Key Points
Question
Does direct transfer to angiography suite (DTAS) improve functional outcomes compared with usual imaging workflow for patients within 6 hours of onset of symptoms for large vessel occlusion ischemic stroke?
Findings
In this randomized clinical trial including 174 patients with suspected large vessel occlusion stroke on admission, large vessel occlusion was confirmed in 147. The use of a DTAS protocol improved in-hospital workflow times, increased the rate of endovascular treatment, and decreased the severity of disability across the range of modified Rankin Scale scores.
Meaning
For patients with acute ischemic stroke due to large vessel occlusion within 6 hours of symptom onset, compared with usual imaging, the use of DTAS led to safe patient triage for acute stroke endovascular treatment, decreased workflow times, and improved outcomes; ongoing international, multicenter clinical trials are exploring the generalizability of these results.
Abstract
Importance
Direct transfer to angiography suite (DTAS) for patients with suspected large vessel occlusion (LVO) stroke has been described as an effective and safe measure to reduce workflow time in endovascular treatment (EVT). However, it is unknown whether DTAS improves long-term functional outcomes.
Objective
To explore the effect of DTAS on clinical outcomes among patients with LVO stroke in a randomized clinical trial.
Design, Setting, and Participants
The study was an investigator-initiated, single-center, evaluator-blinded randomized clinical trial. Of 466 consecutive patients with acute stroke screened, 174 with suspected LVO acute stroke within 6 hours of symptom onset were included. Enrollment took place from September 2018 to November 2020 and was stopped after a preplanned interim analysis. Final follow-up was in February 2021.
Interventions
Patients were randomly assigned (1:1) to follow either DTAS (89 patients) or conventional workflow (85 patients received direct transfer to computed tomographic imaging, with usual imaging performed and EVT indication decided) to assess the indication of EVT. Patients were stratified according to their having been transferred from a primary center vs having a direct admission.
Main Outcomes and Measures
The primary outcome was a shift analysis assessing the distribution of the 90-day 7-category (from 0 [no symptoms] to 6 [death]) modified Rankin Scale (mRS) score among patients with LVO whether or not they received EVT (modified intention-to-treat population) assessed by blinded external evaluators. Secondary outcomes included rate of EVT and door-to-arterial puncture time. Safety outcomes included 90-day mortality and rates of symptomatic intracranial hemorrhage.
Results
In total, 174 patients were included, with a mean (SD) age of 73.4 (12.6) years (range, 19-95 years), and 78 patients (44.8%) were women. Their mean (SD) onset-to-door time was 228.0 (117.9) minutes, and their median admission National Institutes of Health Stroke Scale score was 18 (interquartile range [IQR], 14-21). In the modified intention-to-treat population, EVT was performed for all 74 patients in the DTAS group and for 64 patients (87.7%) in the conventional workflow group (P = .002). The DTAS protocol decreased the median door–to–arterial puncture time (18 minutes [IQR, 15-24 minutes] vs 42 minutes [IQR, 35-51 minutes]; P < .001) and door-to-reperfusion time (57 minutes [IQR, 43-77 minutes] vs 84 minutes [IQR, 63-117 minutes]; P < .001). The DTAS protocol decreased the severity of disability across the range of the mRS (adjusted common odds ratio, 2.2; 95% CI, 1.2-4.1; P = .009). Safety variables were comparable between groups.
Conclusions and Relevance
For patients with LVO admitted within 6 hours after symptom onset, this randomized clinical trial found that, compared with conventional workflow, the use of DTAS increased the odds of patients undergoing EVT, decreased hospital workflow time, and improved clinical outcome.
Trial Registration
ClinicalTrials.gov Identifier: NCT04001738
This randomized clinical trial assesses whether direct transfer to the angiography suite improves clinical outcomes compared with usual imaging workflow among patients within 6 hours from onset of symptoms for large vessel occlusion ischemic stroke.
Introduction
Endovascular treatment (EVT) has become the standard of care for acute ischemic stroke due to a large vessel occlusion (LVO)1,2 after several trials showed its efficacy.3,4,5,6,7 Time from symptom onset to reperfusion is a strong indicator of clinical outcome8,9 and the most relevant modifiable factor together with final reperfusion status.10 Because odds of functional independence decrease with delay in reperfusion,11 strategies to safely reduce this time span are being investigated.
Although the bulk of the workflow time corresponds to the prehospital phase, in-hospital pathways are also continually reassessed to minimize the time from hospital arrival to recanalization. Emergency department admission to artery puncture (door-to-puncture [DTP]) time is a widely used performance metric to evaluate in-hospital workflow efficacy that has been shown to be associated with clinical outcome.12
Despite the efforts dedicated to reduce DTP times, published registries13 and clinical trials have shown the difficulties in decreasing DTP time below 60 minutes, a target that has been set by expert consensus.14 The HERMES meta-analysis15 reported DTP times ranging from 81 minutes for transferred patients to 116 minutes for patients directly admitted to an endovascular center.
Research assessing optimized workflows has led to a newly proposed paradigm in the acute treatment of patients with severe stroke: direct transfer to angiography suite (DTAS). Protocols for DTAS were simultaneously designed in several centers mirroring the ST-segment elevation myocardial infarction strategy of bypassing the emergency department and conventional imaging. On arrival at the angiography suite, the use of flat-panel computed tomography (FPCT)16 enables the ruling out of either an intracranial hemorrhage (ICH) or a large established infarct.17,18 In addition, LVO can be diagnosed with a flat-panel angiography system immediately before arterial puncture or directly with initial diagnostic angiography.17,19,20,21
Regardless of the protocol details, DTAS has been consistently shown to be effective in decreasing DTP time to as low as 16 minutes without safety concerns.22 The effect of DTAS on long-term functional outcomes varies between published nonrandomized studies and is still unclear.
The aim of the present randomized clinical trial ANGIOCAT was to establish whether participants with clinically suspected LVO on admission (established by National Institutes of Health Stroke Scale [NIHSS] score >10) following a DTAS workflow have more favorable long-term clinical outcomes compared with participants following conventional neuroimaging in-hospital workflow.
Methods
Study Design
The ANGIOCAT trial was a prospective, open, randomized clinical trial (NCT04001738)23 with blinded assessment of the primary end point by an independent investigator (trial protocol in Supplement 1). This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The study protocol was approved by the ethics committee of Vall d’Hebron Institut de Recerca, Barcelona, Spain. All patients or their surrogates provided deferred written informed consent that was obtained in a manner consistent with the Declaration of Helsinki.24 No one received compensation or was offered any incentive for participating in this study (eAppendix 1 in Supplement 2).
Whon the emergency medical services team suspected a patient of having acute LVO (Rapid Arterial Occlusion Evaluation [RACE]25 scale, which ranges from 0 to 9, with higher values indicating more severe symptoms, >4), they notified the receiving stroke centers before arrival that the patient had been prescreened. After confirmation of inclusion and exclusion criteria (eAppendix 2 in Supplement 2) by the stroke neurologist on patient arrival, patients were randomly assigned to 1 of 2 study groups:
For the group using the DTAS, FPCT was performed to exclude ICH or large established ischemic lesions that would contraindicate EVT. A diagnostic angiogram was then obtained to confirm the presence of LVO. If indicated, intravenous tissue plasminogen activator could be initiated immediately after FPCT. For patients transferred from a primary stroke center for whom an initial CT scan had already been performed, FPCT was repeated only when considered necessary by the treating physician.
For the group using the direct transfer to CT (DTCT) suite, the usual neuroimaging protocol was followed, including CT and CT angiography. In addition, CT perfusion could be performed if deemed necessary by the treating physician. If indicated, intravenous tissue plasminogen activator could be initiated immediately after CT; indication for EVT was decided after analyzing neuroimages according to institutional and European Stroke Organisation guidelines.2 All patients with a class Ia recommendation of EVT according to current guidelines received EVT. For patients with an Alberta Stroke Program Early Computed Tomography Score (ASPECTS) below 6 or established ischemic signs in the compromised territory (ie, M2 occlusion with an ASPECTS of 6), an individualized decision about EVT was made by the treating team.
Independently of the allocated protocol, all patients received equal care during admission to the stroke unit or the intensive care unit. All thrombectomies were performed with European Medicines Agency–approved thrombectomy devices, at the discretion of the interventionalist. The ANGIOCAT trial was performed in a comprehensive stroke center with a high volume of cases (approximately 300 EVT events per year) and more than 2 years of experience with the DTAS protocol when the study started.
Randomization
Prealert to the Stroke Neurologist Before Randomization
Regional stroke network protocols indicated that emergency medical services should contact the receiving center by telephone on identifying a patient with suspected acute stroke (RACE score >4) and time from symptom onset less than 6 hours. Patients could be categorized as primary admissions or secondary transfers from a primary stroke center. Immediately on patient arrival at the comprehensive stroke center, a stroke neurologist confirmed the inclusion criteria, and the patient was enrolled in the ANGIOCAT trial.
Randomization and Minimization
Patients were randomly assigned to DTAS or DTCT in a 1:1 ratio, following a randomization sequence provided by an independent investigator. To avoid an imbalance in the primary and secondary admission rates between the study groups, a blocked randomization according to transfer provenance was performed: primary (no previous neuroimaging) vs transfer from other centers (previous neuroimaging).
Clinical and Radiologic Assessment
All patients underwent clinical assessment, including prehospital RACE by emergency medical services and NIHSS evaluations by certified stroke neurologists (scores range from 0 to 42, with higher values indicating more severe deficit) on admission, after 24 hours, and at 5 days or prior to discharge. Relevant workflow times (symptoms onset, hospital admission, imaging acquisition, intravenous tissue plasminogen activator bolus, arterial puncture, and reperfusion) were also recorded by stroke neurologists. Functional outcome at 90 days was evaluated using the modified Rankin Scale (mRS; scores range from 0 [no symptoms] to 6 [death]) through a structured telephone-based interview performed by a central, trained, and certified assessor (independent from the center) who was blinded to group assignment.26
Radiologic variables such as baseline ASPECTS27 (range, 0-10, with 1 point subtracted for any evidence of early ischemic change in each defined region on the CT scan), baseline presence and location of LVO (CT angiography or digital subtraction angiography), and 24-hour follow-up CT for ICH detection were assessed by local neuroradiologists. Local interventionalists assessed reperfusion status using the modified Thrombolysis in Cerebral Infarction score, which ranges from 0 (no reperfusion) to 3 (complete reperfusion).28
Masking
Treating physicians could not be blinded to group allocations. Although this inability may lead to performance bias, all involved professionals were encouraged to treat the patients according to best clinical practice. Investigators, who were not blinded to the assigned group, registered secondary outcomes (risk of evaluation bias). However, for all patients, the primary outcome (mRS score at 90 days) was evaluated through a telephone call by a certified central assessor who was blinded to group assignment. Codi Ictus Catalunya (CICAT) is a prospective official mandatory registry of all stroke codes in Catalunya, and 90-day mRS scores of all patients who experience an acute stroke in our territory are centrally evaluated (independent from the hospital) by trained and certified evaluators who are unaware of patient participation in a trial. Results are made public and reported at CICAT. The 90-day mRS scores of patients in the present trial were adjudicated using CICAT registry data.
Outcomes
The primary outcome was the mRS score at 90 days for patients with confirmed LVO (modified intention-to-treat [mITT] population). The primary analysis was a shift analysis assessing the distribution of the 90-day 7-category mRS outcome in the mITT population using an ordinal logistic regression to estimate the common odds ratios (ORs).
The secondary efficacy outcomes included time from hospital admission to arterial puncture, rate of dramatic early recovery (NIHSS score ≤2 or NIHSS score improvement ≥10 points at 24 hours29), rate of mRS score of 2 or lower at 90 days, and rate of undergoing EVT among patients with LVO. Safety outcomes, which were evaluated in the entire study population, included rate of neurologic deterioration (determined as NIHSS score increased ≥4 points at 24 hours30), rate of neurologic complications (mainly symptomatic hemorrhagic transformation), rate of procedural complications, and 90-day mortality.
Sample Size Estimation
For the sample size calculation, we used preliminary published results from our center and previous publications.19 We expected a reduction of 30 to 40 minutes in door–to–arterial puncture time with the DTAS protocol. For patients with confirmed LVO, this reduction may represent a 15% increase in the rate of mRS scores from 0 to 2. Given this estimation, to achieve a statistical power of 75% to detect differences using a bilateral χ2 test with a significance level of 5% and a 1:1 distribution between the 2 study groups, the necessary sample size was 306 patients. Patients were followed up until 90 days after treatment or until death, whichever occurred first.
Trial Termination
An interim analysis was performed as planned after 50% of the maximum sample size (150 patients) had completed 90 days of follow-up (eAppendix 3 in Supplement 2). We assumed a 1-sided familywise error rate of 2.5%, power of 75%, and an OR of 1.70 (ln OR = 0.5306) under the assumption of proportional odds. We tested against an OR of 1 (ln OR = 0), and we used Pocock stopping boundaries for stopping the trial early owing to superiority. We derived z scores by dividing the estimated log OR from the ordinal logistic regression by its standard error. The boundary for superiority of the active treatment over control at the interim analysis is a z score of 2.178 and an α error rate of .029. The steering committee accepted the recommendation of the data and safety monitoring board to stop recruitment because of a significant and substantial difference in the primary outcome (z score of 2.25 and α error of .025). Final follow-up was in February 2021.
Statistical Analysis
The primary efficacy analysis was a shift analysis to assess the improvement in mRS scores in the mITT population using a cumulative ordinal logistic regression to estimate the common OR. For secondary efficacy and safety analyses, statistical significances for intergroup differences were assessed by the Pearson χ2 test or the Fisher exact test for categorical variables and by the t test or the Mann-Whitney test for continuous variables among patients with confirmed LVO for efficacy and among all patients for safety end points. A descriptive analysis of adverse events is presented in aggregate and by event.
Multivariable logistic or ordinal regression analyses were used to determine factors that could be considered independent estimators of good functional outcomes. The analyses were adjusted using variables that presented a trend (P ≤ .10) or a statistically significant association with the explored outcome. The OR and its 95% CI based on logistic or ordinal regression are reported.
Heterogeneity of treatment effect by prespecified clinically relevant variables was tested on the primary outcome using a multiplicative interaction term (treatment × prespecified variable) and mixed-methods modeling. A 2-sided value of P < .05 was considered statistically significant. All statistical analyses were conducted using IBM SPSS Statistics for Windows, version 21 (IBM Corp).
Results
Between September 2018 and November 2020, 2111 patients with a code for stroke were admitted to our center. Of these patients, 466 met the inclusion criteria (premorbid mRS score of 0-2; NIHSS score >10; and time from stroke symptom onset to hospital admission <6 hours). The main reason for excluding patients was off-hours admission, which did not allow sufficient time for the neurovascular team to be onsite before patient arrival.
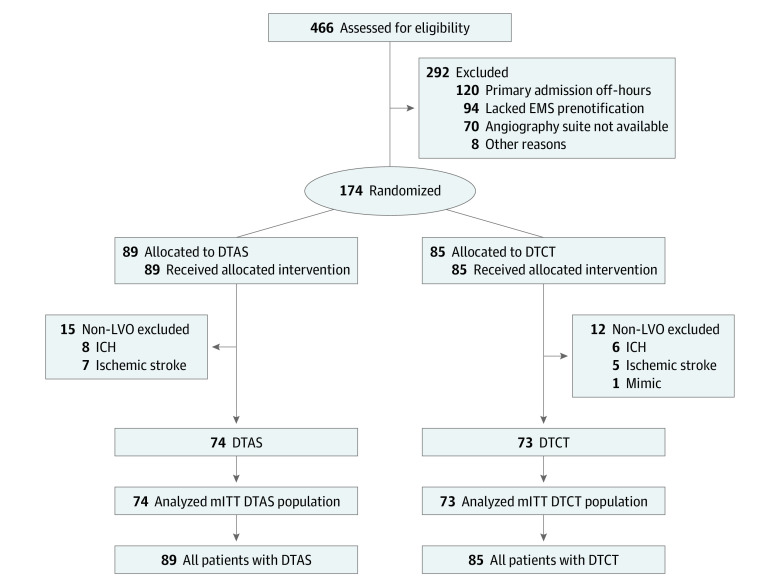
In total, 174 patients (mean [SD] age, 73.4 [12.6] years [range, 19-95 years]; 78 women [44.8%]) were enrolled in the study (safety analysis population: 89 [51.1%] in the DTAS group; 85 [48.9%] in the DTCT group). Of them, 147 patients (84.5%) presented with LVO (mITT population: 74 [50.3%] in the DTAS group; 73 [49.7%] in the DTCT group) (Figure 1). All included patients but 1 had an available evaluation of primary outcome at 90 days, and no crossover occurred.
Figure 1. ANGIOCAT Trial Flow Diagram.
DTCT indicates direct transfer to computed tomography; EMS, emergency medical services; ICH, intracranial hemorrhage; LVO, large vessel occlusion; and mITT, modified intention-to-treat.
Baseline Characteristics
The included 174 patients had a mean (SD) onset-to-door time of 228.0 (117.9) minutes and a median admission NIHSS score of 18 (interquartile range [IQR], 14-21). There were no differences in baseline characteristics in the mITT population (eAppendix 2 in Supplement 2 and Table 1). Of these patients, 107 (61.5%) were admitted during off-hours, and the final diagnosis was ischemic stroke for 159 patients (91.3%) (81 patients [91.0%] in the DTAS group; 78 patients [91.8%] in the DTCT group), ICH in 14 patients (8.0%) (8 patients [9.0%] in the DTAS group; 6 patients [7.1%] in the DTCT group), and stroke mimic in 1 patient. The median ASPECTS in the DTCT group was 9 (IQR, 7-10), and only 3 patients (4.1% of patients with LVO) presented with an ASPECTS of lower than 6.
Table 1. Baseline Characteristics of the Modified Intention-to-Treat Populationa.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| DTAS (n = 74) | DTCT (n = 73) | |
| Age, mean (SD), y | 71.8 (13.1) | 75.6 (12.3) |
| Sex | ||
| Male | 38 (51.4) | 32 (43.8) |
| Female | 36 (48.6) | 41 (56.2) |
| Prestroke mRS score, median (IQR)b | 1 (0-1) | 1 (0-1) |
| Prestroke mRS score | ||
| 0 | 26 (35.1) | 22 (30.1) |
| 1 | 31 (41.9) | 36 (49.3) |
| 2 | 14 (19.4) | 14 (19.2) |
| >2 | 3 (4.1) | 1 (1.4) |
| Risk factors | ||
| Hypertension | 56 (75.7) | 46 (63.0) |
| Diabetes | 10 (13.5) | 19 (26.0) |
| Dyslipidemia | 31 (41.9) | 38 (52.1) |
| Atrial fibrillation | 21 (28.4) | 23 (31.5) |
| Previous stroke | 12 (16.2) | 9 (12.3) |
| Ischemic heart disease | 11 (14.9) | 15 (20.5) |
| Current smoker | 11 (14.9) | 21 (28.8) |
| Previous stroke | 12 (16.2) | 9 (12.3) |
| RACE score, median (IQR)c | 7 (6-8) | 7 (6-8) |
| NIHSS score, median (IQR)d | 17 (14-21) | 18 (14-22) |
| Onset or LTSW to door time, mean (SD), min | 233.6 (106.3) | 240.2 (130.7) |
| Primary/transferred | 19/55 (25.7/74.3) | 21/52 (28.8/71.2) |
| Unknown onset | 15 (20.3) | 7 (9.6) |
| ASPECTS at PSC, median (IQR) | 10 (9-10) | 10 (9-10) |
| Off-hours | 40 (54.0) | 50 (68.5) |
| Intravenous thrombolysis | 37 (50.0) | 39 (53.4) |
| Tandem occlusion | 11 (14.9) | 11 (15.1) |
| Endovascular treatment | 74 (100) | 64 (87.7) |
Abbreviations: ASPECTS, Alberta Stroke Program Early Computed Tomography Score; DTAS, direct transfer to angiography suite; DTCT, direct transfer to computed tomography; IQR, interquartile range; LTSW, last time seen well; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; PSC, primary stroke center; RACE, Rapid Arterial Occlusion Evaluation.
Modified intention-to-treat population was defined as patients with large vessel occlusion. There were no significant differences between groups.
The mRS scores of functional disability range from 0 (no symptoms) to 6 (death). A score of 2 or lower indicates functional independence.
The RACE scale ranges from 0 to 9, with higher values indicating more severe stroke symptoms.
The NIHSS ranges from 0 to 42, with higher values indicating more severe stroke symptoms.
Primary Outcome
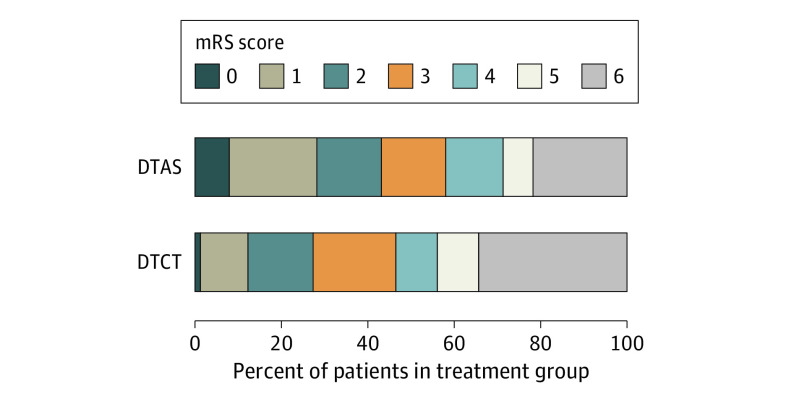
The primary outcome analysis showed an adjusted common OR of improvement of 1 point on the mRS score of 2.2 (95% CI, 1.2-4.1) favoring DTAS (P = .009) (Table 2, Figure 2).
Table 2. Primary and Secondary Clinical Outcomesa.
| Outcome | Patients, % | Effect variable | Odds ratio (95% CI) | ||
|---|---|---|---|---|---|
| DTAS (n = 74) | DTCT (n = 73) | Unadjusted | Adjusted | ||
| Shift analysis | |||||
| 90-d mRS scoreb | NA | NA | Common odds ratio | 2.0 (1.1-3.7) | 2.2 (1.2-4.1) |
| 90-d mRS score (unified 5-6) | NA | NA | Common odds ratio | 2.1 (1.2-3.7) | 2.2 (1.2-4.0) |
| 90-d mRS score | |||||
| 0-2 | 43.2 | 27.4 | Odds ratio | 2.0 (1.0-4.0) | 2.0 (0.8-4.8) |
| 0-3 | 58.1 | 45.2 | Odds ratio | 1.7 (0.9-3.2) | 1.9 (0.8-4.3) |
| 90-d mortalitya | 21.3 | 32.9 | Odds ratio | 0.6 (0.3-1.1) | 0.5 (0.2-1.2) |
Abbreviations: DTAS, direct transfer to angiography suite; DTCT, direct transfer to computed tomography; mRS, modified Rankin Scale; NA, not applicable.
Primary and secondary efficacy outcomes were evaluated in the modified intention-to-treat population, defined as patients with large vessel occlusion, whereas 90-day mortality was evaluated in all patients. The DTAS protocol improved clinical outcome, showing an adjusted common odds ratio of improvement of 1 point on the mRS score of 2.2.
The mRS scores of functional disability range from 0 (no symptoms) to 6 (death), with a score of 2 or lower indicating functional independence.
Figure 2. Percent Distribution of Modified Rankin Scale (mRS) Scores at 90 Days by Treatment in the Modified Intention-to-Treat Population.
Shown are mRS scales for patients in the direct transfer to angiography suite (DTAS) group and the direct transfer to computed tomography (DTCT) group who were evaluated by a central assessor blinded to group assignment. There was a significant difference between the DTAS group and the control DTCT group in the overall distribution of scores after adjustment for age, National Institutes of Health Stroke Scale score at baseline, time from stroke onset to admission, premorbid functional status, and atrial fibrillation (adjusted common odds ratio for improvement of 1 point on mRS, 2.2; 95% CI, 1.2-4.1).
Secondary Outcomes
In the mITT population, the rate of EVT was significantly higher in the DTAS group than in the DTCT group (74 [100%] vs 64 [87.7%]; P = .002) (Table 3). Analysis of the in-hospital treatment delays showed that patients in the DTAS group had a significantly shorter median time from admission to arterial puncture (18 minutes [IQR, 15-24 minutes] vs 42 minutes [IQR, 35-51 minutes]; P < .001) and a shorter median door-to-reperfusion time (57 minutes [IQR, 43-77 minutes] in the DTAS group vs 84 minutes [IQR, 63-117 minutes] in the DTCT group; P < .001). The median time from arterial puncture to reperfusion was similar between groups (37 minutes [IQR, 23-60 minutes] in the DTAS group vs 39 minutes [IQR, 22-62 minutes] in the DTCT group; P = .49). For directly admitted patients who received intravenous tissue plasminogen activator, the median door-to-needle time was similar between groups (20 minutes [IQR, 18-25 minutes] in the DTAS group vs 18 minutes [IQR, 16-38 minutes in the DTCT group]; P = .80).
Table 3. Workflow and Procedural Characteristics of the Modified Intention-to-Treat Populationa.
| Characteristic | Patients, No. (%) | P value | |
|---|---|---|---|
| DTAS (n = 74) | DTCT (n = 73) | ||
| Patients receiving EVT | |||
| Door-to-puncture time, median (IQR), min | 18 (15-24) | 42 (35-51) | <.001 |
| No. of passes, median (IQR) | 2 (1-3) | 2 (1-3) | .22 |
| Onset-to-reperfusion time, mean (SD), min | 290.5 (141.7) | 326.9 (122.2) | .32 |
| Door-to-reperfusion time, median (IQR), min | 57 (43-77) | 84 (63-117) | <.001 |
| Occlusion level | |||
| TICA | 18 (24.3) | 17 (23.3) | .28 |
| M1-MCA | 40 (54.1) | 32 (43.8) | |
| M2-MCA | 11 (14.9) | 22 (30.1) | |
| PCA | 1 (1.35) | 0 | |
| BA | 2 (2.7) | 1 (1.3) | |
| Multiple vessel | 2 (2.7) | ||
| mTICI grade (patients receiving EVT)b | |||
| 0 | 3 | 4 | .82 |
| 1 | 1 (1.35) | 0 | |
| 2a | 6 | 4 | |
| 2b | 20 | 14 | |
| 2c | 16 | 13 | |
| 3 | 28 | 29 | |
| mTICI grades 2b-3 (patients receiving EVT) | 64 (86.4) | 56 (87.5) | .86 |
| Severe procedure complication | 6 (8.1) | 2 (2.7) | .6 |
| 24-h NIHSS score, median (IQR)c | 11 (3-18.5) | 15 (6.5-20) | .24 |
| Dramatic recovery | 25 (33.8) | 21 (28.8) | .51 |
| Symptomatic hemorrhage | 1 (1.4) | 3 (4.1) | .28 |
| Hemicraniectomy | 2 (2.7) | 4 (5.5) | .39 |
| Discharge/5-d NIHSS score, median (IQR) | 6.5 (1-14) | 11 (4-19) | .11 |
| In-hospital mortality | 5 (6.8) | 8 (11.0) | .39 |
| Vascular access complication | 2 (2.7) | 0 | .16 |
Abbreviations: BA, basilar artery; DTAS, direct transfer to angiography suite; DTCT, direct transfer to computed tomography; EVT, endovascular treatment; IQR, interquartile range; MCA, middle cerebral artery; mTICI, modified treatment in cerebral infarction; NIHSS, National Institutes of Health Stroke Scale; PCA, posterior cerebral artery; TICA, terminal internal carotid artery.
Modified intention-to-treat population defined as patients with large vessel occlusion; DTAS showed shorter door-to-puncture and door-to-reperfusion times than DTCT.
The mTICI scale assesses reperfusion status, which ranges from 0 (no reperfusion) to 3 (complete reperfusion).
Scale ranges from 0 to 42, with higher values indicating more severe stroke symptoms.
The rate of dramatic recovery (33.8% in the DTAS group vs 28.8% in the DTCT group; P = .51) and the median NIHSS scores at 24 hours (11 [IQR, 3-18.5] in the DTAS group vs 15 [IQR, 6.5-20] in the DTCT group; P = .24) and at 5 days or discharge (6.5 [IQR, 1-14] in the DTAS group vs 11 [IQR, 4-19] in the DTCT group; P = .11) were not significantly different between groups.
The rates of a good clinical outcome measured by an mRS score of 2 or lower at 90 days were 43.6% for DTAS and 27.8% for DTCT (P = .11). Predefined subgroup analysis showed that the effect of DTAS on outcomes was modified by admission NIHSS score and provenance (eAppendix 2 in Supplement 2). A pure intention-to-treat analysis including all patients showed an adjusted common OR of improvement of 1 point on the mRS score of 1.98 (95% CI, 1.10-3.51) favoring DTAS (P = .02) (eAppendix 2 in Supplement 2).
Safety Outcomes
There were no significant differences between the DTAS group and the DTCT control group in the rates of clinical deterioration (10.1% in the DTAS group vs 17.6% in the DTCT group; P = .15), rates of symptomatic hemorrhagic transformation (1.2% in the DTAS group vs 3.8% in the DTCT group; P = .40), or rates of severe procedural complications (8.1% in the DTAS group vs 2.7% in the DTCT group; P = .60).
Discussion
Our study findings showed that for patients with acute ischemic stroke caused by LVO within 6 hours of symptom onset, the DTAS protocol was safe and led to improved clinical outcomes compared with the DTCT protocol. The DTAS protocol decreased in-hospital delays, achieving shorter times from hospital admission to treatment onset and to reperfusion, which were associated with a significant shift toward better outcomes across the spectrum of disability.
The improvements in clinical outcomes shown in our study are substantial and are likely due to 2 major reasons: increasing the rate of EVT by avoiding overselection in treatment indication and reducing in-hospital workflow. All patients with confirmed LVO who followed the DTAS protocol underwent EVT. The DTAS indication for EVT was based on a simplified imaging protocol that was unable to provide thorough parenchymal assessment compared with conventional neuroimaging, in which identification of a large ischemic lesion led to withholding of EVT in up to 13% of patients with confirmed LVO. The benefit of EVT for patients with large ischemic lesions is unclear; however, several studies have suggested that EVT may improve outcome,31,32 which may have occurred in our study for patients in the DTAS group with large ischemic cores who received EVT. The imaging quality of FPCT may be a concern because it is still inferior to the quality achieved with conventional CT. However, there are several tips that can be implemented to improve imaging quality during flat-panel acquisition, and the latest generation of FPCT allows for the detection of a large infarct core with a significant reduction in artifacts,16 minimizing the number of cases in which FPCT images are deemed insufficient to make decisions about treatment.
Similar to previously published studies,18,19,20,21 DTAS decreased DTP time to as low as 18 minutes and decreased door-to-reperfusion time to less than 1 hour. Improving workflow has been repeatedly associated with improved clinical outcome and with higher rates of successful reperfusion33; odds of functional independence decrease approximately 15% for each additional 30 minutes’ delay in reperfusion.11 These values are in line with the 24-minute decrease in DTP time, the 36-minute decrease in the onset-to-reperfusion time, and the 15% increase in the rate of mRS scores of 0 to 2 in the DTAS group. In our study, DTP time in the DTCT group (43 minutes) was below that typically reported, and 87.5% of patients achieved a DTP time of less than 60 minutes. Therefore, compared with a standard 60-minute performance, implementation of a DTAS protocol with DTP times below 20 minutes may have an even greater effect on outcome.
The rate of mRS scores of 0 to 2 in the DTCT group (27.8%) may appear low when compared with previously published trials with similar imaging criteria (eg, 32.6% in the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands [MR CLEAN]4 and 43.7%3 in the Randomized Trial of Revascularization With Solitaire FR Device versus Best Medical Therapy in the Treatment of Acute Stroke Due to Anterior Circulation Large Vessel Occlusion Presenting Within Eight Hours of Symptom Onset [REVASCAT]). There are several potential explanations for this finding. Contrary to most thrombectomy randomized clinical trials that included only patients with prestroke mRS scores of 0 to 1, the present trial allowed for inclusion of patients with prestroke mRS scores of up to 2. In addition, ANGIOCAT included patients presenting with a large ischemic core, some of whom did not receive EVT.
Our study included primary admissions and secondary transferred patients in a 1:2 proportion. The distinction is relevant because an initial neuroimaging assessment at a primary stroke center enabled identification of an eventual ICH that precluded inclusion in the trial. Given this proportion of primary admissions, the rate of false-positive DTAS candidates defined as patients meeting all the inclusion criteria who finally did not have LVO was 15.5%. Of these patients, 8% presented with ICH, and 7.5% underwent diagnostic angiography, which detected no treatable occlusion. None of the patients experienced a procedural complication, including vascular access, confirming the safety of our DTAS protocol. False-positive activation of DTAS in our study remained similar to that observed in patients with myocardial infarction.34 The present trial also confirmed that DTAS is safe for all patients suspected of acute stroke, including those with no LVO or with ICH.
Our study design included all patients with LVO whether they underwent EVT or not. The benefits of the DTAS protocol are likely multifactorial and must be evaluated as a whole, beyond an isolated decrease in time delays. It is possible that changing the imaging protocol had an effect on EVT indication counterbalancing in the DTAS group, with a potential overselection among patients with low ASPECTS in the DTCT group.
Our previous study results suggested that the quicker treatment was started after symptom onset, the better the treatment effect of DTAS compared with DTCT.22 These findings were not replicated in the present trial. A plausible explanation for this discrepancy is that the early termination of ANGIOCAT underpowered secondary analyses and precluded strong conclusions for those measures.
To offer the clinical benefits of DTAS to the highest number of patients, the angiography suite and interventional team should be immediately available 24 hours a day, 7 days a week. Having a dedicated acute stroke angiography suite and a permanently available team would require organizational and staff efforts that would be justified in large-volume centers if our results are confirmed in a second randomized clinical trial.
Limitations
Our trial had several limitations. The limited funding did not allow for external monitoring of the data other than by the data safety monitoring board. Because ANGIOCAT was a single-center randomized clinical trial performed in a center with sustained experience performing DTAS, results may differ in centers where DTAS is initially adopted, owing to a necessary organizational learning curve before achieving the observed workflow benefits. The early termination of the trial made it unpowered to detect differences between groups in safety variables. Finally, an interim analysis may have overestimated the real treatment effect.35 Multicentric international randomized clinical trials are being developed to determine the replicability of our findings.
Conclusions
In conclusion, findings from this randomized clinical trial indicate that DTAS within 6 hours of symptom onset improved functional outcomes among patients with acute ischemic stroke caused by LVO.
Trial Protocol
eAppendix 1. Investigators and Administrative Staff
eAppendix 2. Supplementary Results
eAppendix 3. First Interim Analysis Focused on the First 150 Patients Evaluated by the DSMB
Data Sharing Statement
References
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 2.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)–European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019;11(6):535-538. doi: 10.1136/neurintsurg-2018-014568 [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 4.Berkhemer OA, Fransen PS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. Published correction appears in N Engl J Med. 2015;372(4):394. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, et al. ; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019-1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafe A, et al. ; SWIFT PRIME Investigators . Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285-2295. doi: 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 7.Campbell BC, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 8.Jahan R, Saver JL, Schwamm LH, et al. Association between time to treatment with endovascular reperfusion therapy and outcomes in patients with acute ischemic stroke treated in clinical practice. JAMA. 2019;322(3):252-263. doi: 10.1001/jama.2019.8286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 10.Dargazanli C, Consoli A, Barral M, et al. Impact of modified TICI 3 versus modified TICI 2b reperfusion score to predict good outcome following endovascular therapy. AJNR Am J Neuroradiol. 2017;38(1):90-96. doi: 10.3174/ajnr.A4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribo M, Molina CA, Cobo E, et al. ; REVASCAT Trial Investigators . Association between time to reperfusion and outcome is primarily driven by the time from imaging to reperfusion. Stroke. 2016;47(4):999-1004. doi: 10.1161/STROKEAHA.115.011721 [DOI] [PubMed] [Google Scholar]
- 12.Sun CH, Ribo M, Goyal M, et al. Door-to-puncture: a practical metric for capturing and enhancing system processes associated with endovascular stroke care, preliminary results from the rapid reperfusion registry. J Am Heart Assoc. 2014;3(2):e000859. doi: 10.1161/JAHA.114.000859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon BK, Xu H, Cox M, et al. Components and trends in door to treatment times for endovascular therapy in Get With The Guidelines-Stroke hospitals. Circulation. 2019;139(2):169-179. doi: 10.1161/CIRCULATIONAHA.118.036701 [DOI] [PubMed] [Google Scholar]
- 14.McTaggart RA, Ansari SA, Goyal M, et al. ; Standards and Guidelines Committee of the Society of NeuroInterventional Surgery (SNIS) . Initial hospital management of patients with emergent large vessel occlusion (ELVO): report of the standards and guidelines committee of the Society of NeuroInterventional Surgery. J Neurointerv Surg. 2017;9(3):316-323. doi: 10.1136/neurintsurg-2015-011984 [DOI] [PubMed] [Google Scholar]
- 15.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 16.Leyhe JR, Tsogkas I, Hesse AC, et al. Latest generation of flat detector CT as a peri-interventional diagnostic tool: a comparative study with multidetector CT. J Neurointerv Surg. 2017;9(12):1253-1257. doi: 10.1136/neurintsurg-2016-012866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Struffert T, Deuerling-Zheng Y, Kloska S, et al. Flat detector CT in the evaluation of brain parenchyma, intracranial vasculature, and cerebral blood volume: a pilot study in patients with acute symptoms of cerebral ischemia. AJNR Am J Neuroradiol. 2010;31(8):1462-1469. doi: 10.3174/ajnr.A2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psychogios MN, Maier IL, Tsogkas I, et al. One-stop management of 230 consecutive acute stroke patients: report of procedural times and clinical outcome. J Clin Med. 2019;8(12):E2185. doi: 10.3390/jcm8122185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez B, Requena M, Aires A, et al. Direct transfer to angio-suite to reduce workflow times and increase favorable clinical outcome. Stroke. 2018;49(11):2723-2727. doi: 10.1161/STROKEAHA.118.021989 [DOI] [PubMed] [Google Scholar]
- 20.Jadhav AP, Kenmuir CL, Aghaebrahim A, et al. Interfacility transfer directly to the neuroangiography suite in acute ischemic stroke patients undergoing thrombectomy. Stroke. 2017;48(7):1884-1889. doi: 10.1161/STROKEAHA.117.016946 [DOI] [PubMed] [Google Scholar]
- 21.Bouslama M, Haussen DC, Grossberg JA, et al. Flat-panel detector CT assessment in stroke to reduce times to intra-arterial treatment: A study of multiphase computed tomography angiography in the angiography suite to bypass conventional imaging. Int J Stroke. 2021;16(1):63-72. doi: 10.1177/1747493019895655 [DOI] [PubMed] [Google Scholar]
- 22.Requena M, Olivé M, García-Tornel Á, et al. Time matters: adjusted analysis of the influence of direct transfer to angiography-suite protocol in functional outcome. Stroke. 2020;51(6):1766-1771. doi: 10.1161/STROKEAHA.119.028586 [DOI] [PubMed] [Google Scholar]
- 23.Evaluation of direct transfer to angiography suite vs. computed tomography suite in endovascular treatment: randomized clinical trial. ClinicalTrials.gov identifier: NCT04001738. Updated January 5, 2021. Accessed June 16, 2021. https://clinicaltrials.gov/ct2/show/NCT04001738
- 24.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 25.Pérez de la Ossa N, Carrera D, Gorchs M, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke. 2014;45(1):87-91. doi: 10.1161/STROKEAHA.113.003071 [DOI] [PubMed] [Google Scholar]
- 26.Wilson JT, Hareendran A, Grant M, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33(9):2243-2246. doi: 10.1161/01.STR.0000027437.22450.BD [DOI] [PubMed] [Google Scholar]
- 27.Barber PA, Demchuk AM, Zhang J, Buchan AM; ASPECTS Study Group. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355(9216):1670-1674. doi: 10.1016/S0140-6736(00)02237-6 [DOI] [PubMed] [Google Scholar]
- 28.Higashida RT, Furlan AJ, Roberts H, et al. ; Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology . Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34(8):e109-e137. doi: 10.1161/01.STR.0000082721.62796.09 [DOI] [PubMed] [Google Scholar]
- 29.Mazighi M, Meseguer E, Labreuche J, et al. Dramatic recovery in acute ischemic stroke is associated with arterial recanalization grade and speed. Stroke. 2012;43(11):2998-3002. doi: 10.1161/STROKEAHA.112.658849 [DOI] [PubMed] [Google Scholar]
- 30.Siegler JE, Boehme AK, Kumar AD, Gillette MA, Albright KC, Martin-Schild S. What change in the National Institutes of Health Stroke Scale should define neurologic deterioration in acute ischemic stroke? J Stroke Cerebrovasc Dis. 2013;22(5):675-682. doi: 10.1016/j.jstrokecerebrovasdis.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desilles JP, Consoli A, Redjem H, et al. ; ETIS (Endovascular Treatment in Ischemic Stroke) Research Investigators . Successful reperfusion with mechanical thrombectomy is associated with reduced disability and mortality in patients with pretreatment diffusion-weighted imaging-Alberta Stroke Program Early Computed Tomography Score ≤6. Stroke. 2017;48(4):963-969. doi: 10.1161/STROKEAHA.116.015202 [DOI] [PubMed] [Google Scholar]
- 32.Kaesmacher J, Chaloulos-Iakovidis P, Panos L, et al. Mechanical thrombectomy in ischemic stroke patients with Alberta Stroke Program Early Computed Tomography Score 0-5. Stroke. 2019;50(4):880-888. doi: 10.1161/STROKEAHA.118.023465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaesmacher J, Maamari B, Meinel TR, et al. ; BEYOND-SWIFT Investigators . Effect of pre- and in-hospital delay on reperfusion in acute ischemic stroke mechanical thrombectomy. Stroke. 2020;51(10):2934-2942. doi: 10.1161/STROKEAHA.120.030208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regueiro A, Fernández-Rodríguez D, Freixa X, et al. False positive STEMI activations in a regional network: comprehensive analysis and clinical impact: results from the Catalonian Codi Infart Network. Rev Esp Cardiol (Engl Ed). 2018;71(4):243-249. doi: 10.1016/j.recesp.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 35.Schulz KF, Grimes DA. Multiplicity in randomised trials II: subgroup and interim analyses. Lancet. 2005;365(9471):1657-1661. doi: 10.1016/S0140-6736(05)66516-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Investigators and Administrative Staff
eAppendix 2. Supplementary Results
eAppendix 3. First Interim Analysis Focused on the First 150 Patients Evaluated by the DSMB
Data Sharing Statement