Abstract
Photodynamic Therapy (PDT) is an externally activated, photochemistry-based approach that generates cytotoxic reactive molecular species (RMS), which kill or modulate biological targets. PDT provides unique opportunities for applications of nanotechnology where light activation can trigger both direct RMS-mediated cytotoxic activity and the release of contents within the nanoconstructs (Figure 1). This process allows several species, working via different mechanisms and molecular targets to be activated or released in the right place and time, thus providing a distinctive approach to combination therapy. With advances in the development of miniaturized, even biodegradable, light sources and delivery systems, exciting possibilities of anatomical reach with PDT are being made possible. This brief article introduces aspects of interfaces of PDT and nanotechnology but, due to space constraints, makes no attempt to be a comprehensive review.
Introduction
The concept of combining light and chemicals for therapy is thousands of years old [1,2]. PDT, in its present form, can be dated back to Raab’s accidental discovery in 1900 that Paramecia combined with acridine orange and exposed to sunlight resulted in cytotoxicity to the organism [3]. Contemporary PDT was developed by contributions from many investigators, notably of Lipson, Schwartz and Dougherty [4–8] and is approved for several cancer and non-cancer applications [2]. PDT involves the light activation of certain chemicals called photosensitizers (PS) to elicit photochemistry that is cytotoxic or deleterious to biologic targets. Inherent to PDT is the dual selectivity imparted by preferential accumulation of the typical photosensitizing agents and by the confinement of light to defined volumes. This photochemistry-based approach is distinct from the more frequently reported laser-activated photothermal approaches where high intensity, often using pulsed lasers, is required to generate thermal effects. PDT typically requires low irradiances in the mW/ cm2 ranges and does not depend on thermally induced “burning” of tissues but rather on the induced photochemistry. It is thus a “kinder, gentler” approach to phototoxicity allowing biological effects to continue after the light trigger has been switched off. Combined with nanotechnology, PDT-Nano provides exceptional opportunities for delivery of therapeutic reagents and newer approaches to combination treatments grounded in cellular mechanisms [9–13] and other advances in photomedicine [14,15]. In addition to enhanced PS delivery, it allows modification of PS physiochemical properties, development of novel, even resorbable light sources, establishment of personalized predictive dosimetry, and the evolution of novel combinatorial therapeutic approaches.
A wide variety of organic and inorganic nanoconstructs (e.g. liposomal, micellar, polymeric, silica and gold nanoparticles) [16] have been introduced to deliver high payloads of PS to the desired sites when combined with targeting moieties. Thus far, the most studied and clinically used nanoconstructs for PS delivery is the liposome [17,18]. Jori et al. demonstrated liposomal delivery of porphyrins and phthalocyanines to the tumors in the 80–90’s [19–21]. In 2001, collaborative work from several laboratories including ours led to the first clinical approval of PDT as a frontline treatment, using Visudyne®, a liposomal verteporfin formulation, and red light for the treatment of age-related macular degeneration [22]. Visudyne is now featured in the listing of nanomaterials approved by the U.S. Food and Drug Administration (FDA). Micelles, with a single layer of polar-nonpolar molecules, are also commonly used to delivery PS [23–25]. TOOKAD delivered in Cremophor micelles (WST09) was in phase II/III clinical trials for the PDT of prostate cancer (ClinicalTrials.gov) [26,27]. Francis et al. modified Bacteriophage MS2 virus capsids with porphyrins and Jurkat-specific aptamers to selectively target and photo-damage Jurkat leukemia T cells in vitro [28]. Packaging of PS into the nanoconstructs can significantly impact the photophysiochemical properties of PS. Pioneered by Zheng, Lovell and colleagues, porphysomes are extraordinarily tightly packed porphyrins within the confined space of the liposomal lipid bilayer [29,30]. These extensively self-quenched porphyrins nanoconstructs (over 1,200-fold greater than the monomerized porphyrins) can be light activated for in vivo photothermal, but not PDT, tumor ablation. Wang et al. demonstrated that reactive oxygen species (ROS) production from protoporphyrin IX can be enhanced up to 4.7 fold when conjugated to gold nanoparticles, thus improving the efficacy of PDT against breast cancer cell in vitro [31]. This enhancement of ROS is likely due to an enhanced electromagnetic field as a result of the localized surface plasmon resonance of gold nanoparticle upon light exposure.
In the context of PDT, nanoconstructs may be built with enough flexibility to be responsive to a broad spectrum of microenvironmental barriers and be more than simple drug carriers. They become photoresponsive entities that act directly, release enclosed materials and yet home in or accumulate at the desired sites preferentially [32]. Polyethylene-glycol (PEG) or monosialoganglioside molecules are widely used to sterically stabilize nanoconstructs and, therefore, can reduce PS uptake by the macrophages in the reticuloendothelial system, prolong circulation half-life, and allow passive accumulation of PS at tumor sites (e.g. via the enhanced permeability and retention effect) [33–36]. Actively targeted nanoconstructs have been developed with the following broad goals:
Inherent targets: where nanoconstructs are driven by moieties (e.g. aptamers, monoclonal antibodies etc.) to direct against specific cancer-associated molecules.
Therapeutically induced targets: where a given treatment triggers or sensitizes the aberrant expression of biomarkers that can serve as molecular targets (e.g. with molecular inhibitors or PDT) [37–40].
Combinations of targets: where cooperatively targeting of both inherent targets and therapeutically induced targets maximizes the treatment benefits by activating several cell death pathways via nanoconstructs.
Photoimmunoconjugates are probably the best examples of focusing on inherent targets. Using non-quenching PS associated with tumor-targeting monoclonal antibodies or antibody fragments, Levy et al. first reported this approach [41]. This has been subsequently developed by several groups including ours in the 80s and the 90s [42–47] and more recently by others [48,49] in different tumor models. We recently reported a “tumor-activatable” photoimmunoconjugate to further improve the safety and selectivity of photoimmunotherapy [50]. In this study, verteporfin was covalently linked to the EGFR-targeting cetuximab at a high payload, which resulted inself-quenching of PS (non-phototoxic). Upon cancer cell internalization, verteporfin phototoxicity and fluorescence was activated via lysosomal proteolysis resulting in de-quenching of verteporfin followed by light irradiation. This enabled the fluorescence imaging of ovarian cancer micrometastases nodules as small as 30 μm and the tumor-specific photo cytotoxicity in a disseminated model of peritoneal cancer micrometastases [50].
The ability to target and control the spatiotemporal release of the PS (e.g. via external activation) has been suggested [51–53]. This approach is extremely useful for applications to tumor where the genetically complex and heterogeneous tumor cells develop multiple mechanisms of survival and resistance to treatments. It enables rational mechanism-based combination of PDT with a secondary mechanistically non-overlapping treatment. Through combinatorial approaches, the molecular responses elicited by PDT (or the other therapy) can sensitize the tumor to the second treatment modality [54,55]. In this context, multicompartmental nanoconstructs provide the opportunity to co-deliver multiple agents, such as PS and conventional or evolving drugs within a single construct (Figure 1),[23, 56]. These constructs are designed with appropriate mechanism-based combinatorial agents and spatiotemporal control release enabled by a combination of appropriate light switch and appropriate chemistries. Such combination treatments customized to deliver the nanoconstruct payloads to the right place at the right time show promise in early studies in pancreatic and ovarian cancer models [47, 50,57–59].
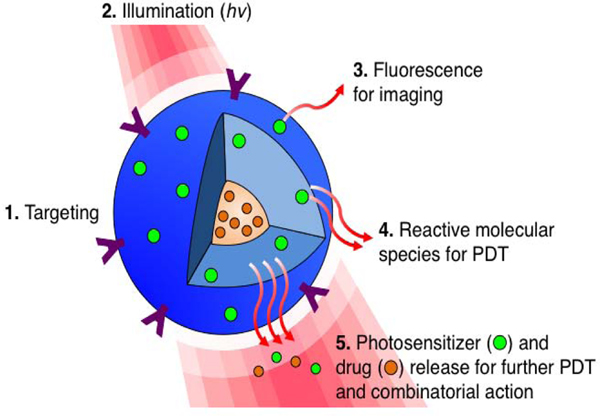
Figure 1:
Targeted, multi-compartment nanoconstruct (blue) co-delivers photosensitizer (PS) and drug to the cell. (1) Surface modification with stabilizers and targeting moieties improves stability and selectively of the nanoconstructs. (2) Upon light irradiation (hv), the (3) fluorescence signal generated from the excited PS can be used for imaging. (4) Light activation of PS results in reactive molecular species production for PDT, and (5) facilitates PS and drug release, allowing for an interactive combination therapy.
With the advent of sophisticated endoscopes and miniaturized light generating and delivery devices, most anatomical sites are accessible for PDT. For example, lung cancer treatments routinely use bronchoscopes incorporating optical fibers. Intra-operative and trans-cutaneous light delivery is also used as in the PDT of pancreatic cancer [60,61]. New approaches exploring nanoconstructs or cells themselves as sources of light are ongoing and have the potential of alleviating the problem of tissue penetration depth. It has been demonstrated that green fluorescent proteins in cells can be used as viable gain medium for optical amplification, creating a laser based on single live cell [62]. Upconverting nanoparticles (i.e. NaYF4 nanocrystals co-doped with Yb3+ and Er3+) use longer wavelength light (i.e. deeper penetrating near-infrared light) to generate shorter (i.e. visible) wavelengths for the activation of PS payloads and the photodynamic destruction of cancers [63–67]. The use of penetrating X-rays to trigger nanoscintillators (i.e. LaF3: Tb3+, quantum dots, Tb2O3 covered by polysiloxane layer) for the excitation of the nanoconstruct coupled PS has been demonstrated [68,69]. This modality where X-rays-activate PS via nanoscintillators can be combined with radiotherapy for combination treatment of diseases that are endoscopically in accessible. Finally, the use of PS fluorescence to detect cancers, guide surgical resection of tumors, and to personalize PDT dose parameters is an ongoing and exciting area of research [2,70]. While several challenges remain, advances in optical technologies combined with actively targeted nanoconstructs containing ’theranostic’ PS offer the potential for personalized photodynamic therapy and combinations for treatment of cancer and other diseases.
Acknowledgment
This work was supported by National Institutes of Health Grants P01CA084203, R01CA156177, and R01CA160998 (to T.H.).
References
- 1.Hasan T, Ortel B, Solban N, Pogue B. Photodynamic therapy of cancer. Kufe DW, Bast RC Jr, Hait WN, Hong WK, Pollock RE, Weichselbaum RR, Holland JF, Frei E III, editors. In: Cancer Medicine 7th edn. Hamilton, Ontario: BC Decker, Inc. 2006; 537–548. [Google Scholar]
- 2.Celli JP, Spring BQ, Rizvi I, Evans CL, Samkoe KS, Verma S, Pogue BW. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010; 110: 2795–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raabe Oscar. Über die Wirkung fluoreszierender Stoffe auf Infusion. Z Biol. 1900; 39: 524–546. [Google Scholar]
- 4.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J. Photodynamic therapy. J Natl Cancer Inst. 1998; 90: 889–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003; 3: 380–387. [DOI] [PubMed] [Google Scholar]
- 6.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011; 61: 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roozeboom MH, Arits AH, Nelemans PJ, Kelleners-Smeets NW. Overall treatment success after treatment of primary superficial basal cell carcinoma: a systematic review and meta-analysis of randomized and nonrandomized trials. British journal of dermatology 2012; 167: 733–756. [DOI] [PubMed] [Google Scholar]
- 8.Pereira S. Photodynamic therapy for pancreatic and biliary tract cancer: the United Kingdom experience. J Natl Compr Canc Netw. 2012; 10 Suppl 2: S48–51. [DOI] [PubMed] [Google Scholar]
- 9.Gomer CJ. Induction of prosurvival molecules during treatment: rethinking therapy options for photodynamic therapy. Journal of the National Comprehensive Cancer Network. 2012; 10: S35–39. [DOI] [PubMed] [Google Scholar]
- 10.Savellano MD. Hasan T. Photochemical targeting of epidermal growth factor receptor: a mechanistic study. Clinical cancer research. 2005; 11: 1658–1668. [DOI] [PubMed] [Google Scholar]
- 11.Kosharskyy B, Solban N, Chang SK, Rizvi I, Chang Y, Hasan T. A mechanism-based combination therapy reduces local tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer research. 2006; 66: 10953–10958. [DOI] [PubMed] [Google Scholar]
- 12.Kessel D, Reiners JJ Jr. Enhanced Efficacy of Photodynamic Therapy via a Sequential Targeting Protocol. Photochem Photobiol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrario A, Gomer CJ. Targeting the tumor microenvironment using photodynamic therapy combined with inhibitors of cyclooxygenase-2 or vascular endothelial growth factor. Methods in molecular biology. 2010; 635: 121–132. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005; 5: 161–171. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008; 60: 1627–1637. [DOI] [PubMed] [Google Scholar]
- 16.Huang YY, Sharma SK, Dai T, Chung H, Yaroslavsky A, Garcia-Diaz MJC, et al. Can nanotechnology potentiate photodynamic therapy? Nanotechnology Reviews. 2012; 1: 111–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen B, Pogue BW, Hasan T. Liposomal delivery of photosensitising agents. Expert Opin Drug Deliv. 2005; 2: 477–487. [DOI] [PubMed] [Google Scholar]
- 18.Jin CS, Zheng G. Liposomal nanostructures for photosensitizer delivery. Lasers Surg Med. 2011; 43: 734–748. [DOI] [PubMed] [Google Scholar]
- 19.Reddi E, Zhou C, Biolo R, Menegaldo E, Jori G. Liposome- or LDL-administered Zn (II)-phthalocyanine as a photodynamic agent for tumours. I. Pharmacokinetic properties and phototherapeutic efficiency. Br J Cancer. 1990; 61: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricchelli F, Biolo R, Reddi E, Tognon G, Jori G. Liposomes as Carriers of Hydrophobic Photosensitizers in Vivo: Increased Selectivity of Tumor Targeting. Proc. of SPIE. 1987; 0847. [Google Scholar]
- 21.Jori G, Tomio L, Reddi E, Rossi E, Corti L, Zorat PL, Calzavara F. Preferential delivery of liposome-incorporated porphyrins to neoplastic cells in tumour-bearing rats. Br J Cancer. 1983; 48: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guidelines for using verteporfin (visudyne) in photodynamic therapy to treat choroidal neovascularization due to age-related macular degeneration and other causes. Retina. 2002; 22: 6–18. [DOI] [PubMed] [Google Scholar]
- 23.Peng CL, Lai PS, Lin FH, Yueh-Hsiu Wu S, Shieh MJ. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38-loaded chlorin-core star block copolymer micelles. Biomaterials. 2009; 30: 3614–3625. [DOI] [PubMed] [Google Scholar]
- 24.Yin R, Wang M, Huang YY, Huang HC, Avci P, Chiang LY, et al. Photodynamic therapy with decacationic [60] fullerene monoadducts: Effect of a light absorbing electron-donor antenna and micellar formulation. Nanomedicine. 2014; 10: 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Nostrum CF. Polymeric micelles to deliver photosensitizers for photodynamic therapy. Adv Drug Deliv Rev. 2004; 56: 9–16. [DOI] [PubMed] [Google Scholar]
- 26.Weersink RA, Bogaards A, Gertner M, Davidson SR, Zhang K, Netchev G, et al. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: clinical experience and practicalities. Journal of photochemistry and photobiology. B, Biology. 2005; 79: 211–222. [DOI] [PubMed] [Google Scholar]
- 27.Lepor H. Vascular Targeted Photodynamic Therapy for Localized Prostate Cancer. Rev Urol. 2008; 10: 254–261. [PMC free article] [PubMed] [Google Scholar]
- 28.Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. Dual-surface modified virus capsids for targeted delivery of photodynamic agents to cancer cells. ACS Nano. 2010; 4: 6014–6020. [DOI] [PubMed] [Google Scholar]
- 29.Lovell JF, Jin CS, Huynh E, Jin H, Kim C, Rubinstein JL, et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nature materials. 2011; 10: 324–332. [DOI] [PubMed] [Google Scholar]
- 30.Jin CS, Lovell JF, Zheng G. One minute, sub-one-watt photothermal tumor ablation using porphysomes, intrinsic multifunctional nanovesicles. Journal of visualized experiments. 2013; e50536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khaing Oo MK, Yang Y, Hu Y, Gomez M, Du H, Wang H. Gold nanoparticle-enhanced and size-dependent generation of reactive oxygen species from protoporphyrin IX. ACS nano. 2012; 6: 1939–1947. [DOI] [PubMed] [Google Scholar]
- 32.Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013; 12: 958–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamblin MR, Miller JL, Rizvi I, Ortel B, Maytin EV, Hasan T. Pegylation of a chlorin(e6) polymer conjugate increases tumor targeting of photosensitizer. Cancer Res. 2001; 61: 7155–7162. [PubMed] [Google Scholar]
- 34.Senior J, Delgado C, Fisher D, Tilcock C, Gregoriadis G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: studies with poly (ethylene glycol)-coated vesicles. Biochimica biophysica acta. 1991; 1062: 77–82. [DOI] [PubMed] [Google Scholar]
- 35.Jain RK. Delivery of molecular and cellular medicine to solid tumors. Adv Drug Deliv Rev. 2001; 46: 149–168. [DOI] [PubMed] [Google Scholar]
- 36.Kessel D. Delivery of photosensitizing agents. Adv Drug Deliv Rev. 2004; 56: 7–8. [DOI] [PubMed] [Google Scholar]
- 37.del Carmen MG, Rizvi I, Chang Y, Moor AC, Oliva E, Sherwood M, et al. Synergism of epidermal growth factor receptor-targeted immunotherapy with photodynamic treatment of ovarian cancer in vivo. Journal of the National Cancer Institute 2005; 97: 1516–1524. [DOI] [PubMed] [Google Scholar]
- 38.Anand S, Wilson C, Hasan T, Maytin EV. Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy. Cancer research. 2011; 71: 6040–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha AK, Anand S, Ortel BJ, Chang Y, Mai Z, Hasan T, et al. Methotrexate used in combination with aminolaevulinic acid for photodynamic killing of prostate cancer cells. British journal of cancer. 2006; 95: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edmonds C, Hagan S, Gallagher-Colombo SM, Busch TM, Cengel KA. Photodynamic therapy activated signaling from epidermal growth factor receptor and STAT3: Targeting survival pathways to increase PDT efficacy in ovarian and lung cancer. Cancer biology & therapy. 2012; 13: 1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mew D, Wat CK, Towers GH, Levy JG. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. Journal of immunology (Baltimore, Md. 1950). 1983; 130: 1473–1477. [PubMed] [Google Scholar]
- 42.Schmidt-Erfurth U, Hasan T, Schomacker K, Flotte T, Birngruber R. In vivo uptake of liposomal benzoporphyrin derivative and photothrombosis in experimental corneal neovascularization. Lasers in surgery and medicine. 1995; 17: 178–188. [DOI] [PubMed] [Google Scholar]
- 43.Goff BA, Bamberg M, Hasan T. Photoimmunotherapy of human ovarian carcinoma cells ex vivo. Cancer Res. 1991; 51: 4762–4767. [PubMed] [Google Scholar]
- 44.Duska LR, Hamblin MR, Miller JL, Hasan T. Combination photoimmunotherapy and cisplatin: effects on human ovarian cancer ex vivo. Journal of the National Cancer Institute. 1999; 91: 1557–63. [DOI] [PubMed] [Google Scholar]
- 45.Molpus KL, Hamblin MR, Rizvi I, Hasan T. Intraperitoneal photoimmunotherapy of ovarian carcinoma xenografts in nude mice using charged photoimmunoconjugates. Gynecologic oncology. 2000; 76: 397–404. [DOI] [PubMed] [Google Scholar]
- 46.Soukos NS, Hamblin MR, Keel S, Fabian RL, Deutsch TF, Hasan T. Epidermal growth factor receptor-targeted immunophotodiagnosis and photoimmunotherapy of oral precancer in vivo. Cancer Res. 2001; 61: 4490–4496. [PMC free article] [PubMed] [Google Scholar]
- 47.Rizvi I, Dinh TA, Yu W, Chang Y, Sherwood ME, Hasan T. Photoimmunotherapy and irradiance modulation reduce chemotherapy cycles and toxicity in a murine model for ovarian carcinomatosis: perspective and results. Israel journal of chemistry. 2012; 52: 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Dongen GA, Visser GW, Vrouenraets MB. Photosensitizer-antibody conjugates for detection and therapy of cancer. Adv Drug Deliv Rev. 2004; 56: 31–52. [DOI] [PubMed] [Google Scholar]
- 49.Mitsunaga Makoto, Ogawa Mikako, Kosaka Nobuyuki, Rosenblum Lauren T, Choyke Peter L, Kobayashi Hisataka. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nature medicine. 2011; 17: 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spring BQ, Abu-Yousif AO, Palanisami A, Rizvi I, Zheng X, Mai Z. et al. Selective treatment and monitoring of disseminated cancer micro metastases in vivo using dual-function, activatable immunoconjugates. Proc Natl Acad Sci USA. 2014; 111: e933–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson DH, Gerasimov OV, Wheeler JJ, Rui Y, Anderson VC. Triggerable plasmalogen liposomes: improvement of system efficiency. Biochimica et biophysica acta. 1996; 1279: 25–34. [DOI] [PubMed] [Google Scholar]
- 52.Qualls MM, Thompson DH. Chloroaluminum phthalocyanine tetrasulfonate delivered via acid-labile diplasmenylcholine-folate liposomes: intracellular localization and synergistic phototoxicity. International journal of cancer. Journal international du cancer. 2001; 93: 384–392. [DOI] [PubMed] [Google Scholar]
- 53.Leung SJ, Romanowski M. Light-activated content release from liposomes. Theranostics. 2012; 2: 1020–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasan T. Using Cellular Mechanisms to Develop Effective Combinations of Photodynamic Therapy and Targeted Therapies. Journal of the National Comprehensive Cancer Network. 2012; 10: S23–S26. [DOI] [PubMed] [Google Scholar]
- 55.Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S. Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg Med. 2006; 38: 516–521. [DOI] [PubMed] [Google Scholar]
- 56.Rahmanzadeh R, Rai P, Celli JP, Rizvi I, Baron-Lühr B, Gerdes J, Hasan T. Ki-67 as a molecular target for therapy in an in vitro three-dimensional model for ovarian cancer. Cancer Res. 2010; 70: 9234–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mir Y, Elrington SA, Hasan T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomedicine: nanotechnology, biology, and medicine. 2013; 9: 1114–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spring QB, Palanisami A, Zheng LK, Blatt EA, Sears RB, Hasan T. Efficient measurement of total tumor microvascularity ex vivo using a mathematical model to optimize volume subsampling. Journal of Biomedical Optics. 2013; 18: 096015. [Google Scholar]
- 59.Abu-Yousif AO, Moor AC, Zheng X, Savellano MD, Yu W, Selbo PK, et al. Epidermal growth factor receptor-targeted photosensitizer selectively inhibits EGFR signaling and induces targeted phototoxicity in ovarian cancer cells. Cancer letters. 2012; 321: 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huggett MT, Jermyn M, Gillams A, Illing R, Mosse S, Novelli M, et al. Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. British journal of cancer. 2014; 110: 1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bown SG, Rogowska AZ, Whitelaw DE, Lees WR, Lovat LB, Ripley P, et al. Photodynamic therapy for cancer of the pancreas. Gut. 2002; 50: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gather Malte C., Seok Hyun Yun. Single-cell biological lasers. Nature Photonics. 2011; 5: 406–410. [Google Scholar]
- 63.Menyuk N, Dwight K, Pierce JW. NaYF4: Yb, Er – an efficient upconversion phosphor. Appl. Phys. Lett. 1972; 21: 159–161. [Google Scholar]
- 64.Chatterjee DK, Yong Z. Upconverting nanoparticles as nanotransducers for photodynamic therapy in cancer cells. Nanomedicine (Lond). 2008; 3: 73–82. [DOI] [PubMed] [Google Scholar]
- 65.Ungun B, Prud’homme RK, Budijon SJ, Shan J, Lim SF, Ju Y, Austin R. Nanofabricated upconversion nanoparticles for photodynamic therapy. Opt Express. 2009; 17: 80–86. [DOI] [PubMed] [Google Scholar]
- 66.Idris NM, Gnanasammandhan MK, Zhang J, Ho PC, Mahendran R, Zhang Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nature medicine. 2012; 18: 1580–1585. [DOI] [PubMed] [Google Scholar]
- 67.Wang C, Tao H, Cheng L, Liu Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials. 2011; 32: 6145–6154. [DOI] [PubMed] [Google Scholar]
- 68.Juzenas P, Chen W, Sun YP, Coelho MA, Generalov R, Generalova N, et al. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv Drug Deliv Rev. 2008; 60: 1600–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bulin Anne-Laure, Truillett Charles, Chouikrat Rima, Lux Francois, Frochot Celine, Amans David, et al. X-ray-Induced Singlet Oxygen Activation with Nanoscintillator-Coupled Porphyrins. The Journal of Physical Chemistry C. 2013; 117: 21583–21589. [Google Scholar]
- 70.Glidden MD, Celli JP, Massodi I, Rizvi I, Pogue BW, Hasan T. Image-Based Quantification of Benzoporphyrin Derivative Uptake, Localization, and Photobleaching in 3D Tumor Models, for Optimization of PDT Parameters. Theranostics. 2012; 2: 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]