Abstract
Fast metabolism of immediate-release tacrolimus (IR-Tac) is associated with decreased kidney function after renal transplantation (RTx) compared to slow metabolizers. We hypothesized, by analogy, that fast metabolism of extended-release tacrolimus (ER-Tac) is associated with worse renal function. We analyzed data from patients who underwent RTx at three different transplant centers between 2007 and 2016 and received an initial immunosuppressive regimen with ER-Tac, mycophenolate, and a corticosteroid. Three months after RTx, a Tac concentration to dose ratio (C/D ratio) < 1.0 ng/ml · 1/mL defined fast ER-Tac metabolism and ≥ 1.0 ng/ml · 1/mL slow metabolism. Renal function (estimated glomerular filtration rate, eGFR), first acute rejection (AR), conversion from ER-Tac, graft and patient survival were observed up to 60-months. 610 RTx patients were divided into 192 fast and 418 slow ER-Tac metabolizers. Fast metabolizers showed a decreased eGFR at all time points compared to slow metabolizers. The fast metabolizer group included more patients who were switched from ER-Tac (p < 0.001). First AR occurred more frequently (p = 0.008) in fast metabolizers, while graft and patient survival rates did not differ between groups (p = 0.529 and p = 0.366, respectively). Calculation of the ER-Tac C/D ratio early after RTx may facilitate individualization of immunosuppression and help identify patients at risk for an unfavorable outcome.
Subject terms: Medical research, Nephrology
Introduction
The calcineurin inhibitor (CNI) tacrolimus (Tac) is highly effective in preventing acute transplant rejection and is consequently recommended as first-line immunosuppressive therapy after renal transplantation (RTx)1. Currently, approximately 95% of RTx recipients are discharged after RTx receiving a Tac-based immunosuppressive regimen2.
While immediate-release Tac (IR-Tac), which is administrated twice daily, has become established over the past 2 decades, once-daily Tac formulations such as extended-release Tac (ER-Tac) and LCP-Tac are gaining traction due to convenience and higher adherence rates3, 4. In the current study, we focus on ER-Tac which has different pharmacokinetics (PK) compared to other Tac products. Replacement of croscarmellose in IR-Tac with ethylcellulose in ER-Tac slows down the diffusion rate of Tac, resulting in prolonged release with 90% absorption after 6–12 h5, 6. While one day after RTx, the mean area under the curve (AUC)0–24 is approximately 30% lower for ER-Tac compared with IR-Tac at comparable dosing, both formulations showed a good correlation between Cmin and AUC0-24 after day 147. ER- and IR-Tac have a narrow therapeutic window and high intra- and interindividual variability, therefore transplant recipients are at risk of underexposure leading to rejection or overexposure causing e.g. CNI-related toxicity8, 9. With this in mind, sequential therapeutic drug monitoring is standard of care, although Cmin-based dose titration is unfortunately limited in predicting individual efficacy9, 10.
On the one hand, non-adherence to Tac remains an underestimated problem; on the other hand, Tac metabolism and PK profiles are increasingly coming into focus11, 12. Once-daily drugs such as ER-Tac can improve treatment adherence, but two recent meta-analyses showed comparable clinical outcomes of ER-Tac and IR-Tac4, 13–15.
Recently, we introduced the C/D ratio for IR-Tac, calculated by dividing the Tac trough concentration (C) by the daily Tac dose (D), as a simple tool to estimate IR-Tac metabolism 16. A low C/D ratio (C/D ratio < 1.05 ng/mL · 1/mg in RTx recipients; < 1.09 ng/mL · 1/mg in liver transplant recipients (LTR)) indicates fast Tac metabolism and is associated with a decreased renal function, a higher rate of biopsy-proven CNI nephrotoxicity (CNIT), and more frequent switching to an alternative immunosuppressive regimen than a higher C/D ratio16–21. Moreover, a low C/D ratio is associated with higher C2 Tac levels and higher rejection rates despite comparable trough levels21–23.
Although the PK profile of ER-Tac is different from the IR-Tac profile, there are also comparable blood concentrations at certain time points (such as Cmax or target trough levels). Therefore, the aim of the study was to evaluate whether the C/D ratio is also suitable for categorization and risk assessment or risk stratification in ER-Tac treated RTx patients9, 24.
Patients and methods
This retrospective observational multicenter study included RTx recipients who underwent transplantation in Cologne and Münster, Germany and Leuven, Belgium between 2007 and 2018. Only patients with an initial immunosuppression consisting of ER-Tac (Astellas), mycophenolate, a corticosteroid, an induction therapy with basiliximab at day 0 and 4, and available C/D ratio at 3 months after transplantation were included. Pregnant women and RTx recipients younger than 18 years of age were excluded. ER-Tac was started at 0.2 mg/kg body weight q.d. with a target trough level of 8–12 ng/mL during the first 3 months, 4–10 ng/mL from month 4 to 6 and 4–8 ng/mL thereafter. Mycophenolate and corticosteroids were given according to local transplant center protocols. The transplant centers Cologne and Münster administered prednisolone and Leuven methylprednisolone.
General demographic data and information on transplantation were obtained from electronic health records of the hospitals (recipients) or from Eurotransplant (donor data). All data were anonymized before analysis. The study was approved by the local ethics committees (Ethik Kommission der Ärztekammer Westfalen-Lippe und der Medizinischen Fakultät der Westfälischen Wilhelms-Universität, No. 2014-381-f-N; Ethik Kommission des Uniklinikums Köln, protocol 14-30; Medical Ethics Committee University Hospitals Leuven, Herestraat 49-B-3000, Leuven, protocol S53364). Methods in this study were carried out in accordance with the current transplantation guidelines, the Declarations of Istanbul and Helsinki, and the International Conference on Harmonization Good Clinical Practice guidelines. All recipients gave written informed consent at the time of transplantation for recording of their clinical data and use in anonymized analyses. No organs or tissue were procured from prisoners.
Patients were divided into two ER-Tac metabolism groups according to their C/D ratio 3 months after RTx (Fig. 1). The C/D ratio was calculated in analogy to previous publications with IR-Tac16, 25. Kidney donor profile index (KDPI) was calculated as previously published using the Organ Procurement and Transplantation Network (OPTN) online calculator26.
Figure 1.
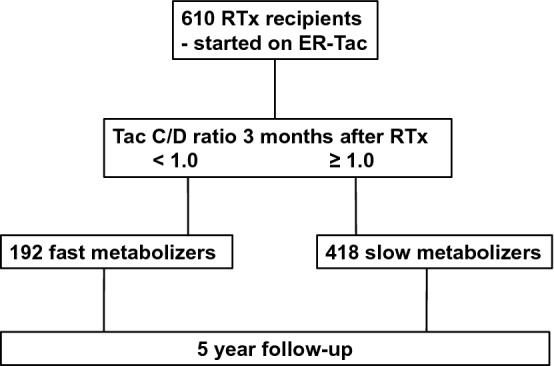
Study recruitment. 610 patients were included on the basis of taking extended-release tacrolimus (ER-Tac) in the first week after transplantation. Three months after RTx, patients were divided in fast and slow metabolizers with regards to their ER-Tac concentration to dose ratio (C/D ratio). RTx recipients were observed up to 5-years after RTx.
A C/D ratio < 1.0 ng/mL · 1/mg at 3 months after transplantation indicated fast ER-Tac metabolizers and a C/D ratio ≥ 1.0 ng/mL 1/mg · slow metabolizers.
Endpoints
The main aim was to investigate the evolution of renal function in the first 5 years after RTx. Therefore, the estimated glomerular filtration rate (eGFR) was calculated from serum creatinine values at day 10 (D10) and months 1, 2, 3, 6, 12, 24, 36, 48, 60 (M1-M60) after RTx. Creatinine was analyzed in a whole blood sample (enzymatic assay; Creatinine-Pap, Roche Diagnostics, Mannheim, Germany). Calculation of the eGFR was performed using the Chronic Kidney Disease Epidemiology Collaboration equation (CKD-EPI). In a first approach, eGFR values were compared between patients with a C/D ratio < 1 ng/mL · 1/mg vs. ≥ 1.0 ng/mL · 1/mg. In the next step, the influence of the M3 C/D ratio on eGFR was evaluated. Therefore, the eGFR changes from each time point to M3 were compared within each connected metabolizer group (eGFR slope) and between both groups.
The time to the occurrence of the first event of “switch of immunosuppression”, “graft failure”, and “death”, whichever occurred first, was determined as further subject of the study. “Switch of immunosuppression” was defined as the conversion from ER-Tac to any other immunosuppression. In a first step, the occurrence of “first AR” in the period RTx until M3 was analyzed of patients who received a Tac C/D ratio at M3 and were subsequently characterized as fast or slow metabolizers. In a second step, the impact of the M3 C/D ratio on further “first AR” events was assessed in a 5-year follow-up, when the time to “first AR” was investigated. Switching of immunosuppression, graft failure or death ended follow-up, so patients who switched, restarted dialysis or died without prior AR were censored at the respective date. Any “first AR” was recorded in case of AR treatment or biopsy-proven acute rejection (BPAR) and subsequent treatment. Histologic results on rejections in the transplant centers Cologne and Münster were obtained from indication biopsies only. The transplant center Leuven performed protocol biopsies 3, 12 and 24 months after RTx. The BANFF 2019 criteria were used to define BPAR. CNIT was assess by the local pathologists according to the histological patterns described in detail by Naesens et al.27. “Graft failure” was defined as irreversible deterioration of kidney function requiring permanent renal replacement therapy.
Statistical analysis
Statistical analyses were performed using IBM SPSS® Statistics 27 for Windows (IBM Corporation, Somers, NY, USA) and SAS software, Version 9.4 TS1M5 of the SAS System for Windows (Copyright © 2021 SAS Institute Inc., Cary, NC, USA). All p-values and confidence limits were two-sided and were intended to be exploratory, not confirmatory. Therefore, no adjustment for multiplicity was made. Exploratory p-values ≤ 0.05 were considered to be statistically noticeable.
In descriptive analysis, normally-distributed continuous variables are reported as mean ± standard deviation and not normally-distributed continuous variables as median (25% quantile–75% quantile, IQR). Absolute and relative frequencies are given for categorical variables. Metabolism groups were compared using Welch’s t-tests for normally-distributed data, Mann–Whitney U-tests for skewed-distributed data, and Fisher’s exact tests for categorical variables. Comparison of the eGFR changes within each metabolism group was performed using Wilcoxon’s signed-rank tests. Boxplots were used for graphical representation.
In order to model renal function (eGFR) over time adjusted for co-variables and dropouts over time, a multivariable linear mixed model was fitted. Recipient’s age, European Senior Program (ESP) (yes/no), the main effects of time (3, 6, 12, 24, 36, 48, 60 months) and metabolizer group (fast/slow), and the interaction between time and group were included as influencing variables. Repeated measurements of each patient were modeled using SAS PROC MIXED by fitting a marginal linear mixed model with an unstructured variance–covariance matrix for the residuals with patient as subject and the order given by time. The empirical sandwich estimator was applied. Missing values were treated as missing at random. Results are reported as least square estimates with corresponding 95% confidence interval (CI), and p-values from the Wald test.
Event rates of the “first AR”, of the component endpoint “switch of the immunosuppression”, “graft failure” and “death as first event” (whatever occurred first), as well as of overall survival were estimated using the Kaplan–Meier method with 95% CI using log-transformation. Patients without an event were censored at their last visit date. In the analysis of “first AR”, patients who switched immunosuppression, showed a graft failure or died without prior AR were additionally censored at the respective date. All time-to-event endpoints started at 3 months after RTx. Consequently, AR and graft failures which occurred until 3 months were not considered in the analysis and patients were excluded from these analyses. Hazard ratios (HR) and 95% CI are given to quantify the effect of fast versus slow metabolizers. A competing risk approach was used to estimate the effect on each component of the combined endpoint. The impact of fast vs. slow metabolizer on the components was thus estimated using Fine and Gray's model leading to subdistribution hazard ratios (sub-HR). Cumulative incidence was estimated using the Aalen-Johansen estimator. Gray's k-sample test was applied to compare the cumulative incidence of the corresponding event type. Additionally, the cause-specific hazard (csH) for each component of the competing event type was compared between the metabolizer groups via cause-specific hazard ratios (csHR) using the methods by Prentice (results not shown).
Results
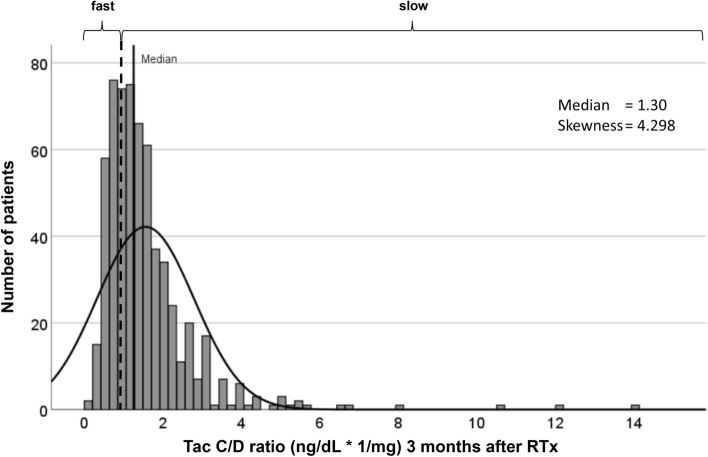
We included and analyzed 610 RTx recipients in a 5-year follow-up. Using the categorization of the C/D ratio of < 1.0 ng/mL · 1/mg and ≥ 1.0 ng/mL · 1/mg at 3 months after RTx, the cohort was divided into approximately 1/3 (n = 192) fast and 2/3 (n = 418) slow metabolizers, similar to previous studies (Figs. 1 and 2)16, 21.
Figure 2.
Empirical distribution of the patients in terms of their concentration to dose ratio (C/D ratio) three months after RTx. Fast ER-Tac metabolizers were defined by a C/D ratio < 1 ng/mL · 1/mg, and slow metabolizers had a C/D ratio ≥ 1 ng/mL · 1/mg.
The RTx cohort showed a skew distribution of the ER-Tac C/D ratio at 3 months with a median of 1.3 (IQR 0.86–1.88) ng/mL · 1/mg.
Table 1 shows the baseline characteristics of the patients. Fast ER-Tac metabolizers were younger (p = 0.003) although more patients within the ESP (p = 0.006) received a graft, albeit with low absolute ESP numbers. There were no other noticeable differences in patient characteristics.
Table 1.
Patients characteristics.
| Fast metabolizers n = 192 | Slow metabolizers n = 418 | p-value | |
|---|---|---|---|
| Body weight (kg) | 74.7 ± 14.5 | 73.6 ± 14.8 | 0.512a |
| Height (m) | 1.71 ± 0.10 | 1.70 ± 0.09 | 0.508a |
| BMI (kg/m2) | 24.7 (22.1–27.4) | 24.5 (22.6–27.1) | 0.810c |
| Age (years) | 52.5 ± 14.3 | 56.0 ± 11.8 | 0.003a |
| Sex (m/f, %) | 120 (62.5%) / 72 (37.5%) | 266 (64%) / 152 (36%) | 0.787b |
| Living donor transpl. | 14 (7.3%) | 23 (5.5%) | 0.465b |
| Cadavaric donor transpl. | 178 (92.7%) | 395 (94.5%) | |
| HBD | 155 (80.7%) | 340 (81.3%) | 0.728b |
| NHBD I | 19 (9.9%) | 42 (10%) | |
| NHBD II | 1 (0.5%) | 0 | |
| NHBD III | 17 (8.9%) | 35 (8.4%) | |
| NHBD IV | 0 | 1 (0.2%) | |
| ESP transpl. | 8 (4.2%) | 3 (0.7%) | 0.006b |
| ABO-incompatible transpl. | 0 | 2 | – |
| Cold ischemia time (h) | 12.1 ± 5.8 | 12.5 ± 5.8 | 0.525a |
| Delayed graft function | 32 (16.7%) | 77 (18.4%) | 0.650b |
| KDPI | 49.0 ± 25.8 | 47.0 ± 25.4 | 0.402a |
| Warm ischemia time (min) | 35 (30–41) | 35 (30–45) | 0.262c |
| HLA MM | |||
| 0 | 8 (4.2%) | 28 (6.7%) | 0.393b |
| 1–3 | 117 (60.9%) | 240 (57.4%) | |
| 4–6 | 59 (30.7%) | 140 (33.5%) | |
| PRA > 20% | 13/177 (6.8%) | 27/394 (6.5%) | 0.860b |
| Combined transpl. | |||
| + Pancreas | 4 (2.1%) | 12 (2.9%) | 0.695b |
| + Liver | 3 (1.6%) | 3 (0.7%) | |
| + Heart | 0 | 1 (0.2%) | |
| Non-combined | 185 (96.4%) | 402 (96.2%) | |
| Donor characteristics | |||
| Donor age (years) | 49.7 ± 16.0 | 49.0 ± 15.1 | 0.601a |
| Donor sex (m/f) % | 82 (52%) / 76 (48%) | 196 (51%) / 188 (49%) | 0.925b |
| Donor height (m) | 1.71 ± 0.09 | 1.72 ± 0.09 | 0.326a |
| Donor weight (kg) | 74.1 ± 14.8 | 74.6 ± 14.4 | 0.718a |
| Donor BMI (kg/m2) | 24.7 (22.6–26.6) | 24.8 (22.5–26.8) | 0.936c |
Data presented as mean ± standard deviation or median (25% quantile-75% quantile), or absolute and relative frequencies.
BMI body mass index, transpl. transplantation, HBD heart-beating donors, NHBD non-heart-beating donors (only from the Leuven-cohort), ESP European Senior Program, KDPI kidney donor profile index, HLA MM human leucocyte antigen mismatch, PRA panel reactive antibodies.
aWelch's t-test.
bFisher's exact test.
cMann–Whitney U test.
Immunosuppression
Compared to slow metabolizers, the group with a C/D ratio < 1.0 ng/mL · 1/mg received higher ER-Tac doses at 1, 3 and 6 months after RTx (all p < 0.001), and even had slightly lower Tac trough levels at month 1 and 3 (both p < 0.001; Table 2). Median Tac blood concentrations were within the target trough level at all three time points. In the Leuven study center, fast metabolizers received slightly more methylprednisolone than slow metabolizers at month 1 after RTx (12 (IQR 9.5–16.0) mg vs. 12 (IQR 8.0–12.0) mg; p = 0.006).
Table 2.
Doses, Tac trough level, Tac C/D ratio.
| Fast metabolizers n = 192 |
Slow metabolizers n = 418 |
p-value | |
|---|---|---|---|
| 1 month after RTx | |||
| Tac daily dose (mg) | 16 (12.0–21.0) | 9 (7–12) | < 0.001a |
| Tac trough level (ng/mL) | 11.0 (9–13) | 12.1 (10.2–15) | < 0.001a |
| Tac C/D ratio | 0.69 (0.52–0.91) | 1.4 (1.0–1.89) | < 0.001a |
| Prednisone dose | 15 (12.5–20) | 15 (10.6–20) | 0.940a |
| Methylprednisolone | 12 (9.5–16) | 12 (8–12) | 0.006a |
| 3 months after RTx | |||
| Tac daily dose (mg) | 14 (11–18) | 7 (5–9) | < 0.001a |
| Tac trough level (ng/mL) | 10.0 (8.0–11.0) | 11.0 (9.3–13.0) | < 0.001a |
| Tac C/D ratio | 0.72 (0.54–0.83) | 1.60 (1.25–2.18) | < 0.001a |
| Prednisone dose | 10 (5–15) | 5 (5–7.5) | 0.117a |
| Methylprednisolone | 4 (4–4) | 4 (4–4) | 0.229a |
| 6 months after RTx | |||
| Tac daily dose (mg) | 11 (9–15) | 6 (4–7.4) | < 0.001a |
| Tac trough level (ng/mL) | 9.9 (7.1–11.9) | 9.4 (6.0–14.0) | < 0.916a |
| Tac C/D ratio | 0.89 (0.63–1.08) | 1.69 (1.29–2.33) | < 0.001a |
| Prednisone dose | 5 (2.5–10) | 5 (1.3–5) | 0.172a |
| Methylprednisolone | 4 (4–4) | 4 (4–4) | 0.835a |
| Initial co-immunosuppression | |||
| Mycophenolate mofetil, n (%) | 190 (99.3%) | 415 (99%) | 0.652b |
| Mycophenolate sodium, n (%) | 2 (0.7%) | 3 (1%) | |
Data presented as mean ± standard deviation or median (25% quantile-75% quantile), or absolute and relative frequencies.
Tac tacrolimus, C/D concentration to dose.
aMann–Whitney U test.
bFisher’s exact test.
Renal function
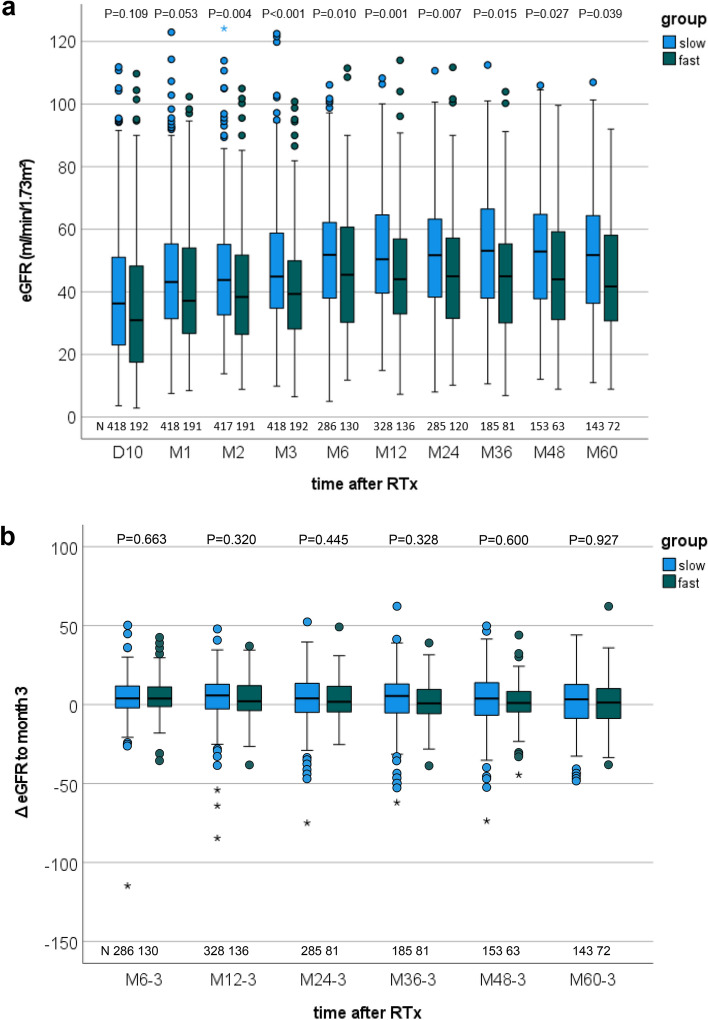
As early as D10, renal function of the fast ER-Tac metabolizers was slightly decreased compared to the slow metabolizers and reached a noticeable difference at M2 (p = 0.004). This difference persisted over the entire time period. (Fig. 3a). In a further step, we analyzed whether the M3 C/D ratio had an influence on the subsequent eGFR change. In contrast to the different ΔeGFR development from D10 to M3 after RTx (p = 0.0362), the subsequent ΔeGFR changes from the M3 eGFR did not differ between both groups (Fig. 3b). However, 5 years after RTx the eGFR in patients with a C/D ratio < 1 ng/mL · 1/mg was lower than in patients with ≥ 1 ng/mL · 1/mg (45.6 ± 19.4 vs. 51.5 ± 19.6 mL/min/1.73 m2; p = 0.039).
Figure 3.
Boxplots of the renal function. Fast ER-tacrolimus metabolizers had a reduced estimated glomerular filtration rate (eGFR) as early as 10 days to 60 months (M60) after renal transplantation (RTx) compared with slow metabolizers (a). Comparison of the eGFR change (ΔeGFR) from subsequent time points to M3 (Mx-3) showed no differences between metabolizer groups (b). P-values are from Welch’s t-test. D day, M month.
The results in the linear mixed model were similar to the univariate analyses (Table 3). The mean eGFR in fast metabolizers was 7.5 (95% CI 4.3–10.6, p < 0.001) mL/min/1.73 m2 lower pooled over all time points (M3–M60). eGFR changed in the whole collective from M3 (p < 0.001) and the changes between both metabolism groups were slightly different (interaction term, p = 0.037). Age at RTx (p < 0.001) had a further potential influence on the renal function. Despite the small p-value the additional effect of age was quite low. Per year of age older the eGFR value declined by -0.4 (95% CI − 0.5 to − 0.3) mL/min/1.73 m2. The influence of transplantation in the ESP indicated only a trend (p = 0.084). In these patients, the eGFR was − 5.7 (95% CI − 12.2 to 0.8) mL/min/1.73 m2 lower than in patients who received an organ in the regular allocation system.
Table 3.
Renal function, eGFR (linear mixed model).
| Model-based estimates | |||||
|---|---|---|---|---|---|
| Mean eGFR | Lower 95% confidence limit | Upper 95% confidence limit | p-value | ||
| ESP transplantation | Yes vs. no | −5.73 | −12.23 | 0.77 | 0.084 |
| Age at RTx | x vs. x − 1 years | −0.36 | −0.46 | −0.26 | < 0.001 |
| Effect of metabolism group combined over all time points | Fast vs. slow | −7.5 | −10.6 | −4.3 | < 0.001 |
| Effect of time combined over both metabolism groups | < 0.001 | ||||
| Interaction term of ER-Tac metabolism groups × time points | 0.037 | ||||
| Least square estimates of the mean difference between fast and slow metabolizer at different time points (combination of main and interaction effects of tacrolimus metabolism group and time points) | |||||
| At 3 months | Fast vs. slow | −7.62 | −10.70 | −4.55 | < 0.001 |
| At 6 months | Fast vs. slow | −6.98 | −10.55 | −3.42 | < 0.001 |
| At 12 months | Fast vs. slow | −8.51 | −11.85 | −5.18 | < 0.001 |
| At 24 months | Fast vs. slow | −8.22 | −11.69 | −4.75 | < 0.001 |
| At 36 months | Fast vs. slow | −9.04 | −12.83 | −5.25 | < 0.001 |
| At 48 months | Fast vs. slow | −7.07 | −11.20 | −2.95 | < 0.001 |
| At 60 months | Fast vs. slow | −4.80 | −9.03 | −0.56 | 0.027 |
| Least square estimates of the mean change between the time points for each metabolism group (combination of main and interaction effects of tacrolimus metabolism group and time points) | |||||
| Fast metabolizer | 6 vs. 3 months | 5.18 | 3.22 | 7.13 | < 0.001 |
| 12 vs. 3 months | 4.08 | 2.11 | 6.05 | < 0.001 | |
| 24 vs. 3 months | 2.92 | 0.76 | 5.09 | 0.008 | |
| 36 vs. 3 months | 2.16 | −0.27 | 4.60 | 0.082 | |
| 48 vs. 3 months | 2.45 | −0.49 | 5.40 | 0.103 | |
| 60 vs. 3 months | 3.87 | 0.84 | 6.89 | 0.012 | |
| Slow metabolizer | 6 vs. 3 months | 4.54 | 3.09 | 5.98 | < 0.001 |
| 12 vs. 3 months | 4.97 | 3.57 | 6.37 | < 0.001 | |
| 24 vs. 3 months | 3.52 | 1.86 | 5.18 | < 0.001 | |
| 36 vs. 3 months | 3.58 | 1.55 | 5.60 | < 0.001 | |
| 48 vs. 3 months | 1.90 | −0.24 | 4.04 | 0.082 | |
| 60 vs. 3 months | 1.04 | −1.26 | 3..33 | 0.375 | |
Results of the linear mixed model. Selected parameter estimates and least square means for estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) are shown. P-values are from Wald tests. Repeated measurements for each patient were modelled using SAS PROC MIXED by fitting a marginal linear mixed model with an unstructured variance–covariance matrix for the residuals with patient as subject and the order given by time.
ESP European Senior Program, RTx renal transplantation.
Time-to-event endpoints
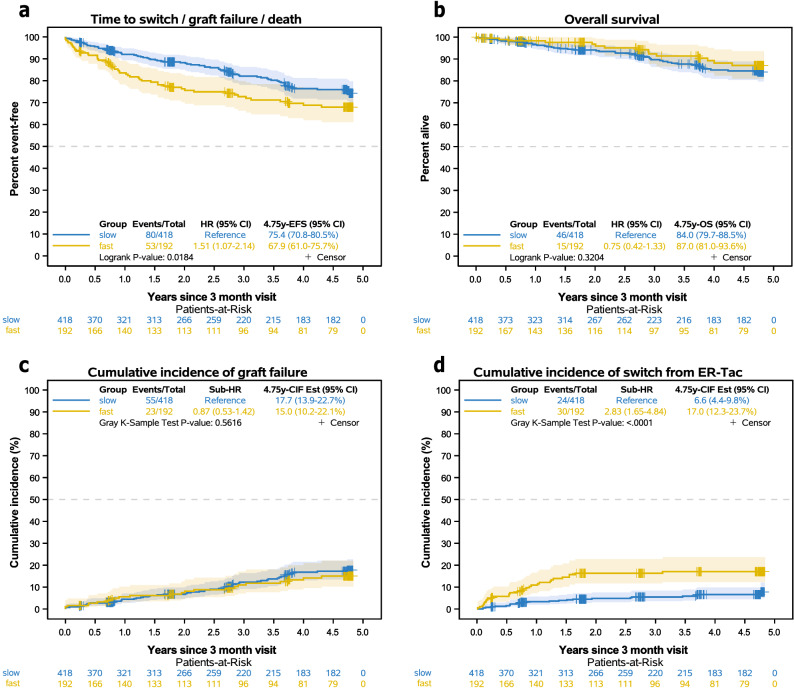
Consideration of the combined endpoint of switch/graft failure/death showed that fast metabolizers had more and earlier events than slow metabolizers over the 5-year follow-up (hazard ratio 1.51 (95% CI 1.07–2.14), Fig. 4a). Competing risk analysis indicated that crucial to these differences was a higher cumulative incidence of fast metabolizers who were switched from ER-Tac to other immunosuppression (p < 0.0001, Table 4, Fig. 4d). In detail, more fast metabolizers were switched from ER-Tac to IR-Tac (p = 0.002) or everolimus (p = 0.021). The main reasons for the change were CNIT (< 0.001) and large variations in Tac trough concentrations (p = 0.036) compared to slow metabolizers. The cumulative incidence of the component “graft failure” of the combined endpoint switch/graft failure/death was not different between the groups (p = 0.562, Fig. 4c). One slow metabolizer died without prior switch or graft failure. A difference in overall survival was not observed (p = 0.320, Fig. 4b). However, analysis of “first AR” events (after M3) showed that fast ER-Tac metabolizers developed more events compared to slow metabolizers (p = 0.0077, Fig. 5, Table 5). In a further analysis, no differences were found in the histological AR subtypes. Considering the period from RTx to M3, fast metabolizers also suffered more frequently from “first AR” than slow metabolizers (24.5% vs. 16.5%; p = 0.026, Table 5).
Figure 4.
Kaplan–Meier curves of the composite endpoint “switch from ER-Tac”, “graft failure”, or “death” as first event by metabolism group starting from three months after RTx (a). Overall survival is shown in (b). Cumulative incidence of the “graft failure” component (c) and “switch from ER-Tac” (c) of the composite endpoint. Since only one death without prior switch or graft failure occurred in the slow metabolism group, the curves were not drawn. The impact of fast vs. slow metabolizer was thus estimated using Fine and Gray's model leading to subdistribution hazard ratios (sub-HR) on the components of the composite endpoint. Cumulative incidence was estimated using the Aalen-Johansen estimator. Gray's k-sample test was applied to compare the cumulative incidence of the corresponding event type. The combined endpoint “switch/graft failure/death” showed more events in the fast metabolizer group. Competing risk analysis revealed that “switch from ER-Tac” occurred more frequently in fast than in slow metabolizers, but no differences were found in regards to graft failure or overall survival.
Table 4.
Switch from ER-Tac to another immunosuppression.
| Fast metabolizers n = 192 | Slow metabolizers n = 418 | p-value | |
|---|---|---|---|
| Switch from ER-Tac between 3 months and 5 years from RTx (events, 5 year-cumulative incidence, 95% CI) | 30 (17.0 [12.3–23.7]%) | 24 (6.6 [4.4–9.8]%) | < 0.0001a |
| Switched to | |||
| IR-Tac | 8 | 2 | 0.002b |
| LCP-T | 1 | 0 | 0.315b |
| Everolimus | 11 | 8 | 0.021b |
| ciclosporin A | 10 | 14 | 0.270b |
| Reason for switch | |||
| CNIT | 23 | 16 | < 0.001b |
| Large Tac level variation | 4 | 1 | 0.036b |
| NODAT | 1 | 3 | – |
| BKVN | 1 | 1 | – |
| Malignancy | 0 | 1 | – |
| NODAT + CNIT | 0 | 1 | – |
| BKVN + CNIT | 1 | 0 | – |
| BKVN + CMV | 0 | 1 | – |
Cumulative incidence was estimated using the Aalen-Johansen estimator.
IR-Tac immediate-release tacrolimus, LCP-T LCP-tacrolimus, CNIT calcineurin inhibitor toxicity, NODAT new onset diabetes after transplantation.
aGray k-sample test.
bFisher's exact test.
Figure 5.
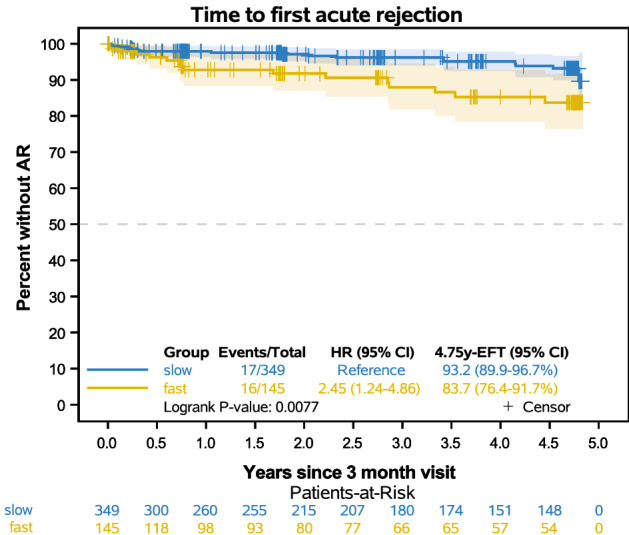
Time to “first acute rejection” (AR) from 3 months after transplantation. Patients who switched immunosuppression, showed a graft failure or died without prior AR were censored at the respective date. Patients with an AR between transplantation and 3 months were excluded (n = 116). Fast ER-Tac metabolizers showed more first rejections compared to slow metabolizers within 5-years after transplantation.
Table 5.
First acute rejection, graft failure and death.
| Fast metabolizers n = 192 | Slow metabolizers n = 418 | p-value | |
|---|---|---|---|
| First acute rejection (from RTx to 3 months after RTx) | 47 (24.5%) | 69 (16.5%) | 0.026a |
| First acute rejection between 3 months and 5 years from RTx (events, 5 year-Est, 95% CI) | 16/145 16.3% (8.3–23.6%) | 17/349 6.8% (3.3–10.1%) | 0.008b |
| Type of first acute rejection (from 3 months to 5 years after RTx) | |||
| ABMR | 0 | 2 | 0.095a |
| TCMR | 7 | 10 | |
| Borderline | 9 | 4 | |
| borderline + ABMR | 0 | 1 | |
| Graft failure as first event between 3 months and 5 years from RTx (events, 5 year-cumulative incidence, 95% CI) |
23 15% (10.2–22.1%) |
56 17.7% (13.9–22.7%) |
0.562c |
| Reasons for graft failure | |||
| Chronic allograft rejection | 5 | 5 | 0.240a |
| Glomerulonephrtis recurrence | 2 | 1 | |
| BKVN | 1 | 4 | |
| Infection | 2 | 11 | |
| Allograft ischemia/renal artery complication | 2 | 4 | |
| Perirenal hematoma | 1 | 0 | |
| Nephrocalcinosis | 0 | 1 | |
| Death with functioning allograft | 10 | 30 | |
| Death 3 months and 5 years from RTx (events, 5 year-Est, 95% CI) |
15 13.0% (6.4–19.0%) |
47 16% (11.5–20.3%) |
0.320b |
| Reasons for death | |||
| Cardiovascular | 2 | 5 | 0.776a |
| Malignancy | 2 | 12 | |
| Infection | 3 | 13 | |
| Encephalopathia | 0 | 1 | |
| Mayor bleeding | 1 | 1 | |
| Intoxication | 0 | 1 | |
| Trauma | 0 | 1 | |
| EuthanasiaA | 0 | 1 | |
| Unknown | 7 | 12 | |
Cumulative incidence was estimated using the Aalen-Johansen estimator.
Est = 1 − Kaplan–Meier estimator, ABMR antibody mediated rejection, TCMR T-cell mediated rejection, BKVN BK virus nephropathy, RTx renal transplantation.
AOnepatient from the Belgian cohort.
P-values: a Fisher’s exact test; bLogrank test; cGray k-sample test.
Discussion
Because IR-Tac and ER-Tac have comparable safety and efficacy, we hypothesized that a C/D ratio-guided analysis would be able to stratify patients treated with ER-Tac analogously to patients treated with IR-Tac with respect to their Tac metabolism.
Previous analysis of IR-Tac metabolism groups after RTx and liver transplantation (LTx) defined by a C/D ratio cut-off showed repeatedly and consistently worse renal outcomes in fast metabolizers compared to slow metabolizers16, 17, 21, 22, 28. Other studies without C/D ratio calculation, but dose and trough data pointed into the same direction29, 30. Interestingly, even studies who included patients with IR-Tac and ER-Tac observed comparable outcomes to pure IR-Tac studies18, 19. An analysis of the Korean Organ Transplantation Registry did not provide information regarding the used Tac formulation but observed the same findings31. Further evidence comes from PK profile analyses and assumptions. When analyzing the PK profiles of RTx patients treated with ER-Tac, one can observe the same mixed PK pattern with different profile types (probably related to the Tac metabolic type of the patients) as in IR-treated RTx recipients32, 33. Interestingly, the Cmax was also not significantly different or only slightly lower than that of patients treated with IR-Tac and was comparable in healthy subjects34, 35. This is important, as we found high Cmax associated with a higher degree of CNIT22. Thus, there was evidence from the literature suggesting a similar C/D ratio-dependent effect in IR-Tac and ER-treated patients.
We have calculated a C/D ratio cutoff of 1.0 ng/mL · 1/mg for the differentiation of metabolism types in ER-Tac treated patients. This fits well with the previously calculated cut-offs 1.05 ng/mL · 1/mg for IR-treated RTx and 1.09 ng/mL · 1/mg for LTx patients16, 17, 21. In addition, the TOMATO study, which included a hybrid cohort of 1029 IR- and ER-Tac patients, and which also used a C/D cutoff of 1.05 ng/mL · 1/mg, found that, on the one hand, the C/D ratio was stable between months 6–12 after RTx. On the other hand, a hazard ratio of 2.25 could be calculated for death-censored graft loss in patients with a C/D ratio < 1.05 ng/mL · 1/mg in contrast to the CYP3A5 genotyping which was not predictive18. Unfortunately, the authors had no data on eGFR in the follow-up. A reduced survival as well as increased incidence of graft failure in fast metabolizers is consistent to our IR-Tac data21, in contrast, we could not observe the same differences in the present study (Fig. 4b,c). This may be related to the fact that during follow-up, 17.0% of patients in the fast ER-Tac metabolizer group were switched to an alternative immunosuppressive regimen, compared to only 6.6% in the slow metabolizer group (p < 0.0001, Table 4, Fig. 4d). The main reason for switching from ER-Tac was CNIT, which was observed more frequently in the group with the C/D ratio < 1.0 ng/mL · 1/mg. This is in congruence with our previous studies on IR-Tac and the observation that Cmax could be critical for its development16, 21, 22, 36. IR- and ER-Tac show similar PK in terms of Cmax and their narrow therapeutic window. Accordingly, adverse effects such as CNIT are common and may occur in similar frequency, especially in fast metabolizers who have higher Cmax than slow metabolizers—at least in IR-Tac treated patients22. One limitation of our present study is that we do not have Cmax data. Interestingly, the major difference in eGFR between both groups developed within 3 months after RTx. We already knew from former studies that CNIT can occur early after RTx, especially in patients treated with high Tac dosages29, 37. Recently, in de novo ER-Tac-treated patients, it was shown that a C/D ratio < 1.05 ng/mL · 1/mg and the presence of interstitial fibrosis and tubular atrophy (IF/TA) in a biopsy 3 months after RTx were associated with future IF/TA progression38. Of note, the authors observed that unlike Tac trough levels, intra-patient variability, or time below the therapeutic range, just a low C/D ratio was associated with IF/TA progression. This is congruent with data from Egeland et al. who identified fast Tac metabolism as a risk factor for the development of IF/TA39. It is important to note in this context that both Tac formulations have high intra- and inter-individual coefficients of variation for AUC, Cmin and Cmax40, 41. Recently, this high intra-patient variability (IPV) in Tac trough levels especially early after RTx has been associated with rejection, de novo donor-specific antibodies (DSA), progressive IF/TA and reduced graft survival42. One explanation could be that patients with high IPV are outside the therapeutic range for extended periods of time since e.g. a single missed Tac dose can greatly affect exposure especially in recipients with fast metabolism43. It has been argued that switching patients from IR-Tac to ER-Tac may be beneficial in terms of IPV, but the data remain inconclusive and clinically limited44, 45. Consistent with these observations, patients with a C/D ratio < 1.0 ng/mL · 1/mg suffered more AR events than patients with a higher C/D ratio (Table 5, Fig. 5). Similar observations have been made in patients with IR-Tac-treated patients21, 23.
Our study has limitations. Since this is a retrospective study, it can only generate new hypotheses. Moreover, we did not assess Cmax and coefficient of variation/IPV in our patients. Therefore, we can only assume effects of fast ER-Tac metabolism (low C/D ratio) of renal outcomes. Further, more patients in the group with a C/D ratio < 1.0 ng/mL · 1/mg were switched mainly due to side effects to an alternative immunosuppression. Thus, we cannot rule out completely that this might have affected the outcomes. However, since we have previously shown that a conversion from Tac to everolimus can be safely done in selected patients with e.g. CNIT this could have stabilized some fast metabolizers and reduced the difference in eGFR loss36. Donor factors may influence graft outcome. Unfortunately, donor eGFR data are not available to us. However, since the donors did not differ significantly between the groups with respect to various factors such as age, BMI, and KDPI, and the rate of DGF was comparable, we do not expect major differences in the eGFR of the donors.
Conclusion
We conclude from our data that patients treated with ER-Tac who have a C/D ratio < 1.0 ng/mL · 1/mg showed a decreased eGFR, especially early after RTx. This was mainly associated with CNIT and a higher rate of first AR, leading to more frequent changes in immunosuppression in these patients. Thus, this group of patients has an increased risk of developing a worse outcome after RTx and therefore deserves special attention. Because it is very simple and without cost, we propose to calculate the C/D ratio early after RTx in patients treated with ER-Tac. Switching rapid ER-Tac metabolizers to other Tac formulations, such as LCP-Tac, or other immunosuppressive agents, such as belatacept or everolimus, is safe and may be beneficial, taking into account the immunologic and metabolic profile of the individual.
Author contributions
G.T., S.R. and D.K. designed the study and wrote the paper; B.F., U.J., K.S.-N., C.K. and D.K. performed data collection and wrote the paper; B.F., R.K., G.T. and S.R. performed analysis and interpretation; H.P. and B.S. wrote the paper. All authors critically reviewed the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. No funding was obtained for data collection, data analysis or writing the manuscript.
Data availability
All authors agree with the all publication's requirements for sharing materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefan Reuter and Dirk Kuypers.
References
- 1.Kidney Disease: Improving Global Outcomes Transplant Work, G. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant.9 Suppl 3, S1–155. 10.1111/j.1600-6143.2009.02834.x (2009). [DOI] [PubMed]
- 2.Hart A, et al. OPTN/SRTR 2018 annual data report: Kidney. Am. J. Transplant. 2020;20 Suppl s1:20–130. doi: 10.1111/ajt.15672. [DOI] [PubMed] [Google Scholar]
- 3.Torabi J, et al. The use of LCP-Tacrolimus (Envarsus XR) in simultaneous pancreas and kidney (SPK) transplant recipients. Am. J. Surg. 2020;219:583–586. doi: 10.1016/j.amjsurg.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 4.Kuypers DR, et al. Improved adherence to tacrolimus once-daily formulation in renal recipients: A randomized controlled trial using electronic monitoring. Transplantation. 2013;95:333–340. doi: 10.1097/TP.0b013e3182725532. [DOI] [PubMed] [Google Scholar]
- 5.Piotti G, Cremaschi E, Maggiore U. Once-daily prolonged-release tacrolimus formulations for kidney transplantation: What the nephrologist needs to know. J. Nephrol. 2017;30:53–61. doi: 10.1007/s40620-016-0316-3. [DOI] [PubMed] [Google Scholar]
- 6.Coilly A, et al. Once-daily prolonged release tacrolimus in liver transplantation: Experts' literature review and recommendations. Liver Transplant. 2015;21:1312–1321. doi: 10.1002/lt.24228. [DOI] [PubMed] [Google Scholar]
- 7.Wlodarczyk Z, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: A randomized, open-label trial. Am. J. Transplant. 2009;9:2505–2513. doi: 10.1111/j.1600-6143.2009.02794.x. [DOI] [PubMed] [Google Scholar]
- 8.Kuypers DRJ. Intrapatient variability of tacrolimus exposure in solid organ transplantation: A novel marker for clinical outcome. Clin. Pharmacol. Ther. 2019 doi: 10.1002/cpt.1618. [DOI] [PubMed] [Google Scholar]
- 9.Schutte-Nutgen K, Tholking G, Suwelack B, Reuter S. Tacrolimus—Pharmacokinetic considerations for clinicians. Curr. Drug Metab. 2018;19:342–350. doi: 10.2174/1389200219666180101104159. [DOI] [PubMed] [Google Scholar]
- 10.Tholking G, Gerth HU, Schuette-Nuetgen K, Reuter S. Influence of tacrolimus metabolism rate on renal function after solid organ transplantation. World J. Transplant. 2017;7:26–33. doi: 10.5500/wjt.v7.i1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuypers DRJ. From nonadherence to adherence. Transplantation. 2020 doi: 10.1097/TP.0000000000003112. [DOI] [PubMed] [Google Scholar]
- 12.Oberbauer, R. et al. Optimization of tacrolimus in kidney transplantation: New pharmacokinetic perspectives. Transplant. Rev. (Orlando)34, 100531. 10.1016/j.trre.2020.100531 (2020). [DOI] [PubMed]
- 13.Obi Y, et al. A single daily dose enhances the adherence to immunosuppressive treatment in kidney transplant recipients: A cross-sectional study. Clin. Exp. Nephrol. 2013;17:310–315. doi: 10.1007/s10157-012-0713-4. [DOI] [PubMed] [Google Scholar]
- 14.Saengram W, Vadcharavivad S, Poolsup N, Chancharoenthana W. Extended release versus immediate release tacrolimus in kidney transplant recipients: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2018;74:1249–1260. doi: 10.1007/s00228-018-2512-7. [DOI] [PubMed] [Google Scholar]
- 15.Vadcharavivad S, Saengram W, Phupradit A, Poolsup N, Chancharoenthana W. Once-daily versus twice-daily tacrolimus in kidney transplantation: A systematic review and meta-analysis of observational studies. Drugs. 2019;79:1947–1962. doi: 10.1007/s40265-019-01217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tholking G, et al. The tacrolimus metabolism rate influences renal function after kidney transplantation. PLoS ONE. 2014;9:e111128. doi: 10.1371/journal.pone.0111128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tholking G, et al. Tacrolimus concentration/dose ratio is associated with renal function after liver transplantation. Ann. Transplant. 2016;21:167–179. doi: 10.12659/aot.895898. [DOI] [PubMed] [Google Scholar]
- 18.Jouve T, et al. The TOMATO study (TacrOlimus MetabolizAtion in kidney TransplantatiOn): Impact of the concentration-dose ratio on death-censored graft survival. Transplantation. 2019 doi: 10.1097/TP.0000000000002920. [DOI] [PubMed] [Google Scholar]
- 19.Nowicka M, et al. Tacrolimus: Influence of the posttransplant concentration/dose ratio on kidney graft function in a two-year follow-up. Kidney Blood Press. Res. 2019;44:1075–1088. doi: 10.1159/000502290. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowska E, et al. C/D ratio in long-term renal function. Transplant Proc. 2019;51:3265–3270. doi: 10.1016/j.transproceed.2019.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Schutte-Nutgen K, et al. Fast tac metabolizers at risk (-) it is time for a C/D ratio calculation. J. Clin. Med. 2019;8:587. doi: 10.3390/jcm8050587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tholking G, et al. A low tacrolimus concentration/dose ratio increases the risk for the development of acute calcineurin inhibitor-induced nephrotoxicity. J. Clin. Med. 2019;8:1586. doi: 10.3390/jcm8101586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egeland EJ, et al. High tacrolimus clearance is a risk factor for acute rejection in the early phase after renal transplantation. Transplantation. 2017;101:e273–e279. doi: 10.1097/TP.0000000000001796. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay S, Nigro V, Weinberg J, Woodle ES, Alloway RR. A steady-state head-to-head pharmacokinetic comparison of all FK-506 (Tacrolimus) formulations (ASTCOFF): An open-label, prospective, randomized, two-arm, three-period crossover study. Am. J. Transplant. 2017;17:432–442. doi: 10.1111/ajt.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tholking G, et al. Influence of tacrolimus metabolism rate on BKV infection after kidney transplantation. Sci. Rep. 2016;6:32273. doi: 10.1038/srep32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahmen M, et al. Validation of the Kidney Donor Profile Index (KDPI) to assess a deceased donor's kidneys' outcome in a European cohort. Sci. Rep. 2019;9:11234. doi: 10.1038/s41598-019-47772-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin. J. Am. Soc. Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 28.Bardou FN, et al. Tacrolimus exposure after liver transplantation for alcohol-related liver disease: Impact on complications. Transpl. Immunol. 2019;56:101227. doi: 10.1016/j.trim.2019.101227. [DOI] [PubMed] [Google Scholar]
- 29.Kuypers DR, et al. Tacrolimus dose requirements and CYP3A5 genotype and the development of calcineurin inhibitor-associated nephrotoxicity in renal allograft recipients. Ther. Drug Monit. 2010;32:394–404. doi: 10.1097/FTD.0b013e3181e06818. [DOI] [PubMed] [Google Scholar]
- 30.Genvigir FD, et al. Influence of the CYP3A4/5 genetic score and ABCB1 polymorphisms on tacrolimus exposure and renal function in Brazilian kidney transplant patients. Pharmacogenet. Genomics. 2016;26:462–472. doi: 10.1097/FPC.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 31.Ro H, et al. The tacrolimus metabolism affect post-transplant outcome mediating acute rejection and delayed graft function: Analysis from Korean Organ Transplantation Registry data. Transpl. Int. 2021;34:163–174. doi: 10.1111/tri.13777. [DOI] [PubMed] [Google Scholar]
- 32.Nakazawa R, et al. Clinically useful limited sampling strategy to estimate area under the concentration-time curve of once-daily tacrolimus in adult Japanese kidney transplant recipients. PLoS ONE. 2019;14:e0225878. doi: 10.1371/journal.pone.0225878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura M, et al. Early phase limited sampling strategy characterizing tacrolimus and mycophenolic acid pharmacokinetics adapted to the maintenance phase of renal transplant patients. Ther. Drug Monit. 2009;31:467–474. doi: 10.1097/FTD.0b013e3181ae44b9. [DOI] [PubMed] [Google Scholar]
- 34.Niioka T, et al. Comparison of pharmacokinetics and pharmacogenetics of once- and twice-daily tacrolimus in the early stage after renal transplantation. Transplantation. 2012;94:1013–1019. doi: 10.1097/TP.0b013e31826bc400. [DOI] [PubMed] [Google Scholar]
- 35.Zaltzman JS, Lai V, Schulz MZ, Moon KH, Cherney DZ. A randomized cross-over comparison of short-term exposure of once-daily extended release tacrolimus and twice-daily tacrolimus on renal function in healthy volunteers. Transplant. Int. 2014;27:1294–1302. doi: 10.1111/tri.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tholking G, et al. Conversion to everolimus was beneficial and safe for fast and slow tacrolimus metabolizers after renal transplantation. J. Clin. Med. 2020;9:328. doi: 10.3390/jcm9020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laskow DA, Vincenti F, Neylan JF, Mendez R, Matas AJ. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: A report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62:900–905. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Chamoun B, et al. Progression of interstitial fibrosis and tubular atrophy in low immunological risk renal transplants monitored by sequential surveillance biopsies: The influence of TAC exposure and metabolism. J. Clin. Med. 2021;10:141. doi: 10.3390/jcm10010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egeland EJ, et al. High tacrolimus clearance—A risk factor for development of interstitial fibrosis and tubular atrophy in the transplanted kidney: A retrospective single-center cohort study. Transpl. Int. 2019;32:257–269. doi: 10.1111/tri.13356. [DOI] [PubMed] [Google Scholar]
- 40.Benkali K, et al. Population pharmacokinetics and Bayesian estimation of tacrolimus exposure in renal transplant recipients on a new once-daily formulation. Clin. Pharmacokinet. 2010;49:683–692. doi: 10.2165/11535950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Vanhove T, Vermeulen T, Annaert P, Lerut E, Kuypers DRJ. High intrapatient variability of tacrolimus concentrations predicts accelerated progression of chronic histologic lesions in renal recipients. Am. J. Transplant. 2016;16:2954–2963. doi: 10.1111/ajt.13803. [DOI] [PubMed] [Google Scholar]
- 42.Kuypers DRJ. Intrapatient variability of tacrolimus exposure in solid organ transplantation: A novel marker for clinical outcome. Clin. Pharmacol. Ther. 2020;107:347–358. doi: 10.1002/cpt.1618. [DOI] [PubMed] [Google Scholar]
- 43.Saint-Marcoux F, et al. How to handle missed or delayed doses of tacrolimus in renal transplant recipients? A pharmacokinetic investigation. Pharmacol. Res. 2015;100:281–287. doi: 10.1016/j.phrs.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: Causes, consequences for clinical management. Transplant Rev. (Orlando) 2015;29:78–84. doi: 10.1016/j.trre.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Stifft F, Stolk LM, Undre N, van Hooff JP, Christiaans MH. Lower variability in 24-hour exposure during once-daily compared to twice-daily tacrolimus formulation in kidney transplantation. Transplantation. 2014;97:775–780. doi: 10.1097/01.TP.0000437561.31212.0e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All authors agree with the all publication's requirements for sharing materials.