Abstract
Trichoderma longibrachiatum is a fungus belonging to the genus Trichoderma. Trichoderma longibrachiatum is not thought as a pathogenic for healthy individuals. However, it has the ability to produce toxic peptides and extracellular proteases and has been described to cause invasive infections in immunocompromised hosts. Trichoderma longibrachiatum has been reported as the causative microorganism of lung infections, skin infections, sinus infections, otitis, stomatitis endocarditis, pericarditis, gastrointestinal infections, mediastinitis and peritonitis. We report the first case of pneumonia with parapneumonic effusion in an old woman with diabetes mellitus due to Trichoderma longibrachiatum.
Abbreviations: ADA, adenosine deaminase; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma glutamyl transferase; CT, computerized tomography; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; FiO2, fraction of inspired oxygen; Hb, hemoglobin; HIV, human immunodeficiency virus; Ht, hematocrit; LDH, serum lactate dehydrogenase; PTLS, platelets; SG, specific gravity; TSH, thyroid-stimulating hormone; WBC, white blood cells
Keywords: Pleural effusion, Trichoderma longibrachiatum, Fungal infection, Diabetes mellitus
Introduction
Trichoderma longibrachiatum is a soil fungus and it is a distinct species in the genus Trichoderma. It can be found all over the world especially in countries with warmer climates. It has been used in many industries because of its ability to produce large amounts of proteins and metabolites [1].
Trichoderma longibrachiatum secretes small toxic peptides which contain amino acids not found in common proteins that are called trilongins and are highly resistant to heat and antimicrobial agents. These peptides are absorbed into cells and produce nano-channels that obstruct vital ion channels which transfer potassium and sodium ions across the cell membrane, leading to conduction defects [2].
Trichoderma longibrachiatum is not considered to be pathogenic for healthy humans, although it has been found to cause indoor contamination and to be potential allergen. It shows increasing clinical importance as opportunistic human pathogen, causing invasive infections mainly in immunocompromised patients [3].
Trichoderma longibrachiatum and other Trichoderma species have been described to have the ability of extracellular protease production to maintain their survival advantage as saprophytic organisms. There is evidence for the possible involvement of proteolytic enzymes in aspergillosis, coccidioidomycosis and sporotrichosis, indicating that the secretion of extracellular proteases may be involved in the pathogenesis mechanisms in cases of opportunistic Trichoderma infections as well [4].
We report the first case of pneumonia with parapneumonic effusion due to Trichoderma longibrachiatum.
Case presentation
A 81-year-old woman, non-smoker, with a history of diabetes mellitus treated with vildagliptin, bronchial asthma treated with inhaled salmeterol/fluticazone, hyperlipidemia treated with atorvastatin, appendectomy and eterozygous beta-thalassemia, presented to our pulmonology department with fever, dyspnea at rest, non-productive cough and pleuritic pain in the left hemithorax over the three last days. She had started receiving treatment with moxifloxacin without improvement.
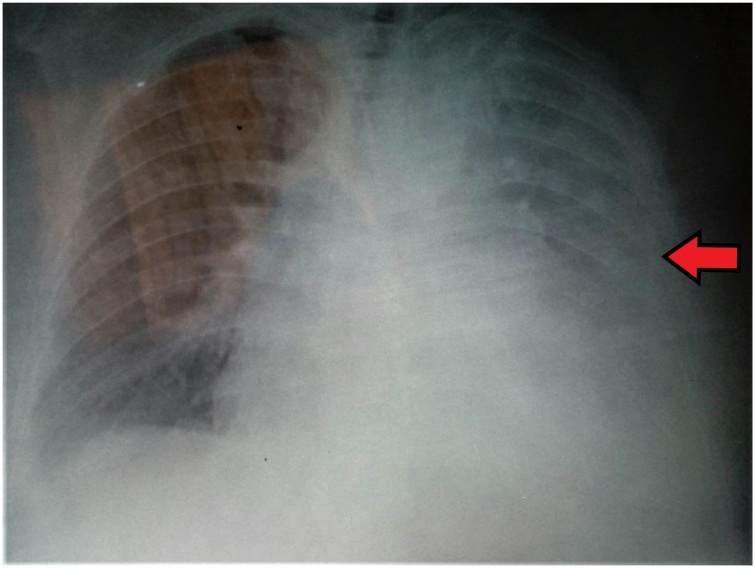
Clinical examination revealed a febrile patient with decreased breath sounds and dullness on percussion at the base of the left lung. Blood pressure was 115/77 mmHg, heart rate was 105 beats per minute, oxygen saturation was 93 % on room air and body temperature 38 °C on admission. Electrocardiography revealed sinus tachycardia. Arterial blood gas analysis showed pO2 64 mmHg, pCO2 32 mmHg, pH 7.49 and HCO3− 24 mmol/L on room air. Chest X-ray showed consolidation in the left lower lobe with left pleural effusion (Fig. 1).
Fig. 1.
Chest X-Ray showing consolidation in the left lower lobe with left pleural effusion.
Laboratory findings included hemoglobin (Hb) 11.2 g/dL (normal 12−15 g/dL), white blood cells (WBC) 17.42 × 103/μL (normal 4−11 × 103 /μL), neutrophils 15.44 × 103/μL (normal 2−8 × 103 /μL), lymphocytes 0.99 × 103/μL (normal 1.1−4 × 103 /μL), platelets (PTLS) 125 × 103/μL (normal 150−400 × 103 /μL), C-reactive protein (CRP) 163.8 mg/L (normal <6 mg/L) and erythrocyte sedimentation rate (ESR) 93 mm/h. Urinalysis revealed red blood cells 2–4 per high power field, white blood cells 20–25 per high power field, protein 30 mg/dl, specific gravity (SG) 1020 and pH 6.
The other blood biochemistry parameters and thyroid-stimulating hormone (TSH) were normal, with the exception of an elevated serum lactate dehydrogenase (LDH) 231 U/L (normal <225 U/L) and elevated glycose 231 mg/dL (normal 60−100 mg/dL). (Table 1)
Table 1.
Laboratory data on admission.
| Serum parameters | Patients data |
|---|---|
| Urea (10−50 mg/dL) | 31 |
| Creatinin (0.5−1.5 mg/dL) | 0.7 |
| Glucose (60−100 mg/dL) | 231 |
| Na (135−148 mEq/L) | 136 |
| K (3.5−5.3 mEq/L) | 4 |
| *AST (5−45 U/L) | 24 |
| *ALT (5−45 U/L) | 14 |
| *GGT (5−45 U/L) | 19 |
| *ALP (42−128 U/L) | 65 |
| *LDH (135−225 U/L) | 231 |
| Albumin (3.5−5.1 g/dL) | 3.7 |
| Proteins (6.5−8.5 g/dL) | 6.5 |
| *CRP (<6 mg/L) | 163.8 |
| *ESR (0−20 mm/h) | 93 |
| *Ht (37−45%)33.1Hb (12−45%) | 33.1 |
| *Hb (12−15 g/L) | 11.2 |
| *PTLS (150−400 × 103.μ/L) | 125 × 103 |
| *WBC (4−11 × 103 μ/L) | 17.42 × 103 |
*AST: aspartate aminotransferase, ALT: alanine transaminase, GGT: gamma-glutamyl transferase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, CRP: C-reactive protein, ESR: erythrocyte sedimentation rate, Ht: hematocrit, Hb: hemoglobin, PTLS: platelets, WBC: white blood cells.
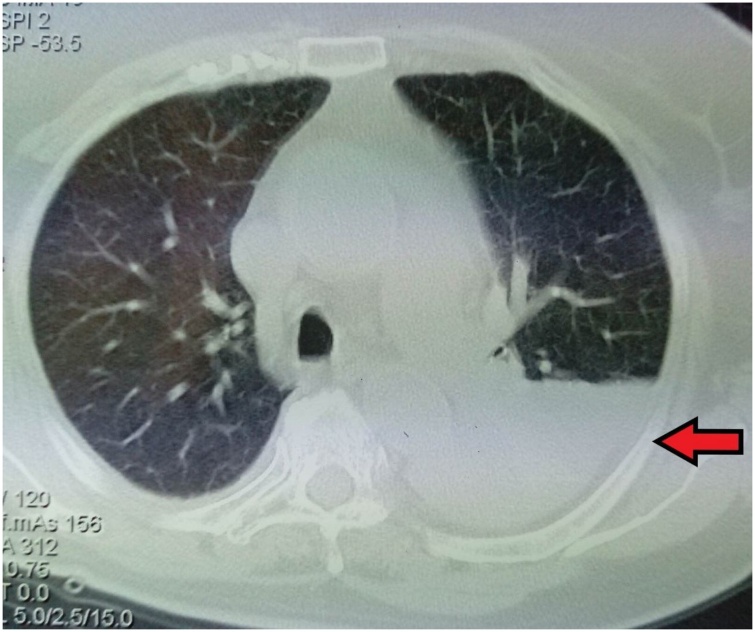
The patient underwent thoracentesis which revealed a polymorphonuclear pleural exudate with normal adenosine deaminase (ADA) 13.10 U/L, pH 7.45, normal cytology and negative stains and cultures (Table 2). The patient received oxygen therapy with Venturi mask delivering 35 % oxygen and intravenous antimicrobial therapy with piperacillin-tazobactam and clindamycin empirically. The patient underwent computerized tomography (CT) of the chest which revealed left pleural effusion with loculates and consolidation in lingula and left lower lobe (Fig. 2). Urinary antigen tests for Legionella pneumonophila and Streptococcus pneumoniae, serological testing for Mycoplasma pneumoniae and three blood cultures were obtained.
Table 2.
Characteristics of the pleural fluid.
| Pleural fluid parameters | Patients data |
|---|---|
| Glucose (mg/dL) | 249.7 |
| *LDH (U/L) | 198 |
| Proteins (g/L) | 4.57 |
| Albumin (g/L) | 3 |
| *ADA (<40 U/L) | 13.10 |
| Lymphocytes (% of total fluid cells) | 36 |
| Neutrophils (% of total fluid cells) | 54 |
*LDH: lactate dehydrogenase, ADA: adenosine deaminase.
Fig. 2.
Computerized tomography (CT) of the chest showing left pleural effusion.
After the first four days of hospitalization, fever persisted, accompanied by respiratory deterioration with new arterial blood gas analysis pO2 52 mmHg, pCO2 38 mmHg, pH 7.49 and HCO3− 34 mmol/L on fraction of inspired oxygen (FiO2) 35 %, increase in amount of left pleural effusion (Fig. 3), increase in total peripheral white cell count to 21.22 × 103 μL and increase in CRP levels 396 mg/L (normal <6 mg/L).
Fig. 3.
Chest X-Ray showing increase in amount of left pleural effusion.
The patient received oxygen therapy with non rebreather mask delivering 100 % oxygen and the treatment with antimicrobials changed empirically to meropenem and vancomycin. The culture of blood with use of Sabouraud dextrose agar at 35 °C yielded a nimatoid fungus identified as a Trichoderma species based on microscopic evaluation which revealed rapidly growing flat green colonies (Fig. 4). Administration of meropenem and vancomycin was stopped and patient received amphotericin B empirically.
Fig. 4.
Trichoderma species on Sabouraud dextrose agar (SDA). Arrow shows rapidly growing flat green colonies.
The fungal cultures obtained from blood were sent to Microbiology Laboratory of National and Kapodistrian University of Athens for species’ identification and sensibility study. The molecular analysis of ribosomal DNA internal transcribed spacer (ITS-1 and ITS-2) sequences revealed that the fungus was Trichoderma longibrachiatum. Susceptibility testing was performed with the use of European Committee on Antimicrobial Susceptibility Testing (EUCAST) methodology. It demonstrated that the fungus was resistant to itraconazole, miconazole, posaconazole, voriconazole, caspofugin and andulafugin and susceptible to amphotericin B.
The patient underwent echocardiography with normal ejection fraction and valve function without vegetations, abdominal and brain CT and fundoscopy without anbnormal findings.
The patient had a history of diabetes mellitus which is considered a type of immunosuppression. In addition, the patient was assessed for any other types of immunodeficiency. The subpopulations of lymphocytes were normal, the ranges of immunoglobulins and complement components were normal and the test for human immunodeficiency virus (HIV) was negative.
The patient after four days of initiation of antifungal therapy presented with absence of fever, clinical, radiological and laboratory improvement. The patient had gradually complete recovery after a therapy with amphotericin B with a total duration of four weeks without development of renal impairment.
Discussion
The current case is, to our knowledge, the first to describe the entity of pneumonia with parapneumonic effusion due to Trichoderma longibrachiatum. Our patient had a history of diabetes mellitus which is a metabolic disorder predisposing individuals to fungal infections
[5]. A parapneumonic effusion is a pleural effusion that results from a lung infection such as pneumonia or lung abscess. It is divided to three types: uncomplicated effusion, complicated effusion, and empyema [6]. Uncomplicated effusion usually responds well to appropriate treatment with antibiotics. Characteristics of complicated parapneumonic effusion include the presence of pus, pleural fluid with positive gram stain or positive culture and pleural fluid with pH < 7.20. These characteristics indicate the need of chest tube drainage. Management of empyemas includes antimicrobials administration, pleural fluid drainage, re-expansion of the lung and in some cases decortication [7].
Pathogens isolated differ between patients with community acquired pneumonia and hospital-acquired pneumonia, with predominance of Streptococcus species and Staphylococci, respectively [7]. Pleural effusion due to fungi is a rare clinical presentation (<1% of pleural infection) and is reported mainly in immunocompromised hosts and patients with underlining diseases such as diabetes mellitus [7]. Candida species are responsible for the most of the cases with high rates of mortality [8,9]. Additionally, Cryptococcus neoformans [10], Aspergillus species [[11], [12], [13], [14], [15], [16]], Trichosporon and Fusarium species [17] have been described as rare causes of pleural effusion is some case reports.
The first case of proven invasive pulmonary infection due to Trichoderma longibrachiatum was described in 2018 in a neutropenic patient with acute leukemia [3]. One year before suspected pulmonary infection due to Trichoderma longibrachiatum after allogenic stem cell transplantation had been reported [18].
Trichoderma longibrachiatum has been associated with infections in other body locations. It has been mentioned as a cause of skin infection in pediatric patients with hematological disorders [19,20]. It has also been described to cause fatal necrotizing stomatitis in a neutropenic patient with malignant lymphoma [21], otitis externa in a 12-year-old child with a long history of otitis [22] and acute invasive sinusitis in a liver and small bowel transplant recipient [23]. Besides, Trichoderma londibrachiatum has been reported as unusual pathogen that caused fungal pericarditis [24], endocarditis in a patient receiving home parenteral nutrition [25] and cardiac implantable electronic device-associated endocarditis in a non-immunocompromised host [26]. Additionally, this pathogen has been related to fatal post-operative mediastinitis and peritonitis in a pediatric patient with complex congenital cardiac disease on peritoneal dialysis [27], inguinal abscess in a renal transplant recipient [28] and a perirectal ulcer in an adult bone marrow transplant patient [29].
Conclusions
This is the first case of pneumonia with parapneumonic effusion due to Trichoderma longibrachiatum. This pathogen can cause invasive infection in several organs especially in immunocompromised patients. Clinicians should be aware of this potential pathogen in patients who present with infectious pleural effusion and who have predisposing factors for fungal infections.
Funding
No sources of funding.
Ethical approval
No ethical approval was required for this publication.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
Vasiliki Epameinondas Georgakopoulou, Despoina Melemeni, Konstantinos Mantzouranis: Conceptualization, Methodology, Writing-Original Draft.
Pagona Sklapani, Serafeim Clapoutakis, Aikaterini Gkoufa, Christos Damaskos, Nikolaos Garmpis, Anna Garmpi: Investigation, Writing-Review & Editing, Visualization.
Nikolaos Trakas, Xanthi Tsiafaki: Supervision, Project administration.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
None.
References
- 1.Samuels G.J., Ismaiel A., Mulaw T.B., Szakacs G., Druzhinina I.S., Kubicek C.P. The Longibrachiatum Clade of Trichoderma: a revision with new species. Fungal Divers. 2012;55(1):77–108. doi: 10.1007/s13225-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mikkola R., Andersson M.A., Kredics L., Grigoriev P.A., Sundell N., Salkinoja-Salonen M.S. 20-Residue and 11-residue peptaibols from the fungus Trichoderma longibrachiatum are synergistic in forming Na+/K+ -permeable channels and adverse action towards mammalian cells. FEBS J. 2012;279(22):4172–4190. doi: 10.1111/febs.12010. [DOI] [PubMed] [Google Scholar]
- 3.Sautour M., Chrétien Ml, Valot S., Lafon I., Basmaciyan L., Legouge C. First case of proven invasive pulmonary infection due to Trichoderma longibrachiatum in a neutropenic patient with acute leukemia. J Mycol Med. 2018;28(4):659–662. doi: 10.1016/j.mycmed.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Kredics L., Antal Z., Szekeres A., Manczinger L., Dóczi I., Kevei F. Production of extracellular proteases by human pathogenic Trichoderma longibrachiatum strains. Acta Microbiol Immunol Hung. 2004;51(3):283–295. doi: 10.1556/AMicr.51.2004.3.6. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues C.F., Rodrigues M.E., Henriques M. Candida sp. infections in patients with diabetes mellitus. J Clin Med. 2019;8(1):76. doi: 10.3390/jcm8010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodríguez Suárez P., Freixinet Gilart J., Hernández Pérez J.M., Hussein Serhal M., López Artalejo A. Treatment of complicated parapneumonic pleural effusion and pleural parapneumonic empyema. Med Sci Monit. 2012;18(7):CR443–CR449. doi: 10.12659/msm.883212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies H.E., Davies R.J.O., Davies C.W.H., on behalf of the BTS Pleural Disease Guideline Group Management of pleural infection in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl. 2):ii41–ii53. doi: 10.1136/thx.2010.137000. ii41. [DOI] [PubMed] [Google Scholar]
- 8.Ko S.C., Chen K.Y., Hsueh P.R., Luh K.T., Yang P.C. Fungal empyema thoracis: an emerging clinical entity. Chest. 2000;117(June (6)):1672–1678. doi: 10.1378/chest.117.6.1672. [DOI] [PubMed] [Google Scholar]
- 9.de Vega Sánchez B., López Ramos I., Ortiz de Lejarazu R., Disdier Vicente C. Fungal empyema: an uncommon entity with high mortality. Arch Bronconeumol. 2017;53(11):641–642. doi: 10.1016/j.arbres.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Chen M., Wang X., Yu X., Dai C., Chen D., Yu C. Pleural effusion as the initial clinical presentation in disseminated cryptococcosis and fungaemia: an unusual manifestation and a literature review. BMC Infect Dis. 2015;15:385. doi: 10.1186/s12879-015-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonatti H., Lass-Floerl C., Angerer K., Singh N., Lechner M., Stelzmueller I. Successful management of postpneumonectomy Aspergillus pleural empyema by combined surgical and anti-fungal treatment with voriconazole and caspofungin. Mycoses. 2010;53(5):448–454. doi: 10.1111/j.1439-0507.2009.01729.x. [DOI] [PubMed] [Google Scholar]
- 12.Goel M.K., Juneja D., Jain S.K., Chaudhuri S., Kumar A. A rare presentation of aspergillus infection as empyema thoracis. Lung India. 2010;27(1):27–29. doi: 10.4103/0970-2113.59265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo F., Ohta H., Nagai Y., Minegishi K., Koyama S. A young immunocompetent patient with spontaneous Aspergillus empyema who developed severe eosinophilia. Respir Med Case Rep. 2017;22:220–223. doi: 10.1016/j.rmcr.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda T., Koreeda Y., Mataki H., Taira T., Noma S., Higashimoto I. A case of Aspergillus empyema successfully treated with combination therapy of voriconazole and micafungin: excellent penetration of voriconazole and micafungin into pleural fluid. Intern Med. 2010;49(12):1163–1169. doi: 10.2169/internalmedicine.49.2860. [DOI] [PubMed] [Google Scholar]
- 15.Rajalingham S., Anshar F.M. Chronic necrotizing pulmonary aspergillosis presenting as bilateral pleural effusion: a case report. J Med Case Rep. 2012;6:62. doi: 10.1186/1752-1947-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takatsuka H., Yamazaki S., Watanabe A., Yokoyama I., Suzuki T., Kamei K. Successful treatment of Aspergillus empyema using combined intrathoracic and intravenous administration of voriconazole: a case report. J Infect Chemother. 2020;26(8):847–850. doi: 10.1016/j.jiac.2020.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Iqbal N., Ammar M., Irfan M., Jabeen K. Trichosporon species and fusarium species as a cause of empyema thoracis in a diabetic patient. Cureus. 2020;12(7):e8973. doi: 10.7759/cureus.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akagi T., Kawamura C., Terasawa N., Yamaguchi K., Kubo K. Suspected pulmonary infection with Trichoderma longibrachiatum after allogeneic stem cell transplantation. Intern Med. 2017;56(2):215–219. doi: 10.2169/internalmedicine.56.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz Fm, Demmler Gj, Travis Wr, Ogden Ak, Rossmann Sn, Rinaldi Mg. Trichoderma longibrachiatum infection in a pediatric patient with aplastic anemia. J Clin Microbiol. 1997;35(2):499–503. doi: 10.1128/JCM.35.2.499-503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Román-Soto S., Álvarez-Rojas E., García-Rodríguez J. Skin infection due to Trichoderma longibrachiatum in a haematological paediatric patient. Clin Microbiol Infect. 2019;25(11):1383–1384. doi: 10.1016/j.cmi.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Myoken Y., Sugata T., Fujita Y., Asaoku H., Fujihara M., Mikami Y. Fatal necrotizing stomatitis due to Trichoderma longibrachiatum in a neutropenic patient with malignant lymphoma: a case report. Int J Oral Maxillofac Surg. 2002;31(6):688–691. doi: 10.1054/ijom.2001.0211. [DOI] [PubMed] [Google Scholar]
- 22.Hennequin C., Chouaki T., Pichon J.C., Strunski V., Raccurt C. Otitis externa due to Trichoderma longibrachiatum. Eur J Clin Microbiol Infect Dis. 2000;19(8):641–642. doi: 10.1007/s100960000326. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa H., Kusne S., Sutton D.A., Manez R., Carrau R., Nichols L. Acute invasive sinusitis due to Trichoderma longibrachiatum in a liver and small bowel transplant recipient. Clin Infect Dis. 1998;26(2):487–489. doi: 10.1086/516317. [DOI] [PubMed] [Google Scholar]
- 24.Recio R., Meléndez-Carmona M.Á, Martín-Higuera M.C., Pérez V., López E., López-Medrano F. Trichoderma longibrachiatum: an unusual pathogen of fungal pericarditis. Clin Microbiol Infect. 2019;25(5):586–587. doi: 10.1016/j.cmi.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez Peralta L.I., Mañas Vera M.R., García Delgado M.J., Pérez de la Cruz A.J. Endocarditis por Trichoderma longibrachiatum en paciente con nutrición parenteral domiciliaria [Endocarditis caused by Trichoderma longibrachiatumin a patient receiving home parenteral nutrition] Nutr Hosp. 2013;28(3):961–964. doi: 10.3305/nh.2013.28.3.6444. [DOI] [PubMed] [Google Scholar]
- 26.Tascini C., Cardinali G., Barletta V., Di Paolo A., Leonildi A., Zucchelli G. Trichoderma longibrachiatum CIED (cardiac implantable electronic device)-associated endocarditis in a non-immunocompromised host: biofilm removal and diagnostic problems in the light of the current literature. Mycopathologia. 2016;181(3–4):297–303. doi: 10.1007/s11046-015-9961-7. [DOI] [PubMed] [Google Scholar]
- 27.Santillan Salas C.F., Joshi A.Y., Dhiman N., Banerjee R., Huskins W.C., Wengenack N.L. Fatal post-operative Trichoderma longibrachiatum mediastinitis and peritonitis in a paediatric patient with complex congenital cardiac disease on peritoneal dialysis. J Med Microbiol. 2011;60(December (Pt 12)):1869–1871. doi: 10.1099/jmm.0.030718-0. [DOI] [PubMed] [Google Scholar]
- 28.Trabelsi S., Hariga D., Khaled S. First case of Trichoderma longibrachiatum infection in a renal transplant recipient in Tunisia and review of the literature. Tunis Med. 2010;88(1):52–57. [PubMed] [Google Scholar]
- 29.Richter S., Cormican M.G., Pfaller M.A., Lee C.K., Gingrich R., Rinaldi M.G. Fatal disseminated Trichoderma longibrachiatum infection in an adult bone marrow transplant patient: species identification and review of the literature. J Clin Microbiol. 1999;37(April (4)):1154–1160. doi: 10.1128/JCM.37.4.1154-1160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]