Abstract
The noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) is a strictly defined thyroid lesion, reclassified in 2016, in order to more accurately reflect the biological behavior of the tumor and thus, modify the way the lesion is clinically approached and perceived both by practitioners and patients. Additionally, this newly specified designation also allows for more uniformity in reporting for general pathologists less comfortable to exclude overt malignancy with certain nuclear features. In recent years, increasing molecular analyses correlated with longitudinal clinical outcomes have fostered improved diagnostic and treatment paradigms. Important revisions made to the definition of NIFTP in 2018 include the prohibition of any true papillae formation and the exclusion of lesions harboring the BRAF V600E mutation and other high-risk genetic abnormalities. These changes reflect the imperfection of the current criteria in outcome prediction and the global efforts for improvement. NIFTP are lesions with a wide range of size and cytomorphology. Although not addressed in the original series, large (≥4 cm) and oncocytic NIFTP have recently been shown to incur no recurrence or metastatic risk. Molecularly, NIFTP have a similar mutational profile as other follicular thyroid neoplasms, with frequent RAS family mutations and PAX8-PPARɤ fusions. However, the transcriptomic landscape is highly heterogenous, adding difficulty to gene expression-based cytopathologic classification. This review summarizes the evolution of the NIFTP concept and important advances in recent literature.
Keywords: Noninvasive follicular thyroid neoplasm with papillary-like nuclear features, Oncocytic, BRAF, TERT, Radioactive iodine, Lobectomy
Introduction
The noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) is a recently defined entity that has been recognized by the current (2017) World Health Organization Classification of Tumors of Endocrine Organs.1 Prior to the introduction of NIFTP, several of the so-called papillary thyroid carcinoma (PTC) nuclear features, namely nuclear enlargement, irregularity and chromatin clearing, had long been considered sufficient for classifying a thyroid neoplasm as malignant, and indeed, papillary thyroid carcinoma. The follicular variant of PTC (FVPTC) is defined by predominantly follicular architectural growth pattern and has accounted for 9 to 23% of PTC.2 FVPTC can be further subclassified into unencapsulated/infiltrative (IFVPTC) and encapsulated (EFVPTC) tumors. Several studies have demonstrated that IFVPTC and EFVPTC are biologically and molecularly distinct.2,3 While IFVPTC frequently harbor the BRAF V600E mutation and present with lymph node metastases, the majority of EFVPTC are organ-confined and associated with RAS family mutations.2,3 In 2016, an international panel of thyroid cancer experts, predominantly pathologists, reviewed 109 curated cases that had been formerly diagnosed as EFVPTC.4 It was noted that this subset of EFVPTC that lack any invasive growth and met a rigorous set of morphologic criteria (Table 1) essentially carried no risk of recurrence or metastasis,4 and the term NIFTP was created as a new designation for this group of non-infiltrative neoplasms. As the subsequent literature has consistently demonstrated the extremely indolent behavior of these tumors,5,6 the NIFTP designation has solidified. This classification, in part, has been intended to remove the stigma and psychological sensitivity for patients of an indolent tumor as well as to provide proper conservative management guidelines for endocrinologists and surgeons.
Table 1.
Comparison of the original (2016) and revised (2018) diagnostic criteria for NIFTP.
| Original Criteria4 | Revised Criteria 20 | |
|---|---|---|
| Tumor boundary | Encapsulation or sharp demarcation | Same as the original |
| Growth pattern | Predominantly follicular with <30% solid/trabecular/insular patterns | Same as the original |
| PTC nuclear features | At least score 2 | Same as the original |
| Capsular invasion | None allowed | Same as the original |
| Vascular invasion | None allowed | Same as the original |
| Psammoma bodies | None allowed | Same as the original |
| Mitotic activity | <3 per 10 high-power fields | Same as the original |
| Tumor necrosis | None allowed | Same as the original |
| True papillae | <1% allowed | None allowed |
| Lack of BRAF V600E mutation a | Not required | Optionalb |
| Lack of RET/PTC rearrangement | Not required | Optionalb |
| Lack of TERT promotor mutations | Not required | Optionalb |
Determined by immunohistochemistry or molecular analysis.
These additional molecular characteristics have proven to be supportive to discern NIFTP from malignant counterparts. Although this information may be helpful, lack of these data does not preclude a diagnosis of NIFTP.
Considering the profound clinical consequence of this nomenclature change, there has been persistent interest toward refining the diagnostic criteria for NIFTP.7 In addition, with the advent of high-throughput genomic technologies, several studies have obtained important findings about the genomic landscape of NIFTP.8,9 This review aims to summarize the literature and highlight the unsolved issues regarding the diagnostic definition and molecular pathogenesis of NIFTP.
Sonographic and cytopathologic presentation
The new classification of NIFTP has led to a large number of radiologic studies aimed at presurgical recognition of NIFTP. Sonographically, most NIFTP cases fall under the low and intermediate suspicion categories based on the American Thyroid Association classification scheme,10 with a typical sonographic appearance of circumscribed, wider-than-tall nodule with a hypoechogenic rim, hypervascular Doppler, and lack of calcifications.10–12 It has proven difficult to distinguish NIFTP from minimally invasive EFVPTC, quite understandably, using ultrasound, due to their largely overlapping appearance.12–14
Although it remains an unsolved challenge to diagnose NIFTP based on fine needle aspiration (FNA) cytopathology, the nomenclature change has a crucial impact, particularly on the surgical approach for cytologically indeterminate thyroid nodules. Several studies have retrospectively reviewed the FNA diagnosis of NIFTP, and found the most common cytologic diagnoses to be atypia of undetermined significance/ follicular lesion of undetermined significance (AUS/FLUS, category III), suspicious for a follicular neoplasm (category IV), or suspicious for malignancy (category V), with only 2–10% of cases designated as benign or malignant based on The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC).15–17 While FNA cytopathology can distinguish most classical PTC from NIFTP, it is currently infeasible to separate follicular-patterned entities (FVPTC, NIFTP, other follicular-patterned thyroid tumors) due to their overlapping cytologic features.18 Nevertheless, the designation of NIFTP as non-cancer has led to significantly lowered positive predictive value for TBSRTC categories III-V thyroid nodules. Accordingly, patients may be counseled towards more conservative surgical options.15,19
Histologic criteria
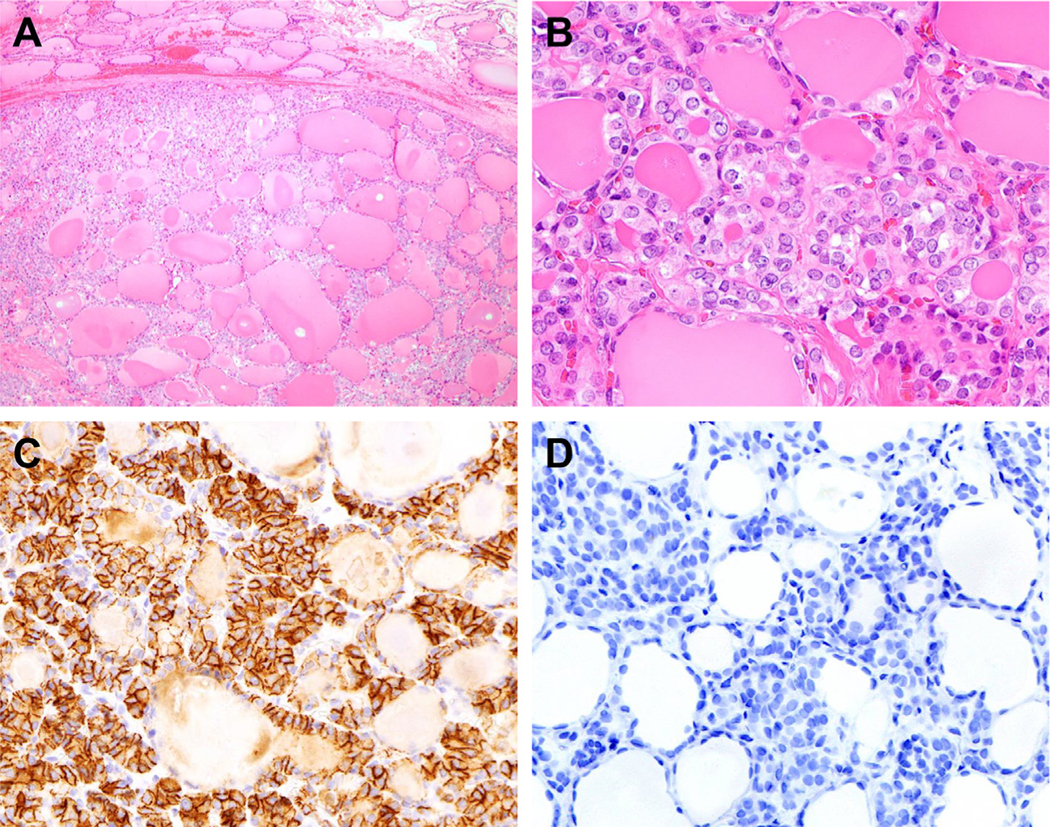
The original definition proposed by Nikiforov et al. in 2016 included rigorously defined sets of inclusion and exclusion criteria. The histologic hallmarks of NIFTP are complete encapsulation or clear demarcation, a predominantly follicular growth pattern, and unequivocal PTC nuclear features (Table 1; Fig. 1A and B). To minimize subjectivity, a scoring system has been introduced to evaluate three aspects of PTC nuclear features: (1) nuclear size and shape, (2) membranous irregularity, and (3) chromatin quality. Each aspect receives a score of 0 to 1, and a total score of at least 2 is required rendering a NIFTP diagnosis.4 The discernibility of PTC nuclear changes often varies between different subsites within the same tumor (the sprinkling phenomenon). When in doubt, an HBME1 immunostain can be helpful as most NIFTP show diffuse membranous positivity (Fig. 1C). The nuclear changes in NIFTP are generally softer (typically score 2) compared to classic PTC. When prominent PTC nuclear features (score 3) are present, the entire lesion should be carefully examined to exclude invasion or papillae formation. A minor (less than 30%) solid, trabecular or insular component is allowable. Regarding the above-mentioned nuclear scoring system, it was derived for pathologists to facilitate threshold determination and uniformity of diagnostic criteria but is not part of the reporting structure.
Fig. 1.
Histologic features of a case of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). (A) At low magnification (40X), the tumor is sharply delineated from the surrounding thyroid parenchyma. (B) Nuclear irregularity and chromatin clearing are evident at high magnification (400X). (C) HBME1 demonstrates prominent membranous staining. (D) Immunohistochemical stain using the BRAF V600E mutant specific antibody (VE1) is negative.
Exclusion criteria include appreciable mitotic activity (≥3 per 10 high-power fields), tumor necrosis, psammoma bodies and invasive growth (capsular or vascular).4 Although the original definition in 2016 allowed <1% of papillary growth, the revised criteria in 2018 demands total absence of true papillae, while rudimentary, hyperplastic-type papilla are permitted.20 This new requirement is based on the observation made by Cho et al. that up to 4% of tumors meeting the 2016 criteria with <1% papillae might still develop regional or distant metastases.21 The distinction between true and rudimentary papilla can be somewhat subjective. Some authors have suggested that a true papilla should have at least three cells on each side of a delicate, well-formed fibrovascular core.22 In order to exclude any papillary growth, the entire tumor including the central portion needs to be microscopically examined. However, the more common practice is evaluating the entire capsule plus representative central tissue. This approach comes with an undetermined risk of over-diagnosing NIFTP/under-diagnosing PTC, particularly in larger tumors.
The revised criteria also require the exclusion of tumors harboring the BRAF V600E mutation, RET/PTC rearrangements, and TERT promotor mutations (Table 1).20 The revision was made after rare BRAF V600E-positive cases were found to develop metastasis.21 The BRAF V600E mutation has commercially available mutant-specific antibody (VE1). The diagnostic utility of the VE1 antibody was recently evaluated by Johnson et al. by comparing a series of NIFTP, invasive EFVPTCs, and classical type PTC with predominantly follicular pattern.23 Of note, the VE1 antibody labeled none of the NIFTP, 14.8% of invasive EFVPTCs and 54.5% of classical PTC with a predominantly follicular growth pattern, reflecting underlying molecular differences.23 Based on the findings, the VE1 immunohistochemical stain appears to be a valuable ancillary test in detecting BRAF V600E mutation in follicular-patterned thyroid tumors.
Even without papillae formation, in one study, up to 6% of tumors that met the revised NIFTP criteria have been reported to be metastatic.24 Cho et al. analyzed 95 BRAF V600E-negative NIFTP with 0% papilla and found central cervical metastasis in 2 (3%) cases.21 As NIFTP is a relatively new concept and the majority of the literature are retrospective, additional studies are crucial for further refinement of the diagnostic criteria.
Ongoing controversies
Large and subcentimeter tumors
The introduction of NIFTP has the greatest clinical impact on large tumors. Prior to the nomenclature shift, non-invasive EFVPTC that are larger than 4 cm used to be staged as T3, and post-operative radioactive iodine (RAI) therapy should be considered per the 2015 American Thyroid Association (ATA) practice guidelines.10 As the new classification intends to encourage conservative management, such as lobectomy alone, it is of paramount importance to evaluate the necessity of RAI for large NIFTP. Most published NIFTP cohorts had a fair representation of large (≥4 cm) lesions and demonstrated consistently favorable outcomes.4, 5 In one study, Xu et al. examined a total of 79 NIFTP ranging 4.0 to 8.0 cm in size, of which 34% had received RAI therapy.6 With a median (range) follow-up of 5.9 (0.3 to 26.0) years, no recurrence or metastasis was observed.6 Another series was reported by Rosario, including 45 NIFTP ranging 4 to 7 cm in diameter.25 With a median (range) follow-up of 72 (18 to 144) months, all the cases remained in clinical remission.25 Although the currently limited data seem to support an indolent clinical course for large NIFTP, additional studies are needed to determine the optimal therapeutic strategy.
Another unresolved issue is the applicability of the NIFTP designation in subcentimeter tumors. The original series that lay the foundation for the design of NIFTP criteria did not include any subcentimeter tumor.4 It therefore remains unclear whether subcentimeter NIFTP follow a similarly indolent course and warrant the removal of microcarcinoma designation. To date, only 78 subcentimeter NIFTP have been reported by three independent studies, with no recurrence or metastasis noted.26–28 The largest study was by Xu et al., who evaluated 52 unifocal subcentimeter tumors that fulfilled all NIFTP diagnostic requirements. Throughout a median (range) follow-up of 4.3 (1.0 to 18.1) years, all subjects remained free of disease.26 The biological potential of subcentimeter NIFTP represents an ongoing need for research to provide proper prognostic guidance.
Oncocytic non-invasive encapsulated follicular variant papillary thyroid carcinoma
As the original study did not specify whether oncocytic features were present in its subjects,4 it is debatable whether tumors with predominantly oncocytic composition but otherwise fulfill the NIFTP criteria may behave indolently. Such tumors are often signed out as oncocytic non-invasive encapsulated follicular variant papillary thyroid carcinoma (O-NI-EFVPTC). In fact, the British guidelines published by the Royal College of Pathologists in 2016 listed oncocytic features as one of the exclusion criteria for NIFTP.29
Xu et al. recently conducted the only study published on O-NI-EFVPTC. Among a total of 61 unifocal O-NI-EFVPTC treated with surgery alone, there was no recurrent or metastatic disease over a median (range) follow-up period of 5.3 years (0.1 to 20.5 years).30 Interestingly, concurrent molecular analysis revealed frequent non-silent mitochondrial DNA (mDNA) mutations in 67% of the cases.30 Disruptive mDNA mutations are commonly seen in oncocytic thyroid neoplasms, including oncocytic hyperplastic nodule, Hürthle cell adenoma and Hürthle cell carcinoma (HCC).31,32 They are believed to cause mitochondrial dysfunction and induce secondary expansion of mitochondrial mass, resulting in the oncocytic cytomorphology. In addition, three O-NI-EFVPTC showed whole chromosomal duplication of chromosome 7, which had been previously reported in HCC.30,32 However, O-NI-EFVPTC lack the widespread loss of heterozygosity characteristic of HCC.32 As for somatic mutations and gene rearrangements, O-NI-EFVPTC did not show any BRAF V600E, TERT promotor mutations, RET, NTRK, or ALK fusions.30 Instead, RAS mutations are found in 33% of these tumors, suggesting a genotype that may overlap with other follicular-patterned thyroid neoplasms.30
Although the lack of high-risk mutations and the excellent prognosis observed in Xu’s cohort seem to support the incorporation of O-NI-EFVPTC into a NIFTP designation, the current literature may still be inadequate to justify a formal nomenclature change. It is anticipated that with further accumulation of data, the behavior of O-NI-EFVPTC may become better defined.
Multifocality
The issue of multifocality in NIFTP raises two questions: (1) whether multifocal (synchronous or metachronous) NIFTP imply the same prognosis as unifocal NIFTP, and (2) whether the finding of NIFTP in a lobectomy specimen indicates an increased risk for NIFTP or carcinomas in the contralateral lobe. The first question remains unaddressed to date. In a recent study by Canini et al., 14.7% of the NIFTP were multifocal, and around 10% were bilateral.33 Unfortunately, no outcome comparison was reported between unifocal and multifocal cases.33 As for whether NIFTP implies increased risk for contralateral tumor, Canberk et al. examined 74 total thyroidectomies with NIFTP as the index lesion.34 Contralateral lesions were found in 13 (18%) cases, including five classical PTC, five follicular variant papillary thyroid microcarcinomas, two NIFTP and one IFVPTC.34 Furthermore, it was noted that NIFTP and IFVPTC cases have statistically similar incidences of contralateral tumors.34 The findings suggest a potential clinical benefit in post-operative follow up for patients treated with lobectomy alone, as suggested by the authors.34
Molecular pathogenesis
Somatic mutations
The mutational profile of NIFTP resembles that of follicular thyroid adenoma, with frequent RAS mutations, most commonly in NRAS (Table 2). While approximately 3–4% of NIFTP carry the BRAF K601E mutation, BRAF V600E should exclude NIFTP from the differential diagnosis according to the revised criteria.20 In a large transcriptomic study by Agrawal et al., BRAF K601E-positive thyroid tumors demonstrated a gene expression profile that aligned with RAS-mutated tumors and differed from BRAF V600E-positive tumors.35 Mutations in PTEN, DICER1, and EIF1AX occur with similar frequencies in NIFTP and follicular thyroid adenomas, but not in PTC (Table 2).
Table 2.
Frequency of various genetic alterations in selected thyroid neoplasmsa.
| NIFTP | EFVPTC | I-FVPTC | CPTC | FA | FC | |
|---|---|---|---|---|---|---|
| Mutations | ||||||
| NRAS41,47–51 | 26–47 | 31 | 22 | 1 | 20–30 | 30 |
| HRAS41,47,48,50–52 | 11–18 | 8 | 17 | <1 | 10 | 8 |
| KRAS47,48,50,52–54 | 2–5 | 0 | 0 | <1 | 3–8 | 6 |
| BRAF K601E47,48,52 | 3–4 | 0 | 0 | - | 3 | 0 |
| BRAF V600E41,48,50 | 0 | 39 | 39 | 71–80 | 0 | 0 |
| TERT promoter1,41,48,55 | 0–7 b | 0 | 13 | 5–15 | 0 | 20 |
| PTEN1,22,45,50 | 4–5 | 0 | 0 | 0 | 5 | 10 |
| DICER22,50,56 | 5 | 0 | 0 | 0 | 8–11 | 7 |
| EIF1AX22,48,50–52 | 5–10 | 0 | 0 | 1 | 3–12 | 8 |
| Rearrangements | ||||||
| THADA30,39,41,52 | 0–22 | 8 | 0 | 0 | 3 | 0 |
| RET41,47,50,52,57 | 0 | 0 | 0 | 5 | 0 | 0 |
| BRAF41,44,50,52 | 0 | 0 | 0 | 4 | 0 | 0 |
| NTRK41,50,52 | 6 | 0 | 17 | 4 | 0 | 0 |
| ALK41,44,50,52 | 0 | 0 | 0 | 1 | 0 | 0 |
| PPARɤ30,41,49,52,58,59 | 6–22 | 0 | 0 | 1 | 4–11 | 20–30 |
| FGFR241,50,52 | 0 | 8 | 0 | 1 | 0 | 0 |
Abbreviations: NIFTP, noninvasive follicular thyroid neoplasm with papillary-like nuclear features; EFVPTC, encapsulated follicular variant papillary thyroid carcinoma; I-FVPTC, infiltrative follicular variant papillary thyroid carcinoma; CPTC, classical type papillary thyroid carcinoma; FA, follicular adenoma; FC, follicular carcinoma.
The percentage of each tumor type harboring a particular mutation is shown as a range reported by the cited studies, per row, in each column.
Although earlier studies have reported TERT promotor mutations in a minor subset of NIFTP, the 2018 revised guidelines have recommended caution be exercised in diagnosing such lesions 20. The finding of pTERT mutation should trigger a careful search for invasion and true papillae formation given its association with more aggressive behavior in thyroid tumors.
Gene rearrangements
PAX8-PPARɤ fusion, a hallmark of follicular thyroid neoplasms, is one of the most common gene rearrangements observed in NIFTP, detected in around 6–22% of the cases (Table 2). PAX8 is an essential protein for the differentiation of thyroid follicular cells, while PPARγ is known to modulate the cell cycle.36 In human thyrocyte-derived cell lines, overexpression of the PAX8-PPARɤ fusion protein has been shown to stimulate cell proliferation and suppress apoptosis,37,38 supporting its tumorigenic function. However, the presence of PAX8-PPARɤ fusion in 4–11% of follicular adenomas makes it unlikely to be sufficient for inducing carcinogenesis. Nevertheless, the high frequency (20–30%) of PPARγ rearrangements in follicular thyroid carcinomas (Table 2) supports a certain survival benefit provided by the fusion.
THADA fusions, observed in 22% of NIFTP, have also been reported in minor subsets of follicular thyroid adenomas and PTC.39,40 It has been demonstrated that THADA-rearranged PTC tend to overexpress IGF2BP3, a translational activator of Insulin-like Growth Factor 2 (IGF2). Elevated intracellular levels of IGF2 have an activating effect on the PI3K and MAPK pathways, and thereby stimulate cellular proliferation and invasion.40 The biological significance of THADA fusions in NIFTP is unclear.
Interestingly, one of 13 (6%) NIFTP cases in the study of Song et al. harbored the ETV6-NTRK3 fusion,41 which is linked to malignancies in various organs presumably through its activating effect on the mitogen activated protein kinase (MAPK) pathway. In the thyroid, the ETV6-NTRK3 fusion is most frequently seen in infiltrative FVPTC and classical PTC (Table 2), particularly in pediatric and radiation-associated cases.42,43 It may therefore be prudent to cautiously examine all ETV6-NTRK3-positive NIFTP to ensure there are no malignant features such as papillary formation or invasion. Other gene rearrangements typical of PTC, such as those involving RET and ALK, are lacking in NIFTP.
Gene expression profiling
Based on a comprehensive transcriptomic dataset generated by RNA sequencing data, Pool et al. were able to classify a cohort of invasive and noninvasive FVPTC and NIFTP into “BRAF-like”, “RAS-like”, and “THADA-like” subtypes.44 Interestingly, the NIFTP cases were distributed in all three categories (5 BRAF-like, 2 RAS-like, one THADA-like).44 The BRAF-like group otherwise included all invasive and BRAF-mutated cases in the cohorts, and showed increased expression of inflammatory receptors and cytokines.44 The RAS-like subtype, represented by most RAS-mutated cases in the study, is characterized by overexpression of genes involved in hydrogen peroxide metabolism. In contrast, the THADA-like group showed upregulated PPAR signaling.44 It is noteworthy that NIFTP show remarkable molecular diversity and do not conform to a uniform transcriptomic signature. This may explain the well-recognized difficulty with classifying thyroid nodules using algorithmic gene expression analysis performed in FNA samples.45
Management considerations
Despite its recent re-classification as non-cancer, NIFTP is still considered to be of low but uncertain biological potential and remains a surgical target, as do follicular adenomas. The overall decreased positive predictive value in cytologically indeterminate thyroid nodules may encourage clinicians and patients to choose thyroid lobectomy over total thyroidectomy and to avoid postoperative RAI. In fact, the pre-NIFTP ATA guidelines published in 2015 had placed non-invasive EFVPTC in the low-risk category and recommended either lobectomy or total thyroidectomy alone.10 This recommendation was reiterated in the ATA position paper in 2017.46 It is still unclear how NIFTP patients should be postoperatively managed. The clinical value of TSH suppression, serum thyroglobulin monitoring and thyroid bed imaging has yet to be determined. Given the previously reported 15% rate of contralateral malignancy in NIFTP patients,34 some form of post-operative surveillance is likely to be of benefit for patients treated with lobectomy, but more data are needed to clarify the best approach.
Conclusion
The recognition of NIFTP as a distinct indolent entity represents an important recent advance in endocrine oncology. With the new non-cancer designation, patients can be spared of unwarranted stress and costs associated with aggressive management. However, the current understanding of the behavior of NIFTP is still based on very limited experience. Additional investigative efforts are essential for solving several persistent issues. The current diagnostic criteria for NIFTP, despite its thoughtful design, may rarely still fail to filter out tumors with metastatic potential.21 Besides, the prognostic significance of tumor size, multicentricity and oncocytic quality remains controversial. Yet another unresolved challenge is the ongoing pursuit for pre-surgical cytopathologic diagnosis of NIFTP using FNA specimens. With the continuously evolving efforts to improve patient outcomes, we look forward to NIFTP becoming an increasingly understood and well-managed condition.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Lloyd RV OR, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. 4 Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 2.Gupta S, Ajise O, Dultz L, et al. Follicular variant of papillary thyroid cancer: encapsulated, nonencapsulated, and diffuse: Distinct biologic and clinical entities. Arch. Otolaryngol. Head Neck Surg 2012;138:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao L, Dias-Santagata D, Sadow PM, Faquin WC. Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol. 2017;125:323–331. [DOI] [PubMed] [Google Scholar]
- 4.Nikiforov YE, Seethala RR, Tallini G, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: A paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson LD. Ninety-four cases of encapsulated follicular variant of papillary thyroid carcinoma: a name change to Noninvasive Follicular Thyroid Neoplasm with Papillary-like Nuclear Features would help prevent overtreatment. Mod. Pathol 2016;29:698–707. [DOI] [PubMed] [Google Scholar]
- 6.Xu B, Tallini G, Scognamiglio T, Roman BR, Tuttle RM, Ghossein RA. Outcome of large noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27:512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seethala RR, Baloch ZW, Barletta JA, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features: a review for pathologists. Mod. Pathol 2018;31:39–55. [DOI] [PubMed] [Google Scholar]
- 8.Strickland KC, Eszlinger M, Paschke R, et al. Molecular testing of nodules with a suspicious or malignant cytologic diagnosis in the setting of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Endocr. Pathol 2018;29:68–74. [DOI] [PubMed] [Google Scholar]
- 9.Basolo F, Macerola E, Ugolini C, Poller DN, Baloch Z. The molecular landscape of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): A literature review. Adv. Anat. Pathol 2017;24:252–258. [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valderrabano P, McGettigan MJ, Lam CA, et al. Thyroid nodules with indeterminate cytology: Utility of the American Thyroid Association sonographic patterns for cancer risk stratification. Thyroid. 2018;28:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang GCH, Fried KO, Scognamiglio T. Sonographic and cytologic differences of NIFTP from infiltrative or invasive encapsulated follicular variant of papillary thyroid carcinoma: A Review of 179 Cases. Diagn. Cytopathol 2017;45:533–541. [DOI] [PubMed] [Google Scholar]
- 13.Brandler TC, Yee J, Zhou F, et al. Does noninvasive follicular thyroid neoplasm with papillary-like nuclear features have distinctive features on sonography. Diagn. Cytopathol 2018;46:139–147. [DOI] [PubMed] [Google Scholar]
- 14.Larouche V, Pusztaszeri MP, Filimon S, Payne R, Hier M, Tamilia M. Preoperative prediction of non-invasive follicular thyroid neoplasm with papillary-like nuclear features: a Canadian single-Centre experience. J. Otolaryngol. Head Neck Surg 2020;49:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faquin WC, Wong LQ, Afrogheh AH, et al. Impact of reclassifying noninvasive follicular variant of papillary thyroid carcinoma on the risk of malignancy in The Bethesda System for Reporting Thyroid Cytopathology. Cancer Cytopathol. 2016;124:181–187. [DOI] [PubMed] [Google Scholar]
- 16.Maletta F, Massa F, Torregrossa L, et al. Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum. Pathol 2016;54:134–142. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim AA, Wu HH. Fine-needle aspiration cytology of noninvasive follicular variant of papillary thyroid carcinoma is cytomorphologically distinct from the invasive counterpart. Am. J. Clin. Pathol 2016;146:373–377. [DOI] [PubMed] [Google Scholar]
- 18.Strickland KC, Vivero M, Jo VY, et al. Preoperative cytologic diagnosis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features: A prospective analysis. Thyroid. 2016;26:1466–1471. [DOI] [PubMed] [Google Scholar]
- 19.Lindeman BM, Nehs MA, Angell TE, et al. Effect of noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on malignancy rates in thyroid nodules: How to counsel patients on extent of surgery. Ann. Surg. Oncol 2019;26:93–97. [DOI] [PubMed] [Google Scholar]
- 20.Nikiforov YE, Baloch ZW, Hodak SP, et al. Change in diagnostic criteria for non-invasive follicular thyroid neoplasm with papillarylike nuclear features. JAMA Oncol. 2018;4:1125–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho U, Mete O, Kim MH, Bae JS, Jung CK. Molecular correlates and rate of lymph node metastasis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features and invasive follicular variant papillary thyroid carcinoma: the impact of rigid criteria to distinguish non-invasive follicular thyroid neoplasm with papillary-like nuclear features. Mod. Pathol 2017;30:810–825. [DOI] [PubMed] [Google Scholar]
- 22.Rossi ED, Faquin WC, Baloch Z, et al. Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): Update and diagnostic considerations-a review. Endocr. Pathol 2019;30:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson DN, Sadow PM. Exploration of BRAFV600E as a diagnostic adjuvant in the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Hum. Pathol 2018;82:32–38. [DOI] [PubMed] [Google Scholar]
- 24.Parente DN, Kluijfhout WP, Bongers PJ, et al. Clinical safety of renaming encapsulated follicular variant of papillary thyroid carcinoma: Is NIFTP truly Benign. World J. Surg 2018;42:321–326. [DOI] [PubMed] [Google Scholar]
- 25.Rosario PW. Long-term outcomes of patients with noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) >/=4cm treated without radioactive iodine. Endocr. Pathol 2017;28:367–368. [DOI] [PubMed] [Google Scholar]
- 26.Xu B, Farhat N, Barletta JA, et al. Should subcentimeter non-invasive encapsulated, follicular variant of papillary thyroid carcinoma be included in the noninvasive follicular thyroid neoplasm with papillary-like nuclear features category. Endocrine. 2018;59:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafique K, LiVolsi VA, Montone K, Baloch ZW. Papillary Thyroid Microcarcinoma: Reclassification to non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): A retrospective clinicopathologic study. Endocr. Pathol 2018;29:339–345. [DOI] [PubMed] [Google Scholar]
- 28.Rosario PW. Subcentimetre non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Histopathology. 2018;73:535–537. [DOI] [PubMed] [Google Scholar]
- 29.Johnson SJ ST, Poller DN NIFTP addendum to the RCPath Dataset for thyroidcancer histopathology reports (2016). https://www.rcpath.org/.
- 30.Xu B, Reznik E, Tuttle RM, et al. Outcome and molecular characteristics of non-invasive encapsulated follicular variant of papillary thyroid carcinoma with oncocytic features. Endocrine. 2019;64:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gasparre G, Porcelli AM, Bonora E, et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc. Natl. Acad. Sci. U.S.A 2007;104:9001–9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganly I, Makarov V, Deraje S, et al. Integrated genomic analysis of hurthle cell cancer reveals oncogenic drivers, recurrent mitochondrial mutations, and unique chromosomal landscapes. Cancer Cell. 2018;34:256–270 e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canini V, Leni D, Pincelli AI, et al. Clinical-pathological issues in thyroid pathology: study on the routine application of NIFTP diagnostic criteria. Sci. Rep 2019;9:13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canberk S, Montezuma D, Tastekin E, et al. The other side of the coin”: understanding noninvasive follicular tumor with papillary-like nuclear features in unifocal and multifocal settings. Hum. Pathol 2019;86:136–142. [DOI] [PubMed] [Google Scholar]
- 35.Integrated genomic characterization of papillary thyroid carcinoma. Cell.2014;159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fajas L, Debril MB, Auwerx J. Peroxisome proliferator-activated receptor-gamma: from adipogenesis to carcinogenesis. J. Mol. Endocrinol 2001;27:1–9. [DOI] [PubMed] [Google Scholar]
- 37.Lui WO, Zeng L, Rehrmann V, et al. CREB3L2-PPARgamma fusion mutation identifies a thyroid signaling pathway regulated by intramembrane proteolysis. Cancer Res. 2008;68:7156–7164. [DOI] [PubMed] [Google Scholar]
- 38.Gregory Powell J, Wang X, Allard BL, et al. The PAX8/PPARgamma fusion oncoprotein transforms immortalized human thyrocytes through a mechanism probably involving wild-type PPARgamma inhibition. Oncogene. 2004;23:3634–3641. [DOI] [PubMed] [Google Scholar]
- 39.Belge G, Roque L, Soares J, et al. Cytogenetic investigations of 340 thyroid hyperplasias and adenomas revealing correlations between cytogenetic findings and histology. Cancer Genet. Cytogenet 1998;101:42–48. [DOI] [PubMed] [Google Scholar]
- 40.Panebianco F, Kelly LM, Liu P, et al. THADA fusion is a mechanism of IGF2BP3 activation and IGF1R signaling in thyroid cancer. Proc. Natl. Acad. Sci. U.S.A 2017;114:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song YS, Won JK, Yoo SK, et al. Comprehensive Transcriptomic and genomic profiling of subtypes of follicular variant of papillary thyroid carcinoma. Thyroid. 2018;28:1468–1478. [DOI] [PubMed] [Google Scholar]
- 42.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–1107. [DOI] [PubMed] [Google Scholar]
- 43.Rabes HM, Demidchik EP, Sidorow JD, et al. Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin. Cancer Res 2000;6:1093–1103. [PubMed] [Google Scholar]
- 44.Pool C, Walter V, Bann D, et al. Molecular characterization of tumors meeting diagnostic criteria for the non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP). Virchows Archiv. 2019;474:341–351. [DOI] [PubMed] [Google Scholar]
- 45.Brandler TC, Liu CZ, Cho M, et al. Does noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) have a unique molecular profile. Am. J. Clin. Pathol 2018;150:451–460. [DOI] [PubMed] [Google Scholar]
- 46.Haugen BR, Sawka AM, Alexander EK, et al. American Thyroid Association Guidelines on the Management of thyroid nodules and differentiated thyroid cancer task force review and recommendation on the proposed renaming of encapsulated follicular variant papillary thyroid carcinoma without invasion to noninvasive follicular thyroid neoplasm with papillary-like nuclear features. Thyroid. 2017;27:481–483. [DOI] [PubMed] [Google Scholar]
- 47.Kim TH, Lee M, Kwon AY, et al. Molecular genotyping of the non-invasive encapsulated follicular variant of papillary thyroid carcinoma. Histopathology. 2018;72:648–661. [DOI] [PubMed] [Google Scholar]
- 48.Jung CK, Kim Y, Jeon S, Jo K, Lee S, Bae JS. Clinical utility of EZH1 mutations in the diagnosis of follicular-patterned thyroid tumors. Hum. Pathol 2018;81:9–17. [DOI] [PubMed] [Google Scholar]
- 49.Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. J. Clin. Endocrinol. Metab 2003;88:2318–2326. [DOI] [PubMed] [Google Scholar]
- 50.Yoo SK, Lee S, Kim SJ, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12:e1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jung SH, Kim MS, Jung CK, et al. Mutational burdens and evolutionary ages of thyroid follicular adenoma are comparable to those of follicular carcinoma. Oncotarget. 2016;7:69638–69648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song YS, Park YJ. Genomic characterization of differentiated thyroid carcinoma. Endocrinol. Metab. (Seoul) 2019;34:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oyama T, Suzuki T, Hara F, et al. N-ras mutation of thyroid tumor with special reference to the follicular type. Pathol. Int 1995;45:45–50. [DOI] [PubMed] [Google Scholar]
- 54.Duan H, Liu X, Ren X, Zhang H, Wu H, Liang Z. Mutation profiles of follicular thyroid tumors by targeted sequencing. Diagn. Pathol 2019;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang N, Liu T, Sofiadis A, et al. TERT promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (FTA) and atypical FTA. Cancer. 2014;120:2965–2979. [DOI] [PubMed] [Google Scholar]
- 56.Poma AM, Condello V, Denaro M, et al. DICER1 somatic mutations strongly impair miRNA processing even in benign thyroid lesions. Oncotarget. 2019;10:1785–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salvatore G, Chiappetta G, Nikiforov YE, et al. Molecular profile of hyalinizing trabecular tumours of the thyroid: high prevalence of RET/PTC rearrangements and absence of B-raf and N-ras point mutations. Eur. J. Cancer (Oxford, England: 1990) 2005;41:816–821. [DOI] [PubMed] [Google Scholar]
- 58.Giordano TJ, Beaudenon-Huibregtse S, Shinde R, et al. Molecular testing for oncogenic gene mutations in thyroid lesions: a case-control validation study in 413 postsurgical specimens. Hum. Pathol 2014;45:1339–1347. [DOI] [PubMed] [Google Scholar]
- 59.Mostoufi-Moab S, Labourier E, Sullivan L, et al. Molecular testing for oncogenic gene alterations in pediatric thyroid lesions. Thyroid. 2018;28:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]