Abstract
Background
The effect of Porphyromonas gingivalis (Pg) infection on oesophageal squamous cell carcinoma (ESCC) prognosis, chemotherapeutic efficacy, and oesophageal cancer cell apoptosis resistance and proliferation remain poorly understood.
Methods
Clinicopathological data from 312 ESCC oesophagectomy patients, along with the computed tomography imaging results and longitudinal cancerous tissue samples from a patient subset (n = 85) who received neoadjuvant chemotherapy (NACT), were analysed. Comparison of overall survival and response rate to NACT between Pg-infected and Pg-uninfected patients was made by multivariate Cox analysis and Response Evaluation Criteria in Solid Tumours v.1.1 criteria. The influence of Pg on cell proliferation and drug-induced apoptosis was examined in ESCC patients and validated in vitro and in vivo.
Results
The 5-year overall survival was lower in Pg-positive patients, and infection was associated with multiple clinicopathological factors and pathologic tumour, node, metastasis stage. Of the 85 patients who received NACT, Pg infection was associated with a lower response rate and 5-year overall survival. Infection with Pg resulted in apoptosis resistance in ESCC and promoted ESCC cell viability, which was confirmed in longitudinal cancerous tissue samples. Pg-induced apoptosis resistance was dependent on fimbriae and STAT3.
Conclusions
Pg infection is associated with a worse ESCC prognosis, reduced chemotherapy efficacy, and can potentiate the aggressive behaviour of ESCC cells.
Subject terms: Pathogenesis, Risk factors, Oesophageal cancer
Background
Genetic predisposition is estimated to account for the induction of 5–10% of all tumours, with the remaining cases due to somatic mutations.1–3 Microbes have been identified as a strong risk factor for some cancers, with an estimated two million new cases attributed to infection each year worldwide.4,5 Since the establishment of Helicobacter pylori as a class I tumorigen, increasing numbers of bacterial species have been identified as causative agents for the progression of certain cancers.6–8 For example, recent studies reported that intratumoural Gammaproteobacteria, and indeed Fusobacterium nucleatum, an oral-derived organism, could aggravate the progression of different cancers in mouse models and in patients with pancreatic ductal adenocarcinoma or colorectal cancer.9–11
Porphyromonas gingivalis, a Gram-negative bacterium, is an overt pathogen in periodontal diseases and other systemic conditions.12,13 Colonisation of P. gingivalis can disrupt the homeostasis of oral bacterial communities, reshape the host immune system, and alter the behaviour and phenotype of host cells by modifying a range of signalling proteins.14–18 Both colonisation of individual organisms and disruption of microbial community homeostasis have been shown to affect the progression of tumorigenesis.8,19–21 Porphyromonas gingivalis infection has also been shown to inhibit apoptosis and stimulate proliferation.22–25 These phenotypes support a mechanistic underpinning for the association of P. gingivalis with several cancers, including oral and pancreatic.8,26,27 Among all cancers, oesophageal cancer is the seventh most common cause of death, and there remains a lack of insight into risk factors and frequent resistance to chemotherapy.28 Thus, there is a pressing need to identify carcinogenic risk factors and improve chemotherapy efficacy in oesophageal squamous cell carcinoma (ESCC). We have recently demonstrated that P. gingivalis can colonise the human oesophageal epithelium, and infection is associated with the progression of ESCC.29 We now address this issue with a larger sample size that allows for better consideration of key confounding factors, and, critically, an assessment of the influence of P. gingivalis infection on ESCC prognosis following surgery and neoadjuvant chemotherapy (NACT).
Apoptosis resistance, accelerated proliferation, and reduced sensitivity to chemotherapy are all hallmarks of cancer cells.30 NACT, in parallel with surgery and radiotherapy, is key to the control of ESCC,31,32 and NACT, administered preoperatively, can significantly improve the overall survival (OS) rates.33 The most commonly used NACT (e.g. paclitaxel, cisplatin, and fluorouracil [5-FU]) are effective at inducing apoptosis and controlling the sustained proliferation of cancer cells.34,35 The majority of patients with advanced ESCC are initially responsive to most NACT treatments. However, tumour recurrence due to drug resistance contributes to a 5-year survival rate of <30%.36 Thus, it is of paramount importance to identify novel factors that lead to chemotherapy resistance in ESCC patients. In vitro evidence demonstrates that P. gingivalis infection induces apoptosis resistance and enhances cell proliferation in epithelial cells.18,22–24 However, it remains to be ascertained whether P. gingivalis infection affects the tumour response to chemotherapy or the growth and apoptosis of oesophageal cancer cells due, in part, to the difficulty in obtaining longitudinal human samples by biopsy.
Using a large clinicopathological data, longitudinal samples from ESCC patients before and after NACT, different oesophageal cancer cell lines, and a xenograft animal model, we demonstrate here, for the first time, that P. gingivalis infection reduces tumour response to chemotherapy, promotes the proliferation of oesophageal cancer cells, and worsens ESCC prognosis. We further found that the fimbrial structure of P. gingivalis and activation of cancer cell STAT3 are critical for the chemotherapy resistance of oesophageal cancer cells.
Methods
The study was approved by the Institutional Review Board of Henan University of Science and Technology (HAUST).
Patients and tissue samples
We retrospectively reviewed the medical records of 330 patients with ESCC treated at the First Affiliated Hospital of HAUST and Anyang People’s Hospital from January 2010 to December 2015. Of these, 312 of 330 had complete medical records and all underwent oesophagectomy surgery and so were selected for analysis. The details of the demographics and clinicopathological features of 312 patients were shown in Supplemental Table S1. Eighty-five of these 312 patients also received NACT, and computed tomography (CT)-scanning examination before and after completion of NACT, which was delivered prior to oesophagectomy. The tumour stages of P. gingivalis-positive and P. gingivalis-negative patients when they started to receive NACT were summarised in Fig. S1D, showing no significant difference between these two patient groups. All the patients received NACT following the Esophageal Cancer Management Guideline developed by the National Health and Family Planning Commission of the People’s Republic of China. The regimen was a two-cycle treatment, where each cycle includes intravenous infusion of paclitaxel (135 mg/m2) and cisplatin (75 mg/m2) for 3 weeks. Importantly, before the initial NACT and CT scanning, this subset of 85 subjects had received biopsy examination followed by a histological confirmation as ESCC. The biopsies from these patients, combined with the subsequent resection samples, were used to assess the influence of P. gingivalis infection on cell apoptosis. Cancerous tissue samples from all surgical resection specimens for immunohistochemistry analysis were obtained from the most representative area, usually from the centre or the middle of cancer lesions, with a minimum size of 0.5 cm3. These samples were divided into two groups: P. gingivalis antigen positive and P. gingivalis antigen negative (Fig. S1). Antigenic data were confirmed by 16S ribosomal RNA V4 and V5 sequencing (Fig. S2) that was carried out by WeGene (Shanghai, China). Demographics (e.g. sex and age) and clinicopathological features (e.g. differentiation status, lymphatic invasion, lymph node metastasis, and tumour, node, metastasis (TNM) stage) were obtained from the medical records. OS rates were determined over 60 months after oesophagectomy.
CT measurement of tumours
Clinical response to treatment was evaluated radiographically using computerised tomography imaging using a CT scanner (LightSpeed; GE Medical Systems, Milwaukee, WI) for the 85 patients who received NACT. CT scans were obtained within 2 weeks preceding chemotherapy and within 4 weeks of NACT completion. Chemotherapy efficacy was retrospectively evaluated according to the Response Evaluation Criteria in Solid Tumours v.1.1. Lesion size was determined by the longest dimension (LD) of the primary tumour. Measurements were performed by a single board-certified radiologist (Dr. Zhao Shijie) who was blinded to follow-up CT changes and patient outcomes.37 The percentage change in the size of the target lesions was calculated using pre- and post-NACT LD differentials. Patients whose lesions disappeared were defined as achieving a complete response to NACT; a >30% decrease in the LD of the target lesions was defined as a partial response, while a >20% increase in LD or the appearance of new lesions was defined as having progressive disease.38 All other outcomes were defined as stable disease. The chemotherapy responses were confirmed by further pathological examination with all patients receiving subsequent oesophagectomy.
Bacterial, cell lines, reagents and antibodies
Porphyromonas gingivalis ATCC 33277 and isogenic ΔfimA mutant39 were cultured anaerobically in trypticase soy broth supplemented with yeast extract (1 mg/ml), hemin (5 μg/ml), and menadione (1 μg/ml) at 37 °C. Oesophageal squamous cell lines (KYSE-30, KYSE-70, KYSE-140, and KYSE-150) were gifted by Dr. QiMin Zhan (State Key Laboratory of Molecular Oncology, Chinese Academy of Medical Sciences (Beijing, China)). All cell lines (KYSE-30, −70, −140, and −150) were routinely tested to confirm the absence of mycoplasma and maintained and propagated in Dulbecco’s modified Eagle’s medium supplemented with 10% foetal bovine serum and 0.1% gentamicin sulfate (Gemini Bio-Products). For bacterial infection, culture supernatants were replaced with antibiotic-free medium and cells were inoculated with P. gingivalis at a multiplicity of infection (MOI) of 10. Paclitaxel, 5-FU, and cisplatin were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell apoptosis, viability, and proliferation assays
Fifty thousand ESCC cells were seeded into 6-well plates for 12 h, then infected with P. gingivalis for 24 h, followed by treatment with paclitaxel (0.5 μM), 5-FU (10 μM), or cisplatin (100 μM) for 24 h. Then, Annexin V and PI were tested to evaluate cell apoptosis, as described in Supplemental methods. For cell viability and proliferation analysis, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays and 5-ethynyl-2-deoxyuridine (Edu) staining were performed, respectively, as described previously.40,41
Immunohistochemistry and P. gingivalis staining
Immunohistochemistry was performed to test the expression of P. gingivalis antigens and apoptotic cells, as described in Supplemental methods and our previous studies.29,41–43
Murine ESCC xenografts
Six-week-old athymic BALB/c nu/nu mice were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). The mice were housed in a pathogen-free environment at a temperature of 25 °C and relative air humidity between 45 and 50%. All mice are healthy with a vendor-provided certificate of physical examination. Thus, we did not carry out the excluding criteria in this study. Based on our previous similar studies,41,43 eight mice per group is able to reject the null hypothesis that the population means of the experimental and control groups are equal, with a power of 0.90. Seventy-two mice, therefore, were randomly divided into nine groups (n = 8 for each group) using the online randomisation tool (random.org). The eight mice in each group were housed in two cages with four mice per cage. For tumour cell inoculation, mice were anaesthetised with isoflurane in a plastic desiccator (Bell jar) that was placed in an externally vented fume hood. One to two millilitres of isoflurane was added in a paper towel in the bottom of the desiccator. Then, each mouse was placed in the desiccator until it was unconscious. Later, the mouse was removed from the desiccator and the tail was marked with different colours. After swabbing the back of the mouse with rubbing alcohol, KYSE-30 cells (1 × 107 mixed with 200 μl Matrigel) with or without pre-treatment of P. gingivalis 33277 or its isogenic ΔfimA mutant (MOI 10) were subcutaneously injected into the flank of the mouse. As the procedure is quite quick, we typically do not confirm the depth of anaesthesia and warm the animals during anaesthesia. The mice inoculated with tumour cells only were considered as the control group, which was used to compare with all other mice with different treatments. P. gingivalis-treated tumour cells were inoculated in mice and rechallenged with P. gingivalis at days 10 and 15. MTZ (30 mg/kg), an antibiotic to which P. gingivalis is susceptible, and a STAT3 inhibitor, WP1066 (20 mg/kg), were administered intraperitoneally from the day the KYSE-30 cells were inoculated. Paclitaxel (30 mg/kg) or the respective solvent control(s) were also administered in the same way from day 10, when the inoculated KYSE-30 cells had developed into a tumour of appreciable size (~60–80 mm3). All the injections were administered every 2 days until day 25. At the time of day 30, all the mice were euthanised by exposure to CO2 and tumour specimens were dissected and weighed. During the whole experiment, all mice were monitored daily for health status and no adverse health events, especially mortality and morbidity were observed. The experiments were blinded to the bacteria and pharmacological treatments when processing the data. Tumour volume was measured using callipers and calculated as length × width × height. All animal studies (including the mouse euthanasia procedure) were carried out in compliance with the regulations and the Henan University of Science and Technology institutional animal care guidelines and conducted according to the AAALAC and IACUC guidelines. All the data relevant to animal experiments are kept in Dr. Gao’s lab. All sections of this study adhere to the ARRIVE Guideline for reporting animal research and an ARRIVE guidelines checklist is included in Checklist S8.
Statistical analysis
The significance of prognostic factors on the OS of ESCC patients was analysed using univariate and multivariate Cox proportional hazards regression model analyses. The Kaplan–Meier method and the log-rank test were employed to examine the influence of P. gingivalis infection on OS and to generate time-to-progression and survival curves. A logistic regression test was used to analyse the association between the infection of P. gingivalis and clinicopathological factors. Moreover, propensity score matching was conducted, as described in Supplemental methods and previous studies.44,45 Student’s t test and analysis of variance (ANOVA) were employed to analyse the data of cell apoptosis in patients and in the xenograft model, respectively. Results are presented as mean ± standard error of the mean, where applicable. Tumour volume, weight, and tumour cell viability were analysed using ANOVA, followed by the Dunnett test for comparison of treatment versus control groups. Propensity score matching and data analysis were conducted in R Studio, version 3.3.3 (non-random package). All other analyses were performed with IBM SPSS Statistics 19 (IBM Corp., Armonk, NY, USA). Differences were considered significant at P < 0.05.
Results
Porphyromonas gingivalis infection exacerbates ESCC prognosis
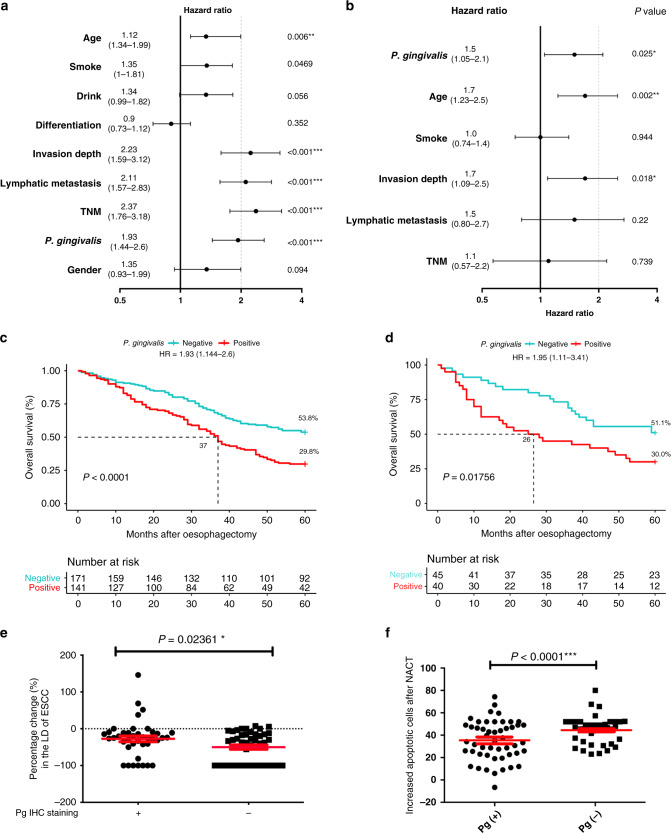
To investigate the clinical significance of P. gingivalis infection, univariate and multivariate Cox proportional hazards models were employed to analyse 5-year OS data from 312 ESCC patients. The patients include 205 male subjects and 107 female subjects, and are divided into two groups, P. gingivalis infection-positive, which has 141 subjects, and P. gingivalis infection negative, which has 171 subjects (Supplemental Table S1). Univariate analysis showed that age, smoking, tumour invasion depth, lymphatic metastasis, TNM stage, and infection of P. gingivalis are significantly associated with poorer OS of the patients (Fig. 1a, n = 312, all P values <0.05). Moreover, Kaplan–Meier analysis showed that patients with P. gingivalis infection had a statistically significantly shorter median OS time compared to patients without P. gingivalis infection (Fig. 1c, log-rank test P < 0.0001; hazard ratio (HR) = 1.93, 95% confidence interval (CI) = 1.44–2.6; Supplemental Table S1). Further analysis with a Cox proportional hazard multivariate model demonstrated that P. gingivalis infection, age, and invasion depth are three independent prognostic factors for survival when adjusted by other factors (Fig. 1b, n = 312, all P values <0.05). In addition, our immunohistochemistry results revealed that infection with P. gingivalis is positively associated with gender, age, smoking, alcohol usage, the severity of lymph node metastasis, depth of muscularis invasion, and TNM stage (Supplemental Table S1 and Fig. S1). To balance measured variables between P. gingivalis-positive and P. gingivalis-negative patients and to minimise the potential interference of confounding factors, propensity score matching was further utilised to ensure comparability of key baseline covariates, including age, gender, smoking, alcohol usage, invasion, metastasis, and TNM stage. For propensity score matching (Supplemental Table S1), 113 pair ESCC patients with all above covariates matched were selected for further analysis. We found that 5-year OS in the P. gingivalis-positive group (37 months) was still significantly lower than that in the P. gingivalis-negative group (51 months) (Fig. S3A; n = 226, P < 0.05). These data suggest infection with P. gingivalis is an independent predictor of shorter OS of patients with ESCC.
Fig. 1. Porphyromonas gingivalis infection exacerbates the prognosis, reduces the efficacy of chemotherapy, and results in apoptosis resistance in ESCC patients.
a, b Forest plot (log scale) showing the univariate and multivariate Cox regression analysis of the influences of different clinical risk factors on the survival of ESCC patients. In multivariate Cox model analysis, the covariates that are adjusted include P. gingivalis infection, age, smoking, invasion depth, lymphatic metastasis, and the TNM stage. Horizontal bars represent the 95% confidence intervals. P values for each variable are indicated, the hazard ratio is stated for each variable and the number in brackets is the exact 95% confidence intervals. c Kaplan–Meier survival analysis reveals that P. gingivalis infection is negatively associated with the overall survival time of ESCC patients. P value was calculated using the log-rank test, and hazard ratio (HR) was calculated using a univariate Cox proportional hazards model (n = 312, P < 0.0001). d Kaplan–Meier survival analysis reveals that P. gingivalis infection is negatively associated with the overall survival time of ESCC patients with NACT (n = 85, P = 0.01756). e The percentage change of the largest diameter (LD) of cancer lesions (based on CT-scanning examination) for 85 patients after neoadjuvant chemotherapy (relative to those of cancer lesions before the neoadjuvant). The baseline (dotted) line indicates that there is no substantial change of the cancer LD after neoadjuvant chemotherapy and “−1” is the maximum change, which means the cancer is invisible after chemotherapy. f The increased apoptotic cells from longitudinal cancerous tissues (before and after receiving neoadjuvant chemotherapy) were detected by immunofluorescence, indicating the average number of apoptotic cells in P. gingivalis-positive patients was significantly lower than that of the P. gingivalis-negative group. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
Porphyromonas gingivalis infection reduces chemotherapy efficacy and promotes apoptosis resistance in ESCC patients
We next evaluated the influence of P. gingivalis infection on tumour response to chemotherapy. The stage of cancer in P. gingivalis-positive and P. gingivalis-negative patients and the regimen and frequency of chemotherapy were described above. As shown in Table 1 and the waterfall plot in Fig. S3B, among the subset of 85 patients with 40 P. gingivalis positive and 45 P. gingivalis negative, totally 46 patients (54.1%) responded to the NACT, with partial response or complete response, while 39 patients (45.9%) were not responsive, with stable disease or progressive disease. In the P. gingivalis-negative group, 30 of 45 patients (66.7%) responded to NACT with partial response (n = 13) or complete response (n = 17), which is a significantly improved response rate, as compared to the 16 of 40 (40.0%) in the P. gingivalis-positive group (P = 0.01371). Moreover, P. gingivalis-positive staining was seen in 24 of 40 (60.0%) patients with progressive disease or stable disease, which is significantly more frequent than the 15 out of 45 (33.3%) in P. gingivalis-negative patients with complete response or partial response (Table 1). In addition, the mean of the reduced percentage of the LD of the primary tumour in P. gingivalis-positive ESCC patients was significantly less than that for the P. gingivalis-negative group (Fig. 1e; n = 85, P = 0.02361). Importantly, utilising Kaplan–Meier survival analysis, we also found that infection with P. gingivalis significantly shortened the median 5-year OS time of NACT patients (51.1 vs 30.0%; Fig. 1d, P = 0.01756; HR = 1.95, 95% CI = 1.11–3.41), with a median of 26 months OS for the P. gingivalis-positive patients, while there is up to 51.1% patients’ survival for the P. gingivalis-negative group, and thus the median OS for the P. gingivalis-negative group will be >60 months. These data demonstrate that infection with P. gingivalis significantly suppresses tumour responses to NACT and impairs chemotherapy efficacy in patients with ESCC.
Table 1.
The response rates of 85 ESCC patients to neoadjuvant chemotherapy.
| Factors | P. gingivalis | χ2 | P value | |
|---|---|---|---|---|
| Positive (n = 40) | Negative (n = 45) | |||
| PD/SD (n = 39) | 24 (60.0%) | 15 (33.3%) | ||
| CR/PR (n = 46) | 16 (40.0%) | 30 (66.7%) | 6.067 | 0.01371 |
Data were analysed by χ2 test.
PD progressive disease, SD stable disease, CR complete response, PR partial response.
We further examined if P. gingivalis infection affected chemotherapy-induced apoptosis and the progression of ESCC using longitudinally acquired samples from ESCC patients who received NACT and oesophagectomy. We found that apoptosis of cancer cells in P. gingivalis-positive patients was significantly lower than that of negative controls (Fig. 1f; n = 85, P < 0.0001; Fig. S3C). These results demonstrate that P. gingivalis infection leads to cancer cell resistance to chemotherapy-induced apoptosis in patients, strongly supporting the notion that P. gingivalis infection exacerbates the progression of ESCC. Combined with the significantly improved 5-year OS in P. gingivalis-negative patients who received NACT treatment (up to 51.1% survival 5 years after oesophagectomy), our results suggest that P. gingivalis could be an interventional target to improve the prognosis of ESCC patients receiving NACT.
Porphyromonas gingivalis infection leads to apoptosis resistance to NACT drugs and promotes the proliferation of ESCC cells
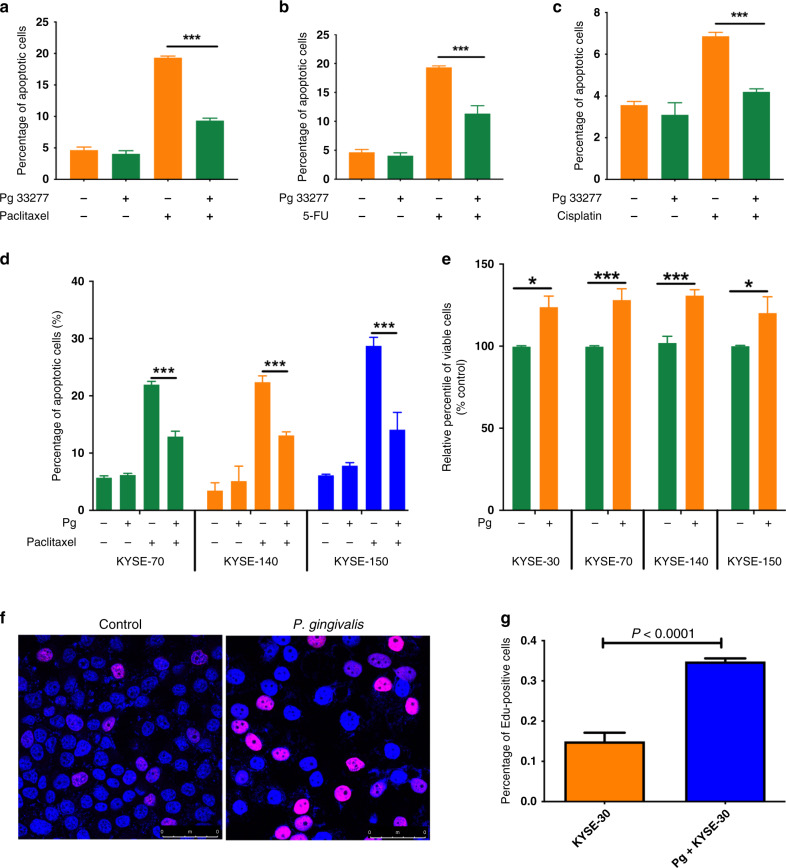
We took an in vitro approach using a widely studied oesophageal cancer cell line, KYSE-30, to test if P. gingivalis infection leads to resistance to apoptosis induced by the NACT drugs paclitaxel, 5-FU, and cisplatin. As shown in Fig. 2, P. gingivalis infection significantly decreased apoptosis in KYSE-30 cells treated with paclitaxel (Fig. 2a, P < 0.001), 5-FU (Fig. 2b, P < 0.001), or cisplatin (Fig. 2c, P < 0.001). Using paclitaxel as a model chemotherapeutic drug to induce apoptosis, we then examined the potential anti-apoptotic properties of P. gingivalis in other ESCC cell lines representing different stages of disease progression. P. gingivalis infection led to significant apoptosis resistance in KYSE-70, KYSE-140, and KYSE-150 cells (Fig. 2d, all P < 0.001). These results demonstrate that P. gingivalis infection efficiently suppresses NACT drug-induced apoptosis in a variety of ESCC cells, reflecting the human clinical data. To further explore the potential pro-tumorigenic effects of P. gingivalis, we investigated whether infection with P. gingivalis affected the viability of different ESCC cells. An MTT colorimetric assay demonstrated that P. gingivalis infection promotes the growth of all tested cancer cells compared with non-infected ESCC cells (Fig. 2e, all P < 0.05). Consistent with the MTT data, immunofluorescence staining with Edu demonstrated that P. gingivalis infection significantly enhanced the proliferation of KYSE-30 cells (Fig. 2f, g, P < 0.0001).
Fig. 2. Porphyromonas gingivalis infection leads to chemotherapy drug-induced apoptosis resistance and promotes proliferation of ESCC cells.
ESCC cells were stimulated with P. gingivalis (MOI 10) for 24 h followed by treatment with chemotherapy drugs for an additional 24 h. a–c Percentages of apoptotic KYSE-30 cells after treatment with paclitaxel (a), 5-FU (b), and cisplatin (c). d The same approach as above was employed to examine the effect of P. gingivalis infection on paclitaxel-induced apoptosis in different ESCC cells, KYSE-70, KYSE-140, and KYSE-150. e The percentage of viable ESCC cells, KYSE-30, KYSE-70, KYSE-140, and KYSE-150, in the presence or absence of P. gingivalis challenge, analysed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. f, g Representative images of Edu-stained ESCC cells (f) and the percentage of Edu-positive ESCC cells, as determined by flow cytometry (g). All data represent the mean ± SEM of three independent experiments in duplicate. * and *** represent P < 0.05 and P < 0.001, respectively.
The fimbrial structure of P. gingivalis is critical to promote the viability of oesophageal cancer cells
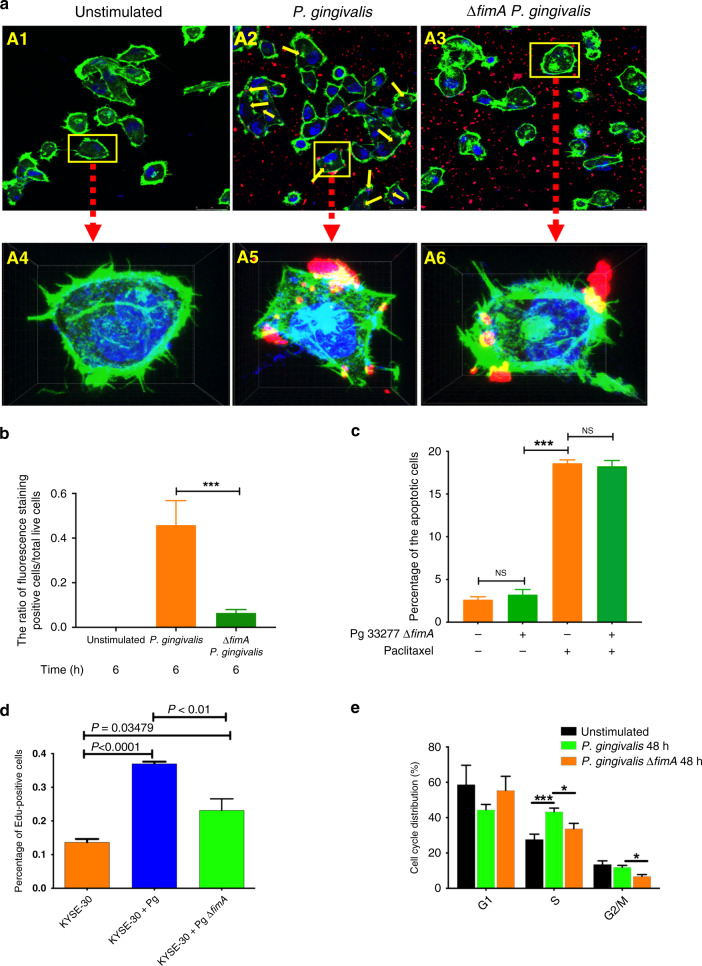
To investigate the mechanistic basis of the anti-apoptosis and pro-survival properties of P. gingivalis, we utilised a ΔfimA mutant of P. gingivalis, which lacks the FimA major fimbrial subunit. This mutant was previously found to have a diminished ability to invade into gingival epithelial cells and to stimulate cell cycle progression.25,46,47 We first examined the invasion of the wild-type and ΔfimA mutant into oesophageal cancer cells. As shown in Fig. 3a, b, the intracellular fluorescence staining of P. gingivalis was significantly lower for the ΔfimA mutant compared to the parental strain. These data were further confirmed by a short video, which dynamically showed the reduced ability of P. gingivalis to invade ESCC cells (Supplemental video clips). Moreover, the absence of FimA leads to the abrogation of the anti-apoptotic and pro-viability properties of P. gingivalis as established by the results of flow cytometry and an MTT assay (Fig. 3c and Supplemental Fig. S4A–E). Edu staining and cell cycle analysis showed that infection of P. gingivalis significantly increases the percentage of S-phase cells, while loss of FimA mitigates this effect (Fig. 3d, e and Fig. S4F). Hence, the influence of P. gingivalis on the apoptosis and proliferation of oesophageal cancer cells is dependent, at least partially, on intact FimA fimbrial structure and related to the existence of intracellular P. gingivalis. Taken together, our results provide direct evidence that P. gingivalis suppresses chemotherapeutic drug-induced apoptosis, and promotes the viability and proliferation of oesophageal cancer cells, in a manner that is dependent on the presence of fimbriae.
Fig. 3. Porphyromonas gingivalis ΔfimA mutant is attenuated for suppression of apoptosis and stimulation of proliferation of ESCC cells.
a Representative images of KYSE-30 cells after challenge with P. gingivalis for 6 h (A1–A3) and amplified images of the cells with yellow rectangle from A1 to A3 (A4–A6). The invasion of P. gingivalis was shown in Supplemental video clips. b The ratio of intracellular fluorescence staining positive cells to total live cells. c, d KYSE-30 cells were challenged with P. gingivalis 33277 or ΔfimA mutant for 24 h. c The percentage of the apoptotic cells shows that P. gingivalis ΔfimA mutant loses the ability to suppress paclitaxel-induced apoptosis. d The percentage of KYSE-30 cells with Edu staining by flow cytometry analysis. e Cell cycle distribution of KYSE-30 cells upon the challenge of P. gingivalis or ΔfimA mutant, showing that P. gingivalis ΔfimA mutant attenuates the ability to increase the cells in the S (synthesis) phase. Data were generated by flow cytometry and analysed with ModFit software. All data are the mean ± SEM of three independent experiments in duplicate. * and *** represent P < 0.05 and P < 0.001, respectively. “NS” represents not significant.
Porphyromonas gingivalis enhances the viability of oesophageal cancer cells through modifying multiple apoptotic and cell cycle signalling pathways
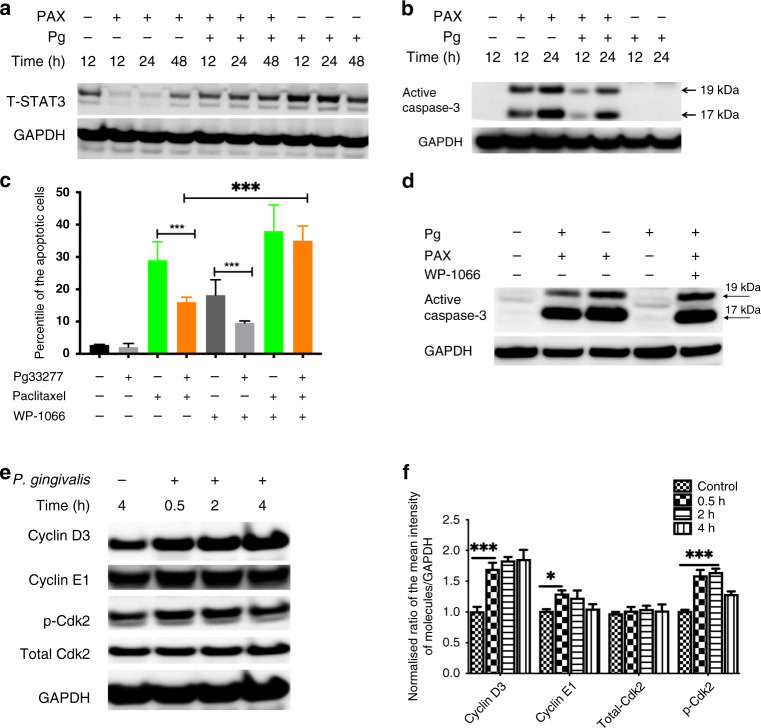
STAT3 has been reported as critical for P. gingivalis-mediated resistance to chemically induced apoptosis in oral epithelial cells, and in the controlled progression of ESCC.24,41 Hence, we examined the possible functions of STAT3 and of caspase-3, the convergent effector enzyme of intrinsic and extrinsic apoptosis pathways, in mediating resistance of P. gingivalis-challenged oesophageal cancer cells to chemotherapeutic drugs. We found that P. gingivalis infection enhanced the expression of STAT3, but reduced the expression of caspase-3 in paclitaxel-treated cells (Fig. 4a, b), as compared with control groups, suggesting that differential regulation of STAT3 and caspase-3 are key signalling circuits for the apoptosis resistance of oesophageal cancer cells challenged with P. gingivalis. Moreover, chemical inhibition of STAT3 with WP1066 leads to cell apoptosis and abrogates the effect of P. gingivalis on paclitaxel-induced apoptosis resistance (Fig. 4c). In addition, inhibition of STAT3 abrogates P. gingivalis-reduced caspase-3 in paclitaxel-treated cells, indicating there could be a crosstalk between STAT3 and caspase-3 (Fig. 4d). Although P. gingivalis infection has been reported to modify apoptosis through other mechanisms, such as manipulation of mitochondrial functions or sequestering of pro-apoptotic effectors,48,49 our data show that STAT3 and caspase-3 signalling are important components of the anti-apoptotic signalling induced by P. gingivalis, and could be exploited as future therapeutic strategies to manipulate the interaction of P. gingivalis with cancer cells.
Fig. 4. Porphyromonas gingivalis infection modifies the activity of different apoptosis and proliferation signalling pathways.
KYSE-30 cells were challenged with P. gingivalis in the presence or absence of paclitaxel for the times indicated, and then whole-cell lysates were collected and analysed by Western blots using antibodies against STAT3, caspase-3, or GAPDH (a, b); cells pre-treated with a STAT3 inhibitor (WP1066, 20 µM) were challenged with P. gingivalis in the presence or absence of paclitaxel, then the staining of annexin V/PI and expression of caspase-3 were used to determine apoptotic or necrotic cell death by flow cytometry and Western blots and, respectively (c, d); inhibition of STAT3 enhances the expression of caspase-3 (d) and cell apoptosis (c) upon challenge with P. gingivalis. e Whole-cell lysates of KYSE-30 cells treated with P. gingivalis at the indicated time points were probed for levels of cyclin D3, E1, and total- and phospho-CDK2; f normalised ratios of different regulatory proteins to GAPDH were determined by densitometry; all results are the average of at least three independent experiments. Error bars represent standard deviation. * and *** represent P < 0.05 and P < 0.001, respectively.
Due to the importance of rapid cell cycle and abnormal DNA content in the progression of cancers, we examined the effect of P. gingivalis on the molecular machinery of cell cycle regulatory proteins and the amount of DNA in different cell cycle stages. As shown in Fig. 4e, f, P. gingivalis challenge substantially enhanced the expression of cyclins D3 and E1, which are established controllers of cell cycle through the S (synthesis) phase. Moreover, infection with P. gingivalis enhanced the phosphorylation of CDK2 at Thr 160 in KYSE-30 cells (Fig. 4e, f), which could increase the activity and promote G1-to-S phase transition and S-phase progression through a combination with cyclin proteins. These results were confirmed by our phenotype data showing that infection of P. gingivalis robustly enhances the amount of S-phase DNA in ESCC cells (Fig. 3e), indicating that P. gingivalis can exploit components of the cell cycle machinery through which it accelerates the proliferation of oesophageal cancer cells.
Porphyromonas gingivalis infection promotes the growth of ESCC in athymic tumour-bearing mice
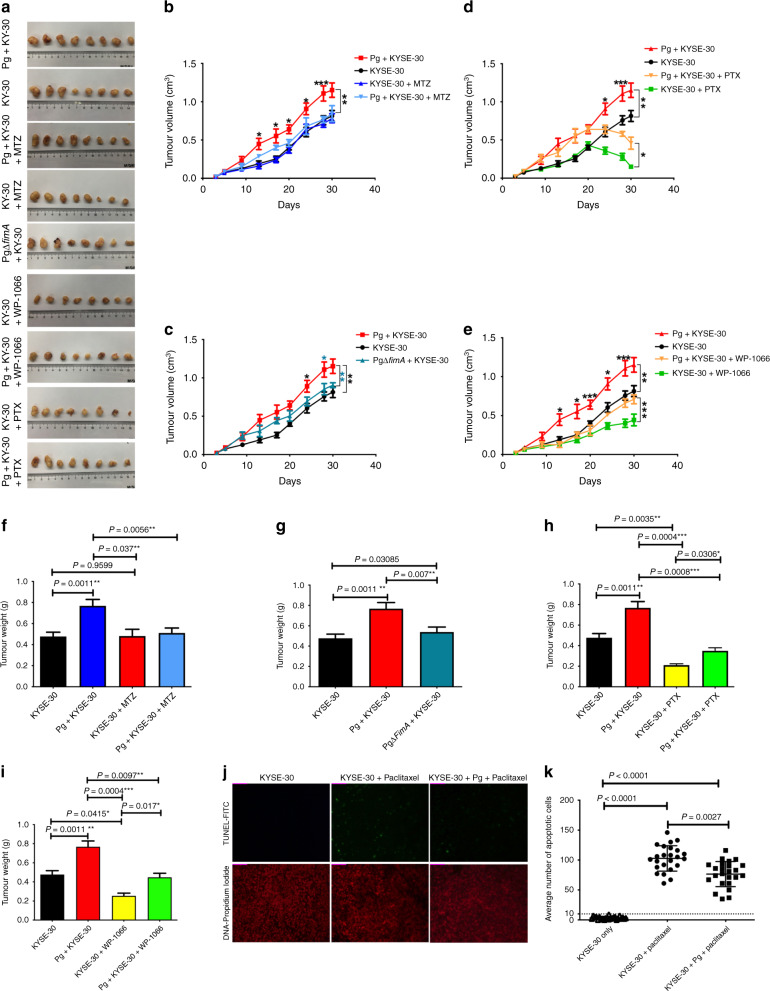
To evaluate the effect of P. gingivalis infection on the growth of ESCC and apoptosis resistance in vivo, KYSE-30 cells were inoculated subcutaneously in athymic nude mice to generate a tumour-bearing mouse model. As shown in Fig. 5a, all tumour specimens were dissected from the athymic nude mice at the end of the experiment. The average tumour volume significantly increased in the mice inoculated with the P. gingivalis-infected KYSE-30 after 14 days, as compared with mice inoculated with uninfected KYSE-30 (Fig. 5b). In contrast, the ΔfimA mutant was unable to significantly increase the tumour volume in mice (Fig. 5c). Interestingly, pre-treatment of mice with metronidazole (MTZ) led to a significant decrease in tumour volumes for the mice inoculated with wild-type P. gingivalis-infected KYSE-30, as compared with the control without MTZ treatment (Fig. 5b; P < 0.05), consolidating the pro-tumorigenic effects of P. gingivalis. Moreover, treatment with paclitaxel or WP1066 significantly decreased the tumour volume of the mice inoculated with P. gingivalis-infected KYSE-30, suggesting that paclitaxel-induced apoptosis and STAT3 are involved in P. gingivalis-promoted tumour growth (Fig. 5d, e, P < 0.05). We also observed similar patterns with the means of tumour weight in all groups as that of volume, and pre-treatment with MTZ abrogated the ability of P. gingivalis to promote the growth of ESCC cells (Fig. 5f–i, P < 0.01). We next examined the anti-apoptotic effects of P. gingivalis infection simulating the in vivo situation through intraperitoneal injection of paclitaxel after 10 days of cell inoculation, when the KYSE-30 had developed into a tumour of appreciable size (~60–80 mm3). Immunohistochemical staining of the sections from the xenograft tumour tissue showed that the number of apoptotic P. gingivalis-infected KYSE-30 cells in paclitaxel-treated mice was significantly decreased compared to mice inoculated with untreated KYSE-30 cells (Fig. 5j, k, P < 0.01), corroborating the anti-apoptotic effects of P. gingivalis in ESCC patients (Fig. 1f, g, P < 0.0001). Collectively, these results demonstrate that P. gingivalis is pro-tumorigenic in the context of ESCC and STAT3 is an important molecular target in this process. Therefore, eradication of P. gingivalis and/or inhibition of STAT3 may enhance chemotherapeutic efficiency in patients with ESCC.
Fig. 5. Porphyromonas gingivalis infection aggravates progression of ESCC through STAT3 signalling in a xenograft tumour-bearing model.
KYSE-30 cells were pre-treated with wild-type P. gingivalis (MOI 10) or P. gingivalis ΔfimA mutant for 24 h and implanted in athymic nude mice (n = 8). Tumour cells were inoculated with the same amount of P. gingivalis at days 10 and 15. Metronidazole (30 mg/kg), WP1066 (20 mg/Kg), paclitaxel (10 mg/kg), and solvent control were administered as described in “Methods”. a Representative tumour specimens dissected from the athymic nude mice xenografted with KYSE-30 cells with different treatments at the end of the study. The average tumour volume (b–e) and tumour weight (f–i) were calculated at the time indicated. As compared with the control group, infection of P. gingivalis significantly promoted tumour growth (a), while loss of FimA (c, g) or metronidazole treatment (b, f) abrogated the pro-tumorigenic ability of P. gingivalis in KYSE-30 inoculated athymic nude mice. Upon treatment with paclitaxel (d, h) or WP1066 (e, i), the volume and weight of xenograft tumours were significantly decreased in mice inoculated with P. gingivalis-infected KYSE-30. Infection with P. gingivalis also significantly reduced the paclitaxel-induced apoptosis in xenograft tumour tissue from mice (j, k). Representative images (j) and an average number (k) of apoptotic cells. The data in (j and k) represent the mean ± standard deviation of apoptotic cells from three non-consecutive tissue sections of each mouse. All results are the average of at least three independent experiments. Error bars represent standard deviation. *, **, and *** represent P < 0.05, P < 0.01, and P < 0.001, respectively.
Discussion
The carcinogenic potential of specific microorganisms and of a dysbiotic microbiome is becoming increasingly recognised. We have previously reported that infection with the oral pathogen, P. gingivalis, is associated with the progression of oesophageal cancer.29 Herein, we demonstrate, for the first time, that P. gingivalis infection is an independent predictor of shorter OS in ESCC patients. Moreover, based on the guidelines of the revised RECIST v.1.1, we also demonstrate that P. gingivalis infection reduces the response of ESCC to NACT in ESCC patients. Porphyromonas gingivalis infection renders ESCC cells resistant to chemotherapy-induced apoptosis and promotes cell growth in culture, in a xenograft animal model, and in longitudinal tissue samples collected from ESCC patients. Fascinatingly, treatment with MTZ, to which P. gingivalis is sensitive, abrogated P. gingivalis-induced apoptosis resistance and reduced overall cancer cell burden in athymic nude mice. In addition, we found P. gingivalis-induced apoptosis resistance is dependent on the intact structure of P. gingivalis fimbriae and activation of cancer cell STAT3. These represent the first data to demonstrate that P. gingivalis infection reduces tumour responses to NACT, promotes the progression of ESCC and exacerbates a poor prognosis. Thus, our findings indicate that eradication of P. gingivalis and/or inhibition of STAT3 has the potential to enhance chemosensitivity and improve the clinical management of this important malignant disease.
Resistance to chemotherapeutic treatments is a major impediment to successful outcomes in medical oncology. In this study, we found that P. gingivalis infection significantly reduces the efficacy of chemotherapy, and exacerbates the prognosis of patients with ESCC. Due to the limited patient number and the difficulty in acquiring longitudinal samples, we were unable to perform propensity score matching for the OS analysis of the patients receiving NACT and oesophagectomy. However, a comparison of the survival rates of P. gingivalis-positive patients with and without chemotherapy highlighted the critical role this organism plays in the efficacy of chemotherapy. Moreover, the finding that P. gingivalis-negative patients with chemotherapy exhibited a significantly improved 5-year OS consolidated this point. Combined with our results from the xenograft animal model showing MTZ diminishes the growth of oesophageal cancer cells, these data suggest eradication of P. gingivalis could enhance the sensitivity of chemotherapy and improve the prognosis of ESCC patients.
The influence of P. gingivalis on the aggressive behaviour of cancer cells in vivo in the tumour milieu of patients with ESCC remains to be determined. Moreover, the composition of the microbiome may influence the properties of P. gingivalis. For example, our recent study has demonstrated that a common oral coloniser Streptococcus gordonii can antagonise the ability of P. gingivalis to participate in the epithelial-to-mesenchymal transition by impeding upregulation of the transcription factor ZEB2.18 In this regard, our supplemental data showed that oesophageal colonisation by P. gingivalis is associated with an increase in Gram-negative anaerobic bacteria such as Treponema lecithinolyticum and Prevotella denticola, and, concurrently, a decrease in Gram-positive bacteria, particularly Propionibacteriaceae (Fig. S2). Hence, P. gingivalis may be able to sculpt the microbial community to ensure a pro-tumorigenic phenotype. Previous studies have reported that there are two distinct oesophageal microbiome subtypes. The healthy type I microbiome is dominated by Gram-positive bacteria, while the pathogenic type II microbiome is dominated by Gram-negative bacteria.50 Moreover, differential oesophageal bacterial communities have been associated with oesophageal inflammatory diseases, esophagitis, and with oesophageal squamous dysplasia, a precursor to ESSC.21,51,52 Thus, defining the influence of P. gingivalis colonisation on the composition of oesophageal microbiome and subsequent pathogenicity warrants more extensive investigation.
Oral administration of MTZ to mice bearing P. gingivalis-exposed oesophageal cancer cell resulted in a significant decrease in the trajectory of tumour growth as compared with mice treated with the vehicle alone. Treatment with MTZ was associated with a significant decrease in tumour volume and tumour weight, as well as an increase in paclitaxel-induced apoptosis efficiency. While these results indicate the potential for control of P. gingivalis infection as a target for ESCC therapy, it may be premature to advocate the use of MTZ. As MTZ targets a group of anaerobic bacteria including some anaerobes with established interactions with P. gingivalis, development of a P. gingivalis-specific antimicrobial agent may achieve a better clinical outcome if the pro-tumorigenic property of P. gingivalis is confirmed by further systemic studies. Additionally, more clinical evidence is required to show the influence of antibiotics on the progression and prognosis of ESCC.
In summary, this study demonstrates, for the first time, that P. gingivalis infection reduces tumour response, impairs chemotherapy efficacy, and is associated with a worse prognosis for ESCC patients. Moreover, infection with P. gingivalis promotes oesophageal cancer cell growth and renders cancer cells resistant to chemotherapy-induced apoptosis in vitro and in vivo. In addition, we found that the intact structure of the P. gingivalis FimA-component fimbriae and activation of cancer cell STAT3 are critical for the pro-tumorigenic property of this organism. Upon confirmation by more clinical studies, particularly relating to the influence of anti-P. gingivalis antibiotic treatment in conjunction with conventional chemotherapy, our findings may contribute to the development of novel strategies to enhance chemotherapy sensitivity, improve clinical management and the prognosis of ESCC patients, and yield innovative insights into the aetiology of oesophageal cancer.
Supplementary information
Acknowledgements
We acknowledge Drs. Mi and Zhang for assisting in the pathological evaluation and for other technical advice.
Author contributions
H.W., S.G., and F.Z conceived the study. Y.L., K.L., X.D., M.M., Z.G., L.Y., and J.R. performed most of the experiments and interpreted the data. X.Y., S.L., D.A.S., R.J.L., and H.W. directed the study and supervised the research. Y.L. and K.L. collected tumour specimens and analysed the clinical data of patients. X.D., J.R., and Z.G. performed all immunofluorescence staining. Y.L. performed animal experiments. S.G., F.Z., and H.W. confirmed the histopathological findings and interpreted the clinical data. H.W. prepared the manuscript. D.A.S. and R.J.L. reviewed and edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Tissue samples and clinicopathological data were obtained from The First Affiliated Hospital of Henan University of Science and Technology and Anyang People’s Hospital. The research was approved by the Research Ethics Committee of Henan University of Science and Technology and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent from each patient was achieved. All animal experiments were authorised through the Animal Care and Use Committee of Henan University of Science and Technology (HAUST). All animal experiments were conducted in accordance with the Guidelines for Animal Health and Use of Henan University of Science and Technology (HAUST).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Funding information
This research was supported by grants DE026727 (H.W.), DE017921, DE011111 (R.J.L.), and DE017680 (D.A.S.) from National Institute of Dental and Craniofacial Research, NIH, USA, and by the Natural Science Foundation of China (NSFC, GS 81472234), and Key Programs of Science and Technology of Henan Province (KPST-HN, GS 161100311200).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shegan Gao, Email: gsg112258@gmail.com.
Fuyou Zhou, Email: ayzhoufuyou@gmail.com.
Huizhi Wang, Email: wangh3@vcu.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01419-5.
References
- 1.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, et al. Environmental and heritable factors in the causation of cancer−analyses of cohorts of twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 2.Gagnaire A, Nadel B, Raoult D, Neefjes J, Gorvel JP. Collateral damage: insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Microbiol. 2017;15:109–128. doi: 10.1038/nrmicro.2016.171. [DOI] [PubMed] [Google Scholar]
- 3.Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene. 2004;23:6445–6470. doi: 10.1038/sj.onc.1207714. [DOI] [PubMed] [Google Scholar]
- 4.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 5.Oh JK, Weiderpass E. Infection and cancer: global distribution and burden of diseases. Ann. Glob. Health. 2014;80:384–392. doi: 10.1016/j.aogh.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffman M, Doorbar J, Wentzensen N, de Sanjose S, Fakhry C, Monk BJ, et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 8.Whitmore SE, Lamont RJ. Oral bacteria and cancer. PLoS Pathog. 2014;10:e1003933. doi: 10.1371/journal.ppat.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170:548–563.e516. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D, et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 2017;358:1443–1448. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010;8:481–490. doi: 10.1038/nrmicro2337. [DOI] [PubMed] [Google Scholar]
- 13.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat. Rev. Microbiol. 2018;16:745–759. doi: 10.1038/s41579-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat. Rev. Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Lamont RJ. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24:477–489. doi: 10.1016/j.tim.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohshima J, Wang Q, Fitzsimonds ZR, Miller DP, Sztukowska MN, Jung YJ, et al. Streptococcus gordonii programs epithelial cells to resist ZEB2 induction by Porphyromonas gingivalis. Proc. Natl Acad. Sci. USA. 2019;116:8544–8553. doi: 10.1073/pnas.1900101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2015;21:172–183. doi: 10.1016/j.molmed.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuboniwa M, Houser JR, Hendrickson EL, Wang Q, Alghamdi SA, Sakanaka A, et al. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat. Microbiol. 2017;2:1493–1499. doi: 10.1038/s41564-017-0021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 2009;7:887–894. doi: 10.1038/nrmicro2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Pilato V, Freschi G, Ringressi MN, Pallecchi L, Rossolini GM, Bechi P. The esophageal microbiota in health and disease. Ann. NY Acad. Sci. 2016;1381:21–33. doi: 10.1111/nyas.13127. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin. Cancer Res. 2012;18:2138–2144. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geng F, Liu J, Guo Y, Li C, Wang H, Wang H, et al. Persistente exposure to Porphyromonas gingivalis promotes proliferative and invasion capabilities, and tumorigenic properties of human immortalized oral epithelial cells. Front. Cell Infect. Microbiol. 2017;7:57. doi: 10.3389/fcimb.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Tang X, Li C, Pan C, Li Q, Geng F, et al. Porphyromonas gingivalis promotes the cell cycle and inflammatory cytokine production in periodontal ligament fibroblasts. Arch. Oral Biol. 2015;60:1153–1161. doi: 10.1016/j.archoralbio.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, et al. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9:1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuboniwa M, Hasegawa Y, Mao S, Shizukuishi S, Amano A, Lamont RJ, et al. P. gingivalis accelerates gingival epithelial cell progression through the cell cycle. Microbes Infect. 2008;10:122–128. doi: 10.1016/j.micinf.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn J, Segers S, Hayes RB. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis. 2012;33:1055–1058. doi: 10.1093/carcin/bgs112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogrendik M. Periodontal pathogens in the etiology of pancreatic cancer. Gastrointest. Tumors. 2017;3:125–127. doi: 10.1159/000452708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013;19:5598–5606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao S, Li S, Ma Z, Liang S, Shan T, Zhang M, et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agents cancer. 2016;11:3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Baba Y, Watanabe M, Yoshida N, Baba H. Neoadjuvant treatment for esophageal squamous cell carcinoma. World J. Gastrointest. Oncol. 2014;6:121–128. doi: 10.4251/wjgo.v6.i5.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J. Gastroenterol. 2010;16:1649–1654. doi: 10.3748/wjg.v16.i13.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S, Liu H, Diao C, Wang X, Gao M, Li Z, et al. Prognosis of surgery combined with different adjuvant therapies in esophageal cancer treatment: a network meta-analysis. Oncotarget. 2017;8:36339–36353. doi: 10.18632/oncotarget.16193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellmunt J, Pons F, Orsola A. Molecular determinants of response to cisplatin-based neoadjuvant chemotherapy. Curr. Opin. Urol. 2013;23:466–471. doi: 10.1097/MOU.0b013e328363de67. [DOI] [PubMed] [Google Scholar]
- 35.Rumiato E, Boldrin E, Amadori A, Saggioro D. Predictive role of host constitutive variants in neoadjuvant therapy of esophageal cancer. Pharmacogenomics. 2016;17:805–820. doi: 10.2217/pgs-2016-0009. [DOI] [PubMed] [Google Scholar]
- 36.Enzinger PC, Mayer RJ. Esophageal cancer. N. Engl. J. Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 37.Krajewski KM, Nishino M, Franchetti Y, Ramaiya NH, Van den Abbeele AD, Choueiri TK. Intraobserver and interobserver variability in computed tomography size and attenuation measurements in patients with renal cell carcinoma receiving antiangiogenic therapy: implications for alternative response criteria. Cancer. 2014;120:711–721. doi: 10.1002/cncr.28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell. Microbiol. 2002;4:305–314. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 40.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl Acad. Sci. USA. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao S, Li S, Duan X, Gu Z, Ma Z, Yuan X, et al. Inhibition of glycogen synthase kinase 3 beta (GSK3beta) suppresses the progression of esophageal squamous cell carcinoma by modifying STAT3 activity. Mol. Carcinog. 2017 doi: 10.1002/mc.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Yakoumatos L, Ren J, Duan X, Zhou H, Gu Z, et al. JAK3 restrains inflammatory responses and protects against periodontal disease through Wnt3a signaling. FASEB J. 2020;34:9120–9140. doi: 10.1096/fj.201902697RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qi YJ, Jiao YL, Chen P, Kong JY, Gu BL, Liu K, et al. Porphyromonas gingivalis promotes progression of esophageal squamous cell cancer via TGFbeta-dependent Smad/YAP/TAZ signaling. PLoS Biol. 2020;18:e3000825. doi: 10.1371/journal.pbio.3000825. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat. Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinberg A, Belton CM, Park Y, Lamont RJ. Role of fimbriae in Porphyromonas gingivalis invasion of gingival epithelial cells. Infect. Immun. 1997;65:313–316. doi: 10.1128/iai.65.1.313-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Ju J, Rigney T, Tribble GD. Fimbriae of Porphyromonas gingivalis are important for initial invasion of osteoblasts, but not for inhibition of their differentiation and mineralization. J. Periodontol. 2011;82:909–916. doi: 10.1902/jop.2010.100501. [DOI] [PubMed] [Google Scholar]
- 48.Yao L, Jermanus C, Barbetta B, Choi C, Verbeke P, Ojcius DM, et al. Porphyromonas gingivalis infection sequesters pro-apoptotic Bad through Akt in primary gingival epithelial cells. Mol. Oral Microbiol. 2010;25:89–101. doi: 10.1111/j.2041-1014.2010.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boisvert H, Duncan MJ. Translocation of Porphyromonas gingivalis gingipain adhesin peptide A44 to host mitochondria prevents apoptosis. Infect. Immun. 2010;78:3616–3624. doi: 10.1128/IAI.00187-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pei Z, Bini EJ, Yang L, Zhou M, Francois F, Blaser MJ. Bacterial biota in the human distal esophagus. Proc. Natl Acad. Sci. USA. 2004;101:4250–4255. doi: 10.1073/pnas.0306398101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pei Z, Yang L, Peek RM, Jr, Levine SM, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett’s esophagus. World J. Gastroenterol. 2005;11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.