Abstract
Introduction and importance
Synovial cell sarcoma (SS) is an extremely rare mesenchymal malignancy, representing nearly 10% of all soft-tissue sarcomas. These high-grade soft tissue sarcomas commonly arise in the para-articular regions of lower extremities. However, 15% of Synovial sarcomas has been described at Unusual locations, including head, neck, and trunk. Herein, we describe the twelfth case of primary synovial cell sarcoma of thyroid with a literature review.
Case presentation
A 43-year-old woman presented with complaint of a progressive neck mass for the last five-months. She developed with dysphagia and dyspnea nearly 2 months prior, without signs of hoarseness, and weight loss. Ultrasonography in which revealed a heterogeneous, hypervascularized thyroid mass. After total thyroidectomy immunohistochemistry was in favor of primary synovial cell sarcoma of thyroid. The diagnosis was confirmed via Molecular genetic analysis of the SYT-SSX fusion gene transcript using the RT- polymerase chain reaction method. Clinical Discussion: Primary thyroid SVS is an extremely rare malignancy with poor biological behavior. SVS has been known for its tendency to local and distal re-occurrence after a few years of treatment. SS can be classified into two subtypes of monophasic or biphasic based on the presence of mesenchymal and/or epithelial components. Accordingly, the most accurate diagnostic tool for SS is considered to be molecular genetic analysis for SYT/SSX fusion transcript.
Conclusion
Herein, we reported an extremely rare case of SVS of thyroid gland. These high-grade soft tissue sarcomas mainly present with an asymptomatic rapid growing neck mass. Unspecific clinical presentations and extreme rarity of this disorder, make the diagnosis of thyroid SVS very challenging. Due to paucity of data, there is not enough evidence to establish a reliable mortality rate. However, the prognosis of thyroid SVS seems unfavorable.
Keywords: Synovial cell sarcoma, Thyroid gland, Case report
Highlights
-
•
Synovial Cell Sarcoma (SS) is an extremely rare mesenchymal malignancy, representing nearly 10% of all soft-tissue sarcomas.
-
•
Primary synovial cell sarcoma of thyroid is exceedingly rare. To our knowledge, only 11 cases have been reported in the English literature
-
•
Herein, we describe the twelfth case of primary synovial cell sarcoma of thyroid with a literature review.
1. Introduction
Synovial cell sarcoma (SS) is an extremely rare mesenchymal malignancy, representing nearly 10% of all soft-tissue sarcomas [1]. These high-grade soft tissue sarcomas commonly arise in the para-articular regions of lower extremities. However, 15% of Synovial sarcomas has been
described at Unusual locations, including head, neck, and trunk [2]. Accordingly, almost all Synovial sarcomas have been associated with specific chromosomal changes [t(X; 18) (p11.2; q11.2)] leading to expression of a chimeric (SYT-SSX) gene transcript [3]. Primary synovial cell sarcoma of thyroid is exceedingly rare. To our knowledge, only 11 cases have been reported in the English literature [1], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]. Herein, we describe the twelfth case of primary synovial cell sarcoma of thyroid with a literature review. This study was reported in line with the SCARE criteria [14].
2. Case presentation
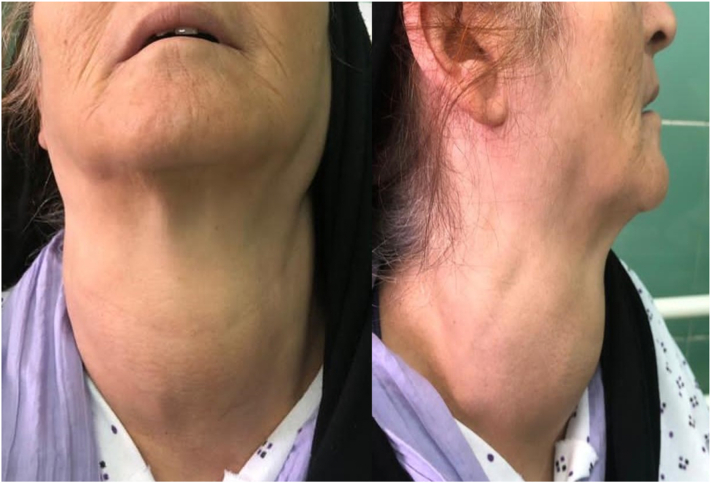
A 43-year-old woman presented with complaint of dysphagia and dyspnea 7 months prior to her admission. In the last five months, she started to have a progressive neck mass, without signs of hoarseness, and weight loss. She was previous case of hypothyroidism, on levothyroxine. Family history revealed diffused goiter in her aunt and first cousin. On examination, there was a firm, non-tender, macronodular, fixed midline mass measuring 10x7cm in diameter without signs of lymph node enlargement (Fig. 1). Routine laboratory studies, including thyroid function tests were unremarkable.
Fig. 1.
Diffuse thyroid enlargement seen in the patient.
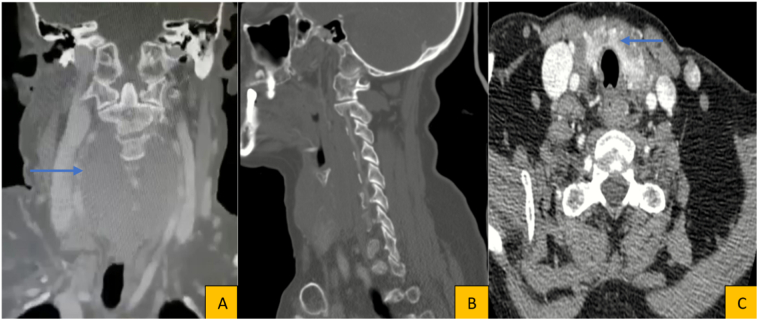
She underwent cricothyroid ultrasonography in which revealed heterogeneous, hypervascularized and mixed (solid and liquid) thyroid mass. There was a 93×46×15mm hypoechoic nodule in lower part of left thyroid gland, suggestive for primary thyroid mass. There were multiple hypoechoic nodules in the right lobe, largest being 34×28 mm in diameter. There was no sign of cervical lymphadenopathy. Furthermore, cervical CT scan with IV contrast revealed a heterogeneous thyroid mass with a 90 ∗ 45 ∗ 65 mm thyroid nodule with internal necrotic view. Accordingly, there were no signs of tumor invasion to larynx, trachea, esophagus, carotid arteries, jugular veins, and cervical spines. The fine needle aspiration (FNA) cytology was in favor of undifferentiated cell carcinoma of thyroid gland. Due to high suspicions for thyroid malignancy, the patient was scheduled for total thyroidectomy. Prior to her surgery, the patient underwent Thoracoabdominal CT scan which showed no signs of distant metastasis (Fig. 2).
Fig. 2.
Cervical CT scan with IV contrast. Blue arrows outline thyroid mass without signs of invasion to surrounding structures.
3. Treatment
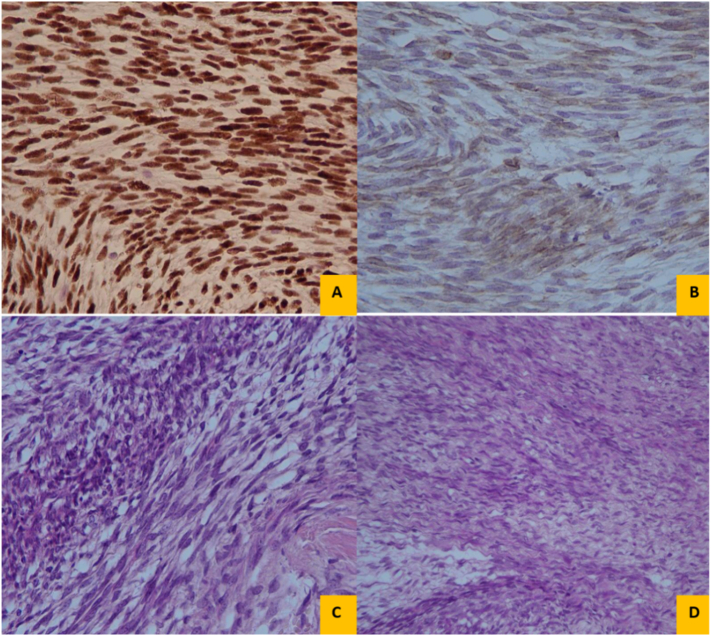
Pre-operative management was considered. Total thyroidectomy was conducted under general anesthesia with the purpose of complete resection of the tumor and involved lymph nodes. There was an encapsulated mass about 100 ∗ 80 ∗ 40 mm diameter. When trying for total excision, the capsule was ruptured and multiple segments of necrotic mass were taken out. No lymphadenopathy or distant metastasis was present. The cut surface showed a creamy, and elastic solid mass with storiform pattern. On microscopy, the tumor was consisted of fascicles and sheets of monophasic spindle cell growth pattern with oval nuclei (Fig. 3). Subsequent immunohistochemistry showed immunoreactivity for EMA, cytokeratin (CK 7, CK 19), BCL-2, and TLE1. However, tumor cells were negative for S-100, CD99, CD-31 and CD-34 with KI-67 index of 5% (Fig. 4). The diagnosis was confirmed via Molecular genetic analysis of the SYT-SSX fusion gene transcript using the RT- polymerase chain reaction method.
Fig. 3.
Histologic evaluation with H&E staining of the thyroid specimen revealed spindle cells with fibrotic-like appearance and focally whorled pattern.
Fig. 4.
Immunohistochemistry showed immunoreactivity for TLE1 (A), BCL-2 (B), and EMA (C, D).
4. Outcome and follow-up
The post-operative course was uneventful without any signs of short-time complications. Patient was started on 6 cycles of doxorubicin and ifosfamide and radiotherapy. She was discharged 6 days following her procedure without any discomfort. The patient was scheduled for monthly cricothyroid ultrasonography, and Contrast-enhanced computed tomography (CT) of thoracic and abdominal every 3 months for evaluation of distant metastasis. Fortunately, the patient showed no signs of local or distant metastasis during her monthly follow-up visits.
5. Discussion
Thyroid sarcoma, a pluripotential mesenchymal neoplasia, is generally a high-grade aggressive tumor [1]. Primary thyroid SVS is an extremely rare malignancy with poor biological behavior. That said, SVS has been known for its tendency to local and distal re-occurrence after a few years of treatment [12]. Prior to 1940, most cases of thyroid anaplastic carcinomas were in fact mistakenly labeled as synovial sarcoma [11]. To best of our knowledge, only 12 cases of primary thyroid synovial sarcomas (including the present case) have been reported in English literature (Table 1). SS typically affect patients during adolescence and adulthood. However, in SVS of thyroid gland the mean age at the time of diagnosis was 39.7 (range 15–72). Accordingly, men are more affected than females with a 2:1 sex ratio.
Table 1.
Review of literature (12 Cases of thyroid synovial sarcoma).
| Study | Sex/age | Initial clinical symptoms | Preoperative diagnosis by FNAB | Initial treatment | Mono- or biphasic | Relapse (month after surgery) | Last follow-up (month after surgery) |
|---|---|---|---|---|---|---|---|
| Roth [1] | M/15 | Excessive salivation/otalgia | No | Total thyroidectomy and cobalt therapy | Biphasic | Not mentioned | Not mentioned |
| Kikuchi [2] | M/60 | Hoarseness/palpable mass | Yes/MTC (medullary thyroid carcinoma) |
Total thyroidectomy neck dissection partial resection of thyroid and cricoid cartilages | Biphasic | Local, lung (18 Months) |
Dead of disease (36 months) |
| Jang [3] | M/15 | Palpable mass | Yes/FL (follicular lesion) | Total thyroidectomy, left Neck dissection | Biphasic | not mentioned | Not mentioned |
| Ryu [4] | F/72 | Dysphagia/hoarseness/palpable mass | Yes/CC (cystic change) |
Thyroidectomy, tracheal fenestration | Monophasic | Local, lung (0.5 months) |
Dead of unknown cause (3 months) |
| Ghafouri [5] | F/44 | Rapidly growing mass | No | Total Thyroidectomy with Incomplete excision | Monophasic | Not mentioned | Not mentioned |
| Boudin [6] | M/55 | Rapidly growing mass | No | Thyroidectomy with, Incomplete excision, CRT | Monophasic | Local/0.5 months Surgery and RT |
Alive without disease (10 months) |
| Bansal [7] | F/28 | Rapidly growing mass/dysphagia/weight loss | No | Total thyroidectomy | Biphasic | Not mentioned | Not mentioned |
| Murro [8] | M/41 | Dysphonia/left neck mass | No | Total thyroidectomy, laryngopharyngoesophagectomy | Biphasic | Not mentioned | Not mentioned |
| Shi [9] | M/31 | Asymptomatic mass | No | Total Thyroidectomy with Incomplete excision | Biphasic | Upper neck cervical lymph nodes (chemoradiation and re-excision) | Alive without disease (36 months) |
| Zahran [10] | M/47 | Rapidly growing mass | No | Total thyroidectomy | Biphasic | Not mentioned | Alive without disease (12 months) |
| Dilamer [11] | M/26 | Neck mass | Yes (papillary carcinoma) | Total thyroidectomy | Biphasic | Local recurrence (chemo immunotherapy) | Dead of disease (41 months) |
| Our case | F/43 | Dysphagia/neck mass | Yes | Total thyroidectomy | Monophasic | Not mentioned | Alive without disease (6 months) |
The most common clinical finding of SVS is an asymptomatic rapid growing neck mass, mimicking anaplastic thyroid carcinoma. Other manifestations include Excessive salivation, hoarseness, dysphagia, and dysphonia which is mostly associated with compression effects by the rapidly growing tumor. Unspecific clinical presentations and extreme rarity of this disorder, make the diagnosis of thyroid SVS very challenging. Imaging studies including ultrasound, magnetic resonance imaging (MRI), and CT scan of the neck could be used to assess the location, size, vascularization, and local invasion of primary tumor to surrounding structures. However, radiologic evaluations fall short in differentiation of SVS from other thyroid malignancies. Pre-operative Fine needle aspiration (FNA) is not a reliable diagnostic tool, and it could be misleading. In fact, three cases of SVS were misdiagnosed as medullary, follicular, and papillary thyroid carcinoma by FNA (Table 1).
Histologic, Immunohistochemistry (IHC), and molecular analyses are considered as the cornerstone of SVS recognition. Histomorphologically, SS can be classified into two subtypes of monophasic or biphasic based on the presence of mesenchymal and/or epithelial components. Biphasic SS, consisted of both spindle and epithelioid cells, is considered to be more common (n = 8, 66.6%) and is diagnostically less challenging especially in common locations [11]. The monophasic subtype on the other hand, is mainly composed of spindle cells, in which accurate diagnosis is extremely challenging. Common differentials of monophasic SS being other spindle cell sarcomas, including leiomyosarcoma, rhabdomyosarcoma, fibrosarcoma, malignant peripheral nerve sheath tumor (MPNST), and spindle epithelial tumor with thymus-like differentiation (SETTLE) [15], [16]. Immunohistochemically, SS typically is positive for epithelial markers including EMA, cytokeratin (CK 7, CK 19), and also the tumors react positively to vimentin, CD 99, and bcl-2 which help distinguish them from other types of sarcomas. However, IHC may yield conflicting results since there have been reports of epithelial cell markers expression in anaplastic cell carcinoma, MPNST, and carcinosarcoma [11], [15].
Accordingly, the most accurate diagnostic tool for SS is considered to be molecular genetic analysis for SYT/SSX fusion transcript. In almost all cases of SS, this chromosomal translocation [t (X; 18) (p11.2; q11.2)] could be detected using frozen or paraffin embedded tissues via real-time reverse transcriptase polymerase chain reaction (RT-PCR) or FISH [1].
Due to rarity of the disease, there is very limited data on the optimal treatment. Since synovial cell sarcomas are considered as highly aggressive and infiltrative tumors, wide excision surgical removal with tumor-free margins is suggested. However, wide margin-free surgical resection cannot always accomplish because of the large tumor size and the proximity to vital structures in the head and neck region. In all 12 cases of SS, total thyroidectomy was the initial treatment option, in which in four patients was the only therapeutic approach (including ours) [7], [10], [11] with two patients underwent cervical lymph node dissection. That said, lymph node dissection is not generally recommended for management of sarcomas, except when nodal involvement is suspected. As usual for sarcomas, chemoradiotherapy is generally recommended given their invasive nature, and high risk of local and distant metastasis. Adjuvant chemotherapy with ifosfomide may be considered which can lead to cancer stabilization and lower chance of recurrence [1], [17].
Due to paucity of data, there is not enough evidence to establish a reliable mortality rate. To best of our knowledge, 3/12 patients passed away and all three had local or distant metastasis. The most common location for distant metastasis was the lungs and local recurrence was reported in two patients. Overall, the prognosis of thyroid SVS seems unfavorable with tumor size, location and radicalness of surgical resection being the most probable prognostic values.
6. Conclusion
Herein, we reported an extremely rare case of SVS of thyroid gland. These high-grade soft tissue sarcomas mainly present with an asymptomatic rapid growing neck mass. Unspecific clinical presentations and extreme rarity of this disorder, make the diagnosis of thyroid SVS very challenging. Due to paucity of data, there is not enough evidence to establish a reliable mortality rate. However, the prognosis of thyroid SVS seems unfavorable.
Ethics approval and consent to participate
The purpose of this research was completely explained to the patient, and was assured that their information will be kept confidential by the researchers. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editor-in-chief of this journal. The present study was approved by the medical ethics committee of the academy.
Source of funding
No funding was obtained for this study.
CRediT authorship contribution statement
SS and M.SH – drafted the manuscript and provided images and histologic evaluations. MS and OO- helped with the draft and reviewed the literature. RS – Supervisor (corresponding author), provided initial feedback and reviewed the final manuscript. The authors read and approved the final manuscript.
Guarantor
Ramin Shekouhi
Research registration
Not applicable.
Patient's perspective
The patient satisfied and was comfortable one month after the operation.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
Not applicable.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Boudin L. Primary synovial sarcoma of the thyroid gland: case report and review of the literature. Case Rep. Oncol. Med. 2014;7(1):6–13. doi: 10.1159/000357913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss S.W., Goldblum J. Enzinger and Weiss's Soft Tissue Tumors. Mosby; St. Louis, MO: 2001. p. 1622. [Google Scholar]
- 3.Sandberg A.A., Bridge J.A. Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. Synovial sarcoma. Cancer Genet. Cytogenet. 2002;133:1–23. doi: 10.1016/s0165-4608(01)00626-4. [DOI] [PubMed] [Google Scholar]
- 4.Bansal N., Ranade R.S., Mishra A. Synovial sarcoma mimicking thyroid carcinoma. ANZ J. Surg. 2015;87(11):E214–E215. doi: 10.1111/ans.13113. [DOI] [PubMed] [Google Scholar]
- 5.Demirel D. Primary synovial sarcoma of the thyroid: challenges in cytologic diagnosis and review of the literature. Acta Cytol. 2020;64(5):498–506. doi: 10.1159/000507312. [DOI] [PubMed] [Google Scholar]
- 6.Ghafouri A. Thyroid synovial sarcoma: a case report. Acta Medica Iranica. 2013:69–72. [PubMed] [Google Scholar]
- 7.Jang K.-S. Primary synovial sarcoma of the thyroid gland. J. Korean Med. Sci. 2007;22(Suppl):S154. doi: 10.3346/jkms.2007.22.S.S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi I. Synovial sarcoma of the thyroid. Report of a case with aspiration cytology findings and gene analysis. Acta Cytol. 2003;47(3):495–500. doi: 10.1159/000326558. [DOI] [PubMed] [Google Scholar]
- 9.Murro D. Fine needle aspiration of secondary synovial sarcoma of the thyroid gland. Diagn. Cytopathol. 2015;43(11):928–932. doi: 10.1002/dc.23327. [DOI] [PubMed] [Google Scholar]
- 10.Roth J.A., Enzinger F., Tannenbaum M. Synovial sarcoma of the neck: a followup study of 24 cases. Cancer. 1975;35(4):1243–1253. doi: 10.1002/1097-0142(197504)35:4<1243::aid-cncr2820350432>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Ryu C.H., Cho K.-J., Choi S.-H. Synovial sarcoma of the thyroid gland. Clin. Exp. Otorhinolaryngol. 2011;4(4):204. doi: 10.3342/ceo.2011.4.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi R.L. Primary synovial sarcoma of the thyroid with locally repeated relapses in short periods: a case report. Biomed. Rep. 2016;5(1):79–82. doi: 10.3892/br.2016.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zahran M. Primary synovial sarcoma of the thyroid gland: a case report and review of literature. Indian J. Otolaryngol. Head Neck Surg. 2020:1–4. doi: 10.1007/s12070-020-01999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agha R.A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 15.Coindre J.M. Should molecular testing be required for diagnosing synovial sarcoma? A prospective study of 204 cases. Cancer. 2003;98(12):2700–2707. doi: 10.1002/cncr.11840. [DOI] [PubMed] [Google Scholar]
- 16.Alberty J., Dockhorn-Dworniczak B. Monophasic synovial sarcoma of the neck in an 8-year-old girl resembling a thyroglossal duct cyst. Int. J. Pediatr. Otorhinolaryngol. 2002;63(1):61–65. doi: 10.1016/s0165-5876(01)00636-x. [DOI] [PubMed] [Google Scholar]
- 17.Le Cesne A. The end of adjuvant chemotherapy (adCT) era with doxorubicin-based regimen in resected high-grade soft tissue sarcoma (STS): pooled analysis of the two STBSG-EORTC phase III clinical trials. J. Clin. Oncol. 2008;26(15_suppl):10525. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.