Abstract
Background/Objectives:
In Samoa, where 80% of the adult population is living with obesity, understanding the determinants of adiposity and growth during infancy may inform prevention efforts. We examined the association of a missense variant, rs373863828, in the CREBRF gene with body composition in Samoan infants. Adults with one or more copies of the rs373863828 minor allele (A) have higher odds of obesity, based on body-mass index (BMI), but paradoxically decreased odds of diabetes compared to those without the allele. Our study may offer novel insight into the natural history and pathogenesis of this unexpected relationship.
Subjects/Methods:
In a prospective study, we measured body composition in early infancy, and at 2- and 4-months of age using anthropometry and Dual-Energy X-Ray Absorptiometry (DXA). We genotyped subjects at the CREBRF rs373863828 locus and compared infants with (AA/AG) and without (GG) the variant. In longitudinal analyses, we calculated the absolute change in each outcome from the early infant to the 4-month assessment, adjusting for baseline and other covariates.
Results:
In cross-sectional analyses, there was no significant difference in infant BMI or fat mass by genotype. After adjusting for covariates, infants with the variant had 4.0 ± 1.8g more bone mass (p=0.026) and 210.9 ±79.6g more lean mass (p=0.009) at 4-months and accumulated 176.9 ± 73.0g more lean mass between the early infant and 4-month assessment (p=0.017).
Conclusions:
The CREBRF rs373863828 minor allele (A) was not associated with increased BMI or adiposity in Samoan infants, but instead with increased lean and bone mass. Our findings suggest that lean (i.e. muscle) and bone mass accretion should be explored as pathways to explain the ‘protective’ effect of the CREBRF variant against diabetes.
Introduction
The dramatic increase in obesity in Samoa over the past fifty years has been well documented, and prevalence is now among the highest in the world1–3. The primary driving force has been globalization, an accompanied increase in sedentary behavior, and access to highly processed and calorie-dense foods2,4. There is also evidence, however, of an underlying genetic susceptibility that may affect Pacific Islander’s metabolic responses to nutritional transition.
A genome-wide association study (GWAS) of adiposity among Samoan adults observed the strong effect of a missense variant, rs373863828 (CREBRF:c.1370G>A p.(R457Q)), on body-mass index (BMI) and abdominal circumference5. This missense variant in CREB3 regulatory factor (CREBRF) is common in Samoans (minor allele frequency 0.259) and has a larger effect on BMI than any previously identified common gene variant. The minor allele, A, increases adult BMI by 1.36 kg/m2 per copy; an effect size ~three-fold greater than variation near FTO (0.39 kg/m2 per copy)6. Interestingly, however, the BMI-increasing allele is associated with decreased odds of type II diabetes and lower fasting serum glucose levels. Several studies in New Zealand Māori and Pacific Islanders (Tongans, Cook Islanders, Niueans and Mariana Islanders, and Micronesians from Guam and Saipan) have reproduced the incongruent effects on BMI and diabetes7–11.
The mechanism by which CREBRF acts remain unclear. One hypothesis is that the variant’s seemingly paradoxical effects on BMI and diabetes operate through a body composition pathway, i.e. that differential proportions of fat mass or distribution of fat stores by genotype may explain the increased BMI but lower odds of diabetes. Specifically, individuals with the variant may have higher adiposity, but store adipose tissue in areas associated with lower risk of metabolic sequelae —that is, subcutaneously rather than viscerally, and or peripherally rather than centrally. Another pathway linking BMI and reduced diabetes risk is through greater bone and lean mass deposition, both of which are shown to be associated with better glucose homeostasis12,13.
It is also unknown when in the life course the ‘increased BMI’ phenotype observed in adults with the variant emerges, underscoring the need to understand the effects of this variant early in life and during critical developmental periods such as infancy and childhood14. Only one study to date, a New Zealand birth cohort of more than six thousand individuals of Māori, Pacific, European, and Asian descent, has examined the CREBRF variant and its association with body size during infancy/childhood (Berry et al., 2018). They found that the variant was not associated with birth weight or weight and height at 2 years, but was positively associated with weight, height, and waist circumference at 4 years9. Notably, the study pooled Pacific ethnicities with Asians and Europeans and no separate analyses were done in the Pacific Islander sample. Further, because the study’s outcomes were anthropometric, any possible association with body composition or regional fat deposition could not be explored. Our aim was to determine the effects of the CREBRF variant, rs373863828, on body composition and regional adiposity among Samoan infants using anthropometry and Dual-Energy X-Ray Absorptiometry (DXA).
Materials/Subjects and Methods
Study Design
We used data from a convenience sample of n=160 Samoan mother-infant dyads participating in a prospective birth cohort study: “Foafoaga O le Ola” (‘Beginning of Life’). We established the cohort to explore determinants of infant growth including maternal diet during pregnancy, infant feeding practices, and presence of the CREBRF rs373863828 variant15. Data collection occurred from June 2017- April 2018 in Samoa. Our analytic sample here was restricted to the n=117 infants for whom we were able to obtain genotype. The availability of anthropometric and DXA data determined sample size for each outcome at each time point (Figure 1).
Figure 1.
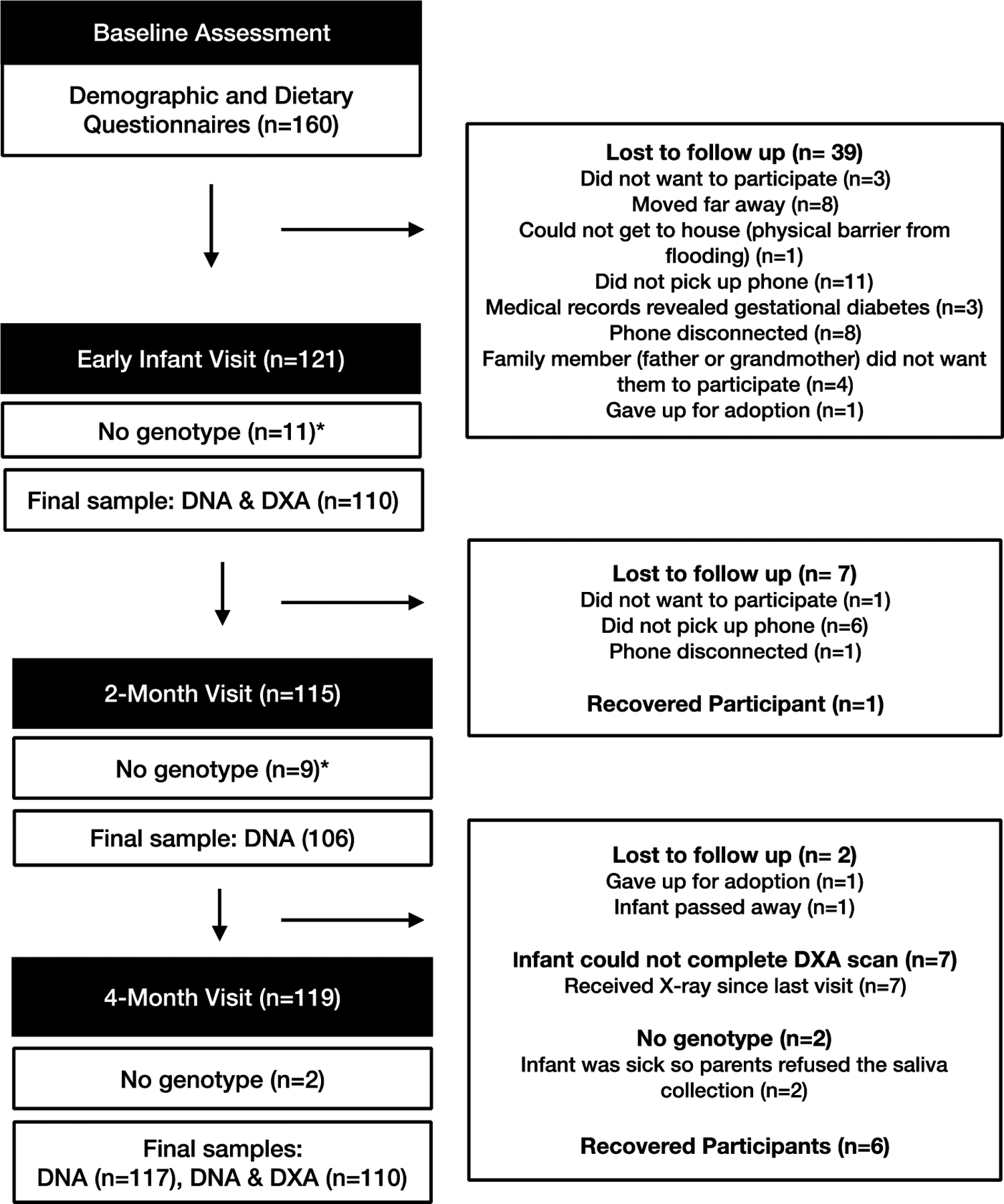
Study design and participant flow diagram.
*We collected saliva for genotyping at the 4-month visit only. Therefore, whether infants had genotype data at the early infant and 2-month visits was dependent on whether they were present at the 4-month visit.
Setting
Samoa is an upper-middle income Pacific Island nation with a Gross Domestic Product (GDP) per capita of $6089 USD16. The island of ‘Upolu, where 75% of the population live, is divided into three census regions with varying degrees of urbanicity and exposure to the nutrition transition, including the Apia Urban Area (AUA; most exposed), sub-urban North West Upolu (NWU; variably exposed), and rural Rest of Upolu (ROU; least exposed). Apia is the capital and largest city on the island, where around 19% of the population live17.
Participants and Procedures
Study participants were recruited from the antenatal care clinic of the Tupua Tamasese Meaole (TTM) Hospital in Apia; the only tertiary care facility in Samoa and the major referral center on ‘Upolu. To be eligible, mothers had to be older than 18 years, 35–40 weeks gestation, have a singleton pregnancy with no complications (including pre-existing or gestational diabetes), and reside within 30 minutes of the AUA to facilitate follow up.
Mothers completed a baseline assessment at the time of recruitment (35–40 weeks gestation) and dyads were invited to participate in three further assessments: in the 2 weeks following their infant’s birth (early infant assessment); and when the infant was 2- and 4-months of age. We faced challenges reaching participants by phone postpartum, which resulted in the age range for the early infant assessment varying between 0–41 days after birth, the 2-month assessment being conducted between 56–103 days, and 111–199 days for the 4-month assessment. DXA scans were only performed at the early infant and 4-month assessments. The recruitment, retention, and study sample size at each assessment is provided in Figure 1.
All participants gave their written informed consent and protocols were granted ethical approval by the Yale University Institutional Review Board (HIC #2000021076) and the Samoa Ministry of Health’s Health Research committee.
DXA scans and measurements
DXA scans were completed by one of three trained operators and analyzed by a single researcher (RLD). A daily quality control scan of a manufacturer-supplied phantom spine was performed to ensure scan reliability. Infants were consistently swaddled in the same brand of blanket and scanned wearing only a clean diaper, in “thin” mode (Lunar iDXA, version enCORE 17, GE Healthcare Medicine, WI, USA). The total body scan provided absolute measures of total body less head (TBLH) fat, lean, bone and total mass (grams) as well as percent fat and fat-free mass (lean mass plus bone mass).
It should be noted that that the x-ray dose for DXA is very low in comparison to other x-ray imaging models (i.e. chest x-ray/CT scan)18–22. For the average infant, radiation exposure associated with a whole body DXA scan using a GE iDXA fan-beam scanner (the model we used) is 0.22–0.25 μSv based on a 90-second scan20,22,23. For comparison, one day’s background radiation (depending on location) is ~8 μSv24, and the total radiation each participant received from this study was less than 1% of that received from natural sources of radiation by a Samoan resident in one year (~2920 μSv). We are aware, however, that any radiation exposure carries potential risk and clearly explained to participants that DXA is not a standard of care procedure and was for research purposes only.
DNA extraction and genotyping
With assistance, mothers collected saliva samples from their infants at the 4-month assessment using Oragene·DISCOVER DNA collection kits (#OGR-575, DNA Genotek). After mixing samples with stabilizing reagents in collection tubes, samples were shipped to the University of Pittsburgh for extraction and purification of DNA using PrepIT L2P DNA Genotek). Resulting DNA was then evaluated for the rs373863828 SNP, per the manufacturer’s instructions, with a pre-designed TaqMan® SNP assay (Assay ID# C_203097374_10, Applied Biosystems) and using an ABI 7900HT instrument and TaqMan® Genotyper Software. All attempted genotypes were successfully measured except one sample, which was not sufficient to produce a result.
Anthropometric measurements
During each assessment trained research assistants collected skinfold thicknesses, head and abdominal circumferences, weight and length of the infants, and height and weight of mothers. All measurements were collected in duplicate and averaged for use in analyses. Infant skinfold thicknesses (triceps, subscapular, iliac crest, thigh) were obtained on the left side of the body using a Harpenden caliper (Harpenden FG1056, West Sussex, UK). Subcutaneous fat mass (mm2) was estimated using the sum of all skinfolds. Head circumference was measured at the widest possible circumference and abdominal circumference above the belly button (to avoid the umbilical cord stump during the early infant assessment) using a standard tape measure (SECA 201, SECA, Hamburg Germany). For infant length and weight measurements, we used a length board (SECA 417, SECA, Hamburg, Germany) and digital scale (SECA 354, SECA, Hamburg, Germany). Infants were weighed in clean diapers, after taring the scale for the diaper weight. Age- and sex-standardized BMI z-scores (zBMI) were calculated using World Health Organization Child Growth Standards25. Abdominal circumference-to-length ratio (abdominal circumference divided by length) was calculated as a measure of abdominal visceral fat26.
We measured maternal height using a portable stadiometer (SECA 217, SECA, Hamburg, Germany) and weight with a digital scale (Tanita HD351, Tanita Corporation of America, IL, USA). Mothers wore light island clothing and were classified as having normal weight, overweight, or obesity based on Polynesian-specific BMI cutpoints27.
Questionnaires
At baseline, we administered a demographic questionnaire to obtain maternal characteristics such as age, census region of residence, gravidity, and smoking during pregnancy (yes/no and number of cigarettes per week). Infant date of birth and sex were reported during the early infant assessment, and infant birth weight and gestational age obtained from hospital medical records. If the recorded infant birth weight was greater than 4000g they were classified as having macrosomia. To control for breastfeeding practices, we used an adapted version of the Centers for Disease Control (CDC) Infant Feeding Practices Survey (IFPS)28, which has modules specific to each of our study time points and had been used previously among Samoans29. All questionnaire measures were administered in Samoan by bilingual, local research assistants.
Statistical Analysis
We conducted cross-sectional analyses of early infant, 2-month, and 4-month body composition and anthropometry, and longitudinal analyses of body composition changes between early infant and 4-month assessments with a focus on the association with the CREBRF c.1370G>A p.(R457Q) genotype. Because of the expected small number of infants with the AA genotype due to small overall sample size and because both AG and AA genotypes increase body size in adults5, we combined AA and AG individuals for all analyses providing a comparison between those with no copies of the variant (GG) and those with one or more (AA and AG).
Infant body composition outcomes were lean, bone, and fat mass, and % fat mass (all measured minus the head30) obtained from DXA scans, weight, length, BMI, zBMI, subcutaneous fat (mm2), and abdominal circumference-to-length ratio. All outcomes met assumptions for normality so no transformation was necessary.
Because of the variation in infant age at each assessment, we used general linear models and estimated least square means to cross-sectionally examine the association of CREBRF genotype and study outcomes at each time point, controlling for infant age only in the first step of analysis. Independent samples t-tests were used for zBMI since this parameter already took into account infant age and sex. For longitudinal analyses we calculated absolute change in each outcome from the early infant assessment to the 4-month assessment. We then calculated the least square means to examine the association between genotype group (AA/AG vs. GG) and each longitudinal ‘change’ variable controlling for the number of days between assessments and adjusted for the value of the respective outcome measurement at the early infant assessment, per Johnson et al.31.
In the next step of our analyses, completed where we observed a significant association between genotype and an outcome of interest using the models described above, we performed multivariable linear regression. We used backward step-wise regressions to identify best-fit models after including covariates identified a priori for their likely association with the outcomes of interest. In the multivariable models we included maternal postpartum BMI (measured at the early infant assessment), maternal age, infant age (days) at the time of assessment, and infant sex as covariates. Maternal gravidity was correlated with maternal age (rs(113) = 0.546, p < 0.001); we used maternal age in analyses because there were less missing data for this variable. To adjust for infant feeding we created categorical variables: for the early infant and 2-month analyses, the variable had two levels (EBF vs. not EBF) because there were very few infants who were formula-feeding exclusively, and for the 4-month and longitudinal analyses, the variable had three levels (EBF, mixed- and formula-feeding). We imputed breastfeeding status at the early infant and 4-month assessment for two participants who only reported feeding status at 2-months (both were EBF at 2 months). Finally, sex-specific effects of the CREBRF variant on body composition outcomes that were significant in the multivariable models were examined by adding an interaction term between sex and CREBRF genotype in the multivariable regressions.
Statistical analyses were performed with PASW software (version 25.0; SPSS Inc., Armonk, NY, USA: IBM Corp) and p-values <0.05 were considered statistically significant. Because this study was exploratory in nature we made no correction for multiple comparisons.
Results
Characteristics of mothers and infants are described in Table 1. Most mothers were residents of the AUA or sub-urban NWU region per study inclusion criteria of residence within 30 minutes of the AUA and had had an average of 2.0 ± 1.7 pregnancies (including the study infant). The majority of mothers had overweight or obesity at the early infant assessment (90.4%), but few (n=10) smoked during pregnancy. The majority of infants were breastfed at all time points (early infant visit, EBF 92%, any breastfeeding (data not shown) 99%; 2-month visit, EBF 66%, any breastfeeding 91%; 4-month visit, EBF 60%, any breastfeeding 85%). Among the n=117 infants who were genotyped the frequency of the minor A allele rs373863828 was 0.218 (n=3, AA; n=45 AG; and n=69 GG) (Table 1).
Table 1.
Participant characteristics.
| Maternal Characteristics | n=117 unless otherwise indicated in parentheses |
|---|---|
| Age, years, mean ± SD | 27.2 ± 5.6 |
| Census region of residence | |
| Apia Urban Area, n (%) | 57 (48.8) |
| Northwest Upolu (peri-urban), n (%) | 59 (51.5) |
| Rest of Upolu (ROU) (rural), n (%) | 1 (0.9) |
| BMI post-pregnancy (measured 6.4 ± 7.0 days after birth), kg/m2, mean ± SD |
34.3 ± 6.6 (n=115) |
| Body mass index (BMI) classificationa | |
| Underweight (<18 kg/m2), n (%) | 0 (0) |
| Normal (18–25.9 kg/m2), n (%) | 11 (9.6) |
| Overweight (26–32 kg/m2), n (%) | 35 (30.4) |
| Obesity (>32 kg/m2), n (%) | 69 (60.0) |
| Gravidity, mean ± SD | 2.0 ± 1.7 (n=115) |
| Smoker during pregnancy, n (%) | 10 (8.5) |
| Number of cigarettes per week (smokers only), mean ± SD | 3.1 ± 2.7 (n=10) |
| Infant Characteristics | n=110 | n=106 | n=117 |
|---|---|---|---|
| Early infant visit (0–41 days) |
2-month visit (56–100 days) |
4-month visit (111–199 days) |
|
| Male, n (%) | 58 (52.7) | 58 (54.7) | 62 (53.0) |
| Genotype AG/AAb, n (%) | 46 (41.4) | 42 (39.6) | 48 (41.0) |
| Infant age, days, mean ± SD | 6.4 ± 7.0 | 72.1 ± 9.8 | 127.9 ± 13.1 |
| Gestational age, weeks, mean ±SD | 40.0 ± 1.0 | 39.9 ± 1.0 | 39.9 ± 1.0 |
| Exclusive breastfeeding, n (%) | 101 (91.8) | 70 (66.0) | 70 (59.8) |
| Birth weight, g, mean ± SD | 3518.7 ± 470.2 | 3513.8 ± 469.5 | 3518.7 ± 470.2 |
| Macrosomia (>4000g), n (%) | 15 (13.6) | 13 (12.2) | 15 (12.8) |
| Weight, g, mean ± SD | 3553.4 ± 5557.2 | 6185.6 ± 798.2 | 7362.8 ± 908.8 |
| Length, cm, mean ± SD | 52.1 ± 2.8 | 60.7 ± 2.0 | 64.8 ± 2.4 |
| BMI, kg/m2, mean ± SD | 13.1 ± 1.4 | 16.7 ± 1.5 | 17.5 ± 1.4 |
| zBMIc, mean ± SD | −0.3 ± 1.0 | 0.3 ± 0.9 | 0.3 ± 0.9 |
| Subcutaneous fatd, mm2, mean ± SD | 20.5 ± 6.4 (108) | 43.2 ± 7.3 | 44.9 ± 7.1 |
| Abdominal circumference, mm, mean ± SD | 34.0 ± 2.5 | 41.2 ± 2.3 | 42.6 ± 2.5 |
| Head circumference, mm, mean ± SD | 35.7 ± 1.5 | 40.7 ± 4.9 | 41.8 ± 1.6 |
| DXA, total body less head (TBLH) | n=110 | n=110 | |
| Fat mass, g, mean ± SD | 504.8 ± 171.2 | --- | 2162.1 ± 463.0 |
| % Fat (%), mean ± SD | 17.8 ± 3.5 | --- | 36.16 ± 4.5 |
| Lean mass, g, mean ± SD | 2240.2 ± 369.1 | --- | 3702.5 ± 480.5 |
| Bone mass, g, mean ± SD | 45.4 ± 7.5 | --- | 76.3 ± 9.8 |
| Fat-free mass, g, mean ± SD | 2285.6 ± 374.0 | --- | 3778.7 ± 488.2 |
Pacific Islander-specific cut-offs for BMI were used.
AA/AG are individuals with 1 or 2 copies of the CREBRF variant rs373863828 (CREBRF:c.1370G>A p.(R457Q)), and GG are infants with no copies.
Age- and sex-standardized BMI z-scores (zBMI) were calculated using World Health Organization Child Growth Standards.
Subcutaneous fat mass (mm2) estimated using the sum of all skinfold measures.
Cross-sectional, infant age-adjusted general linear models (Table 2) did not reveal associations between genotype and weight, length, BMI, fat mass or fat distribution. They did, however, indicate that bone mass was significantly greater, by an average of 2.9 ± 1.4 grams, at the early infant and 4-month (4.1 ± 1.8 grams) assessments in infants with one or more copies of the CREBRF variant. Lean mass was also significantly greater among those with the variant at 4-months (258.3 ± 88.7 grams) compared to those without. At the 2-month assessment (where only anthropometric data was collected) no significant associations were observed. Longitudinal analyses revealed that after adjusting for infant age, infants with the AA or AG genotype accrued more lean mass between the early infant assessment and the 4-month visit, while infants without the variant (GG) had a greater, positive change in % fat (Table 2).
Table 2.
Anthropometric and body composition measures among Samoan infants by CREBRF rs373863828 genotype groups.
| Outcomea | AG/AAb | GGb | Difference | P-valuec | |||
|---|---|---|---|---|---|---|---|
| Mean ± SEM | n | Mean ± SEM | n | Mean ± SEM | |||
| Early infant visit (6.4 days)d | Weight, g | 3553.3 ± 54.3 | 45 | 3553.4 ± 65.3 | 65 | −0.1 ± 85.2 | 0.999 |
| Length, cm | 52.4 ± 0.4 | 45 | 51.9 ± 0.3 | 65 | 0.6 ± 0.5 | 0.253 | |
| BMI, kg/m2 | 12.9 ± 0.2 | 45 | 13.2 ± 0.2 | 65 | −0.3 | 0.229 | |
| zBMIe | −0.4 ± 0.1 | 45 | −0.3 ± 0.1 | 65 | −0.2 ± 0.2 | 0.367 | |
| Subcutaneous fatf, mm2 | 20.2 ± 0.7 | 45 | 20.7 ± 0.6 | 63 | −0.5 ± 0.9 | 0.584 | |
| Abdominal circumference-to-length, mm/cm | 0.7 ± 0.01 | 45 | 0.7 ± 0.01 | 65 | 0 ± 0.01 | 0.473 | |
| DXA measures, total body less head (TBLH) | |||||||
| Fat mass, g | 499.3 ± 19.3 | 45 | 508.6 ± 16.1 | 65 | −9.3 ± 25.2 | 0.713 | |
| % Fat (%) | 17.8 ± 0.5 | 45 | 18.3 ± 0.4 | 65 | −0.5 ± 0.6 | 0.457 | |
| Lean mass, g | 2268.2 ± 48.2 | 45 | 2220.8 ± 39.2 | 65 | 47.4 ± 61.5 | 0.442 | |
| Bone mass, g | 47.1 ± 1.1 | 45 | 44.2 ± 0.9 | 65 | 2.9 ± 1.4 | 0.043 | |
| 2-month visit (72.1 days)g | Weight, g | 6287.2 ± 115.1 | 42 | 6118.9 ± 93.2 | 64 | 168.3 ± 148.1 | 0.259 |
| Length, cm | 60.7 ± 0.3 | 42 | 60.7 ± 0.3 | 64 | 0 ± 0.4 | 1.000 | |
| BMI, kg/m2 | 17.0 ± 0.2 | 42 | 16.6 ± 0.2 | 64 | 0.4 ± 0.3 | 0.118 | |
| zBMIe | 0.4 ± 0.1 | 42 | 0.1 ± 0.1 | 64 | 0.2 ± 0.2 | 0.158 | |
| Subcutaneous fatf, mm2 | 43.3 ± 1.1 | 42 | 43.2 ± 0.9 | 62 | 0.1 ± 1.3 | 0.932 | |
| Abdominal circumference-to-length, mm/cm | 0.7 ± 0.01 | 42 | 0.7 ± 0.01 | 64 | 0 ± 0.01 | 0.135 | |
| 4-month visit (127.6 days)h | Weight, g | 7492.9 ± 129.2 | 48 | 7272.3 ± 107.5 | 69 | 220.6 ± 169.3 | 0.195 |
| Length, cm | 65.0 ± 0.3 | 48 | 64.6 ± 0.3 | 69 | 0.4 ± 0.4 | 0.392 | |
| BMI, kg/m2 | 17.7 ± 0.2 | 48 | 17.4 ± 0.2 | 69 | 0.3 ± 0.3 | 0.317 | |
| zBMIe | 0.4 ± 0.1 | 48 | 0.3 ± 0.1 | 69 | 0.1 ± 0.2 | 0.407 | |
| Subcutaneous fatf, mm2 | 45.1 ± 1.0 | 48 | 44.8 ± 0.9 | 69 | 0.3 ± 1.3 | 0.820 | |
| Abdominal circumference-to-length, mm/cm | 0.7 ± 0.01 | 48 | 0.7 ± 0.01 | 69 | 0 ± 0.01 | 0.362 | |
| DXA measures (TBLH) | |||||||
| Fat mass, g | 2158.2 ± 68.8 | 45 | 2164.7 ± 57.1 | 65 | −6.5 ± 90.0 | 0.942 | |
| % Fat (%) | 35.1 ± 0.7 | 45 | 36.9 ± 0.6 | 65 | −1.8 ± 0.9 | 0.055 | |
| Lean mass, g | 3855.1 ± 67.8 | 45 | 3596.8 ± 56.3 | 65 | 258.3 ± 88.7 | 0.004 | |
| Bone mass, g | 78.7 ± 1.4 | 45 | 74.6 ± 1.2 | 65 | 4.1 ± 1.8 | 0.025 | |
| Difference between early infant and 4-month adjusted for early infant visit (120.2 days)i,j,k | Weight, g | 3832.6 ± 118.7 | 44 | 3732.5 ± 97.3 | 65 | 100.1 ± 154.7 | 0.519 |
| Length, cm | 12.3 ± 0.3 | 44 | 12.8 ± 0.3 | 65 | 0.4 ± 0.5 | 0.327 | |
| BMI, kg/m2 | 4.6 ± 0.3 | 43 | 4.3 ± 0.2 | 62 | 0.3 ± 0.4 | 0.391 | |
| zBMIe | 0.7 ± 0.2 | 43 | 0.6 ± 0.2 | 62 | 0.1 ± 0.2 | 0.736 | |
| Subcutaneous fatf, mm2 | 25.4 ± 1.5 | 41 | 24.3 ± 1.2 | 62 | 1.1 ± 1.9 | 0.561 | |
| Abdominal circumference-to-length, mm/cm | 0.008 ± 0.01 | 43 | 0.006 ± 0.01 | 62 | 0.002 ± 0.01 | 0.845 | |
| DXA measures (TBLH) | |||||||
| Fat mass, g | 1619.0 ± 70.7 | 42 | 1655.8 ± 58.6 | 61 | −36.8 ± 92.4 | 0.691 | |
| % Fat (%) | 17.1 ± 0.7 | 42 | 19.1 ± 0.6 | 61 | −2.0 ± 0.9 | 0.035 | |
| Lean mass, g | 1557.8 ± 61.5 | 42 | 1362.4 ± 50.8 | 61 | 195.4 ± 80.7 | 0.017 | |
| Bone mass, g | 32.1 ± 1.3 | 42 | 29.5 ± 1.1 | 61 | 2.6 ± 1.7 | 0.140 | |
Bolding indicates p-values < 0.05
Values are least square means adjusted for age.
AA/AG are individuals with 1 or 2 copies of the CREBRF variant rs373863828 (CREBRF:c.1370G>A p.(R457Q)), and GG are infants with no copies.
P-values are from pairwise comparisons.
All early infant outcome means were estimated at the mean age 6.4 days except zBMI (which were calculated using WHO age references).
Age- and sex-standardized BMI z-scores (zBMI) were calculated using World Health Organization Child Growth Standards.
Subcutaneous fat mass (mm2) estimated using the sum of all skinfold measures.
All 2-month outcome means were estimated at the mean ages 72.1 days except zBMI (which were calculated using WHO age references).
All 4-month outcome means were estimated at the mean age 127.6 days except zBMI (which were calculated using WHO references).
All outcomes are estimated as the mean difference in body composition measure from 0 to 199 days at a mean age of 120.2 days except zBMI (which was calculated using WHO age references).
To control for infant age in these models, we subtracted number of days old at the 4-month visit from number of days old at the early infant visit.
All models of the difference and rate of change between the 4-month and early infant visit were also adjusted for the corresponding outcome measurement at the early infant visit per Johnson et al.31.
When the significant age-adjusted associations reported in Table 2 were examined in multivariable models taking into account the additional covariates (Table 3), the early infant association between the CREBRF variant and bone mass no longer reached the threshold for statistical significance, although the direction was retained; only maternal postpartum BMI was significantly (positively) associated with early infant bone mass. The association between genotype and bone mass did, however, remain significant at the 4-month assessment after adjustment for the significant positive effects of infant age, sex (males had greater bone mass), and formula feeding (Table 3). The inclusion of these covariates did not appreciably alter the magnitude of the difference between those with and without the variant (4.2 grams) compared to the initial analysis. Similarly, the association between CREBRF genotype and lean mass remained significant at the 4-month assessment after adjustment for the positive associations of infant age and sex (males having higher adjusted lean mass) (Table 3).
Table 3.
Multivariable models for significant outcomes at the early infant and 4-month visit. Model 1 included all covariates (infant age, maternal BMI, sex, breastfeeding status, and maternal age). Model 2 are best-fit models after backwards stepwise elimination.
| Bone Mass at Early infant Visit | %Fat at 4-month Visit | Bone Mass at 4-month Visit | Lean Mass at 4-month Visit | |||||
|---|---|---|---|---|---|---|---|---|
| Predictor | 1 | 2 | 1 | 2c | 1 | 2 | 1 | 2 |
| AA/AG Genotype (Ref: GG genotype) | 2.5 (1.4) (−0.2, 5.2) p=0.071 |
2.5 (1.4) (−0.2, 5.2) p=0.065 |
−1.6 (0.9) (−3.3, 0.2) p=0.078 |
−1.3 (0.9) (−3.0, 0.4) p=0.136 |
4.2 (1.8) (0.6, 7.7) p=0.022 |
4.0 (1.8) (0.5, 7.5) p=0.026 |
202.4 (80.0) (43.6, 361.2) p=0.013 |
210.9 (79.6) (53.0, 368.8) p=0.009 |
| Infant age (days) | 0.2 (0.1) (−0.03, 0.4) p=0.095 |
0.2 (0.1) (−0.02, 0.4) p=0.083 |
0.1 (0.04) (−0.03, 0.1) p=0.195 |
0.2 (0.1) (−0.02, 0.4) p=0.083 |
0.2 (0.1) (0.1, 0.4 p=0.004 |
0.2 (2.8) (0.1, 0.4) p=0.006 |
9.5 (3.4) (2.9, 16.2) p=0.005 |
8.9 (3.3) (2.5, 15.4) p=0.007 |
| Maternal BMI (kg/m2) | 0.3 (0.1) (0.1, 0.5) p=0.010 |
0.3 (0.1) (0.1, 0.5) p=0.007 |
−0.01 (0.07) (−0.1, 0.1) p=0.917 |
0.3 (0.1) (0.1, 0.5) p=0.007 |
−0.03 (0.1) (−0.3, 0.2) p=0.823 |
10.9 (6.0) (−0.9, 22.7) p=0.070 |
||
| Sex (Ref: Male) | −0.2 (1.3) (−2.9, 2.4) p=0.866 |
1.5 (0.9) (−0.2, 3.2) p=0.077 |
1.5 (0.9) (−0.2, 3.2) p=0.085 |
−5.5 (1.7) (−9.0, −2.0) p=0.002 |
−5.7 (1.7) (−9.0, −2.3) p=0.001 |
−404.3 (78.1) (−559.3, −249.3) p<.001 |
−429.8 (76.5) (−581.5, −278.0) p<0.001 |
|
| Not Breastfeeding (Ref: Breastfeeding)a | 4.1 (2.4) (−0.8, 8.9) p=0.099 |
|||||||
| Mixed Feeding (Ref: Breastfeeding) | −2.4 (1.0) (−4.5, −0.4) p=0.022 |
−2.2 (1.0) (−4.2, −0.1) p=0.036 |
−1.4 (2.1) (−5.7, 2.8) p=0.511 |
−58.6 (95.9) (−248.8, 131.7) p=0.543 |
||||
| Formula Feeding (Ref: Breastfeeding) | −2.9 (1.2) (−5.3,−0.5) p=0.021 |
−3.0 (1.2) (−5.5,−0.5) p=0.017 |
5.2 (2.6) (0.1, 10.3) p=0.046 |
5.5 (2.5) (0.7, 10.4) p=0.026 |
35.3 (115.0) (−192.9, 263.5) p=0.760 |
|||
| Maternal age (years) | 0.03 (0.1) (−0.2, 0.3) p=0.791 |
−0.1 (0.2) (−0.2, 0.1) p=0.291 |
0.1 (0.2) (−0.2, 0.4) p=0.414 |
−5.7 (6.9) (−19.4, 8.0) p=0.413 |
||||
| Constant | 32.9 (4.4) (24.1, 41.7) p<.001 |
33.8 (3.5) (26.9, 40.7) p<.001 |
33.5 (5.6) (22.4, 44.5) p<.001 |
37.0 (0.7) (35.5, 38.4) p<.001 |
46.3 (11.4) (23.7, 68.9) p<.001 |
50.5 (9.2) (32.2, 68.8) p<.001 |
2389.5 (509.0) (1379.6, 3399.3) p<.001 |
2279.6 (463.8) (1359.7, 3199.4) p<.001 |
| nb | 109 | 109 | 109 | 109 | 109 | 109 | 109 | 109 |
| R2 | 0.160 | 0.137 | 0.143 | 0.115 | .249 | 0.241 | 0.364 | 0.334 |
| AIC | 428.371 | 425.297 | 323.984 | 321.458 | 478.783 | 473.910 | 1299.406 | 1296.334 |
Beta coefficient (Standard error) (95% CI in parentheses)
For the early infant visit breastfeeding was yes/no.
One male infant was removed because of missing data for maternal postpartum BMI.
The best fit model for %fat excluded genotype, however it was forced back in to test the research question.
The association between genotype and change in lean mass between birth and 4-months was significant after adjusting for infant age and male sex (which were both positively associated with the outcome) and lean mass in early infancy (which was negatively associated) (Table 4). Infants with the AA/AG genotype gained 176.9 grams more lean mass than infants without the variant. The association between GG genotype and change in percent fat no longer met the threshold for statistical significance after controlling for covariates (Table 4). Although it was not significant in the models adjusted only for age, for comparative purposes we present longitudinal analyses of bone mass in Table 4. After adjustment for additional covariates, the association between genotype and change in bone mass remained non-significant.
Table 4.
Longitudinal multivariable models. Model 1 included all covariates (infant age, maternal BMI, sex, breastfeeding status, and maternal age). Model 2 are best-fit models after backwards stepwise elimination.
| Difference in % Fat Mass from Early infant to 4-month Visit | Difference in Bone Mass from Early infant to 4 Month Visit | Difference in Lean Mass from Early infant to 4 Month Visit | ||||
|---|---|---|---|---|---|---|
| Predictor | 1 | 2a | 1 | 2a | 1 | 2 |
| Genotype (Ref: GG genotype) | −1.6 (0.9) (−3.4, 0.2) p=0.072 |
−1.5 (0.9) (−3.3, 0.3) p=0.094 |
2.9 (1.7) (−0.5, 6.2) p=0.090 |
2.3 (1.7) (−1.1, 5.5) p=0.179 |
176.8 (73.3) (31.3, 322.4) p=0.018 |
176.9 (73.0) (32.0, 321.8) p=0.017 |
| % Fat Mass at the early infant visit (%) | −0.7 (0.2) (−1.0, −0.4) p<.001 |
−0.8 (0.1) (−1.0, −0.5) p<.001 |
||||
| Bone mass at the early infant visit, g | −0.4 (0.1) (−0.6, −0.2) p=0.001 |
−0.4 (0.1) (−0.7, −0.2) p<.001 |
||||
| Lean mass at the early infant visit, g | −0.4 (0.1) (−0.6, −0.2) p<0.001 |
−0.4 (0.1) (−0.6, −0.2) p<0.001 |
||||
| Difference in days from early infant to 4-month visit (days) |
0.05 (0.03) (−.02, 0.1) p=0.149 |
0.2 (0.1) (0.06, 0.3) p=0.003 |
0.2 (0.1) (0.1, 0.3) p=0.002 |
11.8 (2.7) (6.5; 17.1) p<0.001 |
11.6 (2.7) (6.3, 17.0) p<0.001 |
|
| Maternal BMI (kg/m2) | −0.05 (0.1) (−0.2, 0.1) p=0.510 |
−0.2 (0.1) (−0.4, 0.1) p=0.176 |
7.5 (5.4) (−3.3, 18.3) p=0.169 |
|||
| Sex (Ref: Male) | 0.5 (1.0) (−1.5, 2.6) p=0.607 |
−4.8 (0.1) (−8.0, −1.6) p=0.004 |
−4.9 (1.6) (−8.0, −1.7) p=0.003 |
−329.3 (72.3) (−472.8, −185.7) p<0.001 |
−354.0 (71.0) (−495.0, 213.1) p<0.001 |
|
| Mixed Feeding (Ref: Breastfeeding) | −2.2 (1.1) (−4.4, −0.1) p=0.040 |
−2.0 (1.1) (−4.1, 0.1) p=0.066 |
−1.7 (1.6) (−5.6, 2.9) p=0.389 |
−53.5 (86.1) (−224.5, 117.4) p=0.536 |
||
| Formula Feeding (Ref: Breastfeeding) | −2.8 (1.3) (−5.4, −0.3) p=0.029 |
−2.9 (1.3) (−5.4, −0.4) p=0.023 |
3.7 (2.3) (−0.9, 8.3) p=0.115 |
116.4 (105.1) (−92.3. 325.2) p=0.271 |
||
| Maternal age (years) | −0.08 (0.1) (−0.3, 0.1) p=0.334 |
0.1 (0.1) (−0.2, 0.4) p=0.452 |
−8.0 (6.2) (−20.4, 4.3) p=0.197 |
|||
| Constant | 30.5 (5.7) (19.2, 41.8) p<.001 |
33.7 (2.3) (29.2, 38.3) p<.001 |
31.3 (10.0) (11.4, 51.2) p=0.002 |
30.3 (8.9) (12.7, 48.0) p=0.001 |
1002.9 (509.9) (−9.6, 2015.4) p=0.052 |
1110.6 (2.7) (177.5, 2043.7) p=0.020 |
| nb | 102 | 102 | 102 | 102 | 102 | 102 |
| R2 | 0.386 | 0.354 | 0.311 | 0.274 | .489 | 0.456 |
| AIC | 309.234 | 306.407 | 430.854 | 432.962 | 1204.195 | 1202.546 |
Beta coefficient (Standard error) (95% CI in parentheses)
The best fit model excluded genotype, however it was forced back in to test the research question.
One male infant was removed because of missing data for maternal postpartum BMI.
Finally, we examined the interaction between sex and genotype in these models by adding in an interaction term, however the interaction was not significant in any models.
Discussion
This is the first study to examine body size, body composition, and the CREBRF rs373863828 variant in infancy. Samoan infants with at least one copy of the minor allele (AA or AG genotype) had marginally greater bone mass at the early infant assessment, significantly greater bone and lean mass at 4-months, and greater lean mass accrual from the early infant to 4-month assessment compared to infants with the GG genotype.
We did not, as we hypothesized, observe differences in fat mass by genotype. In fact, in line with the lean mass and bone findings, infants with the variant had a smaller positive change in % fat during the first 4-months of life compared to those without the variant (borderline significance). Notably, we also did not observe a relationship between the CREBRF variant and infant abdominal-to-length ratio (a proxy for visceral fat) or subcutaneous fat. At least in infancy, this does not support the hypothesis that those with AA or AG genotype have greater adiposity or more metabolically favorable adiposity distribution.
These results support the only other published study examining infant size and CREBRF rs373863828 genotype: a multiethnic birth cohort with children from several Pacific populations9. Although there were questions about the study’s validity based on the unexpectedly high prevalence of the minor allele32 in their multi-ethnic cohort, taken together with our findings, it may suggest that the genotype-phenotype association observed in adults emerges after early infancy, perhaps in response to nutritional or environmental exposures and/or activity patterns (N.B. To date, no environmental exposures have been identified that interact with the CREBRF variant during infancy). A similar, age-related emergence of adiposity has been reported with variants in FTO at 5 years of age33.
Identifying when the phenotypes associated with CREBRF emerge may present health practitioners with an opportunity to intervene. Although the variant appears to lower diabetes risk, the effect of increasing BMI over time may heighten the risk of other cardio-metabolic disease. Minster et al.5 indeed found that the variant does not offer complete protection from obesity-related metabolic disease. Implementing interventions before children with the CREBRF variant experience an increase in BMI may reduce their long-term risk of disease, particularly if we identify environmental factors (e.g., food intake and physical activity) that interact with the genotype. It should be noted that although we had data on infant feeding, we did not have enough power to investigate any interactions. Almost all infants were breastfeeding – at least partially - at all time points, providing an inadequate formula-fed sample for a gene × feeding interaction term in longitudinal models. Future larger studies, perhaps later in infancy when feeding mode is more diverse, should consider whether mixed- or formula-fed infants also show the same variant-associations with fat-free mass.
While no significant associations with adiposity were detected, we do show greater bone and lean mass during infancy associated with the AA or AG CREBRF genotypes. Differences in lean mass by genotype were not evident at birth, but emerged during the first 4-months of life, becoming significant in cross-sectional analyses at 4-months of age and in the longitudinal models. Conversely, the bone phenotype was present at birth and 4-months, but was not significant in longitudinal models, indicating that the magnitude of the difference between genotype groups was only minimally increasing over time. This may indicate that differential deposition of lean mass by genotype is driven by environmental or nutritional status after birth, but that differences in bone mass by genotype are present early in postnatal life and less modifiable. Notably, in the cross-sectional models the effect of the variant on bone mass at the early infant assessment was larger than that of infant sex (and similar at 4-months), which is known to be the strongest predictor of body composition in this age group20. Using air displacement plethysmography at five days of age, a 2019 study found Pacific Islander and Maori infants had greater fat-free mass compared to Asian and European infants34. High fat-free mass may be a unique trait in Pacific Islanders, possibly produced via a pathway associated with the CREBRF variant.
The current findings offer a possible mechanistic explanation for the ‘protective’ association of the CREBRF minor allele with type 2 diabetes. Lean and bone mass have direct effects on diabetes risk, i.e. via effects on whole body glucose utilization. For example, skeletal muscle - the most abundant component of lean mass - is the major source of insulin-independent and insulin-dependent glucose uptake. Not surprisingly then, greater muscle mass in adults is associated with lower serum glucose, greater insulin sensitivity, and lower risk of diabetes13,35. Bone mass also makes substantial contributions to whole body glucose utilization and, at least in mice, perhaps even more so than traditional glucose-utilizing organs such as muscle, adipose tissue, and liver36,37. Lean and bone mass also have indirect effects on diabetes risk via secretion of autocrine, paracrine, or endocrine factors that influence body composition and/or metabolic homeostasis. Skeletal muscle secretes bioactive substances that contribute to insulin sensitivity and glucose homeostasis, including irisin, IL-6, and many other “myokines”38. The endocrine function of bone is exemplified by the osteoblast-specific secreted protein osteocalcin, which regulates pancreatic beta cell proliferation and insulin secretion as well as peripheral insulin sensitivity and energy expenditure12. Studies of adult Samoans have not yet examined body composition and its association with the CREBRF genotype. Ongoing and future studies may lend support to these preliminary findings.
While this study has numerous strengths, including the novelty of its research question, measurement of infant adiposity using DXA, longitudinal evaluation of adiposity from birth to 4-months, and adjustment for important covariates, it also has limitations. First, the sample size is relatively small (driven by time and resources available to PhD student KA). Although we detected bone and lean mass phenotypes associated with the CREBRF variant, it is possible we did not have enough power to detect effects (e.g., on BMI) that would have been present in a larger sample. Second, we were not able to obtain maternal pre-pregnancy weight, which is considered best practice for controlling for maternal BMI. Instead we measured maternal BMI at the time of the early infant assessment, which may instead represent ‘peak’ tissue mass during pregnancy. Third, maternal physical activity during pregnancy, gestational weight gain, and gestational age were not controlled for as confounders in the analysis. Weight monitoring is rare in the Samoan setting and recall of pregnancy physical activity is subject to recall bias. Prospective study designs are needed to objectively measure and adequately examine these maternal characteristics for their association with infant growth, body size, and body composition in this setting.
In conclusion, infants with the CREBRF rs373863828 minor allele (AA/AG) had greater bone and lean mass at 4-months, and greater lean mass accrual from the early infant assessment to the 4-month assessment than those without the variant. We detected no differences in BMI between infants with or without the variant. This suggests that the ‘BMI-increasing’ phenotype observed in adults emerges after 4-months of age. Additional research is required to validate the results of this exploratory study and in particular, to investigate lean and bone mass accretion as a pathway that might explain the protective effect of the variant against diabetes5. Identifying the underlying mechanism by which the CREBRF variant and influence obesity and diabetes risk will be essential for improving cardiometabolic health outcomes in this high-risk population.
Acknowledgments:
This research was funded by grants from MacMillan Center for International Dissertation Research Fellowships at Yale University; the National Institutes of Health grant number [2 R01 HL093093]; and the National Science Foundation grant number [BCS DDRIG 1749911]. The authors thank the participating mothers and their infants and the midwives and doctors at Samoa National Health Services who contributed to the study. The authors would also like to thank Elise Claffey and Madison Rodman for their contributions to data collection, Richard Bribiescas for his support with the study design, and Claudia Valeggia for her feedback on drafts of this manuscript.
Footnotes
Competing Interests: The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results. The authors declare no conflict of interest.
References
- 1.Hawley NL, McGarvey ST. Obesity and diabetes in Pacific Islanders: the current burden and the need for urgent action. Curr Diab Rep 2015;15(5):29. [DOI] [PubMed] [Google Scholar]
- 2.Hodge A, Dowse G, Toelupe P, Collins V, Imo T, Zimmet P. Dramatic increase in the prevalence of obesity in western Samoa over the 13-year period 1978–1991. IInternational J Obes Relat Metab Disord 1994;18(6):419–28. [PubMed] [Google Scholar]
- 3.McGarvey S, Baker PT. The Effects of Modernization and Migration on Samoan Blood Pressures. Hum Biol 1979;51(4):461–79. [PubMed] [Google Scholar]
- 4.Seiden A, Hawley NL, Schulz D, Raifman S, Mcgarvey ST. Long-term trends in food availability, food prices, and obesity in samoa. Am J Hum Biol 2012;24(3):286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minster RL, Hawley NL, Su C, Sun G, Kershaw EE, Cheng H, et al. A thrifty variant in CREBRF strongly influences body mass index in Samoans. Nat Genet 2016;48(9):1049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loos RJF, Yeo GSH. The bigger picture of FTO — the first GWAS - identified obesity gene. Nat Publ Gr 2013;10(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naka I, Furusawa T, Kimura R, Natsuhara K, Yamauchi T, Nakazawa M, et al. A missense variant, rs373863828-A (p.Arg457Gln), of CREBRF and body mass index in Oceanic populations. J Hum Genet. 2017; 62:847–849. [DOI] [PubMed] [Google Scholar]
- 8.Ohashi J, Naka I, Furusawa T, Kimura R, Yamauchi T, Nakazawa M, et al. Association study of CREBRF missense variant (rs373863828 : G > A; p. Arg457Gln) with levels of serum lipid profile in the Pacific populations. Ann Hum Biol 2018;45(3):215–9. [DOI] [PubMed] [Google Scholar]
- 9.Berry SD, Walker CG, Ly K, Snell RG, Atatoa Carr PE, Bandara D, et al. Widespread prevalence of a CREBRF variant amongst Māori and Pacific children is associated with weight and height in early childhood. Int J Obes 2018;42(4):603–7. [DOI] [PubMed] [Google Scholar]
- 10.Krishnan M, Major TJ, Topless RK, Dewes O, Yu L, Thompson JMD. Discordant association of the CREBRF rs373863828 A allele with increased BMI and protection from type 2 diabetes in Māori and Pacific (Polynesian) people living in Aotearoa / New Zealand. Diabetologia 2018;61:1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson RL, Safabakhsh S, Curtis JM, Hsueh WC, Jones LI, Aflague TF, et al. Association of CREBRF variants with obesity and diabetes in Pacific Islanders from Guam and Saipan. Diabetologia 2019;62(9):1647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature 2012;481:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong S, Chang Y, Jung H, Yun KE, Shin H, Ryu S. Relative muscle mass and the risk of incident type 2 diabetes: A cohort study. PLoS One 2017;12(11):e0188650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillman MW. Early infancy – a critical period for development of obesity. J Dev Orig Health Dis 2010;1(05):292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arslanian KJ. Early Life in Samoa: Nutritional and Genetic Predictors of Infant Body Composition and an Analysis of Maternal Attitudes toward Breastfeeding. [Unpublished doctoral dissertation] 2020; Yale University. [Google Scholar]
- 16.World Bank. GDP - Samoa [Internet]. World Bank Natl. accounts data, OECD Natl. Accounts data files 2018;Available from: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?locations=WS
- 17.Samoa Bureau of Statistics. Demographic and Health Survey. 2014.
- 18.Pye D, Hannan W, Hesp R. Effective dose equivalent in dual X-ray absorptiometry. Br J Radiol 1991;63:149. [DOI] [PubMed] [Google Scholar]
- 19.Lewis MK, Blake GM, Fogelman I. Osteoporosis International Original Article Patient Dose in Dual X-ray Absorptiometry. Osteoporos Int 1994;4:11–5. [DOI] [PubMed] [Google Scholar]
- 20.Njeh C, Samat S, Nightingale A, McNeil E, Boivin C. Radiation dose and in vitro precision in paediatric bone mineral density measurement using dual x-ray absorptiometry. Br J Radiol 1997;70:719–27. [DOI] [PubMed] [Google Scholar]
- 21.Thomas S, Kalwalf H, Buckley D, Heubi J. Effective dose of dual energy x-ray absorptiometry scans in children as a function of age. J Clin Densitom 2005;8:415–22. [DOI] [PubMed] [Google Scholar]
- 22.Blake GM, Naeem M, Boutros M. Comparison of effective dose to children and adults from dual X-ray absorptiometry examinations. Bone 2006;38(6):935–42. [DOI] [PubMed] [Google Scholar]
- 23.Steel SA, Baker AJ, Saunderson JR. An assessment of the radiation dose to patients and staff from a Lunar Expert-XL fan beam densitometer. Physiol Meas 1998;19(1):17–26. [DOI] [PubMed] [Google Scholar]
- 24.National Council on Radiation Protection and Measurements: Ionizing radiation exposure of the population of the United States. Bethesda, MD: 1987. [Google Scholar]
- 25.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. 2006.
- 26.Holston A, Stokes T, Olsen C, Choi YS, Curtis J, Higginson J, et al. Novel noninvasive anthropometric measure in preterm and full-term infants: Normative values for waist circumference:length ratio at birth. Pediatr Res 2013;74(3):299–306. [DOI] [PubMed] [Google Scholar]
- 27.Swinburn BA, Ley SJ, Carmichael HE, Plank LD. Body size and composition in Polynesians. Int J Obes 1999;23(11):1178–83. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. The Questionnaires: https://www.cdc.gov/breast-feeding/data/ifps/quest.
- 29.Hawley NL, Johnson W, Nu’usolia O, McGarvey ST. The contribution of feeding mode to obesogenic growth trajectories in American Samoan infants. Pediatr Obes 2012;9:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roubenoff R, Kehayias J, Dawson-Hughes B, Heymsfield S. Use of dual-energy x-ray absorptiometry in body-composition studies: not yet a “gold standard.” Am J Clin Nutr 1993;58(5):589–91. [DOI] [PubMed] [Google Scholar]
- 31.Johnson W Analytical Strategies in Human Growth Research. Am J Hum Biol 2015;83:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Major T, Krishnan M, Topless R, Dewes O, Thompson J, de Zoysa J, et al. Re: “Widespread prevalence of a CREBRF variant among Māori and Pacific children is associated with weight and height in early childhood.” Int J Obes 2018;42(7):1389–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sovio U, Mook-kanamori DO, Warrington NM, Lawrence R, Timpson NJ. Association between Common Variation at the FTO Locus and Changes in Body Mass Index from Infancy to Late Childhood : The Complex Nature of Genetic Association through Growth and Development. PLoS Genet 2011;7(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander T, Conlon CA, Gamble G, von Hurst PR, van Dorp L, Ichhpuniani B, et al. Body composition of New Zealand-born term babies differs by ethnicity, gestational age and sex. Early Hum Dev 2019;140:2–6. [DOI] [PubMed] [Google Scholar]
- 35.Bassett DRJ. Skeletal muscle characteristics: relationships to cardiovascular risk factors. Med Sci Sports Exerc 1994;26(8):957–66. [PubMed] [Google Scholar]
- 36.Liu D mei, Mosialou I, Liu J min. Bone: Another potential target to treat, prevent and predict diabetes. Diabetes, Obes Metab 2018;20(8):1817–28. [DOI] [PubMed] [Google Scholar]
- 37.Zoch ML, Abou DS, Clemens TL, Thorek DLJ, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res 2016;4(January):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckel J Myokines in metabolic homeostasis and diabetes. Diabetologia 2019;62(9):1523–8. [DOI] [PubMed] [Google Scholar]


