Abstract
Na+/H+ exchanger NHE3 of human or primates differs from NHE3 of other animals by having a PY motif, which mediates interaction with the E3 ubiquitin (Ub) ligase Nedd4–2. Ub-conjugation of human NHE3 by Nedd4–2 regulates endocytosis of NHE3 but does not affect its cellular expression. Because Ub-conjugation is a reversible process, the aim of this study is to identify deubiquitinating enzyme (DUB) regulating the post-endosomal fate of human NHE3. Using an activity-based chemical screening, we identified ubiquitin specific protease-7 (USP7) and USP10 that bind NHE3. The roles of DUBs in regulation of NHE3 were studied by determining the effects of silencing of USP7 and USP10. Knockdown of USP7 or USP10 resulted in increased NHE3 ubiquitination and decreased NHE3 expression at the surface membrane and cellular level. The endocytic retrieval of NHE3 was promoted by depletion of USP7 or USP10, with increased association of NHE3 with Rab5a and Rab7. Inhibition of USP7 and USP10 by chemical inhibitors or knockdown had an additive effect on NHE3. In addition, NHE3 half-life was reduced accounting for decreased NHE3 protein abundance. NHE3 is inhibited by protein kinase A. Activation of PKA by forskolin decreased the binding of USP7 and USP10 to NHE3, while increasing ubiquitination of NHE3. Knockdown of USP10 had an additive effect on PKA-dependent inhibition of NHE3. These findings demonstrate that USP7 and USP10 are DUBs that regulate NHE3 ubiquitination and expression, and reveal a new mechanism of NHE3 inhibition involving DUBs.
Keywords: deubiquitinating enzyme, diarrhea, Na+/H+exchange, ubiquitination
1 |. INTRODUCTION
Ubiquitination is a regulated post-translational modification that conjugates ubiquitin (Ub) to Lys residues of target proteins and controls their intracellular fate. The covalent ligation of the 76-amino acid peptide Ub to substrate proteins is a highly conserved process that occurs via the sequential action of three enzymes: Ub-activation enzyme E1, Ub-conjugating enzyme E2, and Ub ligase E3.1 Ubiquitinated proteins are recognized and degraded by the 26S proteasome.2 Like the phosphorylation-dephosphorylation process, Ub-conjugation is reversed by deubiquitinating enzymes (DUBs). DUBs can regulate Ub-dependent cellular pathways by interfering with the formation and the reactivity of the E2-Ub intermediate or by preventing E3 ligases mediated Ub conjugation. DUBs can also remove Ub chains from post-translationally modified proteins to stabilize proteins or rescue proteins from either proteasomal or lysosomal degradation.3,4 Hence, the net ubiquitination levels of substrate proteins are balanced by the combined activities of UB-conjugating enzymes (E1, E2, and E3) and DUBs.3 About 100 DUB genes are in the human genome and DUBs are classified into five distinct families: ubiquitin C-terminal hydrolases (UCHs), ubiquitin-specific proteases (USPs), ovarian tumor proteases (OTUs), Josephin, and JAB1/MPN/MOV34 metalloenzymes (JAMMs). Most DUBs are cysteine proteases, which include the UCH, USP, OTU, and Josephin families. The JAMM family members are zinc metalloproteases.5
Na+/H+ exchanger 3, NHE3, in the brush border membrane (BBM) of the epithelial cells in the small intestine and colon carries out the majority of Na+ absorption between meals.6 NHE3 at the BBM is in flux with NHE3 in intracellular vesicles as such the primary means of NHE3 regulation involves the movement of NHE3 molecules between the two compartments. Previous studies have shown that clathrin, dynamin, or lipid rafts are involved in endocytic recycling of NHE3.7–9 Insight into the possible role of ubiquitination in NHE3 regulation comes from our previous study where we have demonstrated that NHE3 interacts with the E3 Ub ligase neural precursor cell expressed, developmentally down-regulated 4–2 Nedd4–2.10 In this study, NHE3s of human and non-human primates are shown to have a PY motif or PPNY sequence, which enable proteins to interact with Nedd4–2. NHE3s of other animals lack a PY motif and are unable to interact with Nedd4–2. Ubiquitination of NHE3 by Nedd4–2 causes NHE3 to internalize at an increased rate compared with non-primate NHE3s lacking a PY motif. The human NHE3-Nedd4–2 interaction occurs at the surface membrane, decreasing NHE3 protein abundance at the surface membrane, but not the total NHE3 protein expression level in the cells.10 Despite this difference in Nedd4–2 interaction, ubiquitination of non-human NHE3 cannot completely be dismissed. We have reported that rat NHE3 expressed in PS120 cells exhibits a high level of ubiquitination, although ubiquitination of rat NHE3 is not Nedd4–2 dependent.10 In addition, decreased NHE3 expression in opossum kidney cells by ischemia-reperfusion injury is associated with increased ubiquitination.11
Although Nedd4–2 regulates the endocytic internalization of human NHE3, it does not affect total expression of NHE3,10 suggesting that Nedd4–2 has no impact on post-endocytic regulation of NHE3. We postulated that other Ub modulating enzymes might regulate NHE3 protein stability and expression. Recently, the roles of DUBs in regulation of membrane proteins, including the epithelial sodium channel (ENaC), cystic fibrosis transmembrane regulator (CFTR), the glucose transporter GLUT4, and epidermal growth factor receptor, have been reported.12–15 USP10, in particular, deubiquitinates ENaC and CFTR increasing their expression in cell membrane.13,16 The goal of this study is to identify DUB that targets intestinal NHE3 and to determine the role of DUB in NHE3 regulation. We used the chemical screening approach that utilizes N-terminally HA-tagged Ub (HA-Ub) fused with a C-terminal electrophilic trap, such as vinyl methyl ester, which reacts with the active-site Cys residues in most DUBs.17 In the current study, we report identification of two DUBs, USP7, and USP10, that interact and regulate NHE3 ubiquitination and expression.
2 |. MATERIALS AND METHODS
2.1 |. Plasmids
Lentiviral plasmid (pLKO.1-puro) containing short hairpin RNA targeting USP7 (shUSP7; TRCN4057 and 4058), USP10 (shUSP10; TRCN7431 and 7432) or non-targeting scrambled sequences (shCon) were obtained from Sigma-Aldrich (St Louis, MO). The pcDNA3.1 construct carrying human NHE3 fused at the C terminus with an antibody epitope derived from vesicular stomatitis virus glycoprotein (VSVG) has previously been described.10 Plasmid pMT123 expresses HA-tagged Ub.18
2.2 |. Cell lines and cell culture
The human intestine epithelial cell lines, Caco-2bbe and SK-CO15, were cultured in DMEM supplemented with 1 mM sodium pyruvate, 50 units/mL of penicillin, 50 μg/mL of streptomycin, and 10% FBS in a 5% CO2 humidified incubator at 37°C as previously described.19,20 Caco-2bbe cells were stably transfected with human NHE3. Cells infected with lentivirus were selected with 10 μg/mL of puromycin.
2.3 |. Chemical, reagents, and antibodies
The following chemicals were used: HA-tagged ubiquitin-vinyl methyl ester, HA-UbVME (Enzo Life Science, Farmingdale, NY), forskolin (FSK) (Sigma-Aldrich), USP7 inhibitor HBX41108 (Tocris, Minneapolis, MN), USP10 inhibitor spautin-1 (Cayman, Ann Arbor, MI), cycloheximide (Sigma-Aldrich). All other chemicals were purchased from Sigma-Aldrich (St Louis, MO) or EMD Millipore (Billerica, MA), unless otherwise specified. Rabbit polyclonal anti-NHE3 antibody EM450 and mouse monoclonal anti-VSVG antibody P5D4 were previously described.20,21 The following commercial antibodies were used: rabbit anti-HA (Cell Signaling), mouse anti-Ub (Santa Cruz), rabbit anti-USP7 (Cell Signaling), rabbit anti-USP10 (Cell Signaling), rabbit anti-Nedd4–2 (Abcam, Cambridge, MA), mouse anti-Rab5a (Cell signaling), mouse anti-Rab7 (Santa Cruz), rabbit anti-VSVG (Sigma), and mouse anti-β-actin (Sigma).
2.4 |. Identification of active USBs
To identify DUBs targeting NHE3, HA-UbVME that reacts with the active-site Cys residues in DUBs was used as previously described.17,22 Briefly, cells were lysed in buffer (50 mM Tris, pH 7.5, 5 mM EDTA, 150 mM NaCl, and 1.0% NP40) and post-nuclear supernatant (PNS) was obtained by low speed (10 000 g) centrifugation of cell lysate. To PNS, 0.1 μg of the HA-UbVME probe was added and incubated for 1 hour at 37°C. Visualization of DUBs covalently linked to the HA-UbVME probe was achieved by immunoblotting using anti-HA antibody. To identify DUBs interacting with NHE3, the HA-UbVME-DUB complex was immunoprecipitated with anti-VSVG P5D4 antibody or anti-NHE3 EM450 antibody, followed by SDS-PAGE and Western blot analysis using an anti-HA antibody. The specificity of the HA-UbVME probe binding to active DUBs was confirmed with the addition of N-ethylmaleimide (NEM; 10 μM) during the labeling reaction. Identification of each DUB was determined by immunoblotting using specific anti-DUB antibodies.
2.5 |. Isolation of endosome
Endosome isolation was performed using Trident Endosome Isolation Kit (GeneTex Inc., CA) according to the manufacturer’s protocol. Briefly, 20 × 106 of Caco-2bbe/NHE3 cells were harvested and washed with cold PBS. Then the cell pellet was resuspended in 500 μL Buffer A and incubated on ice for 10 minutes followed by vigorous vortex for 10 seconds. Lysate was cartridge filtered and resuspended in Buffer B and incubated at 4°C for 4 hours. The resulting supernatant was centrifuged at 10 000 g for 30 minutes at 4°C and pellet was resuspended in cell lysis buffer to use in SDS-PAGE and immunoblotting.
2.6 |. Coimmunoprecipitation and Western blot analysis
Cells were lysed in cold lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM -glycerophosphate, 2.5 mM sodium pyrophosphate, 1 mM Na2EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM NaF, 10 mM leupeptin, 1% Triton X-100, protease inhibitors mixture, and 2.5 mM N-ethylmaleimide), supplemented with 10 μM MG132 to inhibit proteasomal degradation. Protein concentration was determined by the bicinchoninic acid (BCA) assay (Sigma Aldrich). Equal amounts of cell lysates (typically 500 mg) were incubated overnight with P5D4 or EM450 for Caco-2bbe/NHE3 and SK-CO15 cells, respectively. The immunocomplex was purified by incubating with protein G-Sepharose beads for 1 hour followed by two washes in lysis buffer and one wash in PBS. NHE3-containing immunocomplexes were eluted from the beads in 2x Laemmli buffer, resolved by SDS-PAGE, and immunoblotted.
2.7 |. Detection of NHE3 ubiquitination
Caco-2/NHE3 or SK-CO15 cells were transiently transfected with pMT123 to express HA-Ub. Cells were lysed 2 days after transfection in the lysis buffer. NHE3 was immunoprecipitated as describe above and immunoblotted with anti-HA antibody.
2.8 |. Na+ dependent intracellular pH recovery
The Na+-dependent changes in intracellular pH (pHi) by NHE3 was determined using the ratio-fluorometric, pH-sensitive dye 2',7'-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester (BCECF-AM) as previously described.20 Cells were incubated with NH4+ buffer, followed by sequential perfusion with tetramethylammonium (130 mM TMA-Cl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose) and Na+ buffer that drives Na+-dependent pH recovery. Na+ buffer was supplemented with 50 μM HOE694 to inhibit NHE1 and NHE2 activities. The microfluorometry was performed on an inverted fluorescence microscope and the photometric data were acquired using the Metafluor software (Molecular Devices, Sunnyvale, CA) as previously described.20 Na+/H+ exchange rate was described by the rate of pHi recovery, which was calculated by determining slopes along the pHi recovery by linear least-squares analysis over a minimum of 9 seconds.
2.9 |. Surface biotinylation
Surface biotinylation of NHE3 was performed as described.23 Briefly, cells were rinsed twice in PBS and incubated in borate buffer (154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, and 10 mM H3BO3 (pH 9.0)) for 10 minutes. Cells were then incubated for 40 minutes with 0.5 mg/mL sulfo-NHS-LC-biotin (Pierce, Rockford, IL) in borate buffer. Unbound sulfo-NHS-LC-biotin was quenched with Tris buffer (20 mM Tris, 120 mM NaCl, pH 7.4). Cells were then rinsed with PBS, scraped, lysed in the lysis buffer described above, and sonicated for 2 × 15 seconds. The lysate was agitated for 30 minutes and spun at 14 000 g for 30 minutes at 4°C to remove the insoluble cell debris. An aliquot was retained as the total fraction representing the total cellular NHE3. Protein concentration was determined and 1 mg of lysate was then incubated with streptavidin-agarose beads (Pierce) for 2 hours. The streptavidin-agarose beads were washed three times in lysis buffer and twice in PBS. All the above procedures were performed at 4°C or on ice. Biotinylated surface proteins were then eluted by boiling the beads at 95°C for 10 minutes. Dilutions of the total and surface NHE3 were resolved by SDS-PAGE and immunoblotted with P5D4 or EM450. Densitometric analysis was performed using ImageJ software (National Institutes of Health).
2.10 |. NHE3 half-life
Caco-2bbe/NHE3 cells grown to post-confluence were pretreated with 50 μM cycloheximide (Sigma, St. Louis, MO). At each selected time point, cells were washed with cold PBS and lysed in lysis buffer. The same amounts of cell lysates were resolved by SDS-PAGE and immunoblotted with anti-VSVG (P5D4) antibody as described above. Densitometric analysis was performed using ImageJ software (National Institutes of Health).
2.11 |. Confocal immunofluorescence microscopy
Caco-2bbe/NHE3 cells grown on Transwells (Corning, Lowell, MA) were fixed using 100% methanol at −20°C for 20 minutes. For NHE3 and apical membrane staining, cells were washed three times with PBS, incubated with WGA, Alexa Flour 647-conjugate for 10 minutes, permeabilized using 0.05% Triton X-100 in PBS, and blocked with 5% normal goat serum for 1 hour at RT. Cells were then stained with rabbit anti-VSVG antibody for 16 hours at 4°C. Cells were washed with PBS three times and incubated with Alexa 488-conjugated goat anti-rabbit IgG (Invitrogen) for 1 hour at RT. After three 10 minutes washes with PBS, specimens were mounted with ProLong Glass Antifade Reagent (Invitrogen) and observed under a Nikon A1R HD confocal microscope (Nikon Instruments Inc., NY) coupled to a Plan Apo λ 60x Oli lens. For NHE3 and Rab staining, cells were stained with rabbit anti-VSVG and mouse anti-Rab5a, or mouse anti-Rab7 antibody for 16 hours at 4 C. After three washes for 10 minutes each with PBS, cells were incubated with Alexa 488-conjugated goat anti-rabbit IgG and Alexa 568-conjugated goat anti-mouse IgG (Invitrogen) for 1 hour at room temperature. Pearson’s correlations of NHE3 and Rab were determined using NIS-Elements AR software (Nikon).
2.12 |. Statistical analysis
Statistics was performed using independent samples two-tailed unpaired Student’s t test or ANOVA. Results are presented as mean ± SE mean (SEM). Statistical analysis was performed using GraphPad Prism software (La Jolla, CA). A value of P < .05 was considered significant.
3 |. RESULTS
3.1 |. Identification of active DUBs interacting with NHE3
To identify a DUB that potentially regulates NHE3, we used a HA-UbVME, which acts as an electrophilic trap to covalently link active DUB.17,22 To test the system, the HA-UbVME probe was added to the PNS of Caco-2bbe cells stably expressing human NHE3 tagged with a VSVG epitope at the carboxyl terminus Caco-2bb/NHE3. Several protein bands were identified and the specificity of the HA-tagging was verified by the addition of the cysteine modifier NEM during the binding reaction (Figure 1A, lanes 1–2). NEM inhibits the covalent linkage between DUB and the HA-UbVME probe, and it prevents the binding of active DUB to the HA-UbVME.17 The identical procedure using SK-CO15 PNS resulted in a similar pattern of active DUBs that was blocked by NEM (Figure 1A, lanes 5–6). To identify NHE3-interacting DUBs, we immmunopre-cipitated NHE3 from the HA-UbVME bound PNS using anti-VSVG P5D4 antibody or anti-NHE3 EM450 antibody for Caco-2bbe/NHE3 or SK-CO15, respectively. Of note, SK-CO15 cells have endogenous expression of NHE3.20 Subsequent Western immunoblotting using anti-HA antibody identified two major protein bands with approximate molecular mass of 110 and 130 kDa (Figure 1A, lanes 3–4 for Caco2/NHE3 and lanes 7–8 for SK-CO15). To reveal their identities, we first searched published studies for DUBs of molecular mass of 110 and 130 kDa. This search predicted USP10 of approximately 110 kDa, whereas the 130 kDa band is likely to be either USP7 or USP8. Western immunoblotting using antibodies against USP10 and USP7 resulted in positive bands of approximately 110 and 130 kDa, respectively, in the PNS and NHE3-bound active DUB pool in Caco-2bbe (Figure 1B). Anti-USP8 antibody, however, did not detect any band in the NHE3-bound active DUB pool. To confirm that USP7 or USP10 interact with NHE3, we performed immunoprecipitation of NHE3 using P5D4 or IgG as a negative control, followed by immunoblotting for USP7 or USP10. Both USP7 and USP10 co-immunoprecipitated with NHE3 (Figure 1C), demonstrating their interaction with NHE3. Because we used the PNS to isolate DUBs, their presence in the endosomes was corroborated by immunoblotting of endosomal fraction of Caco-2bbe/NHE3 cells. Both USP7 and USP10 as well as NHE3 were detected in the endosome fraction (Figure 1D).
FIGURE 1.
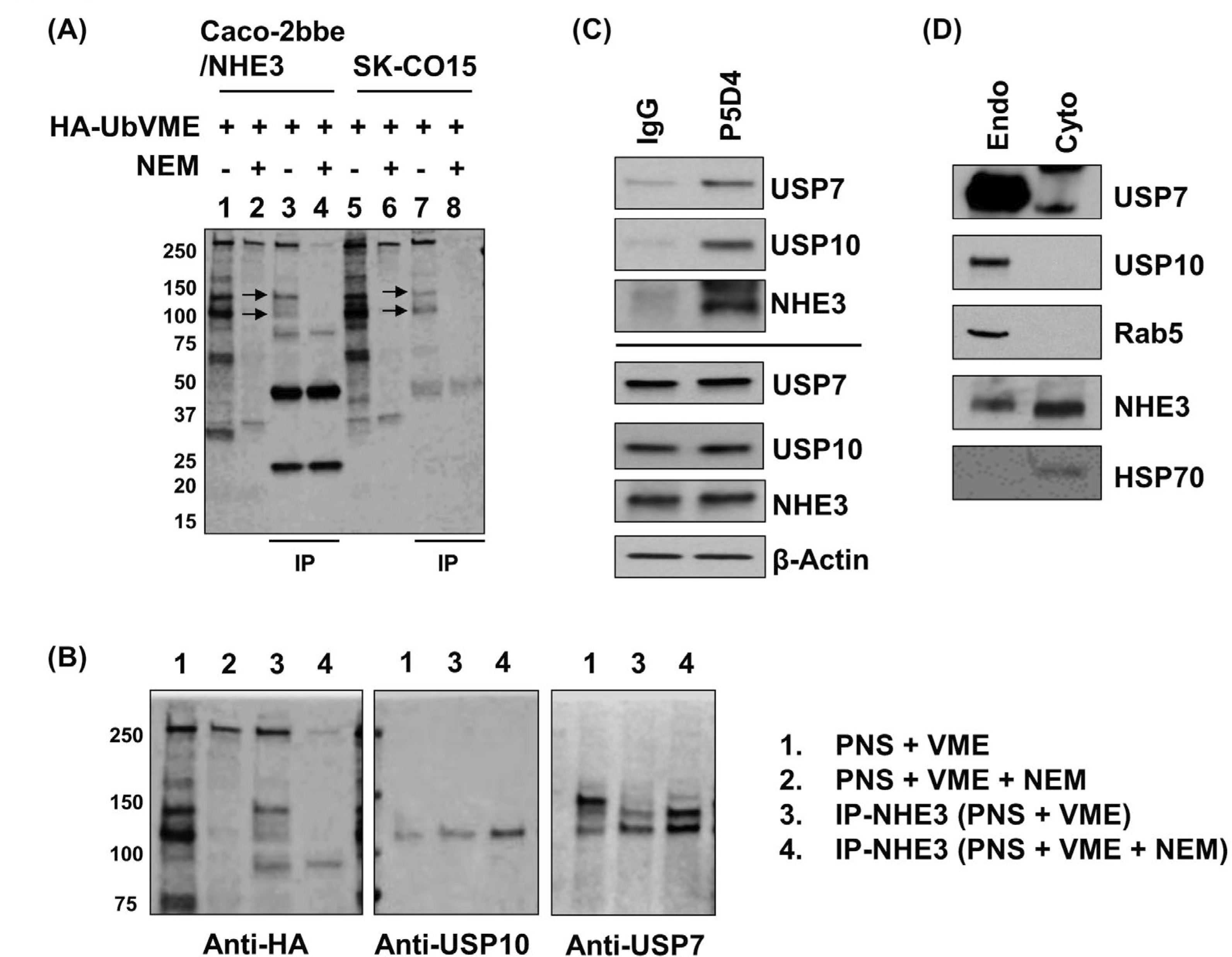
USP7 and USP10 are NHE3 binding DUBs. A, The HA-UbVME probe was used to identify DUBs interacting with NHE3. Post-nuclear supernatant (PNS) of Caco-2bbe/NHE3 and SK-CO15 cells were incubated HA-UbVME with or without NEM, which inhibits the covalent linkage between DUBs and HA-UbVME. Immunoblotting using anti-HA antibody showed the presence of NEM-sensitive 110 and 130 kDa bands (arrows) in both cell lines. B, PNS of Caco-2bbe/NHE3 bound to HA-UbVME was immunoblotted with anti-HA, anti-USP10 or anti-USP7 antibody. C, USP7 and USP10 co-immunoprecipitate with NHE3. Caco-2bbe/NHE3 lysate was immunoprecipitated with anti-VSVG antibody P5D4 or IgG as a control, followed by immunoblotting using anti-USP7, anti-USP10, or anti-NHE3 antibody. Lower four panels show immunoblotting of cell lysates. D, USP7, USP10 and NHE3 are present in early endosomes of Caco-2bbe cells. Early endosomal and cytosomal fractions were immunoblotted using antibodies to the proteins indicated. Rab5a and HSP70 were used as a marker for early endosome and cytoplasm, respectively. Representative blots from three or more independent experiments are shown
3.2 |. Knockdown of DUB increases NHE3 ubiquitination
To determine the effect of these DUBs on NHE3 ubiquitination in Caco-2bbe/NHE3 cells, USP7 and USP10 were knocked down using shUSP7 and shUSP10, respectively. As a control non-targeting control shRNA, shCon, was used. shUSP7 and shUSP10 decreased USP7 and USP10 protein levels by more than 80% compared with shCon (Figure 2A). Transduction with shUSP7 did not alter USP10 expression and vice versa, demonstrating the lack of cross-activity by these shRNAs. In addition, Nedd4–2 expression was not altered by either shRNA constructs. To determine NHE3 ubiquitination levels, Caco-2bbe/NHE3 cells were transfected with a plasmid expressing HA-Ub, followed by immunoprecipitation of NHE3 with anti-VSVG antibody and immunoblotting with anti-HA antibody. Silencing of USP7 or USP10 increased the ubiquitination levels of NHE3 compared with shCon (Figure 2B, upper panel). Normalization of the level of ubiquitination to the amount of NHE3 isolated by immunoprecipitation revealed shUSP7 and shUPS10 increased the ubiquitination levels of NHE3 by 687 ± 34% and 435 ± 44%, respectively, compared with NHE3 in shCon transfected cells (Figure 2B, lower panel). The effect of the DUBs was affirmed by evaluating ubiquitination of endogenously expressed NHE3 in SK-CO15 cells. Knockdown of USP7 or USP10 in SK-CO15 cells increased ubiquitination levels of NHE3 by 358 ± 62% or 396 ± 45%, respectively (Figure 2C). These results demonstrate that USP7 and USP10 regulate NHE3 ubiquitination.
FIGURE 2.
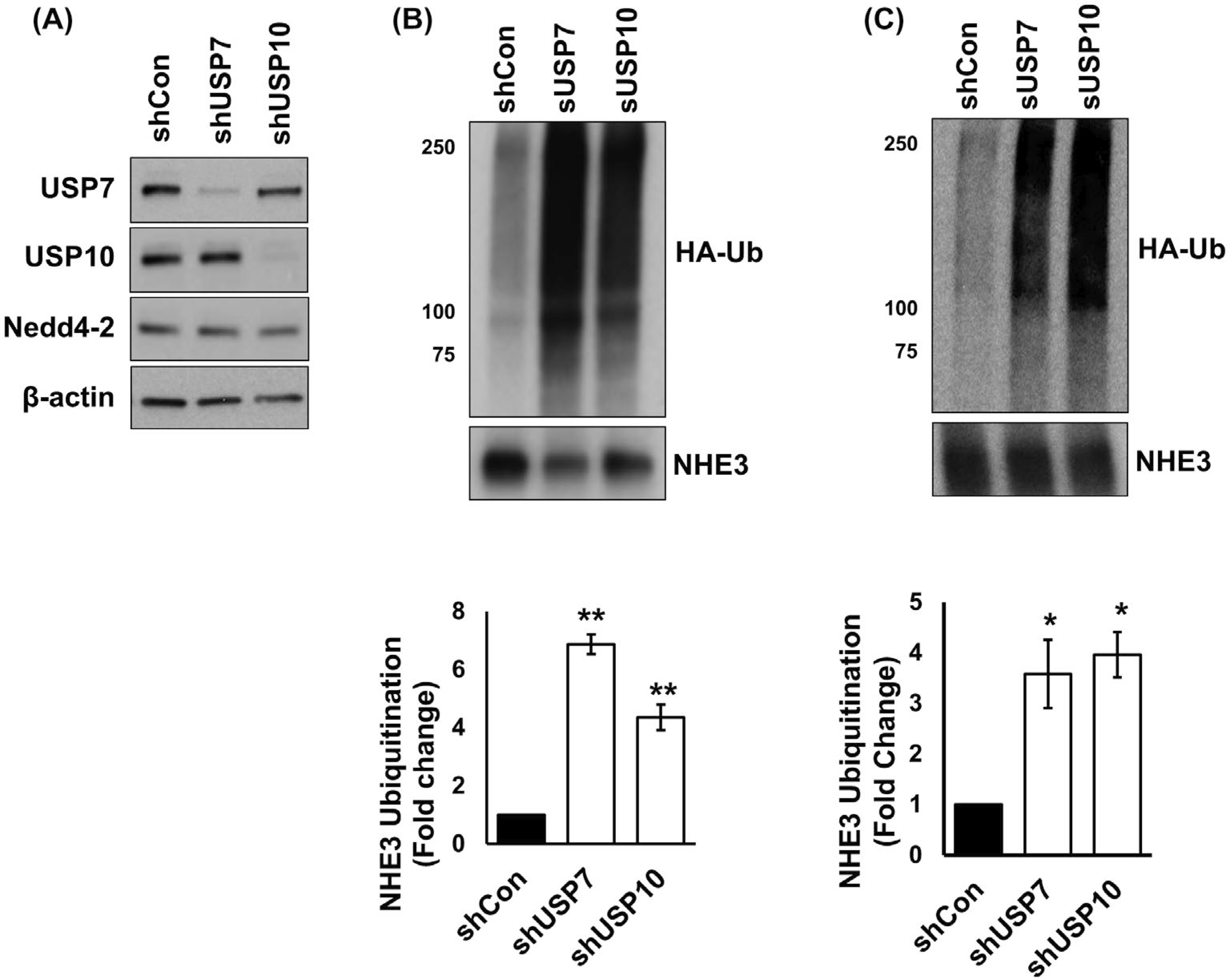
Knockdown of USP7 or USP10 increases ubiquitination levels of NHE3. A, USP7 and USP10 were knocked down using lentiviral shUSP7 and shUSP10, respectively. Non-targeting scrambled shCon was used as a control. Expression levels of USP7, USP10, and Nedd4–2 were determined. B, Ubiquitination of NHE3 in Caco-2bbe/NHE3 cells was determined. The upper panel shows representative blots of NHE3 ubiquitination and immunoprecipitated NHE3 protein from five independent experiments. Lower, quantitation of Ub levels relative to the amount of immunoprecipitated NHE3 proteins. **, P < .01 compared with shCon. C, Ubiquitination level of endogenous NHE3 in SK-CO15 cells was determined. n = 4. *, P < .05 compared with shCon
3.3 |. Knockdown of DUB reduces NHE3 expression and activity
Because DUB knockdown increased the ubiquitination level of NHE3, we questioned whether DUB regulates NHE3 abundance in the cells. To determine NHE3 abundance at the total cellular level and in the apical membrane, we performed surface biotinylation. Knockdown of USP7 or USP10 decreased total NHE3 cellular expression by 44.8 ± 17.4% and 59.8 ± 5.1%, respectively. In addition, NHE3 surface expression was reduced by 70.0 ± 8.6% by shUSP7 and 68.9 ± 4.3% by shUSP10 (Figure 3A). Similarly, shUSP7 or shUSP10 reduced the surface (75 ± 2.8% by shUSP7 and 83.9 ± 4.6% by shUSP10) and total endogenous NHE3 expression (61.1 ± 4.5% by shUSP7 and 75.6 ± 3.1% by shUSP10) in SK-CO15 cells (Figure 3B). The reduced NHE3 abundance at the apical membrane was corroborated by confocal immunofluorescence microscopic analysis. In control Caco-2bbe cells, NHE3 is predominantly located at the apical membrane, which was labeled by WGA (Figure. 3C). In comparison, NHE3 immunofluorescence labeling in cells treated with shUSP7 or shUS10 were markedly reduced, consistent with decreased NHE3 protein expression in these cells. Together, these results demonstrate that USP7 and USP10 are involved in both the endocytic retrieval of NHE3 from the apical membrane and the post-endocytic regulation of NHE3.
FIGURE 3.
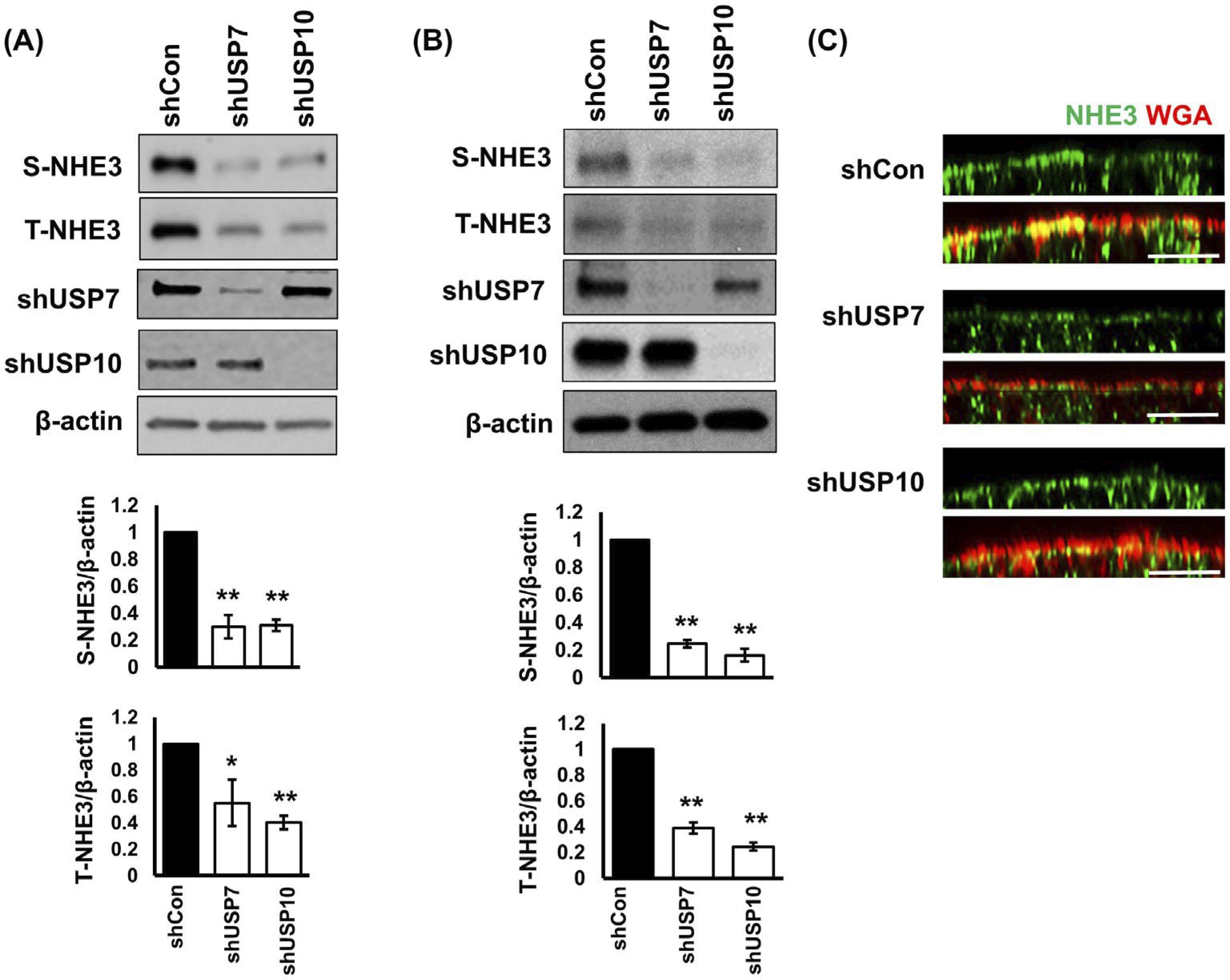
Knockdown of USP7 or USP10 decreased NHE3 abundance. NHE3 expression in the apical membrane (S-NHE3) of Caco-2bbe/NHE3 cells (A) or SK-CO15 cells (B) was determined by surface biotinylation as described in Methods and Materials. Total cellular NHE3 (T-NHE3), USP7, and USP10 expression in cell lysate was determined. Representative immunoblots from three independent experiments are shown. Lower charts show the quantitation of S-NHE3 and T-NHE3 relative to β-actin for each cell line. Results are presented as mean ± SEM. n = 3. *, P < .05, **, P < .01 versus shCon. C, NHE3 cellular localization in Caco-2bbe/NHE3 cells was analyzed by confocal microscopy. NHE3 (green) was immunostained using rabbit anti-VSVG antibody. Wheat germ agglutinin (WGA, red) was used as a marker for the apical membrane. Scale bar, 10 μm
Next, NHE3 activity was determined as Na+-dependent intracellular pH (pHi) recovery in Caco-2bbe/NHE3 cells. USP7 knockdown by shUSP7 reduced NHE3 activity by about 40% from 0.57 ± 0.02 ΔpH unit/min to 0.34 ± 0.02 ΔpH unit/min (Figure 4A). Similarly, shUSP10 resulted in a 44% decrease in NHE3 activity from 0.62 ± 0.02 ΔpH unit/min to 0.35 ± 0.02 ΔpH unit/min (Figure 4B), suggesting that these DUBs regulate Na+/H+ exchange activity by modifying NHE3 expression at the apical membrane.
FIGURE 4.
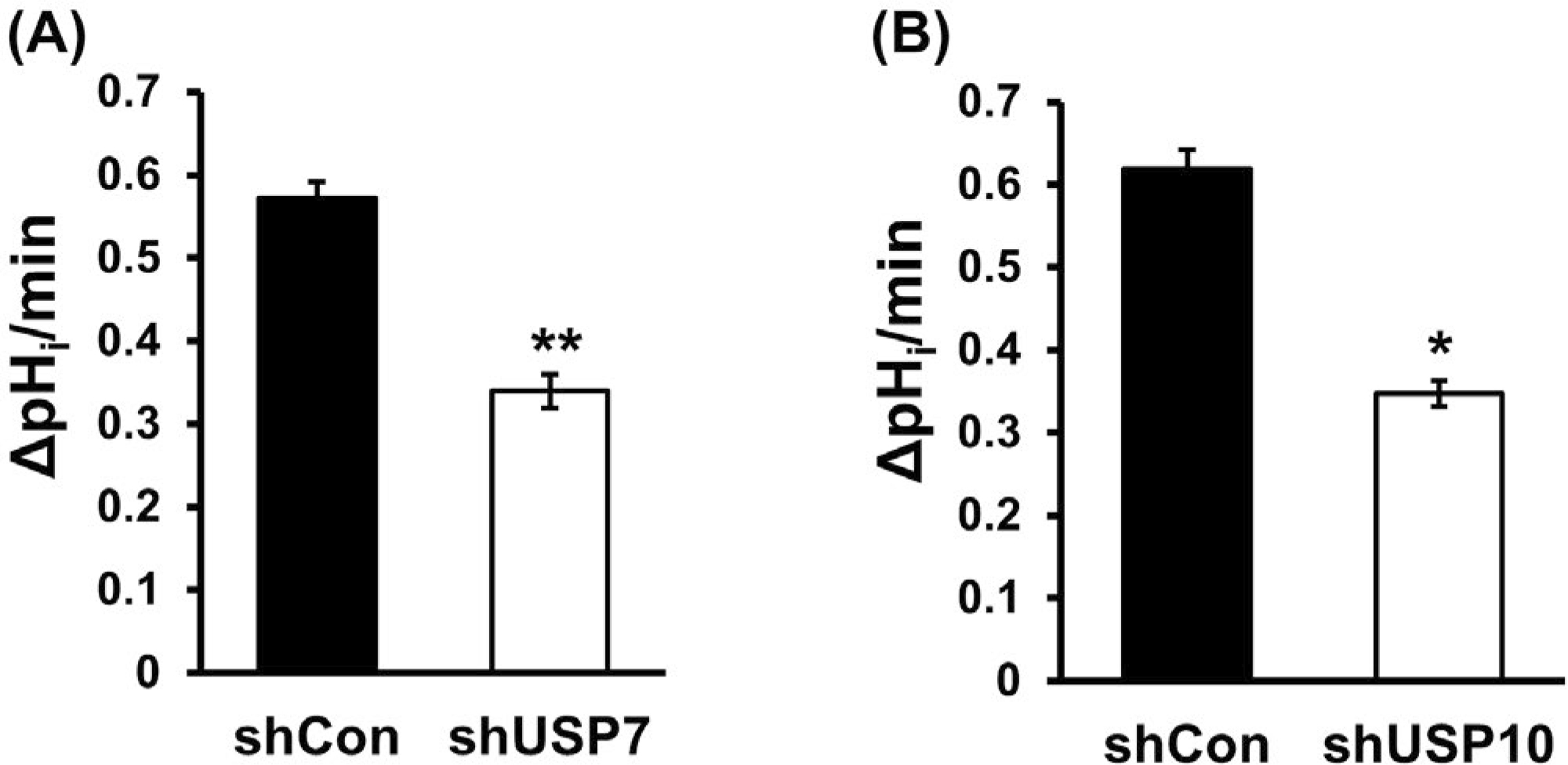
Knockdown of USP7 or USP10 decreased NHE3 abundance. The effect of USP7 (A) or USP10 (B) knockdown on NHE3 activity was determined in Caco-2bbe/NHE3 cells. NHE3 activity was determined as Na+-dependent intracellular pH recovery. Results are presented as mean ± SEM. n ≥ 10. *, P < .05, **, P < .01 compared with shCon
3.4 |. USP7 and USP10 have an additive effect on NHE3
Thus far, the effects of USP7 and USP10 on NHE3 appear similar, but it is not known whether USP7 and USP10 have redundancy or additive effects. Because both shUSP7 and shUSP10 have puromycin resistance, we could not generate a stable cell line with concomitant knockdown of both DUBs. Instead, we used HBX41108 and spautin-1 to inhibit USP7 and USP10, respectively.24,25 Treating Caco-2bbe/NHE3 with HBX41108 or spoutin-1 decreased NHE3 protein abundance by 26.8 ± 2.2% and 14.5 ± 2.4%, respectively, whereas a 43.7 ± 1.9% reduction was achieved with both inhibitors (Figure 5A). These results were confirmed by treating cells with a combination of one shRNA construct and an inhibitor of the other DUB. Treating cells with shUSP7 + spautin-1 had a greater effect on NHE3 protein abundance compared with shUSP7 alone (Figure 5B). Similarly, HBX4118 potentiated the effect of shUSP10, further suggesting that USP7 and USP10 additively regulate NHE3 protein expression.
FIGURE 5.
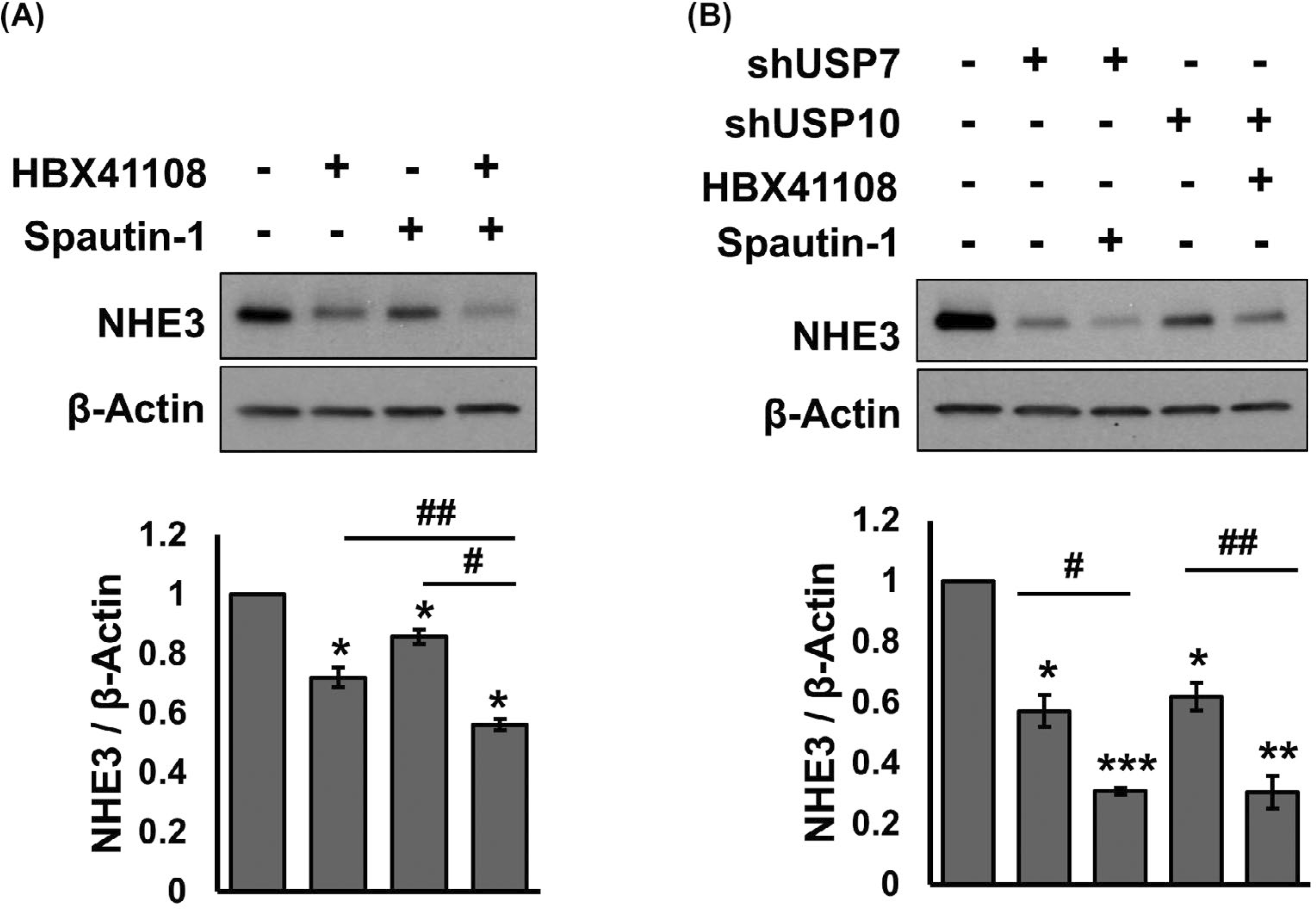
USP7 and USP10 have additive effects on NHE3. A, Caco-2bbe/NHE3 cells were treated with the USP7 inhibitor HBX41108 (5 μM), the USP10 inhibitor spautin-1 (10 μM), or DMSO as a control for 24 hours. NHE3 cellular expression was determined. The chart in the bottom shows the quantitation of NHE3 protein relative to β-actin. *, P < .05 versus DMSO. #, P < .05 versus HBX4118, or spautin-1 alone. n = 3. B, The effects of shUSP7 + spautin-1 or shUSP10 + HBX4118 on NHE3 protein expression were determined. *, P < .05, **, P < .01, ***, P < .001 versus DMSO. #, P < .05, ##, P < .01 versus shUSP7, or shUSP10 alone. n = 3
3.5 |. DUB knockdown increases colocalization of NHE3 with Rab5a and Rab7
Decreased NHE3 surface and total abundance suggest that NHE3 is retrieved from the apical membrane into endosomal compartment for eventual degradation. We, therefore, determined whether USP7 and USP10 modulate NHE3 internalization. The small GTPases Rab5 and Rab7 regulate vesicle fusion with early endosome and late endosome, respectively.26 Western blotting of cells treated with shUSP7 or shUSP10 showed that the expression levels of Ra5a, the Rab5 effector EEA1, or Rab7 were not changed by knockdown of DUBs (Figure 6A). Immunofluorescence analysis of showed infrequent overlap of NHE3 with Rab5a. In comparison to control cells, co-localization of NHE3 with Rab5a was more increased by shUSP7 or shUSP10 (Figure 6B). A quantitative analysis of colocalization of NHE3 and Rab5a fluorescence signals showed a significant increase in Pearson’s colocalization coefficient by shUSP7 or shUSP10 (Figure 6C). It has been shown previously that internalized CFTR co-immunoprecipates with Rab5.13 Although NHE3 may not bind directly to Rab proteins, it is reasonable to assume that NHE3 and Rab5a are in a macrocomplex so that NHE3 and Rab5a are indirectly linked together. To test this idea, we immunoprecipitated NHE3 from cell lysate with a limiting amount of anti-VSVG antibody so that similar amounts of NHE3 could be immunoprecipitated despite the decreased NHE3 expression by DUB knockdown. As shown in Figure 6D, Rab5a co-immunoprecipitated with NHE3 in all cells. Normalization of the amount of Rab5a to NHE3 showed a several-fold increase in Rab5a pulled down by NHE3 in shUSP7 or shUPS10 treated cells, corroborating the results of the immunofluorescence analysis. Because NHE3 protein expression was reduced by shUSP7 and shUSP10, these DUBs are likely to regulate the post-internalization sorting of NHE3. Accordingly, knockdown of USP7 or USP10 markedly increased colocalization of NHE3 with the late endosome marker Rab7 as demonstrated in Figure 6E,F. These results collectively demonstrate that knockdown of USP7 or USP10 increases NHE3 trafficking from the surface membrane to the late endosomes.
FIGURE 6.
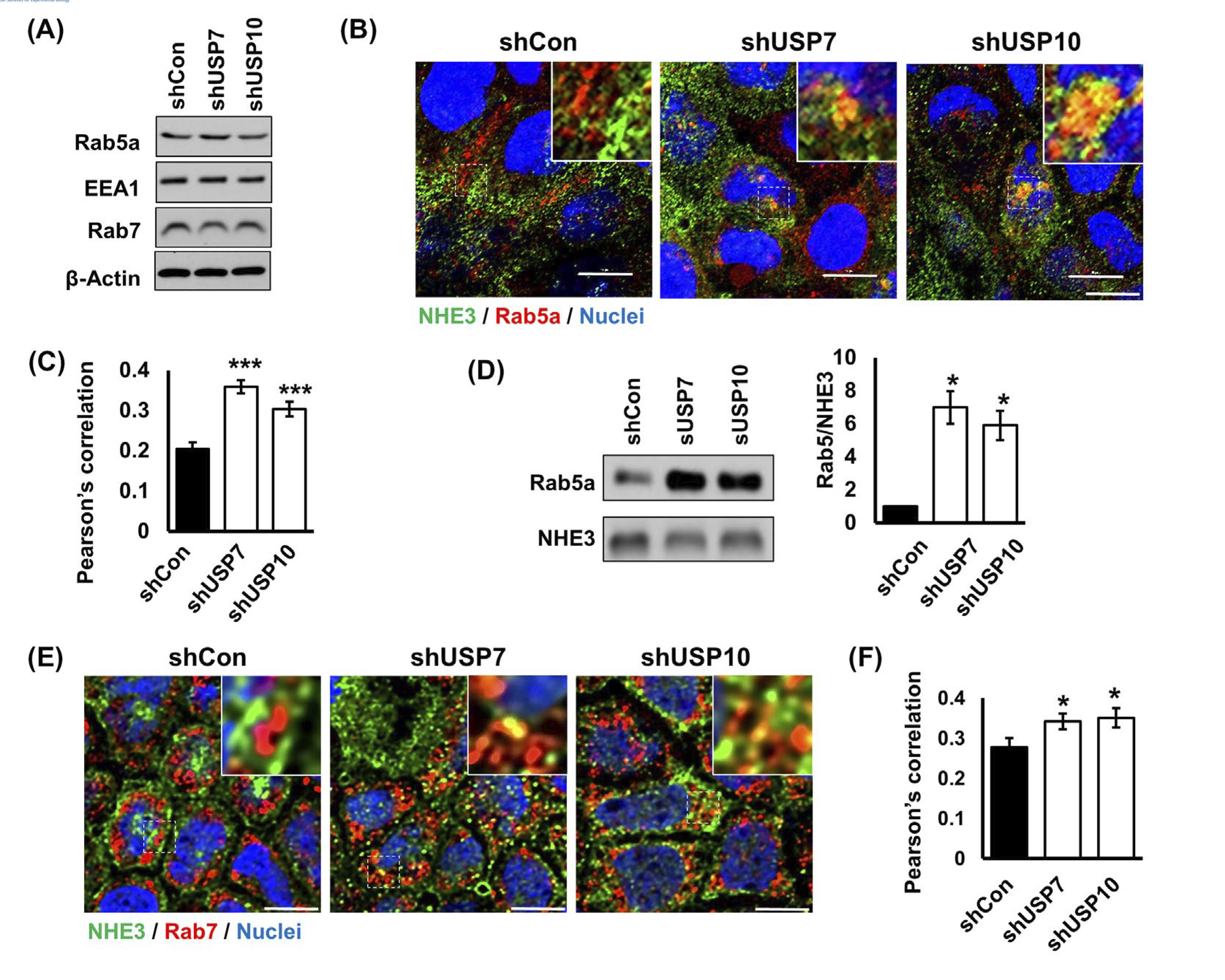
Colocalization of NHE3 with Rab5a and Rab7 is increased by knockdown of USP7 or USP10. A, Expression levels of Rab5a, EE1, and Rab7 were determined in shUSP7 and shUPS10 transduced cells. B, Expression of NHE3 (green) and Rab5a (red) in Caco-2bbe/NHE3 cells was assessed by confocal immunofluorescence microscopy. DAPI was used for nuclear counterstaining (blue). Representative images from three or more independent experiments are shown. Bars, 10 μm. Insets shown magnified view of the boxed regions. Scale bar, 10 μm. C, Graphs represent Pearson’s coefficient of colocalization of NHE3 and Rab5a from more than 22 independent fields of cells. ***, P < .001 versus shCon. D, Western blot shows Rab5a co-immunoprecipitated with NHE3. right, Rab5 expression normalized to β-actin. *, P < .05 versus shCon. E, NHE3 (green) and Rab7 (red) in Caco-2bbe/NHE3 cells were visualized by confocal immunofluorescence microscopy. DAPI (blue), nuclei. Bars, 10 μm. F, Pearson’s coefficient of colocalization of NHE3 and Rab7 from more than 20 independent fields of cells is shown. *, P < .05 versus shCon
3.6 |. DUBs regulate the half-life of NHE3
A decrease in total NHE3 expression and colocalization of NHE3 with Rab7 by DUB knockdown suggests that NHE3 protein stability is regulated by the deubiquitination process. To determine the steady-state NHE3 expression, cells transduced with shCon or shUSP10 were treated with cycloheximide to block translation, and total NHE3 expression in the cell lysate was determined for up to 48 hours. The change in NHE3 expression in control cells was relatively slow with a steady-state half-life of NHE3 estimated to be about 20 hours, consistent with previous studies (Figure 7A).10,27 In comparison, there was a greater reduction of NHE3 expression over time in shUSP10 cells (Figure 7B). In cells transduced with shUSP10, the half-life was estimated to be about 12 hours, which indicated that the rate of NHE3 degradation was almost doubled.
FIGURE 7.
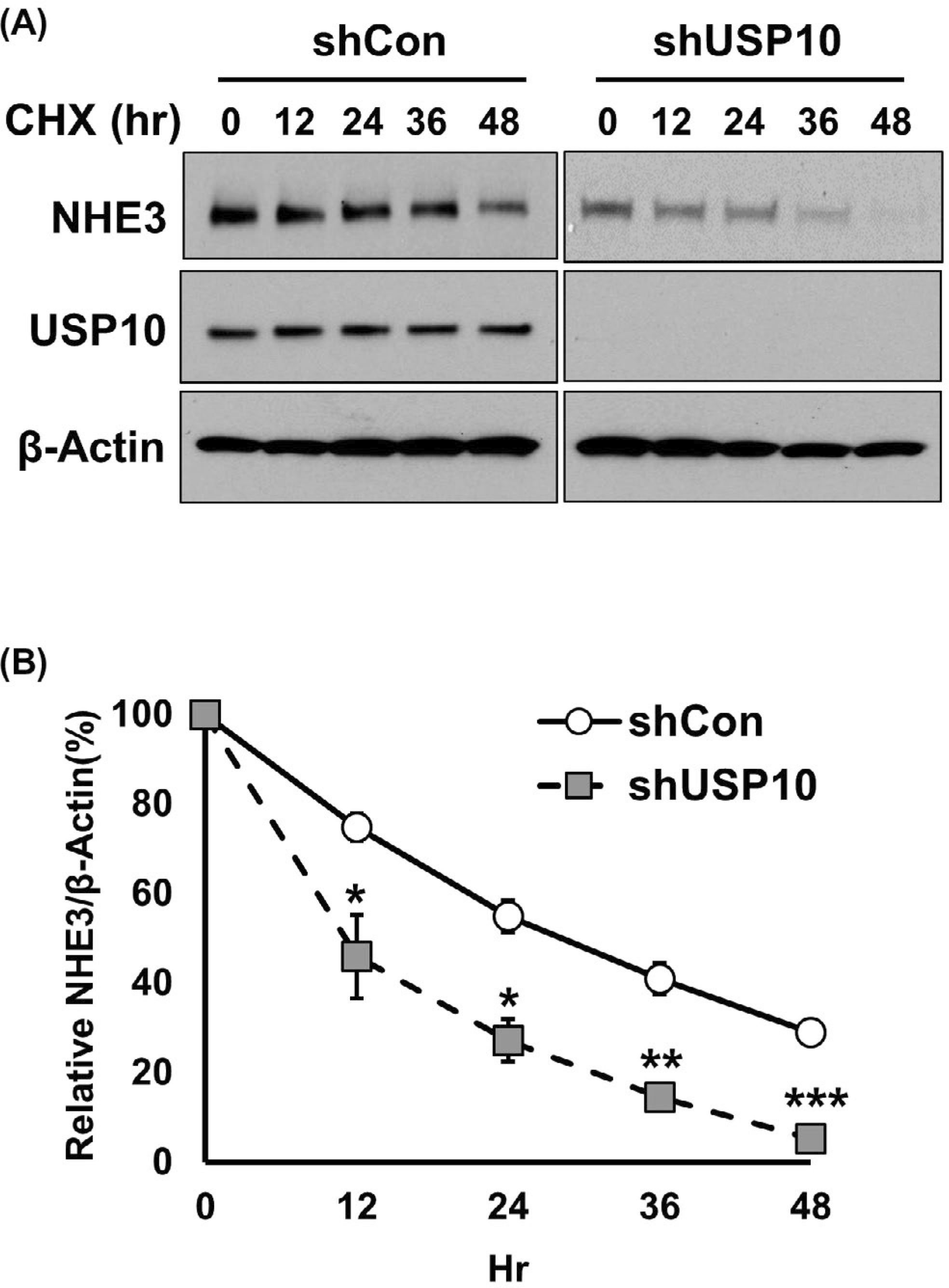
Knockdown of USP10 decreases the half-life of NHE3. A, Caco-2bbe/NHE3 expressing shCon or shUSP10 were treated with cycloheximide for indicated time duration. NHE3 expression was determined by Western blotting. Representative blots from three independent experiments are shown. B, The graph represents NHE3 expression levels normalized to β-actin. *, P < .05, **, P < .01 versus shCon
3.7 |. The NHE3-DUB interaction is regulated by PKA
Our studies above demonstrated the role of USP7 and USP10 on NHE3 expression and activity under basal conditions. To assess whether DUBs modulate NHE3 under regulatory conditions, we determined their role in PKA-dependent inhibition of NHE3. FSK, which activates adenylyl cyclase and PKA, inhibits NHE3 activity via increasing NHE3 phosphorylation and internalization.28 We postulated that inhibition of NHE3 by the FSK-PKA signaling involves a change in NHE3 ubiquitination and that knockdown of DUB should potentiate NHE3 inhibition. As shown in Figure 8A, FSK treatment resulted in an increase in NHE3 ubiquitination level. Because DUBs can directly bind to target proteins, we assessed whether the affinity of USP7 or USP10 for NHE3 is regulated by FSK treatment by co-immunoprecipitation. FSK treatment decreased the amount of USP7 and USP10 co-immunoprecipitated with NHE3 (Figure 8B), indicating PKA negatively regulates the NHE3-USP7/10 interaction. Of note, the reduction in their interaction was not a result of decreased USP7 or USP10 expression as the abundance of USP7 or USP10 was not altered by FSK. To ensure that the increased NHE3 ubiquitination by FSK was a direct consequence of decreased activity of the DUBs, we determined FSK-induced NHE3 ubiquitination in cells transfected with shUSP7 or shUSP10. FSK-mediated NHE3 ubiquitination was significantly augment in cells with USP7 or USP10 knockdown (Figure 8C), demonstrating that the overall level of Ub conjugation on NHE3 is determined in part by the presence of USP7 and USP10. We have shown above (Figures 3 and 4) that increased NHE3 ubiquitination levels correlated with reduced activity of NHE3. Therefore, we next determined the effect of FSK on NHE3 activity in cells with shUSP10 knockdown. FSK inhibited NHE3 in both shCon and shUSP10 cells, but shUSP10 and FSK had an additive effect, resulting in significantly greater inhibition compared with FSK or shUSP10 alone (Figure 8D). These results demonstrate that FSK mitigates the USP7/10 binding to NHE3, thereby increasing NHE3 ubiquitination and reducing NHE3 activity.
FIGURE 8.
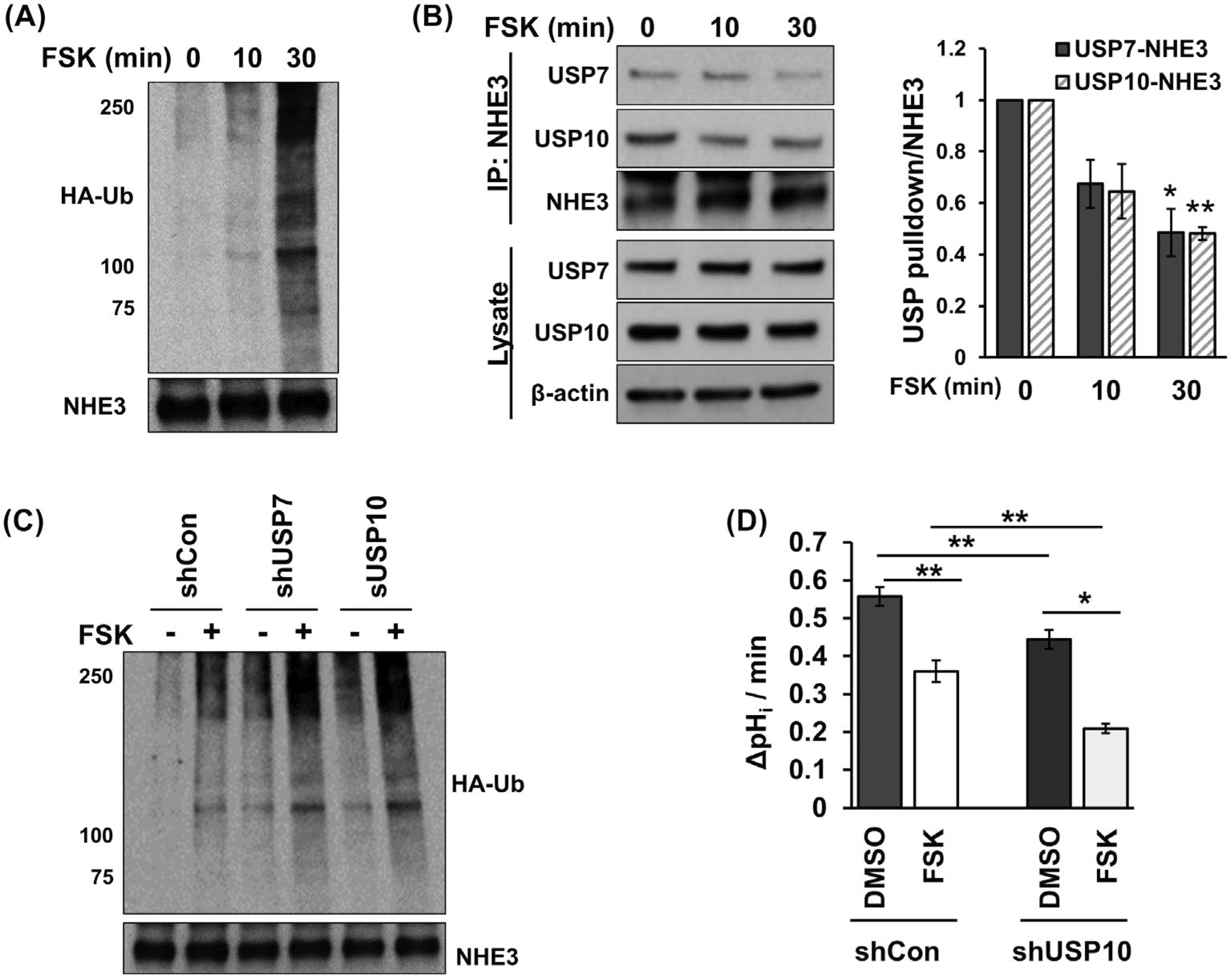
Forskolin (FSK) reduces the interaction of DUBs with NHE3 and potentiates NHE3 ubiquitination. A, NHE3 ubiquitination in response to FSK treatment was determined. The bottom panel shows immunoprecipitated NHE3 proteins. A representative Western immunoblot from four independent experiments is shown. B, Co-immunoprecipitation of USP7 and USP10 with NHE3 was determined in cells treated with FSK. Top two rows show co-immunoprecipitated USP7 and USP10. Third row shows immunoprecipitated NHE3. Lower panels show expression levels of USP7 and USP10 in cells treated with FSK. β-actin was used a loading control. The chart on the left shows the quantitation of co-immunoprecipitated USP7 or USP10 relative to immunoprecipitated NHE3. *, P < .05, **, P < .01 versus 0 minute. n = 3. C, NHE3 ubiquitination was determined in cells treated with FSK. NHE3 was immunoprecipitated from various Caco-2bbe/NHE3 cell lines transiently expressing HA-Ub, and Ub levels were determined using anti-HA antibody. The bottom panel shows immunoprecipitated NHE3 proteins. A representative Western immunoblot from three independent experiments is shown. D, The effect of FSK with or without shUSP10 on NHE3 activity was determined in Caco-2bbe/NHE3 cells. NHE3 activity was determined as Na+-dependent intracellular pH recovery as described in Methods and Materials. Results are presented as mean ± SEM. n ≥ 10. *, P < .05, **, P < .01
4 |. DISCUSSION
In this study, we have identified USP7 and USP10 as DUBs regulating NHE3 using a chemical trap that reacts with active DUBs. We postulated that DUBs targeting NHE3 would physically interact with NHE3. USP7 and USP10 were isolated from the immunocomplexes containing NHE3, indicating that these are active DUBs interacting with NHE3. The binding of USP7 and USP10 to NHE3 was confirmed by co-immunoprecipitation. Knockdown of either USP7 or USP10 increased ubiquitination level of NHE3, demonstrating that these proteins deubiquitinate NHE3. USP7 and USP10 are both members of the USP family of cysteine proteases. USP proteases have a structurally conserved catalytic domain, a variable N-terminal tumor necrosis factor receptor-like domain, which is involved in mainly protein-protein interaction, and a flexible C-terminal tail that regulates the catalytic state of DUB.29 USP7, which is also known as herpesvirus-associated ubiquitin-specific protease (HAUSP), is mainly found in the nucleus but also in the cytoplasm.30,31 USP7 has a role in regulation of the stability of proteins involved in a broad range of cellular processes, including DNA damage and repair, DNA dynamics and epigenetic regulation.29 Previously, USP10 has been demonstrated to regulate the endocytic recycling of CFTR in airway epithelial cells.13 Additionally, USP10 indirectly regulates ENaC by deubiquitinating soring nexin 3.16 The current study adds NHE3 to the list of electrolyte carriers targeted by USP10, while it is the first to imply USP7 in the regulation of a protein primarily expressed in the BBM.
NHE3 regulation involves internalization and recycling by endocytosis.7,8 In this study, we found that knockdown USP7 or USP10 decreased the surface membrane abundance of NHE3. Knockdown of USP7 or USP10 increased the association of NHE3 with Rab5a, which suggested that endocytic trafficking of NHE3 to the early endosomes was enhanced. These results are in line with our previous study that ubiquitination of human NHE3 by Nedd4–2 facilitates the movement of NHE3 molecules from the plasma membrane to the endosomal compartment.10 However, unlike Nedd4–2, knockdown of USP7 or USP10 resulted in a significant decrease in total NHE3 protein abundance. Internalized cargoes destined for degradation move from early to late endosomes, a process requiring fusion with lysosome in a Rab7-dependent manner.32 Accordingly, we found increased association of NHE3 with Rab7 when USP7 or USP10 was depleted. In addition, USP10 knockdown decreased the half-life of NHE3 by about a half from approximately 20 hours to 12 hours. The differential effect of Nedd4–2 versus USP7/10 on the total NHE3 expression was unexpected. The lack of effect on NHE3 degradation by Nedd4–2 suggests that Nedd4–2 is involved in the initial ubiquitination of NHE3 that promotes its trafficking to the early endosomes. In fact, we have previously shown that Nedd4–2 interacts with human NHE3 at the surface membrane.10 In the current study, we suggest that USP7 and USP10 decouple Ub chains from internalized NHE3, resorting it back to the apical membrane. In the absence of USP7 or USP10, Ub-conjugated NHE3 advances to the late endosomes and eventually to lysosomes or proteasomes for degradation.
The similar effects of USP7 and USP10 on NHE3 activity and protein abundance raised the possibility that these DUBs have redundancy. Inhibition of USP7 and USP10 by either chemically or a combination of genetic and chemical inhibitor resulted in an additive effect on NHE3 inhibition. We reasoned that if USP7 and USP10 have redundant regulatory functions on NHE3, knockdown of one USP, for example, USP7, would not impact NHE3 as USP10 would compensate for the loss of USP7. Moreover, given that the knockdown efficiency of each USP was more than 80%, the additive effect of a chemical inhibitor appears to support our conclusion of their additivity. A previous study in a human renal proximal tubular cell line has identified USP48 as a DUB targeting NHE3.33 USP48 is ubiquitously expressed, but our chemical trap assay did not yield USP48 perhaps in part due to low expression in Caco-2 cells.34,35 It remains to be determined whether USP48 is involved in regulation of intestinal NHE3, but these findings raise a question why more than one DUB is needed for the regulation of NHE3. Ubiquitination typically occurs on Lys, and Ub may be conjugated to an individual Lys, exists in multimeric chains linked to Lys residues, or is linked to other Ub-modifiers.1 Many DUBs show selectivity for particular Ub linkage types and show variability in Ub-chain recognition and catalytic activity.36 There are precedents where two DUBs associate with the same protein.37 Interestingly, both USP7 and USP10 are involved in regulation of the tumor suppressor protein p53 where USP7 and USP10 cooperatively deubiquitinate p53.38,39 USP10 translocates to the nucleus, aiding deubiquitination of p53 by USP7.39 Our study appears to be in line with the idea that USP7 and USP10 cooperatively target NHE3, exerting additive effects on NHE3 regulation. However, additional experiments will be required to demonstrate their additive or cooperative roles on NHE3 and to elucidate the specific roles they play in NHE3 regulation.
In the intestinal track, NHE3 is a frequent target of enteropathogenic agents and inhibition of NHE3 is a major cause of diarrhea.40 Infection by Vibrio cholera increases Cl− secretion and decreases Na+ absorption as a result from the presence of cholera toxin secreted by the bacteria, which activates PKA.41 FSK, which activates adenylyl cyclase, inhibits NHE3 via a PKA-dependent mechanism. Mechanistically, PKA-induced inhibition of NHE3 involves NHE3 phosphorylation, interaction with scaffold proteins, and internalization.28,42,43 Our current study identified the deubiquitinating proteases USP7 and USP10 as new regulators of NHE3 in this pathological process. We showed that FSK attenuated the interaction of USP7 and USP10 with NHE3, resulting in increased ubiquitination of NHE3. We did not investigate how FSK regulates Nedd4–2, but we conditionally assumed that Nedd4–2 activity was not altered by FSK treatment because Nedd4–2 expression was not affected by the DUB depletion. Both USP7 and USP10 are subjected to phosphorylation-dependent regulation.39,44 Particularly, phosphorylation of USP7 by the protein kinase CK2 is shown to stabilize USP7.44 Although USP7 or USP10 expression was not altered by acute FSK treatment, any effect on USP7 and USP10 phosphorylation remains to be determined.
In summary, we have demonstrated that USP7 and USP10 physically interact and ubiquitinate NHE3. USP7 and USP10 have a similar mode of NHE3 regulation, altering NHE3 endocytosis and post-endocytic process. USP7 and USP10 additively regulate NHE3 expression, although the exact mechanism of their cooperativity needs additional investigation. In addition, the current study showed that the current picture of NHE3 regulation by PKA is incomplete, and it adds USP7 and UPS10 as additional players in this regulatory process.
ACKNOWLEDGMENT
The current study was supported by the National Institutes of Health Grant DK107719 and the VA Merit Award I01BX004459 to CCY. Confocal microscopic analyses were supported in part by the Integrated Cellular Imaging Shared Resources of Winship Cancer Institute of Emory University and NIH/NCI under award P30CA138292.
Funding information
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Grant/Award Number: DK107719; U.S. Department of Veterans Affairs (VA), Grant/Award Number: I01BX004459
Abbreviations:
- BBM
brush border membrane
- CFTR
cystic fibrosis transmembrane regulator
- DUB
deubiquitinating enzyme
- ENaC
epithelial sodium channel
- FSK
forskolin
- HA
hemagglutin
- HA-UbVME
N-terminally HA-tagged ubiquitin fused with vinyl methyl ester
- JAMM
Josephin, and JAB1/MPN/MOV34 metalloenzymes
- Nedd4–2
Neural precursor cell Expressed, Developmentally Down-regulated 4–2
- NEM
N-ethylmaleimide
- NHE3
Na+/H+ exchanger 3
- OTU
ovarian tumor proteases
- pHi
intracellular pH
- PKA
protein kinase A
- PNS
post-nuclear supernatant
- shRNA
short hairpin RNA
- Ub
ubiquitin
- UCH
ubiquitin C-terminal hydrolases
- USP
ubiquitin-specific protease
- VME
vinyl methy ester
- VSVG
vesicular stomatitis virus glycoprotein
- WGA
wheat germ agglutinin
Footnotes
DISCLOSURES
The authors disclose no conflict of interests.
REFERENCES
- 1.Piper RC, Lehner PJ. Endosomal transport via ubiquitination. Trends Cell Biol. 2011;21:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thrower JS, Hoffman L, Rechsteiner M, Pickart CM. Recognition of the polyubiquitin proteolytic signal. EMBO J. 2000;19:94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clague MJ, Urbe S, Komander D. Breaking the chains: deubiquitylating enzyme specificity begets function. Nat Rev Mol Cell Biol. 2019;20:338–352. [DOI] [PubMed] [Google Scholar]
- 4.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. [DOI] [PubMed] [Google Scholar]
- 5.Hutchins AP, Liu S, Diez D, Miranda-Saavedra D. The repertoires of ubiquitinating and deubiquitinating enzymes in eukaryotic genomes. Mol Biol Evol. 2013;30:1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher MM, Gontarek JD, Bess RS, Donowitz M, Yeo CJ. The Na+/H+ exchange isoform NHE3 regulates basal canine ileal Na+ absorption in vivo. Gastroenterology. 1997;112:174–183. [DOI] [PubMed] [Google Scholar]
- 7.Chow CW, Khurana S, Woodside M, Grinstein S, Orlowski J. The epithelial Na(+)/H(+) exchanger, NHE3, is internalized through a clathrin-mediated pathway. J Biol Chem. 1999;274:37551–37558. [DOI] [PubMed] [Google Scholar]
- 8.Collazo R, Fan L, Hu MC, Zhao H, Wiederkehr MR, Moe OW. Acute regulation of Na+/H+ exchanger NHE3 by parathyroid hormone via NHE3 phosphorylation and dynamin-dependent endocytosis. J Biol Chem. 2000;275:31601–31608. [DOI] [PubMed] [Google Scholar]
- 9.Murtazina R, Kovbasnjuk O, Donowitz M, Li X. Na+/H+ exchanger NHE3 activity and trafficking are lipid raft-dependent. J Biol Chem. 2006;281:17845–17855. [DOI] [PubMed] [Google Scholar]
- 10.No YR, He P, Yoo BK, Yun CC. Unique regulation of human Na+/H+ exchanger 3 (NHE3) by Nedd4–2 ligase that differs from non-primate NHE3s. J Biol Chem. 2014;289:18360–18372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Sole F, Hu M-C, Zhang J, et al. The reduction of Na/H exchanger-3 protein and transcript expression in acute ischemia-reperfusion injury is mediated by extractable tissue factor(s). Kidney Int. 2011;80:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. J Biol Chem. 2007;282:37885–37893. [DOI] [PubMed] [Google Scholar]
- 13.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284:18778–18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadler JBA, Lamb CA, Welburn CR, et al. The deubiquitinating enzyme USP25 binds tankyrase and regulates trafficking of the facilitative glucose transporter GLUT4 in adipocytes. Sci Rep. 2019;9:4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savio MG, Wollscheid N, Cavallaro E, et al. USP9X controls EGFR fate by deubiquitinating the endocytic adaptor Eps15. Curr Biol. 2016;26:173–183. [DOI] [PubMed] [Google Scholar]
- 16.Boulkroun S, Ruffieux-Daidie D, Vitagliano JJ, et al. Vasopressin-inducible ubiquitin-specific protease 10 increases ENaC cell surface expression by deubiquitylating and stabilizing sorting nexin 3. Am J Physiol Renal Physiol. 2008;295:F889–F900. [DOI] [PubMed] [Google Scholar]
- 17.Borodovsky A, Ovaa H, Kolli N, et al. Chemistry-based functional proteomics reveals novel members of the deubiquitinating enzyme family. Chem Biol. 2002;9:1149–1159. [DOI] [PubMed] [Google Scholar]
- 18.Treier M, Staszewski LM, Bohmann D. Ubiquitin-dependent C-Jun degradation in-vivo is mediated by the delta-domain. Cell. 1994;78:787–798. [DOI] [PubMed] [Google Scholar]
- 19.Lin S, Yeruva S, He P, et al. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology. 2010;138:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo BK, Yanda MK, No YR, Yun CC. Human intestinal epithelial cell line SK-CO15 is a new model system to study Na(+)/H(+) exchanger 3. Am J Physiol Gastrointest Liver Physiol. 2012;303:G180–G188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatis virus glycoprotein block its tranport to the cell surface. EMBO J. 1986;5:931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemelaar J, Galardy PJ, Borodovsky A, Kessler BM, Ploegh HL, Ovaa H. Chemistry-based functional proteomics: mechanism-based activity-profiling tools for ubiquitin and ubiquitin-like specific proteases. J Proteome Res. 2004;3:268–276. [DOI] [PubMed] [Google Scholar]
- 23.He P, Klein J, Yun CC. Activation of Na+/H+ exchanger NHE3 by angiotensin II is mediated by inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3 (IRBIT) and Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2010;285:27869–27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J, Zhang J, Liang L, et al. Potent USP10/13 antagonist spautin-1 suppresses melanoma growth via ROS-mediated DNA damage and exhibits synergy with cisplatin. J Cell Mol Med. 2020;24:4324–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colland F, Formstecher E, Jacq X, et al. Small-molecule inhibitor of USP7/HAUSP ubiquitin protease stabilizes and activates p53 in cells. Mol Cancer Ther. 2009;8:2286–2295. [DOI] [PubMed] [Google Scholar]
- 26.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. [DOI] [PubMed] [Google Scholar]
- 27.Cavet ME, Akhter S, Murtazina R, Sanchez de Medina F, Tse CM, Donowitz M. Half-lives of plasma membrane Na(+)/H(+) exchangers NHE1–3: plasma membrane NHE2 has a rapid rate of degradation. Am J Physiol Cell Physiol. 2001;281:C2039–C2048. [DOI] [PubMed] [Google Scholar]
- 28.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem. 1999;274:3978–3987. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya S, Chakraborty D, Basu M, Ghosh MK. Emerging insights into HAUSP (USP7) in physiology, cancer and other diseases. Signal Transduct Target Ther. 2018;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Everett RD, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daubeuf S, Singh D, Tan Y, et al. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood. 2009;113: 3264–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanlandingham PA, Ceresa BP. Rab7 regulates late endocytic trafficking downstream of multivesicular body biogenesis and cargo sequestration. J Biol Chem. 2009;284:12110–12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armando I, Villar VA, Jones JE, et al. Dopamine D3 receptor inhibits the ubiquitin-specific peptidase 48 to promote NHE3 degradation. FASEB J. 2014;28:1422–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thul PJ, Akesson L, Wiking M, et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321. [DOI] [PubMed] [Google Scholar]
- 35.Uhlen M, Fagerberg L, Hallstrom BM, et al. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. [DOI] [PubMed] [Google Scholar]
- 36.Mevissen TET, Komander D. Mechanisms of deubiquitinase specificity and regulation. Annu Rev Biochem. 2017;86:159–192. [DOI] [PubMed] [Google Scholar]
- 37.Kee Y, Huang TT. Role of deubiquitinating enzymes in DNA repair. Mol Cell Biol. 2016;36:524–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cummins JM, Vogelstein B. HAUSP is required for p53 destabilization. Cell Cycle. 2004;3:689–692. [PubMed] [Google Scholar]
- 39.Yuan J, Luo K, Zhang L, Cheville JC, Lou Z. USP10 regulates p53 localization and stability by deubiquitinating p53. Cell. 2010;140:384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurney MA, Laubitz D, Ghishan FK, Kiela PR. Pathophysiology of Intestinal Na(+)/H(+) exchange. Cell Mol Gastroenterol Hepatol. 2017;3:27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yun CH, Oh S, Zizak M, et al. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA. 1997;94:3010–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh V, Yang J, Yin J, et al. Cholera toxin inhibits SNX27-retromer-mediated delivery of cargo proteins to the plasma membrane. J Cell Sci. 2018;131:jcs218610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoronenkova SV, Dianova II, Ternette N, Kessler BM, Parsons JL, Dianov GL. ATM-dependent downregulation of USP7/HAUSP by PPM1G activates p53 response to DNA damage. Mol Cell. 2012;45:801–813. [DOI] [PMC free article] [PubMed] [Google Scholar]


