Abstract
Tumor microenvironment (TME) can serve as the ‘soil’ for the growth and survival of tumor cells and function synergically with tumor cells to mediate tumor progression and therapeutic resistance. Reactive oxygen species (ROS) is somewhat of a double-edged sword for tumors. Accumulating evidence has reported that regulating ROS levels can serve an anti-tumor role in the TME, including the promotion of cancer cell apoptosis, inhibition of angiogenesis, preventing immune escape, manipulating tumor metabolic reorganization and improving drug resistance. In the present review, the potential role of ROS in anti-tumor therapy was summarized, including the possibility of directly or indirectly targeting the TME.
Keywords: tumor microenvironment, reactive oxygen species, tumor angiogenesis, immune escape, metabolism recombination, tumor drug resistance
1. Introduction
Reactive oxygen species (ROS) is a general term used to describe molecules with high oxidative reactivity. They are mainly produced by the electron transport chain during aerobic respiration in the mitochondria or as a byproduct of the activity of several metabolic enzymes, including xanthine oxidase, lipoxygenase and cytochrome P450 (1). In addition, exogenous stimuli, such as stress, ultraviolet radiation, tumor chemotherapy and radiotherapy (RT), can stimulate ROS production (2). Under physiological conditions, cells can scavenge intracellular ROS using antioxidants, including catalase, glutathione and ascorbic acid, to maintain the dynamic redox balance (3). Once the level of ROS exceed the tolerance threshold of cells, a variety of pathological disorders occur. Previous studies have shown that abnormal ROS levels are closely associated with the occurrence of tumors and neurodegenerative diseases (4,5). During the moderate redox state, ROS can induce tumorigenesis by activating the MAPK and ERK signaling pathway or promoting mutations in the genomic DNA (6). However, previous studies have demonstrated that ROS production is actually inhibited during breast and colon tumor progression, where tumor cells attempt to reduce or eliminate the adverse effects of ROS by potently activating their antioxidant systems (7,8). This leads to resistance to treatments, including chemotherapy, RT and immunotherapy (9,10). In addition, an elevation in ROS levels in breast cancer and human multiple myeloma has been found to promote tumor cell death in different signaling pathways and increase sensitivity to anti-tumor therapy (11,12).
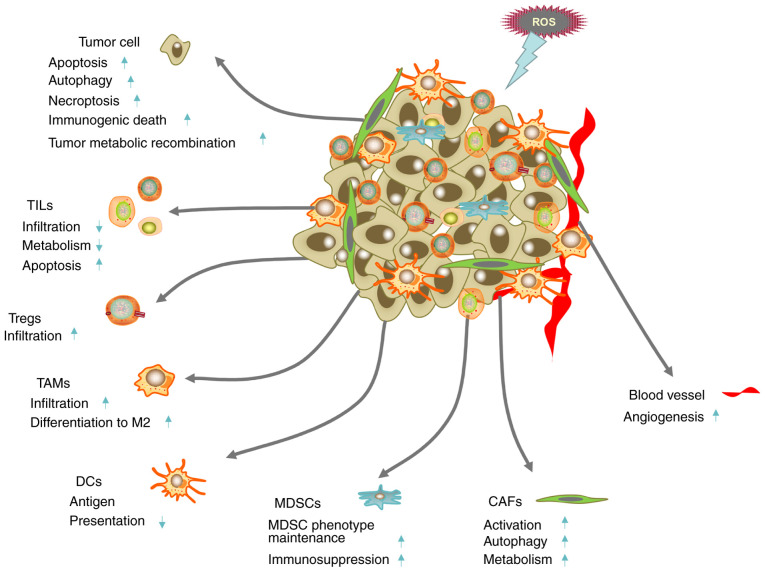
The tumor microenvironment (TME) mainly includes tumor cells and their surrounding immune cells, cancer-associated fibroblasts (CAFs) and vascular endothelial cells (13). It is characterized by hypoxia, low pH, high interstitial pressure, overexpression of glutathione, redox imbalance and immunosuppression (14,15). Paget first proposed the hypothesis of ‘seed and soil’ in 1989, where tumor cells were known as ‘seeds’ and the surrounding microenvironment were known as ‘soil’ (16). Langley and Fidler (17) then revisited this theory and reviewed the close relationship between tumor and angiogenesis in organ metastasis. They found that angiogenesis can promote the metastasis of tumor organs, which provides a theoretical basis for antiangiogenic therapy. Recent studies in stomach and lung cancer have found that in the TME, immune and metabolic reorganization can also promote the occurrence, development, invasion, metastasis of tumors (Fig. 1) (18,19). Manipulating the TME may therefore be more beneficial for controlling the progression of tumors and reverse the drug resistance of tumors. Over the past decade, an increasing number of studies have revealed that regulation of the levels of ROS can exert anti-tumor effects by acting on the TME (20,21). These effects include promoting tumor cell apoptosis, inhibiting angiogenesis, inhibiting immune escape, regulating tumor metabolic reorganization and reversing drug resistance (20,22). The present review analyzes the complex role of ROS in anti-tumor therapy in relation to the TME.
Figure 1.
Complexity of the interaction between ROS and the TME. The up and down arrows represent cell responses to ROS treatment. ROS can promote tumor cell death, promote tumor metabolic reprogramming, induce tumor angiogenesis and activate CAFs and autophagy. ROS can also reduce lymphocyte infiltration, reduce the antigen presenting ability of DCs and maintain the immunosuppressive function of Treg cells and MDSCs to promote the formation of the immunosuppressive microenvironment. CAF, cancer-associated fibroblast; DC, dendritic cell; MDSC, myelogenic suppressor cell; ROS, reactive oxygen species; TAM, tumor-associated macrophage; TIL, tumor-infiltrating lymphocyte; TME, tumor microenvironment; Treg, T regulatory cells.
2. ROS and TME
ROS and tumor cells
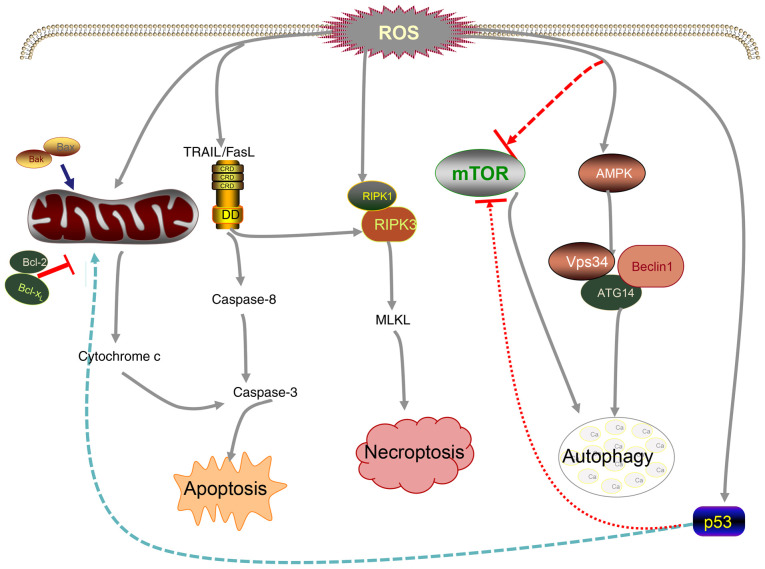
Compared with normal cells, cancer cells have a higher level of oxidation (23,24). Excessive ROS renders tumor cells that are already under oxidative stress more fragile, which eventually leads to apoptosis, autophagy and necrosis (Fig. 2).
Figure 2.
Mechanism of cancer cell death induced by ROS. Elevated ROS can reduce Bcl-2 activity, promote the release of cytochrome c and activate death-related ligands TRAIL/FasL, thereby promoting caspase-mediated apoptosis. High levels of ROS can also promote the formation of the RIPK-1/RIPK-3 complex and eventually lead to necrosis. Moreover, ROS can inhibit the activity of mTOR, promote the activation of AMPK and subsequently induce autophagy. p53 is involved in ROS-mediated cancer cell death. ATG14, autophagy related 14; 5AMPK, AMP-activated protein kinase; FasL, Fas ligand; MLKL, mixed lineage kinase domain-like pseudokinase; RIPK, receptor interacting serine/threonine kinase; ROS, reactive oxygen species; TRAIL, TNF-related apoptosis-inducing ligand; Vps34, vacuolar protein sorting 34.
ROS and apoptosis
Apoptosis is also termed type I programmed cell death and is a form of programmed cell death that serves to clear damaged cells in an orderly manner, which are mainly divided into two types, namely exogenous and endogenous apoptosis (25). The former primarily involves the FasL/FasR, TNF-α/TNF receptor 1 (TNF-α/TNFR1), TNF ligand superfamily member 12/death receptor (DR)3, TNF-related apoptosis-inducing ligand (Apo2L)/DR4, and Apo2L/DR5 signaling pathways (26–29). By contrast, the endogenous signaling pathway is also termed the mitochondrial pathway and mainly entails increasing the permeability of mitochondria, which elevates the concentration of intracellular Ca2+ and regulates the activity of the Bcl-2 family of proteins (30). This effect is accompanied by the release of cytochrome c (Cyt c), apoptosis-inducing factor and endonuclease G, leading to tumor cell apoptosis (31,32). The final node of apoptosis is mainly initiated by the activation of enzymes in the caspase family (33,34). Under normal conditions, the production and clearance of intracellular ROS are maintained in a dynamic balance. Previous studies have shown that low levels of ROS can promote cell proliferation, whereas excessive accumulation of ROS will lead to colon cancer cell apoptosis (35,36). Tumor cells proliferate at a high rate and are frequently in a state of high oxidative stress (23). Therefore, they tend to be more sensitive to internal and external oxidative stimuli (24). A potential anti-cancer strategy is to aggravate oxidative stress in the cancer cells further by increasing the intracellular ROS levels or by inhibiting the antioxidant capacity of cells (37,38). It has been demonstrated that oxidation of the mitochondrial membrane by ROS can release Cyt c into the cytosol more easily to promote apoptosis (11).
Bcl-2 is a key regulator of apoptosis and as such, ROS levels can influence the functionality of Bcl-2. In a previous study with lung cancer, it was found that excessive ROS production in H460 lung cancer cells inhibit the expression of Bcl-2, whilst increasing the expression Bcl-2 served the effect of inhibiting the increase of ROS (39). In addition, ROS can also regulate a number of exogenous signaling pathways. As the product of the FasL/FasR pathway by the NADPH oxidase system, ROS can activate protein tyrosine kinase, which further promotes Fas-mediated apoptosis (40). A functional relationship between ROS and several signaling pathways has also been found. Zhu et al (41) found that overproduction of ROS in gastric cancer cells can effectively increase the expression of JNK, which then participate in apoptosis mediated by the MAPK pathway. In another study with breast cancer, Zang et al (42) found that ROS can activate the NF-κB and STAT3 signaling pathways to mediate tumor cell apoptosis. Many chemotherapeutic drugs function by increasing the production of ROS, which leads to irreversible apoptosis. Sulindac is a nonsteroidal anti-inflammatory drug. In the treatment of lung cancer, it has been shown to increase ROS production and subsequent mitochondrial membrane damage, which promoted tumor cell apoptosis (43). Doxorubicin can also increase the production of ROS and activate the tumor suppressor p53, resulting in tumor cell death (44). In addition, photodynamic therapy, RT and emerging sonodynamic therapy, chemodynamic therapy, enzyme dynamic therapy and ROS-based nanomedicine therapy have all been documented to serve anti-tumor roles by increasing the levels of cellular ROS (22,45,46).
ROS and autophagy
Autophagy is termed type II programmed cell death and is a process in which cells remove intracellular damage, senescent organelles and structural and biological macromolecules, such as proteins and lipids, by lysosome-mediated degradation (47). Autophagy is highly conserved and is regulated by the autophagy-related (ATG) family of proteins (47). It is now considered to be not only a mechanism of cell survival, but also an inhibitory mechanism that can induce the death of transformed cancer cells (47). ROS is a classical autophagy inducer and a key component for the interaction between apoptosis and autophagy (48). In general, autophagy induced by moderate levels of ROS can reduce the damage caused by oxidative stress and protect cells (49). By contrast, high levels of ROS can activate autophagic cell death and have destructive effects on cells (49). Wu et al (50) previously showed that H2O2 pretreatment triggered autophagy in hepatocellular carcinoma (HCC) cells, where high concentration of H2O2 could stimulate autophagic apoptosis in HCC cell lines. Another study has also shown that excessive ROS may induce autophagic cell death in human oral cancer CAL 27 cells by promoting Unc51-like kinase 1 protein ubiquitination and upregulating the expression of the autophagy-related protein Beclin-1 (51). In addition, ROS can also alter the activity of signaling pathways that regulate autophagy. Activation of mTOR kinase, an enzyme in the autophagic pathway, is inhibited by the AKT and MAPK signaling pathways (52). AKT induces protective autophagy whilst sustaining the degradation of p53 and the expression of NF-κB in HCC cells (53). Therefore, pathways that negatively regulate mTOR, including the protein kinase 5AMP-activated protein kinase and p53, which are sensitive to oxidative stress, can promote autophagy (53,54).
ROS and necroptosis
Cell necrosis is currently considered to be a type III programmed cell death (55). It is initiated by TNFR1 and transmits cell death signals through receptor-interacting serine-threonine kinase 1 (RIP1). RIP3 and mixed lineage kinase domain-like pseudokinase (MLKL) (55). It has been previously reported that ROS can promote the autophosphorylation of Serine 161 on RIP1 (56,57). Phosphorylated RIP1 can then recruit RIP3 to form the programmed necrosis complex, which activate programmed necrosis to increase the intracellular ROS content further, completing the positive feedback loop (58). A previous study with melanoma have found that increasing ROS can regulate RIP1 to promote melanoma cell necrosis, where the JNK signaling pathway was involved (59). Yang et al (60) demonstrated that RIP3 can phosphorylate the pyruvate dehydrogenase complex to promote cell oxygen consumption and ROS production in murine fibroblast L929 cells, which enhanced the formation of necrotic bodies following stimulation by TNF. Additionally, p53 has also been implicated in ROS-induced programmed necrosis. Tu et al (61) showed that etoposide can induce the necrosis of fibroblasts in a BAX/BAK double-knockout mouse embryonic model. This was found to be the result of a synergistic interaction between DNA damage-induced ROS and p53-induced elevation of cathepsin Q (61). In a study of mouse embryonic fibroblasts, during oxidative stress, p53 has been reported to accumulate in the mitochondrial matrix and interact with the cyclophilin D regulator located in the intima of the mitochondrial permeability transition pore (62). This resulted in mitochondrial damage, ROS production and finally programmed cell necrosis (62).
ROS and angiogenesis
Serving as the ‘soil’ for the growth of cancer cells, the TME must provide sufficient nutrition for them. Neovascularization is the main method used for the transport of nutrients during tumor occurrence and metastasis (63). It has been shown that ROS can regulate tumor angiogenesis and promote angiogenesis by targeting transcription factors or tumor suppressors, such as activating protein 1, hypoxia inducible factor-1α (HIF-1α), NF-κB, and p53 (64). However, in recent years, it has been found that the increase of ROS production in the TME can reduce neovascularization and inhibit tumor progression (65). Inducing apoptosis in vascular endothelial cells is generally considered to be the core strategy for inhibiting angiogenesis and treating related diseases, including cancer, neovascular age-related macular degeneration and diabetic retinopathy (66). ROS is a promoter of vascular and endothelial cell death in colon and breast tumors (67,68). Owing to the atypical metabolic environment, vascular endothelial cells in the TME generally exhibit higher levels of ROS compared with those in normal vascular endothelial cells and are more vulnerable to cytotoxicity caused by a further increment in ROS levels (69). Therefore, increasing the ROS levels further is more likely to aggravate cell death (69). Topalovski et al (70) found that fibulin-5 can promote ROS production in vascular endothelial cells by acting on the fibronectin receptor β1 to exert its anti-angiogenic effects in pancreatic cancer cells. In addition, N-benzyl-2-nitro-1-imidazole-acetamide, a therapeutic agent for Chagas disease, has been found to exert anti-tumor effects in Ehrlich tumor cells by increasing ROS levels and inhibiting angiogenesis (71). Synthesis of redox regulators using silver nanoframe technology has been shown to induce excessive ROS generation and enhance cytotoxicity in the vascular endothelium (72). This prevented formation of the tubular network in the endothelial cells and poly-ADP ribose modification of VEGF, thereby inhibiting angiogenesis (72). Furthermore, Cao et al (73) found that decylubiquinone can increase ROS to inhibit the formation of the tubular structure through the ROS/p53/brain-specific angiogenesis inhibitor 1 signaling pathway in the chicken embryo chorioallantoic membrane model.
ROS and CAFs
CAFs are particularly abundant in the matrix and can be derived from a variety of sources (74). They serve an important role in tumor growth, metastasis and drug resistance (74,75), by secreting a variety of cytokines and growth factors. The specific mechanisms involved in these effects include the maintenance of cancer stem cell (CSC) stemness, promotion of epithelial-mesenchymal transformation (EMT), remodeling of the vascular system and regulation of tumor immunity (76–79). In previous years, it was found that the autophagy of CAFs can also serve an important role in the occurrence and development of tumors (80,81). Activation of CAFs is largely dependent on the stimulation of TME by local hypoxia, oxidative stress, growth factors released by adjacent tumor cells and infiltrating immune cells (82). A previous study with ovarian cancer has shown that ROS produced by tumors can induce the expression of chloride intracellular channel 4, chemokine (C-C motif) ligand 2, TGF-β1, NF-κB and STAT3 in CAFs to induce their activation (83). In addition, ROS can also induce myofibroblast differentiation by upregulating the expression of chemokine (C-X-C motif) ligand-12 whilst downregulating that of caveolin 1 (CAV1) (84–86). ROS can also play an important role in the regulation of CAF autophagy. Tumor cells have been observed to induce oxidative stress in adjacent CAFs (87). In breast cancer, an increase in the levels of ROS can activate the expression of HIF-1α and NF-κB in CAFs to subsequently induce CAF autophagy, which may lead to a decrease in ROS-dependent CAV1 expression (88). The downregulation of CAV1 promotes mitochondrial dysfunction and oxidative stress further in CAFs, thereby forming a positive feedback loop (88,89). In addition, ROS can also regulate the metabolism of CAFs. In tumor cells, even under aerobic conditions, glycolysis is highly active, which is characterized by increased glucose uptake and increased lactic acid secretion (90). This is known as the ‘Warburg effect’ (90). CAFs will also produce a similar phenomenon of aerobic glycolysis under the influence of tumor cells, which is called the ‘reverse Warburg effect’ (86). During this phenomenon, oxidative phosphorylation of tumor cells produces a large quantity of ROS, which specifically triggers oxidative stress in CAFs and disrupts the oxidative phosphorylation system (86). This results in the additive accumulation of ROS and induces a chain reaction of oxidative stress (86). The mode of glucose metabolism in CAFs changes from oxidative phosphorylation to glycolysis, which produces high-energy raw materials, such as lactic acid and ketone, which supply tumors to promote cell division and support Krebs cycle (86). Additionally, an acidic microenvironment with high lactate content is also created during this event, which inhibits the growth of normal cells whilst promoting tumor cell proliferation and metastasis (86). Inhibition of CAV1 by ROS is known to be one of the driving factors for changing in the metabolic pattern of CAFs (91). Martinez-Outschoorn et al (92) previously found that when the MCF7 breast cancer cells were co-cultured with fibroblasts, tumor cell apoptosis occurred after the removal of H2O2. This effect was proposed to be caused by the lack of lactic acid produced by CAFs in tumor cells (92).
ROS and the immune microenvironment
In recent years, tumor immunotherapy, including immune checkpoint inhibitors (93), chimeric antigen receptor-T cell therapy and tumor vaccines (94), has emerged as another promising anti-cancer therapeutic strategy after surgery, chemotherapy and RT. Although clear therapeutic effects have been achieved, in current clinical practice immunotherapy can only offer lasting survival benefits to 20–30% of the patients, since most patients will face the problems of insensitivity or resistance to immunotherapy (95). For example, ipilimumab, a cytotoxic T-cell lymphocyte antigen-4 inhibitor, has a positive response rate of only 15% in patients with advanced melanoma (96). Similarly, the positive response rate of drugs that target programmed cell death 1/programmed cell death ligand 1 (PD-1/PD-L1) signaling rarely exceeds 40% (97). Previous studies have found that limited T lymphocyte infiltration or low immunogenicity of the TME is the main reason for the failure of treatment (98,99). The interaction between tumor cells and immune cells is a core feature of the TME (100,101). Owing to the high plasticity of immune cells, tumor cells promote the maturation, differentiation and recruitment of immunosuppressive cells by secreting IL-10, TGF-β, and VEGF to construct an immunosuppressive TME (102,103). In turn, the immunosuppressive cells can promote tumor growth, metastasis and escape from immune surveillance (103). ROS was found to be an effector of cytotoxicity induced by numerous anti-cancer drugs, which is a byproduct of cellular oxidative metabolism (104). As signal mediators, ROS serves a key role in the immune monitoring of regulatory (Tregs) and effector T cells, which relies on classical receptors, such as toll-like receptors, and perception of the metabolic environment (104,105). The concentration of ROS in tumor tissues is typically higher compared with that recorded in surrounding normal tissues (23). In the TME with persistently high ROS levels, both immune and tumor cells are affected. The increase of ROS is one of the main causes of immunosuppression in the TME (106). However, some studies have shown that increasing ROS levels can induce immunogenic death (ICD) in tumor cells (107,108), which exhibits a synergistic effect with immunotherapy (107). A growing body of evidence suggests that targeting the redox levels of immune cells can result in a variety of phenotypes and functions, which can overcome immunosuppression in the TME to ultimately inhibit tumor growth (21,22). In this section, the function and effect of ROS on the different tumor-infiltrating immune cell types were explored, with focus on the effect of ROS in tumor immunotherapy (Fig. 1).
ROS and lymphocytes
T cells (also known as T lymphocytes) consist of a heterogeneous group of lymphocytes in the tumor matrix, which includes cytotoxic T lymphocytes, helper T lymphocytes and Tregs (109). They mediate immune surveillance and the killing of cancer cells (109). ROS participate in the regulation of T cells in the TME. Murphy and Siegel (110) previously reported that following reduction in mitochondrial ROS produced by complex III, T cells could no longer be continuously activated even after stimulation by CD3 or CD28. Activation of T cells requires stimulation of the T-cell receptor (TCR) through the induction of the MAPK signal transduction pathway and transcription factors such as nuclear factor of activated T cells (NFAT), NF-κB and activator protein-1 (111). Studies (112,113) have shown that mitochondrial ROS can be transferred to T cell immune synapses. After stimulation by antigens, H2O2 in the mitochondria can enhance the MAPK signaling pathway, leading to T cell activation and proliferation (112). However, a number studies have also shown that high levels of ROS in the TME can inhibit the activation, proliferation and anti-tumor function of T cells (114–117). H2O2-mediated activation of TCR can promote the production of mitochondrial superoxides, which can enhance the expression of FasL in T cells and contribute to T cell activation-induced cell death (114,115). Recent studies have shown that chronic oxidative stress can cause T cell weakness or failure (116,117).
PD-1 is an immunosuppressive receptor that is mainly expressed in activated T cells and can exert negative immunoregulatory effects after activation by the PD-L1 ligand on the surfaces of antigen-presenting cells (118). Kumar et al (119) and Chamoto et al (120) previously found that ROS and mitochondrial activation serve an important role in T cell immunity induced by PD-1 blockade. PD-1 blocking treatment can increase the ROS content in T cells (119). Using a ‘bilateral tumor model’, it was found that boosting mitochondrial activity of T cells by the addition of bezafibrate, a pan-peroxisome proliferator-activated receptor agonist, can partially improve the efficacy of PD-1 blockade in a lung cancer model with systemic immunosuppressive properties (119). These findings suggest that regulation of mitochondria-derived ROS in T cells may have an impact on PD-1 blocking therapy. Another study (121) found that use of the phenothiazine calmodulin inhibitor trifluoperazine could increase the levels of ROS and stimulate the expression of PD-L1 in colorectal cancer cells, tumor-infiltrating CD4+ and CD8+ T cells. However, other studies have shown that enhanced ROS levels in neck squamous carcinoma cells can also reduce the expression of PD-L1 (122,123). Therefore, ROS can exert different biological effects in a manner that is dependent on its quantity, where different levels of ROS can mediate different immune cell responses.
Treg cells belong to a typical class of immunosuppressive cells. In particular, the CD4+ subset of forkhead box P3 (FOXP3) Tregs can play an important role in mediating tumor immune tolerance (124). It has been shown that the levels of ROS in the microenvironment are associated with immune tolerance mediated by Treg cells (125). By contrast, ROS can promote the differentiation of Treg cells (126). It was found that bile acid can promote the differentiation of Treg cells by increasing the levels of mitochondrial ROS, which subsequently increased the acetylation of H3K27 in the Foxp3 promoter (126). Kunisada et al (127) demonstrated that metformin blocked the differentiation of immature CD4+ T cells into Treg cells by inhibiting mitochondrial ROS, which subsequently downregulated the expression of FOXP3 and reduced the number of tumor-infiltrating Treg cells. Conversely, ROS can also maintain the function of Treg cells. Yu et al (128) found that ROS induced by TCR signaling specifically inhibited the protein degradation of deubiquitin-like enzyme SUMO-specific peptidase 3 (SENP3), which preserved the immunosuppressive activity of Treg cells. Interfering with the levels of ROS can specifically inhibit the expression of SENP3, resulting in the weakening of Treg cell function and consequently improve the tumor immune response. Maj et al (129) revealed the relationship between ROS and immunosuppression by Treg cells in the TME of ovarian cancer. The results of this previous study showed that ROS in the TME may cause the apoptosis of Treg cells, where the apoptotic cells can subsequently release large quantities of adenosine triphosphate (ATP) (129). Although ATP is beneficial to body function under normal circumstances, early apoptotic Treg cells can rapidly convert ATP to adenosine by CD39 and CD73 (129). These adenosines are specific to T cells and can bind to their cell surface adenosine A2A receptors to inhibit T cell activation (129). In conclusion, ROS can serve an important role in the function of T cells, whereby high levels of ROS in T cells may confer anti-tumor effects, whereas ROS in Treg cells appear to be associated with immunosuppression.
ROS and natural killer (NK) cells
NK cells are a type of effector lymphocytes that play an important role in the anti-tumor process and are profoundly influenced by hypoxia and oxidative stress in the TME (130). Zheng et al (131) previously found that hypoxia in the TME led to an increase in the levels of ROS, which could continuously activate the mTOR/dynamin-related protein 1 pathway in NK cells, resulting in excessive mitochondrial fission. After mitochondrial fragmentation, the production of ROS was accelerated and a positive feedback loop was established (131). This process ultimately leaded to apoptosis and mitochondrial autophagy, which decreased the activity and tumor killing ability of NK cells (131). Therefore, the aberrant increase in ROS levels in the TME may be one of the mechanisms underlying the failure of NKs. Supporting this, reducing ROS levels in the TME or improving the tolerance of NK cells to ROS have been reported to prevent this failure (132–134).
ROS and antigen-presenting cells (APC)
ROS and dendritic cells (DCs)
DCs are professional antigen-presenting cells that play an important role in both innate and adaptive immunity. Immature DCs have strong migratory ability, whilst mature DCs can effectively activate initial T cells to initiate, regulate and maintain the immune response (135). The relationship between ROS and DCs is complex, which involve both metabolic and transcriptional changes (136). Previous studies have found that an increase in the environmental redox potential can hinder cross presentation (137,138). Excessive ROS can lead to the chronic activation of the endoplasmic reticulum stress response and oxidative damage to intracellular lipids, which inhibit the ability of DCs to present local antigens to T cells (137,138). These effects aforementioned can impede the development of an effective anti-tumor immune response. However, low levels of ROS can act as a key signaling component to promote the maturation of antigen-presenting cells through the activation of signaling pathways, including NF-κB, mTOR and ERK, in addition to the activation of intracellular Ca2+ channels (139). It has been demonstrated that ROS can promote cytoplasmic antigen transmission in DCs by lysosome escape and antigen protection, resulting in effective antigen cross presentation and strong CD8+ T cell responses (140,141).
ROS and macrophages
Macrophages are mainly derived from myeloprogenitor cells in the bone marrow and serve the innate immune system (142). Tumor-associated macrophages (TAMs) have been frequently observed to infiltrate the tumor tissue, which serve an ‘accomplice’ role in tumor development and metastasis (142). They can be divided into the M1 and M2 subtypes, which are thought to inhibit and promote cancer progression, respectively (142). Reprogramming or repolarization of TAMs to an anti-tumor phenotype may be an effective method for enhancing the efficacy of immunotherapy (143). Previous studies have shown that continuously increasing the levels of ROS in the TME can contribute to the differentiation of TAMs into the M2 subtype (144,145). TAMs that were isolated from melanoma after high ROS treatment appeared to show a more aggressive phenotype, which may be associated with the secretion of ROS-dependent TNF-α in mouse melanoma B16F1 and B16F10 cell lines (146). Griess et al (147) found that ROS elimination can selectively inhibit the polarization and tumor-promoting function of M2 macrophages through the STAT3 signaling pathway. In addition, it was found that M2 TAMs can express PD-1, but ROS clearance can polarize the TAM balance towards the M1 phenotype and reduced the expression of PD-L1 (148). However, the effects of ROS on TAM differentiation and regulation of the PD-1 immune checkpoint pathway are worthy of further study.
ROS and B cells
B cells are derived from bone marrow and are specialized antigen-presenting cells that can also mediate the humoral immune response by producing antibodies (149). A number of previous studies found a positive correlation between B lymphocyte infiltration and patient response to immunotherapy in various types of tumors, such as sarcoma, melanoma and renal cell carcinoma, which highlights the important role of B cells in anti-tumor immunity (150–152). A study has also found that increasing ROS levels in the microenvironment can promote the expression of HIF-1α, nuclear factor erythroid 2-related factor 2 (NRF2) and C-X-C chemokine receptor type 4, which in turn regulate the multiple stages of B cell development (153). Feng et al (154) previously found that B-cell receptor (BCR)-induced B cell activation also required ROS (154), similar to T cells. Mechanistically, ROS mediates the activation and proliferation of B cells by activating the NF-κB and PI3K signaling pathways (154). After treatment with N-acetylcysteine for 3 h, the proliferation of B cells was significantly inhibited (154). ROS has also been found to determine cell fate after B-cell activation. B cells treated with high levels of ROS can undergo class switch recombination, whereas low levels of ROS can induce differentiation into plasma cells (155). In addition, ROS can regulate apoptosis and autophagy in B cells (156,157). The p66SHC protein not only antagonized BCR survival signals and promoted apoptosis, but also prevented B cell survival through selective autophagy/mitochondrial autophagy, by increasing ROS production (156,157). In conclusion, ROS can be considered to be involved in multiple stages of B cell development, including activation, differentiation and death.
ROS and myeloid-derived suppressor cells (MDSCs)
MDSCs are heterogeneous cell groups that consist of myeloid progenitor cells and immature bone marrow cells (IMCs). They form an important part of the TME and possess potent immunosuppressive activity (158). ROS serve an important role in maintaining the undifferentiated state of MDSCs (159). In mice transplanted with colon cancer and sarcoma, scavenging of H2O2 induced the differentiation of immature myelocytes into macrophages (160). Of note, in the absence of NADPH oxidase (NOX) activity, MDSCs differentiated into macrophages and DCs (160). Therefore, endogenous oxidative stress may be a mechanism of MDSC inhibition to suppress its differentiation in tumors. MDSCs can act on other immune cells through ROS. It has been previously found that ROS produced by MDSCs can permanently inactivate T cells and destroy their ability to initiate the immune response (161). Inhibition of ROS in MDSCs can reverse immunosuppression and exert anti-tumor effects (161). In addition, not only the T cell response is a target of ROS-mediated MDSC inhibition. MDSCs can also inhibit the response of NK cells to adenovirus vectors and vaccinia virus infection by releasing ROS (162,163). Recent studies have shown that MDSCs can also negatively regulate B cell-mediated immune response through ROS (164,165). Lelis et al (166) found that MDSCs can inhibit B cell proliferation and antibody production by cell contact through argininase, nitric oxide and ROS. In addition to playing a role in MDSC-mediated immunosuppression, ROS has also been found to be intrinsically involved with the activation of transcription factors, including NRF2 and HIF-1α (167). This process induces the transcriptional and metabolic reprogramming of MDSCs, which affects their differentiation and maintenance (167). Therefore, in the TME, ROS act as inducers of oxidative stress and a medium of immune regulation, which is an important process in the formation of cancer cells (159).
ROS and ICD
Tumors maintain the microenvironment of immune suppression by displaying low immunogenicity and secreting immunosuppressive cytokines, including IL-10, TGF-β, and VEGF (168). ICD can be triggered by various treatments, such as chemotherapy, RT and photodynamic therapy (169). Various cell death-related molecules, such as damage-associated molecular patterns, are released to enhance the immunogenicity of tumor cells and the initial immune response, which is an innovative measure in immunotherapy (169). At present, the prevailing notion is that there is a positive association between ROS production and ICD induction in anti-tumor therapy (170). Excessive levels of ROS are frequently used for the oxidative killing of tumors and induction of ICD. These processes can provide potential antigen stimulation to the immune system. A nano-study based on sonodynamic therapy found that enhanced continuous ultrasonic-triggered inertial cavitation increased ROS production and induced strong ICD (107). This was characterized by increased antigen exposure and presentation, enhanced maturation of DCs and increased infiltration of active-effector CD8+ T cells (107). Li et al (171) previously explored the potential use of a fluorine-assembled nanocluster to reverse immunosuppression and reawaken the immune system. Following the production of sufficient ROS levels by fluorine assembly@ photodynamic immunotherapy for tumor (PMPt) to break ROS-sensitive connectors under laser irradiation, cisplatin-coupled PMPt is released to penetrate the tumor and kill Treg cells and MDSCs (171). Additionally, ROS can strongly induce ICD by increasing infiltration by DCs and T cells to turn a cold tumor into a hot tumor and stimulate an effective anti-tumor immune response (171). However, a number of studies have shown that the increase of ROS in the TME can markedly reduce ICD and the number of tumor-infiltrating T lymphocytes (172,173). In a previous study using a breast cancer model, Deng et al (172) found that the elimination of ROS from the TME using nano-scavengers could alleviate immunosuppressive ICD induced by oleandrin anticancer drug and prolong the survival time of T cells in breast cancer. Elimination of ROS also lead to an increase in anti-tumor immunity and T lymphocyte infiltration, resulting in a potent anti-tumor effect (172). It is hypothesized that these contradictory results may be related to the different levels of ROS. Therefore, further studies are warranted to confirm these findings aforementioned.
ROS and tumor metabolic recombination
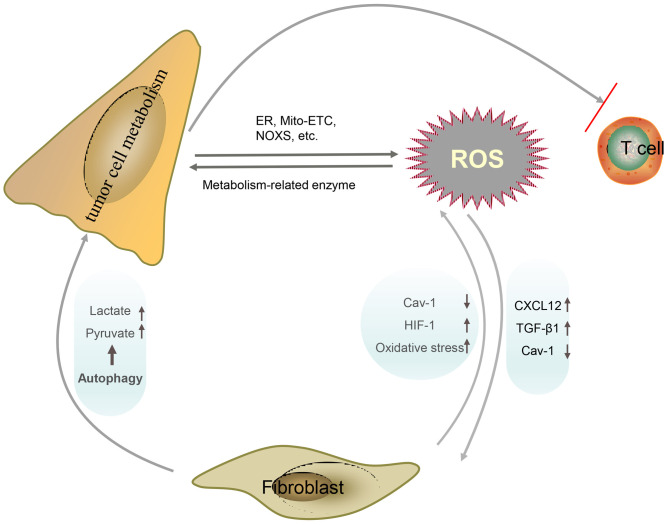
Glucose deficiency is a characteristic of the TME (174). For the maintenance of survival and rapid proliferation, tumor cells will undergo a series of metabolic reprogramming to improve their adaptability to nutritional deficiency, particularly to glucose (174). Metabolic reprogramming of tumor cells frequently leads to the excessive production of ROS and oxidative stress, but tumor cells can maintain ROS homeostasis and prevent ROS-mediated cell death by enhancing their antioxidant system (175). Low concentrations of ROS can promote tumor metabolism by altering the activity of key enzymes, including pyruvate kinase M2, GAPDH and α-ketoglutarate, inducing metabolism-related genomic changes and activating a number of signaling pathways (Fig. 3) (176). It has been found that overlapping with the m-AAA protease 1 homolog can promote the production of ROS, improve the stability of HIF-1α, and increase the expression of glucose transporters and glycolytic enzymes, including hexokinase 2 and lactate dehydrogenase (LDH) A, under hypoxic conditions (177). These effects lead to an increase in the glycolytic ability of colorectal cancer cells (177). To investigate the role of the non-classical glutamine pathway in the development of pancreatic cancer, Wang et al (178) found an imbalance in ROS in the occurrence and development of the disease. ROS inhibited arginine methylation enzyme coactivator associated arginine methyltransferase 1, which in turn inhibited the activity of malate dehydrogenase malate dehydrogenase 1. These observations suggest that ROS activated the non-classical glutamine metabolism to promote the growth of pancreatic cancer cells. ROS can also promote tumor metabolic reprogramming by activating NRF2, which increases the expression of NADPH-generating enzymes, such as glucose-6-phosphate dehydrogenase, isocitrate dehydrogenase 1 and malic enzyme 1 (ME1), and supports lung cancer growth by increasing NADPH and purine biosynthesis (179). In addition, ROS can also mediate the metabolic interaction between tumor cells and their microenvironment. As aforementioned, tumor cells can produce a large quantity of ROS through metabolism (86). This triggers the ‘reverse Warburg effect’ in CAFs to produce lactic acid, ketone and other high-energy raw materials to improve the metabolic plasticity of tumor (86). ROS can also reduce the expression of CAV1 and promote the transformation of myofibroblasts (87). In pancreatic, breast, lung and prostate cancer, it has been demonstrated that downregulation of CAV1 expression is associated with the overexpression of key metabolic enzymes, including pyruvate kinase isozymes M2 and LDH (180–182). In addition, the transport of lactic acid and ketone bodies, which are glycolytic products, can also be detected at the same time of CAV-1 downregulation (180–182). ROS can also mediate the interaction between tumor metabolism and the immune microenvironment. In a previous study with ovarian cancer (183), it was found that tumors can mediate effector T cell dysfunction by inhibiting the expression of the histone-lysine N-transmethylase 2 enhancer of zeste homolog 2 by glucose restriction. T cells isolated from malignant ascites in patients with ovarian cancer were found to activate the inositol requiring kinase 1α-X-box binding protein-1 endoplasmic reticulum stress response, which reduced glucose uptake and inhibited mitochondrial activity (184). This finding suggests that oxidative stress and glucose deprivation in the TME may contribute to lymphocyte dysfunction in human tumors.
Figure 3.
Regulation of ROS in tumor cell metabolism. Specifically, metabolic alterations in cancer cells cause the accumulation of ROS, which in turn acts on metabolic enzymes to promote the metabolic programming of cancer cells. The accumulation of ROS can also activate CAFs and promote autophagy, which can further increase the accumulation of ROS and provide raw materials for tumor metabolism. In addition, ROS are also involved in the inhibition of T cell function by tumor metabolism. CAFs, carcinoma associated fibroblasts; Cav-1, caveolin-1; CXCL12, chemokine (C-X-C motif) ligand 12; ER, endoplasmic reticulum; HIF-1, hypoxia inducible factor 1; Mito-ETC, mitochondrial electron transport chain; NOXS, NADPH oxidases; ROS, reactive oxygen species.
3. ROS and tumor drug resistance
The prognosis of cancer has markedly improved due to the advent of targeted therapy and immunotherapy (185). However, drug resistance remains to be a challenge for the treatment of cancer. The mechanisms of drug resistance include: i) Heterogeneity of tumor cells; ii) the TME, including hypoxia, abnormal angiogenesis, EMT, tumor metabolic recombination and the immunosuppressive microenvironment; iii) tumor stem cells; iv) mutation of the drug target gene or signaling compensation; v) detoxification mechanism; vi) pharmacological changes, such as drug inactivation, decreased drug absorption, enhanced drug metabolic activity and increased expression of drug efflux transporter; vii) reduction in the sensitivity of apoptosis; and 8) increase in the ability to repair DNA damage (185). Previous studies have confirmed that a high concentration of ROS is one of the characteristics of drug-resistance in cancer cells (186,187). ROS can promote drug resistance in tumors through a variety of mechanisms. As such, ROS can promote the formation of an immunosuppressive microenvironment and mediate resistance to immunotherapy by promoting the phenotypes of MDSCs, DCs and TAMs (106). ROS can also promote EMT, where cells undergoing EMT typically exhibit characteristics of cancer stem cells, with high rates of self-renewal and resistance to drugs and radiation (188). In addition, ROS can regulate the expression of multidrug resistance genes, such as the transmembrane drug efflux protein P-glycoprotein (P-gp), and ATP-dependent substrate transport on both mRNA and protein levels (186). Therefore, blocking ROS has been proposed to overcome resistance to chemotherapy. However, ROS has also been found to promote the sensitivity of tumor cells to drug treatment (11,12). The levels of ROS in tumors are generally higher than those observed in normal cells obtained from the same tissue source (24). Therefore, once the levels of ROS exceed the threshold through continuous accumulation, the cells will undergo apoptosis (35). RT and a number of chemotherapeutic drugs, including cisplatin, 5-fluorouracil and oxaliplatin can kill tumor cells by promoting the excessive accumulation of ROS. However, tumor cells can initiate the mechanism for the inhibition of the excessive accumulation of ROS, to develop drug resistance (189). Wang et al (190) found that inhibition of solute carrier family 7 member 11 using vorinostat, an inhibitor of histone deacetylase, can lead to an increase in ROS in drug-resistant melanoma cells to lethal levels, which lead to apoptosis only in drug-resistant cells. CSCs consist of a subpopulation of tumor cells that is resistant to chemotherapy and are characterized by high invasiveness and metastasis (191). Choi et al (192) previously demonstrated that CSCs can maintain low ROS levels by coupling forkhead box M1-dependent peroxiredoxin 3 expression and fatty acid oxidation-mediated NADPH regeneration, both of which are essential for maintaining the biological characteristics of CSCs. The accumulation of ROS in vivo and in vitro can render CSCs sensitive to RT and chemotherapy (193,194). In addition, the production of ROS, especially mitochondrial-derived ROS, is essential for the induction of apoptosis, autophagy and ICD of tumor cells. Therefore, ROS-based nanotechnology can increase the sensitivity of tumor cells to RT, chemotherapy, targeted therapy and immunotherapy (195–198). The relationship between ROS and drug-resistant tumors is highly complex, where various studies yielded conflicting findings. For example, P-gp was found to be overexpressed in MCF-7 cells after treatment with a low concentration of H2O2 (1 M) (199). However, a high concentration of H2O2 (10 M) downregulated the expression of P-gp in human myelogenous leukemia K562/DOX cells (200). These studies were conducted in different cell lines, such that some conclusions remained contradictory. Therefore, the relationship between ROS and multidrug resistance warrants further comprehensive investigation (Fig. 4).
Figure 4.
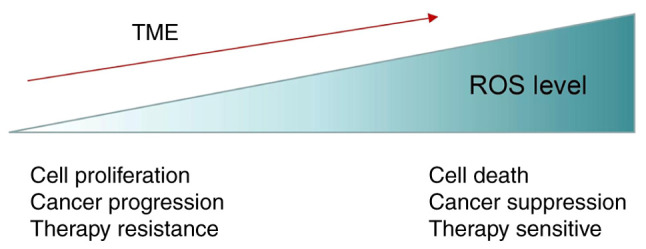
Different levels of ROS exert varied biological effects on tumors. Low levels of ROS may play a more tumor-promoting role, whereas lethal levels of ROS have the opposite effect on promoting the death of tumor cells and increasing their sensitivity to treatment. ROS, reactive oxygen species; TME, tumor microenvironment.
4. Conclusions
If a malignant tumor is known as a ‘seed’, the TME can be termed as the ‘soil’ that allows the ‘seed’ to grow. The TME serves a key role in several steps of tumor development, including local drug resistance, immune escape and distant metastasis (15,18,19). The combination of immune checkpoint inhibitors or cell therapy with microenvironment-targeted therapy is expected to improve the prognosis of patients with cancer in the future (201). Tumor cells are characterized by indefinite proliferative potential, which is frequently accompanied with local tissue hypoxia, abnormal angiogenesis and metabolic reprogramming (202). In addition, persistent endoplasmic reticulum stress appears to be a new feature of tumors, which allows tumor cells to adapt to carcinogenic and environmental challenges to coordinate different immunomodulatory mechanisms and promote tumor progression (203,204). ROS production caused by hypoxia, metabolic reprogramming and endoplasmic reticulum stress altogether serve an important role in the cross-dialogue between the tumor and the surrounding microenvironment. ROS plays a dual role in intracellular signal transduction and cell fate regulation in this process (Fig. 4), the levels of which in cancer cells are largely dependent on the antioxidant defense system (186). Therefore, increasing the levels ROS to accurately break the redox balance is the key prerequisite for the effective treatment of cancer. However, ROS can also promote tumor cell proliferation, vascular proliferation, CAF differentiation, immune escape and drug resistance. It has been suggested that there are different pools of ROS in cancer cells with differing functions (205). ROS derived from NADPH oxidase can promote the proliferation of small intestinal crypt cells, whereas ROS induced by p53-induced glycolysis and apoptosis regulator (TIGAR) deletion can exert the opposite effect (206). NADPH oxidase produces extracellular superoxides, while TIGAR protects against intracellular ROS damage by supporting the pentose phosphate pathway (206). The beneficial or harmful effects of ROS in cells are not necessarily mutually exclusive. Further studies on the different tumor cell types, ROS levels and even the level-effect relationship between ROS and tumor cells are required. Collectively, these findings indicate that the combination of ROS-based redox regulators with standard RT and chemotherapy or even immunotherapy may be of great significance in tumor therapy.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Authors contributions
YCS conceptualized the design of the present review. WL and XYH performed the literature search wrote the paper. JQB and TTH made several revisions of the text, making crucial contributions to the scientific analysis and discussion of the thesis presented in the review. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Nosaka Y, Nosaka AY. Generation and detection of reactive oxygen species in photocatalysis. Chem Rev. 2017;117:11302–11336. doi: 10.1021/acs.chemrev.7b00161. [DOI] [PubMed] [Google Scholar]
- 2.Kumari S, Badana AK, G MM GS, Malla RR. Reactive oxygen species: A key constituent in cancer survival. Biomark Insights. 2018;13:1177271918755391. doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- 5.Cruces-Sande A, Rodríguez-Pérez AI, Herbello-Hermelo P, Bermejo-Barrera P, Méndez-Álvarez E, Labandeira-García JL, Soto-Otero R. Copper increases brain oxidative stress and enhances the ability of 6-hydroxydopamine to cause dopaminergic degeneration in a rat model of parkinsons disease. Mol Neurobiol. 2019;56:2845–2854. doi: 10.1007/s12035-018-1274-7. [DOI] [PubMed] [Google Scholar]
- 6.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee R, Chatterjee J. ROS and oncogenesis with special reference to EMT and stemness. Eur J Cell Biol. 2020;99:151073. doi: 10.1016/j.ejcb.2020.151073. [DOI] [PubMed] [Google Scholar]
- 8.Okon IS, Zou MH. Mitochondrial ROS and cancer drug resistance: Implications for therapy. Pharmacol Res. 2015;100:170–174. doi: 10.1016/j.phrs.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parekh A, Das S, Parida S, Das CK, Dutta D, Mallick SK, Wu PH, Kumar BNP, Bharti R, Dey G, et al. Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo. Oncogene. 2018;37:4546–4561. doi: 10.1038/s41388-018-0272-6. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Guo D, Yin X, Ding S, Shen M, Zhang R, Wang Y, Xu R. Zinc oxide nanoparticles induce human multiple myeloma cell death via reactive oxygen species and Cyt-C/Apaf-1/Caspase-9/Caspase-3 signaling pathway in vitro. Biomed Pharmacother. 2020;122:109712. doi: 10.1016/j.biopha.2019.109712. [DOI] [PubMed] [Google Scholar]
- 12.Xia B, Wang J. Effects of adenosine on apoptosis of ovarian cancer a2780 cells via ROS and caspase pathways. Onco Targets Ther. 2019;12:9473–9480. doi: 10.2147/OTT.S216620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 14.Guo X, Cheng Y, Zhao X, Luo Y, Chen J, Yuan WE. Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnology. 2018;16:74. doi: 10.1186/s12951-018-0398-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng J, Gao P. Toward normalization of the tumor microenvironment for cancer therapy. Integr Cancer Ther. 2019;18:1534735419862352. doi: 10.1177/1534735419862352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 17.Langley RR, Fidler IJ. The seed and soil hypothesis revisited-the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akhtar M, Haider A, Rashid S, Al-Nabet ADMH. Pagets ‘seed and soil’ theory of cancer metastasis: An idea whose time has come. Adv Anat Patho. 2019;26:69–74. doi: 10.1097/PAP.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Li J, Li D, Wang Z, Zhao J, Wu X, Sun Q, Lin PP, Plum P, Damanakis A, et al. Tumor biology and multidisciplinary strategies of oligometastasis in gastrointestinal cancers. Semin Cancer Biol. 2020;60:334–343. doi: 10.1016/j.semcancer.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 20.Malla R, Surepalli N, Farran B, Malhotra SV, Nagaraju GP. Reactive oxygen species (ROS): Critical roles in breast tumor microenvironment. Crit Rev Oncol Hematol. 2021;160:103285. doi: 10.1016/j.critrevonc.2021.103285. [DOI] [PubMed] [Google Scholar]
- 21.Kuo CL, Chou HY, Chiu YC, Cheng AN, Fan CC, Chang YN, Chen CH, Jiang SS, Chen NJ, Lee AY. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020;474:138–150. doi: 10.1016/j.canlet.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 22.An J, Hu YG, Cheng K, Li C, Hou XL, Wang GL, Zhang XS, Liu B, Zhao YD, Zhang MZ. ROS-augmented and tumor-microenvironment responsive biodegradable nanoplatform for enhancing chemo-sonodynamic therapy. Biomaterials. 2020;234:119761. doi: 10.1016/j.biomaterials.2020.119761. [DOI] [PubMed] [Google Scholar]
- 23.Arfin S, Jha NK, Jha SK, Kesari KK, Ruokolainen J, Roychoudhury S, Rathi B, Kumar D. Oxidative stress in cancer cell metabolism. Antioxidants (Basel) 2021;10:642. doi: 10.3390/antiox10050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirzaei S, Hushmandi K, Zabolian A, Saleki H, Torabi SMR, Ranjbar A, SeyedSaleh S, Sharifzadeh SO, Khan H, Ashrafizadeh M, et al. Elucidating role of reactive oxygen species (ROS) in cisplatin chemotherapy: A focus on molecular pathways and possible therapeutic strategies. Molecules. 2021;26:2382. doi: 10.3390/molecules26082382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igney FH, Krammer PH. Death and anti-death: Tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 26.Saxena N, Yadav P, Kumar O. The Fas/Fas ligand apoptotic pathway is involved in abrin-induced apoptosis. Toxicol Sci. 2013;135:103–118. doi: 10.1093/toxsci/kft139. [DOI] [PubMed] [Google Scholar]
- 27.Jo E, Jang HJ, Yang KE, Jang MS, Huh YH, Yoo HS, Park JS, Jang IS, Park SJ. Cordyceps militaris induces apoptosis in ovarian cancer cells through TNF-α/TNFR1-mediated inhibition of NF-κB phosphorylation. BMC Complement Med Ther. 2020;20:1. doi: 10.1186/s12906-019-2780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang P, Wang H, Chen Y, Lodhi A, Sun C, Sun F, Yan L, Deng Y, Ma H. DR5 related autophagy can promote apoptosis in gliomas after irradiation. Biochem Biophys Res Commun. 2020;522:910–916. doi: 10.1016/j.bbrc.2019.11.161. [DOI] [PubMed] [Google Scholar]
- 29.Bergeron S, Beauchemin M, Bertrand R. Camptothecin- and etoposide-induced apoptosis in human leukemia cells is independent of cell death receptor-3 and −4 aggregation but accelerates tumor necrosis factor-related apoptosis-inducing ligand-mediated cell death. Mol Cancer Ther. 2004;3:1659–1669. [PubMed] [Google Scholar]
- 30.Brenner C, Cadiou H, Vieira HL, Zamzami N, Marzo I, Xie Z, Leber B, Andrews D, Duclohier H, Reed JC, Kroemer G. Bcl-2 and Bax regulate the channel activity of the mitochondrial adenine nucleotide translocator. Oncogene. 2000;19:329–336. doi: 10.1038/sj.onc.1203298. [DOI] [PubMed] [Google Scholar]
- 31.Sun KX, Xia HW. Pachymic acid inhibits growth and induces cell cycle arrest and apoptosis in gastric cancer SGC-7901 cells. Oncol Lett. 2018;16:2517–2524. doi: 10.3892/ol.2018.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque M, Islam M. Pleurotus mushroom induces apoptosis by altering the balance of proapoptotic and antiapoptotic genes in breast cancer cells and inhibits tumor sphere formation. Medicina (Kaunas) 2019;55:716. doi: 10.3390/medicina55110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, Cho IA, Kang KR, Lim H, Kim TH, Yu SK, Kim HJ, Lee SA, Moon SM, Chun HS, et al. Reversine induces caspase-dependent apoptosis of human osteosarcoma cells through extrinsic and intrinsic apoptotic signaling pathways. Genes Genomics. 2019;41:657–665. doi: 10.1007/s13258-019-00790-1. [DOI] [PubMed] [Google Scholar]
- 34.Kuranaga E. Beyond apoptosis: Caspase regulatory mechanisms and functions in vivo. Genes Cells. 2012;17:83–97. doi: 10.1111/j.1365-2443.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- 35.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 36.Lin S, Li Y, Zamyatnin AA, Jr, Werner J, Bazhin AV. Reactive oxygen species and colorectal cancer. J Cell Physiol. 2018;233:5119–5132. doi: 10.1002/jcp.26356. [DOI] [PubMed] [Google Scholar]
- 37.Lin B, Chen H, Liang D, Lin W, Qi X, Liu H, Deng X. Acidic pH and high-H2O2 dual tumor microenvironment-responsive nanocatalytic graphene oxide for cancer selective therapy and recognition. ACS Appl Mater Interfaces. 2019;11:11157–11166. doi: 10.1021/acsami.8b22487. [DOI] [PubMed] [Google Scholar]
- 38.Choi EJ, Jeon SM. NRF2-driven redox metabolism takes center stage in cancer metabolism from an outside-in perspective. Arch Pharm Res. 2020;43:321–336. doi: 10.1007/s12272-020-01224-3. [DOI] [PubMed] [Google Scholar]
- 39.Um HD. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: A review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget. 2016;7:5193–5203. doi: 10.18632/oncotarget.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.You L, Dong X, Ni B, Fu J, Yang C, Yin X, Leng X, Ni J. Triptolide induces apoptosis through fas death and mitochondrial pathways in HepaRG cell line. Front Pharmacol. 2018;9:813. doi: 10.3389/fphar.2018.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Q, Guo Y, Chen S, Fu D, Li Y, Li Z, Ni C. Irinotecan induces autophagy-dependent apoptosis and positively regulates ROS-related JNK- and p38-MAPK pathways in gastric cancer cells. Onco Targets Ther. 2020;13:2807–2817. doi: 10.2147/OTT.S240803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang YQ, Feng YY, Luo YH, Zhai YQ, Ju XY, Feng YC, Sheng YN, Wang JR, Yu CQ, Jin CH. Quinalizarin induces ROS-mediated apoptosis via the MAPK, STAT3 and NF-κB signaling pathways in human breast cancer cells. Mol Med Rep. 2019;20:4576–4586. doi: 10.3892/mmr.2019.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang KE, Park C, Kwon SJ, Kim YS, Park DS, Lee MK, Kim BR, Park SH, Yoon KH, Jeong ET, et al. Synergistic induction of apoptosis by sulindac and simvastatin in A549 human lung cancer cells via reactive oxygen species-dependent mitochondrial dysfunction. Int J Oncol. 2013;43:262–270. doi: 10.3892/ijo.2013.1933. [DOI] [PubMed] [Google Scholar]
- 44.Zhang T, He WH, Feng LL, Huang HG. Effect of doxorubicin-induced ovarian toxicity on mouse ovarian granulosa cells. Regul Toxicol Pharmacol. 2017;86:1–10. doi: 10.1016/j.yrtph.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Jiang W, Wang Q, Hang L, Wang Y, Wang Y. ROS-sensitive biomimetic nanocarriers modulate tumor hypoxia for synergistic photodynamic chemotherapy. Biomater Sci. 2019;7:3706–3716. doi: 10.1039/C9BM00634F. [DOI] [PubMed] [Google Scholar]
- 46.Lopes TZ, de Moraes FR, Tedesco AC, Arni RK, Rahal P, Calmon MF. Berberine associated photodynamic therapy promotes autophagy and apoptosis via ROS generation in renal carcinoma cells. Biomed Pharmacother. 2020;123:109794. doi: 10.1016/j.biopha.2019.109794. [DOI] [PubMed] [Google Scholar]
- 47.Mowers EE, Sharifi MN, Macleod KF. Functions of autophagy in the tumor microenvironment and cancer metastasis. FEBS J. 2018;285:1751–1766. doi: 10.1111/febs.14388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao L, Loveless J, Shay C, Teng Y. Targeting ROS-mediated crosstalk between autophagy and apoptosis in cancer. Adv Exp Med Biol. 2020;1260:1–12. doi: 10.1007/978-3-030-42667-5_1. [DOI] [PubMed] [Google Scholar]
- 49.Li L, Tan J, Miao Y, Lei P, Zhang Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell Mol Neurobiol. 2015;35:615–621. doi: 10.1007/s10571-015-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Z, Wang H, Fang S, Xu C. Roles of endoplasmic reticulum stress and autophagy on H2O2-induced oxidative stress injury in HepG2 cells. Mol Med Rep. 2018;18:4163–4174. doi: 10.3892/mmr.2018.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lien JC, Lin MW, Chang SJ, Lai KC, Huang AC, Yu FS, Chung JG. Tetrandrine induces programmed cell death in human oral cancer CAL 27 cells through the reactive oxygen species production and caspase-dependent pathways and associated with beclin-1-induced cell autophagy. Environ Toxicol. 2017;32:329–343. doi: 10.1002/tox.22238. [DOI] [PubMed] [Google Scholar]
- 52.Kim KY, Park KI, Kim SH, Yu SN, Park SG, Kim YW, Seo YK, Ma JY, Ahn SC. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int J Mol Sci. 2017;18:1088. doi: 10.3390/ijms18051088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei B, Huang Q, Huang S, Mai W, Zhong X. Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is dependent on reactive oxygen species (ROS) and NF-κB/p53 pathway. J Pharmacol Sci. 2016;131:77–83. doi: 10.1016/j.jphs.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signa. 2013;25:50–65. doi: 10.1016/j.cellsig.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 55.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 56.Schenk B, Fulda S. Reactive oxygen species regulate Smac mimetic/TNFα-induced necroptotic signaling and cell death. Oncogene. 2015;34:5796–5806. doi: 10.1038/onc.2015.35. [DOI] [PubMed] [Google Scholar]
- 57.Li Y, Gong P, Kong C, Tian X. Bufalin engages in RIP1-dependent and ROS-dependent programmed necroptosis in breast cancer cells by targeting the RIP1/RIP3/PGAM5 pathway. Anticancer Drugs. 2019;30:e0770. doi: 10.1097/CAD.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Su SS, Zhao S, Yang Z, Zhong CQ, Chen X, Cai Q, Yang Z, Huang D, Wu R, Han J. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat Commun. 2017;8:14329. doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pawlikowska M, Piotrowski J, Jędrzejewski T, Kozak W, Slominski AT, Brożyna AA. Coriolus versicolor-derived protein-bound polysaccharides trigger the caspase-independent cell death pathway in amelanotic but not melanotic melanoma cells. Phytother Res. 2020;34:173–183. doi: 10.1002/ptr.6513. [DOI] [PubMed] [Google Scholar]
- 60.Yang Z, Wang Y, Zhang Y, He X, Zhong CQ, Ni H, Chen X, Liang Y, Wu J, Zhao S, et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat Cell Biol. 2018;20:186–197. doi: 10.1038/s41556-017-0022-y. [DOI] [PubMed] [Google Scholar]
- 61.Tu HC, Ren D, Wang GX, Chen DY, Westergard TD, Kim H, Sasagawa S, Hsieh JJ, Cheng EH. The p53-cathepsin axis cooperates with ROS to activate programmed necrotic death upon DNA damage. Proc Natl Acad Sci USA. 2009;106:1093–1098. doi: 10.1073/pnas.0808173106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ying Y, Padanilam BJ. Regulation of necrotic cell death: p53, PARP1 and cyclophilin D-overlapping pathways of regulated necrosis? Cell Mol Life Sci. 2016;73:2309–2324. doi: 10.1007/s00018-016-2202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng Q, Hou W. Regulation of angiogenesis by microRNAs in cancer. Mol Med Rep. 2021;24:583. doi: 10.3892/mmr.2021.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu B, Cui LS, Zhou B, Zhang LL, Liu ZH, Zhang L. Monocarbonyl curcumin analog A2 potently inhibits angiogenesis by inducing ROS-dependent endothelial cell death. Acta Pharmacol Sin. 2019;40:1412–1423. doi: 10.1038/s41401-019-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson EC, Grant ZL, Coultas L. Endothelial cell apoptosis in angiogenesis and vessel regression. Cell Mol Life Sci. 2017;74:4387–4403. doi: 10.1007/s00018-017-2577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakamaki K. Regulation of endothelial cell death and its role in angiogenesis and vascular regression. Curr Neurovasc Res. 2004;1:305–315. doi: 10.2174/1567202043362072. [DOI] [PubMed] [Google Scholar]
- 68.Miao Y, Cui L, Chen Z, Zhang L. Gene expression profiling of DMU-212-induced apoptosis and anti-angiogenesis in vascular endothelial cells. Pharm Biol. 2016;54:660–666. doi: 10.3109/13880209.2015.1071414. [DOI] [PubMed] [Google Scholar]
- 69.Li GH, Lin XL, Zhang H, Li S, He XL, Zhang K, Peng J, Tang YL, Zeng JF, Zhao Y, et al. Ox-Lp(a) transiently induces HUVEC autophagy via an ROS-dependent PAPR-1-LKB1-AMPK-mTOR pathway. Atherosclerosis. 2015;243:223–235. doi: 10.1016/j.atherosclerosis.2015.09.020. Corrigendum in: Atherosclerosis 250: 189, 2016. [DOI] [PubMed] [Google Scholar]
- 70.Topalovski M, Hagopian M, Wang M, Brekken RA. Hypoxia and transforming growth factor β cooperate to induce fibulin-5 expression in pancreatic cancer. J Biol Chem. 2016;291:22244–22252. doi: 10.1074/jbc.M116.730945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeferino RC, Mota NSRS, Grinevicius VMAS, Filipe KB, Sulis PM, Silva FRMB, Filho DW, Pich CT, Pedrosa RC. Targeting ROS overgeneration by N-benzyl-2-nitro-1-imidazole-acetamide as a potential therapeutic reposition approach for cancer therapy. Invest New Drugs. 2020;38:785–799. doi: 10.1007/s10637-019-00820-5. [DOI] [PubMed] [Google Scholar]
- 72.Duraipandy N, Dharunya G, Lakra R, Korapatti PS, Syamala Kiran M. Fabrication of plumbagin on silver nanoframework for tunable redox modulation: Implications for therapeutic angiogenesis. J Cell Physiol. 2019;234:13110–13127. doi: 10.1002/jcp.27981. [DOI] [PubMed] [Google Scholar]
- 73.Cao J, Liu X, Yang Y, Wei B, Li Q, Mao G, He Y, Li Y, Zheng L, Zhang Q, et al. Decylubiquinone suppresses breast cancer growth and metastasis by inhibiting angiogenesis via the ROS/p53/ BAI1 signaling pathway. Angiogenesis. 2020;23:325–338. doi: 10.1007/s10456-020-09707-z. [DOI] [PubMed] [Google Scholar]
- 74.Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146:895–905. doi: 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liao Z, Tan ZW, Zhu P, Tan NS. Cancer-associated fibroblasts in tumor microenvironment-Accomplices in tumor malignancy. Cell Immunol. 2019;343:103729. doi: 10.1016/j.cellimm.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Pereira BA, Vennin C, Papanicolaou M, Chambers CR, Herrmann D, Morton JP, Cox TR, Timpson P. CAF Subpopulations: A new reservoir of stromal targets in pancreatic cancer. Trends Cancer. 2019;5:724–741. doi: 10.1016/j.trecan.2019.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Kim BG, Sung JS, Jang Y, Cha YJ, Kang S, Han HH, Lee JH, Cho NH. Compression-induced expression of glycolysis genes in CAFs correlates with EMT and angiogenesis gene expression in breast cancer. Commun Biol. 2019;2:313. doi: 10.1038/s42003-019-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eiro N, González L, Martínez-Ordoñez A, Fernandez-Garcia B, González LO, Cid S, Dominguez F, Perez-Fernandez R, Vizoso FJ. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell Oncol (Dordr) 2018;41:369–378. doi: 10.1007/s13402-018-0371-y. [DOI] [PubMed] [Google Scholar]
- 79.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, Chikamatsu K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8:8633–8647. doi: 10.18632/oncotarget.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yan Y, Chen X, Wang X, Zhao Z, Hu W, Zeng S, Wei J, Yang X, Qian L, Zhou S, et al. The effects and the mechanisms of autophagy on the cancer-associated fibroblasts in cancer. J Exp Clin Cancer Res. 2019;38:171. doi: 10.1186/s13046-019-1172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang X, Schönrogge M, Eichberg J, Wendt EHU, Kumstel S, Stenzel J, Lindner T, Jaster R, Krause B, Vollmar B, Zechner D. Blocking autophagy in cancer-associated fibroblasts supports chemotherapy of pancreatic cancer cells. Front Oncol. 2018;8:590. doi: 10.3389/fonc.2018.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Attieh Y, Vignjevic D. The hallmarks of CAFs in cancer invasion. Eur J Cell Biol. 2016;95:493–502. doi: 10.1016/j.ejcb.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Yao Q, Qu X, Yang Q, Wei M, Kong B. CLIC4 mediates TGF-beta1-induced fibroblast-to-myofibroblast transdifferentiation in ovarian cancer. Oncol Rep. 2009;22:541–548. doi: 10.3892/or_00000469. [DOI] [PubMed] [Google Scholar]
- 84.Sampson N, Brunner E, Weber A, Puhr M, Schäfer G, Szyndralewiez C, Klocker H. Inhibition of Nox4-dependent ROS signaling attenuates prostate fibroblast activation and abrogates stromal-mediated protumorigenic interactions. Int J Cancer. 2018;143:383–395. doi: 10.1002/ijc.31316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Lisanti MP, Martinez-Outschoorn UE, Chiavarina B, Pavlides S, Whitaker-Menezes D, Tsirigos A, Witkiewicz A, Lin Z, Balliet R, Howell A, Sotgia F. Understanding the ‘lethal’ drivers of tumor-stroma co-evolution: Emerging role(s) for hypoxia, oxidative stress and autophagy/mitophagy in the tumor micro-environment. Cancer Biol Ther. 2010;10:537–542. doi: 10.4161/cbt.10.6.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: Role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle. 2010;9:3515–3533. doi: 10.4161/cc.9.17.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bernard M, Yang B, Migneault F, Turgeon J, Dieudé M, Olivier MA, Cardin GB, El-Diwany M, Underwood K, Rodier F, Hébert MJ. Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy. 2020;16:2004–2016. doi: 10.1080/15548627.2020.1713640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Urbano AM. Otto Warburg: The journey towards the seminal discovery of tumor cell bioenergetic reprogramming. Biochim Biophys Acta Mol Basis Dis. 2021;1867:165965. doi: 10.1016/j.bbadis.2020.165965. [DOI] [PubMed] [Google Scholar]
- 91.Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou X, Zhang J, Zhao S, Zhou BP, Mi J. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep. 2015;10:1335–1348. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Outschoorn UE, Goldberg A, Lin Z, Ko YH, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol Ther. 2011;12:924–938. doi: 10.4161/cbt.12.10.17780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Feng X, Xu W, Li Z, Song W, Ding J, Chen X. Immunomodulatory nanosystems. Adv Sci (Weinh) 2019;6:1900101. doi: 10.1002/advs.201900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hamieh M, Dobrin A, Cabriolu A, van der Stegen SJC, Giavridis T, Mansilla-Soto J, Eyquem J, Zhao Z, Whitlock BM, Miele MM, et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568:112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strickler JH, Hanks BA, Khasraw M. Tumor mutational burden as a predictor of immunotherapy response: Is more always better? Clin Cancer Res. 2021;27:1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carreau N, Pavlick A. Revolutionizing treatment of advanced melanoma with immunotherapy. Surg Oncol. 2019 Jan 12; doi: 10.1016/j.suronc.2019.01.002. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 97.Boyero L, Sánchez-Gastaldo A, Alonso M, Noguera-Uclés JF, Molina-Pinelo S, Bernabé-Caro R. Primary and acquired resistance to immunotherapy in lung cancer: Unveiling the mechanisms underlying of immune checkpoint blockade therapy. Cancers (Basel) 2020;12:3729. doi: 10.3390/cancers12123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anichini A, Perotti VE, Sgambelluri F, Mortarini R. Immune escape mechanisms in non small cell lung cancer. Cancers (Basel) 2020;12:3605. doi: 10.3390/cancers12123605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marshall LA, Marubayashi S, Jorapur A, Jacobson S, Zibinsky M, Robles O, Hu DX, Jackson JJ, Pookot D, Sanchez J, et al. Tumors establish resistance to immunotherapy by regulating Treg recruitment via CCR4. J Immunother Cancer. 2020;8:e000764. doi: 10.1136/jitc-2020-000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mima K, Kosumi K, Baba Y, Hamada T, Baba H, Ogino S. The microbiome, genetics, and gastrointestinal neoplasms: The evolving field of molecular pathological epidemiology to analyze the tumor-immune-microbiome interaction. Hum Genet. 2021;140:725–746. doi: 10.1007/s00439-020-02235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ali AMR, Tsai JW, Leung CH, Lin H, Ravi V, Conley AP, Lazar AJ, Wang WL, Nathenson MJ. The immune microenvironment of uterine adenosarcomas. Clin Sarcoma Res. 2020;10:5. doi: 10.1186/s13569-020-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosmaczewska A, Ciszak L, Potoczek S, Frydecka I. The significance of Treg cells in defective tumor immunity. Arch Immunol Ther Exp (Warsz) 2008;56:181–191. doi: 10.1007/s00005-008-0018-1. [DOI] [PubMed] [Google Scholar]
- 103.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Augustin RC, Delgoffe GM, Najjar YG. Characteristics of the tumor microenvironment that influence immune cell functions: Hypoxia, oxidative stress, metabolic alterations. Cancers (Basel) 2020;12:3802. doi: 10.3390/cancers12123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lötscher J, Balmer ML. Sensing between reactions-how the metabolic microenvironment shapes immunity. Clin Exp Immunol. 2019;197:161–169. doi: 10.1111/cei.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kotsafti A, Scarpa M, Castagliuolo I, Scarpa M. Reactive oxygen species and antitumor immunity-from surveillance to evasion. Cancers (Basel) 2020;12:1748. doi: 10.3390/cancers12071748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yin Y, Jiang X, Sun L, Li H, Su C, Zhang Y, Xu G, Li X, Zhao C, Chen Y, Xu H, Zhang K. Continuous inertial cavitation evokes massive ROS for reinforcing sonodynamic therapy and immunogenic cell death against breast carcinoma. Nano Today. 2021;36:101009. doi: 10.1016/j.nantod.2020.101009. [DOI] [Google Scholar]
- 108.Yang J, Ma S, Xu R, Wei Y, Zhang J, Zuo T, Wang Z, Deng H, Yang N, Shen Q. Smart biomimetic metal organic frameworks based on ROS-ferroptosis-glycolysis regulation for enhanced tumor chemo-immunotherapy. J Control Release. 2021;334:21–33. doi: 10.1016/j.jconrel.2021.04.013. [DOI] [PubMed] [Google Scholar]
- 109.Nakamura Y, Zhenjie Z, Oya K, Tanaka R, Ishitsuka Y, Okiyama N, Watanabe R, Fujisawa Y. Poor lymphocyte infiltration to primary tumors in acral lentiginous melanoma and mucosal melanoma compared to cutaneous melanoma. Front Oncol. 2020;10:524700. doi: 10.3389/fonc.2020.524700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murphy MP, Siegel RM. Mitochondrial ROS fire up T cell activation. Immunity. 2013;38:201–202. doi: 10.1016/j.immuni.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 111.Kaminski MM, Sauer SW, Klemke CD, Süss D, Okun JG, Krammer PH, Gülow K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: Mechanism of ciprofloxacin-mediated immunosuppression. J Immunol. 2010;184:4827–4841. doi: 10.4049/jimmunol.0901662. [DOI] [PubMed] [Google Scholar]
- 112.Li Y, Liang R, Zhang X, Wang J, Shan C, Liu S, Li L, Zhang S. Copper chaperone for superoxide dismutase promotes breast cancer cell proliferation and migration ROS-Mediated MAPK/ERK signaling. Front Pharmacol. 2019;10:356. doi: 10.3389/fphar.2019.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ball JA, Vlisidou I, Blunt MD, Wood W, Ward SG. Hydrogen peroxide triggers a dual signaling axis to selectively suppress activated human T lymphocyte migration. J Immunol. 2017;198:3679–3689. doi: 10.4049/jimmunol.1600868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang L, Kuang Z, Zhang D, Gao Y, Ying M, Wang T. Reactive oxygen species in immune cells: A new antitumor target. Biomed Pharmacother. 2021;133:110978. doi: 10.1016/j.biopha.2020.110978. [DOI] [PubMed] [Google Scholar]
- 115.Belikov AV, Schraven B, Simeoni L. T cells and reactive oxygen species. J Biomed Sci. 2015;22:85. doi: 10.1186/s12929-015-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, Peralta R, Wang Y, Wang Y, DePeaux K, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22:205–215. doi: 10.1038/s41590-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Franco F, Jaccard A, Romero P, Yu YR, Ho PC. Metabolic and epigenetic regulation of T-cell exhaustion. Nat Metab. 2020;2:1001–1012. doi: 10.1038/s42255-020-00280-9. [DOI] [PubMed] [Google Scholar]
- 118.Salas-Benito D, Conde E, Tamayo-Uria I, Mancheño U, Elizalde E, Garcia-Ros D, Aramendia JM, Muruzabal JC, Alcaide J, Guillen-Grima F, et al. The mutational load and a T-cell inflamed tumour phenotype identify ovarian cancer patients rendering tumour-reactive T cells from PD-1+tumour-infiltrating lymphocytes. Br J Cancer. 2021;124:1138–1149. doi: 10.1038/s41416-020-01218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumar A, Chamoto K, Chowdhury PS, Honjo T. Tumors attenuating the mitochondrial activity in T cells escape from PD-1 blockade therapy. Elife. 2020;9:e52330. doi: 10.7554/eLife.52330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chamoto K, Chowdhury PS, Kumar A, Sonomura K, Matsuda F, Fagarasan S, Honjo T. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc Natl Acad Sci USA. 2017;114:E761–E770. doi: 10.1073/pnas.1620433114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia Y, Jia C, Xue Q, Jiang J, Xie Y, Wang R, Ran Z, Xu F, Zhang Y, Ye T. Antipsychotic drug trifluoperazine suppresses colorectal cancer by inducing G0/G1 arrest and apoptosis. Front Pharmacol. 2019;10:1029. doi: 10.3389/fphar.2019.01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bailly C. Regulation of PD-L1 expression on cancer cells with ROS-modulating drugs. Life Sci. 2020;246:117403. doi: 10.1016/j.lfs.2020.117403. [DOI] [PubMed] [Google Scholar]
- 123.Liu K, Du S, Gao P, Zheng J. Verteporfin suppresses the proliferation, epithelial-mesenchymal transition and stemness of head and neck squamous carcinoma cells via inhibiting YAP1. J Cancer. 2019;10:4196–4207. doi: 10.7150/jca.34145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marangoni F, Zhakyp A, Corsini M, Geels SN, Carrizosa E, Thelen M, Mani V, Prüßmann JN, Warner RD, Ozga AJ, et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell. 2021 Jun 21; doi: 10.1016/j.cell.2021.05.027. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ni D, Tang T, Lu Y, Xu K, Shao Y, Saaoud F, Saredy J, Liu L, Drummer C, 4th, Sun Y, et al. Canonical secretomes, innate immune caspase-1-, 4/11-gasdermin D non-canonical secretomes and exosomes may contribute to maintain treg-ness for treg immunosuppression, tissue repair and modulate anti-tumor immunity via ROS pathways. Front Immunol. 2021;12:678201. doi: 10.3389/fimmu.2021.678201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, Ha S, Nelson BN, Kelly SP, Wu L, et al. Bile acid metabolites control T(H)17 and T(reg) cell differentiation. Nature. 2019;576:143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kunisada Y, Eikawa S, Tomonobu N, Domae S, Uehara T, Hori S, Furusawa Y, Hase K, Sasaki A, Udono H. Attenuation of CD4 + CD25 + regulatory T cells in the tumor microenvironment by metformin, a type 2 diabetes drug. EBioMedicine. 2017;25:154–164. doi: 10.1016/j.ebiom.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yu X, Lao Y, Teng XL, Li S, Zhou Y, Wang F, Guo X, Deng S, Chang Y, Wu X, et al. SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat Commun. 2018;9:3157. doi: 10.1038/s41467-018-05676-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–1341. doi: 10.1038/ni.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Betten A, Dahlgren C, Mellqvist UH, Hermodsson S, Hellstrand K. Oxygen radical-induced natural killer cell dysfunction: Role of myeloperoxidase and regulation by serotonin. J Leukoc Biol. 2004;75:1111–1115. doi: 10.1189/jlb.1103595. [DOI] [PubMed] [Google Scholar]
- 131.Zheng X, Qian Y, Fu B, Jiao D, Jiang Y, Chen P, Shen Y, Zhang H, Sun R, Tian Z, Wei H. Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol. 2019;20:1656–1667. doi: 10.1038/s41590-019-0511-1. [DOI] [PubMed] [Google Scholar]
- 132.Mimura K, Kua LF, Shimasaki N, Shiraishi K, Nakajima S, Siang LK, Shabbir A, So J, Yong WP, Kono K. Upregulation of thioredoxin-1 in activated human NK cells confers increased tolerance to oxidative stress. Cancer Immunol Immunother. 2017;66:605–613. doi: 10.1007/s00262-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aydin E, Johansson J, Nazir FH, Hellstrand K, Martner A. Role of NOX2-derived reactive oxygen species in NK cell-mediated control of murine melanoma metastasis. Cancer Immunol Res. 2017;5:804–811. doi: 10.1158/2326-6066.CIR-16-0382. [DOI] [PubMed] [Google Scholar]
- 134.Aurelius J, Martner A, Riise RE, Romero AI, Palmqvist L, Brune M, Hellstrand K, Thorén FB. Chronic myeloid leukemic cells trigger poly(ADP-ribose) polymerase-dependent inactivation and cell death in lymphocytes. J Leukoc Biol. 2013;93:155–160. doi: 10.1189/jlb.0512257. [DOI] [PubMed] [Google Scholar]
- 135.Gu FF, Zhang K, Ma LL, Liu YY, Li C, Hu Y, Yang QF, Liang JY, Zeng YL, Wang Y, Liu L. The superior ability of human BDCA3 + (CD141 +) dendritic cells (DCs) to cross-present antigens derived from necrotic lung cancer cells. Front Immunol. 2020;11:1267. doi: 10.3389/fimmu.2020.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paardekooper LM, Vos W, van den Bogaart G. Oxygen in the tumor microenvironment: Effects on dendritic cell function. Oncotarget. 2019;10:883–896. doi: 10.18632/oncotarget.26608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Giovanelli P, Sandoval TA, Cubillos-Ruiz JR. Dendritic cell metabolism and function in tumors. Trends Immunol. 2019;40:699–718. doi: 10.1016/j.it.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 138.Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, Janssen EM. Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. J Immunol. 2015;195:2624–2632. doi: 10.4049/jimmunol.1501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mao D, Hu F, Yi Z, Kenry, Xu S, Yan S, Luo Z, Wu W, Wang Z, Kong D, et al. AIEgen-coupled upconversion nanoparticles eradicate solid tumors through dual-mode ROS activation. Sci Adv. 2020;6:eabb2712. doi: 10.1126/sciadv.abb2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang C, Li P, Liu L, Pan H, Li H, Cai L, Ma Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials. 2016;79:88–100. doi: 10.1016/j.biomaterials.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 141.Oberkampf M, Guillerey C, Mouriès J, Rosenbaum P, Fayolle C, Bobard A, Savina A, Ogier-Denis E, Enninga J, Amigorena S, et al. Mitochondrial reactive oxygen species regulate the induction of CD8 T cells by plasmacytoid dendritic cells. Nature Commun. 2018;9:2241. doi: 10.1038/s41467-018-04686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.DeNardo D, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi: 10.1038/s41577-019-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allavena P, Anfray C, Ummarino A, Andón FT. Therapeutic manipulation of tumor-associated macrophages: Facts and hopes from a clinical and translational perspective. Clin Cancer Res. 2021;27:3291–3297. doi: 10.1158/1078-0432.CCR-20-1679. [DOI] [PubMed] [Google Scholar]
- 144.Zhang J, Li H, Wu Q, Chen Y, Deng Y, Yang Z, Zhang L, Liu B. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. 2019;22:101116. doi: 10.1016/j.redox.2019.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li L, Sun F, Han L, Liu X, Xiao Y, Gregory AD, Shapiro SD, Xiao G, Qu Z. PDLIM2 repression by ROS in alveolar macrophages promotes lung tumorigenesis. JCI Insight. 2021;6:e144394. doi: 10.1172/jci.insight.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin X, Zheng W, Liu J, Zhang Y, Qin H, Wu H, Xue B, Lu Y, Shen P. Oxidative stress in malignant melanoma enhances tumor necrosis factor-α secretion of tumor-associated macrophages that promote cancer cell invasion. Antioxid Redox Signal. 2013;19:1337–1355. doi: 10.1089/ars.2012.4617. [DOI] [PubMed] [Google Scholar]
- 147.Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med. 2020;147:48–60. doi: 10.1016/j.freeradbiomed.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ruan J, Ouyang M, Zhang W, Luo Y, Zhou D. The effect of PD-1 expression on tumor-associated macrophage in T cell lymphoma. Clin Transl Oncol. 2021;23:1134–1141. doi: 10.1007/s12094-020-02499-0. [DOI] [PubMed] [Google Scholar]
- 149.Wei Y, Huang CX, Xiao X, Chen DP, Shan H, He H, Kuang DM. B cell heterogeneity, plasticity, and functional diversity in cancer microenvironments. Oncogene. 2021 Jun 29; doi: 10.1038/s41388-021-01918-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 150.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 152.Petitprez F, de Reyniès A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 153.Jang JW, Thuy PX, Lee JW, Moon EY. CXCR4 promotes B cell viability by the cooperation of nuclear factor (erythroid-derived 2)-like 2 and hypoxia-inducible factor-1α under hypoxic conditions. Cell Death Dis. 2021;12:330. doi: 10.1038/s41419-021-03615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Feng YY, Tang M, Suzuki M, Gunasekara C, Anbe Y, Hiraoka Y, Liu J, Grasberger H, Ohkita M, Matsumura Y, et al. Essential role of NADPH oxidase-dependent production of reactive oxygen species in maintenance of sustained B Cell receptor signaling and b cell proliferation. J Immunol. 2019;202:2546–2557. doi: 10.4049/jimmunol.1800443. [DOI] [PubMed] [Google Scholar]
- 155.Jang KJ, Mano H, Aoki K, Hayashi T, Muto A, Nambu Y, Takahashi K, Itoh K, Taketani S, Nutt SL, et al. Mitochondrial function provides instructive signals for activation-induced B-cell fates. Nat Commun. 2015;6:6750. doi: 10.1038/ncomms7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Onnis A, Cianfanelli V, Cassioli C, Samardzic D, Pelicci PG, Cecconi F, Baldari CT. The pro-oxidant adaptor p66SHC promotes B cell mitophagy by disrupting mitochondrial integrity and recruiting LC3-II. Autophagy. 2018;14:2117–2138. doi: 10.1080/15548627.2018.1505153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Onnis A, Cassioli C, Finetti F, Baldari CT. Regulation of selective B cell autophagy by the pro-oxidant adaptor p66SHC. Front Cell Dev Biol. 2020;8:193. doi: 10.3389/fcell.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yin K, Xia X, Rui K, Wang T, Wang S. Myeloid-derived suppressor cells: A new and pivotal player in colorectal cancer progression. Front Oncol. 2020;10:610104. doi: 10.3389/fonc.2020.610104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ohl K, Tenbrock K. Reactive oxygen species as regulators of MDSC-mediated immune suppression. Front Immunol. 2018;9:2499. doi: 10.3389/fimmu.2018.02499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J Leukoc Biol. 2003;74:186–196. doi: 10.1189/jlb.0103010. [DOI] [PubMed] [Google Scholar]
- 161.Park MJ, Lee SH, Kim EK, Lee EJ, Baek JA, Park SH, Kwok SK, Cho ML. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci Rep. 2018;8:3753. doi: 10.1038/s41598-018-21856-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fortin C, Yang Y, Huang X. Monocytic myeloid-derived suppressor cells regulate T-cell responses against vaccinia virus. Eur J Immunol. 2017;47:1022–1031. doi: 10.1002/eji.201646797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu J, Huang X, Yang Y. Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer. J Virol. 2012;86:13689–13696. doi: 10.1128/JVI.01595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dong G, Yang Y, Li X, Yao X, Zhu Y, Zhang H, Wang H, Ma Q, Zhang J, Shi H, et al. Granulocytic myeloid-derived suppressor cells contribute to IFN-I signaling activation of B cells and disease progression through the lncRNA NEAT1-BAFF axis in systemic lupus erythematosus. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165554. doi: 10.1016/j.bbadis.2019.165554. [DOI] [PubMed] [Google Scholar]
- 165.Jaufmann J, Lelis FJN, Teschner AC, Fromm K, Rieber N, Hartl D, Beer-Hammer S. Human monocytic myeloid-derived suppressor cells impair B-cell phenotype and function in vitro. Eur J Immunol. 2020;50:33–47. doi: 10.1002/eji.201948240. [DOI] [PubMed] [Google Scholar]
- 166.Lelis FJN, Jaufmann J, Singh A, Fromm K, Teschner AC, Pöschel S, Schäfer I, Beer-Hammer S, Rieber N, Hartl D. Myeloid-derived suppressor cells modulate B-cell responses. Immunol Lett. 2017;188:108–115. doi: 10.1016/j.imlet.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 167.Satoh H, Moriguchi T, Taguchi K, Takai J, Maher JM, Suzuki T, Winnard PT, Jr, Raman V, Ebina M, Nukiwa T, Yamamoto M. Nrf2-deficiency creates a responsive microenvironment for metastasis to the lung. Carcinogenesis. 2010;31:1833–1843. doi: 10.1093/carcin/bgq105. [DOI] [PubMed] [Google Scholar]
- 168.Saleh R, Elkord E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin Cancer Biol. 2020;65:13–27. doi: 10.1016/j.semcancer.2019.07.017. [DOI] [PubMed] [Google Scholar]
- 169.Hernández ÁP, Juanes-Velasco P, Landeira-Viñuela A, Bareke H, Montalvillo E, Góngora R, Fuentes M. Restoring the immunity in the tumor microenvironment: Insights into immunogenic cell death in onco-therapies. Cancers (Basel) 2021;13:2821. doi: 10.3390/cancers13112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 171.Li Z, Zhu L, Sun H, Shen Y, Hu D, Wu W, Wang Y, Qian C, Sun M. Fluorine assembly nanocluster breaks the shackles of immunosuppression to turn the cold tumor hot. Proc Natl Acad Sci USA. 2020;117:32962–32969. doi: 10.1073/pnas.2011297117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Deng H, Yang W, Zhou Z, Tian R, Lin L, Ma Y, Song J, Chen X. Targeted scavenging of extracellular ROS relieves suppressive immunogenic cell death. Nat Commun. 2020;11:4951. doi: 10.1038/s41467-020-18745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Garg AD, Dudek AM, Ferreira GB, Verfaillie T, Vandenabeele P, Krysko DV, Mathieu C, Agostinis P. ROS-induced autophagy in cancer cells assists in evasion from determinants of immunogenic cell death. Autophagy. 2013;9:1292–1307. doi: 10.4161/auto.25399. [DOI] [PubMed] [Google Scholar]
- 174.Buono R, Longo VD. Starvation, stress resistance, and cancer. Trends Endocrinol Metab. 2018;29:271–280. doi: 10.1016/j.tem.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li XX, Wang ZJ, Zheng Y, Guan YF, Yang PB, Chen X, Peng C, He JP, Ai YL, Wu SF, et al. Nuclear receptor Nur77 facilitates melanoma cell survival under metabolic stress by protecting fatty acid oxidation. Mol Cell. 2018;69:480–492 e7. doi: 10.1016/j.molcel.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 176.Sullivan LB, Chandel NS. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014;2:17. doi: 10.1186/2049-3002-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wu Z, Zuo M, Zeng L, Cui K, Liu B, Yan C, Chen L, Dong J, Shangguan F, Hu W, et al. OMA1 reprograms metabolism under hypoxia to promote colorectal cancer development. EMBO Rep. 2020;22:e50827. doi: 10.15252/embr.202050827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang YP, Zhou W, Wang J, Huang X, Zuo Y, Wang TS, Gao X, Xu YY, Zou SW, Liu YB, et al. Arginine Methylation of MDH1 by CARM1 inhibits glutamine metabolism and suppresses pancreatic cancer. Mol Cell. 2016;64:673–687. doi: 10.1016/j.molcel.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 179.Panieri E, Telkoparan-Akillilar P, Suzen S, Saso L. The NRF2/KEAP1 axis in the regulation of tumor metabolism: Mechanisms and therapeutic perspectives. Biomolecules. 2020;10:791. doi: 10.3390/biom10050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shao S, Qin T, Qian W, Yue Y, Xiao Y, Li X, Zhang D, Wang Z, Ma Q, Lei J. Positive feedback in Cav-1-ROS signalling in PSCs mediates metabolic coupling between PSCs and tumour cells. J Cell Mol Med. 2020;24:9397–9408. doi: 10.1111/jcmm.15596. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 181.Ilkhani K, Bastami M, Delgir S, Safi A, Talebian S, Alivand MR. The engaged role of tumor microenvironment in cancer metabolism: Focusing on cancer-associated fibroblast and exosome mediators. Anticancer Agents Med Chem. 2021;21:254–266. doi: 10.2174/1871520620666200910123428. [DOI] [PubMed] [Google Scholar]
- 182.Zhai Y, Chai L, Chen J. The relationship between the expressions of tumor associated fibroblasts Cav-1 and MCT4 and the prognosis of papillary carcinoma of breast. Pak J Pharm Sci. 2017;30(Suppl 1):S263–S372. [PubMed] [Google Scholar]
- 183.Ngwa VM, Edwards DN, Philip M, Chen J. Microenvironmental metabolism regulates antitumor immunity. Cancer Res. 2019;79:4003–4008. doi: 10.1158/0008-5472.CAN-19-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Song M, Sandoval TA, Chae CS, Chopra S, Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C, et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562:423–428. doi: 10.1038/s41586-018-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gottesman MM, Lavi O, Hall MD, Gillet JP. Toward a better understanding of the complexity of cancer drug resistance. Annu Rev Pharmacol Toxicol. 2016;56:85–102. doi: 10.1146/annurev-pharmtox-010715-103111. [DOI] [PubMed] [Google Scholar]
- 186.Cui Q, Wang JQ, Assaraf YG, Ren L, Gupta P, Wei L, Ashby CR, Jr, Yang DH, Chen ZS. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. 2018;41:1–25. doi: 10.1016/j.drup.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 187.Jadhao M, Tsai EM, Yang HC, Chen YF, Liang SS, Wang TN, Teng YN, Huang HW, Wang LF, Chiu CC. The long-term DEHP exposure confers multidrug resistance of triple-negative breast cancer cells through ABC transporters and intracellular ROS. Antioxidants (Basel) 2021;10:949. doi: 10.3390/antiox10060949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. doi: 10.1186/s12943-016-0577-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ge W, Zhao K, Wang X, Li H, Yu M, He M, Xue X, Zhu Y, Zhang C, Cheng Y, et al. iASPP is an antioxidative factor and drives cancer growth and drug resistance by competing with Nrf2 for Keap1 Binding. Cancer Cell. 2017;32:561–573.e6. doi: 10.1016/j.ccell.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 190.Wang L, Leite de Oliveira R, Huijberts S, Bosdriesz E, Pencheva N, Brunen D, Bosma A, Song JY, Zevenhoven J, Los-de Vries GT, et al. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell. 2018;173:1413–1425.e14. doi: 10.1016/j.cell.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 191.Menéndez ST, Gallego B, Murillo D, Rodríguez A, Rodríguez R. Cancer stem cells as a source of drug resistance in bone sarcomas. J Clin Med. 2021;10:2621. doi: 10.3390/jcm10122621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Choi HJ, Jhe YL, Kim J, Lim JY, Lee JE, Shin MK, Cheong JH. FoxM1-dependent and fatty acid oxidation-mediated ROS modulation is a cell-intrinsic drug resistance mechanism in cancer stem-like cells. Redox Biol. 2020;36:101589. doi: 10.1016/j.redox.2020.101589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Banerjee S, Mukherjee S, Bhattacharya A, Basak U, Chakraborty S, Paul S, Khan P, Jana K, Hazra TK, Das T. Pyridoxine enhances chemo-responsiveness of breast cancer stem cells via redox reconditioning. Free Radic Biol Med. 2020;152:152–165. doi: 10.1016/j.freeradbiomed.2020.02.031. [DOI] [PubMed] [Google Scholar]
- 194.Li D, Fu Z, Chen R, Zhao X, Zhou Y, Zeng B, Yu M, Zhou Q, Lin Q, Gao W, et al. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget. 2015;6:31151–31163. doi: 10.18632/oncotarget.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tsai TL, Lai YH, Hw Chen H, Su WC. Overcoming radiation resistance by iron-platinum metal alloy nanoparticles in human copper transport 1-overexpressing cancer cells via mitochondrial disturbance. Int J Nanomedicine. 2021;16:2071–2085. doi: 10.2147/IJN.S283147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Q, Zhang J, Li J, Ye H, Li M, Hou W, Li H, Wang Z. Glutathione-activated NO-/ROS-generation nanoparticles to modulate the tumor hypoxic microenvironment for enhancing the effect of HIFU-combined chemotherapy. ACS Appl Mater Interfaces. 2021;13:26808–26823. doi: 10.1021/acsami.1c07494. [DOI] [PubMed] [Google Scholar]
- 197.Chen W, Yu D, Sun SY, Li F. Nanoparticles for co-delivery of osimertinib and selumetinib to overcome osimertinib-acquired resistance in non-small cell lung cancer. Acta Biomater. 2021;29:258–268. doi: 10.1016/j.actbio.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Banstola A, Poudel K, Pathak S, Shrestha P, Kim JO, Jeong JH, Yook S. Hypoxia-mediated ROS amplification triggers mitochondria-mediated apoptotic cell death via PD-L1/ROS-responsive, dual-targeted, drug-laden thioketal nanoparticles. ACS Appl Mater Interfaces. 2021;13:22955–22969. doi: 10.1021/acsami.1c03594. [DOI] [PubMed] [Google Scholar]
- 199.Cen J, Zhang L, Liu F, Zhang F, Ji BS. Long-term alteration of reactive oxygen species led to multidrug resistance in MCF-7 cells. Oxid Med Cell Longev. 2016;2016:7053451. doi: 10.1155/2016/7053451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang J, Liu L, Cen J, Ji B. BME, a novel compound of anthraquinone, down regulated P-glycoprotein expression in doxorubicin-resistant human myelogenous leukemia (K562/DOX) cells via generation of reactive oxygen species. Chem Biol Interact. 2015;239:139–145. doi: 10.1016/j.cbi.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 201.Murciano-Goroff YR, Warner AB, Wolchok JD. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020;30:507–519. doi: 10.1038/s41422-020-0337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Reina-Campos M, Moscat J, Diaz-Meco M. Metabolism shapes the tumor microenvironment. Curr Opin Cell Biol. 2017;48:47–53. doi: 10.1016/j.ceb.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen X, Cubillos-Ruiz J. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer. 2021;21:71–88. doi: 10.1038/s41568-020-00312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Andrews AM, Tennant MD, Thaxton JE. Stress relief for cancer immunotherapy: Implications for the ER stress response in tumor immunity. Cancer Immunol Immunother. 2020;70:1165–1175. doi: 10.1007/s00262-020-02740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Harris IS, DeNicola GM. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020;30:440–451. doi: 10.1016/j.tcb.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 206.Cheung EC, Lee P, Ceteci F, Nixon C, Blyth K, Sansom OJ, Vousden KH. Opposing effects of TIGAR- and RAC1-derived ROS on Wnt-driven proliferation in the mouse intestine. Genes Dev. 2016;30:52–63. doi: 10.1101/gad.271130.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.