Abstract
Ethnopharmacological relevance
Traditional Chinese medicine (TCM) has a long history in the prevention and treatment of pandemics. The TCM formula Lung Cleansing and Detoxifying Decoction (LCDD), also known as Qing Fei Pai Du Decoction, has been demonstrated effective against Coronavirus Disease 2019 (COVID-19).
Aim of the study
This work aimed to elucidate the active ingredients, targets and pathway mechanism of LCDD related to suppression of inflammatory, immunity regulation and relaxation of airway smooth muscle for the treatment of COVID-19.
Materials and methods
Mining chemical ingredients reported in LCDD, 144 compounds covering all herbs were selected and screened against inflammatory-, immunity- and respiratory-related GPCRs including GPR35, H1, CB2, B2, M3 and β2-adrenoceptor receptor using a label-free integrative pharmacology method. Further, all active compounds were detected using liquid chromatography-tandem mass spectrometry, and an herb-compound-target network based on potency and content of compounds was constructed to elucidate the multi-target and synergistic effect.
Results
Thirteen compounds were identified as GPR35 agonists, including licochalcone B, isoliquiritigenin, etc. Licochalcone B, isoliquiritigenin and alisol A exhibited bradykinin receptor B2 antagonism activities. Atractyline and shogaol showed as a cannabinoid receptor CB2 agonist and a histamine receptor H1 antagonist, respectively. Tectorigenin and aristofone acted as muscarinic receptor M3 antagonists, while synephrine, ephedrine and pseudoephedrine were β2-adrenoceptor agonists. Pathway deconvolution assays suggested activation of GPR35 triggered PI3K, MEK, JNK pathways and EGFR transactivation, and the activation of β2-adrenoceptor mediated MEK and Ca2+. The herb-compound-target network analysis found that some compounds such as licochalcone B acted on multiple targets, and multiple components interacted with the same target such as GPR35, reflecting the synergistic mechanism of Chinese medicine. At the same time, some low-abundance compounds displayed high target activity, meaning its important role in LCDD for anti-COVID-19.
Conclusions
This study elucidates the active ingredients, targets and pathways of LCDD. This is useful for elucidating multitarget synergistic action for its clinical therapeutic efficacy.
Keywords: COVID-19, Lung cleansing and detoxifying decoction, Dynamic mass redistribution, Target and pathway, GPCR
Graphical abstract
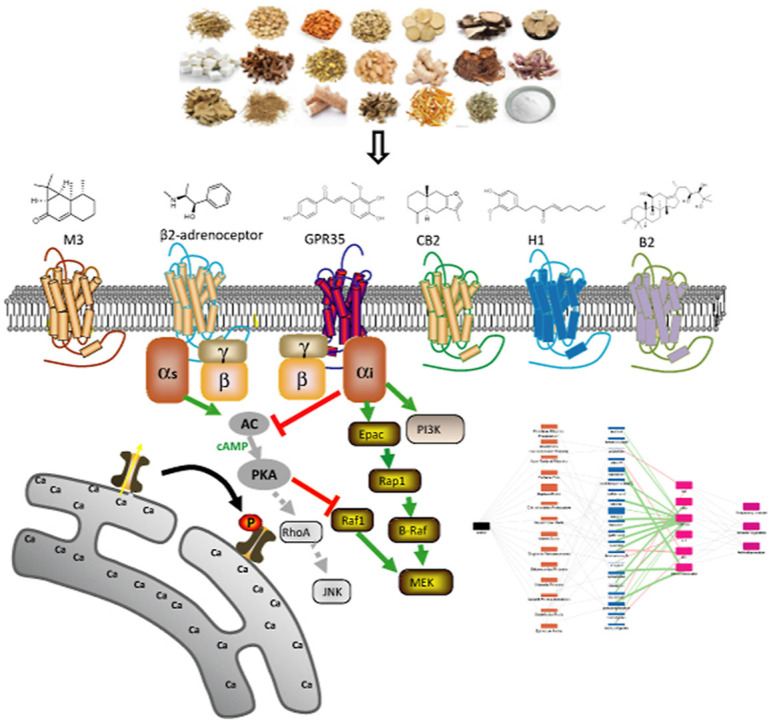
1. Introduction
Traditional Chinese medicine (TCM) has been used in the prevention and treatment of pandemics for thousands of years. A lot of clinical experiences and effective prescriptions have been accumulated. The severe acute respiratory syndrome named Coronavirus Disease 2019 (COVID-19) has caused widespread infection across China and around the world since the end of 2019. In the process of fighting COVID-19, a lot of Chinese herbal preparations represented by Lung Cleansing and Detoxifying Decoction (LCDD) have been widely used in clinical practice and have shown good efficacy (Gao et al., 2020; Liu et al., 2020b; Wang et al., 2020). LCDD, also named Qing Fei Pai Du Decoction, has been widely used for the treatment of exogenous fever since 200 CE in China. It is an optimized combination of 4 classic TCM recipes, including Maxing Shigan Decoction, Wuling Powder, Xiaochaihu Decoction and Shegan Mahuang Decoction. The whole formula included 20 herbs and 1 mineral (Table 1 ). Maxing Shigan Decoction has been used to treat epidemics caused by viruses, such as H1N1 influenza (Wang et al., 2011) and RSV pneumonia (Jiang et al., 2015). The extracts of Glycyrrhiza uralensis Fisch. (Glycyrrhizae Radix) have beneficial effects in antiviral activities, such as human immunodeficiency virus (HIV), herpes simplex virus (HSV), SARS-CoV and etc (Yang et al., 2015). The extracts of Ephedra sinica Stapf (Ephedrae Herba) has inhibition effect on H1N1 virus (Abourashed et al., 2003). Although the components of LCDD have been shown to play a role in a variety of viral diseases, the targets, active ingredients and pathway mechanism for treatment of COVID-19 remain unknown.
Table 1.
Information for lung cleansing and detoxifying decoction (LCDD).
| NO. | Names | Uses |
|---|---|---|
| 1 | Ephedra sinica Stapf (English: Ephedrae Herba, Chinese: Ma Huang) | Coughs, bronchial, asthma, fever, inhibition of H1N1 virus (Abourashed et al., 2003) |
| 2 | Glycyrrhiza uralensis Fisch. (English: Glycyrrhizae Radix, Chinese: Zhi Gan Cao) | Antiviral (HIV, SARS-CoV, etc.), antimicrobial, anti-inflammatory, and immunoregulatory activities (Yang et al., 2015) |
| 3 | Prunus armeniaca L. (English: Armeniacae Semen, Chinese: Xing Ren) | Pain and inflammatory diseases, reduce fever, relieve cough and quench thirst.(Jung et al., 2008) |
| 4 | Gypsum fibrosum (Chinese: Sheng Shi Gao) | / |
| 5 | Cinnamomum cassia (L.) J.Presl (English: Cinnamomi Ramulus, Chinese: Gui Zhi) | Antibacterial, anti-inflammatory, antiviral, antitumour, antipyretic and analgesic (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Liu et al., 2020d) |
| 6 | Alisma plantago-aquatica L. (English: Alismatis Rhizoma, Chinese: Ze Xie) | Anti-inflammatory and cardiovascular regulatory (Fong et al., 2007) |
| 7 | Polyporus umbellatus(Pers.) Fries (English: Polyporus, Chinese: Zhu Ling) | Treating edema, promoting diuretic processes, immune system enhancement and antimicrobial activities (Bandara et al., 2015) |
| 8 | Atractylodes lancea (Thunb.) DC. (English: Atractylodis macrocephalae Rhizoma, Chinese: Bai Zhu) | Immune and anti-inflammatory activity (Ruqiao et al., 2020) |
| 9 | Poria cocos (Schw.) Wolf (English: Poria, Chinese: Fu Ling) | Anti-inflammatory, antioxidant, and antiviral activities (Huang et al., 2020a, Huang et al., 2020b) |
| 10 | Bupleurum chinense DC. (English: Bupleuri Radix, Chinese: Chai Hu) | Evacuating fever and pyretolysis, soothing liver and relieving depression and lifting the spirit (Yang et al., 2017) |
| 11 | Scutellaria baicalensis Georgi (English: Scutellariae Radix, Chinese: Huang Qin) | Antipyretic, hemostatic, virus-related diseases (Ming et al., 2007) |
| 12 | Pinellia ternata (Thunb.) Makino (English: Pinellinae Rhizoma Praeparatum, Chinese: Jiang Ban Xia) | Treatment of insomnia (Lin et al., 2019) |
| 13 | Zingiber officinale Roscoe (English: Zingiberis Rhizoma recens, Chinese: Sheng Jiang) | Anti-inflammatory (Liao, 2015) |
| 14 | Aster tataricus L.f. (English: Asteris Radix, Chinese: Zi Wan) | Anti-expectorant, antitussive and anti-inflammatory (Rho et al., 2020) |
| 15 | Tussilago farfara L. (English: Farfarae Flos, Chinese: Kuan Dong Hua) | Treating cough, tuberculosis, asthma and obstructive lung diseases (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Liu et al., 2020d) |
| 16 | Belamcanda chinensis (L.) DC. (English: Belamcandae Rhizoma, Chinese: She Gan) | Healing respiratory diseases, anti-mutagenic, and anti-inflammatory (Wozniak and Matkowski, 2015) |
| 17 | Asarum sieboldii Miq. (English: Asari Radix et Rhizoma, Chinese: Xi Xin) | Dispel wind, dissipate cold, and relieve pain (Liu et al., 2020a, Liu et al., 2020b, Liu et al., 2020c, Liu et al., 2020d) |
| 18 | Dioscorea japonica Thunb. (English: Dioscoreae Rhizoma, Chinese: Shan Yao) | Treatment of inflammatory diseases, such as asthma, rheumatoid arthritis and bronchitis (Chiu et al., 2013) |
| 19 | Citrus × aurantium L. (English: Aurantii Fructus immaturus, Chinese: Zhi Shi) | Anti-ulcer, anti-inflammatory and antioxidant (Tan et al., 2017) |
| 20 | Citrus × aurantium L. (English: Citri reticulatae Pericarpium, Chinese: Chen Pi) | Coughs, anti-inflammatory and antioxidant (Yu et al., 2018) |
| 21 | Pogostemon cablin (Blanco) Benth. (English: Pogostemonis Herba, Chinese: Huo Xiang) | Treatment of common cold, nausea, diarrhea, headaches and fever (Li et al., 2011) |
Computational methods have been adopted to predict drug-target interactions of LCDD. Using the network pharmacology method, it was found that LCDD balanced immunity and eliminated inflammation by regulating several proteins co-expressed with angiotensin-converting enzyme 2 (ACE2) and signaling pathways strongly linked to the development of COVID-19 (Zhao et al., 2020a, Zhao et al., 2020b). Using the similar methods, other studies have shown that LCDD protected against COVID-19 via regulating anti-inflammatory, antiviral activity and cytokine storms level (Chen et al., 2020; Yang et al., 2020). Several key compounds (baicalin, glycyrrhizic acid, hesperidin, and hyperoside) and key targets (AKT1, TNF-α, IL-6, PTGS2, HMOX1, IL-10, and TP53) has been predicted (Zhao et al., 2020a, Zhao et al., 2020b). However, the targets of LCDD for treatment of COVID-19 are mostly based on assumptions. Experiment based identification of the target of the main ingredient is crucial to elucidate its actual therapeutic mechanism.
The COVID-19 patients showed mild or acute respiratory syndrome accompanied by release of pro-inflammatory cytokines (Conti et al., 2020). Besides, cytokine storm is associated with clinical deterioration of COVID-19 (Huang et al., 2020a, Huang et al., 2020b). Therefore, suppression of inflammatory, immunity regulation and relaxation of airway smooth muscle may have therapeutic effect on COVID-19. G protein-coupled receptors (GPCRs) are targets of approximately 33 % of the marketed human medicine, and play an important role in regulating physiological functions, such as immunity regulation, anti-inflammatory effects and respiratory regulation (Santos et al., 2017). Thus, LCDD may target a panel of GPCRs for treatment of COVID-19. The orphan GPCR GPR35 is expressed by human immune cells and its agonists play an important role in immunity regulation and anti-inflammatory activity (Quon et al., 2020). The antagonists of histamine 1 receptor (H1) are effective in suppressing inflammation (Qu et al., 2021). Cannabinoid receptor 2 (CB2) is highly expressed in multiple immune cells and its agonists show potency for the treatment of inflammatory and immune regulation (Tang et al., 2021). Bradykinin receptor 2 (B2) antagonists prevent inflammatory responses (Terzuoli et al., 2014). The muscarinic receptor 3 (M3) and β2-adrenoceptor are also involved in immunomodulatory and inflammation (Kistemaker et al., 2015; Kolmus et al., 2015). Moreover, these two receptors also play a critical role in respiratory disease, such as asthma and chronic obstructive pulmonary disease (COPD) (Patel et al., 2017; Pera and Penn, 2014). Therefore, targeting these two receptors would benefit COVID-19 patients with breathing difficulties.
In this study, we hypothesized that the active components of LCDD may function through targeting GPR35, H1, CB2, B2, M3 and β2-adrenoceptor due to their critical roles in immunomodulatory, anti-inflammation and respiratory disease. These effects are highly consistent with the symptoms of COVID-19. We used a label-free integrative pharmacology method to identify active ingredients, targets and pathways of LCDD. This method uses label-free resonant waveguide grating biosensors to convert drug-induced dynamic redistribution of cellular constituents into an integrated and kinetic response, called the dynamic mass redistribution (DMR) (Schroder et al., 2011). The DMR is recorded as a shift in resonant wavelength and represents a cellular phenotypic response which covers a wide range of targets/pathways. (Grundmann and Kostenis, 2015; Ye, 2006). This assay has been extensively applied in target identification of multiple TCMs (Wang et al., 2019; Zhang et al., 2014). Herb-compound-target network was also built based on experimental data to elucidate therapeutic mechanism of LCDD. This study is useful for elucidating multitarget synergistic action of LCDD, and provides a theoretical foundation for global application in anti-COVID-19.
2. Materials and methods
2.1. Materials
Natural compounds listed in Table S1 were purchased from Shanghai Yuanye Bio-technology (Shanghai, China), Shanghai Sidend Technical Service (Shanghai, China), National Institutes for Food and Drug Control (Beijing, China), Chengdu Profa Technology Development (Chengdu, China) and Chengdu Push Bio-technology (Chengdu, China). Zaprinast, epinephrine, histamine, bradykinin, CP55940 and acetylcholine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Carbachol was obtained from Tocris Bioscience Co. (St. Louis, MO, USA). Hank's balanced salt solution (HBSS), HEPES, fetal bovine serum (FBS) were from Thermo Fisher Scientific (Waltham, MA). Ham's F12K medium, Dulbecco's modified Eagle's medium (DMEM) and McCoy's 5A medium were bought from Sigma-Aldrich (St Louis, MO, USA). HPLC grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). Water was purified from a MilliQ water system (Billerica, MA, USA). Formic acid was obtained from J&K Scientific (Beijing, China). All natural compounds were prepared in 100 mM and were dissolved in 100 % dimethyl sulfoxide (DMSO) and diluted with the assay buffer (1 × Hank's balanced salt solution (HBSS) buffer, 10 mM HEPES, pH 7.4) to the desired concentrations before DMR assays.
2.2. Cell culture
Human colorectal adenocarcinoma HT-29 cells, human epidermoid carcinoma A431 cells and human lung carcinoma A549 cells were obtained from the Type Culture Collection of the Chinese Academy of Science (Shanghai, China). HEK293-M3 cells and CHO-CB2 cells were stably transfected as previously reported (Zhou et al., 2020a, Zhou et al., 2020b). The cell culture medium was used as follows: 1) McCoy's 5A supplemented with 10 % FBS for HT-29 cells; 2) DMEM supplemented with 10 % FBS for A431, A549 and HEK293-M3 cells; 3) Ham's F12K medium supplemented with 10 % FBS for CHO-CB2 cells.
2.3. DMR assays
All DMR assays were performed using an Epic® BT system (Corning, NY, USA). HT-29 cells (32,000 cells per well), HEK293-M3 cells (25,000 cells per well), A431 cells (25,000 cells per well), A549 cells (20,000 cells per well) and CHO-CB2 cells (15,000 cells per well) were seeded in Epic 384-well biosensor microplates for ~ 20 h, respectively, and cells formed a monolayer on the bottom of the well in the culture medium with a confluence of ~95 %. Then they were directly washed with the assay buffer and maintained in 30 μL assay buffer for 1 h for DMR assay except A431 cells. The A431 cells were further incubated in the serum-free medium for 24 h. After starvation, the cells were conducted similar treatment for DMR assay.
To screen natural compounds against GPR35, H1, CB2, B2, M3 and β2-adrenoceptor receptor in these five cell lines, a 2-min baseline was first established, followed by compound addition and DMR recording for 1 h. A 2-min baseline was then re-established, followed by adding zaprinast (2 μM, in HT-29 cells), carbachol (200 nM, in HEK293-M3 cells), epinephrine (5 nM, in A431 cells), histamine (10 μM, in A431 cells), bradykinin (500 nM, in A549 cells) and CP55940 (1 μM, in CHO-CB2) and recording DMR signals for 1 h.
For pathway deconvolution assays, HT-29 cells and A431 cells were initially treated with pathway modulators or control for 1 h. Afterwards, the baseline was re-established, followed by adding licochalcone B and ephedrine at a fixed concentration and monitoring the cellular responses induced by the compounds for 1 h.
2.4. Preparation of LCDD sample, standard solution and calibration tests
The powder sample of LCDD (20.15 mg)-gypsum fibrosum (15 g) was dissolved in 1 mL water, followed by filtration through a 0.22-μm membrane. Stock solutions of the reference compounds were prepared in proper solvent separately at the concentration of about 0.2 mg/mL. After analysis of stock solution (injection volume 0.2 μL), the EIC peak area was used to calculate the approximate concentration of each compound in LCDD. Then the single reference substance stock solution was diluted to the approximate concentration and reanalyzed along with LCDD (injection volume 2 μL), using EIC peak area to calculate the exact concentration of each compound in LCDD. The sample dissolution and dilution process and the quantitative peak area were shown in Table S2.
Experiments were performed on an Agilent UPLC 1290 system coupled with a high resolution quadrupole time-of-flight MS/MS 6545 system (Agilent Technologies, Santa, Clara, CA, USA), which equipped with a JetStream technology ESI interface. For chromatographic analysis, an Acquity UPLC® BEH Shield RP18 column (100 mm × 2.1 mm, 1.7 μm, Waters, Milford, MA, USA) was used. Mobile phase A was water containing 0.1 % (v/v) formic acid. Mobile phase B was acetonitrile. The linear gradient elution program was optimized as follows: 0–2 min, 100 % mobile phase A, 2–7 min, 100%–70 % mobile phase A, 7–10.5 min, 70%–40 % A, 10.5–11.5 min, 40%–10 % A, 11.5–15 min, 10 % A. The flow rate was 0.4 mL/min. The column temperature was maintained at 30 °C. The injection volume was 2 μL. Mass detection was operated both in negative and positive mode. The source parameters were as follows: gas temperature 320 °C, drying gas flow rate 8 L/min, nebulizer pressure 35 psi, sheath gas temperature 350 °C, sheath gas flow rate 11 L/min, nozzle voltage (Expt) 1000 V, fragmentor voltage 135 V, skimmer voltage 65 V, collision energy 20 eV, capillary voltage 4000 V in positive mode and 3500 V in negative mode. The scan range was m/z 100–1500 for the MS scan and m/z 50–1000 for the MS/MS scan.
2.5. Networking of LCDD related to COVID-19
The recommended prescription of each herb in LCDD was collected from Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). The content and potency value of active compounds were from HPLC-MS/MS and DMR assays, respectively. The herb attribution information corresponding to the active compounds came from the database and literatures. Import the relationship between herbs, active compounds, and targets into the Cytoscape 3.8.0, then an herb-compound-target network for LCDD was established.
2.6. Data analysis
All DMR data were acquired by EpicImager 1.20S (Corning, NY, USA) and processed by Epic Filtration v3.9.5 (Corning, NY, USA), Microsoft Excel 2010, and GraphPad Prism 6.02 (GraphPad Software Inc., San Diego, CA, USA). DMR signals were background corrected; EC50 and IC50 values were calculated by fitting the concentrations-DMR response curves with nonlinear regression.
3. Results
3.1. Label-free cell phenotypic profiling of 144 compounds of LCDD
Based on chemical components in herbs of LCDD reported in literature (Zhou et al., 2020a, Zhou et al., 2020b), we first chose 144 compounds available in our natural product library or market. The selected compounds and their corresponding herb information were shown in Table S1. These compounds were profiled against five cell lines, including HT-29 cells (endogenously express GPR35 receptor) (Deng et al., 2011), HEK293-M3 cells, A431 cells (endogenously express β2-adrenoceptor and H1 receptor) (Tran and Ye, 2008), A549 cells (endogenously express B2 receptor) (Izumi et al., 2018) and CHO-CB2 cells. The DMR arising from the stimulation of a specific cell line with each compound was obtained individually to determine its agonist or antagonist activity. The DMR responses of each compound were then converted into a similarity analysis (Fig. 1 ). According to the physiological role of these receptors, GPR35/CB2/β2 agonists or M3/H1/B2 antagonists are effective components for anti-COVID-19. In profiling, the compound is considered as an agonist if it induces significant DMR signal and blocks the receptor probe-induced DMR signal. While it is an antagonist if it does not induce obvious DMR responses and reduces the receptor probe-induced DMR signal. Herein, the agonist probe of GPR35, CB2, β2, M3, H1 and B2 was zaprinast, CP55940, epinephrine, carbachol, histamine and bradykinin, respectively.
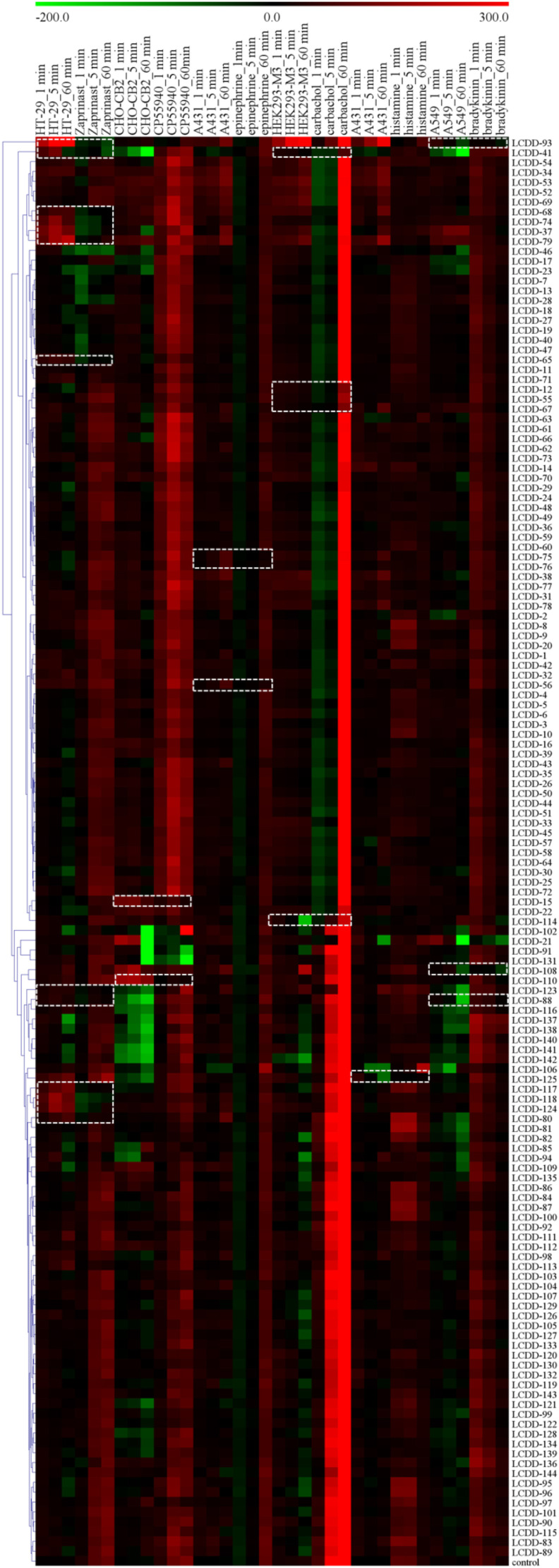
Fig. 1.
Label-free cell phenotypic heat map of 144 compounds in five cell lines including HT-29, CHO-CB2, A431, HEK293-M3 and A549 cells. This heat map was obtained using similarity analysis of the DMR signals of the compounds in these cell lines, and the DMR of the GPR35 agonist zaprinast at 2 μM in HT-29, the CB2 agonist CP55940 at 1 μM in CHO-CB2, the β2-adrenoceptor agonist epinephrine at 5 nM in A431, the M3 agonist carbachol at 200 nM in HEK293-M3, the H1 agonist histamine at 10 μM in A431 and the B2 agonist bradykinin at 500 nM in A549 after the pretreatment with compounds. For each compound profile, the real amplitudes at 1, 5 and 60 min after compounds treatment were used and color-coded as follows: red, positive; black, zero; green, negative. Each compound was assayed at 100 μM. All data represent mean ± s.d. from two independent measurements, each in duplicate (n = 4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
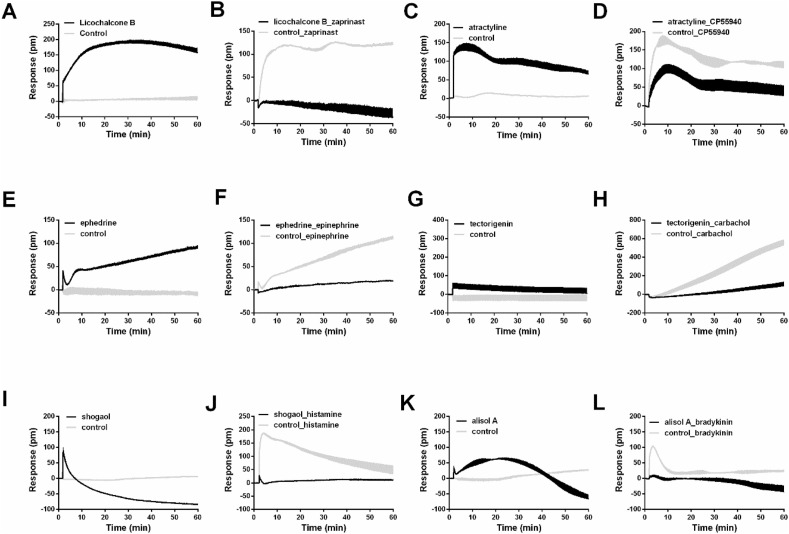
As shown in Fig. 1, compounds led to remarkable DMR signal and attenuated zaprinast-induced DMR signal in HT-29 cells, including LCDD-93 (licochalcone B), LCDD-41 (tectorigenin), LCDD-68 (taxifolin), LCDD-74 (caffeic acid), LCDD-37 (gallic acid), LCDD-79 (isochlorogenic acid C), LCDD-65 (kynurenic acid), LCDD-123 (luteolin), LCDD-88 (isoliquiritigenin), LCDD-117 (baicalin), LCDD-118 (baicalein), LCDD-124 (scutellarin) and LCDD-80 (quercetin). The results suggested that these 13 compounds were potential GPR35 agonists. For CHO-CB2 cells, LCDD-15 (atractyline) and LCDD-110 (alisol A 24-acetate) displayed the similar characteristics, meaning that they had agonistic activity on CB2. In addition, LCDD-75 (ephedrine), LCDD-76 (pseudoephedrine) and LCDD-56 (synephrine) were possible β2-adrenoceptor agonists. LCDD-41 (tectorigenin), LCDD-12 (alismoxide), LCDD-55 (nomilin), LCDD-67 (herbacetin) and LCDD-114 (aristofone) resulted in negligible DMR signal and blocked the DMR signal induced by carbachol, indicating that these five compounds were M3 receptor antagonists. The similar behavior was observed with LCDD-125 (shogaol) for H1 receptor in A431 cells, and LCDD-93 (licochalcone B), LCDD-108 (alisol A) and LCDD-88 (isoliquiritigenin) for B2 receptor in A549 cells. Due to different patterns of active compounds detected in each receptor, the holistic view of representative active compounds for six receptors was shown in Fig. 2 . Take licochalcone B as an example, it induced a positive DMR response in HT-29 cells (Fig. 2A), and the response of zaprinast was totally inhibited after cells were pretreated with licochalcone B (Fig. 2B). These results suggested that licochalcone B was a GPR35 agonist. Together, 24 potential active compounds were discovered from LCDD, suggesting that they were probably effective components in the treatment of COVID-19.
Fig. 2.
Characteristic real-time DMR response. (A) Real-time DMR response of 100 μM licochalcone B and control in HT-29 cells. (B) Real-time DMR response of 2 μM zaprinast after HT-29 cells pretreated with 100 μM licochalcone B and control for 1 h. (C) Real-time DMR response of 100 μM atractyline and control in CHO-CB2 cells. (D) Real-time DMR response of 1 μM CP55940 after CHO-CB2 cells pretreated with 100 μM atractyline and control for 1 h. (E) Real-time DMR response of 100 μM ephedrine and control in A431 cells. (F) Real-time DMR response of 5 nM epinephrine after A431 cells pretreated with 100 μM ephedrine and control for 1 h. (G) Real-time DMR response of 100 μM tectorigenin and control in HEK293-M3 cells. (H) Real-time DMR response of 200 nM carbachol after HEK293-M3 cells pretreated with 100 μM tectorigenin and control for 1 h. (I) Real-time DMR response of 100 μM shogaol and control in A431 cells. (J) Real-time DMR response of 10 μM histamine after A431 cells pretreated with 100 μM shogaol and control for 1 h. (K) Real-time DMR response of 100 μM alisol A and control in A549 cells. (L) Real-time DMR response of 500 nM bradykinin after A549 cells pretreated with 100 μM alisol A and control for 1 h. All data represent mean ± s.d. from two independent measurements, each in duplicate (n = 4).
3.2. Validation of activities of potential compounds on six targets
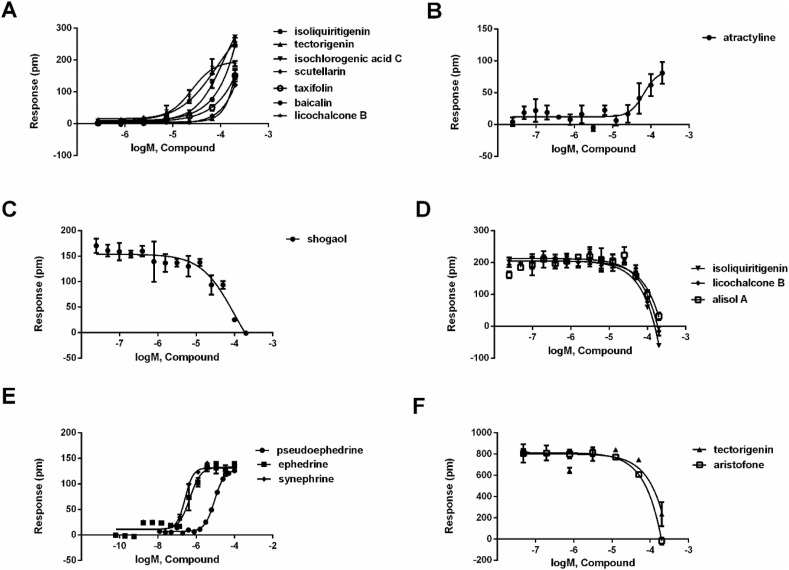
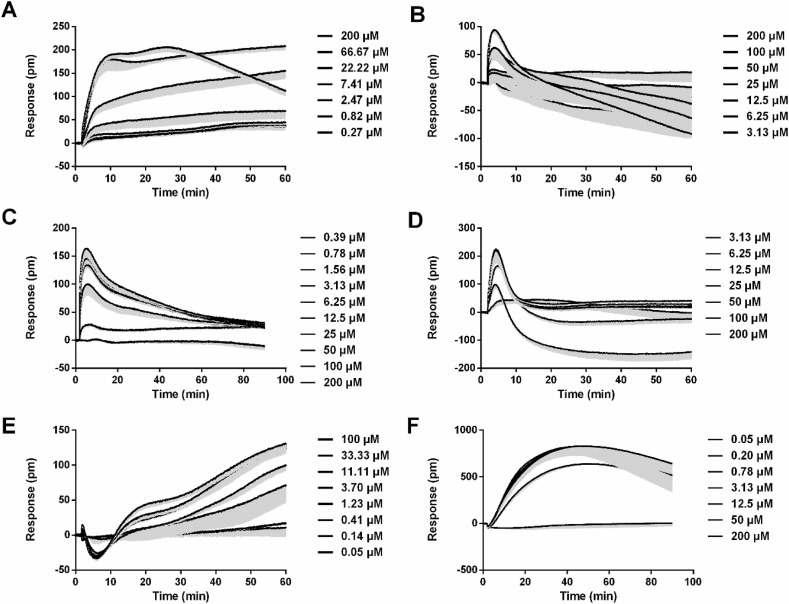
Since the experiment in Section 3.1 was conducted using a single concentration, further multi-concentration experiments were required to confirm the activity of the candidate compounds, so as to determine if their activity were concentration dependent. Compounds that showed activity in the general screening assay (section 3.1) and caused DMR responses in a concentration-dependent manner would be determined to be agonistic or antagonistic compounds. We next evaluated the potency of 24 potential compounds on six targets. Follow-up multiple concentration tests, it was shown that licochalcone B, tectorigenin, taxifolin, isochlorogenic acid C, isoliquiritigenin, baicalin and scutellarin stimulated concentration-dependent response on HT-29 cells (Fig. 3 A and Table 2 ). The real-time multiple concentration DMR response of licochalcone B on HT-29 cells was shown in Fig. 4 A. In addition, caffeic acid (Deng and Fang, 2012), gallic acid (Deng and Fang, 2012), baicalein (Deng et al., 2012b), luteolin (Deng et al., 2012b), quercetin (Deng et al., 2012b) and kynurenic acid (Deng et al., 2012a) were reported GPR35 agonist, and their activities were also measured by DMR assay in literatures (Table 2). These results demonstrated that all potential compounds were GPR35 agonists. It is worth mentioning that licochalcone B, tectorigenin, isochlorogenic acid C and isoliquiritigenin were novel GPR35 agonist. For CHO-CB2 cells, only atractyline led to a concentration-dependent DMR agonism response, suggesting that it was a CB2 agonist (Fig. 3B and Table 2). The real-time multiple concentration DMR response of atractyline was shown in Fig. 4B. Shogaol concentration-dependently inhibited the response of histamine in A431 cells, suggesting that it was a H1 antagonist (Fig. 3C and Table 2). The real-time multiple concentration DMR response of histamine after A431 cells were pretreated with shogaol was shown in Fig. 4C. For B2 receptor in A549 cells, licochalcone B, alisol A and isoliquiritigenin also blocked bradykinin-induced response in a concentration-dependent manner, suggesting that these three compounds were B2 antagonists (Fig. 3D and Table 2). The real-time multiple concentration DMR response of bradykinin after cells were pretreated with alisol A was shown in Fig. 4D.
Fig. 3.
Characterization of potential compounds on six targets. (A) The DMR amplitudes of licochalcone B, tectorigenin, taxifolin, isochlorogenic acid C, isoliquiritigenin, baicalin and scutellarin as a function of their concentrations in HT-29 cells. (B) The DMR amplitudes of atractyline as a function of its concentrations in CHO-CB2 cells. (C) The DMR amplitudes of histamine after A431 cells were pretreated with different concentrations of shogaol. (D) The DMR amplitudes of bradykinin after A549 cells were pretreated with different concentrations of licochalcone B, alisol A and isoliquiritigenin, respectively. (E) The DMR amplitudes of ephedrine, pseudoephedrine and synephrine as a function of their concentrations in A431 cells. (F) The DMR amplitudes of acetylcholine after HEK293-M3 cells were pretreated with different concentrations of tectorigenin and aristofone, respectively. All data represent mean ± s.d. from two independent measurements, each in triplicate (n = 6).
Table 2.
Potency value and content of active compounds.
| NO. | Compound | Target | EC50/IC50 (μM) | Agonist/antagonist | Content μg/mg |
|---|---|---|---|---|---|
| 1 | licochalcone B | GPR35 | 25.12 | agonist | 0.000822 |
| 2 | tectorigenin | GPR35 | >200 | agonist | 0.004408 |
| 3 | taxifolin | GPR35 | >200 | agonist | 0.000486 |
| 4 | isochlorogenic acid C | GPR35 | 114.30 | agonist | 0.183710 |
| 5 | isoliquiritigenin | GPR35 | >200 | agonist | 0.000073 |
| 6 | baicalin | GPR35 | >200 | agonist | 2.571457 |
| 7 | scutellarin | GPR35 | 86.26 | agonist | 0.171974 |
| 8 | caffeic acid | GPR35 | 290 a | agonist | 0.0344684 |
| 9 | gallic acid | GPR35 | 1.16 a | agonist | 0.008892 |
| 10 | baicalein | GPR35 | 10.3 a | agonist | 0.003157 |
| 11 | luteolin | GPR35 | 7.24 a | agonist | 0.001091 |
| 12 | quercetin | GPR35 | 8.02 a | agonist | 0.003465 |
| 13 | kynurenic acid | GPR35 | 152 a | agonist | 0.008850 |
| 14 | atractyline | CB2 | 65.23 | agonist | undetected |
| 15 | shogaol | H1 | >200 | antagonist | undetected |
| 1 | licochalcone B | B2 | 139.70 | antagonist | 0.000822 |
| 16 | alisol A | B2 | >200 | antagonist | 0.001993 |
| 5 | isoliquiritigenin | B2 | >200 | antagonist | 0.000073 |
| 17 | ephedrine | β2-adrenoceptor | 0.49 | agonist | 1.391095 |
| 18 | pseudoephedrine | β2-adrenoceptor | 9.00 | agonist | 0.823086 |
| 19 | synephrine | β2-adrenoceptor | 0.25 | agonist | 0.162534 |
| 2 | tectorigenin | M3 | >200 | antagonist | 0.0044075 |
| 20 | aristofone | M3 | >200 | antagonist | undetected |
The superscript letter a in column four indicates that the EC50/IC50 value measured using the DMR technique comes from literatures.
Fig. 4.
Characteristics of the real-time DMR signals induced by representative compounds. (A) The DMR responses of licochalcone B in HT-29 cells. (B) The DMR responses of atractyline in CHO-CB2 cells. (C) The DMR responses of histamine after A431 cells were pretreated with shogaol. (D) The DMR responses of bradykinin after A549 cells were pretreated with alisol A. (E) The DMR responses of ephedrine in A431 cells. (F) The DMR responses of acetylcholine after HEK293-M3 cells were pretreated with aristofone. The data represent mean ± s.d. from two independent measurements, each in triplicate (n = 6).
For β2-adrenoceptor in A431 cells, ephedrine, pseudoephedrine and synephrine all triggered concentration-dependent DMR response (Fig. 3E and Table 2), suggesting that these compounds were β2-adrenoceptor agonists. Although ephedrine, pseudoephedrine and synephrine were not the first reported β2-adrenoceptor agonists, their activities were further confirmed using DMR assay. The real-time multiple concentration DMR response of ephedrine was displayed in Fig. 4E. For M3 potential antagonists, only tectorigenin and aristofone showed concentration-dependent inhibition effects on acetylcholine-induced DMR signal, suggesting that these two compounds were M3 antagonists (Fig. 3F and Table 2). Importantly, tectorigenin and aristofone were new M3 antagonists. The real-time multiple concentration DMR response of acetylcholine after HEK293-M3 cells were pretreated with aristofone was shown in Fig. 4F. After validation, 20 compounds were identified as the active components, which had agonistic or antagonistic activities on target in a concentration-dependent manner.
3.3. Pathway deconvolution of licochalcone B in HT-29 cells and ephedrine in A431 cells
Among all active compounds, the signaling pathways of agonists were deconvoluted. Licochalcone B and ephedrine with the potency lower than 50 μM were selected as representative agonists for exploring signaling pathways of GPR35 in HT-29 cells and β2-adrenoceptor in A431 cells, respectively. DMR pathway deconvolution assays were used to examine the effects of the pretreatment of cells with different pathway modulators on DMR response induced by licochalcone B or ephedrine.
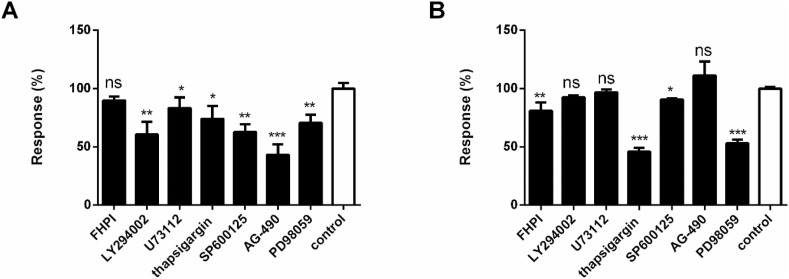
For the DMR response of licochalcone B in HT-29 cells, results showed that the phospholipase C (PLC) inhibitor U73122 and Ca2+ ATPase inhibitor thapsigargin slightly inhibited its signals (Fig. 5 A), indicating that Gq-PLC-Ca2+ only played a small role in the DMR signal induced by licochalcone B. The PI3K inhibitor LY294002, JNK inhibitor SP600125, EGFR inhibitor AG-490 and MEK inhibitor PD98059 partially inhibited the DMR of licochalcone B. In contrast, the p38 MAPK inhibitor FHPI almost had no effect on the DMR of licochalcone B. Taken together, these results suggested that the activation of licochalcone B on GPR35 primarily triggered PI3K, JNK, MEK pathway, and EGFR transactivation.
Fig. 5.
Effects of pathway inhibitors FHPI (20 μM), LY294002 (20 μM), U73122 (20 μM), thapsigargin (10 μM), SP600125 (10 μM), AG-490 (50 μM), PD98059 (10 μM) or control on DMR induced by licochalcone B (A), ephedrine (B). The data represent mean ± s.d. from two independent measurements, each in triplicate (n = 6). *, P < 0.05 versus control; **, P < 0.01 versus control; ***, P < 0.001 versus control, t-test.
For the DMR response of ephedrine in A431 cells, results showed that U73122 had no effect on inhibiting DMR response of ephedrine (Fig. 5B), indicating that PLC was not involved in the DMR signal induced by ephedrine. However, thapsigargin partially reduced the DMR signal, indicating that Ca2+ involved in the DMR signal induced by ephedrine. LY294002 had no suppression of DMR signal of ephedrine. Similar results were observed for AG-490. FHPI and SP600125 slightly inhibited the signals of ephedrine. In contrast, PD98059 had almost 50 % inhibition of effect on the DMR. Therefore, these results suggested that the activation of ephedrine on β2-adrenoceptor primarily mediated MEK and Ca2+ pathways.
3.4. Herb-compound-target-effect network for COVID-19
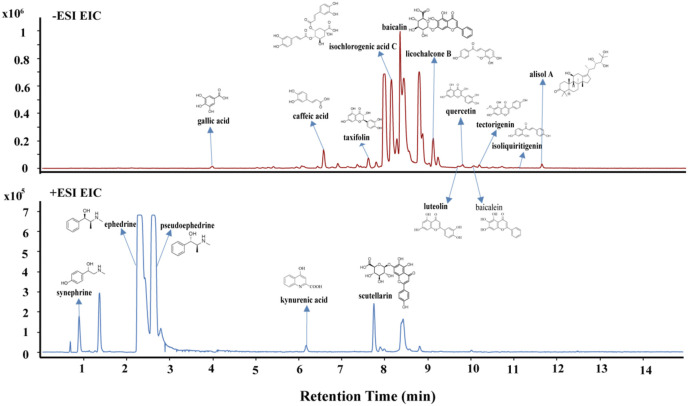
The content and potency value of active compounds as well as recommended prescription mass of each herb were considered in the construction of the network. To determine the content of active compounds, a HPLC-MS/MS method was established for the quantification of active compound in the LCDD samples (Fig. 6 ). The detailed quantitative process was shown in method section and Table S2. The quantitative results of each active compound were displayed in Table 2. A total of 17 active compounds were detected, while atractyline, shogaol and aristofone were not detected. Among these detected active compounds, the abundance of baicalin, ephedrine, pseudoephedrine, isochlorogenic acid C, scutellarin and synephrine was relatively high.
Fig. 6.
Extracted ion chromatogram of Lung Cleansing and Detoxifying Decoction.
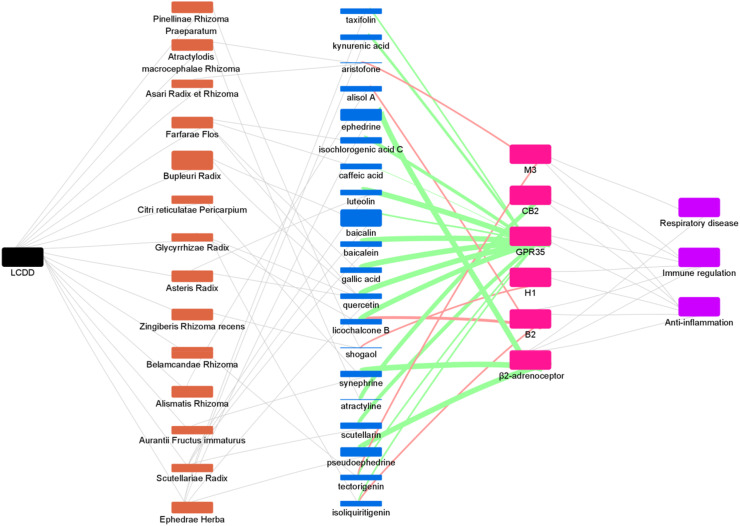
After determining the content of the active compounds, we constructed an herb-compound-target network (Fig. 7 ). We used the size of the rectangle to distinguish the proportion of herbs and the content of compounds in LCDD. Although Bupleuri Radix accounted for a large proportion of LCDD, we found that it contained few active compounds targeting these six GPCRs, and only baicalin and quercetin were GPR35 agonists. Ephedrae Herba occupied a medium proportion in LCDD, but we could see that there were 5 edges connecting to active compounds, which is the most among these herbs, suggesting that Ephedrae Herba is very important in the treatment of COVID-19. Additionally, Scutellariae Radix, Farfarae Flos and Glycyrrhizae Radix may also play a critical role in the treatment of COVID-19, because there were also many edges connecting active compounds. We used the color of the edge between the active compound and the target to distinguish the agonistic (green) and antagonistic (red) activities and the thickness of the edge to represent the potency of the activity. We found that the content of ephedrine and pseudoephedrine both from Ephedrae Herba was relatively high, and their β2-adrenoceptor agonistic activity was also strong as indicated by the size of the rectangle and thickness of the edge, which further confirmed the important role of Ephedrae Herba. Moreover, there were two edges of licochalcone B, isoliquiritigenin and tectorigeniniso connecting to targets, meaning that these compounds act on multiple targets. At the same time, there are multi-edges of compounds converging to a target, suggesting that multiple components interact with the same target such as GPR35, reflecting the synergistic mechanism of Chinese medicine. Although some compounds are very low in content in LCDD, such as licochalcone B, gallic acid, synephrine and luteolin, they may also play an important role because of their high target activity.
Fig. 7.
The herb-compound-target network. Black rectangle: Lung Cleansing and Detoxifying Decoction (LCDD); orange rectangle: herb; blue rectangle: active compound; pink rectangle: target; purple rectangle: disease. Note: although atractyline, shogaol and aristofone were not detected by HPLC-MS/MS, they were represented by a very thin line. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
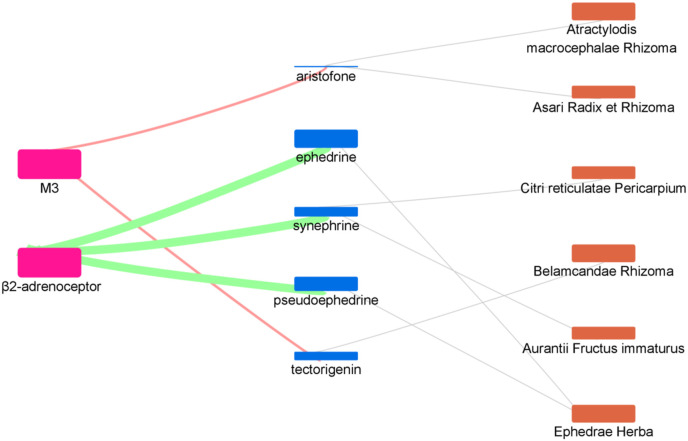
The SARS-CoV-2 infection inducing airway hyperresponsiveness raised the possibility the dyspnoea and respiratory failure in severe COVID-19 patients. Therefore, regulating respiration during the disease process was the crucial intervention in treating COVID-19. M3 antagonists and β2-adrenoceptor agonists could inhibit bronchoconstriction, targeting these two receptors would benefit COVID-19 patients with breathing difficulties (Bornstein et al., 2021; De Virgiliis and Di Giovanni, 2020). We enriched the results of the respiratory disease related nodes (Fig. 8 ). As a result, we found that six herbs played a role in respiratory regulation, including Citri reticulatae Pericarpium, Atractylodis macrocephalae Rhizoma, Asari Radix et Rhizoma, Ephedrae Herba, Aurantii Fructus immaturus and Belamcandae Rhizoma. The active compounds in LCDD here targeted all of the six GPCRs involved in suppression of inflammatory, immunity regulation and relaxation of airway smooth muscle. These results suggested that LCDD could be used as a promising formula to intervene in the pathological process of COVID-19.
Fig. 8.
The enriched herb-compound-target network of respiratory disease related nodes.
4. Discussion
TCM has played an important role in the prevention and treatment of several pandemics in history. Clinical results have demonstrated the efficacy of TCMs in the treatment of COVID-19. LCDD is considered as a “universal prescription” that was recommended for COVID-19. However, its mechanism and active components remain unknown. In this study, we used a label-free integrative pharmacology method to identify the target and pathway mechanisms of active components of LCDD. As a number of COVID-19 patients showed high levels of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α, it was proposed that down-regulation of inflammatory responses may improve outcome. Numerous reports have suggested a possible therapeutic role of antagonists to cytokines in the treatment of COVID-19, while some studies explored GPCRs as therapeutic targets against COVID-19. GPCRs are involved in most physiological processes in mammals, including inflammatory responses. Growing evidence demonstrated that H1 antagonists may be effective in suppressing inflammation caused by the SARS-CoV-2 infection (Qu et al., 2021). CB2 activation may have therapeutic benefit in treating COVID-19 by limiting inflammatory cytokine release (Rossi et al., 2020). B2 receptors are also possible targets for COVID-19 for regulating inflammatory responses (Kaplan and Ghebrehiwet, 2021). The SARS-CoV-2 infection induced airway hyperresponsiveness and thus raised the possibility of dyspnoea and respiratory failure in severe COVID-19 patients (De Virgiliis and Di Giovanni, 2020). M3 antagonists could limit bronchoconstriction and vasodilation and limit the inflammatory response (Patel et al., 2017). For these reasons, we screened active component of LCDD against six GPCRs related to inflammation, immunity and respiration, including GPR35, H1, CB2, B2, M3 and β2-adrenoceptor.
A significant finding is that licochalcone B and isoliquiritigenin both from Glycyrrhizae Radix showed dual activity against GPR35 and B2 receptors, and tectorigenin from Belamcandae Rhizoma showed dual activity against GPR35 and M3 receptor. Previous reports indicated that licochalcone B and isoliquiritigenin played an indispensable role in the potent anti-inflammatory effect of Glycyrrhiza uralensis Fisch. (Furusawa et al., 2009; Zhu et al., 2019). In addition, tectorigenin which is an effective component derived from Belamcanda chinensis, has attracted considerable interest because of its anti-inflammatory activity (Ha le et al., 2013; Pan et al., 2008). However, their targets for anti-inflammatory activity were not clear. Our findings provide a target mechanism for explaining the anti-inflammatory effect of these active components. This multi-target feature of the single compound may produce a synergistic effect in the treatment of COVID-19.
A single targeted drug may be not suitable to cure a complex disease, such as COVID-19. TCM has a good potential to complement the medical treatment for COVID-19. This may partly because of its multi-component and multi-target effects. We explored the mechanism of LCDD against COVID-19 by pharmacology and combination strategy. A double-weighted network pharmacology, taking into account of the content weight and potency value of active compounds, was established for the first time. Previous studies have explained the treatment mechanism of LCDD through the target network model, established by predicting and collecting the targets of compounds (Chen et al., 2020; Yang et al., 2020; Zhao et al., 2020a, Zhao et al., 2020b). The network constructed in this study is entirely based on the results of experimental tests. It may be helpful for us to understand the actual mechanism of LCDD, especially the mechanism of GPCRs related to inflammation, immunity and respiration.
From the herb-compound-target networks, Ephedrae Herba and Glycyrrhizae Radix were considered to be very important in the treatment of COVID-19. It had been reported that Glycyrrhizae Radix was the most frequently used in prescriptions for the treatment of respiratory disease (Fu et al., 2013). The prescriptions for treatment of dyspnoea often is a combination of Herba ephedrae and Glycyrrhizae Radix (Fu et al., 2013). Interestingly, as a fundamental part of LCDD, Maxing Shigan Decoction which is recommended as a basic prescription and applied widely in the clinical treatment of COVID-19 also consists of Herba Ephedrae and Radix Glycyrrhizae. Our research also confirmed the importance of Ephedrae Herba and Glycyrrhizae Radix from the molecular mechanism level. Not only in Maxing Shigan Decoction, the active ingredients targeting GPCRs were also present in all four formulas of LCDD. As a combination recipe, LCDD completely exert the efficacy of being a multi-component and multi-target medicine. Though we discovered 20 potential active ingredients, this work is subject to a few limitations that should be noticed. First, further research would be required for the synergistic effects and metabolism of the active compounds in vivo. Second, due to the complexity of the human body and limited biotargets tested in this work, we only partially explained the therapeutic mechanism of LCDD, and more therapeutic targets should be included so as to explain the mechanism of action from a more holistic perspective. Despite the limitations, findings of this study provided new ideas and evidence for further researches on the treatment of COVID-19 using LCDD.
The label-free DMR technology has broad signaling-pathway coverage (Rocheville et al., 2013), facilitating studies of multiple types of signaling pathways, and has the advantage in deconvoluting the signaling pathways of endogenous GPCRs. Here, we dissected the signaling pathways of GPR35 in HT-29 cells and β2-adrenocennptor in A431 cells. Previous studies have shown that GPR35 is a Gi and G16 coupled receptor (Taniguchi et al., 2008). The β2-adrenoceptor is a prototypic Gs-coupled receptor (Ferrie et al., 2014). We showed that the activation of GPR35 by licochalcone B primarily triggered PI3K, MEK, JNK pathway, EGFR transactivation, and Gq-PLC-Ca2+ is basically not activated. The activation of ephedrine on β2-adrenoceptor primarily mediated MEK and Ca2+ pathways. Currently, the identified signaling pathways are relatively upstream, and more studies are needed to establish a complete signaling pathway for the regulation of inflammation and dyspnoea caused by the SARS-CoV-2 infection.
5. Conclusions
In this study, the targets and pathways of LCDD were deconvoluted using a label-free integrative pharmacology method. 144 compounds from LCDD were profiled against six inflammatory-, immunity- and respiratory-related GPCRs. Twenty active compounds were identified for each target. Moreover, their potency and content in LCDD were determined, and the signaling pathways of licochalcone B and ephedrine were dissected. It is worth noting that a content and potency value of active compounds was both considered in the construction of the experiment-based herb-compound-target network for COVID-19. This study showed that LCDD had a protection effect on COVID-19 by regulating a panel of GPCRs and was helpful for elucidating its actual therapeutic mechanism. These results indicated that the effective treatment of LCDD for COVID-19 may be through a holistic treatment of multi-components acting on multi-targets. Our research provides an experimental basis and research ideas for further discovery of TCM formula and botanical drug for the treatment of COVID-19.
CRediT authorship contribution statement
Fangfang Xu: Formal analysis, Writing – original draft, contributed equally to this work. designed the research; analysed the data; drafted the manuscript. All authors approved the final version of the manuscript. Tao Hou: Formal analysis, contributed equally to this work. performed the research; analysed the data; All authors approved the final version of the manuscript. Aijin Shen: performed the research; All authors approved the final version of the manuscript. Hongli Jin: performed the research; All authors approved the final version of the manuscript. Yuansheng Xiao: performed the research; All authors approved the final version of the manuscript. Wenyi Yu: performed the research; All authors approved the final version of the manuscript, performed the research; All authors approved the final version of the manuscript. Jixia Wang: Writing – original draft, designed the research; drafted the manuscript. All authors approved the final version of the manuscript. Yanfang Liu: designed the research; All authors approved the final version of the manuscript. Xinmiao Liang: designed the research; All authors approved the final version of the manuscript.
Declaration of competing interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgements
This work was supported by National Key R&D Program of China (2020YFC0845400), National Science and Technology Major Project (2018ZX09735-002), and the innovation program of science and research from DICP, CAS (DICP, CAS201803 and DICP I201933).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2021.114488.
Abbreviations
- COVID-19
Coronavirus Disease 2019
- TCM
traditional Chinese medicine
- LCDD
Lung Cleansing and Detoxifying Decoction
- HIV
human immunodeficiency virus
- HSV
herpes simplex virus
- ACE2
angiotensin-converting enzyme 2
- GPCR
G-protein coupled receptor
- H1
histamine receptor 1
- CB2
cannabinoid receptor 2
- B2
bradykinin receptor 2
- M3
muscarinic receptor 3
- COPD
chronic obstructive pulmonary disease
- DMR
dynamic mass redistribution
- HBSS
Hank's balanced salt solution
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Abourashed E.A., El-Alfy A.T., Khan I.A., Walker L. Ephedra in perspective--a current review. Phytother Res. : PTR. 2003;17(7):703–712. doi: 10.1002/ptr.1337. [DOI] [PubMed] [Google Scholar]
- Bandara A.R., Rapior S., Bhat D.J., Kakumyan P., Chamyuang S., Xu J.C., Hyde K.D. Polyporus umbellatus, an edible-medicinal cultivated mushroom with multiple developed health-care products as Food, medicine and cosmetics: a review. Cryptog. Mycolog. 2015;36(1):3–42. [Google Scholar]
- Bornstein S.R., Voit-Bak K., Donate T., Rodionov R.N., Gainetdinov R.R., Tselmin S., Kanczkowski W., Muller G.M., Achleitner M., Wang J., Licinio J., Bauer M., Young A.H., Thuret S., Bechmann N., Straube R. Chronic post-COVID-19 syndrome and chronic fatigue syndrome: is there a role for extracorporeal apheresis? Mol. Psychiatr. 2021:1–4. doi: 10.1038/s41380-021-01148-4. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Y.K., Gao Y., Hu L.S., Yang J.W., Wang J.R., Sun W.J., Liang Z.Q., Cao Y.M., Cao Y.B. Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.S., Deng J.S., Chang H.Y., Chen Y.C., Lee M.M., Hou W.C., Lee C.Y., Huang S.S., Huang G.J. Antioxidant and anti-inflammatory properties of Taiwanese yam (Dioscorea japonica Thunb. var. pseudojaponica (Hayata) Yamam.) and its reference compounds. Food Chem. 2013;141(2):1087–1096. doi: 10.1016/j.foodchem.2013.04.031. [DOI] [PubMed] [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- De Virgiliis F., Di Giovanni S. Lung innervation in the eye of a cytokine storm: neuroimmune interactions and COVID-19. Nat. Rev. Neurol. 2020;16(11):645–652. doi: 10.1038/s41582-020-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Fang Y. Anti-inflammatory gallic Acid and wedelolactone are G protein-coupled receptor-35 agonists. Pharmacology. 2012;89(3–4):211–219. doi: 10.1159/000337184. [DOI] [PubMed] [Google Scholar]
- Deng H., Hu H., Fang Y. Tyrphostin analogs are GPR35 agonists. FEBS Lett. 2011;585(12):1957–1962. doi: 10.1016/j.febslet.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Deng H., Hu H., Fang Y. Multiple tyrosine metabolites are GPR35 agonists. Sci. Rep. 2012;2:373. doi: 10.1038/srep00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Hu H., Ling S., Ferrie A.M., Fang Y. Discovery of natural phenols as G protein-coupled receptor-35 (GPR35) agonists. ACS Med. Chem. Lett. 2012;3(2):165–169. doi: 10.1021/ml2003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrie A.M., Sun H., Zaytseva N., Fang Y. Divergent label-free cell phenotypic pharmacology of ligands at the overexpressed beta(2)-adrenergic receptors. Sci. Rep. 2014;4:3828. doi: 10.1038/srep03828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong W.F., Wang C., Zhu G.Y., Leung C.H., Yang M.S., Cheung H.Y. Reversal of multidrug resistance in cancer cells by Rhizoma Alismatis extract. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2007;14(2–3):160–165. doi: 10.1016/j.phymed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Fu X.J., Song X.X., Wei L.B., Wang Z.G. Study of the distribution patterns of the constituent herbs in classical Chinese medicine prescriptions treating respiratory disease by data mining methods. Chin. J. Integr. Med. 2013;19(8):621–628. doi: 10.1007/s11655-012-1090-2. [DOI] [PubMed] [Google Scholar]
- Furusawa J., Funakoshi-Tago M., Mashino T., Tago K., Inoue H., Sonoda Y., Kasahara T. Glycyrrhiza inflata-derived chalcones, Licochalcone A, Licochalcone B and Licochalcone D, inhibit phosphorylation of NF-kappaB p65 in LPS signaling pathway. Int. Immunopharm. 2009;9(4):499–507. doi: 10.1016/j.intimp.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Gao K., Song Y.P., Chen H., Zhao L.T., Ma L. Therapeutic efficacy of Qingfei Paidu decoction combined with antiviral drugs in the treatment of corona virus disease 2019: a protocol for systematic review and meta analysis. Medicine (Baltim.) 2020;99(22) doi: 10.1097/MD.0000000000020489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann M., Kostenis E. Label-free biosensor assays in GPCR screening. Methods Mol. Biol. 2015;1272:199–213. doi: 10.1007/978-1-4939-2336-6_14. [DOI] [PubMed] [Google Scholar]
- Ha le M., Que do T.N., Huyen do T.T., Long P.Q., Dat N.T. Toxicity, analgesic and anti-inflammatory activities of tectorigenin. Immunopharmacol. Immunotoxicol. 2013;35(3):336–340. doi: 10.3109/08923973.2013.770521. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.J., Hsu N.Y., Lu K.H., Lin Y.E., Lin S.H., Lu Y.S., Liu W.T., Chen M.H., Sheen L.Y. Poria cocos water extract ameliorates the behavioral deficits induced by unpredictable chronic mild stress in rats by down-regulating inflammation. J. Ethnopharmacol. 2020;258 doi: 10.1016/j.jep.2020.112566. [DOI] [PubMed] [Google Scholar]
- Izumi S., Higa-Nakamine S., Nishi H., Torihara H., Uehara A., Sugahara K., Kakinohana M., Yamamoto H. Phosphorylation of epidermal growth factor receptor at serine 1047 in cultured lung alveolar epithelial cells by bradykinin B2 receptor stimulation. Pulm. Pharmacol. Therapeut. 2018;48:53–61. doi: 10.1016/j.pupt.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Jiang L., Gao M., Qu F., Li H.-l., Yu L.-b., Rao Y., Wang Y.-s., Xu G.-l. Pharmacokinetics of Maxing Shigan decoction in normal rats and RSV pneumonia model rats by HPLC-MS/MS. China J. Chin. Mater. Med. 2015;40(13):2649–2655. [PubMed] [Google Scholar]
- Jung H.J., Kim Y.S., Shin M.S., Kim C.J., Kim Y.S. Effects of armeniacae semen and amygdalin on the lipopolysaccaride-induced prostaglandin E2 synthesis and nitric oxide production in mouse BV2 microglial cells. Experimental Neurobiology. 2008;17(2) [Google Scholar]
- Kaplan A.P., Ghebrehiwet B. Pathways for bradykinin formation and interrelationship with complement as a cause of edematous lung in COVID-19 patients. J. Allergy Clin. Immunol. 2021;147(2):507–509. doi: 10.1016/j.jaci.2020.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistemaker L.E., van Os R.P., Dethmers-Ausema A., Bos I.S., Hylkema M.N., van den Berge M., Hiemstra P.S., Wess J., Meurs H., Kerstjens H.A., Gosens R. Muscarinic M3 receptors on structural cells regulate cigarette smoke-induced neutrophilic airway inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(1):L96–L103. doi: 10.1152/ajplung.00259.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmus K., Tavernier J., Gerlo S. beta2-Adrenergic receptors in immunity and inflammation: stressing NF-kappaB. Brain Behav. Immun. 2015;45:297–310. doi: 10.1016/j.bbi.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Li Y.C., Xian Y.F., Ip S.P., Su Z.R., Su J.Y., He J.J., Xie Q.F., Lai X.P., Lin Z.X. Anti-inflammatory activity of patchouli alcohol isolated from Pogostemonis Herba in animal models. Fitoterapia. 2011;82(8):1295–1301. doi: 10.1016/j.fitote.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Liao H. Effects of shengjiang (zingiberis rhizoma recens) and its processed products on nitric oxide production in macrophage RAW 264.7 cells. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/828156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Nie B., Yao G., Yang H., Ye R., Yuan Z. Pinellia ternata (Thunb.) Makino Preparation promotes sleep by increasing REM sleep. Nat. Prod. Res. 2019;33(22):3326–3329. doi: 10.1080/14786419.2018.1474466. [DOI] [PubMed] [Google Scholar]
- Liu C., Wu H., Wang L., Luo H., Lu Y., Zhang Q., Tang L., Wang Z. Farfarae Flos: a review of botany, traditional uses, phytochemistry, pharmacology, and toxicology. J. Ethnopharmacol. 2020;260 doi: 10.1016/j.jep.2020.113038. [DOI] [PubMed] [Google Scholar]
- Liu D., You Y., Chen Y., Tang S. Efficacy of integrative Traditional Chinese and Western medicine for the treatment of patients infected with 2019 novel coronavirus (COVID-19): a protocol for systematic review and meta analysis. Medicine (Baltim.) 2020;99(29) doi: 10.1097/MD.0000000000020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.X., Xu F., Shang M.Y., Wang X., Cai S.Q. The Relative Content and Distribution of Absorbed Volatile Organic Compounds in Rats Administered Asari Radix et Rhizoma Are Different between Powder- and Decoction-Treated Groups. Molecules. 2020;25(19) doi: 10.3390/molecules25194441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang Q., Li R.L., Wei S.J., Huang C.Y., Gao Y.X., Pu X.F. The traditional uses, phytochemistry, pharmacology and toxicology of Cinnamomi ramulus: a review. J. Pharm. Pharmacol. 2020;72(3):319–342. doi: 10.1111/jphp.13189. [DOI] [PubMed] [Google Scholar]
- Ming C.H.U., Zhengyun C.H.U., Dandan W. The extract of compound Radix scuteilariae on mRNA replication and IFN expression of influenza virus in mice. J. Chin. Med. Mater. 2007;30(1):63–65. [PubMed] [Google Scholar]
- Pan C.H., Kim E.S., Jung S.H., Nho C.W., Lee J.K. Tectorigenin inhibits IFN-gamma/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells. Arch Pharm. Res. (Seoul) 2008;31(11):1447–1456. doi: 10.1007/s12272-001-2129-7. [DOI] [PubMed] [Google Scholar]
- Patel K.R., Bai Y., Trieu K.G., Barrios J., Ai X. Targeting acetylcholine receptor M3 prevents the progression of airway hyperreactivity in a mouse model of childhood asthma. Faseb. J. 2017;31(10):4335–4346. doi: 10.1096/fj.201700186R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera T., Penn R.B. Crosstalk between beta-2-adrenoceptor and muscarinic acetylcholine receptors in the airway. Curr. Opin. Pharmacol. 2014;16:72–81. doi: 10.1016/j.coph.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu C., Fuhler G.M., Pan Y. Could histamine H1 receptor antagonists Be used for treating COVID-19? Int. J. Mol. Sci. 2021;22(11) doi: 10.3390/ijms22115672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quon T., Lin L.C., Ganguly A., Tobin A.B., Milligan G. Therapeutic opportunities and challenges in targeting the orphan G protein-coupled receptor GPR35. ACS Pharmacol Transl Sci. 2020;3(5):801–812. doi: 10.1021/acsptsci.0c00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho J., Seo C.S., Park H.S., Jeong H.Y., Moon O.S., Seo Y.W., Son H.Y., Won Y.S., Kwun H.J. Asteris Radix et Rhizoma suppresses testosterone-induced benign prostatic hyperplasia in rats by regulating apoptosis and inflammation. J. Ethnopharmacol. 2020;255 doi: 10.1016/j.jep.2020.112779. [DOI] [PubMed] [Google Scholar]
- Rocheville M., Martin J., Jerman J., Kostenis E. Mining the potential of label-free biosensors for seven-transmembrane receptor drug discovery. Progress in molecular biology and translational science. 2013;115:123–142. doi: 10.1016/B978-0-12-394587-7.00003-8. [DOI] [PubMed] [Google Scholar]
- Rossi F., Tortora C., Argenziano M., Di Paola A., Punzo F. Cannabinoid receptor type 2: a possible target in SARS-CoV-2 (CoV-19) infection? Int. J. Mol. Sci. 2020;21(11) doi: 10.3390/ijms21113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruqiao L., Yueli C., Xuelan Z., Huifen L., Xin Z., Danjie Z., Le S., Yanxue Z. Rhizoma Atractylodis macrocephalae: a review of photochemistry, pharmacokinetics and pharmacology. Pharmazie. 2020;75(2):42–55. doi: 10.1691/ph.2020.9738. [DOI] [PubMed] [Google Scholar]
- Santos R., Ursu O., Gaulton A., Bento A.P., Donadi R.S., Bologa C.G., Karlsson A., Al-Lazikani B., Hersey A., Oprea T.I., Overington J.P. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017;16(1):19–34. doi: 10.1038/nrd.2016.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder R., Schmidt J., Blattermann S., Peters L., Janssen N., Grundmann M., Seemann W., Kaufel D., Merten N., Drewke C., Gomeza J., Milligan G., Mohr K., Kostenis E. Applying label-free dynamic mass redistribution technology to frame signaling of G protein-coupled receptors noninvasively in living cells. Nat. Protoc. 2011;6(11):1748–1760. doi: 10.1038/nprot.2011.386. [DOI] [PubMed] [Google Scholar]
- Tan W., Li Y., Wang Y., Zhang Z., Wang T., Zhou Q., Wang X. Anti-coagulative and gastrointestinal motility regulative activities of Fructus Aurantii Immaturus and its effective fractions. Biomed. Pharmacother. 2017;90:244–252. doi: 10.1016/j.biopha.2017.03.060. [DOI] [PubMed] [Google Scholar]
- Tang Y., Wolk B., Nolan R., Scott C.E., Kendall D.A. Characterization of subtype selective cannabinoid CB2 receptor agonists as potential anti-inflammatory agents. Pharmaceuticals. 2021;14(4) doi: 10.3390/ph14040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y., Tonai-Kachi H., Shinjo K. 5-Nitro-2-(3-phenylpropylamino)benzoic acid is a GPR35 agonist. Pharmacology. 2008;82(4):245–249. doi: 10.1159/000157625. [DOI] [PubMed] [Google Scholar]
- Terzuoli E., Meini S., Cucchi P., Catalani C., Cialdai C., Maggi C.A., Giachetti A., Ziche M., Donnini S. Antagonism of bradykinin B2 receptor prevents inflammatory responses in human endothelial cells by quenching the NF-kB pathway activation. PloS One. 2014;9(1) doi: 10.1371/journal.pone.0084358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E., Ye F. Duplexed label-free G protein--coupled receptor assays for high-throughput screening. J. Biomol. Screen. 2008;13(10):975–985. doi: 10.1177/1087057108326141. [DOI] [PubMed] [Google Scholar]
- Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L., Dong J.P., Liang L.R., Li X.W., Hu K., He X.S., Sun Y.H., An Y., Yang T., Cao Z.X., Guo Y.M., Wen X.M., Wang Y.G., Liu Y.L., Jiang L.D. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann. Intern. Med. 2011;155(4):217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- Wang J., Chen L., Qu L., Li K., Zhao Y., Wang Z., Li Y., Zhang X., Jin Y., Liang X. Isolation and bioactive evaluation of flavonoid glycosides from Lobelia chinensis Lour using two-dimensional liquid chromatography combined with label-free cell phenotypic assays. J. Chromatogr. A. 2019;1601:224–231. doi: 10.1016/j.chroma.2019.04.073. [DOI] [PubMed] [Google Scholar]
- Wang R.Q., Yang S.J., Xie C.G., Shen Q.L., Li M.Q., Lei X., Li J.K., Huang M. Clinical observation of qingfei paidu decoction in treating COVID-19. Pharmacology and Clinics of Chinese Materia Medica. 2020;36(1):13–18. [Google Scholar]
- Wozniak D., Matkowski A. Belamcandae chinensis rhizome--a review of phytochemistry and bioactivity. Fitoterapia. 2015;107:1–14. doi: 10.1016/j.fitote.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Yang F., Dong X., Yin X., Wang W., You L., Ni J. Radix Bupleuri: a review of traditional uses, botany, phytochemistry, pharmacology, and toxicology. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/7597596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Liu H., Bai C., Wang Y., Zhang X., Guo R., Wu S., Wang J., Leung E., Chang H., Li P., Liu T., Wang Y. Chemical composition and pharmacological mechanism of qingfei paidu decoction and ma xing shi Gan decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol. Res. 2020;157 doi: 10.1016/j.phrs.2020.104820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Wang L.-q., Yuan B.-c., Liu Y. The pharmacological activities of licorice. Planta Med. 2015;81(18):1654–1669. doi: 10.1055/s-0035-1557893. [DOI] [PubMed] [Google Scholar]
- Ye F. Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev. Technol. 2006;4(5):583–595. doi: 10.1089/adt.2006.4.583. [DOI] [PubMed] [Google Scholar]
- Yu X., Sun S., Guo Y., Liu Y., Yang D., Li G., Lu S. Citri Reticulatae Pericarpium (Chenpi): botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J. Ethnopharmacol. 2018;220:265–282. doi: 10.1016/j.jep.2018.03.031. [DOI] [PubMed] [Google Scholar]
- Zhang X.L., Deng H.Y., Xiao Y.S., Xue X.Y., Ferrie A.M., Tran E., Liang X.M., Fang Y. Label-free cell phenotypic profiling identifies pharmacologically active compounds in two traditional Chinese medicinal plants. RSC Adv. 2014;4(50):26368–26377. [Google Scholar]
- Zhao J., Tian S., Lu D., Yang J., Zeng H., Zhang F., Tu D., Ge G., Zheng Y., Shi T., Xu X., Zhao S., Yang Y., Zhang W. Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine : Int. j. phytotherap. Phytopharmacol. 2020 doi: 10.1016/j.phymed.2020.153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Tian S.S., Yang J., Liu J.F., Zhang W.D. Investigating mechanism of Qing-Fei-Pai-Du-Tang for treatment of COVID-19 by network pharmacology. Chin. Tradit. Herb. Drugs. 2020;51(4):829–835. [Google Scholar]
- Zhou H., Peng X., Hou T., Zhao N., Qiu M., Zhang X., Liang X. Identification of novel phytocannabinoids from Ganoderma by label-free dynamic mass redistribution assay. J. Ethnopharmacol. 2020;246 doi: 10.1016/j.jep.2019.112218. [DOI] [PubMed] [Google Scholar]
- Zhou Y.Y., Gao W.Y., Gu X.R., Chen Z.Q., Zhao H.Y., Bian B.L., Yang L.X., Si N., Wang H.J., Tan Y. Identification and attribution of chemical constituents of Qingfei Paidu Decoction based on UHPLC-LTQ-Orbitrap-MS technology. China J. Chin. Mater. Med. 2020:1–15. doi: 10.19540/j.cnki.cjcmm.20200423.202. [DOI] [PubMed] [Google Scholar]
- Zhu X., Liu J., Chen S., Xue J., Huang S., Wang Y., Chen O. Isoliquiritigenin attenuates lipopolysaccharide-induced cognitive impairment through antioxidant and anti-inflammatory activity. BMC Neurosci. 2019;20(1):41. doi: 10.1186/s12868-019-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.