannot be detected. The clinical use of fMRI, which allows the measurement of blood oxygenation relative to the paramagnetic properties of deoxyhaemoglobin also lags behind its use in research.136 In animal models, however, good results were obtained monitoring vessel normalisation using, for example, imaging biomarkers with fMRI or by photoacoustic imaging. Both methods were able to denote the normalisation window in mice and rats after low-dose anti-angiogenic treatment.47,48,137 The practical advantages of photoacoustic imaging over approaches that demand large and expensive instruments renders this technique a likely choice for monitoring the (tumour) vasculature in humans and in the clinic in the near future.138
A further approach to determine the beginning and end of the vessel normalisation process involves non-invasive imaging of the tumour redox state and energy metabolism. Monitoring the partial pressure of oxygen (pO2) in murine tumours using electron paramagnetic resonance (EPR) and fMRI imaging identified a normalisation window 2–4 days after anti-angiogenic treatment with sunitinib. The perfusion of the tissue was improved and reduced the occurrence of acute hypoxia. The oxygen levels in the tissues were stabilised to normal and an oxidative shift of the tumour redox status in the metabolic pyruvate/lactate flux measured by an exogenous nitroxide probe and 13C-NMR was revealed.31,139 In principle, it would be possible to measure metabolic changes using PET—for example, fluorodeoxyglucose (FDG)-PET could be used to measure glucose uptake in endothelial cells. A reduced glucose uptake can lead to vessel normalisation and hence be a valuable indicator of this process.77,78,140 At the same time, a widespread goal is to step away from costly and time-consuming methods towards strategies that are more amenable to routine clinical practice.
Few molecular markers that indicate the presence of a normalised TME have been identified. A potential candidate, however, is apelin, a peptide ligand for the apelin receptor (APJ), which, when activated, regulates blood pressure and encourages the formation of new blood vessels.141 The expression of apelin is driven by hypoxia and hence is comparably high in cancers.142 Low apelin mRNA or protein serum levels were shown to indicate vessel normalisation in the mouse model; here, the lowest apelin levels were seen around day 5 after treatment, suggesting a normalisation window between days 3 and 5.143
Outlook
So much evidence now exists for the benefit of a normalised tumour vascular network as part of any cancer treatment that it offers an extraordinary opportunity to improve treatment outcomes. Immunotherapy, for instance, has progressed rapidly in the past five years, but not all types of tumour respond to this approach; it is now therefore vital to complement this treatment, and others, with well controlled adjustments to the vasculature. Finding the opportune moment to normalise the vasculature remains a challenge, and the discovery of a reliable and universally measurable indicator has yet to be made. However, some approaches to assess the problem are emerging. Measuring hypoxia-driven biomarkers, such as apelin, in a blood test might be more feasible than measuring the expression levels of a protein like CD109 in dormant endothelial cells or assessing blood oxygenation by fMRI each day over weeks.144
An additional challenge lies in how best to create such a ‘window of opportunity’ therapeutically and to keep it open for as long as necessary. Systemic and oral anti-angiogenic treatments can come with severe side effects. The range of clinically approved anti-angiogenic medication is limited, and most anti-angiogenic drugs will be given after conventional treatments have failed or if a patient is diagnosed with advanced stages of cancer (see Fig. 3). Both factors can be restrictive in the design of combination therapy. Applying precision radiotherapy in early stages to create vessel normalisation has considerable potential. Endothelial cells are, by definition, normal cells, albeit heterogeneic, and are more efficient in their DNA repair than tumour cells. Consequently, precision radiotherapy can promote a reduction in angiogenic factors through the death of cancer cells whilst permitting the surviving endothelial cells to relax and remodel under normalised angiogenic conditions. This vascular reset in turn can serve as a base for almost any other subsequent treatment intervention.
Whereas the normalisation effect achieved by medication or radiotherapy can be short-lived and expensive, physical activity is cost-effective and discretionary, virtually free of side effects and applicable over extended periods of time. The benefit of creating a vessel normalisation window simply through moderate aerobic activity would be undeniable. The rate of activity that yielded promising results in exercising mice in a laboratory setting would translate to a daily brisk walk in humans, which is achievable even under life-changing circumstances.81 Physical activity during or after cancer treatment has long been associated with overall well-being and improved quality of live. Now biological evidence can be added that increased blood flow will aid any following treatment and should be explored further. Nearly 2000 clinical trials are currently actively studying the behavioural impact of exercise on cancer patients, and valuable data can be obtained from these studies (Refer to https://clinicaltrials.gov/).
In conclusion, cancer researchers are increasingly appreciating that only a complete understanding of tumour plasticity and the evolutionary biology supporting tumorigenesis will achieve durable treatment responses,145 with evidence indicating that in-depth analyses of the vascular architecture and the dynamics in vascular function will provide the most robust indications regarding tumour adaptation. The classification of evolutionary stages in cancer would allow, for instance, a range of approved end-stage cancer therapies to be administered to patients at earlier stages of cancer without raising the risk for side effects.
Acknowledgements
The authors wish to acknowledge the support of our research group and close collaborators for helpful discussions on related primary research.
Author contributions
The review article was conceived by A.L.M. A.L.M. and I.G.M. co-wrote the article and the figures were conceived and developed by A.L.M.
Ethics approval and consent to participate
The review article does not contain experimental data and consequently no ethical approvals or consents were necessary.
Consent to publish
Not applicable.
Data availability
As a review article access to all associated primary data relating to the article is the responsibility of the authors of the cited primary research. The review article does not contain any primary data.
Competing interests
The authors declare no competing interests.
Funding information
A.L.M. is funded by a grant from the Rosetrees Trust and the John Black Foundation. I.G.M. is the John Black Associate Professor of Prostate Cancer (University of Oxford), Professor of Translational Prostate Cancer Biology (Queen’s University of Belfast) and an Adjunct Professor at the University of Bergen. I.G.M. is supported by the John Black Foundation, Prostate Cancer UK, the Rosetrees Trust and the Norwegian Research Council. All of this funding supports primary research.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Franses JW, Edelman ER. The evolution of endothelial regulatory paradigms in cancer biology and vascular repair. Cancer Res. 2011;71:7339. doi: 10.1158/0008-5472.CAN-11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey W. An anatomical disquisition on the motion of the heart and blood in animals*. Ann. Noninvasive Electrocardiol. 2020;5:196–203. doi: 10.1111/j.1542-474X.2000.tb00387.x. [DOI] [Google Scholar]
- 3.Meyer J. Über die Neubildung von Blutgefäßen in plastischen Exudaten serösen Membranen und in Hautwunden. Ann Charité. 1852;4:41–140. [Google Scholar]
- 4.Clark ER, Clark EL. Observations on changes in blood vascular endothelium in the living animal. Am. J. Anat. 1935;57:385–438. doi: 10.1002/aja.1000570303. [DOI] [Google Scholar]
- 5.Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am. J. Anat. 1939;64:251–301. doi: 10.1002/aja.1000640203. [DOI] [Google Scholar]
- 6.Ribatti, D., Nico, B. & Crivellato, E. The development of the vascular system: a historical overview. in Vascular Morphogenesis: Methods and Protocols (ed. Ribatti, D.) 1–14 (Springer New York, 2015). [DOI] [PubMed]
- 7.Patan S. Vasculogenesis and angiogenesis. Cancer Treat. Res. 2004;117:3–32. doi: 10.1007/978-1-4419-8871-3_1. [DOI] [PubMed] [Google Scholar]
- 8.Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 2000;50:1–15. doi: 10.1023/A:1006493130855. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Jiang L, Li C, Hu D, Bu J-W, Cai D, et al. Haemodynamics-driven developmental pruning of brain vasculature in zebrafish. PLoS Biol. 2012;10:e1001374. doi: 10.1371/journal.pbio.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 11.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat. Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- 12.Larrivée B, Niessen K, Pollet I, Corbel SY, Long M, Rossi FM, et al. Minimal contribution of marrow-derived endothelial precursors to tumor vasculature. J. Immunol. 2005;175:2890. doi: 10.4049/jimmunol.175.5.2890. [DOI] [PubMed] [Google Scholar]
- 13.Marçola M, Rodrigues CE. Endothelial progenitor cells in tumor angiogenesis: another brick in the wall. Stem Cells Int. 2015;2015:832649. doi: 10.1155/2015/832649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moschetta M, Mishima Y, Sahin I, Manier S, Glavey S, Vacca A, et al. Role of endothelial progenitor cells in cancer progression. Biochimica et Biophysica Acta. 2014;1846:26–39. doi: 10.1016/j.bbcan.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Ueno T, Ishiguro H, Morita S, Toi M. The lack of increases in circulating endothelial progenitor cell as a negative predictor for pathological response to neoadjuvant chemotherapy in breast cancer patients. npj Precis. Oncol. 2017;1:6. doi: 10.1038/s41698-017-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mentzer SJ, Konerding MA. Intussusceptive angiogenesis: expansion and remodeling of microvascular networks. Angiogenesis. 2014;17:499–509. doi: 10.1007/s10456-014-9428-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burri PH, Djonov V. Intussusceptive angiogenesis–the alternative to capillary sprouting. Mol. Aspects Med. 2002;23:S1–S27. doi: 10.1016/S0098-2997(02)00096-1. [DOI] [PubMed] [Google Scholar]
- 18.Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev. Dyn. 2004;231:474–488. doi: 10.1002/dvdy.20184. [DOI] [PubMed] [Google Scholar]
- 19.Algire GHC, Vascular HW. reactions of normal and malignant tissues in vivo. J. Natl Cancer Inst. 1945;6:73. doi: 10.1093/jnci/6.1.73. [DOI] [Google Scholar]
- 20.Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. Br. J. Cancer. 1968;22:258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971;133:275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 23.Plouët J, Schilling J, Gospodarowicz D. Isolation and characterization of a newly identified endothelial cell mitogen produced by AtT-20 cells. EMBO J. 1989;8:3801–3806. doi: 10.1002/j.1460-2075.1989.tb08557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 25.Kerbel RS. Tumor angiogenesis. New Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat. Rev. Cancer. 2012;12:699–709. doi: 10.1038/nrc3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin. Radiat. Oncol. 1996;6:10–21. doi: 10.1016/S1053-4296(96)80032-4. [DOI] [PubMed] [Google Scholar]
- 28.Krzywinska E, Stockmann C. Hypoxia, metabolism and immune cell function. Biomedicines. 2018;6:56. doi: 10.3390/biomedicines6020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br. J. Cancer. 2009;100:865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohga N, Ishikawa S, Maishi N, Akiyama K, Hida Y, Kawamoto T, et al. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am. J. Pathol. 2012;180:1294–1307. doi: 10.1016/j.ajpath.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto S, Batra S, Saito K, Yasui H, Choudhuri R, Gadisetti C, et al. Antiangiogenic agent sunitinib transiently increases tumor oxygenation and suppresses cycling hypoxia. Cancer Res. 2011;71:6350–6359. doi: 10.1158/0008-5472.CAN-11-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claffey KP, Brown LF, del Aguila LF, Tognazzi K, Yeo KT, Manseau EJ, et al. Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res. 1996;56:172–181. [PubMed] [Google Scholar]
- 34.Le Guelte A, Dwyer J, Gavard J. Jumping the barrier: VE-cadherin, VEGF and other angiogenic modifiers in cancer. Biol. Cell. 2011;103:593–605. doi: 10.1042/BC20110069. [DOI] [PubMed] [Google Scholar]
- 35.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 2003;163:1801–1815. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nina Kristine R, Aphirak J, Tore L, Catharina de Lange D. Macromolecular diffusion in the extracellular matrix measured by fluorescence correlation spectroscopy. J. Biomed. Optics. 2008;13:1–9. doi: 10.1117/1.2982530. [DOI] [PubMed] [Google Scholar]
- 37.Ariffin AB, Forde PF, Jahangeer S, Soden DM, Hinchion J. Releasing pressure in tumors: what do we know so far and where do we go from here? A review. Cancer Res. 2014;74:2655–2662. doi: 10.1158/0008-5472.CAN-13-3696. [DOI] [PubMed] [Google Scholar]
- 38.Le Serve AW, Hellmann K. Metastases and the normalization of tumour blood vessels by ICRF 159: a new type of drug action. Br. Med. J. 1972;1:597–601. doi: 10.1136/bmj.1.5800.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rybak SM, Sanovich E, Hollingshead MG, Borgel SD, Newton DL, Melillo G, et al. “Vasocrine” formation of tumor cell-lined vascular spaces: implications for rational design of antiangiogenic therapies. Cancer Res. 2003;63:2812–2819. [PubMed] [Google Scholar]
- 40.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 41.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 42.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 2011;10:417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 43.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz MA, Vestweber D, Simons M. A unifying concept in vascular health and disease. Science. 2018;360:270–271. doi: 10.1126/science.aat3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreiter J, Meyer S, Schmidt C, Schulz RM, Langer S. Dorsal skinfold chamber models in mice. GMS Interdiscip Plast. Reconstr. Surg. DGPW. 2017;6:Doc10. doi: 10.3205/iprs000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Liao C, Liu Y, Yang G, Ke T, Ding Y, et al. MR imaging biomarkers evaluating vascular normalization window after anti-vessel treatment. Oncotarget. 2017;9:11964–11976. doi: 10.18632/oncotarget.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bohndiek SE, Sasportas LS, Machtaler S, Jokerst JV, Hori S, Gambhir SS. Photoacoustic tomography detects early vessel regression and normalization during ovarian tumor response to the antiangiogenic therapy trebananib. J. Nucl. Med. 2015;56:1942–1947. doi: 10.2967/jnumed.115.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol. Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winkler F, Kozin SV, Tong RT, Chae S-S, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Willett CG, Kozin SV, Duda DG, di Tomaso E, Kozak KR, Boucher Y, et al. Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice. Semin. Oncol. 2006;33:S35–S40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collet G, Lamerant-Fayel N, Tertil M, El Hafny-Rahbi B, Stepniewski J, Guichard A, et al. Hypoxia-regulated overexpression of soluble VEGFR2 controls angiogenesis and inhibits tumor growth. Mol. Cancer Ther. 2014;13:165–178. doi: 10.1158/1535-7163.MCT-13-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boudria A, Abou Faycal C, Jia T, Gout S, Keramidas M, Didier C, et al. VEGF165b, a splice variant of VEGF-A, promotes lung tumor progression and escape from anti-angiogenic therapies through a β1 integrin/VEGFR autocrine loop. Oncogene. 2019;38:1050–1066. doi: 10.1038/s41388-018-0486-7. [DOI] [PubMed] [Google Scholar]
- 54.Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 55.Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and growth factors. J. Cell Sci. 2008;121:3487. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mavrou A, Oltean S. SRPK1 inhibition in prostate cancer: A novel anti-angiogenic treatment through modulation of VEGF alternative splicing. Pharmacol Res. 2016;107:276–281. doi: 10.1016/j.phrs.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magnussen AL, Rennel ES, Hua J, Bevan HS, Beazley Long N, Lehrling C, et al. VEGF-A165b is cytoprotective and antiangiogenic in the retina. Investig. Ophthalmol. Vis. Sci. 2010;51:4273–4281. doi: 10.1167/iovs.09-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Liao S, Diop-Frimpong B, Chen W, Goel S, Naxerova K, et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA. 2012;109:16618–16623. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dieterich LC, Mellberg S, Langenkamp E, Zhang L, Zieba A, Salomäki H, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J. Pathol. 2012;228:378–390. doi: 10.1002/path.4072. [DOI] [PubMed] [Google Scholar]
- 60.Qayum N, Muschel RJ, Im JH, Balathasan L, Koch CJ, Patel S, et al. Tumor vascular changes mediated by inhibition of oncogenic signaling. Cancer Res. 2009;69:6347–6354. doi: 10.1158/0008-5472.CAN-09-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sawada J, Urakami T, Li F, Urakami A, Zhu W, Fukuda M, et al. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell. 2012;22:235–249. doi: 10.1016/j.ccr.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hida K, Maishi N, Annan DA, Hida Y. Contribution of tumor endothelial cells in cancer progression. Int. J. Mol. Sci. 2018;19:1272. doi: 10.3390/ijms19051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 64.Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat. Med. 2015;21:121–131. doi: 10.1038/nm.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falcón BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park JS, Kim IK, Han S, Park I, Kim C, Bae J, et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer Cell. 2016;30:953–967. doi: 10.1016/j.ccell.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 67.Alto LT, Terman JR. Semaphorins and their signaling mechanisms. Methods Mol. Biol. 2017;1493:1–25. doi: 10.1007/978-1-4939-6448-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, et al. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler. Thromb. Vasc. Biol. 2011;31:741–749. doi: 10.1161/ATVBAHA.110.211920. [DOI] [PubMed] [Google Scholar]
- 69.Serini G, Bussolino F, Maione F, Giraudo E. Class 3 semaphorins: physiological vascular normalizing agents for anti-cancer therapy. J. Intern. Med. 2013;273:138–155. doi: 10.1111/joim.12017. [DOI] [PubMed] [Google Scholar]
- 70.Fogal V, Sugahara KN, Ruoslahti E, Christian S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis. 2009;12:91–100. doi: 10.1007/s10456-009-9137-5. [DOI] [PubMed] [Google Scholar]
- 71.Baron M. An overview of the Notch signalling pathway. Semin. Cell Dev. Biol. 2003;14:113–119. doi: 10.1016/S1084-9521(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 72.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 73.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev. Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Maes H, Kuchnio A, Peric A, Moens S, Nys K, De Bock K, et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell. 2014;26:190–206. doi: 10.1016/j.ccr.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Trindade A, Djokovic D, Gigante J, Mendonça L, Duarte A. Endothelial Dll4 overexpression reduces vascular response and inhibits tumor growth and metastasization in vivo. BMC Cancer. 2017;17:189-. doi: 10.1186/s12885-017-3171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polet F, Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J. Intern. Med. 2013;273:156–165. doi: 10.1111/joim.12016. [DOI] [PubMed] [Google Scholar]
- 77.Zhang L, Li S, Li L, Chen Z, Yang Y. COX‑2 inhibition in the endothelium induces glucose metabolism normalization and impairs tumor progression. Mol. Med. Rep. 2018;17:2937–2944. doi: 10.3892/mmr.2017.8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, Pircher A, et al. Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30:968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kocher M, Treuer H, Voges J, Hoevels M, Sturm V, Muller RP. Computer simulation of cytotoxic and vascular effects of radiosurgery in solid and necrotic brain metastases. Radiother. Oncol. 2000;54:149–156. doi: 10.1016/S0167-8140(99)00168-1. [DOI] [PubMed] [Google Scholar]
- 80.Potiron VA, Abderrahmani R, Clément-Colmou K, Marionneau-Lambot S, Oullier T, Paris F, et al. Improved functionality of the vasculature during conventionally fractionated radiation therapy of prostate cancer. PLoS ONE. 2013;8:e84076. doi: 10.1371/journal.pone.0084076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koelwyn GJ, Quail DF, Zhang X, White RM, Jones LW. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer. 2017;17:620. doi: 10.1038/nrc.2017.78. [DOI] [PubMed] [Google Scholar]
- 82.Di Francescomarino S, Sciartilli A, Di Valerio V, Di Baldassarre A, Gallina S. The effect of physical exercise on endothelial function. Sports Med. 2009;39:797–812. doi: 10.2165/11317750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 83.Betof, A. S., Lascola, C. D., Weitzel, D., Landon, C., Scarbrough, P. M., Devi, G. R. et al. Modulation of murine breast tumor vascularity, hypoxia and chemotherapeutic response by exercise. J. Natl Cancer Inst.107, djv040, 10.1093/jnci/djv040 (2015). [DOI] [PMC free article] [PubMed]
- 84.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, et al. Fluid shear stress and the vascular endothelium: for better and for worse. Prog. Biophys. Mol. Biol. 2003;81:177–199. doi: 10.1016/S0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 85.Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget. 2016;7:65429–65440. doi: 10.18632/oncotarget.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCarthy M. Antiangiogenesis drug promising for metastatic colorectal cancer. Lancet. 2003;361:1959. doi: 10.1016/S0140-6736(03)13603-3. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Al-Soudi A, Kaaij MH, Tas SW. Endothelial cells: from innocent bystanders to active participants in immune responses. Autoimmun. Rev. 2017;16:951–962. doi: 10.1016/j.autrev.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 89.Funaro A, Ferrero E, Mehta K, Malavasi F. Schematic portrait of human CD38 and related molecules. Chem. Immunol. 2000;75:256–273. doi: 10.1159/000058773. [DOI] [PubMed] [Google Scholar]
- 90.Rothermel AL, Wang Y, Schechner J, Mook-Kanamori B, Aird WC, Pober JS, et al. Endothelial cells present antigens in vivo. BMC Immunol. 2004;5:5. doi: 10.1186/1471-2172-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mai J, Virtue A, Shen J, Wang H, Yang X-F. An evolving new paradigm: endothelial cells—conditional innate immune cells. J. Hematol. Oncol. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 93.Huang Y, Kim BYS, Chan CK, Hahn SM, Weissman IL, Jiang W. Improving immune–vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 2018;18:195. doi: 10.1038/nri.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barzilai S, Yadav SK, Morrell S, Roncato F, Klein E, Stoler-Barak L, et al. Leukocytes breach endothelial barriers by insertion of nuclear lobes and disassembly of endothelial actin filaments. Cell Rep. 2017;18:685–699. doi: 10.1016/j.celrep.2016.12.076. [DOI] [PubMed] [Google Scholar]
- 95.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 96.Dvorak HF. Tumors: wounds that do not heal—a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin. Thromb. Hemost. 2019;45:576–592. doi: 10.1055/s-0039-1687908. [DOI] [PubMed] [Google Scholar]
- 97.Mpekris F, Voutouri C, Baish JW, Duda DG, Munn LL, Stylianopoulos T, et al. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl Acad. Sci. USA. 2020;117:3728. doi: 10.1073/pnas.1919764117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, et al. Hypoxia: a key player in antitumor immune response. A Review in the theme: cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 2015;309:C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, et al. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McEver RP, Zhu C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 2010;26:363–396. doi: 10.1146/annurev.cellbio.042308.113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang H, Langenkamp E, Georganaki M, Loskog A, Fuchs PF, Dieterich LC, et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-kappaB-induced endothelial activation. Faseb J. 2015;29:227–238. doi: 10.1096/fj.14-250985. [DOI] [PubMed] [Google Scholar]
- 103.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Couzin-Frankel J. Cancer immunotherapy. Science. 2013;342:1432. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 107.Li, Y., Li, F., Jiang, F., Lv, X., Zhang, R., Lu, A. et al. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway: translational blockade of immune checkpoints. Int. J. Mol. Sci.17, 1151 (2016). [DOI] [PMC free article] [PubMed]
- 108.Carter, T., Shaw, H., Cohn-Brown, D., Chester, K. & Mulholland, P. Ipilimumab and bevacizumab in glioblastoma. Clin. Oncol.28, 622–626 (2016). [DOI] [PubMed]
- 109.Wu X, Li J, Connolly EM, Liao X, Ouyang J, Giobbie-Hurder A, et al. Combined anti-VEGF and anti-CTLA-4 therapy elicits humoral immunity to galectin-1 which is associated with favorable clinical outcomes. Cancer Immunol. Res. 2017;5:446–454. doi: 10.1158/2326-6066.CIR-16-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Di Tacchio, M., Macas, J., Weissenberger, J., Sommer, K., Bähr, O., Steinbach, J. P. et al. Tumor vessel normalization, immuno-stimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol. Res.7, 1910–1927 (2019). [DOI] [PubMed]
- 111.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol. Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 112.Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8+ T cells. Nat. Immunol. 2015;16:609. doi: 10.1038/ni.3159. [DOI] [PubMed] [Google Scholar]
- 113.Sanchez LR, Borriello L, Entenberg D, Condeelis JS, Oktay MH, Karagiannis GS. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019;106:259–274. doi: 10.1002/JLB.MR0218-056RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 2018;23:1239–1248. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harney AS, Karagiannis GS, Pignatelli J, Smith BD, Kadioglu E, Wise SC, et al. The selective Tie2 inhibitor rebastinib blocks recruitment and function of Tie2(Hi) macrophages in breast cancer and pancreatic neuroendocrine tumors. Mol. Cancer Ther. 2017;16:2486–2501. doi: 10.1158/1535-7163.MCT-17-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nishide S, Matsunaga S, Shiota M, Yamaguchi T, Kitajima S, Maekawa Y, et al. Controlling the phenotype of tumor-infiltrating macrophages via the PHD-HIF axis inhibits tumor growth in a mouse model. iScience. 2019;19:940–954. doi: 10.1016/j.isci.2019.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544:250–254. doi: 10.1038/nature21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sato N, Nariuchi H, Tsuruoka N, Nishihara T, Beitz JG, Calabresi P, et al. Actions of TNF and IFN-gamma on angiogenesis in vitro. J. Investig. Dermatol. 1990;95:85s–89ss. doi: 10.1111/1523-1747.ep12874809. [DOI] [PubMed] [Google Scholar]
- 119.Sun T, Yang Y, Luo X, Cheng Y, Zhang M, Wang K, et al. Inhibition of tumor angiogenesis by interferon-gamma by suppression of tumor-associated macrophage differentiation. Oncol. Res. 2014;21:227–235. doi: 10.3727/096504014X13890370410285. [DOI] [PubMed] [Google Scholar]
- 120.Kwee BJ, Seo BR, Najibi AJ, Li AW, Shih T-Y, White D, et al. Treating ischemia via recruitment of antigen-specific T cells. Sci. Adv. 2019;5:eaav6313. doi: 10.1126/sciadv.aav6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Johansson-Percival A, He B, Ganss R. Immunomodulation of tumor vessels: it takes two to tango. Trends Immunol. 2018;39:801–814. doi: 10.1016/j.it.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 122.Batchelor TT, Sorensen AG, di Tomaso E, Zhang W-T, Duda DG, Cohen KS, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sorensen AG, Batchelor TT, Zhang W-T, Chen P-J, Yeo P, Wang M, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69:5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Batchelor TT, Gerstner ER, Emblem KE, Duda DG, Kalpathy-Cramer J, Snuderl M, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc. Natl Acad. Sci. USA. 2013;110:19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.von Baumgarten L, Brucker D, Tirniceru A, Kienast Y, Grau S, Burgold S, et al. Bevacizumab has differential and dose-dependent effects on glioma blood vessels and tumor cells. Clin. Cancer Res. 2011;17:6192–6205. doi: 10.1158/1078-0432.CCR-10-1868. [DOI] [PubMed] [Google Scholar]
- 126.Keunen O, Johansson M, Oudin A, Sanzey M, Rahim SA, Fack F, et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl Acad. Sci. USA. 2011;108:3749–3754. doi: 10.1073/pnas.1014480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li Y, Ali S, Clarke J, Cha S. Bevacizumab in recurrent glioma: patterns of treatment failure and implications. Brain Tumor Res. Treat. 2017;5:1–9. doi: 10.14791/btrt.2017.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Claes A, Wesseling P, Jeuken J, Maass C, Heerschap A, Leenders WP. Antiangiogenic compounds interfere with chemotherapy of brain tumors due to vessel normalization. Mol. Cancer Ther. 2008;7:71–78. doi: 10.1158/1535-7163.MCT-07-0552. [DOI] [PubMed] [Google Scholar]
- 129.Boujelben A, Watson M, McDougall S, Yen Y-F, Gerstner ER, Catana C, et al. Multimodality imaging and mathematical modelling of drug delivery to glioblastomas. Interface Focus. 2016;6:20160039. doi: 10.1098/rsfs.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Donnem T, Reynolds AR, Kuczynski EA, Gatter K, Vermeulen PB, Kerbel RS, et al. Non-angiogenic tumours and their influence on cancer biology. Nat. Rev. Cancer. 2018;18:323–336. doi: 10.1038/nrc.2018.14. [DOI] [PubMed] [Google Scholar]
- 131.García-Gómez P, Valiente M. Vascular co-option in brain metastasis. Angiogenesis. 2020;23:3–8. doi: 10.1007/s10456-019-09693-x. [DOI] [PubMed] [Google Scholar]
- 132.Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 133.Reymond N, Im JH, Garg R, Vega FM, Borda d’Agua B, Riou P, et al. Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J. Cell Biol. 2012;199:653–668. doi: 10.1083/jcb.201205169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tolaney SM, Boucher Y, Duda DG, Martin JD, Seano G, Ancukiewicz M, et al. Role of vascular density and normalization in response to neoadjuvant bevacizumab and chemotherapy in breast cancer patients. Proc. Natl Acad. Sci. USA. 2015;112:14325–14330. doi: 10.1073/pnas.1518808112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jezzard, P., Matthews, P. M. & Smith, S. M. Functional Magnetic Resonance Imaging: An Introduction to Methods (Oxford University Press, 2001).
- 137.Yang J, Zhang G, Li Q, Liao C, Huang L, Ke T, et al. Photoacoustic imaging for the evaluation of early tumor response to antivascular treatment. Quant. Imaging Med. Surg. 2019;9:160–170. doi: 10.21037/qims.2018.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brown E, Brunker J, Bohndiek SE. Photoacoustic imaging as a tool to probe the tumour microenvironment. Dis. Model. Mech. 2019;12:dmm039636. doi: 10.1242/dmm.039636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Matsumoto S, Saito K, Takakusagi Y, Matsuo M, Munasinghe JP, Morris HD, et al. In vivo imaging of tumor physiological, metabolic, and redox changes in response to the anti-angiogenic agent sunitinib: longitudinal assessment to identify transient vascular renormalization. Antioxid. Redox Signal. 2014;21:1145–1155. doi: 10.1089/ars.2013.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu S, Han P, Huang M, Wu JC, Chang C, Tsao PS, et al. In vivo, ex vivo, and in vitro studies on apelin’s effect on myocardial glucose uptake. Peptides. 2012;37:320–326. doi: 10.1016/j.peptides.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 141.Cox CM, D’Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev. Biol. 2006;296:177–189. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 142.Eyries M, Siegfried G, Ciumas M, Montagne K, Agrapart M, Lebrin F, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ Res. 2008;103:432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 143.Zhang L, Takara K, Yamakawa D, Kidoya H, Takakura N. Apelin as a marker for monitoring the tumor vessel normalization window during antiangiogenic therapy. Cancer Sci. 2016;107:36–44. doi: 10.1111/cas.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamakawa D, Jia W, Kidoya H, Hosojima S, Torigata M, Zhang L, et al. Visualization of proliferative vascular endothelial cells in tumors in vivo by imaging their partner of Sld5-1 promoter activity. Am. J. Pathol. 2018;188:1300–1314. doi: 10.1016/j.ajpath.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 145.Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev. Drug Discov. 2020;19:39–56. doi: 10.1038/s41573-019-0044-1. [DOI] [PubMed] [Google Scholar]
- 146.Standring, S., Standring, S. & Gray, H. Gray’s Anatomy E-Book: The Anatomical Basis of Clinical Practice (Elsevier, 2015).
- 147.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hida K, Hida Y, Amin DN, Flint AF, Panigrahy D, Morton CC, et al. Tumor-associated endothelial cells with cytogenetic abnormalities. Cancer Res. 2004;64:8249. doi: 10.1158/0008-5472.CAN-04-1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As a review article access to all associated primary data relating to the article is the responsibility of the authors of the cited primary research. The review article does not contain any primary data.