Abstract
The purpose of this study was to explore the association of the MCT1 gene Glu490Asp polymorphism (rs1049434) with athletic status and performance of endurance athletes. A total of 1,208 Brazilians (318 endurance athletes and 890 non-athletes) and 867 Europeans (315 endurance athletes and 552 non-athletes) were evaluated in a case–control approach. Brazilian participants were classified based on self-declared ethnicity to test whether the polymorphism was different between Caucasians and Afro-descendants. Moreover, 66 Hungarian athletes underwent an incremental test until exhaustion to assess blood lactate levels, while 46 Russian athletes had their maximum oxygen uptake ( ) compared between genotypes. In the Brazilian cohort, the major T-allele was more frequent in Caucasian top-level competitors compared to their counterparts of lower competitive level (P = 0.039), and in Afro-descendant athletes compared to non-athletes (P = 0.015). Similarly, the T-allele was more frequent in European athletes (P = 0.029). Meta-analysis of the Brazilian and European cohorts confirmed that the T-allele is over-represented in endurance athletes (OR: 1.48, P = 0.03), especially when Afro-descendant athletes were included in the meta-analysis (OR: 1.58, P = 0.005). Furthermore, carriers of the T/T genotype accumulated less blood lactate in response to intense effort (P < 0.01) and exhibited higher (P = 0.04). In conclusion, the Glu490Asp polymorphism was associated with endurance athletic status and performance. Our findings suggest that, although ethnic differences may exist, the presence of the major T-allele (i.e., the Glu-490 allele) favours endurance performance more than the mutant A-allele (i.e., the 490-Asp allele).
Keywords: Aerobic Performance, Aerobic Power, Athletes, Erythrocytes, Genetics, Monocarboxylate Transporter
INTRODUCTION
At higher exercise intensities, the increased demand for glycolysis leads to excessive cellular lactate production and accumulation of protons (H+), which need to be buffered or expelled from the muscle to regulate cellular pH and maintain a fast glycolytic flow [1]. The 1:1 transmembrane cotransport of lactate and H+ is facilitated by proton-linked monocarboxylate transporters (MCTs) via a facilitated diffusion mechanism between different cells within a given muscle or between muscle and blood [2, 3]. The MCTs form part of the solute carrier 16 (SLC16) gene family that contains 14 related proteins (isoforms), wherein the first isoform (MCT1) is particularly important for the transport of lactate and one of the most well-studied and functionally characterized transporters, largely due to its broader tissue distribution [4].
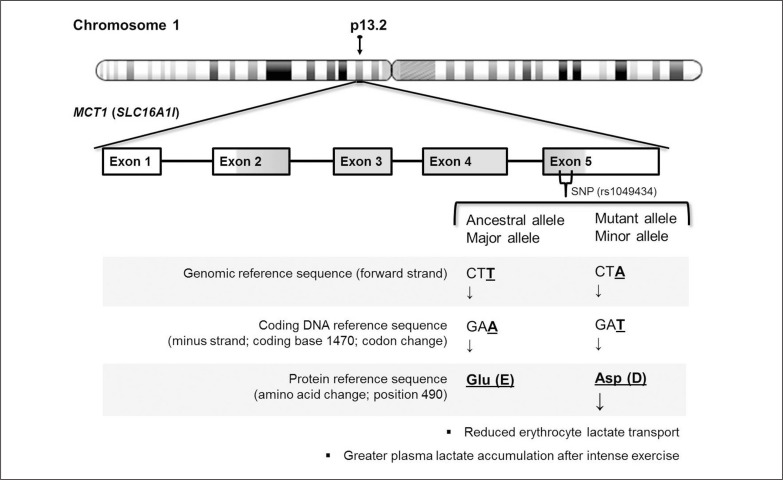
The MCT1 is found in almost all tissues, sometimes in conjunction with other MCT isoforms (e.g., in skeletal muscle) and sometimes as the only transporter (e.g., in erythrocytes) [5]. Erythrocytes play a fundamental role in supporting muscle lactate efflux because their rapid uptake of lactate flowing from muscle to plasma prevents the rise of plasma lactate levels, while maintaining a favourable gradient concentration difference between muscle and plasma [6]. Therefore, it is reasonable to assume that a deficiency in erythrocyte lactate transport capacity could influence the muscle metabolism during intense exercise. Indeed, some individuals were shown to have a reduced rate of erythrocyte lactate transport and consequently, a failure to decrease muscle lactate (and H+) levels normally after intense exercise [7, 8]. Since MCT1 is the only isoform found in erythrocytes, in order to identify mutations that could explain the reduced rate of lactate transport, Merezhinskaya et al. [8] sequenced the full-length MCT1 coding sequence of five individuals previously identified as having reduced erythrocyte lactate transport. Three coding variants were found; however, while two of these variants were absent or rare in a replication cohort, a third mutation was identified as a common gene polymorphism [8]. This polymorphism involves a transversion substitution at coding base 1470 (c.1470A > T), which converts amino acid #490 of the protein encoded by the MCT1 gene from glutamic acid to aspartic acid (p.Glu490Asp; see Figure 1).
FIG. 1.
Representation of the MCT1 gene Glu490Asp polymorphism
Several subsequent studies assessing healthy moderately active men [9, 10] and elite male athletes [11, 12] demonstrated that those carriers of the mutant allele exhibit a higher blood lactate concentration after different protocols of intense exercise, as well as a lower decrease in blood lactate levels after physical exertion [13]. Furthermore, in an assessment of a Russian cohort, endurance-oriented rowers showed a lower frequency of the mutant allele and the homozygous genotype for the mutant allele compared to the control group, suggesting that the major allele may be preferred for performance [11]. However, this association was not replicated in other endurance athlete cohorts [14–16]. In a study assessing Brazilian athletes, a strong association was found between the major allele and power athletic status, but the endurance group did not differ from the control group [17].
As recently highlighted, African populations have a marked lower frequency of the Glu490Asp mutant allele [18]. According to the 1000 Genomes Project Phase 3 [19], the minor allele frequency (MAF) in African populations ranges from 7.1% to 13.1%, whereas the MAF in European populations ranges from 38.9% to 47.2%. Moreover, a study assessing Israeli top-level runners showed that the MAF was significantly lower in those runners of Ethiopian origin compared to those of Caucasian origin or controls [20]. The homozygous genotype for the mutant allele was absent among the 37 Israeli runners of Ethiopian origin, suggesting an association between the polymorphism and endurance athletes of African descent [20], which needs to be confirmed in larger and independent samples.
The Brazilian population is heterogeneous. After the arrival of millions of Europeans during the second half of the 19th century and the first half of the 20th century [21], the European component of ancestry has become uniformly predominant in Brazil [22]. Nevertheless, this massive immigration of Europeans did not conceal some previous existing components of African ancestry [21]. Therefore, the purpose of this study was to further explore the Brazilian cohort of endurance athletes to test whether the MCT1 gene Glu490Asp polymorphism differs between athletes and non-athletes according to ethnic background, using a case–control approach. In addition, the polymorphism was evaluated in an independent cohort of European endurance athletes. A secondary purpose of this study was to evaluate the aerobic performance of athletes with different Glu490Asp genotypes. We tested the hypothesis that the major allele (i.e., the Glu-490 allele) is preferred for endurance performance because, in theory, it favours the individual to sustain higher exercise intensities.
MATERIALS AND METHODS
Research Design
For the case–control association study, two independent cohorts were used: a Brazilian cohort stratified by athletic status and self-declared ethnicity, and a European cohort composed of Russian and Hungarian athletes of Caucasian descent. For the study of aerobic performance, athletes underwent a physical test to assess circulating lactate levels (Hungarians) or maximum oxygen uptake ( ; Russians). The Brazilian study was approved by the research ethics committee of the School of Physical Education and Sport, University of São Paulo, São Paulo, Brazil. The study performed on the Hungarian sample was approved by the Hungarian Ethics Committee. The study performed on the Russian sample was approved by the ethics committee of the Physiological Section of the Russian National Committee for Biological Ethics. All procedures adopted were conducted according to the World Medical Association Declaration of Helsinki ethical principles for research involving human subjects. Written informed consent was obtained from all individual participants.
Subjects
Brazilian cohort: A total of 1,208 Brazilians participated in the study, 318 of which were endurance athletes (237 men and 81 women; age: 28.8 ± 9.0 years), and 890 were adult non-athletic individuals (506 men and 384 women; age: 32.8 ± 12.3 years). The endurance group comprised athletes from the following sport disciplines: road cycling and mountain biking (n = 106), running ≥ 1,500 m (n = 51), rowing (n = 87), swimming ≥ 400 m (n = 18) and triathlon (n = 56). Those who represented Brazil in international competitions such as the Olympics, World or Continental Championships were classified as international-level athletes. Non-athletes were those never engaged in sports competition and were representative of the general Brazilian population. These data were derived from a previous study [17].
All participants answered a questionnaire that included a multiplechoice question about self-declared ethnicity, based on the classification used by the Brazilian Institute of Geography and Statistics (IBGE). Those who self-declared as white were classified as Caucasians, whereas individuals self-declared as black or brown were classified as Afro-descendants. For those whose self-declared ethnicity is white, the main genomic contribution is from European ancestry, whereas for those whose self-declared ethnicity is black or brown, the main genomic contribution is from African ancestry [23]. There was a similar proportion of Caucasians and Afro-descendants between athletes and non-athletes. Of the 1,208 participants, 422 were classified as Afro-descendants (118 athletes and 304 non-athletes, ≈ 35%), and 786 were classified as Caucasians (200 athletes and 586 nonathletes, ≈ 65%).
European cohort: A total of 382 Russians and 196 Hungarians were enrolled in the case–control study. The Russian sample was composed of 208 long-distance endurance athletes (age: 22.6 ± 3.8 years) and 174 controls (age: 44.5 ± 4.1 years). These Russian data are independent of our previous publication [11], and included the following sport disciplines: biathlon (n = 60), cross-country skiing (n = 95), marathon running (n = 1), orienteering (n = 3), race walking (n = 9), swimming 5–25 km (n = 19) and triathlon (n = 21). All athletes were internationallevel competitors who represented Russia in international competitions and have been tested negative for doping substances. The control group included unrelated citizens, without any competitive sport experience. The Hungarian sample was composed of 107 endurance athletes (age: 26.1 ± 5.1 years) from the following sport disciplines: rowing (n = 29), canoeing (n = 39), triathlon (n = 29), ultramarathon (n = 5) and road cycling (n = 5), and 89 controls (age: 29.8 ± 8.9 years). All Hungarian athletes were national team members and international-level competitors. The control group included sedentary, healthy individuals with no competitive sport experience. In addition, data of European populations (n = 289) from the 1000 Genomes Project were included as controls. More specifically, data from 4 European populations that did not differ statistically from our control group composed of Russians and Hungarians (1000 Genomes population code: CEU, FIN and GBR) were included.
Regarding the study of aerobic performance, 66 Hungarian elite athletes (football players, rowers, triathletes and water polo players; 47 men and 19 women; age: 25.4 ± 5.4 years) underwent an incremental treadmill test to exhaustion to assess blood lactate levels, while 46 male athletes from the Russian endurance group (rowers, kayakers, biathletes and cross-country skiers; age 25.4 ± 3.6 years) and members of the Russian national teams had their determined.
DNA extraction and genotyping
The genomic DNA of the Brazilians was isolated from buccal epithelial cells obtained from mouthwashes with a 0.9% saline solution prepared with DNA- and DNAse-free water and extracted using chloroform followed by alcohol precipitation. The rs1049434 was genotyped using a pre-designed specific TaqMan® SNP Genotyping Assay (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions and using a Rotor-Gene Q PCR cycler (Qiagen, Hilden, Germany). The VIC dye-labelled probe indicates the genomic A-allele, and the FAM dye-labelled probe indicates the genomic T-allele. An increase in VIC or FAM fluorescent signal indicates homozygosity for the VIC- or FAM-specific allele, while an increase in both signals indicates heterozygosity. A scatter plot showing the endpoint fluorescence signals was used to discriminate the genotypes.
The TaqMan assay describes the genomic alleles instead of the transcript alleles (i.e., based on the coding sequence; Figure 1), which were described in initial studies [8, 9] and other previous studies [11, 15, 24] with a genotyping procedure based on conventional PCR. Here, the genomic alleles were described similar to the method used by the 1000 Genomes Project [19]. It is worth noting that the transcript alleles are complementary (and inverse) to the genomic alleles and can cause some confusion in the interpretation of the data. Therefore, the amino acid change (i.e., Glu490Asp) was taken into account when appropriate to avoid misinterpretation [25].
The genomic DNA of the Russian samples was isolated from leukocytes (venous blood). DNA extraction and purification were performed using a commercial kit according to the manufacturer’s instructions (Technoclon, Moscow, Russia). The rs1049434 was genotyped using HumanOmni1-Quad BeadChips (Illumina Inc., Hayward, CA, USA) according to the instructions of the Infinium High-Density Assay. The genomic DNA of the Hungarian samples was isolated from EDTA stabilized blood using a commercial kit according to the manufacturer’s instructions (QIAamp DNA kit, Qiagen, Hilden, Germany). The rs1049434 was genotyped using the TaqMan® SNP Genotyping Assay pre-loaded on OpenArray™ plates and using the OpenArray™ NT Cycler System (BioTrove Inc., Pleasanton, CA, USA). In both Russian and Hungarian samples, genotypes were described based on the genomic alleles as used in the Brazilian sample.
Performance analysis
To assess the blood lactate levels of Hungarian athletes, an incremental test was performed on a treadmill. A modified Bruce protocol was applied, in which the test started with a speed of 8 km/h and increased by 2 km/h after 2 minutes. Thereafter, the exercise intensity was increased by adjusting the treadmill incline by 1.5% every minute until volitional exhaustion. Capillary blood lactate (mmol/L) was drawn from the ear lobe before (LAr), immediately after (LAmax) and 5 minutes after (LA5) the interruption of the exercise for the assessment of the lactate concentration (Biosen C-Line Lactate analyzer, EKF Diagnostics, Cardiff, United Kingdom). The blood lactate accumulation was calculated by a subtraction of the resting concentration from the value measured immediately after the test (i.e., LAacc = LAmax – LAr).
The of Russian athletes was determined during an incremental exercise test to exhaustion using a specific ergometer or a motor-driven treadmill according to the athlete’s specialty. A detailed description of testing protocols can be found elsewhere [26]. In all cases, a spiroergometry system with breath-by-breath technology was used to measure oxygen consumption during the entire test (Cortex, Leipzig, Germany). The was the highest mean value observed over a 30 s period.
Statistical Analysis
The Chi-square test (χ2) analysis was used to evaluate Hardy-Weinberg Equilibrium (HWE) expectations. A deviation from HWE was observed when χ2 > 3.84 (i.e., P > 0.05). Genotype and allele frequencies were also compared using the χ2 or Fisher’s exact test when appropriate. To determine the strength of the associations, a binary logistic regression analysis was used to compare cases and controls under three genetic models: codominant (T/T vs. T/A or A/A), dominant (T/T vs. T/A+A/A) and recessive (T/T+T/A vs. A/A). Each logistic regression reports an odds ratio (OR), a 95% confidence interval (95% CI) and a P-value. A meta-analysis of the Brazilian and European cohorts was performed using the Review Manager (RevMan) computer program version 5.3. The DerSimonian and Laird random-effects model was used to calculate weighted OR and its 95% CI. The overall effect was assessed using the Z score with the significance level established at P < 0.05. Heterogeneity between studies was assessed using the Cochran Q test (χ2).
Blood lactate levels between genotypes were compared using one-way analysis of variance (ANOVA) and Tukey post hoc test. For the comparison under the recessive model, the difference between means was compared using an unpaired t-test. Spearman correlation (non-parametric) was used to evaluate the relationship between and genotypes, which were coded as a dummy variable, as follows: A/A = 1, T/A = 2 and T/T = 3. Differences with P < 0.05 were considered significant. Statistical analyses were performed using SPSS software version 22.0 (IBM Corp., Armonk, NY, USA) or GraphPad Prism version 6.01 (GraphPad Software, San Diego, CA, USA) was used.
Results
The genotype distribution of the Glu490Asp polymorphism met the HWE expectations in Brazilians for both self-declared ethnicities. Furthermore, there was no statistical evidence of heterogeneity within the general Brazilian population. Genotype distribution was not significantly different in non-athletes according to self-declared ethnicity (χ2 = 2.53, P = 0.283), nor was the allele frequency (P = 0.123). Table 1 shows the genotype distribution and allele frequency of the polymorphism in the Brazilian cohort. Genotype distribution and allele frequency did not differ between non-athletes and Caucasian athletes as a whole. However, further analysis of the Caucasian endurance group showed an intragroup difference. Caucasian international-level competitors had a lower frequency of the A/A genotype compared to Caucasian national- or regional-level competitors (14.5% vs. 19.4% vs. 22%, P = 0.034). Consequently, the major T-allele was more frequent in Caucasian international-level athletes compared to their counterparts of lower competitive level (63% vs. 59% vs. 57%, P = 0.039), that is, there is a progressive increase in the frequency of the T-allele as the competitive level increases.
TABLE 1.
Genotype distribution and allele frequency of the MCT1 gene Glu490Asp polymorphism in the Brazilian cohort according to self-declared ethnicity.
| Status | Group | n | Genotypes (%) | Alleles ( %) | |||
|---|---|---|---|---|---|---|---|
| T/T | T/A | A/A | T-allele | A-allele | |||
| Control | Caucasian non-athletes | 586 | 36.9 | 47.3 | 15.9 | 60.5 | 39.5 |
| Control | Afro-descendant non-athletes | 304 | 41.1 | 46.4 | 12.5 | 64.3 | 35.7 |
| Control | Non-athletes† | 890 | 38.3 | 47.0 | 14.7 | 61.8 | 38.2 |
| Endurance | Caucasian athletes | 200 | 38.0 | 43.5 | 18.5 | 59.8 | 40.2 |
| Endurance | Afro-descendant athletes | 118 | 47.5 | 44.9 | 7.6 a | 69.9 | 30.1 a |
Genotypes were described based on the genomic alleles.
Pooled data of non-athletes (i.e., this group includes data from both self-declared ethnicities).
The genotype distribution (Codominant model: χ2 = 6.08, P = 0.048) and allele frequency (P = 0.015) in Afro-descendant athletes were significantly different from those of non-athletes.
Regarding Afro-descendant athletes, they differed from the general population, especially under the recessive model (i.e., T/T+T/A vs. A/A genotypes). The A/A genotype was underrepresented in Afrodescendant athletes. Afro-descendant carriers of the A/A genotype rather than T/T+T/A had a decreased odds ratio of being an endurance athlete compared with non-athletes (OR: 0.48, 95% CI: 0.24–0.97, P = 0.04). The A/A genotype was even more underrepresented in Afro-descendant international-level athletes. The genotype distribution in Afro-descendant international-level athletes was: T/T = 51.0%, T/A = 44.9% and A/A = 4.1% (n = 49), and the presence of the A/A genotype rather than T/T decreased the odds ratio of being an international-level endurance athlete compared with non-athletes (OR: 0.21, 95% CI: 0.05–0.89, P = 0.034). A similar trend was observed when comparing Afro-descendant international-level athletes and Afro-descendant non-athletes (A/A vs. T/T; OR: 0.26, 95% CI: 0.06–1.16, P = 0.078). Therefore, the major allele was more frequent in Afro-descendant athletes, especially in international-level athletes. The T-allele was found in only 61.8% of non-athletes compared to 69.9% of Afro-descendant athletes (P = 0.015) or 73.5% of Afrodescendant international-level athletes (P = 0.02).
The genotype distribution of the Glu490Asp polymorphism also met the HWE expectations in Europeans. Table 2 shows the genotype distribution and allele frequency of the polymorphism in the European cohort. The presence of the A/A genotype (recessive model; OR: 0.61, 95% CI: 0.41–0.92, P = 0.019) decreased the odds ratio of being an endurance athlete. Therefore, the major T-allele was more frequent in European athletes (P = 0.029). Meta-analysis of the Brazilian (Caucasians) and European cohorts confirmed that carriers of the T-allele (i.e., T/T+T/A genotypes) are over-represented in Caucasian endurance athletes (OR: 1.48), while the A/A genotype is under-represented (OR: 0.68, P = 0.03). Moreover, the association became stronger when Afro-descendant athletes were included in the meta-analysis (Table 3). There was no statistical evidence of heterogeneity between cohorts.
TABLE 2.
Genotype distribution and allele frequency of the MCT1 gene Glu490Asp polymorphism in the European cohort.
| Status | Group | n | Genotypes (%) | Alleles (%) | |||
|---|---|---|---|---|---|---|---|
| T/T | T/A | A/A | T-allele | A-allele | |||
| Control | Non-athletes | 552 | 35.3 | 47.3 | 17.4 | 59.0 | 41.0 |
| Endurance | Athletes | 315 | 40.0 | 48.6 | 11.4 a | 64.3 | 35.7 a |
Genotypes were described based on the genomic alleles.
The genotype distribution (Codominant model: χ2 = 5.94, P = 0.05) and allele frequency (P = 0.029) in endurance athletes were significantly different from those of non-athletes.
TABLE 3.
Meta-analysis of the association between MCT1 gene Glu490Asp polymorphism and endurance athletic status.
| Group | Total number | T/T + T/A genotypes | A/A genotype | |
|---|---|---|---|---|
| Athletes | Controls | OR (95% CI) | OR (95% CI) | |
| Caucasian Brazilians† | 77 | 586 | 1.13 (0.58–2.22) | 0.88 (0.45–1.74) |
| European Cohort | 315 | 552 | 1.63 (1.08–2.46) | 0.61 (0.41–0.92) |
| Total | 392 | 1138 | 1.48 (1.04–2.10) | 0.68 (0.48–0.96) |
| Test for overall effect | – | – | Z = 2.18 (P = 0.03) | Z = 2.18 (P = 0.03) |
| Heterogeneity | – | – | χ² = 0.82 (P = 0.36) | χ² = 0.82 (P = 0.36) |
| Brazilian Cohort‡ | 195 | 890 | 1.51 (0.92–2.49) | 0.66 (0.40–1.09) |
| European Cohort | 315 | 552 | 1.63 (1.08–2.46) | 0.61 (0.41–0.92) |
| Total | 510 | 1442 | 1.58 (1.15–2.17) | 0.63 (0.46–0.87) |
| Test for overall effect | – | – | Z = 2.83 (P = 0.005) | Z = 2.83 (P = 0.005) |
| Heterogeneity | – | – | χ² = 0.06 (P = 0.81) | χ² = 0.06 (P = 0.81) |
Only data from Caucasian international-level athletes were considered for the meta-analysis, given that a difference was identified between Caucasian athletes of international-level and their counterparts of lower competitive level.
Data from Afro-descendant Brazilians were added to the previous analysis, containing only Caucasian Brazilians. All Afro-descendant athletes were considered for the meta-analysis.
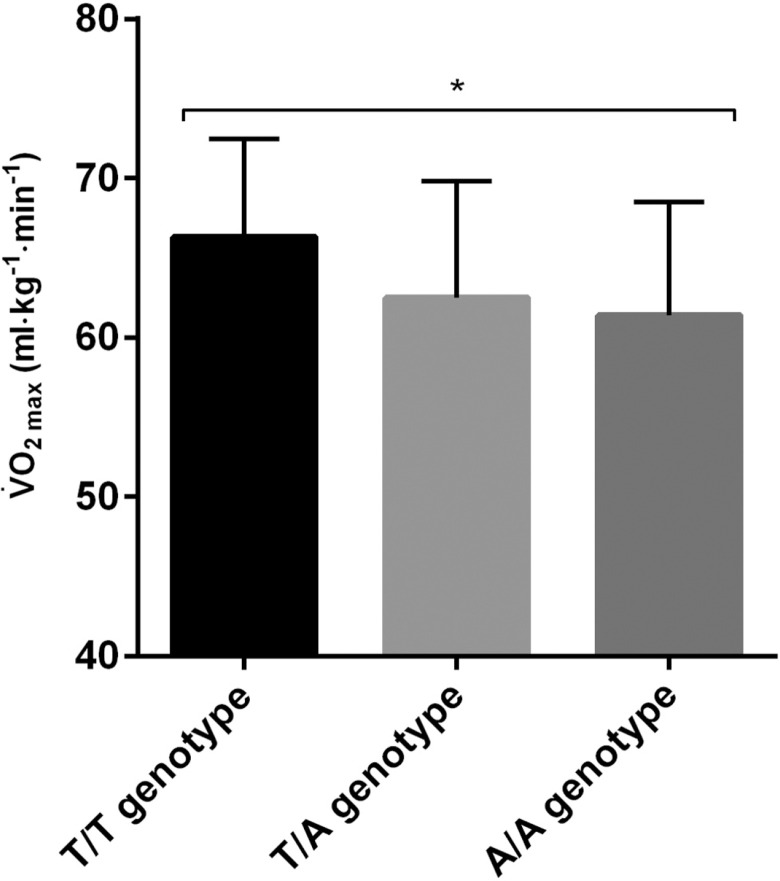
Table 4 shows the blood lactate levels of Hungarian athletes with different Glu490Asp genotypes and subjected to an incremental test until exhaustion. Carriers of the A/A genotype showed higher lactate accumulation during exercise and a greater peak 5 minutes after maximum effort, as evidenced by the analysis under the recessive model (T/T+T/A vs. A/A). On the other hand, the major T-allele was positively correlated with (ml·kg-1·min-1) in Russian male endurance athletes, with the T/T genotype showing the highest values (T/T = 66.3 ± 6.2, n = 14; T/A = 62.5 ± 7.3, n = 23; A/A = 61.4 ± 7.1, n = 9; r = 0.3, P = 0.04), as shown in Figure 2.
TABLE 4.
Blood lactate responses (mmol/L) of Hungarian athletes before (LAr), immediately after (LAmax) and 5 minutes after (LA5) to an incremental treadmill test until exhaustion.
| Genotypes (n) | P- values | ||||
|---|---|---|---|---|---|
| T/T (26) | T/A (34) | A/A (6) | Codominant | Recessive | |
| LAr | 1.52 ± 0.62 | 1.60 ± 0.40 | 1.46 ± 0.06 | 0.89 | 0.75 |
| LAmax | 8.5 ± 1.40 | 9.88 ± 1.18 | 13.6 ± 2.26 | < 0.01 | < 0.01 |
| LA5 | 11.00 ± 3.67 | 11.59 ± 3.95 | 17.50 ± 1.98 | 0.11 | 0.04 |
| LAacc | 6.98 ± 1.13 | 8.27 ± 1.20 | 12.14 ± 2.32 | < 0.01 | < 0.01 |
Genotypes were described based on the genomic alleles. Values are expressed as mean ± SD. LAacc (blood lactate accumulation) = LAmax – LAr.
FIG. 2.
VO2 max in Russian athletes.
DISCUSSION
In the present study, we explore the relevance of the MCT1 gene Glu490Asp polymorphism (also known as A1470T; rs1049434) for endurance athletes. The main finding of the present study was that the major T-allele (i.e., the Glu-490 allele) is associated with athletic status and performance. Meta-analysis of 510 endurance athletes compared with 1,442 non-athletes showed that carriers of the Glu490 allele were more likely to be an athlete. In particular, the consideration of Afro-descendant athletes made the association stronger.
In the initial study of Merezhinkaya et al. [8], three of five individuals with marked reductions in the rate of erythrocyte lactate transport were carriers of the Glu490Asp mutant allele: two homozygotes and one heterozygote. Interestingly, one homozygote was a black male with ≈ 51% reduced erythrocyte lactate transport, whereas the other homozygote was a white male with ≈ 34% reduced erythrocyte lactate transport; the heterozygote was a black male with ≈ 41% reduced transport [8]. Therefore, the black male homozygous for the mutant allele showed greater lactate transport deficit than the white male homozygous for the mutant allele, although with the available data was not possible to state whether this is an effect of ethnicity or other variables (e.g., age or training status). More recently, it was found that Caucasian non-athletes [9, 10], Caucasian athletes [11] and Japanese athletes [12] carriers of the mutant allele exhibit a higher blood lactate concentration after intense exercise, which is in line with the current study of Hungarian athletes. However, to date, no study has evaluated the extent of the influence of this polymorphism on lactate response to intense exercise in Afrodescendant individuals.
Differences in the responses to endurance exercise between African or Afro-descendants and Caucasians have been evaluated mainly among runners, where compelling evidence indicates that African/Afro-descendant distance runners exhibit lower blood lactate concentrations during exercise [27–31] and superior fatigue resistance [27, 31]. African runners’ exhibit a lower plasma lactate accumulation, which means that these athletes accumulate lactate at a slower rate as the exercise intensity increases [31]. This difference is clearer at higher running intensities, performed at or above 80% of maximum intensity [28, 29], as well as after maximum exertion [32]. These nearmaximal efforts are commonly applied by well-trained endurance athletes running distances ranging from 5,000 m to the marathon [33], and plasma lactate levels are an important indicator of the individual ability to sustain intense exercise [34, 35]. Therefore, the lower plasma lactate accumulation observed in African/Afro-descendant runners may be an inherent trait that, in part, is the advantage that these individuals have over Caucasians [28]. The lower frequency of the Glu490Asp mutant allele and the homozygous genotype for the mutant allele in African populations (as indicated by the 1000 Genomes Project) and Afro-descendant athletes ([20] and the present study) were consistent with this hypothesis. The mutant allele frequency in Brazilian Afro-descendants is not as low as that observed in African populations, probably due to the considerable admixture of Brazilians; however, its frequency was lower among Afro-descendant athletes.
There are critical factors for MCT1 function, such as activation of transporter and substrate affinity [36]. Topology predictions suggest that the MCT1 protein (like all other MCT isoforms) has 12 transmembrane-spanning helices with intracellular terminal domains: the N-terminal domain, important for membrane insertion and correct structure maintenance, and the C-terminal domain, important for the determination of substrate specificity [36]. Since the Glu490Asp polymorphism is just ten amino acids from the C-terminus (amino acid #500), it is assumed that this gene variant may affect the substrate specificity and transport activity.
Indeed, the Glu490Asp polymorphism was shown to affect the transporter activity, given that the negatively charged 490-Asp allele was shown to lead to a change in the transporter conformation and a reduction in transporter-substrate affinity [37]. A reduced affinity of MCT1 for lactate may limit its rate of transport and cause plasma lactate accumulation [3, 4], especially when considering skeletal muscle fibre-type proportions and metabolic enzyme activities of the individual. Some studies have shown that Africans may have a higher percentage of type IIa fibres [29, 32, 38], which can serve as important power generators for the high intensities applied by welltrained endurance athletes [31]. However, type IIa fibres have higher glycolytic enzyme activities and greater ability to produce lactate relative to type I fibres [39, 40]. Based on this, Afro-descendant athletes are able to produce equal or even more muscle lactate, although they may have lower plasma lactate levels during exercise [28]. Since a less efficient metabolism could raise plasma lactate levels in those most susceptible to its production, the Glu-490 allele is probably part of a genetic architecture that prevents plasma lactate accumulation in Afro-descendant athletes, favouring the maintenance of intense endurance exercise, and therefore, is more frequent in these individuals.
On the other hand, some evidence suggests that Caucasians may have a higher percentage of type I fibres [27, 29, 31, 38], which have higher capillary density and greater abundance of mitochondria and oxidative enzymes than type II fibres [40]. Moreover, there is a positive linear relationship between percentage of type I fibres and MCT1 content [41]. Since the biological effect of the mutant allele becomes evident at higher lactate levels [7], when a saturation phenomenon for lactate transport can create a tendency for lactate accumulation [3], it is reasonable to assume that a favourable background could prevent lactate levels from rising and the 490-Asp allele interference, especially in highly trained individuals. Notwithstanding, the above explanation does not rule out that the Glu-490 allele is advantageous for Caucasian individuals, as evidenced by the meta-analysis and performance tests on Caucasian athletes (blood lactate and analysis).
In an assessment of Caucasian endurance athletes, we found an association between MCT1 genotypes and , with the homozygous genotype for the Glu-490 allele (T/T genotype) showing the highest values. Since there is a positive correlation between and endurance-related phenotypes [42], the ≈ 7% higher value in homozygotes for the Glu-490 allele is not negligible. As oxygen consumption increases linearly with exercise intensity, individuals with higher could reach and sustain higher exercise intensities prior to excessive lactate accumulation and fatigue [43], which is typically accompanied by H+ accumulation, resulting in cellular and blood acidosis [44]. Assuming that individuals homozygous for the Glu-490 allele have an inherent increased plasma lactate removal capacity, these individuals are expected to have superior fatigue resistance and higher , regardless ethnicity. However, it appears reasonable to surmise that this effect may be lower in Caucasians or restricted to specific groups of Caucasian athletes. Consequently, when using larger samples and varied approaches, the association is found. Further studies including molecular, biochemical or physiological analyses will be of paramount importance to better elucidate the functional underpinnings of the Glu490Asp polymorphism in lactate metabolism and endurance performance among individuals with different biological backgrounds.
The main limitation of this study was the statistical power of some individual comparisons. Although the study involved 510 athletes and 1,442 non-athletes, when stratified by ethnicity or competitive level, the available sample size was not adequate for powerful comparisons. For example, doubling the size of the Brazilian sample would be more appropriate. However, it is noteworthy that the athletes evaluated are representative of the best Brazilian competitive scenario. For the European cohort, we used data from European populations as controls to increase our statistical power. In the absence of this data retrieved from the 1000 Genomes Project, we would have a low ratio of cases to controls. Although these controls are not exactly from the same regions of the athletes evaluated, we performed the χ2 to assess the heterogeneity of proportions to ensure that this procedure did not affect the association tests. Only data consistent with our cohort were used. Taken together, the meta-analysis of all cohorts provided a broader view of the association between the Glu490Asp polymorphism and endurance athletic status.
It is worth mentioning that this study was based on the genomic reference sequence, that is, the major T-allele represents the Glu-490 allele, whereas the minor (or mutant) A-allele represents the 490-Asp allele. Since alleles can be assigned in the opposite way depending on the reference used (i.e., the genomic or transcript alleles), this clarification becomes important. Different designations have the potential to confuse the interpretation of published data if not adequately described. For example, recently Onali et al. [18] conducted an interesting study describing the higher frequency of the Glu-490 allele in African populations compared with other world regions, but there was a misconception when comparing data from the 1000 Genomes Project (based on the genomic alleles) with associations previously described in athlete cohorts (based on the transcript alleles)–opposite alleles were considered the same.
In conclusion, the major allele of the Glu490Asp polymorphism found in the MCT1 gene was associated with endurance athletic status, lower lactate accumulation during intense endurance exercise and higher . Based on the present findings, it is plausible to assume that the presence of the major allele (i.e., the Glu-490 allele) favours endurance performance more than the mutant allele (i.e., the 490-Asp allele). Nonetheless, the physiological mechanisms underlying the polymorphism still need to be better explored, taking into account that differences between ethnicities may exist. Once these mechanisms are better elucidated, nutritional strategies to control cellular and blood pH and appropriate training programmes can be used accordingly. It is noteworthy that other factors can contribute to lower lactate and pH levels during exercise. Each of these traits has its own genetic predisposition, which can vary based on population background and environmental stimuli.
Acknowledgments
The authors are grateful to all participants who kindly provided their samples for DNA analysis. The Brazilian study was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq #151189/2018-8 and #301213/2015-1) and the Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP #12/22516-6).
Conflict of interest
All authors declare having no conflict of interest.
REFERENCES
- 1.Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R502–516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118(4):691–728. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 3.Thomas C, Bishop DJ, Lambert K, Mercier J, Brooks GA. Effects of acute and chronic exercise on sarcolemmal MCT1 and MCT4 contents in human skeletal muscles: current status. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R1–14. doi: 10.1152/ajpregu.00250.2011. [DOI] [PubMed] [Google Scholar]
- 4.Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3(4):1611–1643. doi: 10.1002/cphy.c130008. [DOI] [PubMed] [Google Scholar]
- 5.Halestrap AP, Wilson MC. The monocarboxylate transporter family--role and regulation. IUBMB Life. 2012;64(2):109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 6.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558(Pt 1):5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbein WN. Lactate transporter defect: a new disease of muscle. Science. 1986;234(4781):1254–1256. doi: 10.1126/science.3775384. [DOI] [PubMed] [Google Scholar]
- 8.Merezhinskaya N, Fishbein WN, Davis JI, Foellmer JW. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle Nerve. 2000;23(1):90–97. doi: 10.1002/(sici)1097-4598(200001)23:1<90::aid-mus12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Cupeiro R, Benito PJ, Maffulli N, Calderon FJ, Gonzalez-Lamuno D. MCT1 genetic polymorphism influence in high intensity circuit training: a pilot study. J Sci Med Sport. 2010;13(5):526–530. doi: 10.1016/j.jsams.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Cupeiro R, Gonzalez-Lamuno D, Amigo T, Peinado AB, Ruiz JR, Ortega FB, Benito PJ. Influence of the MCT1-T1470A polymorphism (rs1049434) on blood lactate accumulation during different circuit weight trainings in men and women. J Sci Med Sport. 2012;15(6):541–547. doi: 10.1016/j.jsams.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Fedotovskaya ON, Mustafina LJ, Popov DV, Vinogradova OL, Ahmetov II. A common polymorphism of the MCT1 gene and athletic performance. Int J Sports Physiol Perform. 2014;9(1):173–180. doi: 10.1123/ijspp.2013-0026. [DOI] [PubMed] [Google Scholar]
- 12.Kikuchi N, Fuku N, Matsumoto R, Matsumoto S, Murakami H, Miyachi M, Nakazato K. The Association Between MCT1 T1470A Polymorphism and Power-Oriented Athletic Performance. Int J Sports Med. 2016;38(1):76–80. doi: 10.1055/s-0042-117113. [DOI] [PubMed] [Google Scholar]
- 13.Cupeiro R, Perez-Prieto R, Amigo T, Gortazar P, Redondo C, Gonzalez-Lamuno D. Role of the monocarboxylate transporter MCT1 in the uptake of lactate during active recovery. Eur J Appl Physiol. 2016;116(5):1005–1010. doi: 10.1007/s00421-016-3365-3. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Zaken S, Eliakim A, Nemet D, Rabinovich M, Kassem E, Meckel Y. Differences in MCT1 A1470T polymorphism prevalence between runners and swimmers. Scand J Med Sci Sports. 2015;25(3):365–371. doi: 10.1111/sms.12226. [DOI] [PubMed] [Google Scholar]
- 15.Sawczuk M, Banting LK, Cieszczyk P, Maciejewska-Karlowska A, Zarebska A, Leonska-Duniec A, Jastrzebski Z, Bishop DJ, Eynon N. MCT1 A1470T: a novel polymorphism for sprint performance? J Sci Med Sport. 2015;18(1):114–118. doi: 10.1016/j.jsams.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Yvert T, Miyamoto-Mikami E, Murakami H, Miyachi M, Kawahara T, Fuku N. Lack of replication of associations between multiple genetic polymorphisms and endurance athlete status in Japanese population. Physiol Rep. 2016;4(20):e13003. doi: 10.14814/phy2.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilherme JPLF, Bertuzzi R, Lima-Silva AE, Pereira AC, Lancha Junior AH. Analysis of Sports-Relevant Polymorphisms in a large Brazilian Cohort of Top-Level Athletes. Ann Hum Genet. 2018;82(5):254–264. doi: 10.1111/ahg.12248. [DOI] [PubMed] [Google Scholar]
- 18.Onali F, Calo CM, Massidda M, Alvarez-Alvarez MM, Esteban ME. An unexpected world population variation of MCT1 polymorphism 1470T > A involved in lactate transport. Eur J Sport Sci. 2018;18(10):1376–1382. doi: 10.1080/17461391.2018.1491629. [DOI] [PubMed] [Google Scholar]
- 19.Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature. 2015;526(7571):68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Zaken S, Meckel Y, Nemet D, Kassem E, Eliakim A. Genetic Basis for the Dominance of Israeli Long-Distance Runners of Ethiopian Origin. J Strength Cond Res. 2019 doi: 10.1519/JSC.0000000000002989. In press. [DOI] [PubMed] [Google Scholar]
- 21.Kehdy FS, Gouveia MH, Machado M, Magalhaes WC, Horimoto AR, Horta BL, Moreira RG, Leal TP, Scliar MO, Soares-Souza GB, Rodrigues-Soares F, Araujo GS, Zamudio R, Sant Anna HP, Santos HC, Duarte NE, Fiaccone RL, Figueiredo CA, Silva TM, Costa GN, Beleza S, Berg DE, Cabrera L, Debortoli G, Duarte D, Ghirotto S, Gilman RH, Goncalves VF, Marrero AR, Muniz YC, Weissensteiner H, Yeager M, Rodrigues LC, Barreto ML, Lima-Costa MF, Pereira AC, Rodrigues MR, Tarazona-Santos E. Origin and dynamics of admixture in Brazilians and its effect on the pattern of deleterious mutations. Proc Natl Acad Sci U S A. 2015;112(28):8696–8701. doi: 10.1073/pnas.1504447112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pena SD, Di Pietro G, Fuchshuber-Moraes M, Genro JP, Hutz MH, Kehdy Fde S, Kohlrausch F, Magno LA, Montenegro RC, Moraes MO, de Moraes ME, de Moraes MR, Ojopi EB, Perini JA, Racciopi C, Ribeiro-Dos-Santos AK, Rios-Santos F, Romano-Silva MA, Sortica VA, Suarez-Kurtz G. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6(2):e17063. doi: 10.1371/journal.pone.0017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardena MM, Ribeiro-Dos-Santos A, Santos S, Mansur AJ, Pereira AC, Fridman C. Assessment of the relationship between self-declared ethnicity, mitochondrial haplogroups and genomic ancestry in Brazilian individuals. PLoS One. 2013;8(4):e62005. doi: 10.1371/journal.pone.0062005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Haro C, Soria M, Vicente J, Fanlo AJ, Sinues B, Escanero JF. Variants of the Solute Carrier SLC16A1 Gene (MCT1) Associated With Metabolic Responses During a Long-Graded Test in Road Cyclists. J Strength Cond Res. 2015;29(12):3494–3505. doi: 10.1519/JSC.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 25.Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, Lyon E, Ward BE. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. 2008;10(4):294–300. doi: 10.1097/GIM.0b013e31816b5cae. [DOI] [PubMed] [Google Scholar]
- 26.Ahmetov I, Kulemin N, Popov D, Naumov V, Akimov E, Bravy Y, Egorova E, Galeeva A, Generozov E, Kostryukova E, Larin A, Mustafina L, Ospanova E, Pavlenko A, Starnes L, Zmijewski P, Alexeev D, Vinogradova O, Govorun V. Genome-wide association study identifies three novel genetic markers associated with elite endurance performance. Biol Sport. 2015;32(1):3–9. doi: 10.5604/20831862.1124568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coetzer P, Noakes TD, Sanders B, Lambert MI, Bosch AN, Wiggins T, Dennis SC. Superior fatigue resistance of elite black South African distance runners. J Appl Physiol (1985) 1993;75(4):1822–1827. doi: 10.1152/jappl.1993.75.4.1822. [DOI] [PubMed] [Google Scholar]
- 28.Harley YX, Kohn TA, St Clair Gibson A, Noakes TD, Collins M. Skeletal muscle monocarboxylate transporter content is not different between black and white runners. Eur J Appl Physiol. 2009;105(4):623–632. doi: 10.1007/s00421-008-0942-0. [DOI] [PubMed] [Google Scholar]
- 29.Kohn TA, Essen-Gustavsson B, Myburgh KH. Do skeletal muscle phenotypic characteristics of Xhosa and Caucasian endurance runners differ when matched for training and racing distances? J Appl Physiol (1985) 2007;103(3):932–940. doi: 10.1152/japplphysiol.01221.2006. [DOI] [PubMed] [Google Scholar]
- 30.Saltin B, Larsen H, Terrados N, Bangsbo J, Bak T, Kim CK, Svedenhag J, Rolf CJ. Aerobic exercise capacity at sea level and at altitude in Kenyan boys, junior and senior runners compared with Scandinavian runners. Scand J Med Sci Sports. 1995;5(4):209–221. doi: 10.1111/j.1600-0838.1995.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 31.Weston AR, Karamizrak O, Smith A, Noakes TD, Myburgh KH. African runners exhibit greater fatigue resistance, lower lactate accumulation, and higher oxidative enzyme activity. J Appl Physiol (1985) 1999;86(3):915–923. doi: 10.1152/jappl.1999.86.3.915. [DOI] [PubMed] [Google Scholar]
- 32.Santos-Concejero J, Tucker R, Myburgh KH, Essen-Gustavsson B, Kohn TA. Greater performance impairment of black runners than white runners when running in hypoxia. Int J Sports Med. 2014;35(10):809–816. doi: 10.1055/s-0034-1367012. [DOI] [PubMed] [Google Scholar]
- 33.Joyner MJ, Coyle EF. Endurance exercise performance: the physiology of champions. J Physiol. 2008;586(1):35–44. doi: 10.1113/jphysiol.2007.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demarle AP, Heugas AM, Slawinski JJ, Tricot VM, Koralsztein JP, Billat VL. Whichever the initial training status, any increase in velocity at lactate threshold appears as a major factor in improved time to exhaustion at the same severe velocity after training. Arch Physiol Biochem. 2003;111(2):167–176. doi: 10.1076/apab.111.2.167.14003. [DOI] [PubMed] [Google Scholar]
- 35.Midgley AW, Mc Naughton LR, Wilkinson M. The relationship between the lactate turnpoint and the time at VO2max during a constant velocity run to exhaustion. Int J Sports Med. 2006;27(4):278–282. doi: 10.1055/s-2005-865668. [DOI] [PubMed] [Google Scholar]
- 36.Juel C, Halestrap AP. Lactate transport in skeletal muscle – role and regulation of the monocarboxylate transporter. J Physiol. 1999;517(Pt 3):633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki S, Futagi Y, Kobayashi M, Ogura J, Iseki K. Functional characterization of 5-oxoproline transport via SLC16A1/MCT1. J Biol Chem. 2015;290(4):2303–2311. doi: 10.1074/jbc.M114.581892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ama PF, Simoneau JA, Boulay MR, Serresse O, Theriault G, Bouchard C. Skeletal muscle characteristics in sedentary black and Caucasian males. J Appl Physiol (1985) 1986;61(5):1758–1761. doi: 10.1152/jappl.1986.61.5.1758. [DOI] [PubMed] [Google Scholar]
- 39.Fishbein WN, Merezhinskaya N, Foellmer JW. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve. 2002;26(1):101–112. doi: 10.1002/mus.10168. [DOI] [PubMed] [Google Scholar]
- 40.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 41.Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol. 1999;276(5):E843–848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- 42.Lundby C, Robach P. Performance Enhancement: What Are the Physiological Limits? Physiology (Bethesda) 2015;30(4):282–292. doi: 10.1152/physiol.00052.2014. [DOI] [PubMed] [Google Scholar]
- 43.San-Millan I, Brooks GA. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018;48(2):467–479. doi: 10.1007/s40279-017-0751-x. [DOI] [PubMed] [Google Scholar]
- 44.Brooks GA. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018;27(4):757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]