Abstract
The role of lipoprotein-A [Lp (a)] as a risk factor for stroke is less well documented than for coronary heart disease. Hence, we conducted a systematic review and meta-analysis for the published observational studies in order to investigate the association of Lp (a) levels with the risk of stroke and its subtypes. In our meta-analysis, 41 studies involving 7874 ischemic stroke (IS) patients and 32,138 controls; 13 studies for the IS subtypes based on TOAST classification and 7 studies with 871 Intracerebral hemorrhage (ICH) cases and 2865 control subjects were included. A significant association between increased levels of Lp (a) and risk of IS as compared to control subjects was observed (standardized mean difference (SMD) 0.76; 95% confidence interval (CIs) 0.53–0.99). Lp (a) levels were also found to be significantly associated with the risk of large artery atherosclerosis (LAA) subtype of IS (SMD 0.68; 95% CI 0.01–1.34) as well as significantly associated with the risk of ICH (SMD 0.65; 95% CI 0.13–1.17) as compared to controls. Increased Lp (a) levels could be considered as a predictive marker for identifying individuals who are at risk of developing IS, LAA and ICH.
Subject terms: Neuroscience, Biomarkers
Introduction
Stroke is reported as the most common cause of long term disability and the second most leading cause of death worldwide1. Almost 80% of strokes are ischemic stroke (IS) and 15–20% are haemorrhagic stroke (HS) in origin2,3. According to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification; IS has been categorised according to the presumed etiological mechanism into five groups: large artery atherosclerosis (LAA), small vessel disease (SVD), cardioembolic disease (CE), other determined etiology (ODE), and undetermined etiology (UDE)4.
Lipoprotein (a) or Lp (a) is a lipoprotein moiety that consists of core lipoprotein molecule, containing apolipoprotein B (apo-B100), to which a glycoprotein of variable molecular weight, apolipoprotein (a) [apo(a)], is covalently attached via a cysteine-cysteine disulfide bond5,6. By binding LDL, calcium, and other components into an atherosclerotic plaque on the walls of blood arteries, Lp (a) is hypothesised to speed up the development of atherosclerosis7. The LPA gene regulates the variation in Lp (a) plasma concentrations genetically, ranging from 36% in the PROCARDIS8 consortium to 70–90% in genome-wide association studies, with larger apo(a) isoforms related with lower values of Lp (a)9,10. The concentrations of Lp (a) range from 0.1 mg/dl to more than 200 mg/dl11.
On a cellular level, apo(a) undergoes post-translational changes in the endoplasmic reticulum as a secretory protein (ER). The length of time it takes to modify larger apo(a) isoforms is determined by the size of the apo(a) isoform. As a result, larger apo(a) molecules are produced at a slower rate per unit of time, resulting in lower Lp (a) plasma concentrations6,12. Plasma Lp (a) concentrations appear to be regulated by synthesis rather than catabolism, according to kinetic studies. Concentration and pathological responses may be influenced by apo(a) sequence polymorphisms. Lp (a)/apo(a) functions may also be affected by changes in circulating Lp (a). Importantly, the relevance of apo(a) in cardiovascular diseases (CVD s) and peripheral vascular disorders, as well as its physiological function, remain unknown, and there is no effective therapeutic option for decreasing increased Lp levels (a)11,13–15.
Other large, population-based cohort studies on stroke have produced mixed results, with some research associating raised Lp (a) levels to a higher incidence of IS16–19, while others have found no link20–22. This could be due to a lack of discrimination across incidence stroke subtypes22, as well as ethnic or other disparities in cohort composition. A growing number of epidemiological studies have found a link between dyslipidemia and atherosclerosis-related stroke. Indeed, the lipid metabolism of different stroke types and IS subtypes differs dramatically23–26. Two previously published meta-analyses27,28 had confirmed that elevated Lp (a) is an independent risk factor for IS, however, IS subtypes based on TOAST classification as well as HS remains to be explored further. Hence, we conducted a systematic review and meta-analysis for the published observational studies in order to investigate the association of Lp (a) levels with the risk of stroke and its subtypes.
Methods
Search strategy
This systematic literature review was performed using the guidelines of the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-analyses)29. A comprehensive search for all the published articles was performed in electronic databases including PubMed, EMBASE, Cochrane Library, Trip Databases, Worldwide Science, and Google Scholar from 01st January 1950 to 30th April 2020. Following search terms: ‘Lipoprotein (a)’ OR ‘Lp (a) Levels’ OR ‘Lipid Biomarkers’ AND ‘Stroke’ OR ‘Subtypes’ OR ‘TOAST Classification’ OR ‘Ischemic Stroke’ AND ‘Haemorrhagic Stroke’ OR Intracerebral Haemorrhage’ OR ‘ICH’ AND ‘Cerebrovascular Disease’ AND ‘cerebral infarction’ were used. Reference lists of the selected studies were also searched manually to obtain any additional eligible studies on human subjects. No restrictions related to language, sex and publication year was applied.
Eligibility criteria
Inclusion criteria
(1) Observational studies including case–control, nested case–control, cross sectional and cohort design investigating the association of Lp (a) levels with the risk of stroke or stroke types or IS sub-types based on TOAST classification compared to control subjects; (2) studies with clinically confirmed diagnosis of stroke (ischemic or haemorrhagic) using CT or MRI scans; (3) patients aged ≥ 18 years (adult population); (4) studies reporting numbers for patients and control groups as well as raw values for Lp (a) levels.
Exclusion criteria
(1) Duplicates, case reports, case series, systematic reviews, conference abstracts, preprints and editorials; (2) Studies not reporting relevant outcomes; (3) Unavailability of full-texts.
Risk of bias in individual studies
The risk of bias was assessed by Newcastle–Ottawa Scale (NOS) for quality assessment of all the included studies in the meta-analysis30. The assessment criteria involving NOS uses three broad criteria viz. selection, comparability and exposure. Selection criteria defines and analyses the cases and control subjects included in the study, comparability defines the matching or comparison of cases and control subjects for better empirical investigation and exposure determines whether the study was conducted in a blinded or unbiased manner along with the response of the subjects. Publication bias was assessed using Begg’s and Egger’s funnel plot analysis31,32.
Data extraction
All relevant studies were analysed separately by two reviewers (PK and PS) based on the inclusion criteria listed above. The analysis was done first at the title and abstract level and then at the full-text level. Any disagreement was resolved by discussion with a third reviewer. Following data were extracted from the studies which included: First Author’s Name, Published Year, Ethnicity, Country, Study Design, Number of Cases and Controls, Mean Age, mean and standard deviation values of biochemical parameters including Lp (a), methods of Lp (a) assay and follow up duration. Data was extracted independently by two authors (PK and PS) using a standardized extraction table. Lp (a) concentrations were reported with different units in the included studies and were converted to similar units for analysis purpose using online unit conversion tools (http://unitslab.com/node/85).
Statistical analysis
A random or fixed effect model was used to calculate the pooled Standardized Mean Difference (SMD) or Odds Ratio (OR) with 95% confidence interval (CI). Heterogeneity was calculated with the I2 statistic and was adjusted by subgroup analysis followed by meta-regression using the quality score of the included studies. The heterogeneity was considered as significant in case of I2 more than 50% for which random-effects model was applied, on the other hand, if I2 was less than 50%, then fixed-effect model was applied. A sensitivity analysis was performed by sequentially omitting a single study in each turn, to validate the pooled observed effect. Tests were considered statistically significant at a p-value less than 0.05. Data were analyzed using STATA, version 13.0 (Stata Statistical Software, Release 13; StataCorp LP, College Station, TX).
Statement of ethics
Ethical approval was not required for this manuscript as it was a systematic review and meta-analysis done by using the existing published data and the research was not directly conducted in any human subjects.
Results
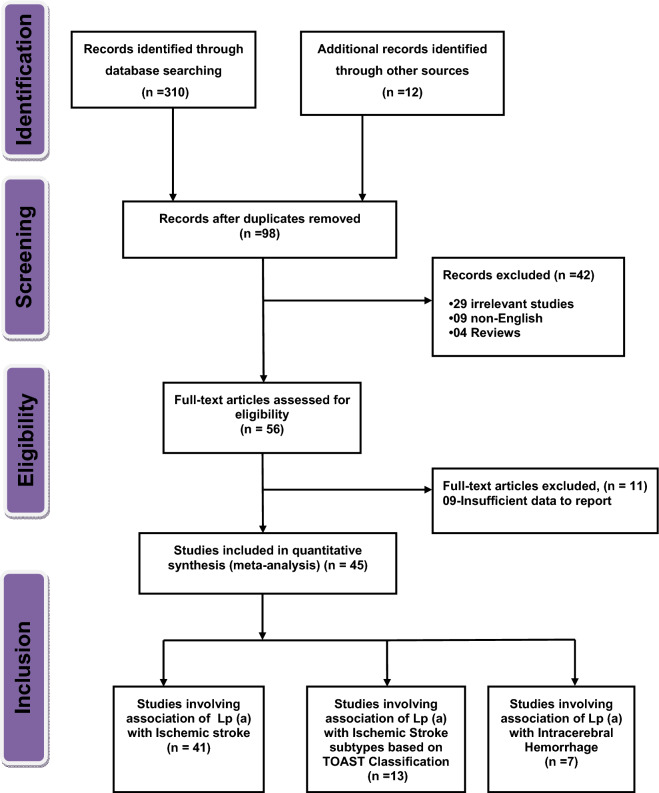
Figure 1 represents the PRISMA flow diagram listing the detailed reasons for exclusion and inclusion of studies in our systematic review and meta-analysis. Initially, a total of 322 studies were identified after searching in six different databases. PRISMA checklist has been provided in the supplementary table (Table S1). After removing duplicate articles, 98 articles were found and on further exclusion, a total of 56 full text articles were reviewed for eligibility and finally 45 studies were included in the systematic review and meta-analysis. The baseline characteristics of all the included 45 studies (41 studies for investigating the association of LP (a) with the risk of IS; 13 studies for IS subtypes and 7 studies for ICH) are given in Tables 1, 2 and 3.
Figure 1.
Flow diagram for the selection of studies and specific reasons for exclusion from the present meta-analysis.
Table 1.
Baseline characteristics of studies included in the systematic review and meta-analysis for the relationship between serum Lp (a) levels and risk of ischemic stroke.
| S. no. | Author name and year | Ethnicity | Study design | Source of control | Sample size (IS/control) | IS age (mean ± SD) | Control age (mean ± SD) | Matching criteria | LPA assay method | LPA cut off value | LPA timepoint | Follow up duration | NOS quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Shintani et al., 199333 | Asian | Case–control study | HB | 54/81 | 62 ± 8.1 | 61.1 ± 8.6 | NA | ELISA | ≥ 42.6 mg/dl | 4 weeks | NA | 5 |
| 2. | More et al., 201734 | Asian | Case–control study | HB | 100/50 | NA | NA | NA | NA | ≥ 30 mg/dl | NA | NA | 4 |
| 3. | Albala et al., 201035 | Caucasian | Case–control study | PB | 317/413 | 69.7 ± 12.3 | 69.7 ± 11.7 | Age, sex and race/ethnicity | Immunonephelometric procedure | ≤ 30 mg/dl | Within 72 h | NA | 6 |
| 4. | Kiechl et al., 200736 | Caucasian | Prospective cohort study | PB | 82/683 | 70.2 ± 10.3 | 61.8 ± 10.9 | Age and sex | ELISA | ≥ 24 mg/dl | NA | 7 | |
| 5. | Christopher et al., 199637 | Asian | Case–control study | HB | 50/50 | 27 ± 5 | 27 ± 5 | Age, sex and socioeconomic status | ELISA | NA | NA | NA | 5 |
| 6. | Fu et al., 202038 | Asian | Case–control study | HB | 1953/1953 | 62.3 ± 11.8 | 59.9 ± 11.1 | Age and sex | Latex agglutination turbidimetric method | 23.2 mg/dl | NA | NA | 7 |
| 7. | Dhamija et al., 200939 | Asian | Case–control study | HB | 66/72 | 54.43 ± 13 | 54.4 ± 13 | Age and sex | Immunoturbidimetric immunoassay | ≤ 30 mg/dl | Within 12 h | NA | 6 |
| 8. | Shao-yi-Li et al., 201440 | Asian | Prospective cohort study | PB | 181/120 | 63 ± 4.6 | 62.5 ± 5.7 | Age and sex | Immunoprecipitation techniques | ≥ 30 mg/dl | Within 24 h | NA | 7 |
| 9. | Milionis et al., 200541 | Caucasian | Case–control study | PB | 163/166 | 77.6 ± 4.8 | 77.7 ± 4.8 | Age and sex | Immunoprecipitation techniques | ≥ 30 mg/dl | Within 24 h | No | 7 |
| 10. | Peng et al., 199942 | Asian | Case–control study | HB | 90/90 | 62.6 ± 8.9 | 63.1 ± 8.3 | NA | ELISA | NA | Within 24 h | No | 4 |
| 11. | Jurgens et al., 199543 | Caucasian | Case–control study | HB | 42/288 | 51.4 ± 7.2 | 51 ± 7.1 | NA | ELISA | 20 mg/dl | Within 48 h | NA | 7 |
| 12. | Ridker et al., 199520 | Caucasian | Nested case–control study | PB | 198/198 | 62.5 ± 5 | 62.1 ± 5 | Age, sex and smoking | NA | 19.68 mg/dl | NA | 7 | |
| 13. | Rigal et al., 200644 | Caucasian | Case–control study | PB | 100/100 | 45.3 ± 7.7 | 45.1 ± 6.8 | Age and sex | Immunoturbidimetric method | 30 mg/dl | Within 4 days | NA | 6 |
| 14. | Sun et al., 200345 | Asian | Case–control study | PB | 1326/1817 | 61.1 ± 9.2 | 59.6 ± 8.5 | NA | ELISA | NA | Within 6 weeks | NA | 7 |
| 15. | Tascilar et al., 200846 | Caucasian | Case–control study | PB | 85/77 | 61.6 ± 13.5 | 54.7 ± 8.4 | NA | Latex agglutination assay | NA | NA | NA | 5 |
| 16. | Zenker et al., 198647 | Caucasian | Case–control study | HB | 46/37 | 53.6 ± 9.7 | 54.4 ± 7.7 | NA | Electro immunoassay | NA | NA | NA | 4 |
| 17. | Botet et al., 199248 | Caucasian | Case–control study | HB | 100/100 | 64.4 ± 6 | 64.4 ± 6 | Age | Electro immunoassay | NA | NA | NA | 4 |
| 18. | Glader et al., 199921 | Caucasian | Case–control study | PB | 101/201 | 55.6 ± 6.9 | 55.6 ± 6.8 | Age and sex | ELISA | 30 mg/dl | NA | NA | 4 |
| 19. | Poitrine et al., 201049 | Caucasian | Prospective cohort study | PB | 98/8978 | 55.6 ± 3 | 54.8 ± 2.8 | NA | Selective bi-site immunoenzymatic assay | NA | Within 12 h | 7 | |
| 20. | Albucher et al., 200050 | Caucasian | Case–control study | PB | 94/111 | 35.8 ± 8.2 | 35.8 ± 8.2 | Age | Rocket immunoelectrodiffusion | NA | NA | NA | 5 |
| 21. | Markus et al., 199751 | Caucasian | Case–control study | HB | 164/91 | 66.1 ± 9.8 | 64.6 ± 8.2 | NA | ELISA | 40 mg/dl | NA | NA | 6 |
| 22. | Alfthan et al., 199452 | Caucasian | Prospective cohort study | PB | 74/269 | 54 ± 4 | 54 ± 4 | NA | Two-site immunoradiometric method | NA | NA | 5 | |
| 23. | Chakraborty et al., 201353 | Asian | Case–control study | HB | 100/120 | 54 ± 10.9 | 52.5 ± 9.8 | Age and sex | Immunoturbidimetric method | NA | At 1, 7 days, 3 and 6 months | 6 | |
| 24. | Jones et al., 200754 | Caucasian | Case–control study | PB | 184/230 | 71.9 ± 10 | 70.3 ± 6.9 | NA | ELISA | > 45 nmol/L | NA | NA | 7 |
| 25. | Jones et al., 200955 | Caucasian | Case–control study | PB | 245/439 | 71.4 ± 10.5 | 68.8 ± 6.6 | NA | ELISA | NA | NA | NA | 6 |
| 26. | Denti et al., 200356 | Caucasian | Case–control study | HB | 79/98 | 82.9 ± 7.4 | 82.9 ± 7.4 | Age and sex | ELISA | NA | Within 48 h | NA | 5 |
| 27. | Hiraga et al., 199657 | Asian | Case–control study | HB | 83/39 | 67.6 ± 10.5 | 65.3 ± 6.8 | NA | Latex immunosorbent assay | NA | NA | NA | 4 |
| 28. | Pena-Diaz et l., 200358 | Caucasian | Case–control study | HB | 52/91 | 53.4 ± 10.5 | 40.2 ± 13.1 | NA | Immunonephelometric method | > 22.45 mg/dl | NA | NA | 4 |
| 29. | Karttunen et al., 200259 | Caucasian | Case–control study | PB | 46/104 | 41.5 ± 3.1 | 43.7 ± 3.2 | NA | ELISA | NA | NA | NA | 5 |
| 30. | Kario et al., 199460 | Asian | Case–control study | PB | 31/50 | 83 ± 5 | 84 ± 5 | NA | ELISA | > 30 mg/dl | Within 4 days | NA | 4 |
| 31. | Ma Lijuan et al., 201361 | Asian | Case–control study | HB | 124/64 | 60.6 ± 12.1 | 62 ± 9.1 | NA | Sandwich ELISA | NA | Within 12 h | NA | 6 |
| 32. | Murai et al.,, 198562 | Asian | Case–control study | HB | 156/99 | 64.8 ± 9 | 61.5 ± 13.4 | Age | Single radial immunodiffusion method | 17 mg/dl | NA | NA | 4 |
| 33. | Lindgren A et al., 199263 | Caucasian | Case–control study | PB | 119/159 | 70.7 ± 9.1 | 60 ± 11.5 | Age | Radioimmunoassay | NA | NA | NA | 5 |
| 34. | Kooten et al., 199664 | Caucasian | Case–control study | HB | 119/274 | 66.3 ± 15.4 | 50.2 ± 7.4 | NA | Two-site immunoradiometric assay | NA | NA | NA | 6 |
| 35. | Peynet et al., 199965 | Caucasian | Case–control study | PB | 90/84 | 37.4 ± 8.7 | 37.4 ± 8.7 | Age and sex | Immunonephelometric assay | NA | After 3 months of stroke | NA | 6 |
| 36. | Petersen et al., 200766 | Caucasian | Case–control study | HB | 253/63 | 63 ± 14 | 60.2 ± 10.6 | Age and sex | Double-antibody ELISA | 30 mg/dl | NA | NA | 6 |
| 37. | Saito et al., 199767 | Asian | Case–control study | HB | 118/95 | 71 ± 10 | NA | Sandwich ELISA | NA | NA | NA | 4 | |
| 38. | Santos-silva et al., 200268 | Caucasian | Case–control study | HB | 50/29 | 20–79 | Age | Electro immunodiffusion | NA | NA | NA | 4 | |
| 39. | Seki et al., 199769 | Asian | Case–control study | HB | 64/37 | 72.1 ± 8.4 | 61 ± 20 | NA | ELISA | NA | NA | NA | 5 |
| 40. | Schreiner et al., 1994 (Black)70 | Caucasian | Prospective cohort study | PB | 324/14,818 | 56.6 ± 6 | 53 ± 6 | NA | ELISA | 30 mg/dl | NA | NA | 5 |
| 41. | Zhang et al., 201371 | Asian | Case–control study | HB | 153/100 | 63 ± 12.7 | 63 ± 12.7 | Age and sex | Immunoturbidimetric method | NA | NA | NA | 6 |
Table 2.
Baseline characteristics of studies included in the systematic review and meta-analysis for the relationship between serum Lp(a) levels and risk of ischemic stroke subtypes based on TOAST classification.
| S. no. | Author name and year | Ethnicity | Study design | Sample size (IS) | Sample size (LAA) | Sample size (SVD) | Sample size (CE) | Sample size (UDE) | Sample size (ODE) | Sample size (control) | LPA assay method | LPA cut off value | LPA timepoint | Follow up duration | NOS quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Shintani et al., 199333 | Asian | Case–control study | 45 | 9 | 34 | NA | NA | NA | 81 | ELISA | ≥ 42.6 mg/dl | Within 4 weeks | 5 | |
| 2. | Tang et al., 201972 | Asian | Retrospective cohort study | 226 | 119 | 107 | NA | NA | NA | NA | Immunoturbidimetry | 30 mg/dl | Within 2 h | NA | 7 |
| 3. | Cerrato et al., 200273 | Caucasian | Prospective cohort study | 202 | 119 | 83 | NA | NA | NA | NA | NA | NA | Within 3 months | NA | 6 |
| 4. | Sun et al., 200345 | Asian | Case–control study | 1326 | 809 | 517 | NA | NA | NA | 1817 | ELISA | Within 6 weeks | 7 | ||
| 5. | Botet et al., 199248 | Caucasian | Case–control study | 76 | 48 | 28 | NA | NA | NA | 100 | Electro immunoassay | NA | NA | NA | 5 |
| 6. | Markus et al., 199751 | Caucasian | Case–control study | 163 | 49 | 37 | 62 | 15 | 91 | ELISA | 40 mg/dl | NA | NA | 5 | |
| 7. | Chakraborty et al., 201353 | Asian | Case–control study | 100 | 35 | 21 | 19 | 22 | 3 | 120 | Immunoturbidimetric method | At 1, 7 days, 3 and 6 months | NA | 6 | |
| 8. | Lindgren et al., 199263 | Caucasian | Case–control study | 119 | NA | 41 | 33 | 35 | 10 | 159 | Radioimmunoassay | NA | NA | NA | 5 |
| 9. | Slowik et al., 200274 | Caucasian | Case–control study | 71 | 30 | 41 | NA | NA | NA | 30 | Immunonephelometric assay | > 30 mg/ml | Within 8 months | NA | 4 |
| 10. | Kooten et al., 199664 | Caucasian | Case–control study | 119 | 71 | 48 | 20 | 12 | 274 | Two-site immunoradiometric assay | NA | NA | NA | 6 | |
| 11. | Petersen et al., 200766 | Caucasian | Case–control study | 254 | 71 | 53 | 62 | 51 | 17 | 63 | Double-antibody ELISA | 30 mg/dl | NA | NA | 6 |
| 12. | Saito et al., 199767 | Asian | Case–control study | 118 | 13 | 35 | 21 | 17 | 95 | Sandwich ELISA | NA | NA | NA | 5 | |
| 13. | Yokohawa et al., 200875 | Asian | Cross-sectional study | 161 | 87 | 55 | 19 | NA | NA | NA | ELISA | NA | NA | NA | 4 |
Table 3.
Baseline characteristics of studies included in the systematic review and meta-analysis for the relationship between serum Lp(a) levels and risk of intracerebral haemorrhage (ICH).
| S. no. | Author name and year | Ethnicity | Study design | Sample size (ICH/control) | ICH age, years | Control age, years | Source of control | Matching criteria | LPA assay method | LPA cut -off value | LPA assay timepoint | Follow up duration | NOS quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Fu et al., 202038 | Asian | Case–control study | 196/392 | 57.9 ± 13.7 | 57.9 ± 13.7 | HB | Age and sex | Latex agglutination turbidimetric method | 23.2 mg/dl | NA | NA | 7 |
| 2. | Sun et al., 200345 | Asian | Case–control study | 499/1817 | 58.2 ± 9.7 | 59.6 ± 8.5 | PB | NA | ELISA | Within 6 weeks | NA | NA | 7 |
| 3. | Pena-Diaz et al., 200358 | Caucasian | Case–control study | 105/91 | 62.5 ± 10.6 | 40.2 ± 13.1 | HB | NA | Immunonephelometric method | > 22.45 mg/dl | NA | NA | 6 |
| 4. | Lindgren et al., 199263 | Caucasian | Case–control study | 12/159 | 68.9 ± 11.6 | 60 ± 11.5 | PB | Age | Radioimmunoassay | NA | NA | NA | 5 |
| 5. | Kooten et al., 199664 | Caucasian | Case–Control Study | 21/274 | 50.2 ± 7.4 | HB | NA | Two-site immunoradiometric assay | NA | NA | NA | 4 | |
| 6 | Saito et al., 199767 | Asian | Case–control study | 32/95 | 64 ± 11 | HB | NA | Sandwich ELISA | NA | NA | NA | 4 | |
| 7 | Seki et al.,199769 | Asian | Case–control study | 64/37 | 62 ± 9 | 61 ± 20 | HB | NA | ELISA | NA | NA | NA | 5 |
Characteristics of included studies for ischemic stroke
Out of 45 studies, 41 studies investigated the association of Lp (a) with the risk of IS as compared to control subjects with a total 7874 IS cases and 32,138 control subjects20,21,33–71. Thirty-five studies were case–control, one was nested case–control and five were population-based cohort studies. The publication years of the studies included in our meta-analysis ranged from 1985 to 2020. The studies were divided into two groups of populations based on ethnicity; 25 studies were conducted in Caucasian population and 16 studies were in Asian population. The sample size for IS cases ranged from 31 to 1953. Twenty-two studies used hospital-based (HB) source of control and nineteen studies used population-based (PB) source of control. The quality score was high in nine articles, medium in 22 articles and low in 10 articles. The detailed quality scores (NOS scores) of the included studies ranging from low to high are represented in supplementary tables (Table S2).
ELISA technique was found to be most common methods for LPA assay and reported in 18 studies, follow-up duration and LPA timepoints were reported in limited studies. No information was available for the stroke patients undergoing any LPA treatments in all the included studies in our meta-analysis. Only 11 studies21,35,38,40–42,44,45,52,54,55 reported calculated OR with 95% CI for the association of Lp (a) with the risk of IS as compared to control subjects; which have been used directly for estimating pooled ORs with 95% CI.
Characteristics of included studies for Ischemic Stroke subtypes
Thirteen studies33,45,48,51,53,63,64,66,67,72–75 reported the association of Lp (a) with the risk of IS subtypes based on TOAST classification. The publication years ranged from 1992 to 2019. Out of 13 studies, eight studies reported data for LAA subtypes33,45,48,51,53,64,66,74; nine studies for SVD subtypes33,45,48,51,53,63,64,66,74 and five studies for CE subtypes51,53,63,64,66 with control subjects. Seven studies were conducted in Caucasian population and six studies were in Asian population. The sample size for IS subtypes ranged from 09 to 1809. Ten studies were case–control, one was cross-sectional and two were population-based cohort studies. The quality score was medium in nine studies, high in two studies and low in two studies as represented in supplementary tables (Table S3).
Characteristics of included studies for Intracerebral hemorrhage
Only seven studies involving 871 Intracerebral hemorrhage (ICH) cases and 2865 control subjects were identified for the association of Lp (a) levels with the risk of ICH as compared to control subjects38,45,58,63,64,67,69. The publication years ranged from 1992 to 2020. Out of seven studies, three studies were conducted in Caucasian population and four studies were in Asian population. The sample size for ICH ranged from 06 to 499. Six studies were case–control and one was a nested case–control study. The quality score was medium in three studies, high in two studies and low in two studies as represented in supplementary tables (Table S4).
Association of Lp (a) levels with risk of ischemic stroke
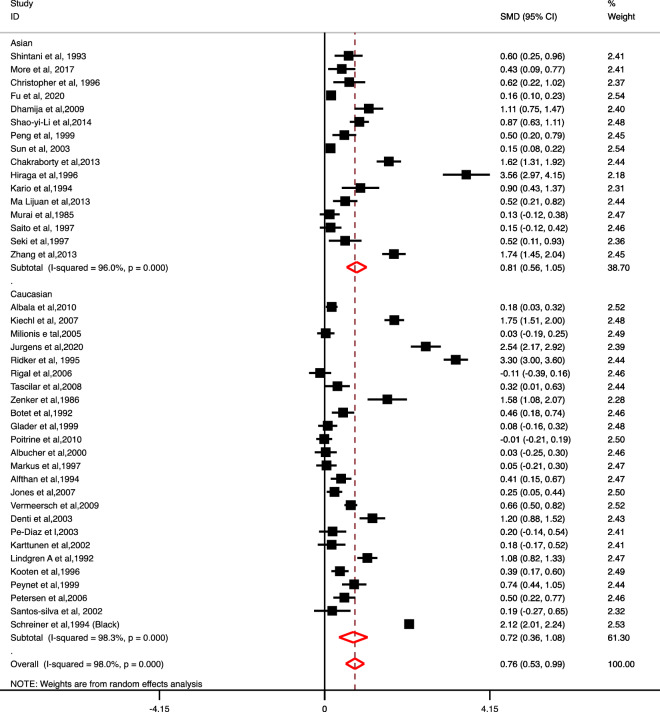
A significant association between increased levels of Lp (a) and risk of IS as compared to control subjects was observed (SMD 0.76; 95% CI 0.53–0.99). A subgroup analysis based on ethnicity also showed a significant association between increased levels of Lp (a) and the risk of IS in 16 Asian (SMD 0.81; 95% CI 0.56–1.05) as well as 25 Caucasian studies (SMD 0.72; 95% CI 0.36–1.08) respectively (Fig. 2).
Figure 2.
Forest plot for the association of Lp (a) level with the risk of Ischemic stroke vs. control based on ethnicity.
Based on study design, further subgroup analysis also showed a significant association between increased levels of Lp (a) and the risk of IS as compared to control groups in 35 case–control studies (SMD 0.64; 95% CI 0.48–0.80) and one nested case–control study (SMD 3.30; 95% CI 3.00–3.60) (Table 4). However, we did not observe any significant association between Lp (a) levels and risk of IS in the subgroup consisting of five prospective cohort studies (SMD 0.96; 95% CI − 0.01 to 1.93).
Table 4.
Summary of findings for the association of LP (a) with the risk of stroke types and subtypes.
| Variable | IS vs. control (no. of studies = 41) | LAA vs. control (no. of studies = 08) | SVD vs. control (no. of studies = 09) | CE vs. control (no. of studies = 05) | ICH vs. control (no. of studies = 07) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMD (95% CI) | I2 (%) | p-value | SMD (95% CI) | I2 (%) | p-value | SMD (95% CI) | I2 (%) | p-value | SMD (95% CI) | I2 (%) | p-value | SMD (95% CI) | I2 (%) | p-value | |
| Based on study design | |||||||||||||||
| Case–control studies | 0.64 (0.48 to 0.80) | 94.7 | < 0.0001 | 0.32 (0.00 to 0.64) | 89.1 | < 0.0001 | − 0.06 (− 0.46 to 0.34) | 93 | < 0.0001 | 0.05 (− 1.11 to − 1.21) | 97.2 | < 0.0001 | 0.39 (− 0.07 to 0.84) | 94.5 | < 0.0001 |
| Nested case control studies | 3.30 (3.00 to 3.60) | – | – | – | – | – | – | – | – | – | – | – | 2.24 (1.76 to 2.72) | – | – |
| Prospective cohort studies | 0.96 (− 0.01 to 1.93) | 99.2 | < 0.0001 | – | – | – | – | – | – | – | – | – | – | – | – |
| Overall | 0.76 (0.53 to 0.99) | 98 | < 0.0001 | 0.32 (0.00 to 0.64) | 89.1 | < 0.0001 | − 0.06 (− 0.46 to 0.34) | 93 | < 0.0001 | 0.05 (− 1.11 to − 1.21) | 97.2 | < 0.0001 | 0.65 (0.13 to 1.17) | 96 | < 0.0001 |
| Based on ethnicity | |||||||||||||||
| Asian | 0.81 (0.56 to 1.05) | 96 | < 0.0001 | 0.08 (− 0.22 to 0.39) | 59.1 | 0.087 | − 0.56 (− 1.98 to 0.85) | 98 | < 0.0001 | − 2.58 (− 3.15 to − 2.00) | – | – | 0.41 (− 0.24 to − 1.06) | 96.6 | < 0.0001 |
| Caucasian | 0.72 (0.36 to 1.08) | 98.3 | < 0.0001 | 0.45 (− 0.10 to 0.99) | 98.1 | < 0.0001 | 0.16 (− 0.08 to 0.40) | 57.1 | 0.04 | 0.08 (− 0.18 to 1.54) | 94.2 | < 0.0001 | 0.98 (− 0.32 to 2.28) | 94.2 | < 0.0001 |
| Overall | 0.76 (0.53 to 0.99) | 98 | < 0.0001 | 0.32 (0.00 to 0.64) | 89.1 | < 0.0001 | − 0.06 (− 0.46 to 0.34) | 93 | < 0.0001 | 0.05 (− 1.11 to − 1.21) | 97.2 | < 0.0001 | 0.65 (0.13 to 1.17) | 96 | < 0.0001 |
| Based on NOS quality score | |||||||||||||||
| High | 0.99 (0.53 to 1.44) | 98.9 | < 0.0001 | 0.16 (0.08 to 0.25) | – | – | 0.14 (0.04 to 0.24) | – | – | – | – | – | 0.61 (− 0.34 to 1.56) | 98.8 | < 0.0001 |
| Medium | 0.66 (0.32 to 1.00) | 97.6 | < 0.0001 | 0.99 (− 0.23 to 0.74) | 89.8 | < 0.0001 | − 0.12 (− 0.79 to 0.56) | 94.7 | < 0.0001 | 0.05 (− 1.11 to − 1.21) | 97.2 | < 0.0001 | 0.27 (0.03 to 0.52) | 0 | 0.48 |
| Low | 0.77 (0.33 to 1.21) | 93.8 | < 0.0001 | 0.93 (0.55 to 1.32) | – | – | 0.00 (− 0.33 to 0.33) | – | – | – | – | – | 1.06 (− 1.27 to 3.38) | 98.2 | < 0.0001 |
| Overall | 0.76 (0.53 to 0.99) | 98 | < 0.0001 | 0.32 (0.00 to 0.64) | 89.1 | < 0.0001 | − 0.06 (− 0.46 to 0.34) | 93 | < 0.0001 | 0.05 (− 1.11 to − 1.21) | 97.2 | < 0.0001 | 0.65 (0.13 to 1.17) | 96 | < 0.0001 |
SMD standardized mean difference, CI confidence interval, IS ischemic stroke, LAA large artery atherosclerosis, SVD small vessel disease, CE cardioembolism, ICH intracerebral haemorrhage.
Bold values of OR represent statistically significant results (p-value < 0.05).
On the basis of NOS quality grading, we also observed a significant association between increased levels of Lp (a) and the risk of IS as compared to control groups in high-quality studies (SMD 0.99; 95% CI 0.53–1.44); medium (SMD: 0.66; 95% CI 0.32–1.00) and low-quality studies (SMD 0.77; 95% CI 0.33–1.21) (Table 4).
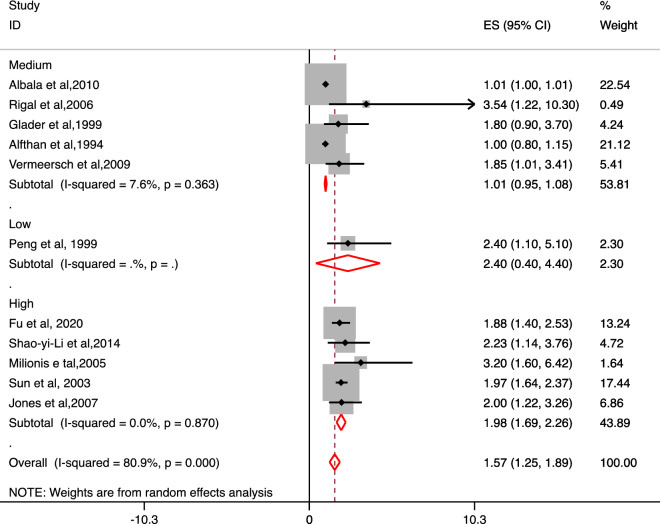
Based on extraction of reported calculated OR values directly from eleven studies, we observed an overall a significant association of increased levels of Lp (a) with the risk of IS as compared to control groups (OR 1.57; 95% CI 1.25–1.89). Based on ethnicity, a significant association of increased levels of Lp (a) with the risk of IS as compared to control groups was observed for Asian population (OR 1.97; 95% CI 1.67–2.26) but not for Caucasian population (OR 1.10; 95% CI 0.89–1.29). A significant association of increased levels of Lp (a) with the risk of IS as compared to control groups (OR 1.54; 95% CI 1.21–1.86) was observed for case–control studies but not for prospective cohort studies (OR 2.23; 95% CI 0.92–3.54). High-quality studies confirmed a significant association of increased levels of Lp (a) with the risk of IS as compared to control groups (OR 1.98; 95% CI 1.69–2.26) but not the medium (OR 1.01; 95% CI 0.95–1.08) and low-quality studies (OR 2.40; 95% CI 0.40–4.40) (Fig. 3).
Figure 3.
Forest plot for the association of Lp (a) level with the risk of Ischemic stroke vs. control for the reported Odds ratio in the included studies based on NOS quality grading.
Association of Lp (a) levels with the risk of large artery atherosclerosis
The association of Lp (a) levels with the risk of LAA stroke subtype vs. control was investigated in eight studies and our findings reveal an overall significant association of increased levels of Lp (a) with the risk of LAA as compared to control groups (SMD 0.32; 95% CI 0.00–0.64). Based on ethnicity, no significant association for the increased levels of Lp (a) with the risk of LAA in Asian population (SMD 0.08; 95% CI − 0.22 to 0.39) as well as Caucasian population (SMD 0.45; 95% CI − 0.10 to 0.64) was observed. All included eight studies were of case–control design which showed a significant association and based on NOS quality grading, no association was observed for medium quality studies (SMD 0.99; 95% CI − 0.23 to 0.74). Single studies were found based on high and low NOS quality and reported significant association for the increased levels of Lp (a) with the risk of LAA as compared to control groups (Table 4).
Association of Lp (a) levels with the risk of small vessel disease
No significant association for the increased Lp (a) levels with the risk of SVD subtype vs. control (SMD − 0.06; 95% CI − 0.46 to 0.34) was observed. Moreover, based on ethnicity, study design and NOS quality grading, similar non-significant association was observed except for high quality study which included only a single study for SVD vs. control subjects (SMD 0.14; 95% CI 0.04 to 0.24) (Table 4).
Association of Lp (a) levels with the risk of cardioembolic stroke
No significant association for the increased Lp (a) levels with the risk of CE stroke of IS subtype vs. control (SMD − 0.06; 95% CI − 0.46 to 0.34) was observed. Subgroup analysis based on ethnicity, study design and NOS quality grading also revealed a non-significant association for the increased Lp (a) levels with the risk of CE subtype vs. control subjects (Table 4).
Association of Lp (a) levels with the risk of intracerebral hemorrhage
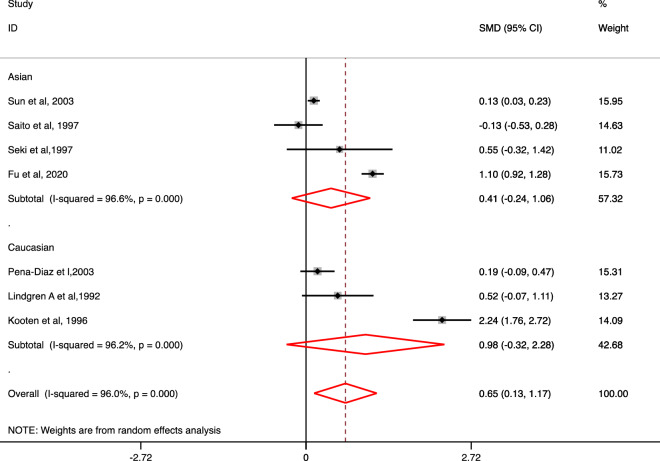
Overall, a significant association for the association of increased Lp (a) levels with the risk of ICH as compared to control subjects (SMD 0.65; 95% CI 0.13–1.17) was observed (Fig. 4). Non-significant association for the increased Lp (a) levels with the risk of ICH vs. control was observed based on ethnicity and study design. However, after conducting a subgroup analysis based on NOS quality grading, a significant association for the increased Lp (a) levels with the risk of ICH as compared to control subjects (SMD 0.27; 95% CI 0.03–0.52) was only observed in medium quality studies (Table 4).
Figure 4.
Forest plot for the association of Lp (a) level with the risk of Intracerebral hemorrhage (ICH) vs. control based on ethnicity.
Publication bias analysis
The shape of the funnel plots indicated the presence of publication bias while analysing the Lp (a) levels with the overall risk of IS. After conducting the Begg’s test, we observed that a significant publication bias was present in the included studies for Lp (a) levels with the overall risk of IS (p-value: 0.002) (Figure S-1a). The shape of other funnel plots for the included studies of IS subtypes and ICH in the meta-analysis did not indicate the presence of any publication bias (Figure S-1b–e).
Meta-regression analysis
To further explore the amount of heterogeneity present in our meta-analysis, we conducted meta-regression analysis based on NOS quality score, study design and ethnicity for determining the impact of heterogeneity. Significant heterogeneity was observed for overall IS vs. control based on NOS quality (p = 0.005) (see Supplementary figure S-2a). We observed that NOS quality score and ethnicity was not associated with the overall effect size in any of the outcomes measured in the meta-analysis (Supplementary Figure S-2b–e, S-3 and S-4a–e). For LAA, SVD, CE and ICH, all studies were of case–control design, hence meta-regression was not possible for these groups based on study design.
Sensitivity analysis
A sensitivity analysis was conducted by omitting a single study in each turn to determine if the overall effect size was influenced by the exclusion of a single study. Overall, no impact was observed for IS vs. control group. The sensitivity analysis suggested significant outliers for the included studies by two studies (Sun et al. 200345 and Petersen et al. 200766) investigating for the association of Lp (a) with LAA vs. control; two studies (Sun et al. 200345 and Chakraborty et al. 201353) for SVD vs. control; three studies (Kooten et al. 199664 Chakraborty et al. 201353 and Petersen et al. 200766) for CE vs. control; three studies (Kooten et al. 199664 Sun et al. 200345 and Fu et al. 202038) for ICH vs. Control which could have potentially affected the overall effect size estimates (Figure S-3a–e).
Discussion
The present systematic review and meta-analysis of 45 studies analysed the potential role of Lp (a) levels and its association with the risk of IS, IS subtypes based on TOAST classification and ICH compared to control subjects. To the best of our knowledge, this is the most robust and the largest meta-analysis conducted till date which comprised of both Asian and Caucasian ethnicities to ascertain the risk of IS, IS subtypes and HS with increased levels of Lp (a). Our meta-analysis revealed that increased levels of Lp (a) are significantly associated with the risk of IS in Asian as well as Caucasian population. Also a significant association for the increased level of Lp (a) with the risk of LAA and ICH as compared to control subjects was observed. Lp (a) levels were found to be greater in Asian population as compared to Caucasian population confirming greater risk of stroke as compared to control group. However, on the basis of subtypes, no significant association was observed in Asian as well as Caucasian population separately for increased levels of Lp (a) in LAA, SVD and CE subtypes when compared to control groups.
Two previous meta-analyses had also established the association of elevated Lp (a) levels and risk of stroke by pooling data from case–control, prospective cohort and nested case–control studies27,28. A total of thirty-one studies were included in the meta-analysis by Smolders et al. 200727 in which the association of overall stroke was found to be statistically significant with Lp (a) increment levels (SMD 0.39; 95% CI 0.23–0.54) which is in agreement with the findings of our meta-analysis. Another meta-analysis by Nave et al. 201528 observed a significant association between Lp (a) and IS with OR of 1.41 (95% CI 1.26–1.57) for case–control studies (n = 11) and the pooled estimated risk ratio was 1.29 (95% CI 1.06–1.58) for prospective studies (n = 9).
Despite the fact that this systematic review and meta-analysis was undertaken comprehensively with defined inclusion and exclusion criteria along with uniform measured-effect across all analyses, the study has some following limitations: (1)included studies had a wide range of incorporated variables like age, ethnicity, sample size, study-design; (2) mean and standard deviations of Lp (a) levels obtained from few studies were converted from either the actual reported median values or the inter-quartile range values, inferring that they did not actually represent the original mean and standard deviation values of Lp (a) levels. (c) Subgroup analysis based on cut-off values of Lp (a) levels was not performed owing to non-availability of cut-off values of Lp (a) in majority of the included articles as represented in Tables 1, 2 and 3. (d) A random-effects model was used to account for the significant heterogeneity arising out of the studies. Therefore, large scale population based observational studies with defined clinical characteristics of the stroke-affected subjects and healthy controls are needed to ascertain the association of Lp (a) with either IS, subtypes of IS or ICH in a statistically significant manner.
Conclusion
Increased Lp (a) levels could be considered as a predictive marker for identifying individuals who are at risk of developing IS, LAA and ICH.
Supplementary Information
Author contributions
P.K. and P.S. were involved in study selection and data extraction for the included study; S.M., and M.B. contributed in writing the manuscript to its final version. P.K. contributed to the concept, designing, statistical analysis and writing the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any grants from funding agencies in the public, commercial, or not-for profit sectors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-95141-0.
References
- 1.Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Bevan S, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke. 2012;43:3161–3167. doi: 10.1161/STROKEAHA.112.665760. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LB, et al. Primary prevention of ischemic stroke: A statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2001;32:280–299. doi: 10.1161/01.STR.32.1.280. [DOI] [PubMed] [Google Scholar]
- 4.Adams HP, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 5.Aronis KN, et al. Associations of lipoprotein(a) levels with incident atrial fibrillation and ischemic stroke: The ARIC (Atherosclerosis Risk in Communities) Study. J. Am. Heart Assoc. 2017;6:e007372. doi: 10.1161/JAHA.117.007372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lippi G, Favaloro EJ, Sanchis-Gomar F. Antisense lipoprotein[a] therapy: State-of-the-art and future perspectives. Eur. J. Intern. Med. 2020;76:8–13. doi: 10.1016/j.ejim.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 7.Manocha A, Srivastava LM. Lipoprotein (a): A unique independent risk factor for coronary artery disease. Indian J. Clin. Biochem. 2016;31:13–20. doi: 10.1007/s12291-015-0483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke R, et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 9.Boerwinkle E, et al. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J. Clin. Investig. 1992;90:52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt K, Noureen A, Kronenberg F, Utermann G. Structure, function, and genetics of lipoprotein (a) J. Lipid Res. 2016;57:1339–1359. doi: 10.1194/jlr.R067314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fogacci F, et al. Serum lipoprotein(a) level as long-term predictor of cardiovascular mortality in a large sample of subjects in primary cardiovascular prevention: Data from the Brisighella Heart Study. Eur. J. Intern. Med. 2017;37:49–55. doi: 10.1016/j.ejim.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Dieplinger H, Utermann G. The seventh myth of lipoprotein(a): Where and how is it assembled? Curr. Opin. Lipidol. 1999;10:275–283. doi: 10.1097/00041433-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: Potential sites for therapeutic targets. Metabolism. 2013;62:479–491. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gencer B, Kronenberg F, Stroes ES, Mach F. Lipoprotein(a): The revenant. Eur. Heart J. 2017;38:1553–1560. doi: 10.1093/eurheartj/ehx033. [DOI] [PubMed] [Google Scholar]
- 15.Ferretti G, et al. Lipoprotein(a): A missing culprit in the management of athero-thrombosis? J. Cell Physiol. 2018;233:2966–2981. doi: 10.1002/jcp.26050. [DOI] [PubMed] [Google Scholar]
- 16.Bostom AG, et al. A prospective investigation of elevated lipoprotein (a) detected by electrophoresis and cardiovascular disease in women. The Framingham Heart Study. Circulation. 1994;90:1688–1695. doi: 10.1161/01.CIR.90.4.1688. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TT, et al. Predictive value of electrophoretically detected lipoprotein(a) for coronary heart disease and cerebrovascular disease in a community-based cohort of 9936 men and women. Circulation. 1997;96:1390–1397. doi: 10.1161/01.CIR.96.5.1390. [DOI] [PubMed] [Google Scholar]
- 18.Ariyo AA, Thach C, Tracy R, Cardiovascular Health Study Investigators Lp(a) lipoprotein, vascular disease, and mortality in the elderly. N. Engl. J. Med. 2003;349:2108–2115. doi: 10.1056/NEJMoa001066. [DOI] [PubMed] [Google Scholar]
- 19.Ohira T, et al. Lipoprotein(a) and incident ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2006;37:1407–1412. doi: 10.1161/01.STR.0000222666.21482.b6. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, Stampfer MJ, Hennekens CH. Plasma concentration of lipoprotein(a) and the risk of future stroke. JAMA. 1995;273:1269–1273. doi: 10.1001/jama.1995.03520400039041. [DOI] [PubMed] [Google Scholar]
- 21.Glader CA, et al. Chlamydia pneumoniae antibodies and high lipoprotein(a) levels do not predict ischemic cerebral infarctions. Results from a nested case–control study in Northern Sweden. Stroke. 1999;30:2013–2018. doi: 10.1161/01.STR.30.10.2013. [DOI] [PubMed] [Google Scholar]
- 22.Price JF, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Lipoprotein (a) and development of intermittent claudication and major cardiovascular events in men and women: The Edinburgh Artery Study. Atherosclerosis. 2001;157:241–249. doi: 10.1016/S0021-9150(00)00719-X. [DOI] [PubMed] [Google Scholar]
- 23.Yuan B-B, et al. Variance of serum lipid levels in stroke subtypes. Clin. Lab. 2015;61:1509–1514. doi: 10.7754/Clin.Lab.2015.150118. [DOI] [PubMed] [Google Scholar]
- 24.Wityk RJ, et al. Lipoprotein (a) and the risk of ischemic stroke in young women. Atherosclerosis. 2000;150:389–396. doi: 10.1016/S0021-9150(99)00388-3. [DOI] [PubMed] [Google Scholar]
- 25.Arora P, et al. Lipoprotein(a) and risk of ischemic stroke in the REGARDS study. Arterioscler. Thromb. Vasc. Biol. 2019;39:810–818. doi: 10.1161/ATVBAHA.118.311857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laloux P, Galanti L, Jamart J. Lipids in ischemic stroke subtypes. Acta Neurol. Belg. 2004;104:13–19. [PubMed] [Google Scholar]
- 27.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: A meta-analysis of observational studies. Stroke. 2007;38:1959–1966. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 28.Nave AH, et al. Lipoprotein (a) as a risk factor for ischemic stroke: A meta-analysis. Atherosclerosis. 2015;242:496–503. doi: 10.1016/j.atherosclerosis.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Moher D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 31.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shintani S, Kikuchi S, Hamaguchi H, Shiigai T. High serum lipoprotein(a) levels are an independent risk factor for cerebral infarction. Stroke. 1993;24:965–969. doi: 10.1161/01.STR.24.7.965. [DOI] [PubMed] [Google Scholar]
- 34.More PP, Itkelwar BJ, Patil DR. Lipoprotein (a) as a risk factor of ischemic stroke: A case–control study. Int. J. Adv. Med. 2017;4:1138–1143. doi: 10.18203/2349-3933.ijam20173247. [DOI] [Google Scholar]
- 35.Boden-Albala B, et al. Increased stroke risk and lipoprotein(a) in a multiethnic community: The Northern Manhattan Stroke Study. Cerebrovasc. Dis. 2010;30:237–243. doi: 10.1159/000319065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiechl S, et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: Prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 2007;27:1788–1795. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 37.Christopher R, Kailasanatha KM, Nagaraja D, Tripathi M. Case–control study of serum lipoprotein(a) and apolipoproteins A-I and B in stroke in the young. Acta Neurol. Scand. 1996;94:127–130. doi: 10.1111/j.1600-0404.1996.tb07042.x. [DOI] [PubMed] [Google Scholar]
- 38.Fu H, et al. Association between lipoprotein(a) concentration and the risk of stroke in the Chinese Han population: A retrospective case–control study. Ann. Transl. Med. 2020;8:212. doi: 10.21037/atm.2020.01.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhamija RK, et al. Homocysteine and lipoprotein (a) correlation in ischemic stroke patients. J. Neurol. Sci. 2009;281:64–68. doi: 10.1016/j.jns.2009.02.341. [DOI] [PubMed] [Google Scholar]
- 40.Li S, et al. The relationship between serum lipoprotein (a) levels and ischemic stroke risk: A cohort study in the Chinese population. Inflammation. 2014;37:686–693. doi: 10.1007/s10753-013-9785-x. [DOI] [PubMed] [Google Scholar]
- 41.Milionis HJ, et al. Serum lipoprotein(a) levels and apolipoprotein(a) isoform size and risk for first-ever acute ischaemic nonembolic stroke in elderly individuals. Atherosclerosis. 2006;187:170–176. doi: 10.1016/j.atherosclerosis.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Peng DQ, Zhao SP, Wang JL. Lipoprotein (a) and apolipoprotein E epsilon 4 as independent risk factors for ischemic stroke. J. Cardiovasc. Risk. 1999;6:1–6. doi: 10.1177/204748739900600101. [DOI] [PubMed] [Google Scholar]
- 43.Jürgens G, et al. Lipoprotein(a) serum concentration and apolipoprotein(a) phenotype correlate with severity and presence of ischemic cerebrovascular disease. Stroke. 1995;26:1841–1848. doi: 10.1161/01.STR.26.10.1841. [DOI] [PubMed] [Google Scholar]
- 44.Rigal M, et al. Lipoprotein (a) and risk of ischemic stroke in young adults. J. Neurol. Sci. 2007;252:39–44. doi: 10.1016/j.jns.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Sun L, et al. Pentanucleotide TTTTA repeat polymorphism of apolipoprotein(a) gene and plasma lipoprotein(a) are associated with ischemic and hemorrhagic stroke in Chinese: A multicenter case–control study in China. Stroke. 2003;34:1617–1622. doi: 10.1161/01.STR.0000078370.12085.02. [DOI] [PubMed] [Google Scholar]
- 46.Tascilar N, et al. Relationship of apoE polymorphism with lipoprotein(a), apoA, apoB and lipid levels in atherosclerotic infarct. J. Neurol. Sci. 2009;277:17–21. doi: 10.1016/j.jns.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 47.Zenker G, et al. Lipoprotein(a) as a strong indicator for cerebrovascular disease. Stroke. 1986;17:942–945. doi: 10.1161/01.STR.17.5.942. [DOI] [PubMed] [Google Scholar]
- 48.Pedro-Botet J, et al. Lipoprotein and apolipoprotein profile in men with ischemic stroke. Role of lipoprotein(a), triglyceride-rich lipoproteins, and apolipoprotein E polymorphism. Stroke. 1992;23:1556–1562. doi: 10.1161/01.STR.23.11.1556. [DOI] [PubMed] [Google Scholar]
- 49.Canouï-Poitrine F, et al. Relative contribution of lipids and apolipoproteins to incident coronary heart disease and ischemic stroke: The PRIME Study. Cerebrovasc. Dis. 2010;30:252–259. doi: 10.1159/000319067. [DOI] [PubMed] [Google Scholar]
- 50.Albucher JF, et al. Serum lipids in young patients with ischaemic stroke: A case–control study. J. Neurol. Neurosurg. Psychiatry. 2000;69:29–33. doi: 10.1136/jnnp.69.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markus HS, Kapadia R, Sherwood RA. Relationship between lipoprotein (a) and both stroke and carotid atheroma. Ann. Clin. Biochem. 1997;34(Pt 4):360–365. doi: 10.1177/000456329703400404. [DOI] [PubMed] [Google Scholar]
- 52.Alfthan G, et al. Relation of serum homocysteine and lipoprotein(a) concentrations to atherosclerotic disease in a prospective Finnish population based study. Atherosclerosis. 1994;106:9–19. doi: 10.1016/0021-9150(94)90078-7. [DOI] [PubMed] [Google Scholar]
- 53.Chakraborty B, et al. Lipoprotein(a), ferritin, and albumin in acute phase reaction predicts severity and mortality of acute ischemic stroke in North Indian patients. J. Stroke Cerebrovasc. Dis. 2013;22:e159–e167. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Jones GT, et al. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin. Chem. 2007;53:679–685. doi: 10.1373/clinchem.2006.079947. [DOI] [PubMed] [Google Scholar]
- 55.Jones GT, Deng M, Hammond-Tooke GD, McCormick SPA, van Rij AM. Increased plasma lipoprotein(a) found in large-artery atherosclerotic, but not small-artery occlusive, stroke. Clin. Chem. 2009;55:1888–1890. doi: 10.1373/clinchem.2009.126771. [DOI] [PubMed] [Google Scholar]
- 56.Denti L, et al. The role of lipid profile in determining the risk of ischemic stroke in the elderly: A case–control study. Arch. Gerontol. Geriatr. 2003;37:51–62. doi: 10.1016/S0167-4943(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 57.Hiraga T, et al. Lipoprotein(a) is an independent risk factor for multiple cerebral infarctions. Atherosclerosis. 1996;122:29–32. doi: 10.1016/0021-9150(95)05743-9. [DOI] [PubMed] [Google Scholar]
- 58.de la Peña-Díaz A, et al. Functional approach to investigate Lp(a) in ischaemic heart and cerebral diseases. Eur. J. Clin. Investig. 2003;33:99–105. doi: 10.1046/j.1365-2362.2003.01114.x. [DOI] [PubMed] [Google Scholar]
- 59.Karttunen V, et al. Risk factors for cryptogenic ischaemic stroke. Eur. J. Neurol. 2002;9:625–632. doi: 10.1046/j.1468-1331.2002.00464.x. [DOI] [PubMed] [Google Scholar]
- 60.Kario K, et al. Close relation between lipoprotein (a) levels and atherothrombotic disease in Japanese subjects > 75 years of age. Am. J. Cardiol. 1994;73:1187–1190. doi: 10.1016/0002-9149(94)90179-1. [DOI] [PubMed] [Google Scholar]
- 61.Ma L, et al. Serum lipoprotein(a) complexes with beta2-glycoprotein I levels in patients with ischemic stroke. Clin. Chim. Acta. 2014;429:163–167. doi: 10.1016/j.cca.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 62.Murai A, Miyahara T, Fujimoto N, Matsuda M, Kameyama M. Lp(a) lipoprotein as a risk factor for coronary heart disease and cerebral infarction. Atherosclerosis. 1986;59:199–204. doi: 10.1016/0021-9150(86)90048-1. [DOI] [PubMed] [Google Scholar]
- 63.Lindgren A, Nilsson-Ehle P, Norrving B, Johansson BB. Plasma lipids and lipoproteins in subtypes of stroke. Acta Neurol. Scand. 1992;86:572–578. doi: 10.1111/j.1600-0404.1992.tb05489.x. [DOI] [PubMed] [Google Scholar]
- 64.van Kooten F, van Krimpen J, Dippel DW, Hoogerbrugge N, Koudstaal PJ. Lipoprotein(a) in patients with acute cerebral ischemia. Stroke. 1996;27:1231–1235. doi: 10.1161/01.STR.27.7.1231. [DOI] [PubMed] [Google Scholar]
- 65.Peynet J, et al. Apolipoprotein(a) size polymorphism in young adults with ischemic stroke. Atherosclerosis. 1999;142:233–239. doi: 10.1016/S0021-9150(98)00232-9. [DOI] [PubMed] [Google Scholar]
- 66.Petersen NH, et al. Lp(a) lipoprotein and plasminogen activity in patients with different etiology of ischemic stroke. CED. 2007;23:188–193. doi: 10.1159/000097640. [DOI] [PubMed] [Google Scholar]
- 67.Saito T, Ookubo R, Kuriyama M, Sano R, Ichinose A. Lipoprotein(a) concentration and molecular weight of apolipoprotein(a) in patients with cerebrovascular disease and diabetes mellitus. Thromb. Res. 1997;87:527–538. doi: 10.1016/S0049-3848(97)00182-5. [DOI] [PubMed] [Google Scholar]
- 68.Santos-Silva A, et al. Erythrocyte damage and leukocyte activation in ischemic stroke. Clin. Chim. Acta. 2002;320:29–35. doi: 10.1016/S0009-8981(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 69.Seki Y, Takahashi H, Shibata A, Aizawa Y. Plasma levels of thrombomodulin and lipoprotein (a) in patients with cerebral thrombosis. Blood Coagul. Fibrinolysis. 1997;8:391–396. doi: 10.1097/00001721-199710000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Schreiner PJ, et al. Lipoprotein(a) as a correlate of stroke and transient ischemic attack prevalence in a biracial cohort: The ARIC Study. Atherosclerosis Risk in Communities. Ann. Epidemiol. 1994;4:351–359. doi: 10.1016/1047-2797(94)90068-X. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Zhang X-A. Prognostic value of serum lipoprotein(a) levels in patients with acute ischemic stroke. NeuroReport. 2014;25:262–266. doi: 10.1097/WNR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 72.Tang Y, Geng D. Associations of plasma LP(a), Hcy and D-D levels with the subtype of ischemic cerebrovascular disease. Medicine (Baltimore) 2019;98:e14910. doi: 10.1097/MD.0000000000014910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cerrato P, et al. Higher lipoprotein (a) levels in atherothrombotic than lacunar ischemic cerebrovascular disease. Neurology. 2002;58:653–655. doi: 10.1212/WNL.58.4.653. [DOI] [PubMed] [Google Scholar]
- 74.Slowik A, et al. LDL phenotype B and other lipid abnormalities in patients with large vessel disease and small vessel disease. J. Neurol. Sci. 2003;214:11–16. doi: 10.1016/S0022-510X(03)00166-7. [DOI] [PubMed] [Google Scholar]
- 75.Yokokawa H, et al. Prevalence of metabolic syndrome and serum marker levels in patients with four subtypes of cerebral infarction in Japan. J. Clin. Neurosci. 2008;15:769–773. doi: 10.1016/j.jocn.2006.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.