Abstract
目的
探讨人矮小同源盒基因2(SHOX2)对人膀胱癌细胞迁移、侵袭能力和干细胞特性的影响及其可能机制。
方法
分析TCGA肿瘤数据库中SHOX2基因在膀胱癌标本和癌旁对照标本的表达情况,利用GEPIA网站对TCGA膀胱数据中的SHOX2基因表达量进行单因素生存分析,并利用GSEA软件预测其可能的生物学功能。选取对数生长期的人膀胱癌细胞T24,采用SHOX2 shRNA及SHOX2过表达质粒分别敲低、过表达SHOX2基因,用Western blot检测SHOX2蛋白表达量,通过划痕实验、Transwell侵袭实验检测细胞的迁移、侵袭能力,悬浮成球、克隆形成实验检测细胞的干细胞特性。在光镜下观察细胞形态变化,并用Western blot检测细胞上皮间充质转化(EMT)标志分子E-cadherin、Vimentin蛋白表达量的变化和TGF-β信号通路重要组件TβR-I蛋白表达量的变化。
结果
与癌旁膀胱组织相比,SHOX2基因在TCGA膀胱癌组织中表达升高(P=0.01),在配对的膀胱癌组织中明显升高(P=0.001),SHOX2基因表达与总生存率呈负相关(P=0.01);GSEA显示SHOX2基因表达与EMT(P=0.002,P=0.03)及干细胞特性(P=0.006,P=0.008)呈正相关。在膀胱癌细胞T24中,两种不同的SHOX2 shRNA均能降低SHOX2的蛋白表达水平(shSHOX2-1,P=0.004;shSHOX2-2,P=0.03),SHOX2过表达质粒则能提高SHOX2的蛋白表达水平(P=0.007)。划痕实验、Transwell侵袭实验结果显示,抑制SHOX2表达后膀胱癌细胞的迁移(P < 0.001,P=0.02)、侵袭(P=0.002,P=0.003)能力明显降低,而SHOX2过表达则提高膀胱癌细胞的迁移(P < 0.001)、侵袭(P=0.004)能力。悬浮成球、克隆形成实验结果显示,抑制SHOX2表达后明显减弱膀胱癌细胞的干细胞特性(P=0.002,P=0.007,P < 0.001);而SHOX2过表达则增强膀胱癌细胞的干细胞特性(P=0.002,P < 0.001)。此外,抑制SHOX2表达可使细胞发生上皮样形态改变,而SHOX2过表达可使细胞发生间充质样形态改变;Western blot结果显示,抑制SHOX2表达可使膀胱癌细胞中的E-cadherin蛋白表达水平升高(P < 0.001),Vimentin(P < 0.001,P=0.01)、TβR-I(P=0.03,P=0.02)蛋白表达水平降低;而SHOX2过表达可使膀胱癌细胞中的Vimentin(P=0.04)、TβR-I(P=0.003)蛋白表达水平升高。
结论
SHOX2在膀胱癌中扮演致癌基因:SHOX2增强膀胱癌细胞的迁移、侵袭能力和干细胞特性,其作用机制可能与TGF-β信号通路参与调控的EMT有关。
Keywords: SHOX2, 膀胱癌, 迁移, 侵袭, 干细胞特性, 上皮间充质转化, TGF-β信号通路
Abstract
Objective
To explore the role of human short stature homeobox 2 (SHOX2) in regulating the migration, invasion and stemness of human bladder cancer cells.
Methods
We analyzed SHOX2 gene expression in bladder cancer and adjacent tissues based on TCGA database. Univariate survival analysis of SHOX2 gene expression in TCGA-BLCA data was performed using GEPIA. The probable function of SHOX2 was predicted using GSEA. Human bladder cancer T24 cell models of SHOX2 knockdown or overexpression were assessed for changes in migration and invasion abilities using wound healing assay and Transwell assay, and their cancer stem cell-like characteristics were evaluated using tumorsphere formation assay and colony formation assay. Western blotting was used to detect the expressions of epithelial mesenchymal transition (EMT) markers Ecadherin and vimentin and the TGF-β signaling network component TβR-I in the cells.
Results
SHOX2 expression was significantly higher in bladder cancer tissues than in the adjacent tissues (P < 0.05), especially in paired tissue specimens (P < 0.01), and was negatively correlated with the overall survival of the patients (P < 0.05). SHOX2 gene expression was correlated positively with EMT-related (P < 0.05) and stemness-related gene signatures (P < 0.01). In T24 cells, SHOX2 knockdown significantly suppressed cell migration and invasion, which was significantly enhanced by SHOX2 overexpression (P < 0.01). The cancer stem cell-like characteristics of T24 cells was repressed by SHOX2 knockdown but significantly enhanced by SHOX2 overexpression (P < 0.01). SHOX2 knockdown induced morphological changes of the cells into epithelioid cells, whereas SHOX2 overexpression induced a mesenchymal morphology of the cells. SHOX2 knockdown increased E-cadherin expression and decreased vimentin and TβR-I expression, while SHOX2 overexpression increased the expressions of vimentin and TβR-I in the cells.
Conclusion
SHOX2 promotes the migration, invasion and stemness of human bladder cancer cells possibly by regulating EMT via the TGF-β signaling pathway.
Keywords: SHOX2, bladder cancer, migration, invasion, stemness, EMT, TGF-β
膀胱癌是泌尿系统第2常见的恶性肿瘤,已成为严重的公共卫生威胁之一[1, 2]。约25%的膀胱癌为肌层浸润性膀胱癌,约75%为非肌层浸润性膀胱癌[3]。尽管非肌层浸润性膀胱癌可以通过经尿道膀胱肿瘤电切术等外科学方式进行治疗,但术后复发率高达70%,其中约20%进展为肌层浸润性膀胱癌,最终导致远处转移,预后不良[4]。因此,了解膀胱癌侵袭转移和复发的分子机制,探索新的治疗靶点极具临床意义和研究价值。
人矮小同源盒基因2(SHOX2)是人矮小同源盒基因的同源物[5],主要参与脊椎动物胚胎形成期的转录调控[6]。近年来,越来越多证据表明SHOX2在肿瘤的发生发展过程中扮演了重要角色。在肺癌中,SHOX2可以作为诊断和预后的生物标志物[7-9];在食管鳞状细胞癌、胃腺癌、口腔鳞状细胞癌中,SHOX2可以促进肿瘤侵袭转移[10-12];在肝癌中,高表达的SHOX2与术后复发密切相关[13]。然而,SHOX2在膀胱癌细胞中的生物学功能和作用机制目前国内外尚未见报道。因此,本研究分析了TCGA数据库中SHOX2基因在膀胱癌与癌旁膀胱组织中的表达差异及其对膀胱癌预后的影响,利用基因集富集分析GSEA软件对SHOX2的生物学功能进行预测,并通过功能缺失性及功能获得性实验,在细胞水平观察SHOX2对膀胱癌细胞迁移、侵袭能力和干细胞特性的影响。本研究探索SHOX2在膀胱癌细胞中对上皮间充质转化(EMT)的调控作用及其可能机制,为深入研究探讨膀胱癌侵袭转移和复发的机制提供重要的理论基础与实验依据。
1. 材料和方法
1.1. 细胞株、主要试剂和耗材
人膀胱癌细胞株T24(美国典型物保藏中心)。RPMI 1640培养基、DMEM/F12培养基、胎牛血清和胰蛋白酶(BI);SHOX2鼠单克隆抗体(Abcam);Ecadherin兔多克隆抗体、Vimentin兔多克隆抗体、β-actin鼠单克隆抗体、辣根过氧化物酶标记的羊抗兔抗体、辣根过氧化物酶标记的羊抗鼠抗体(proteintech);基质胶、Transwell小室、超低吸附六孔板(Corning);人EGF、人bFGF(Peprotech);B-27(Thermo Fisher);人胰岛素(Meilunbio);总蛋白提取试剂盒、蛋白浓度测定试剂盒、蛋白上样缓冲液、PVDF膜、ECL发光试剂盒、细胞培养皿、六孔板、PBS、甲醇、4%多聚甲醛、结晶紫、TBS、吐温等(碧云天)。
1.2. 膀胱癌细胞培养、SHOX2 shRNA及SHOX2过表达质粒转染
1.2.1. 膀胱癌细胞培养
膀胱癌细胞培养于含10%胎牛血清、100 U/mL青霉素和100 μg/mL链霉素的RPMI 1640培养基中,于37 ℃、5% CO2温育箱中培养,取对数生长期的细胞用于实验。
1.2.2. SHOX2 shRNA转染
提前1 d将T24细胞铺于6 cm培养皿,在37 ℃、5% CO2温育箱中孵育过夜。待细胞融合度达70%~80%,更换含有8~10 μg/mL polybrene的新鲜培养基,添加1 mL慢病毒液,在37 ℃、5%CO2温育箱中孵育过夜。
每次实验设3个组:对照组(Scramble)、实验组1、实验组2;实验组1:shSHOX2-1:正义链为5'-CCGGgg accaatttcaccctggaacCTCGAGgttccagggtgaaattggtccTT TTTG-3',反义链为5'-AATTCAAAAAggaccaatttcaccc tggaacCTCGAGgttccagggtgaaattggtcc-3';实验组2:shSHOX2-2,正义链为5'-CCGGggatgcgaaagggatggag gaCTCGAGtcctccatccctttcgcatccTTTTTG-3',反义链为5'-AATTCAAAAAggatgcgaaagggatggaggaCTCGAGtc ctccatccctttcgcatcc-3'。质粒载体为PLKO.1。
转染后24 h更换为新鲜培养基,向培养基中添加终浓度为3.5 μg/mL的嘌呤霉素进行筛选,药筛24 h后更换新鲜培养基,提取各组细胞的蛋白质,检测各项相关指标。
1.2.3. SHOX2过表达质粒转染
提前1 d将T24细胞铺于6 cm培养皿,在37 ℃、5%CO2温育箱中孵育过夜。待细胞融合度达70%~80%,将pc-DNA4-SFB-SHOX2和PEF-IRES-rtTA 1∶1比例用转染试剂PEI共转染入细胞,每次实验设2个组:对照组(Vector)、实验组(pSHOX2),转染12 h后更换新鲜培养基继续培养,36 h后,终浓度为3.5 μg/mL的嘌呤霉素药筛24 h后更换新鲜培养基,提取各组细胞的蛋白质,检测各项相关指标。
1.3. Western blot
细胞总蛋白通过总蛋白提取试剂盒提取,加入蛋白上样缓冲液,95 ℃下变性15 min,并保存于-20 ℃。凝胶电泳由10% SDS电泳液分离蛋白、湿法将蛋白转印至PVDF膜;5%BSA室温封闭1 h;加入一抗4 ℃孵育过夜;TBST液洗膜3次,洗5 min/次;加入二抗室温孵育1 h;TBST液洗膜4次,洗5 min/次;ECL发光试剂盒显影;GeneGnome XRQ化学发光成像系统获取图像。
1.4. 划痕实验
细胞以4×105/孔接种于6孔板,培养细胞使汇合度接近100%,用100 µL枪头在6孔板内画1条宽度均匀的直线,使两侧细胞不相连接,PBS洗涤清除漂浮细胞,加入完全培养基,放入37 ℃、5% CO2温育箱中培养,分别于0、24 h在同一部位用倒置显微镜观察并拍照,计算划痕愈合率,划痕愈合率=(0 h划痕面积-24 h划痕面积)/0 h划痕面积,实验重复3次。
1.5. Transwell侵袭实验
用基质胶包被Transwell小室底部膜,于4 ℃风干,再水化基底膜。细胞提前用无血清RPMI 1640培养基饥饿处理12 h后,收集细胞制成细胞悬液,将200 µL含1×105细胞数的悬液加入上室,下室加入500 µL含10% 胎牛血清的RPMI 1640培养基,放入37 ℃、5% CO2温育箱中培养24 h,取出小室,弃上室培养基,用干棉签轻拭去上室细胞,4%多聚甲醛固定,结晶紫染色,PBS洗涤,在显微镜下随机选取3个视野拍照计数。
1.6. 悬浮成球实验
将细胞分散接种于超低吸附六孔板中(2000细胞/ 孔),使用无血清DMEM/F12培养基,其中添加20 ng/mL人EGF、20 ng/mL人bFGF、5 ng/mL胰岛素、2% B-27。培养1~2周后,显微镜下观察并计算肿瘤球数目。
1.7. 克隆形成实验
将细胞分散接种于6孔板中(1000细胞/孔),培养1~2周。PBS洗涤后,4%多聚甲醛固定,结晶紫染色,PBS洗涤。直接观察,计算克隆数。
1.8. 生物信息学分析
利用GEPIA(<a href="http://gepia.cancer-pku.cn/" target="_blank">http://gepia.cancer-pku.cn/</a>)网站对TCGA膀胱癌数据集进行Kaplan-Meier生存分析;使用基因集富集分析(GSEA, <a href="http://software.broadinstitute.org/gsea/msigdb/index.jsp" target="_blank">http://software.broadinstitute.org/gsea/msigdb/index.jsp</a>)软件分析TCGA膀胱癌数据集。
1.9. 统计学分析
应用SPSS 22.0统计软件进行统计学检验,计量资料采用均数±标准差表示,组间比较用两样本t检验,以P < 0.05为差异有统计学意义。图片数据的采集及处理用Graphpad Prism5和Image J软件进行。每次实验重复3次。
2. 结果
2.1. SHOX2基因在膀胱癌组织中高表达并与不良预后相关
TCGA膀胱癌标本数据分析显示,与癌旁膀胱组织相比,SHOX2基因在膀胱癌组织中的表达量升高(P=0.01,图 1A),此趋势在配对的膀胱癌组织中更为明显(P=0.001,图 1B)。GEPIA网站Kaplan-Meier生存分析显示,SHOX2基因表达水平与膀胱癌患者总体生存率呈负相关(P=0.01,图 1C)。
1.
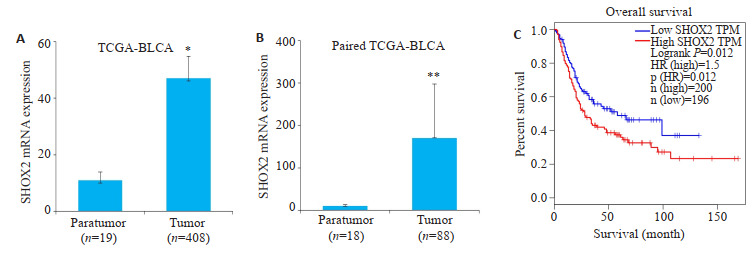
SHOX2基因在TCGA数据库膀胱癌组织中高表达并与不良预后相关
SHOX2 gene expression was significantly elevated in bladder cancer tissues based on TCGA-BLCA datasets and was associated with poor survival of the patients. A: Relative SHOX2 mRNA expression in bladder cancer tissues (n=408) and adjacent bladder tissues (n=19, *P=0.01). B: Relative SHOX2 mRNA expression in bladder cancer tissues (n=18) and paired adjacent tissues (n=18, **P=0.001). C: Kaplan-Meier survival analysis of overall survival of patients with bladder cancer with high and low SHOX2 expression level using GEPIA(P=0.01).
2.2. SHOX2基因表达水平与EMT及肿瘤干细胞特性呈正相关
SHOX2基因表达水平与EMT(P=0.01,P=0.03,图 2A)及肿瘤干细胞相关的基因集呈正相关(P=0.006,P=0.008,图 2B)。
2.
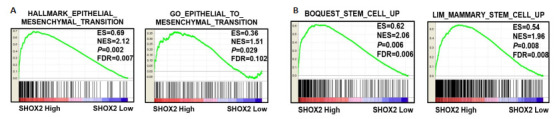
SHOX2基因在GSEA数据库中与膀胱癌的EMT及干细胞特性呈正相关
SHOX2 gene expression is positively correlated with EMT-related and stemness-related gene signatures of bladder cancer based on GSEA database. A: Data from GSEA indicate significant correlations between SHOX2 gene expression and EMT-related gene signatures of bladder cancer (HALLMARK_EPITHELIAL_MESENCHYMAL_TRANSITION, P=0.002; GO_EPITHELIAL_TO_MESENCHYMAL_ TRANSITION, P=0.03). B: SHOX2 gene expression was significantly correlated with stemness-related gene signatures of bladder cancer (BOQUEST_STEM_CELL_UP, P=0.006; LIM_MAMMARY_STEM_CELL_UP, P=0.008).
2.3. 构建SHOX2敲低、过表达的稳定细胞株
Western blot分析显示,在转染SHOX2 shRNA的膀胱癌细胞T24中SHOX2蛋白表达水平显著降低(shSHOX2-1,P=0.004;shSHOX2-2,P=0.03,图 3A);而转染SHOX2过表达质粒的T24细胞中,SHOX2的蛋白表达水平显著升高(P=0.007,图 3B)。
3.
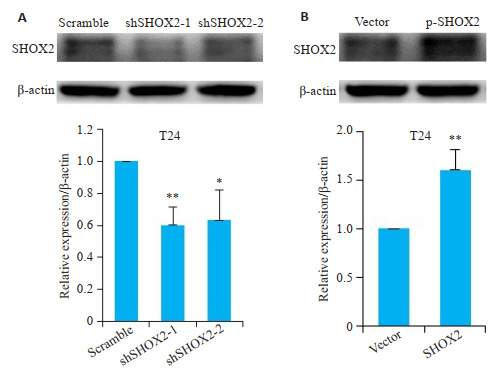
SHOX2 shRNA及SHOX2过表达质粒转染T24细胞后SHOX2蛋白的表达水平
Expression of SHOX2 in T24 cells after transfection with SHOX2 shRNA or SHOX2 lentivirus. A: Effect of SHOX2 shRNA and scramble shRNA on SHOX2 expression in T24 cells detected by Western blotting (**P=0.004, *P=0.03 vs scramble). B: Effect of SHOX2 lentivirus and vector control on SHOX2 expression in T24 cells detected by Western blotting (**P=0.007).
2.4. SHOX2促进膀胱癌细胞的迁移、侵袭
划痕实验结果显示,shSHOX2敲低组细胞划痕面积愈合百分比与对照组Scramble相比明显减少(shSHOX2-1,P < 0.001;shSHOX2-2,P=0.02,图 4A);过表达SHOX2组细胞划痕面积愈合百分比与对照组Vector相比明显增加(P < 0.001,图 4B)。
4.
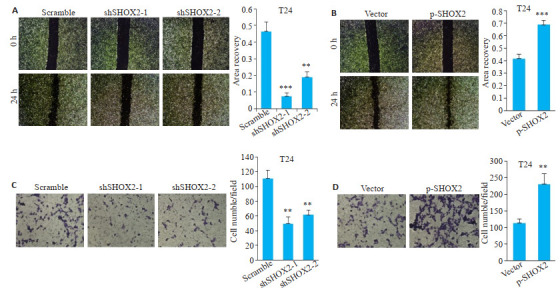
SHOX2促进膀胱癌细胞的迁移、侵袭
SHOX2 promotes migration and invasion of bladder cancer cells. A: Wound healing assay of T24 cells transfected with SHOX2 shRNA or scramble shRNA (Original magnification: × 40) and quantitative analysis (***P < 0.001, **P=0.02 vs scramble). B: Wound healing assay of T24 cells transfected with SHOX2 lentivirus or the control vector (× 40) and quantitative analysis (***P < 0.001). C: Transwell assay in T24 cells transfected with SHOX2 shRNA or scramble shRNA (×200) and quantitative analysis (shSHOX2-1, **P=0.002 vs scramble; shSHOX2-2, **P=0.003 vs scramble). D: Transwell assay ofn T24 cells transfected with SHOX2 lentivirus or the control vector (×200) and quantitative analysis (**P=0.004).
Transwell侵袭实验结果显示,shSHOX2敲低组细胞穿透至下层的数量较Scramble组明显减少(shSHOX2-1,P=0.002;shSHOX2-2,P=0.003,图 4C);过表达SHOX2组细胞穿透至下层的数量较Vector组明显增加(P=0.004,图 4D)。
2.5. SHOX2增强膀胱癌细胞的干细胞特性
悬浮成球实验结果显示,shSHOX2敲低组细胞形成肿瘤球的数目与对照组Scramble相比减少(shSHOX2-1,P=0.002;shSHOX2-2,P=0.007,图 5A);过表达SHOX2组细胞形成肿瘤球的数目与对照组Vector相比增加(P=0.002,图 5B)。
5.
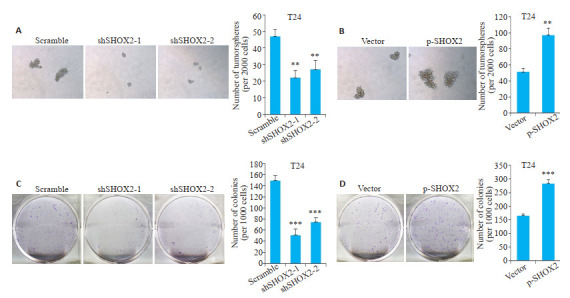
SHOX2增强膀胱癌细胞的干细胞特性
SHOX2 enhances cancer stem cell-like characteristics of bladder cancer cells. A: Tumorsphere formation assay of T24 cells transfected with SHOX2 shRNA or scramble shRNA (×100) (shSHOX2-1, **P=0.002 vs scramble; shSHOX2-2, **P=0.007 vs scramble); B: Tumorsphere formation assay of T24 cells transfected with SHOX2 lentivirus or the control vector (× 100) (**P=0.002). C: Colony formation assay of T24 cells transfected with SHOX2 shRNA or scramble shRNA (***P < 0.001 vs scramble); D: Colony formation assay of T24 cells transfected with SHOX2 lentivirus or the control vector (***P < 0.001 vs vector).
克隆形成实验结果显示,shSHOX2敲低组细胞克隆形成数与对照组Scramble相比减少(shSHOX2-1,P < 0.001;shSHOX2-2,P < 0.001,图 5C);过表达SHOX2组细胞克隆形成数与对照组Vector相比增加(P < 0.001,图 5D)。
2.6. SHOX2通过TGF-β信号通路诱导EMT
在光镜下观察膀胱癌细胞T24的形态变化,结果显示抑制SHOX2表达可使细胞呈现连接紧密的卵圆形的上皮样形态(图 6A),而SHOX2过表达可使细胞呈现连接松散的长条形的间充质样形态(图 6B)。
6.
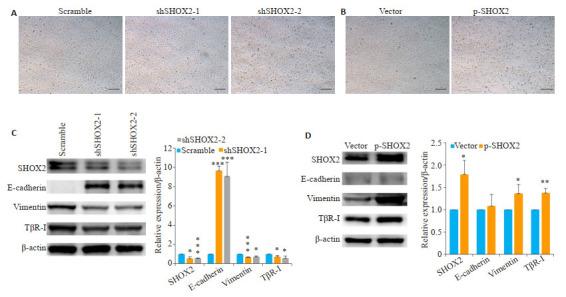
SHOX2通过TGF-β信号通路诱导EMT
SHOX2 induces EMT through TGF-β signaling pathway. A: Morphology of T24 cells transfected with SHOX2 shRNA or scramble shRNA (× 200); B: Morphology of T24 cells transfected with SHOX2 lentivirus or the control vector (Original magnification: ×200); C: Effect of SHOX2 shRNA and scramble shRNA on E-cadherin (***P < 0.001), vimentin (***P < 0.001, *P=0.01) and TβR-I (shSHOX2-1, *P=0.03; shSHOX2-2, *P=0.02) expressions in T24 cells detected by Western blotting; D: Effect of SHOX2 lentivirus and the control vector on vimentin (*P=0.04 vs vector) and TβR-I (**P=0.003 vs vector) expression in T24 cells detected by Western blotting.
Western blot结果显示,抑制SHOX2的表达可使膀胱癌细胞T24中的EMT标志分子E-cadherin蛋白(shSHOX2-1,P < 0.001;shSHOX2-2,P < 0.001,图 6C)表达水平升高、Vimentin蛋白(shSHOX2-1,P < 0.001;shSHOX2-2,P=0.01,图 6C)表达水平降低,而过表达SHOX2的膀胱癌细胞中Vimentin蛋白(P=0.04,图 6D)表达水平升高。此外,抑制SHOX2的表达可使T24细胞中的TGF-β信号通路重要组件TβR-I蛋白(shSHOX2-1,P=0.03;shSHOX2-2,P=0.02,图 6C)表达水平降低,而过表达SHOX2的T24细胞中TβR-I蛋白(P=0.003,图 6D)表达水平升高。
3. 讨论
SHOX2最早在肺癌中被证实与肿瘤的发生发展有关,SHOX2的表达量在肺癌早期即可发生变化,多项研究表明SHOX2启动子甲基化可作为肺癌诊断和预后的生物标志物[7-9]。有研究在原发性肝癌临床手术标本中对比分析SHOX2表达量和患者术后复发的关系,发现SHOX2与肝癌的术后复发密切相关[13]。近年来,越来越多的证据表明SHOX2可以通过促进肿瘤的侵袭转移来影响肿瘤的预后[10-12]。本研究通过分析TCGA膀胱癌标本数据,发现SHOX2基因在膀胱癌组织中高表达,并与膀胱癌患者临床预后不良密切相关,这支持了SHOX2在膀胱癌中的致癌作用。同时,我们通过对TCGA数据库中膀胱癌样本进行GSEA富集分析,预测SHOX2可能的生物学功能。结果显示,SHOX2基因表达水平与上皮间充质转化(EMT)及肿瘤干细胞特性呈正相关。
为了进一步验证SHOX2在膀胱癌细胞中的生物学功能与作用机制,我们采用SHOX2 shRNA及SHOX2过表达质粒转染膀胱癌细胞T24,构建SHOX2敲低、过表达的稳定细胞株。划痕实验、Transwell侵袭实验结果显示,下调SHOX2的表达后,T24细胞的迁移、侵袭能力明显下降,而上调SHOX2的表达则显著增强T24细胞的迁移、侵袭能力,这表明SHOX2可以促进膀胱癌细胞的迁移及侵袭。EMT在肿瘤的侵袭转移中起关键作用[14, 15]。EMT是指具有极性的上皮样细胞转化为运动能力较强的间充质样细胞,从而使细胞在细胞外基质中的移动能力增强[16, 17],EMT对膀胱癌的侵袭转移起着极为重要的作用[18, 19]。有研究证实了SHOX2在食管鳞状细胞癌细胞中可以促进EMT的发生[10],本研究也发现,在膀胱癌细胞T24中抑制SHOX2的表达可使细胞呈现上皮样形态,且EMT标志分子E-cadherin蛋白表达水平升高、Vimentin蛋白表达水平降低;而过表达SHOX2可使T24细胞呈现间充质样形态,且Vimentin蛋白表达水平升高,说明SHOX2在膀胱癌细胞中可以促进EMT,这与我们的生物信息学分析结果一致。
肿瘤干细胞是指具有自我更新和多向分化潜能的肿瘤细胞亚群[20, 21],干细胞特性的维持与膀胱癌的侵袭转移和复发密切相关[22-24]。越来越多的证据表明,EMT过程可以使肿瘤干细胞富集,从而使肿瘤细胞的干细胞特性增强[25, 26]。有研究发现,EMT也可以增强膀胱癌细胞的干细胞特性[27, 28]。本研究通过悬浮成球实验和克隆形成实验,发现下调SHOX2的表达后,T24细胞的成球数和克隆形成数均下降,而上调SHOX2的表达则可显著增加T24细胞的成球数和克隆形成数,说明SHOX2确实可以在一定程度上增强膀胱癌细胞的干细胞特性,与生物信息学分析结果一致。
TGF-β信号通路在诱导肿瘤细胞EMT过程中起重要作用[29-31],许多研究证明TGF-β信号通路可以促进膀胱癌细胞EMT[32-33]。有研究发现SHOX2在乳腺癌细胞中可以通过调控TGF-β信号通路中的重要组件TβR-I来诱导EMT过程[34]。本研究发现,在膀胱癌细胞T24中抑制SHOX2的表达可使TβR-I蛋白表达水平降低,而过表达SHOX2可使T24细胞中的TβR-I蛋白表达水平升高,说明SHOX2可能参与调控TGF-β信号通路。结合实验结果,本研究认为SHOX2可能通过激活TGF-β信号通路,参与调控EMT过程,进而增强膀胱癌细胞的迁移、侵袭能力和干细胞特性。
综上所述,本研究首先通过生物信息学分析,发现SHOX2基因在膀胱癌组织中高表达且与膀胱癌患者临床预后不良相关,其生物学功能与EMT和肿瘤干细胞特性密切相关。通过功能缺失性及功能获得性实验证实SHOX2能增强膀胱癌细胞迁移、侵袭能力及肿瘤干细胞特性,其作用机制可能与TGF-β信号通路参与调控的EMT有关。这表明针对SHOX2靶点的基因治疗可能成为减少膀胱癌侵袭转移和复发的潜在治疗手段,但SHOX2是如何激活TGF-β信号通路,最终诱导膀胱癌细胞EMT,值得进一步研究。
Biographies
植曦,医师,E-mail: 503691370@qq.com
周俊豪,医师,Email: zhouatian@163.com
Funding Statement
国家自然科学基金(81772257)
Supported by National Natural Science Foundation of China (81772257)
Contributor Information
植 曦 (Xi ZHI), Email: 503691370@qq.com.
周 俊豪 (Junhao ZHOU), Email: zhouatian@163.com.
刘 存东 (Cundong LIU), Email: cundongliu@163.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA: a Cancer J Clin, 2018, 68(6): 394-424.] [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. Cancer of the bladder-cancer stat facts [OL]. SEER. https://seer.cancer.gov/statfacts/html/urinb.html.
- 3.Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. http://www.sciencedirect.com/science/article/pii/S030228382030230X. Eur Urol. 2021;79(1):82–104. doi: 10.1016/j.eururo.2020.03.055. [Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines[J]. Eur Urol, 2021, 79(1): 82-104.] [DOI] [PubMed] [Google Scholar]
- 4.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J Urol. 2016;196(4):1021–9. doi: 10.1016/j.juro.2016.06.049. [Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline[J]. J Urol, 2016, 196(4): 1021-9.] [DOI] [PubMed] [Google Scholar]
- 5.Liu H, Espinoza-Lewis RA, Chen C, et al. The role of Shox2 in SAN development and function. Pediatr Cardiol. 2012;33(6):882–9. doi: 10.1007/s00246-012-0179-x. [Liu H, Espinoza-Lewis RA, Chen C, et al. The role of Shox2 in SAN development and function[J]. Pediatr Cardiol, 2012, 33(6): 882-9.] [DOI] [PubMed] [Google Scholar]
- 6.Rosin JM, McAllister BB, Dyck RH, et al. Mice lacking the transcription factor SHOX2 display impaired cerebellar development and deficits in motor coordination. Dev Biol. 2015;399(1):54–67. doi: 10.1016/j.ydbio.2014.12.013. [Rosin JM, McAllister BB, Dyck RH, et al. Mice lacking the transcription factor SHOX2 display impaired cerebellar development and deficits in motor coordination[J]. Dev Biol, 2015, 399(1): 54-67.] [DOI] [PubMed] [Google Scholar]
- 7.Song L, Yu H, Li Y. Diagnosis of lung cancer by SHOX2 gene methylation assay. Mol Diagn Ther. 2015;19(3):159–67. doi: 10.1007/s40291-015-0144-5. [Song L, Yu H, Li Y. Diagnosis of lung cancer by SHOX2 gene methylation assay[J]. Mol Diagn Ther, 2015, 19(3): 159-67.] [DOI] [PubMed] [Google Scholar]
- 8.Zhang CZ, Yu WJ, Wang L, et al. DNA methylation analysis of the SHOX2 and RASSF1A panel in bronchoalveolar lavage fluid for lung cancer diagnosis. J Cancer. 2017;8(17):3585–91. doi: 10.7150/jca.21368. [Zhang CZ, Yu WJ, Wang L, et al. DNA methylation analysis of the SHOX2 and RASSF1A panel in bronchoalveolar lavage fluid for lung cancer diagnosis [J]. J Cancer, 2017, 8(17): 3585-91.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss G, Schlegel A, Kottwitz D, et al. Validation of the SHOX2/ PTGER4 DNA methylation marker panel for plasma-based discrimination between patients with malignant and nonmalignant lung disease. J Thorac Oncol. 2017;12(1):77–84. doi: 10.1016/j.jtho.2016.08.123. [Weiss G, Schlegel A, Kottwitz D, et al. Validation of the SHOX2/ PTGER4 DNA methylation marker panel for plasma-based discrimination between patients with malignant and nonmalignant lung disease[J]. J Thorac Oncol, 2017, 12(1): 77-84.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi J, Jin L, Chen J, et al. MiR-375 suppresses invasion and metastasis by direct targeting of SHOX2 in esophageal squamous cell carcinoma. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=swhxyswwlxb201702007. Acta Biochim Biophys Sin. 2017;49(2):159–69. doi: 10.1093/abbs/gmw131. [Yi J, Jin L, Chen J, et al. MiR-375 suppresses invasion and metastasis by direct targeting of SHOX2 in esophageal squamous cell carcinoma[J]. Acta Biochim Biophys Sin, 2017, 49(2): 159-69.] [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Chen YX, Zhang B. IGF2-AS affects the prognosis and metastasis of gastric adenocarcinoma via acting as a CeRNA of miR-503 to regulate SHOX2. Gastric Cancer. 2020;23(1):23–38. doi: 10.1007/s10120-019-00976-2. [Huang J, Chen YX, Zhang B. IGF2-AS affects the prognosis and metastasis of gastric adenocarcinoma via acting as a CeRNA of miR-503 to regulate SHOX2[J]. Gastric Cancer, 2020, 23(1): 23-38.] [DOI] [PubMed] [Google Scholar]
- 12.Sun C, Liu XH, Sun YR. MiR-223-3p inhibits proliferation and metastasis of oral squamous cell carcinoma by targeting SHOX2. http://www.ncbi.nlm.nih.gov/pubmed/31486492. Eur Rev Med Pharmacol Sci. 2019;23(16):6927–34. doi: 10.26355/eurrev_201908_18732. [Sun C, Liu XH, Sun YR. MiR-223-3p inhibits proliferation and metastasis of oral squamous cell carcinoma by targeting SHOX2[J]. Eur Rev Med Pharmacol Sci, 2019, 23(16): 6927-34.] [DOI] [PubMed] [Google Scholar]
- 13.Yang T, Zhang H, Cai SY, et al. Elevated SHOX2 expression is associated with tumor recurrence of hepatocellular carcinoma. http://europepmc.org/abstract/med/23851611. Ann Surg Oncol. 2013;20(Suppl 3):S644-9. doi: 10.1245/s10434-013-3132-1. [Yang T, Zhang H, Cai SY, et al. Elevated SHOX2 expression is associated with tumor recurrence of hepatocellular carcinoma[J]. Ann Surg Oncol, 2013, 20(Suppl 3): S644-9.] [DOI] [PubMed] [Google Scholar]
- 14.Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–50. doi: 10.1038/nature04872. [Christofori G. New signals from the invasive front [J]. Nature, 2006, 441(7092): 444-50.] [DOI] [PubMed] [Google Scholar]
- 15.Liang X. EMT: new signals from the invasive front. Oral Oncol. 2011;47(8):686–7. doi: 10.1016/j.oraloncology.2011.04.016. [Liang X. EMT: new signals from the invasive front[J]. Oral Oncol, 2011, 47(8): 686-7.] [DOI] [PubMed] [Google Scholar]
- 16.Smith B, Bhowmick N. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5(2):17–25. doi: 10.3390/jcm5020017. [Smith B, Bhowmick N. Role of EMT in metastasis and therapy resistance[J]. J Clin Med, 2016, 5(2): 17-25.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. http://annonc.oxfordjournals.org/external-ref?access_num=19262571&link_type=MED. Nat Rev Cancer. 2009;9(4):265–73. doi: 10.1038/nrc2620. [Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits[J]. Nat Rev Cancer, 2009, 9(4): 265-73.] [DOI] [PubMed] [Google Scholar]
- 18.McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-tomesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer. http://www.ncbi.nlm.nih.gov/pubmed/20012924?dopt=AbstractPlus. Cancer Metastasis Rev. 2009;28(3/4):335–44. doi: 10.1007/s10555-009-9194-7. [McConkey DJ, Choi W, Marquis L, et al. Role of epithelial-tomesenchymal transition (EMT) in drug sensitivity and metastasis in bladder cancer [J]. Cancer Metastasis Rev, 2009, 28(3/4): 335-44.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo CC, Majewski T, Zhang L, et al. Dysregulation of EMT drives the progression to clinically aggressive sarcomatoid bladder cancer. Cell Rep. 2019;27(6):1781–93. doi: 10.1016/j.celrep.2019.04.048. [Guo CC, Majewski T, Zhang L, et al. Dysregulation of EMT drives the progression to clinically aggressive sarcomatoid bladder cancer [J]. Cell Rep, 2019, 27(6): 1781-93.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–61. doi: 10.1056/NEJMra061808. [Jordan CT, Guzman ML, Noble M. Cancer stem cells[J]. N Engl J Med, 2006, 355(12): 1253-61.] [DOI] [PubMed] [Google Scholar]
- 21.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–34. doi: 10.1038/nm.4409. [Batlle E, Clevers H. Cancer stem cells revisited[J]. Nat Med, 2017, 23(10): 1124-34.] [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Lin K, Yang Z, et al. Bladder cancer stem cells: clonal origin and therapeutic perspectives. http://pubmedcentralcanada.ca/pmcc/articles/PMC5630446/ Oncotarget. 2017;8(39):668–79. doi: 10.18632/oncotarget.19112. [Li Y, Lin K, Yang Z, et al. Bladder cancer stem cells: clonal origin and therapeutic perspectives [J]. Oncotarget, 2017, 8(39): 668-79.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aghaalikhani N, Rashtchizadeh N, Shadpour P, et al. Cancer stem cells as a therapeutic target in bladder cancer. J Cell Physiol. 2019;234(4):3197–206. doi: 10.1002/jcp.26916. [Aghaalikhani N, Rashtchizadeh N, Shadpour P, et al. Cancer stem cells as a therapeutic target in bladder cancer[J]. J Cell Physiol, 2019, 234(4): 3197-206.] [DOI] [PubMed] [Google Scholar]
- 24.王 芳, 王 志平. 膀胱肿瘤干细胞研究进展. https://www.cnki.com.cn/Article/CJFDTOTAL-SCYX200702010.htm. 中华泌尿外科杂志. 2013;(6):474–8. [王芳, 王志平. 膀胱肿瘤干细胞研究进展[J]. 中华泌尿外科杂志, 2013(6): 474-8.] [Google Scholar]
- 25.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. http://www.tandfonline.com/servlet/linkout?suffix=CIT0085&dbid=8&doi=10.1517%2F14728220903544507&key=18485877. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. [Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells[J]. Cell, 2008, 133(4): 704-15.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floor S, van Staveren WC, Larsimont D, et al. Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. http://carcin.oxfordjournals.org/external-ref?access_num=10.1038/onc.2011.184&link_type=DOI. Oncogene. 2011;30(46):4609–21. doi: 10.1038/onc.2011.184. [Floor S, van Staveren WC, Larsimont D, et al. Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations[J]. Oncogene, 2011, 30(46): 4609-21.] [DOI] [PubMed] [Google Scholar]
- 27.Islam SS, Mokhtari RB, Noman AS, et al. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. http://onlinelibrary.wiley.com/doi/10.1002/mc.22300. Mol Carcinog. 2016;55(5):537–51. doi: 10.1002/mc.22300. [Islam SS, Mokhtari RB, Noman AS, et al. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer[J]. Mol Carcinog, 2016, 55(5): 537-51.] [DOI] [PubMed] [Google Scholar]
- 28.Migita T, Ueda A, Ohishi T, et al. Epithelial-mesenchymal transition promotes SOX2 and NANOG expression in bladder cancer. http://www.nature.com/articles/labinvest201717. Lab Invest. 2017;17:102–11. doi: 10.1038/labinvest.2017.17. [Migita T, Ueda A, Ohishi T, et al. Epithelial-mesenchymal transition promotes SOX2 and NANOG expression in bladder cancer[J]. Lab Invest, 2017, 17: 102-11.] [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. http://europepmc.org/abstract/MED/19153598. Cell Res. 2009;19(2):156–72. doi: 10.1038/cr.2009.5. [Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition[J]. Cell Res, 2009, 19(2): 156-72.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed V. TGF-beta signaling in cancer. http://smartsearch.nstl.gov.cn/paper_detail.html?id=11f72511ce1621540d627d3cf226d6fc. J Cell Biochem. 2016;117(6):1279–87. doi: 10.1002/jcb.25496. [Syed V. TGF-beta signaling in cancer [J]. J Cell Biochem, 2016, 117(6): 1279-87.] [DOI] [PubMed] [Google Scholar]
- 31.刘 丽华, 代 勤, 闵 志刚, et al. 转化生长因子β1通过ERK/MAPK信号转导通路促进脑胶质瘤细胞侵袭. http://www.j-smu.com/CN/Y2013/V33/I12/1744. 南方医科大学学报. 2013;33(12):1744–7. [刘丽华, 代勤, 闵志刚, 等. 转化生长因子β1通过ERK/MAPK信号转导通路促进脑胶质瘤细胞侵袭[J]. 南方医科大学学报, 2013, 33(12): 1744-7.] [PubMed] [Google Scholar]
- 32.Chen Z, He S, Zhan Y, et al. TGF-beta-induced transgelin promotes bladder cancer metastasis by regulating epithelial-mesenchymal transition and invadopodia formation. http://www.sciencedirect.com/science/article/pii/S2352396419305286. EBio Med. 2019;151:47208–220. doi: 10.1016/j.ebiom.2019.08.012. [Chen Z, He S, Zhan Y, et al. TGF-beta-induced transgelin promotes bladder cancer metastasis by regulating epithelial-mesenchymal transition and invadopodia formation[J]. EBio Med, 2019, 151: 47208-220.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan M, Zhang D, Zhang E, et al. SENP2 suppresses epithelialmesenchymal transition of bladder cancer cells through deSUMOylation of TGF-betaRI. Mol Carcinog. 2017;56(10):2332–41. doi: 10.1002/mc.22687. [Tan M, Zhang D, Zhang E, et al. SENP2 suppresses epithelialmesenchymal transition of bladder cancer cells through deSUMOylation of TGF-betaRI[J]. Mol Carcinog, 2017, 56(10): 2332-41.] [DOI] [PubMed] [Google Scholar]
- 34.Hong S, Noh H, Teng Y, et al. SHOX2 is a direct miR-375 target and a novel epithelial-to-mesenchymal transition inducer in breast cancer cells. Neoplasia. 2014;16(4):279–90. doi: 10.1016/j.neo.2014.03.010. [Hong S, Noh H, Teng Y, et al. SHOX2 is a direct miR-375 target and a novel epithelial-to-mesenchymal transition inducer in breast cancer cells [J]. Neoplasia, 2014, 16(4): 279-90.] [DOI] [PMC free article] [PubMed] [Google Scholar]


