Abstract
Various coronavirus disease 2019 (COVID-19) vaccines are being developed, which show practical preventive effects. Here, we report a 51-year-old healthy man with nephrotic syndrome secondary to minimal change disease (MCD) after Ad26.COV.2 (Janssen) vaccination. He had no comorbid disease and received Ad26.COV.2 on April 13, 2021. Seven days after vaccination, he developed edema and foamy urine. Edema rapidly aggravated with decreased urine volume. He was admitted to the hospital 28 days after vaccination, and his body weight increased by 21 kg after vaccination. His serum creatinine level was 1.54 mg/dL, and 24-h urinary protein excretion was 8.6 g/day. Kidney biopsy revealed no abnormality in the glomeruli and interstitium of the cortex and medulla under the light microscope. Electron microscopy revealed diffuse effacement of the podocyte foot processes, thus, he was diagnosed with MCD. High-dose steroid therapy was applied, and his kidney function improved three days after steroid therapy. Three weeks after steroid use, his serum creatinine decreased to 0.95 mg/dL, and spot urine protein-to-creatine decreased to 0.2 g/g. This case highlights the risk of new-onset nephrotic syndrome secondary to MCD after vectored COVID-19 vaccination. Although the pathogenesis is uncertain, clinicians need to be careful about adverse renal effects of COVID-19 vaccines.
Keywords: Minimal Change Disease, Nephrotic Syndrome, COVID-19, Vectored Vaccine
Graphical Abstract
INTRODUCTION
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which originated in Wuhan, China, in December 2019, is still a pandemic. Recently, various coronavirus disease 2019 (COVID-19) vaccines are being developed, which show retentive effects and are used worldwide, including Janssen, Pfizer-BioNTech, Moderna, Oxford-AstraZeneca, Novavax, and Sputnik V.
In this case report, we present a case of a patient who suffered full-blown nephrotic syndrome secondary to minimal change disease (MCD) after being administered the vectored COVID-19 vaccine, Ad26.COV.2 (Janssen).
CASE DESCRIPTION
A 51-year-old healthy Korean man without comorbid diseases was admitted to our hospital with complaints of generalized edema on May 12, 2021. On April 13, 2021, he received Ad26.COV.2. The next day after vaccination, he had fatigue and mild myalgia, then he did not feel any discomfort and did not take analgesics or herbal medication. After seven days of vaccination, he recognized peripheral edema on both lower extremities and edema had been aggravated. As edema developed, the total daily amount of urine decreased by a quarter of the usual amount. On May 3, 2021 (20 days after vaccination), his body weight increased from 77 kg to 91 kg. He visited the local nephrology clinic, and urinalysis revealed protein 4+ and urinary sediment revealed 3−5 red blood cells per high power field. Serum albumin level was 1.6 g/dL and creatinine level was 1.13 mg/dL. The nephrologist suspected nephrotic syndrome and transferred him to the tertiary referral hospital.
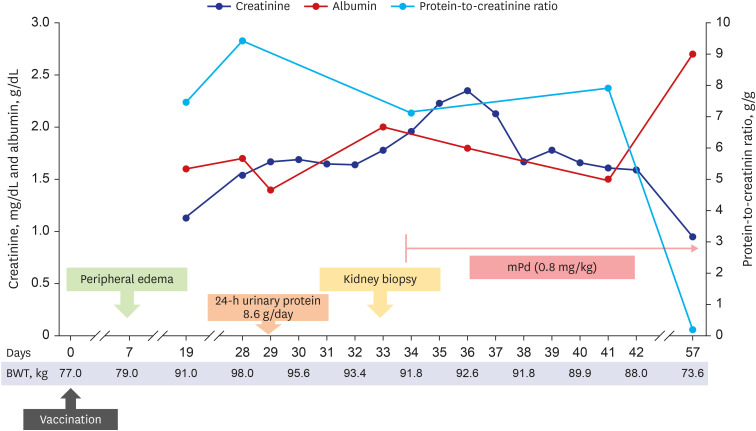
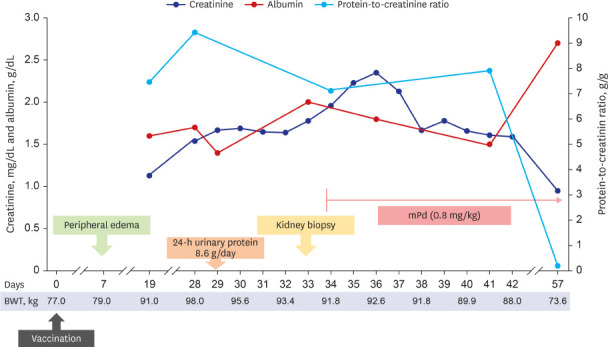
He was admitted 28 days after vaccination and the clinical course is shown in Fig. 1. His body weight increased to 98 kg with generalized edema, including lower and upper extremities, trunk, and face in a physical examination. Initial blood pressure was 146/98 mmHg; pulse rate, 60 beats/min; and axillary temperature, 36.5ºC. Abdominal computed tomography revealed a normal size and shape of both kidneys. In the laboratory findings to identify glomerulonephritis, anti-nuclear antibody, anti-neutrophil cytoplasmic antibody, anti-glomerular basement membrane (GBM) antibody, and anti-phospholipase A2 receptor antibody were all negative. Both complement C3 and C4 levels were within the normal range. Hepatitis B surface antigen, hepatitis C antibody, and human immunodeficiency virus antigen/antibody tests were negative. Serum immunoglobulin (Ig) G level decreased to 578.0 mg/dL (reference: 700−1,600 mg/dL). Real-time reverse transcriptase-polymerase chain reaction assay for SARS-CoV-2 (Seegen, Seoul, Korea) was negative. Total amount of collected 24-h urinary protein was 8,600.0 mg/day. Serum total cholesterol level was 416.0 mg/dL, albumin level was 1.6 g/dL, and creatinine level was 1.54 mg/dL.
Fig. 1. Clinical course after vaccination.
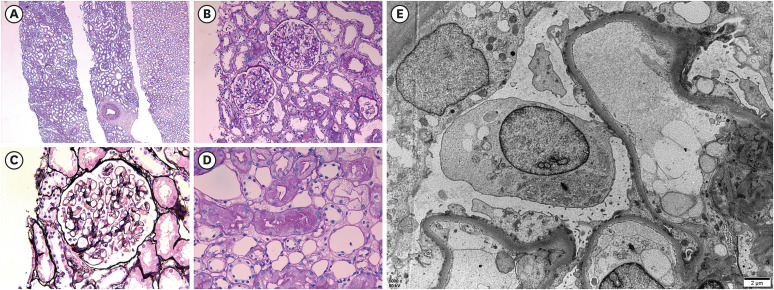
To control edema, intravenous furosemide was applied, then daily urine volume increased to over 2,000.0 mL/day, and edema gradually improved. However, his creatinine level increased to 1.96 mg/dL for seven days, and a kidney biopsy was performed 33 days after vaccination (Fig. 2). In the periodic acid-Schiff stain, there were 28 glomeruli having a normal appearance. In the interstitium, there was no inflammation or injury; and no chronic changes, such as tubular atrophy and interstitial fibrosis in the cortex and medulla were observed. Some proximal tubules contained protein droplets. Immunofluorescence revealed that IgG, IgA, IgM, C3, and fibrinogen were all negative. Electron microscopy revealed that GBM thickness was normal (average 375.2 nm) with diffuse effacement of the podocyte foot processes. There was no electron-dense deposit. According to the histopathological findings, he was diagnosed with MCD.
Fig. 2. Histopathological findings of kidney biopsy. (A) No tubular injury and tubular atrophy/interstitial fibrosis (periodic acid-Schiff; original magnification ×40). (B) Normal appearance glomeruli (periodic acid-Schiff; original magnification ×200). (C) Normal glomerular basement membrane without deposits (periodic acid-methenamine silver; original magnification ×400). (D) Protein reabsorption droplet accumulation in proximal tubules, which indicates heavy protein leakage across the glomerular filter and increased reabsorption of protein (periodic acid-Schiff; original magnification ×400). (E) Diffuse effacement of the podocyte foot processes and microvilli formation (electron microscopy; original magnification ×5,000).
High-dose steroid therapy was initiated immediately after checking the histopathology result by light microscopy. Considering the body weight, 64 mg parenteral methylprednisolone (the equivalence of 1 mg/kg prednisone) was administered. At three days after steroid use, his serum creatinine level started to improve. He was discharged at nine days after kidney biopsy (42 days after vaccination) with oral steroids. Two week after discharge, the follow-up examination showed decreased serum creatinine level to 0.95 mg/dL, increased serum albumin to 2.7 g/dL, and decreased spot urine protein-to-creatinine ratio to 0.2 g/g.
Ethics statement
Informed consent for data collection and publication of the study was obtained from the patient.
DISCUSSION
Here, we report the case of a healthy person with MCD after being administered Ad26.COV2.S, showing nephrotic syndrome with severe proteinuria and acute kidney injury. To the best of our knowledge, this is the first case of the adverse event of new-onset MCD after vectored vaccine injection for COVID-19.
Ad26.COV2.S is a replication-incompetent adenovirus type 26 vectored COVID-19 vaccine encoding a stabilized variant of the SARS-CoV-2 S protein.1 The recommended dosing regimen is a single intramuscular injection with a dose of 5 × 1010 viral particles.1 The vaccine efficacy of Ad26.COV.2 was 66.1% (95% confidence interval, 55.0−74.8%) to prevent moderate to severe/critical COVID-19. Some adverse events were reported, such as thromboembolic events, tinnitus, and vaccination site hypersensitivity, but there were no reported adverse kidney events.1
Foot process effacement of podocytes, visible by electron microscopy, are the hallmark of MCD. Although the exact pathogenesis is still unelucidated, podocyte injury by circulating factors released by T cells is considered the main mechanism.2 The increased proportion of circulating CD8+ T suppressor cells and type 2 T helper cells that secrete various cytokines, such as interleukin 4, 5, 9, 10, and 13, has been confirmed in studies.2 There are several cases of new-onset nephrotic syndrome by podocytopathies associated with COVID-19.3,4 The presence of angiotensin-converting enzyme 2, which helps SARS-CoV-2 invasion presents in podocytes, may be related to the podocytopathies.3 Also, the mechanism is unclear, but this case suggests that the adenovirus-based vectored vaccine for COVID-19 can affect T-cell function, and it can also occur rapidly within days after vaccination.
There are several cases of new-onset and relapsing MCD after COVID-19 vaccination and Table 1 summarizes these cases.5,6,7,8,9,10,11,12,13,14 All cases of MCD occurred within 10 days after vaccination with the first dose and the histopathological findings showed typical MCD findings. In addition, most cases of MCD were from mRNA vaccination, and our case is the first case of new-onset MCD after vectored vaccination. Fortunately, steroid treatment was effective in most cases except the patient who had underlying chronic kidney disease. Taken together, both vectored and mRNA COVID-19 vaccines can affect immune regulation, especially T cell-related, so there is a risk of new-onset and relapsing MCD.
Table 1. Summary of minimal change disease cases after coronavirus disease 2019 vaccination.
| Study | Age/sex | Types of vaccine/manufacturer | Symptom onset time after vaccination, days | Renal pathology | Treatment | Response | |
|---|---|---|---|---|---|---|---|
| New-onset | |||||||
| Lebedev et al.5 | 50/M | mRNA/Pfizer | 4 | Podocyte foot process effacement, acute tubular injury | High dose steroid | CR | |
| Maas et al.6 | 80's/M | mRNA/Pfizer | 7 | Podocyte foot process effacement | High dose steroid | CR | |
| D'Agati et al.7 | 77/M | mRNA/Pfizer | 7 | Podocyte foot process effacement, acute tubular injury, segmental mesangial sclerosis, glomerular basement membrane thickening | High dose steroid | NR | |
| Holzworth et al.8 | 63/F | mRNA/Moderna | < 7 | Podocyte foot process effacement, acute tubular injury, acute interstitial nephritis | High dose steroid | NA | |
| Weijers et al.9 | 61/F | mRNA/Pfizer | 8 | Podocyte foot process effacement | High dose steroid | CR | |
| Relapse | |||||||
| Kervella et al.10 | 34/F | mRNA/Pfizer | 10 | NA | High dose steroid | CR | |
| Morlidge et al.11 | 30/M | Vector/AstraZeneca | 2 | NA | Moderate dose steroid | CR | |
| Morlidge et al.11 | 40/F | Vector/AstraZeneca | 1 | NA | Moderate dose steroid + TAC | CR | |
| Manchianti et al.12 | 39/M | mRNA/Pfizer | 3 | Podocyte foot process effacement | High dose steroid | CR | |
| Komaba et al.13 | 60's/M | mRNA/Pfizer | 8 | NA | Moderate dose steroid + CsA | CR | |
| Schworzer et al.14 | 22/M | mRNA/Pfizer | 3 | NA | High dose steroid + TAC | CR | |
NR = no response, NA = not available, CR = complete remission, TAC = tacrolimus, CsA = cyclosporine.
Also, vaccination-related nephrotic syndrome secondary to MCD was reported in other vaccines, such as influenza, hepatitis B, pneumococcus, and measles.15 The pathogenic mechanism of podocyte foot process effacement after vaccination has been suggested that vaccination stimulates antigen-presenting and B cells. Stimulated antigen-presenting and B cells lead to antigen presentation and cytokine production, thereby activating T cells causing podocyte injury.16 Most MCD cases after vaccination occurred within two weeks after vaccination and many cases were accompanied by severe acute kidney injury. Therefore, clinicians need to be careful about the occurrence of proteinuria or edema after vaccination.
Here, we report the adverse effect of new-onset nephrotic syndrome secondary to MCD following Ad26.COV2.S. Clinicians need to be aware of the possibility of uncommon adverse effects after COVID-19 vaccination and prepare for proper management.
ACKNOWLEDGMENTS
The authors thank Dr. Jun Chul Kim in CHA Gumi Medical Center, CHA University, for his assistance for this study.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lim JH, Park SH.
- Data curation: Lim JH, Han MH, Kim YJ, Kim MS, Park SH.
- Investigation: Lim JH, Han MH, Kim YJ, Kim MS, Jung HY, Choi JY, Cho JH, Kim CD, Kim YL, Park SH.
- Methodology: Lim JH, Park SH.
- Visualization: Lim JH, Han MH, Kim YJ, Kim MS, Park SH.
- Writing - original draft: Lim JH.
- Writing - review & editing: Lim JH, Park SH.
References
- 1.U.S. Food & Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting. [Updated 2021]. [Accessed May 24, 2021]. https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcement.
- 2.Vivarelli M, Massella L, Ruggiero B, Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12(2):332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta RK, Bhargava R, Shaukat AA, Albert E, Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21(1):326. doi: 10.1186/s12882-020-01970-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SA, Carter HP. New-onset nephrotic syndrome in a child associated with COVID-19 infection. Front Pediatr. 2020;8:471. doi: 10.3389/fped.2020.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebedev L, Sapojnikov M, Wechsler A, Varadi-Levi R, Zamir D, Tobar A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(1):142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maas RJ, Gianotten S, van der Meijden WA. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(2):312. doi: 10.1053/j.ajkd.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agati VD, Kudose S, Bomback AS, Adamidis A, Tartini A. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021;100(2):461–463. doi: 10.1016/j.kint.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzworth A, Couchot P, Cruz-Knight W, Brucculeri M. Minimal change disease following the Moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;100(2):463–464. doi: 10.1016/j.kint.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weijers J, Alvarez C, Hermans MM. Post-vaccinal minimal change disease. Kidney Int. 2021;100(2):459–461. doi: 10.1016/j.kint.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kervella D, Jacquemont L, Chapelet-Debout A, Deltombe C, Ville S. Minimal change disease relapse following SARS-CoV-2 mRNA vaccine. Kidney Int. 2021;100(2):457–458. doi: 10.1016/j.kint.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morlidge C, El-Kateb S, Jeevaratnam P, Thompson B. Relapse of minimal change disease following the AstraZeneca COVID-19 vaccine. Kidney Int. 2021;100(2):459. doi: 10.1016/j.kint.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mancianti N, Guarnieri A, Tripodi S, Salvo DP, Garosi G. Minimal change disease following vaccination for SARS-CoV-2. J Nephrol. 2021 doi: 10.1007/s40620-021-01091-1. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komaba H, Wada T, Fukagawa M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021 doi: 10.1053/j.ajkd.2021.05.006. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwotzer N, Kissling S, Fakhouri F. Letter regarding “Minimal change disease relapse following SARS-CoV-2 mRNA vaccine”. Kidney Int. 2021;100(2):458–459. doi: 10.1016/j.kint.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel C, Shah HH. Vaccine-associated kidney diseases: a narrative review of the literature. Saudi J Kidney Dis Transpl. 2019;30(5):1002–1009. doi: 10.4103/1319-2442.270254. [DOI] [PubMed] [Google Scholar]
- 16.Colucci M, Corpetti G, Emma F, Vivarelli M. Immunology of idiopathic nephrotic syndrome. Pediatr Nephrol. 2018;33(4):573–584. doi: 10.1007/s00467-017-3677-5. [DOI] [PubMed] [Google Scholar]