Abstract
Decellularization of xenogeneic heart valves might lead to excellent regenerative implants, from which many patients could benefit. However, this material carries various xenogeneic epitopes and thus bears a considerable inherent immunological risk. Here, we investigated the regenerative and immunogenic potential of xenogeneic decellularized heart valve implants using pigs deficient for the galactosyltransferase gene (GGTA1-KO) as novel large animal model. Decellularized aortic and pulmonary heart valves obtained from sheep, wild-type pigs or GGTA1-KO pigs were implanted into GGTA1-KO pigs for 3, or 6 months, respectively. Explants were analyzed histologically, immunhistologically (CD3, CD21 and CD172a) and anti-αGal antibody serum titers were determined by ELISA. Xenogeneic sheep derived implants exhibited a strong immune reaction upon implantation into GGTA1-KO pigs, characterized by massive inflammatory cells infiltrates, presence of foreign body giant cells, a dramatic increase of anti-αGal antibody titers and ultimately destruction of the graft, whereas wild-type porcine grafts induced only a mild reaction in GGTA1-KO pigs. Allogeneic implants, wild-type/wild-type and GGTA1-KO/GGTA1-KO valves did not induce a measurable immune reaction. Thus, GGTA1-KO pigs developed a ‘human-like’ immune response toward decellularized xenogeneic implants showing that immunogenicity of xenogeneic implants is not sufficiently reduced by decellularization, which detracts from their regenerative potential.
Keywords: αGal-KO pig, decellularization, heart valves, xenoantibodies, large animal model
Introduction
Recent advances in genetic engineering of pigs, improved immunosuppressive treatments and organ preservation techniques have enabled a resurgence of xenotransplantation, i.e. the live supporting discordant transplantation of porcine hearts into baboons [1] (reviewed in [2, 3]). A promising approach to make xenogeneic tissues available for clinical application is decellularization, which dramatically reduces antigenicity by removing major cellular components (reviewed in [4]). Xenogeneic, decellularized grafts such as heart valves or small intestinal submucosa patches might enable novel therapeutic strategies for multiple diseases (reviewed in [5]). Results from first clinical trials indicate that acellular (non-fixed) matrices are superior to the currently available glutaraldehyde-fixed bioprostheses [6, 7], because they might be remodeled by host cells and might resume regenerative capacity (reviewed in [8]). However, xenogeneic acellular (non-fixed) grafts, usually are rejected by the human immune system upon implantation as observed for two decellularized porcine heart valve products, Synergraft® [9] and Matrix P® [10, 11]. The reason, why xenogeneic implants induce a detrimental immune response has yet to be investigated. Preformed human antibodies recognizing matrix-bound xenoantigens that remain on the surface even after decellularization are thought to be critically involved in this process [12–14]. To become clinically applicable, xenoantigens have to be completely eliminated from decellularized xenogeneic matrices and those matrices have to be evaluated in suitable animal models for potential reaction with preformed xenoantibodies, also found in humans.
Carbohydrate xenoantigens, incl. αGal, N-glycolylneuraminic acid (Neu5Gc) or the Sda blood group antigen (SDa) can be removed by genetic engineering of the donor animal such that they lack any enzymes producing antigens [15, 16]. Recently, viable pigs with a genetic knockout of the GGTA1 (αGal), CMAH (Neu5Gc), B4GALNT2 (SDa) and SLA class I genes have been produced [17]. However, this straightforward genetic approach is only compatible with known antigens and there is considerable risk that neo-antigens will emerge because other enzymes will modify the now available glycans which otherwise would have been modified by the now inactivated enzymes [18]. Therefore, specific enzymatic treatments have been developed to remove or modify whole families of glycans, including xenoantigens [19]. Recently, we have shown that the number of N-linked glycans (including αGal positive glycans) present on wild-type (wt) porcine heart valve matrices can be significantly reduced by applying PNGaseF [20]. When implanted into wt sheep, PNGaseF treated decellularized porcine valves exhibited excellent hemodynamics and showed less inflammation than untreated decellularized valves [21]. However, unlike humans, sheep do not produce preformed anti-pig xenoantibodies and therefore the acute and hyperacute immune reaction observed in humans caused by preformed xenoantibodies cannot be investigated.
Baboons or other non-human primates (NHP) are considered to be the ultimate large animal model for xenotransplantation research, mainly based on the close phylogenetic relationship to humans, including the immunological system, and the presence of preformed anti-αGal and anti-Sda xenoantibodies. However, research with NHPs is significantly hampered by serious ethical concerns, high costs and limited availability that detracts from pre- and clinical testing of decellularized xenogeneic implants. Hence, a cost and time-effective, easy-to-handle, and ethically acceptable, clinically relevant large animal model is urgently needed.
Here, we hypothesized that pigs, deficient for the major xenoantigen αGal, will produce considerable amounts of preformed antibodies against αGal-positive ovine aortic and pulmonary heart valve conduits, thereby mimicking pig-to-human implantation and could therefore be used as suitable animal model for preclinical evaluation of the immunological potential of decellularized, PNGaseF treated xenogeneic and ovine aortic and pulmonary heart valve conduit implants.
Materials and methods
Study design
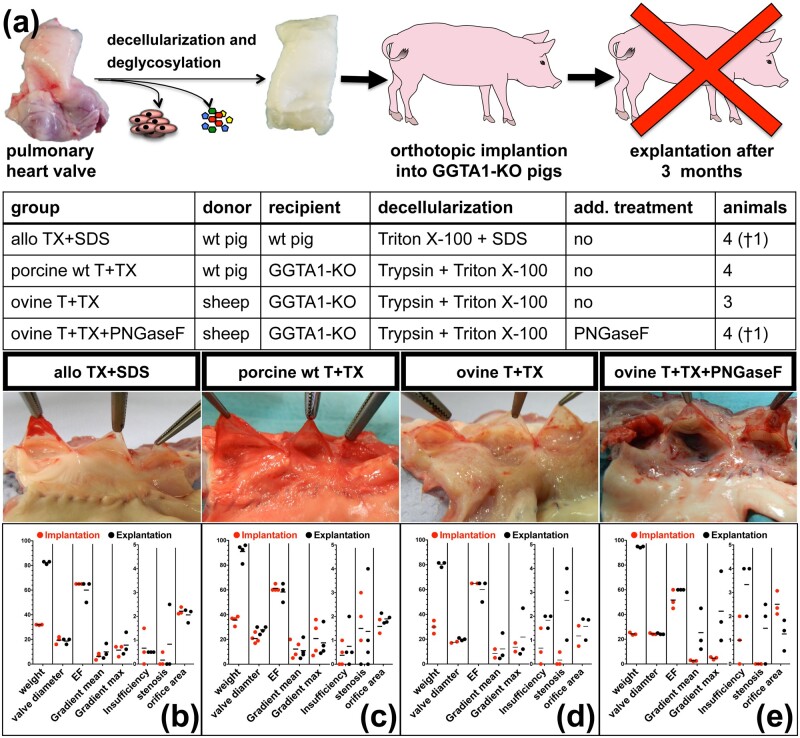
The investigation of decellularized cardiovascular implants using GGTA1-KO pigs as recipients was carried out in two steps. At first, implants derived from decellularized aortic heart valves and aortic tissue, isolated either from GGTA1-KO pigs, wt pigs or sheep (ovine), were implanted as aorto–aortic bypass into GGTA1-KO pigs for 3, or 6 months, respectively. Second, decellularized porcine (wt pig) and ovine (sheep) pulmonary heart valves were orthotopically implanted into GGTA1-KO pigs and their function evaluated after 3 months. To clarify if pigs would react to allogeneic decellularized implants in a similar way as sheep and humans do, decellularized wt pig pulmonary heart valves were implanted into wt pigs.
The experiments had been approved by an external ethics committee (Lower Saxony State Office for Consumer Protection and Food Safety, LAVES, Oldenburg/Germany; AZ 33.12-42502-04-14/1536).
Donor and recipient animals
Porcine wt (German Landrace) and ovine heart valves and aortae were harvested from local slaughterhouses. Homozygous GGTA1-KO donor and recipient pigs and all wt recipient pigs were German Landrace pigs from the Experimental herd of the Institute of Farm Animal Genetics in Mariensee (Germany) [22, 23] and were treated according to the German laws for animal welfare, and genetically modified organisms. Only pigs with a bi-allelic (homozygous) knockout of the GGTA1 gene (GGTA1-KO) were used in this study, the animals did not have any additional transgenes. These animals were initially produced by somatic cloning, followed by conventional breeding for propagation of the animals. We used a total of 21 male and female GGTA1-KO recipient pigs from different litters. The animals were 4–6 months of age for the aorto–aortic bypass model and 3 months of age for the pulmonary valve replacements.
Decellularization and manufacturing of implants
Aortic heart valves and aortae were decellularized employing 0.5% sodium dodecylsulfate (SDS) and 0.5% sodium deoxycholate (SD) as previously described [21]. Aortic bypass grafts were made from decellularized conduits (anulus, valve cusps, ascending aorta and part of the aortic arch) and individual pieces of the descending aorta (Table 1) [6, 24]. All branching points of arteries from the graft (mainly the coronary and carotid arteries) were closed via sutures (Fig. 1). The grafts were stored in PBS supplemented with antibiotics (1% penicillin/streptomycin and 1% gentamycin), until use.
Table 1.
Applied detergent- and enzyme-based decellularization protocols
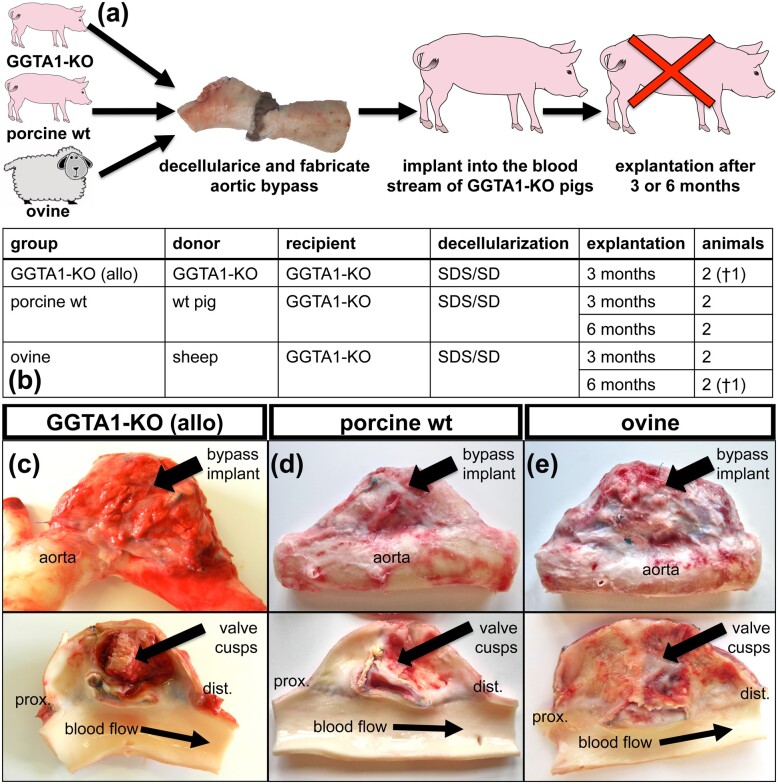
Figure 1.
(a) Experimental set-up of decellularized aorto–aortic bypass grafts made from aorta and aortic valves implanted into GGTA1-KO pigs. Decellularized grafts were implanted into the descending thoracic aorta, allowing continuous blood flow through the grafts and naive aorta. (b) 10 animals, distributed into 5 groups, underwent surgery and were explanted after 3 and 6 months, respectively. Two animals died shortly after surgery (†). Gross morphology of aorto–aortic bypass grafts derived from GGTA1-KO pigs (c), grafts derived from wt pigs (d) and ovine grafts (e) after 3 months in vivo.
For the allogeneic controls in wt pigs, wt pulmonary heart valves (PHV) were decellularized with Triton X-100 followed by SDS (TX+SDS) (Table 1). For implantation into GGTA1-KO pigs, ovine and wt porcine PHV were decellularized with 0.125% Trypsin/0.05% EDTA followed by TX (T+TX) (Table 1). Both decellularization methods had been successfully applied in a similar sheep implantation model [21].
PNGaseF and Benzonase digestion
Enzymatic treatment of decellularized PHV (dPHV) with PNGaseF and Benzonase was performed to remove N-linked glycans and residual nucleic acids. The dPHV were incubated in Benzonase buffer (50 mM TRIS, 6.1 mM MgCl2, pH 8.45) for 12 h for equilibrium and subsequently exposed to 150 ml Benzonase in Benzonase buffer (64 U/ml, Benzonase®, EMPROVE® bio, Merck, Darmstadt, Germany) for 12 h at 37°C. After four washing cycles in PBS (12 h each), the Benzonase treated dPHV were exposed to 50 ml PNGaseF (1000 U/ml, PNGaseF, NEB, Frankfurt, Germany) in G7 buffer for 24 h at 37°C. The enzyme treated dPHVs were subjected to nine washing cycles in PBS of 12 h each, supplemented with antibiotics (1% penicillin/streptomycin and 1% gentamycin) and then stored herein.
Quantification of DNA, hydroxyproline and GAGs
Ovine (n = 5) and porcine (n = 6) PHVs treated exactly like the putative implants were used for biochemical and histological characterization. To determine the effects of the PNGaseF treatment, Trypsin+TX dPHVs were cut into two pieces, one piece was treated with PNGaseF, while the other piece was further washed with PBS. PHVs [pieces of pulmonary artery (PA)] were frozen in liquid nitrogen, crushed, freeze-dried and were then used to quantify DNA, hydroxyproline and GAGs. Triplicates of 2–10 mg dried matrix granulates per heart valve were subjected to biochemical assays which are described in detail elsewhere [25]. Briefly, Hoechst 33258 was used to quantify DNA, hydroxyproline was quantified according to Stegemann et al. [26] and GAGs were measured according to Farndale et al. [27].
Quantification of αGal epitopes by inhibition ELISA of M86
Matrix resident αGal epitopes were quantified using a modified inhibition ELISA previously described [12]. Briefly, binding of anti-αGal antibody M86 (ALX-801-090-1, Enzo Life Sciences) to αGal-BSA coated (Galα1-3Galβ1-4GlcNAc-BSA, NGP1334, Dextra, Reading, UK) ELISA plates was inhibited by different amounts of decellularized matrices crushed in liquid nitrogen. The amount of matrix needed to bind 50% of the present M86 anti-αGal (IC50) was quantified for comparison. Low IC50 corresponds to high amounts of αGal epitopes, whereas high IC50 corresponds to a low amount of αGal epitopes.
Animal housing and medical treatment
Animals were acclimated to their environment in the experimental facility for 7 d prior to surgery and were fasted overnight prior to intervention. For anesthesia, a premedication mixture of Ketamine 10% (20.0 mg/kg bodyweight), Stresnil 4% (2.0 mg/kg bodyweight), Xylariem 2% (2.0 mg/kg bodyweight) and Atropine 1% (0.10 mg/kg bodyweight) was injected intramuscularly. Lidocaine spray was used before intubation. After induction, the animals were endotracheally intubated and anesthesia was maintained with the aid of isoflurane (2.0–3.0%), oxygen (1.8–2.5 l/min) and compressed air (0.8–1.5 l/min).
All animals received sufficient analgesia with Novacen 500 mg (50 mg/kg bodyweight) prior to the intervention and for an additional 6 d post-surgery. Furthermore, the animals got Buprenodale 0.3 mg (10 µg/kg bodyweight) for 3 d post-surgery. Antibiotic treatment with Floxibac 50 mg (2.5 mg/kg bodyweight) was maintained for 4 d post-surgery. All animals received an anticoagulation treatment starting 24 h after surgery: Fragmin 5000 I.E. (0.2 ml/d, s.c.) for 14 d and ASS (500 mg/d, p.o.), until euthanasia and autopsy.
Surgery
In the first experimental group, an aortic bypass made from decellularized aorta and aortic heart valve was sutured into the descending thoracic aorta. The thorax cavity was opened via left lateral thoracotomy. The dorsal half of the descending aorta was partially clamped using a bow-shaped aortic-anastomosis clamp, incised using a scalpel blade and the graft (aorta) was connected to the aorta using a running suture. The graft was now cross-clamped and the aortic clamp was opened to allow blood flow into the graft. The procedure was repeated with the distal anastomosis and the graft evacuated from air, before the clamps were removed.
In the second experiment, the surgical procedure applied to orthotopically replace the PHV was adapted from previous studies in sheep and had been reported previously from our group [28, 29]. Briefly, all three native pulmonary valve cusps were removed, and the dPHV grafts were orthotopically implanted (in supravalvular position) using running sutures.
Blood was collected at 0, 2, 5, 8, 12, 15, 21, 28, 56, 90 days, respectively, and in two cases 180 days after surgery.
Transesophageal echocardiography and explantation
To investigate the physiological function of dPHV implants, transesophageal echocardiography (TEE) was performed shortly after implantation and then immediately prior to explantation 3 months post-surgery. The same physician performed all examinations. The following parameters were determined: Diameter [mm] (annular diameter), orifice area [mm2], ejection fraction (EF) [%], mean valvular gradient [mmHg], maximum valvular gradient [mmHg], grade of insufficiency [0°–4°] and grade of stenosis [0°–4°]. Immediately after TEE, the sedated animals were euthanized, hearts were excised and the grafts were dissected ex vivo. After macroscopic examination, the graft was split into several pieces for further analysis.
Histological analysis and immunofluorescence staining
For paraffin embedding and sectioning, three separate pieces containing cusps, heart muscle and aorta/PA per explant and specimen of all anastomosis were prepared. Calcified specimens were decalcified prior to embedding, using decalcifier soft solution (6484, Carl Roth, Karlsruhe, Germany). Sections of 2 µm thickness were cut and stained according to standard procedures using Hematoxylin and Eosin (H&E) and Elastica van Gieson (EvG) stain.
For immunofluorescent analyses, one piece containing cusps, heart muscle and aorta/pulmonary artery per explant and specimen from all anastomoses was embedded and frozen in Tissue-Tek O.C.T. (Sakura, Alphen aan den Rijn, The Netherlands). Cryosections of 10 µm thickness were fixed in acetone at −20°C for 8 min. To prevent unspecific antibody binding cuts were blocked with 10% donkey serum/PBS solution (D9663, Sigma-Aldrich, Munich, Germany) for 1 h. Primary antibodies: rabbit anti-CD3 (1:500, A0452, Dako), biotinylated mouse anti-CD21 (1:100, NBP1-28247, clone BB6-11C9.6, Novus, Wiesbaden, Germany), mouse anti-CD172a (1:100, MCA2312, clone BL1H7, BioRad, Puchheim, Germany), and secondary antibodies: Cy3 donkey anti-rabbit IgG (1:300, 711-165-152, Jackson ImmunoResearch, Pennsylvania, USA) Cy3 donkey anti-mouse IgG (1:300, 715-165-150, Jackson ImmunoResearch) and AvidinD-Texas Red (1:300, A-2006, Vector laboratories, Burlingame, USA) were diluted in 10% donkey serum/PBS and applied for 2 h at RT and o.n. at 4°C, respectively. Nuclei were counterstained using DAPI (Invitrogen) and mounted using ShandonTM Immu-Mount (Thermo Fischer Scientific, Waltham, USA).
Quantitative evaluation of histological images
Infiltration of grafts by inflammatory cells, foreign body giant cells and blood-filled capillaries was assessed on H&E-stained sections made from three individual pieces of the graft. The adventitia, intima and sinus region of the PA and the heart valve cusps were graded separately. The arbitrary score used was 0 = no cells or vessels, 1 = few interspersed cells or vessels, and 2 = many cells or vessels. Values for each part of the grafts were added and displayed as stacked bar graph.
Pig anti-αGal ELISA
Porcine anti-αGal IgG were quantified using a modified ELISA, based on a previously published human anti-αGal ELISA [30]. A biotin conjugated goat anti-porcine IgG antibody (ABIN637873, antibodies-online, Aachen, Germany) diluted 1:40 000 in 1% BSA/PBS was used to detect porcine antibodies. To normalize the samples, one serum was selected as standard and simultaneously run on all plates. The standard was used to calculate arbitrary units, given 100 000 units/ml for the standard.
Results
Aortic bypass in GGTA1-KO pigs
Surgeries and post-operative development
A total of 10 GGTA1-KO pigs underwent successful aortic bypass implantation without any complications. Two pigs died 5 and 9 days, respectively, after surgery (overview in Fig. 1a and b). One pig had an insufficient anastomosis between graft and aorta causing bleeding and the other animal died suddenly with an edema in the left lung. The remaining pigs developed normally.
Macroscopical appearance
The pigs were euthanized three months after implantation, all grafts were covered by fibrotic tissue and in some cases, the graft strongly adhered to lung tissue (Fig. 1c–e). All grafts had become partially, or even completely stenotic. Allogeneic GGTA1-KO grafts exhibited a shiny and smooth surface and an intact aortic tissue (Fig. 1c).
All four porcine wt explants (n = 2 after 3 months and n = 2 after 6 months), exhibited a smooth and shiny surface, an intact aortic tissue similar to the allogeneic GGTA1-KO graft (Fig. 1d). The three ovine grafts explanted after 3 months (n = 2), or 6 months (n = 1), were completely deformed. After 3 months, the luminal surface was covered by thick and rough fibrotic tissue (Fig. 1e). The aortic tissue looked yellowish and was partially dissolved. After 6 months in vivo, the wall of the aorta appeared very thin and a large cavern had formed distal to the proximal anastomosis (SupplementaryFig. S1).
Histology
H&E stain revealed extensive cellular repopulation in all decellularized grafts. The allogeneic GGTA1-KO implants exhibited the lowest density of cells, especially the media of the aorta was only partially repopulated (Fig. 2a and b). The cells displayed a fibroblast-like morphology. Porcine implants derived from wt pigs showed a higher degree of repopulation, predominantly with mesenchymal and inflammatory cells (Fig. 2c and d). The sheep derived implants had the highest density of cells, with inflammatory cells being most prominent (Fig. 2e and f).
Figure 2.
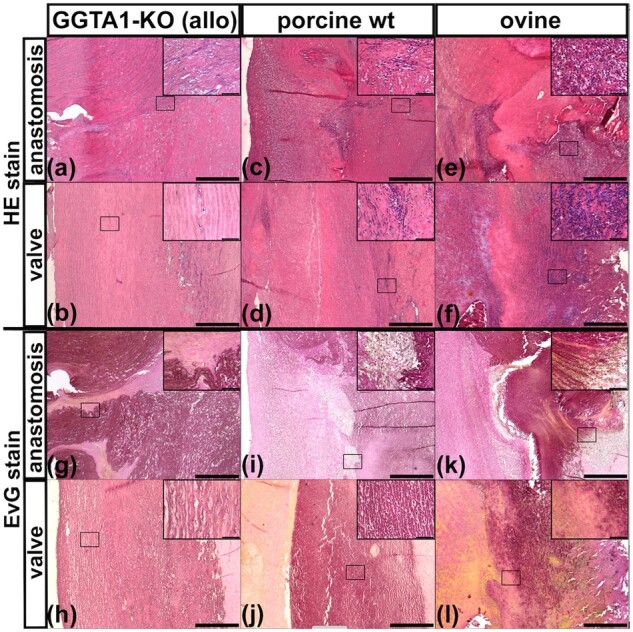
Histology of explanted aortic bypasses after 3 months in vivo. (a–f) H&E staining to visualize cells and (g–l) Elastica van gieson (EvG) staining to visualize elastic fibers and other ECM components. Position of figure depicted in inserts is marked by a black rectangle. Cuts made from graft to recipient anastomosis (anastomosis) and of the graft at the level of the former aortic valve (valve) have been stained and are depicted here. (a, b, g, h) Porcine graft derived from GGTA1-KO pig. (c, d, i, j) Porcine graft derived from wt pig. (e, f, k, l) Ovine xenogeneic graft derived from sheep. Scale bars are 500 µm and 50 µm for inserts.
The extracellular matrix (ECM) was mainly composed of intact elastic fibers, and appeared dark with Elastica van Gieson stain, in both the allogeneic GGTA1-KO (Fig. 2g and h) and porcine wt (Fig. 2i and j) explants. In xenogeneic ovine explants, only patches of intact elastic fibers could be found, whereas most of the tissue was composed of fibrotic tissue (Fig. 2k). In addition, a yellowish substance, presumably fibrin, was found on all explants (Fig. 2l). After 6 months in vivo, fewer immune cells could be found in the wt pig and sheep derived implants. The single ovine graft showed herds of calcification (SupplementaryFig.S2).
Orthotopic replacement of PHVs
Characterization of decellularized implants
The main purpose of this study was to develop an animal model for preclinical and functional testing of heart valve implants and the initial experiment shown above, had revealed that 3 months were sufficient to observe changes of the valves and hemodynamics initiated by the inflammatory processes.
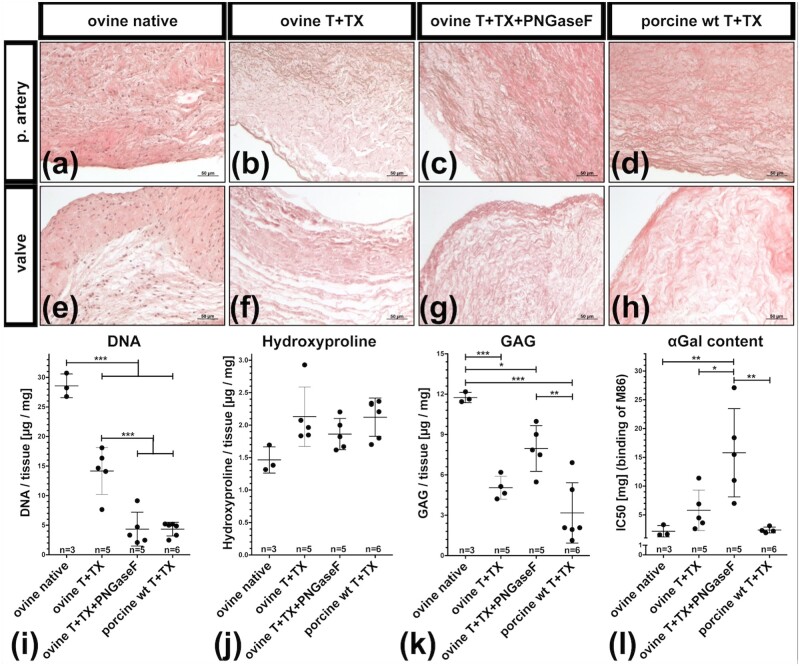
Histological evaluation of ovine and porcine PHVs decellularized by Trypsin+TX showed that intact cells could not be found in the valves’ cusps and pulmonary arteries (Fig. 3a–h). Quantification of DNA revealed that the decellularized matrices contained much less DNA when compared to native ovine PHVs (28.6 ± 2.0 µg/mg) (Fig. 3i). After PNGaseF treatment, the amount of DNA was reduced from 14.2 ± 4.0 µg/mg to 4.3 ± 2.9 µg/mg, which was comparable to porcine dPHVs (4.3 ± 1.2 µg/mg). The hydroxyproline content slightly increased, whereas GAG content decreased due to decellularization (Fig. 3j and k). Amounts of αGal epitopes were also reduced after decellularization, as indicated by an increased IC50 in the inhibition ELISA. Native ovine and decellularized porcine PHVs contained the highest amount of αGal epitopes, coinciding with a very low IC50 (2.2 ± 1.0 mg, respectively, 2.4 ± 0.5 mg, Fig. 3l). Ovine dPHVs contained slightly fewer αGal epitopes (IC50 = 5.8 ± 3.5), which were further reduced by the PNgaseF treatment (IC50 = 15.8 ± 7.7 mg). Graphs used to calculate the IC50 are depicted in SupplementaryFig.S3.
Figure 3.
Histological and biochemical characterization of decellularized valves (a–h) H&E-stained sections showing pulmonary arteries (p. artery) and valve cusps (valve) of native ovine PHVs (a, e), trypsin+TX decellularized ovine PHVs before (b–f) and after (c–g) PNGaseF treatment and trypsin+TX decellularized porcine PHVs (d, h). DNA (i), hydroxyproline (j) and GAG (k) contents were quantified in freeze-dried tissues. (l) The αGal epitopes in fresh tissues are depicted as IC50, determined an inhibitory ELISA. Low IC50 corresponds to high amounts of αGal, whereas high IC50 corresponds to low amounts of αGal epitopes present in the tested tissue. (i–l) Each dot represents an individual biological replicate. Scale bars represent 50 µm.
Surgery and post-operative development
The first aorto–aortic bypass experiment revealed that a 3 months observation period was sufficient to uncover antigenicity mediated changes of the decellularized xenogeneic implants. Therefore, in the second experiment, all animals were explanted after 3 months. A total of 15 pigs underwent orthotopic heart valve replacement. One pig died 7 days after surgery, due to bleeding and/or a pericardial tamponade. Another pig had to be euthanized 32 days after surgery and postmortem examination revealed a severe (almost complete) stenosis of the implanted pulmonary heart valve, caused by thrombus formation. The other 13 pigs survived without complications three months after surgery until euthanasia. Venal catheters used for regular blood collection were removed few days after surgery in all pigs. After surgery, animals showed normal weight gain, starting from 30.4 ± 4.7 kg and reaching 87.1 ± 6.9 kg after 3 months (i.e. euthanasia) (SupplementaryTableS4).
TEE analysis
TEE analysis revealed a normal EF of 60% in all animals shortly after implantation and for 9 out of 12 pigs also at the time of slaughter (Fig. 4), the remaining three pigs exhibited a reduced EF of 50% (Fig. 4a, b, and d). Two of three wt implants into wt hosts maintained a normal physiological function (Fig. 4a). However, one implant, which was strongly attached to the thoracic cavity, became stenotic, resulting in increased valvular gradients and reduced orifice area as well EF (Fig. 4a).
Figure 4.
(a) Schematic depiction of the experimental set-up, gross morphology and TEE results of dPHVs, orthotopically implanted into pigs for 3 months. TEE analysis was performed shortly after implantation and immediately prior to death. Valve diameter [mm], ejection fraction (EF) [%], transvalvular gradients (mean and max) [mmHG], grade of insufficiency and stenosis [0–4, 4 = total] and orifice area [cm2] were determined. (b) Porcine pulmonary grafts derived from wt pigs and decellularized with the aid of TX+SDS after 3 months implanted into wt pigs (allo) TX+SDS). (c) porcine pulmonary heart valves obtained from wt pigs and decellularized with the aid of T+TX 3 months after implantation into GGTA1-KO pigs (porcine wt T+TX). (d) xenogeneic ovine PHVs decellularized with T+TX after 3 months implanted into GGTA1-KO pigs (ovine T+TX). (e) xenogeneic ovine PHVs decellularized with T+TX and additionally treated with PNGaseF 3 months after implantation into GGTA1-KO pigs (ovine T+TX+PNGaseF).
With the exception of the wt into GGTA1-KO pig group, which showed an EF increase from 20.8 ± 3.9 mm to 27.8 ± 2.9 mm (Fig. 4b), the valve diameter remained unchanged in the other groups (Fig. 4c–e). The increased diameter found in the wt into GGTA1-KO group resulted in decreased valvular gradients (mean: 11.0 ± 7.5 mmHg, max: 17.3 ± 11.7 mmHg); gradients were relatively high immediately after implantation (mean: 12.3 ± 6.9 mmHg, max: 20.8 ± 14.0 mmHg). Three out of four porcine wt implants into GGTA1-KO hosts maintained normal function at termination of the experiment, while one implant became stenotic and non-functional (Fig. 4b). All six xenogeneic, ovine derived implants decellularized with T+TX with or without PNGaseF and implanted into GGTA1-KO pigs, became non-functional and five of six were even stenotic (Fig. 4d and e). Decellularized xenogeneic valves additionally treated with PNGaseF showed even higher gradients (mean: 25.3 ± 17.4 vs. 12.2 ± 11.2, max: 42.7 ± 29.0 vs. 21.7 ± 20.2 mmHg) and a higher degree of insufficiency (1.8 ± 0.3 vs. 0.5 ± 0) compared to PNGaseF untreated ovine implants (Fig. 4d and e). Individual TEE data are listed for each animal in Supplementary Table S4.
Macroscopical appearance
After 3 months, wt grafts from the wt hosts (allogeneic control) exhibited thin, translucent and pliable leaflets as well a white, smooth and shiny surface of the pulmonary artery (Fig. 4b). Porcine wt grafts from GGTA-1 hosts exhibited slightly reddish and thickened leaflets. PAs itself exhibited a slightly reddish, but overall smooth surface (Fig. 4c). Small thrombotic adhesions were detected within the sinus of the cusps and in two of four valves also at the site of the anastomosis. Two of three ovine T+TX grafts exhibited translucent and pliable leaflets, whereas one valve had degenerated and calcified leaflets. In all three valves, the pulmonary artery was altered and appeared fibrotic (Fig. 4d). Ovine grafts additionally treated with PNGaseF exhibited a similar phenotype as the altered pulmonary artery, whereas all leaflets appeared to be calcified and covered with thrombotic adhesions (Fig. 4e).
Histological analysis
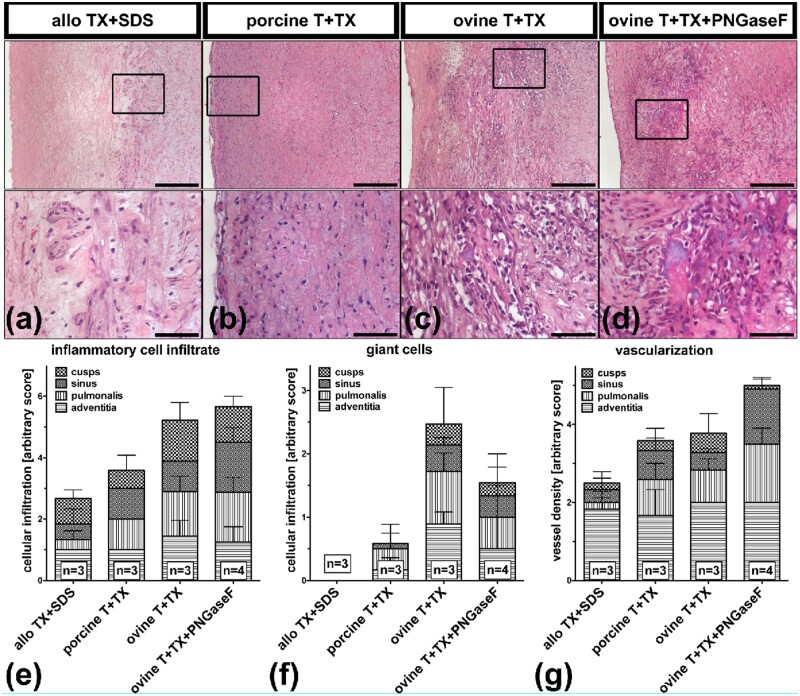
All decellularized implants were at least partially repopulated by host cells after 3 months in vivo. Allogeneic control implants exhibited cellular repopulation of the luminal surfaces and of the intimal tissue of cusps and PA. However, intimal repopulation was not complete and most pronounced close to the adventitia (Fig. 5a). In porcine wt grafts, implanted into GGTA1-KO pigs, cells were found throughout the entire PA (Fig. 5b). Xenogeneic ovine implants untreated or treated with PNGaseF had the highest density of cells, with clusters of cells within the PA, sinus and even cusps of the implants (Fig. 5c and d). In the allogeneic controls, most of the repopulating cells exhibited a mesenchymal phenotype (Fig. 5e), whereas in porcine wt and xenogeneic ovine explants abundant inflammatory cells were observed (Fig. 5e). Abundant foreign body giant cells were detected in all ovine explants, fewer in porcine wt explants, but not in allogeneic control explants (Fig. 5f). Compared to allogeneic controls, porcine wt explants exhibited more blood-filled capillaries and ovine explants had even a higher number of capillaries, mainly within the PA and the sinus region (Fig. 5g).
Figure 5.
Histology of decellularized ovine PHV orthotopically implanted into GGTA1-KO pigs. dPHV derived from wt pigs that were implanted into wt pigs as allogeneic controls are also shown. (a–d) H&E-stained paraffin sections depicting the PA of the explanted grafts. Position of inlet-contents is marked in the corresponding low magnification picture using a rectangular. Scale bars are 500 µm and 50 µm (inlet). (a) Allogeneic pulmonary graft derived from wt pigs and decellularized with TX+SDS 3 months after implantation into wt pigs (allo TX+SDS). (b) porcine PHV obtained from wt pigs and decellularized with T+TX 3 months after implantation into GGTA1-KO pigs (porcine wt T+TX). (c) xenogeneic ovine PHV decellularized using T+TX 3 months after implantation into GGTA1-KO pigs (ovine T+TX). (d) xenogeneic ovine PHV decellularized with the aid of T+TX and additionally deglycosylated with PNGaseF 3 months after implantation into GGTA1-KO pigs (ovine T+TX+PNGaseF). (e–g) H&E-stained sections of the explants were visually inspected for the presence of inflammatory cells, foreign body giant cells (giant cells) and newly formed blood-filled capillaries (vascularization). Cellular infiltrates (inflammatory cells, foreign body giant cells) and newly formed blood-filled capillaries (vascularization) of the explants cusps, sinus, PA and adventitia were scored according to an arbitrary score (0 = no cells, 1 = few cells or 2 = many cells). For each explant, three individual sections were scored. The numbers (n) displayed represent the number of animals per group. Mean values and error bars (SD) are displayed in stacked bar graph.
Identification of infiltrating cells
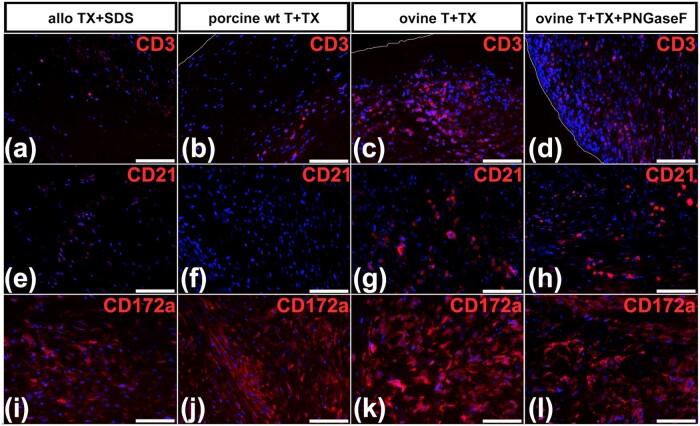
Only few CD3-positive T cells were found in allogeneic controls (Fig. 6a) and porcine wt (Fig. 6b) explants. In xenogeneic ovine explants, abundant CD3 positive cells could be detected (Fig. 6c and d). Fewer CD21-positive B cells were observed within a similar pattern (Fig. 6e–h). Abundant CD172a-positive myeloid cells were identified in all explants (Fig. 6i–l), with the highest density in xenogeneic explants (Fig. 6k and l).
Figure 6.
Immunofluorescence analysis of explanted grafts. All pictures feature a part of the PA of the explants. (a–d) Anti-CD3 (T cells), (e–h) anti CD21 (B cells) and (i–l) anti-CD172a (myeloid cells) antibodies were used for staining. (b, c, d) White lines indicate the luminal surface of the grafts. Scale bars represent 50 µm. (a, e, i) Representative pictures of allogeneic pulmonary graft tissue derived from wt pigs and decellularized with TX+SDS after 3 months (allo TX+SDS). (b, f, j) representative pictures of porcine pulmonary heart valves obtained from wt pigs and decellularized with T+TX 3 months after implantation into GGTA1-KO pigs (porcine wt T+TX). (c, g, k) representative pictures of xenogeneic ovine pulmonary heart valves decellularized using T+TX 3 months after implantation into GGTA1-KO pigs (ovine T+TX). (d, h, l) representative pictures of xenogeneic ovine pulmonary heart valves decellularized with T+TX and additionally deglycosylated with the aid of PNGaseF 3 months after implantation into GGTA1-KO pigs (ovine T+TX+PNGaseF).
Quantification of anti-αGal antibodies
Monitoring of anti-αGal antibodies in GGTA1-KO pigs treated with GGTA1-KO implants (allo) revealed that anti-αGal remained at a constantly low titer of about 500 for the entire duration of the experiment (Fig. 7a). Animals treated with wt porcine (allogeneic but αGal-positive) implants showed a slight increase of anti-αGal antibodies within 6–10 days post-surgery with maximum titers of ∼1000 (Fig. 7a and b). A dramatic increase of anti-αGal titers with peaks between 4000 and 8000 occurred around day 20 after surgery in all GGTA1-KO pigs implanted with xenogeneic ovine implants (Fig. 7a and b). The additional PNGaseF treatment of ovine implants did not affect anti-αGal antibody titers. In general, animals treated with aortic bypasses showed a stronger reaction as those treated with pulmonary valves. In the present study, all pigs were 3 months old or even older, and exhibited similar levels of anti-αGal antibodies that did not further increase after implantation of αGal-negative implants (Supplementary Fig. S5). Therefore, the increase of anti-αGal antibodies was caused by the implants.
Figure 7.
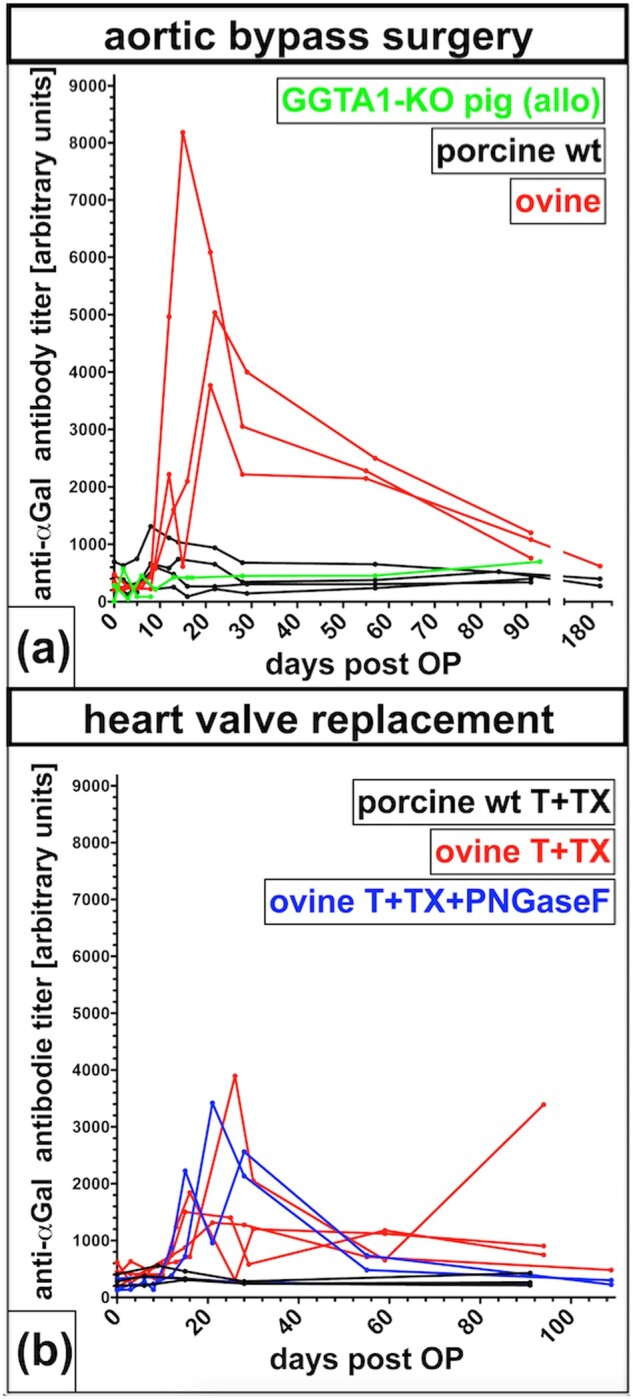
Anti-αGal IgG measured in sera of GGTA1-KO pigs. (a) GGTA1-KO pigs, which underwent aortic bypass surgery with decellularized grafts obtained from either GGTA1-KO pigs (allo), wt pigs (porcine wt) or sheep (ovine). (b) GGTA1-KO pigs with orthotopic replacement of the pulmonary heart valves decellularized with T+TX obtained from either wt pigs (porcine wt T+TX) or sheep (ovine T+TX) and ovine T+TX decellularized grafts additionally deglycosylated with PNGaseF (ovine T+TX+PNGaseF).
Discussion
Antigenicity and immunogenicity are major hurdles for a putative clinical application of xenogeneic implants, incl. heart valves, but are neglected in most studies. One of the reasons might be the lack of a reliable, cost efficient and practical large animal model that is able to display a human-like immune response toward the xenogeneic implants. The immunological response of GGTA1-KO pigs toward xenogeneic and αGal-positive decellularized ovine heart valves observed in the present study was largely similar to the human immune response toward decellularized porcine SynergraftTM heart valves [9] and was therefore called a human-like rejection response.
Here, we report for the first time the use of genetically modified GGTA1-KO pigs as suitable large animal model, and provide compelling evidence that in contrast to previous experiments using classical, i.e. mainly non-transgenic large animal models, decellularized xenogeneic and αGal-positive heart valves fail upon implantation due to massive immune reactions.
The immunological response of GGTA1-KO pigs toward xenogeneic and αGal-positive decellularized ovine heart valves observed in the present study was largely similar to the human immune response toward decellularized porcine SynergraftTM heart valves [9]. Simon et al. found massive infiltrations of granulocytes and macrophages already 2 and 7 days after implantation of decellularized porcine grafts. One graft explanted 1 year after implantation exhibited additional infiltrations with lymphocytes, massive neovascularization and formation of a pseudointima [9]. These typical features of an adaptive immune reaction and acute humoral, foreign body type reaction could also be observed against xenogeneic ovine, but not allogeneic or allogeneic and αGal-positive grafts in the present study.
The absence of an adaptive immune response in GGTA1-KO pigs exposed to allogeneic porcine, but αGal-positive grafts, might also explain the weak anti-αGal antibody response observed in these animals. The observed peaks of IgG already 14 days after implantation indicated that in this setting, the anti-αGal immune response involved immune cells directed against αGal epitopes still present on the valves. In contrast, after implantation of xenogeneic and αGal-positive ovine implants, anti-αGal antibodies peaked after 30 days indicating the generation of additional anti-αGal directed B cells that were not yet present at the time of implantation. Similar observations have been made in GGTA1-KO mice immunized with allogeneic or xenogeneic αGal-positive cells. The critical involvement of T-cells was crucial for the xenograft response, but was completely lacking in mice exposed to αGal epitopes present on allogeneic cells [31].
The observed acute and chronic xenograft responses revealed that the immunological processes observed in humans studies using decellularized porcine heart valves and in pig-to-baboon xenotransplantation experiments are fully recapitulated in GGTA1-KO pigs (reviewed in [32]). Classical animal models, such as the domestic sheep, used to study the function of cardiovascular implants usually do not have preformed antibodies and thus do not show a hyperacute rejection of porcine organs [33], Furthermore, sheep exhibited only a mild immune reaction when challenged with a xenogeneic porcine decellularized heart valve [21]. Overall, this indicates that results obtained in GGTA1-KO pigs are much closer to clinical reality as results obtained in sheep experiments.
It is conceivable that for some studies, genetically engineered large farm animals could replace NHPs, thus allowing for example, to study the effects of decellularization or enzymatic modifications on immunogenicity, mediated by carbohydrate antigens. However, it remains to be determined if also other genetically engineered farm animals, such as the domestic sheep could be used as predictive large animal model. Here, we observed the typical rapid growth of the recipient pigs, which enforced the implanted heart valves to rapid adaptation. Sheep grow much slower as pigs and have emerged as an established model for studying cardiovascular implants. This, together with the fact that most xenogeneic implants are of porcine origin, could favor genetically modified sheep as useful animal model for functional testing of xenogeneic cardio vascular implants.
Current protocols for the production of genetically modified farm animals are nowadays compatible with targeting essentially any gene and the known xenoantigens, incl. αGal, Neu5Gc or SDs antigen, can be reliably be deleted by modern gene editing protocols. Tissue from these donor pigs will likely form a major source for future clinical application of porcine xenografts. However, numerous other, still unknown xenoantigens do exist, with various carbohydrates being most prominent. This requires new strategies to remove the entire population of carbohydrates to abolish antigenicity of xenogeneic materials. In previous in vitro experiments, we demonstrated that the PNGaseF treatment reduced the number of carbohydrates, including αGal epitopes [20]. However, the present in vivo study revealed that the PNGaseF treatment did not have any beneficial effects, most likely because the number of the remaining antigens was too high. Therefore, further treatments, targeting other antigens, not just N-linked antigens, have to be developed in order to arrive at immune tolerant porcine heart valves.
Here, we report for the first time the use of GGTA1-KO pigs as humanized large animal model with promising features for the preclinical testing of xenogeneic decellularized heart valves to significantly prolong long-term survival in human patients.
However, several limitations were also apparent from the present results. As the main purpose of the aorto–artic-bypass model was to determine the ‘optimal’ observation period, we used our well-established SDS/SD as decellularization protocol. For the pulmonary valve replacement, we used T+TX, because it had turned out to be a better decellularization protocol. Unfortunately, the usage of different decellularization protocols limits the comparability between both study groups. Furthermore, wt pigs were used as recipients and donors for allogeneic pulmonary heart valve implants and these valves were treated with a different decellularization protocol. The purpose of including an allogenic group was to investigate if allogeneic decellularized pulmonary heart valves also have normal functional properties when implanted into pigs as we observed in humans or sheep. The present results show that this is the case and we speculate that the reaction of GGTA1-KO pigs to allogeneic implants will not differ from the reaction of wt pigs.
In the present study, the allogeneic, αGal positive wt pig derived heart valves were implanted into GGTA1-KO pigs to elucidate the role of preformed anti-αGal antibodies in this specific animal model. However, the reaction of wt pigs toward ovine decellularized heart valves would have been the best possible control to discriminate changes made by the GGTA1-KO. We can only speculate that the reaction would be as mild as observed in pig-to-sheep experiments [21].
Furthermore, we acknowledge that the GGTA1-KO animals used in this study were a rather heterogenous group of animals initially produced by somatic cloning, followed by conventional breeding that came from different litters (SupplementaryTable S6). When looking into a possible correlation of anti-αGal antibody titers, litter and experimental group, we found that in most cases animals from each study group were siblings exhibiting similar titers of anti-αGal antibodies at day 0 that in turn differed between litters and therefore study groups (SupplementaryTableS6). Nevertheless, we think that maternal antibodies did not significantly affect the titers of antibodies, because the anti-αGal titers at day 30 did not correlate with titers observed at day 0. This may have increased the variability of the current data and in the future, an even better defined genetic background could be used to further substantiate our findings.
Conclusion
In conclusion, results from the present study provide compelling evidence that genetically modified GGTA1-KO pigs mimic acute and chronic pig-to-human xenograft rejection processes to a large extent and show that decellularization in combination with PNGaseF treatment is not sufficient to reduce the antigenicity of animal derived heart valves to an acceptable level for clinical application.
Supplementary data
Supplementary data are available at REGBIO online.
Supplementary Material
Acknowledgments
We are very grateful to the staff of the Institute in Mariensee and at HTTG-MHH, including Iris Stelter, Rita Lechler, Ronald Wittig, Meike Stuenkel, Willi Hasselbring, Isabel Smart, Doreen Lenz, Karolina Theodoridis, Astrid Diers-Ketterkat, Karin Peschel, Mikhail Magdei and Leonie Gädert, for their competent and enthusiastic contribution in the present work. We acknowledge the excellent support from the pathologist Dr. Sabine Delventhal.
Funding
This work was funded by the Fördergemeinschaft Deutsche Kinderherzzentren e. V., the Deutsche Herzstiftung e. V., and the German Research Foundation DFG via the Cluster of Excellence ‘From regenerative biology to reconstructive therapy’ (REBIRTH) and via projects B1 and C7 of TRR127 (Biology of xenogeneic cell and organ transplantation—from bench to bedside).
Conflict of interest statement. A.H. is shareholder of Corlife oHG, a company producing decellularized human heart valves using one of the decellularization protocols described in the manuscript. All other authors declare to have no conflict of interest.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplemental information files. The reader may contact the corresponding authors with any request.
References
- 1. Langin M, Mayr T, Reichart B. et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 2018;564:430–3. [DOI] [PubMed] [Google Scholar]
- 2. Cowan PJ, Tector AJ.. The resurgence of xenotransplantation. Am J Transplant 2017;17:2531–6. [DOI] [PubMed] [Google Scholar]
- 3. Perkel JM. Xenotransplantation makes a comeback. Nat Biotechnol 2016;34:3–4. [DOI] [PubMed] [Google Scholar]
- 4. Wong ML, Griffiths LG.. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta Biomater 2014;10:1806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salvatori M, Peloso A, Katari R. et al. Semi-xenotransplantation: the regenerative medicine-based approach to immunosuppression-free transplantation and to meet the organ demand. Xenotransplantation 2015;22:1–6. [DOI] [PubMed] [Google Scholar]
- 6. Horke A, Tudorache I, Laufer G. et al. Early results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement: the ARISE study and ARISE Registry data. Eur J Cardiothorac Surg 2020;58:1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boethig D, Horke A, Hazekamp M. et al. A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR Trial and ESPOIR Registry data. Eur J Cardiothorac Surg 2019;56:503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouten CVC, Smits A, Baaijens FPT.. Can we grow valves inside the heart? Perspective on material-based in situ heart valve tissue engineering. Front Cardiovasc Med 2018;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simon P, Kasimir MT, Seebacher G. et al. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 2003;23:1002–6. discussion 1006. [DOI] [PubMed] [Google Scholar]
- 10. Perri G, Polito A, Esposito C. et al. Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur J Cardiothorac Surg 2012;41:1320–5. [DOI] [PubMed] [Google Scholar]
- 11. Ruffer A, Purbojo A, Cicha I. et al. Early failure of xenogenous de-cellularised pulmonary valve conduits–a word of caution. Eur J Cardiothorac Surg 2010;38:78–85. [DOI] [PubMed] [Google Scholar]
- 12. Ramm R, Niemann H, Petersen B. et al. Decellularized GGTA1-KO pig heart valves do not bind preformed human xenoantibodies. Basic Res Cardiol 2016;111:39. [DOI] [PubMed] [Google Scholar]
- 13. Bastian F, Stelzmuller ME, Kratochwill K. et al. IgG deposition and activation of the classical complement pathway involvement in the activation of human granulocytes by decellularized porcine heart valve tissue. Biomaterials 2008;29:1824–32. [DOI] [PubMed] [Google Scholar]
- 14. Kasimir MT, Rieder E, Seebacher G. et al. Presence and elimination of the xenoantigen gal (alpha1, 3) gal in tissue-engineered heart valves. Tissue Eng 2005;11:1274–80. [DOI] [PubMed] [Google Scholar]
- 15. Wang R-G, Ruan M, Zhang R-J. et al. Antigenicity of tissues and organs from GGTA1/CMAH/beta4GalNT2 triple gene knockout pigs. J Biomed Res 2018;33:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Estrada JL, Martens G, Li P. et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischer K, Rieblinger B, Hein R. et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation 2020;27:e12560. [DOI] [PubMed] [Google Scholar]
- 18. Ariyoshi Y, Takeuchi K, Pomposelli T. et al. Antibody reactivity with new antigens revealed in multi-transgenic triple knockout pigs may cause early loss of pig kidneys in baboons. Xenotransplantation 2021;28:e12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. LaVecchio JA, Dunne AD, Edge AS.. Enzymatic removal of alpha-galactosyl epitopes from porcine endothelial cells diminishes the cytotoxic effect of natural antibodies. Transplantation 1995;60:841–7. [PubMed] [Google Scholar]
- 20. Findeisen K, Morticelli L, Goecke T. et al. Toward acellular xenogeneic heart valve prostheses: histological and biomechanical characterization of decellularized and enzymatically deglycosylated porcine pulmonary heart valve matrices. Xenotransplantation 2020;27:e12617. [DOI] [PubMed] [Google Scholar]
- 21. Ramm R, Goecke T, Theodoridis K. et al. Decellularization combined with enzymatic removal of N-linked glycans and residual DNA reduces inflammatory response and improves performance of porcine xenogeneic pulmonary heart valves in an ovine in vivo model. Xenotransplantation 2020;27:e12571. [DOI] [PubMed] [Google Scholar]
- 22. Petersen B, Frenzel A, Lucas-Hahn A. et al. Efficient production of biallelic GGTA1 knockout pigs by cytoplasmic microinjection of CRISPR/Cas9 into zygotes. Xenotransplantation 2016;23:338–46. [DOI] [PubMed] [Google Scholar]
- 23. Hauschild J, Petersen B, Santiago Y. et al. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A 2011;108:12013–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Theodoridis K, Tudorache I, Cebotari S. et al. (*) Six-year-old sheep as a clinically relevant large animal model for aortic valve replacement using tissue-engineered grafts based on decellularized allogenic matrix. Tissue Eng Part C Methods 2017;23:953–63. [DOI] [PubMed] [Google Scholar]
- 25. Theodoridis K, Muller J, Ramm R. et al. Effects of combined cryopreservation and decellularization on the biomechanical, structural and biochemical properties of porcine pulmonary heart valves. Acta Biomater 2016;43:71–7. [DOI] [PubMed] [Google Scholar]
- 26. Stegemann H, Stalder K.. Determination of hydroxyproline. Clin Chim Acta 1967;18:267–73. [DOI] [PubMed] [Google Scholar]
- 27. Farndale RW, Buttle DJ, Barrett AJ.. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986;883:173–7. [DOI] [PubMed] [Google Scholar]
- 28. Lichtenberg A, Tudorache I, Cebotari S. et al. Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation 2006;114:I559–65. [DOI] [PubMed] [Google Scholar]
- 29. Theodoridis K, Tudorache I, Calistru A. et al. Successful matrix guided tissue regeneration of decellularized pulmonary heart valve allografts in elderly sheep. Biomaterials 2015;52:221–8. [DOI] [PubMed] [Google Scholar]
- 30. Ramm R, Hartmann T, Tudorache I. et al. No evidence for alphaGal epitope transfer from media containing FCS onto human endothelial cells in culture. Xenotransplantation 2015;22:345–55. [DOI] [PubMed] [Google Scholar]
- 31. Tanemura M, Yin D, Chong A. et al. Differential immune response to carbohydrate epitopes on allo- and xenografts: implications for accommodation. Transplant Proc 2000;32:991–3. [DOI] [PubMed] [Google Scholar]
- 32. Rosales IA, Colvin RB.. The pathology of solid organ xenotransplantation. Curr Opin Organ Transplant 2019;24:535–42. [DOI] [PubMed] [Google Scholar]
- 33. Beschorner WE, Sudan DL, Radio SJ. et al. Heart xenograft survival with chimeric pig donors and modest immune suppression. Ann Surg 2003;237:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its supplemental information files. The reader may contact the corresponding authors with any request.