Abstract
The gold standard for classification of neurodegenerative diseases is postmortem histopathology; however, the diagnostic odyssey of this case challenges such a clinicopathologic model. We evaluated a 60-year-old woman with a 7-year history of a progressive dystonia–ataxia syndrome with supranuclear gaze palsy, suspected to represent Niemann–Pick disease Type C. Postmortem evaluation unexpectedly demonstrated neurodegeneration with 4-repeat tau deposition in a distribution diagnostic of progressive supranuclear palsy (PSP). Whole-exome sequencing revealed a new heterozygous variant in TGM6, associated with spinocerebellar ataxia type 35 (SCA35). This novel TGM6 variant reduced transglutaminase activity in vitro, suggesting it was pathogenic. This case could be interpreted as expanding: (1) the PSP phenotype to include a spinocerebellar variant; (2) SCA35 as a tau proteinopathy; or (3) TGM6 as a novel genetic variant underlying a SCA35 phenotype with PSP pathology. None of these interpretations seem adequate. We instead hypothesize that impairment in the crosslinking of tau by the TGM6-encoded transglutaminase enzyme may compromise tau functionally and structurally, leading to its aggregation in a pattern currently classified as PSP. The lessons from this case study encourage a reassessment of our clinicopathology-based nosology.
Keywords: cerebellar ataxia, neurogenetics, movement disorders, postmortem
1. Introduction
The gold standard for classification of neurodegenerative diseases is postmortem histopathology. Tau is a microtubule-associated protein, which, when hyperphosphorylated, accumulates in unique patterns dependent on selective regional vulnerability [1,2,3]. Tau accumulation is associated with neurodegenerative diseases currently labeled as tauopathies. Phenotypes associated with abnormal tau accumulation include, but are not limited to, progressive supranuclear palsy (PSP), corticobasal degeneration, Pick’s disease, argyrophilic grain disease, and several less common conditions [4]. The range of “tau proteinopathy” disorders, defined by the accumulation of filamentous tau at autopsy, is continuously growing and has begun to include clinically unrelated disorders [5].
Here, we describe the intellectual odyssey of a case with initially discrepant clinical, genetic, and pathologic information in whom the confirmation of pathogenicity of a novel genetic variant in TGM6, a genotype without prior reported neuropathology, prompted reinterpretation of the data and a reappraisal of the clinicopathology model on which the nosology of tau proteinopathies is based.
2. Case Report
A 59-year-old right-handed Caucasian woman presented to our center after a five-year history of jerks and stiffness in the left limbs, frequent falls, dysphagia, and mild emotional lability. She had first noticed loss of fine motor skills in her left hand while typing. This progressed over a year to include “spasms” when attempting to utilize that hand. Within two years, she exhibited jerky movements and intermittent painless posturing of her left foot, lasting from seconds to minutes. Four years after symptom onset, she developed progressive dysphagia, falls, worsened rigidity, and a left leg that was “not following instructions.” She manifested compulsive behaviors and inappropriate crying and laughing. She denied dietary or bowel habit changes, anosmia, dream-enactment behaviors, or any other sleep-related symptoms. She occasionally repeated questions but had no cognitive complaints. While many aspects of her family history could not be confirmed, her deceased maternal grandmother had apparent dysphonia and tremor. Her parents and siblings had no neurological diseases.
Neurological examination revealed a complex hyperkinetic syndrome consistent with dysarthria, orofacial dystonia, eye movement abnormalities (oculomotor apraxia, slowed horizontal saccades, severe vertical gaze palsy, overcome with oculocephalic maneuvers), and multidirectional, jerky, low-frequency head movements that were variably interpreted as tremor or stereotypies against a background of generalized dystonia that was greater in the left limbs and included a right hand tremor with cerebellar and dystonic features (Supplementary Video S1). The Montreal Cognitive Assessment revealed predominantly frontal cognitive impairment (21/30) with deficits in visuospatial acuity (2/5), serial subtractions (0/3), verbal fluency (0/1), and delayed recall (3/5) tasks.
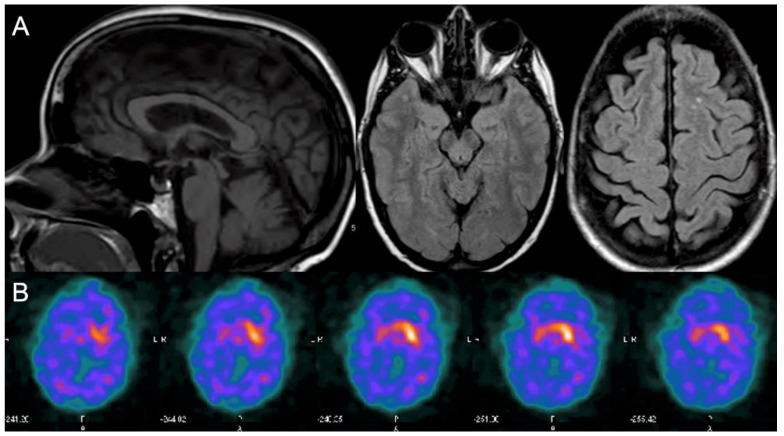
Laboratory investigations revealed normal serum copper, ceruloplasmin, heavy metals, vitamin E, comprehensive metabolic profile (including liver function tests), mildly high ferritin with normal iron, and no acanthocytes. She had no antibodies against phospholipids, double-stranded DNA, glutamic acid decarboxylase, and gliadin. A serum paraneoplastic antibody panel was negative. Neither copper urine levels nor cerebrospinal fluid analyses were performed. Gene testing for Huntington’s disease was normal. She had a normal electroencephalogram, electromyogram, and nerve conduction studies. Brain magnetic resonance imaging (MRI), obtained four years after symptom onset, revealed mild cerebellar and tegmental midbrain atrophy and scattered white matter hyperintensities (Figure 1A). Cervical spine MRI showed minor spondylotic changes without cord abnormalities. Single-photon emission computed tomography (DaTscan) revealed marked decreased uptake in the bilateral putamina (right > left) and right caudate (Figure 1B). The striatum-binding ratio was 85% below the mean in the right and 68% below in the left.
Figure 1.
Brain magnetic resonance imaging (MRI) and single-photon emission computed tomography (DaTscan) obtained four years after symptom onset. (A) Brain MRI Left panel: mid-sagittal T1-weighted sequence with mild vermal cerebellar atrophy and midbrain atrophy (midbrain anteroposterior diameter, 8.6 mm; midbrain-to-pons ratio 0.54) [6]; central panel: axial T2 FLAIR sequence showing mild tegmental midbrain atrophy; right panel: axial T2 FLAIR sequence showing two small white matter hyperintensities. (B) DaTscan showed asymmetric (right > left), abnormally decreased uptake in the bilateral putamen and right caudate.
Based on the clinical features, Niemann–Pick Disease Type-C (NPC) was suspected, but oxysterol levels (<0.02 nmol/mL) and NPC1 and NPC2 gene sequencing were normal (skin biopsy not performed). There was relentless progression. By the sixth year of her illness, she had become immobile and lost substantial weight (the last measured weight was below 30 kg) due to dysphagia to both liquids and solids (Supplementary Video S2), with subsequent malnutrition. Trihexyphenidyl and diazepam attenuated the dystonia severity; levodopa, escalated to 600 mg/day, had no effect. She died at the age of 60, 7 years after symptom onset.
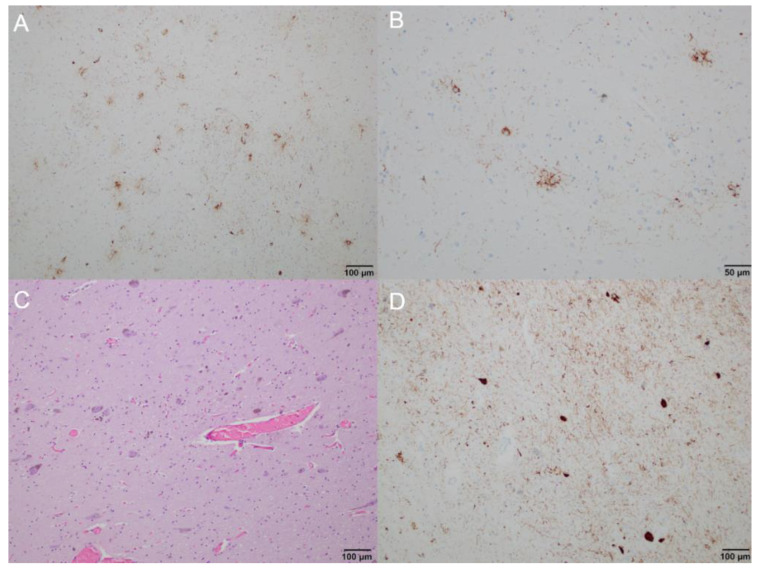
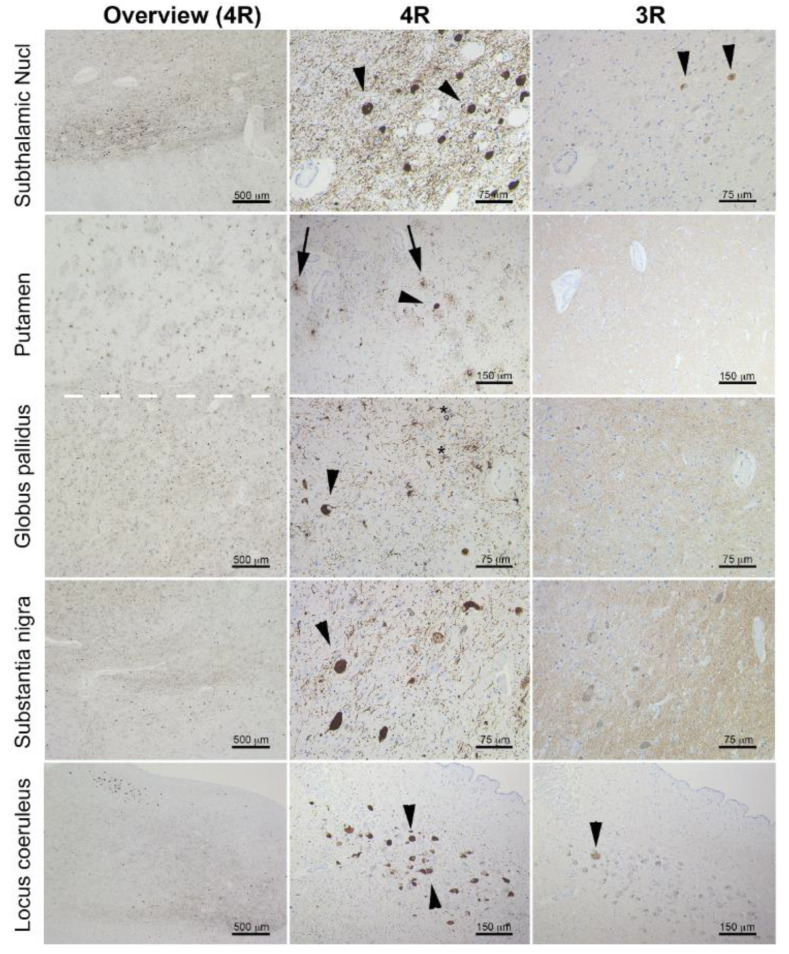
Postmortem neuropathology. On gross examination, the brain was mildly atrophic (1050 g); there was marked hypopigmentation in the substantia nigra and mild atrophy of the anterior cerebellar vermis and brainstem. Neuropathology examination was performed using formalin-fixed paraffin-embedded tissue blocks of several cortical, subcortical, and brainstem regions, as well as the cerebellum. The following monoclonal antibodies were used for immunohistochemistry: anti-tau (clone AT8), anti-4 repeat(R)tau (RD4), anti-3R tau (RD3), anti-phospho-TDP-43 (pS409/410; GTX82580), anti-α-synuclein (5G4; SPM451), and anti-Aβ (6F/3D; 2C8). Immunostaining for AT8 revealed neurofibrillary tangles (seen also on hematoxylin-eosin staining), fine granular cytoplasmic immunoreactivity in neuronal cytoplasm, neuropil threads, tufted astrocytes, oligodendrocytic-coiled bodies, and threads in the white matter (Figure 2A–D). The bulk of neuronal and oligodendrocytic tau pathology was in subcortical areas, including the subthalamic nucleus, midbrain, pons, medulla oblongata, tegmentum, and substantia nigra. The dentate nucleus and the anterior horn of the spinal cord were also affected. Astrocytic tau pathology predominated in the striatum and cortical regions, particularly in the frontal cortex, but was also seen in the occipital cortex. The extensive immunohistochemical staining for beta amyloid on the left superior and middle temporal gyri, left middle frontal gyrus, left inferior parietal lobule, and right cerebellum did not reveal any amyloid angiopathy, diffuse deposits, or senile/neuritic plaques. Immunostaining for 4R tau isoform was positive, while immunostaining for 3R tau isoform was not (Figure 3). TDP-43, beta-amyloid, and alpha-synuclein staining were negative. In summary, the neuropathological features were diagnostic of the 4R tau proteinopathy, PSP.
Figure 2.
Brain neuropathology, microscopic anatomy: (A) immunohistochemistry staining showing tau accumulation in the putamen, predominantly in the form of tufted astrocytes and tau-positive neurons (100× magnification); (B) immunohistochemistry staining showing tufted astrocytes in the caudate nucleus (200× magnification); (C,D) substantia nigra showing neuronal cell loss, tau-positive neurons, and numerous neuropil threads ((C) hematoxylin and eosin staining; (D) immunohistochemistry staining; 100× magnification).
Figure 3.
Immunostaining for 4R (left and middle column) and 3R (right column) tau isoforms in the subthalamic nucleus, putamen, globus pallidus, substantia nigra, and locus coeruleus. Note that the pathology is predominated by 4R tau deposition and shows tufted astrocytes (few examples indicated by arrows), globose neurofibrillary tangles (few examples indicated by arrowheads), and coiled bodies (few examples indicated by asterisks). Only a single 3R tau immunoreactive neurofibrillary tangle is seen in the locus coeruleus (arrowhead), and two are seen weakly stained in the subthalamic nucleus (arrowheads).
Genetic evaluation. Whole-exome sequencing revealed a heterozygous variant of unknown significance (NM_198994 c.616A > C, p.T206P) in TGM6, associated with spinocerebellar ataxia type 35 (SCA35) [2] but previously unreported.
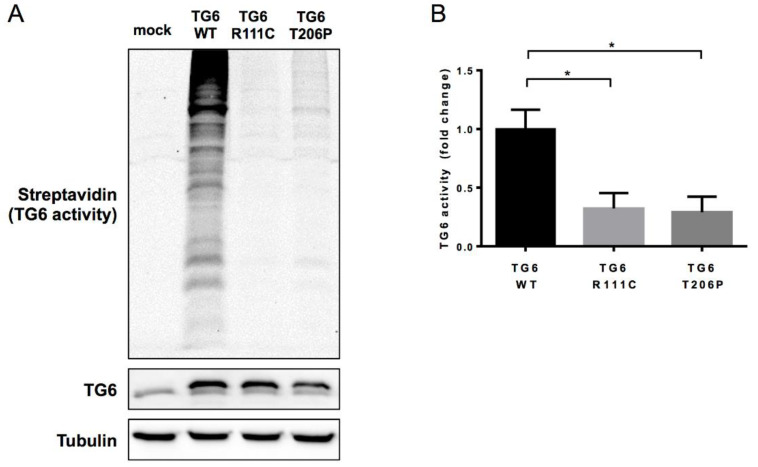
Functional evaluation of the T206P-TGM6 variant. The TGM6 protein transglutaminase 6 (TG6) catalyzes transamidation (crosslinking) within and between proteins. Pathogenic TGM6 variants have shown a loss in transamidase activity and increased neuronal toxicity in vitro and in vivo [7,8]. Therefore, to assess for pathogenicity, we tested whether the T206P variant affected the TG6 enzymatic activity involved in the transamidation of glutamine. We cloned the T206P variant in a mammalian expression vector, transfected HEK293T cells, and tested TG6 transamidase activity in vitro [8]. T206P significantly reduced TG6 activity when compared to wild-type TG6 (Figure 4), and the reduction in transamidase activity was similar to that observed in the R111C variant previously identified in SCA35 patients and reported as toxic in primary neurons and drosophila. The loss in enzymatic function supported the pathogenic role of T206P in our patient.
Figure 4.
In vitro assay showing TG6 transamidase activity: (A) Western blotting analysis showed significantly compromised transamidase activity of the TGM6 T206P variant compared to wild-type TG6 protein. (B) Quantification of TG6 enzymatic activity from experiment shown in panel A. Graph, mean ± SEM, * p <0.05, one-way ANOVA with Tukey’s post hoc test. Y-Axis represents the fold change.
3. Discussion
The clinical picture of a markedly asymmetric, progressive ataxia–dystonia syndrome with oculomotor apraxia and vertical supranuclear gaze palsy resembled NPC but was instead associated with a new pathogenic variant (T206P) in TGM6, previously reported as the genetic etiology of SCA35, and with an unanticipated pathology consistent with PSP. While the clinical features were broadly compatible with the syndrome of spinocerebellar ataxia, SCA35 has never been reported to be associated with supranuclear gaze palsy (as per a systematic review of 66 cases reported; Supplementary Table S1 [9,10,11,12,13,14,15] and Figure S1), nor with an underlying 4R tau proteinopathy compatible with PSP pathology. That said, in the background of severe DaT deficit on imaging and vertical supranuclear gaze palsy on examination, PSP was clinically excluded by the hyperkinetic rather than the parkinsonian phenotype, as well as the presence of appendicular and axial ataxia, which are exclusionary [16]. While a cerebellar variant of PSP has been reported [17], these patients, mostly from Asia, have not manifested dystonia or other hyperkinesias, nor the marked dopamine transporter loss exhibited by our patient. Moreover, the distribution of tau pathology did not show a cerebellum-predominant pattern; instead, it met staging five (of six) for PSP–Richardson syndrome [18]. We recognize that the clinical perspective on what represents a “tau proteinopathy”, with the accumulation of filamentous tau, continues to evolve, given the growing range of clinically unrelated disorders with documented tau accumulation at autopsy [5].
TG6 mutations have been reported to decrease its transamidase activity and increase susceptibility to apoptosis in neuronal [8] and non-neuronal cells [19]. TGM6 codes for TG6, a calcium-dependent enzyme expressed in the central nervous system, particularly in the olfactory lobe, cerebral cortex, cerebellum, and brainstem [20]. TG6 activity is responsible for creating stable isopeptide bonds, also known as transglutaminase-catalyzed protein crosslinking [20,21]. Transglutaminases can also polyaminate proteins, namely they add primary amines to lysines and glutamines [1]. Polyamination of tubulin has been shown to be essential for the stabilization of axon microtubules [1,2]. Alterations in tubulin polyamination by transglutaminases could favor the formation of neurofibrillary tangles, a feature of several tau-related disorders [3].
Abnormal non-specific transglutaminase activity has been previously identified in Alzheimer’s disease, Huntington’s disease, and PSP [2,3,22]. Under physiologic conditions, transglutaminase activity and the corresponding stability of microtubules are essential for neural structure and function, as well as early neurite development [13]. The inhibition of transglutaminase activity reduces microtubules’ stability in culture [1]. Although transglutaminase activity and levels of microtubules are relatively low in immature cells, they concurrently increase with differentiation, facilitating extension and stabilization of neurites [1,2,3]. These changes in cytoskeletal dynamics and stability in neurodegenerative processes, while incompletely understood, result in polymerization of the protein with subsequent loss of their function [1,2,3]. The observable result is the accumulated protein, the anchor used to classify and approach neurodegenerative disorders; the unobservable event is the loss of the functional, soluble precursors of the same protein (as they turn into tangles), which is invisible at autopsy.
Our case highlights a nosology challenge: whether to describe it as expanding the phenotypic spectrum associated with PSP pathology or with TGM6-SCA35. On one hand, our observation of an NPC-like phenotype adds to the variability of clinical presentations associated with PSP-type tau pathology (interestingly, 8.5% PSP patients harbor heterozygous variants in NPC1 or NPC2) [23]. On the other hand, since we detected a genetic variant associated with SCA35, and this gene is related to an enzyme associated with functional impairment of tau crosslinking, we theorize that aberrant TG6 might contribute to the initiation of tau pathology in a distribution currently classified as PSP.
The present case has several limitations. Unfortunately, we were unable to study the deceased maternal grandmother or her healthy siblings to evaluate for TGM6 carrier status. We also acknowledge that a single case study cannot address the wider question of whether the pathogenetic mechanisms of tau proteinopathies are associated with normal tau depletion (loss-of-function model) [24] or abnormal tau accumulation (gain-of-function model), and the extent to which either or both of these contribute to the underlying clinical phenotype [25]. Further in vivo studies on mouse models will be needed to clarify the interaction between TGM6 and tau pathology. Tentatively, our observations lend support to the notion that tau pathology, even in a pattern currently classified as PSP, may be the result of several upstream disrupted pathogenic abnormalities (TG6-associated impaired tau crosslinking among them) but not the pathogenic abnormality. The clinico-genetic-pathology discrepancies highlighted by this case may serve to inform a future reassessment of the clinicopathology-based nosology of neurodegenerative disorders.
Supplementary Materials
The following are available online at https://drive.google.com/drive/folders/1Db_4b5sBaL-DwtCYdkcwHWatiFYV5hFS, Supplementary Video S1. Neurologic examination of the patient at the first visit, five years after symptom onset. Supplementary Video S2. Second neurologic examination, six month after the first video. The patient is increasingly immobile, exhibiting marked weight loss due to dysphagia. The left arm has become nearly fixed. Supplementary Table S1. TGM6 variants reported in literature and related clinical features. Supplementary Figure S1. Lollipop graph depicting the TGM6 gene with the known variants located on their specific positions.
Author Contributions
(1) Research Project: A. Conception, L.M.; A.J.E., B. Organization, L.M.; J.S.; A.J.E.; M.B.; A.S., C. Execution, L.M.; J.S.; A.M.; M.B.; E.A.; E.J.H.; K.R.D. (2): Statistical Analysis: A. Design, A.M.; M.B., B. Execution, A.M.; G.G.K.; A.E.L.; M.B., C. Review and Critique, A.M.; M.B.; (3) Manuscript Preparation: A. Writing of the First Draft, L.M.; J.S.; A.J.E.; M.B.; M.A.K., B. Review and Critique, L.M.; J.S.; A.J.E.; A.M.; E.A., E.J.H.; K.R.D.; M.C.H.; C.D.S.; G.G.K.; A.E.L.; M.H.; M.B.; M.A.K.; A.S.; M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by The Parkinson’s Patient Diagnostic Genetic Assistance Fund at the University of Cincinnati and by the Gardner Family Foundation (CCBP Study), both located in Cincinnati, OH, USA. Rossy Foundation, Safra Foundation, and Porridge for Parkinson’s Foundation supported the contributions of G.G.K. and A.E.L.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the University of Cincinnati (Protocol code #2017-5985; approved on 20 December 2018).
Informed Consent Statement
Written informed consent has been obtained from the patient and from her next of kin to publish this paper and the related materials (video and images).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest related to the research covered in this article. Luca Marsili has received honoraria from the International Association of Parkinsonism and Related Disorders (IAPRD) Society for social media and web support. Alberto J Espay has received grant support from the NIH and the Michael J Fox Foundation; personal compensation as a consultant/scientific advisory board member for Abbvie, Neuroderm, Neurocrine, Amneal, Adamas, Acadia, Acorda, Kyowa Kirin, Sunovion, Lundbeck, and USWorldMeds; publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer; and honoraria from USWorldMeds, Acadia, and Sunovion. Espay is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies. Christopher D. Stephen has provided scientific advisory for Xenon Pharmaceuticals and SwanBio Therapeutics and received research funding from Sanofi-Genzyme for a study of video oculography in late-onset GM2 gangliosidosis. Gabor G. Kovacs has served as an advisor for Biogen and received publishing royalties from Elsevier, Wiley-Blackwell, and Cambridge University Press, and shares a patent for the a-synculein antibody 5G4. Anthony E. Lang has served as an advisor for Abbvie, Acorda, AFFiRis, Biogen, Denali, Janssen, Lilly, Lundbeck, Maplight, Paladin, Retrophin, Roche, Sun Pharma, Sunovion, Theravance, and Corticobasal Degeneration Solutions; received honoraria from Sun Pharma, AbbVie and Sunovion; received grants from Brain Canada, Canadian Institutes of Health Research, Corticobasal Degeneration Solutions, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, the Ontario Brain Institute, Parkinson Foundation, Parkinson Canada, and W. Garfield Weston Foundation; received publishing royalties from Elsevier, Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press. Marcelo A. Kauffman is an employee of the CONICET and has received grant support from the Ministry of Science and Technology of Argentina and the Ministry of Health of Buenos Aires. Andrea Sturchio is cofounder of REGAIN Therapeutics, owner of a provisional patent on compositions and methods for treatment and/or prophylaxis of proteinopathies.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song Y., Kirkpatrick L.L., Schilling A.B., Helseth D.L., Chabot N., Keillor J.W., Johnson G.V., Brady S.T. Transglutaminase and polyamination of tubulin: Posttranslational modification for stabilizing axonal microtubules. Neuron. 2013;78:109–123. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeitner T.M., Battaile K., Cooper A.J. γ-Glutamylamines and neurodegenerative diseases. Amino Acids. 2013;44:129–142. doi: 10.1007/s00726-011-1209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeitner T.M., Pinto J.T., Krasnikov B.F., Horswill M., Cooper A.J. Transglutaminases and neurodegeneration. J. Neurochem. 2009;109(Suppl. 1):160–166. doi: 10.1111/j.1471-4159.2009.05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kovacs G.G. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol. 2015;41:3–23. doi: 10.1111/nan.12208. [DOI] [PubMed] [Google Scholar]
- 5.Mulroy E., Jaunmuktane Z., Balint B., Erro R., Latorre A., Bhatia K.P. Some New and Unexpected Tauopathies in Movement Disorders. Mov. Disord. Clin. Pract. 2020;7:616–626. doi: 10.1002/mdc3.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massey L.A., Jäger H.R., Paviour D.C., O’Sullivan S.S., Ling H., Williams D.R., Kallis C., Holton J., Revesz T., Burn D.J., et al. The midbrain to pons ratio: A simple and specific MRI sign of progressive supranuclear palsy. Neurology. 2013;80:1856–1861. doi: 10.1212/WNL.0b013e318292a2d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J.L., Yang X., Xia K., Hu Z.M., Weng L., Jin X., Jiang H., Zhang P., Shen L., Guo J.F., et al. TGM6 identified as a novel causative gene of spinocerebellar ataxias using exome sequencing. Brain. 2010;133:3510–3518. doi: 10.1093/brain/awq323. [DOI] [PubMed] [Google Scholar]
- 8.Tripathy D., Vignoli B., Ramesh N., Polanco M.J., Coutelier M., Stephen C.D., Canossa M., Monin M.L., Aeschlimann P., Turberville S., et al. Mutations in TGM6 induce the unfolded protein response in SCA35. Hum. Mol. Genet. 2017;26:3749–3762. doi: 10.1093/hmg/ddx259. [DOI] [PubMed] [Google Scholar]
- 9.Chen K., Lu Y., Peng F., Yu H.L., Wu J.Y., Tan Y., Zhao Y.X. TGM6 variants in Parkinson’s disease: Clinical findings and functional evidence. J. Integr. Neurosci. 2020;19:51–64. doi: 10.31083/j.jin.2020.04.283. [DOI] [PubMed] [Google Scholar]
- 10.Fung J.L.F., Tsang M.H.Y., Leung G.K.C., Yeung K.S., Mak C.C.Y., Fung C.W., Chan S.H.S., Yu M.H.C., Chung B.H.Y. A significant inflation in TGM6 genetic risk casts doubt in its causation in spinocerebellar ataxia type 35. Parkinsonism Relat. Disord. 2019;63:42–45. doi: 10.1016/j.parkreldis.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y.C., Lin J.J., Liao Y.C., Tsai P.C., Lee Y.C., Soong B.W. Spinocerebellar ataxia 35: Novel mutations in TGM6 with clinical and genetic characterization. Neurology. 2014;83:1554–1561. doi: 10.1212/WNL.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z.H., Shi M.M., Liu Y.T., Wang Y.L., Luo H.Y., Wang Z.L., Shi C.H., Xu Y.M. TGM6 gene mutations in undiagnosed cerebellar ataxia patients. Parkinsonism Relat. Disord. 2018;46:84–86. doi: 10.1016/j.parkreldis.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Li M., Pang S.Y., Song Y., Kung M.H., Ho S.L., Sham P.C. Whole exome sequencing identifies a novel mutation in the transglutaminase 6 gene for spinocerebellar ataxia in a Chinese family. Clin. Genet. 2013;83:269–273. doi: 10.1111/j.1399-0004.2012.01895.x. [DOI] [PubMed] [Google Scholar]
- 14.Fasano A., Hodaie M., Munhoz R.P., Rohani M. SCA 35 presenting as isolated treatment-resistant dystonic hand tremor. Parkinsonism Relat. Disord. 2017;37:118–119. doi: 10.1016/j.parkreldis.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Manini A., Bocci T., Migazzi A., Monfrini E., Ronchi D., Franco G., De Rosa A., Sartucci F., Priori A., Corti S., et al. A case report of late-onset cerebellar ataxia associated with a rare p.R342W TGM6 (SCA35) mutation. BMC Neurol. 2020;20:408. doi: 10.1186/s12883-020-01964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Höglinger G.U., Respondek G., Stamelou M., Kurz C., Josephs K.A., Lang A.E., Mollenhauer B., Müller U., Nilsson C., Whitwell J.L., et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanazawa M., Shimohata T., Toyoshima Y., Tada M., Kakita A., Morita T., Ozawa T., Takahashi H., Nishizawa M. Cerebellar involvement in progressive supranuclear palsy: A clinicopathological study. Mov. Disord. 2009;24:1312–1318. doi: 10.1002/mds.22583. [DOI] [PubMed] [Google Scholar]
- 18.Kovacs G.G., Lukic M.J., Irwin D.J., Arzberger T., Respondek G., Lee E.B., Coughlin D., Giese A., Grossman M., Kurz C., et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. 2020;140:99–119. doi: 10.1007/s00401-020-02158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan W.J., Wang J.L., Liu Y.T., Ma Y.T., Zhou Y., Jiang H., Shen L., Guo J.F., Xia K., Li J.D., et al. Spinocerebellar ataxia type 35 (SCA35)-associated transglutaminase 6 mutants sensitize cells to apoptosis. Biochem. Biophys. Res. Commun. 2013;430:780–786. doi: 10.1016/j.bbrc.2012.11.069. [DOI] [PubMed] [Google Scholar]
- 20.Thomas H., Beck K., Adamczyk M., Aeschlimann P., Langley M., Oita R.C., Thiebach L., Hils M., Aeschlimann D. Transglutaminase 6: A protein associated with central nervous system development and motor function. Amino Acids. 2013;44:161–177. doi: 10.1007/s00726-011-1091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basso M., Ratan R.R. Transglutaminase is a therapeutic target for oxidative stress, excitotoxicity and stroke: A new epigenetic kid on the CNS block. J. Cereb. Blood Flow Metab. 2013;33:809–818. doi: 10.1038/jcbfm.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zemaitaitis M.O., Kim S.Y., Halverson R.A., Troncoso J.C., Lee J.M., Muma N.A. Transglutaminase activity, protein, and mRNA expression are increased in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 2003;62:173–184. doi: 10.1093/jnen/62.2.173. [DOI] [PubMed] [Google Scholar]
- 23.Picillo M., Amboni M., Bruni A., Maletta R., Barone P. Prevalence of heterozygous mutations in Niemann-Pick type C genes in a cohort of progressive supranuclear palsy. Parkinsonism Relat. Disord. 2020;79:9–10. doi: 10.1016/j.parkreldis.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Malmberg M., Malm T., Gustafsson O., Sturchio A., Graff C., Espay A.J., Wright A.P., El Andaloussi S., Lindén A., Ezzat K. Disentangling the Amyloid Pathways: A Mechanistic Approach to Etiology. Front. Neurosci. 2020;14:256. doi: 10.3389/fnins.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Espay A.J., Vizcarra J.A., Marsili L., Lang A.E., Simon D.K., Merola A., Josephs K.A., Fasano A., Morgante F., Savica R., et al. Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology. 2019;92:329–337. doi: 10.1212/wnl.0000000000006926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.