Abstract
Intervertebral disc (IVD) degeneration is associated with elevated levels of inflammatory cytokines implicated in disease aetiology and matrix degradation. Toll-like receptor-4 (TLR4) has been shown to participate in the inflammatory responses of the nucleus pulposus (NP) and its levels are upregulated in disc degeneration. Activation of TLR4 in NP cells leads to significant, persistent changes in cell biophysical properties, including hydraulic permeability and osmotically active water content, as well as alterations to the actin cytoskeleton. The study hypothesis was that inflammation-induced changes to cellular biomechanical properties and actin cytoskeleton of NP cells could be prevented by inhibiting TLR4 signalling. Isolated NP cells from bovine discs were treated with lipopolysaccharide (LPS), the best studied TLR4 agonist, with or without treatment with the TLR4 inhibitor TAK-242. Cellular volume regulation responses to step osmotic loading were measured and the transient volume-response was captured by time-lapse microscopy. Volume-responses were analysed using mixture theory framework to investigate hydraulic permeability and osmotically active intracellular water content. Hydraulic permeability and cell radius were significantly increased with LPS treatment and these changes were blocked in cells treated with TAK-242. LPS-induced remodelling of cortical actin and IL-6 upregulation were also mitigated by TAK-242 treatment. These findings indicated that TLR4 signalling participated in NP cell biophysical regulation and may be an important target for mitigating altered cell responses observed in IVD inflammation and degeneration.
Keywords: Intervertebral disc, mechanobiology, inflammation, cell mechanics, toll-like receptor-4, actin cytoskeleton, biophysical properties
Introduction
Back pain stemming from IVD degeneration is a leading cause of disability worldwide and causes significant economic and societal burden (Cheung et al., 2009; Diseases and Injuries, 2020; Hurwitz et al., 2018). Back pain from DD is conventionally treated with immunosuppressive (steroid) injections or surgery, both of which are only partially effective (Lurie et al., 2014; Weinstein et al., 2007; Weinstein et al., 2006; Weinstein et al., 2008; Weinstein et al., 2009; Weinstein et al., 2010). Therefore, there is a significant clinical need for better approaches to mitigate degeneration and promote recovery of spine function. DD is defined by changes to ECM (Antoniou et al., 1996; Keshari et al., 2008; Millward-Sadler et al., 2009; Pearce et al., 1987; Roughley et al., 2002), decreased disc height and altered mechanical function (Fujita et al., 1997; Fujiwara et al., 2000; Gu et al., 1999a; Gu et al., 1999b; Iatridis et al., 2013; Johannessen and Elliott, 2005; O’Connell et al., 2011). Proteoglycan degradation in DD occurs in part due to increased catabolic enzymes induced by inflammatory mediators (e.g. MMPs, ADAMTs). Increases in expression of NO, PGE2 (Kang et al., 1996; Kang et al., 1997; Liu et al., 2001) and pro-inflammatory cytokines, especially TNFα, IL-1β and IL-6, have been shown to occur during DD (Ahn et al., 2002; Bachmeier et al., 2007; Le Maitre et al., 2005; Le Maitre et al., 2006; Le Maitre et al., 2007; Seguin et al., 2005; Shamji et al., 2010; Zhang et al., 2016). Recent studies have highlighted a significant role of innate immune activation, particularly of TLRs in DD (Klawitter et al., 2014; Maidhof et al., 2014; Quero et al., 2013; Rajan et al., 2013), where a pro-inflammatory signalling can trigger DD in the absence of a traumatic insult (Lai et al., 2016; Rajan et al., 2013). Activation of TLR4 by LPS has been shown to induce a pro-inflammatory cascade in IVD cells, with subsequent loss of IVD matrix integrity (Rajan et al., 2013). TLRs are members of a receptor family activated by DAMPs that results in persistent pro-inflammatory signalling (Lin et al., 2011; Risbud and Shapiro, 2014). While inflammation is a necessary step in wound healing, persistent inflammation can damage tissues and may contribute to pain (Tracey, 2007). IVD expression of TLR2 and TLR4 increases with increasing DD severity and mediates catabolic and inflammatory processes (Klawitter et al., 2014; Maidhof et al., 2014; Quero et al., 2013; Rajan et al., 2013).
Innate inflammation also has consequences on cell mechanobiology. Activation of TLR4 in NP cells from the gelatinous central region of the IVD alters the actin cytoskeleton and cell mechanotransduction by increasing cellular hydraulic permeability and cell size (Maidhof et al., 2014). The cytoskeleton participates in sensing and transmission of mechanical signals from the ECM to the cells (Blain, 2009; Chen et al., 2004; Hayes et al., 1999; Li et al., 2008). NP cells are spherical and their cytoskeleton is composed of actin microfilaments (F-actin), microtubules and vimentin intermediate filaments. NP cells are derived from the notochord and, in immature NP cells, F-actin has a punctuate structure that is constrained to the cell cortex (Li et al., 2008). This diffuse cytoskeleton regulates the cell response to compressive loading (Chen et al., 2004). NP cells are continuously subjected to hydrostatic and osmotic pressure induced by spinal loading, and mechanical loading in the disc is known to regulate cell volume (Mavrogonatou and Kletsas, 2012), gene expression (Neidlinger-Wilke et al., 2006; Wuertz et al., 2007), protein synthesis (Neidlinger-Wilke et al., 2012; Sivan et al., 2006) as well as being critical during growth and development (Urban, 2002). Maintenance of biomechanical properties at the cellular level also prevents degenerative changes within the disc at the tissue level (Hernandez et al., 2020). Protection against changes in cellular mechanical properties, using inhibitors of actomyosin contractility, mitigates degenerative changes at the tissue level, including the prevention of GAG loss and inhibition of pro-inflammatory cytokine and MMPs expression (Hernandez et al., 2020). As such, the maintenance of cellular mechanical properties of disc cells is a promising strategy for the prevention of degenerative effects within the disc.
The goal of the present study was to investigate the role of TLR4 in mediating cell morphological and cytoskeletal changes in NP cells and on biomechanical properties, specifically cellular hydraulic permeability. TLR4 activation has long-term consequences on NP cells, as cells do not spontaneously recover to baseline properties upon activation in vitro (Maidhof et al., 2014). Therefore, the TLR4 inhibitor, TAK-242, was evaluated for protection against alterations of pro-inflammatory signalling, cell biomechanical properties and cytoskeleton. The study hypothesis was that TLR4 inhibition could protect against changes to NP cell biomechanical properties by mitigating alterations to the actin cytoskeleton and cell size.
Materials and Methods
Cell isolation and culture
NP tissue was harvested under sterile conditions from the lumbar spines (L1/2-L5/6) of 4 slaughtered juvenile cows (6–9 weeks old) that were obtained from an abattoir (Green Village Packing Company, Green Village, NJ, USA; permission was obtained to use these animal parts for research). Cells were isolated from pooled (L1/2-L5/6), minced NP tissue using 0.3 mg/mL collagenase type I + 0.3 mg/mL collagenase type II (Sigma) in complete medium [DMEM (Gibco) +10 % FBS (Atlanta Biologicals, Flowery Branch, GA, USA) + 1 % penicillin-streptomycin solution (Corning)] for up to 4 h. Then, digests were strained using a 70 μm cell strainer and cells were collected from digests by centrifugation (400 ×g, 5 min). Next, isolated cells were cultured in complete medium and standard culture conditions (37 °C, 5 % CO2) and expanded to passage 2 prior to further treatment and analysis. Medium was changed twice weekly.
Part 1: evaluating dose-response of TAK-242 on LPS-stimulated NP cells
LPS (Sigma-Aldrich) was suspended in sterile dH2O by sonication (10 mg/mL) and diluted in DMEM to 0.1 μg/mL. TAK-242 (TLR4 inhibitor, EMD Millipore) was solubilised in pure DMF (Thermo Scientific) (25 mmol/L). TAK-242 stock was diluted in DMEM to create treatment solutions of varying doses: 0.1, 1, 10 or 100 μmol/L TAK-242. DMF equivalent to the highest concentration of TAK-242 (0.4 % DMF) was added to untreated controls and LPS groups to account for the possible effects of the solvent in TAK-242-treated groups.
To evaluate TAK-242 dose-response on LPS-stimulated cells, NP cells were transferred to 24-well plates (105 cells/well) and cultured overnight in complete medium. Then, complete medium was replaced with either DMEM alone (untreated group), DMEM supplemented with LPS (0.1 μg/mL, LPS group) or DMEM supplemented with LPS (0.1 μg/mL) + TAK-242 at 0.1, 1, 10 or 100 μmol/L. In the LPS + TAK-242 groups, TAK-242 was added to the cells 1 h prior to the addition of LPS, to allow time for the intracellular inhibitor to act on the cells. Then, TAK-242 was maintained in the culture for 24 h along with the LPS co-treatment. After 24 h, medium supernatants were collected and assayed for nitrite and LDH release. Nitrite release, a breakdown product of released NO, was measured in cell supernatants by Griess reaction using a commercially available assay (Griess Reagent System, Promega), according to manufacturer’s protocol. The NO release response was analysed to determine the IC50, the concentration of TAK-242 inhibitor at which the NO release was reduced by half. The IC50 was determined as an absolute IC50 using a linear least square fit of percentage inhibition of NO release by TAK-242. Results were computed using GraphPad Prism software. LDH levels, an indicator of cell death, was measured using the Roche Cytotoxicity Detection KitPLUS according to manufacturer’s protocols. For LDH assay, a positive cytotoxic kill control was used by lysing an untreated group of cells with Triton X-100 (10×). Cytotoxicity (%) was determined using untreated and Triton X-100-treated cells as negative (0 %) and positive (100 %) cytotoxicity controls, respectively.
To further characterise the TAK-242 dose-response, IL-6 expression was examined at the end of the 24 h treatment. Total RNA was extracted from treated NP cells using RNeasy kit (QIAGEN) following manufacturer’s instructions. Concentration and purity were measured by NanoDrop, with 260/280 ratios between 1.8 and 2.0 for all samples. Primers listed in Table 1 were designed using the Universal Probe Library v 2.45 (Roche). One-step quantitative PCR was performed using ABI7900 instrument and Eurogentec kit (RT-QPRT-032X, Eurogentec, Liège, Belgium) with 100 ng RNA and the following amplification protocol: 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Analysis of gene expression was performed with RQ Manager 1.2 software using the 2− (ΔΔCt) method to calculate fold change relative to LPS treatment group.
Table 1.
Part 1 primers.
| Gene | Forward | Reverse |
|---|---|---|
| IL-6 | TGATCCAGATCCTGAAGCAAA | CATCTTCTCCAGCAGGTCAGT |
| GAPDH (housekeeping) | GCATCGTGGAGGGACTTATG | GGGGCCATCCACAGTCTT |
To confirm the specificity of NP cell responses to LPS stimulation and not due to other potential contaminates in the culture, nitrite levels were measured in NP cells co-treated with PmB, an antibiotic that binds to LPS in solution. PmB (1, 25 and 50 μg/mL) was added to the medium 1 h prior to treatment of cells with LPS to ensure its binding to LPS.
Part 2: evaluating the effects of TLR4 inhibition on cell mechanobiology
Biophysical properties and cytoskeletal imaging
Based on part 1 results, subsequent experiments employed 1 μmol/L TAK-242 to achieve more than 50 % inhibition of TLR4 activation by LPS. In part 2 studies, NP cells were cultured in one of the following 4 groups: untreated, LPS (0.1 μg/mL), TAK-242 (1 μmol/L) or LPS + TAK-242 (Fig. 1). As in part 1, TAK-242 was added to the cells first as a pre-treatment, 1 h prior to the addition of LPS. Then, LPS or LPS + TAK-242 co-treatment was continued for 24 h.
Fig. 1. LPS and TAK-242 treatment study design and outcome measures.
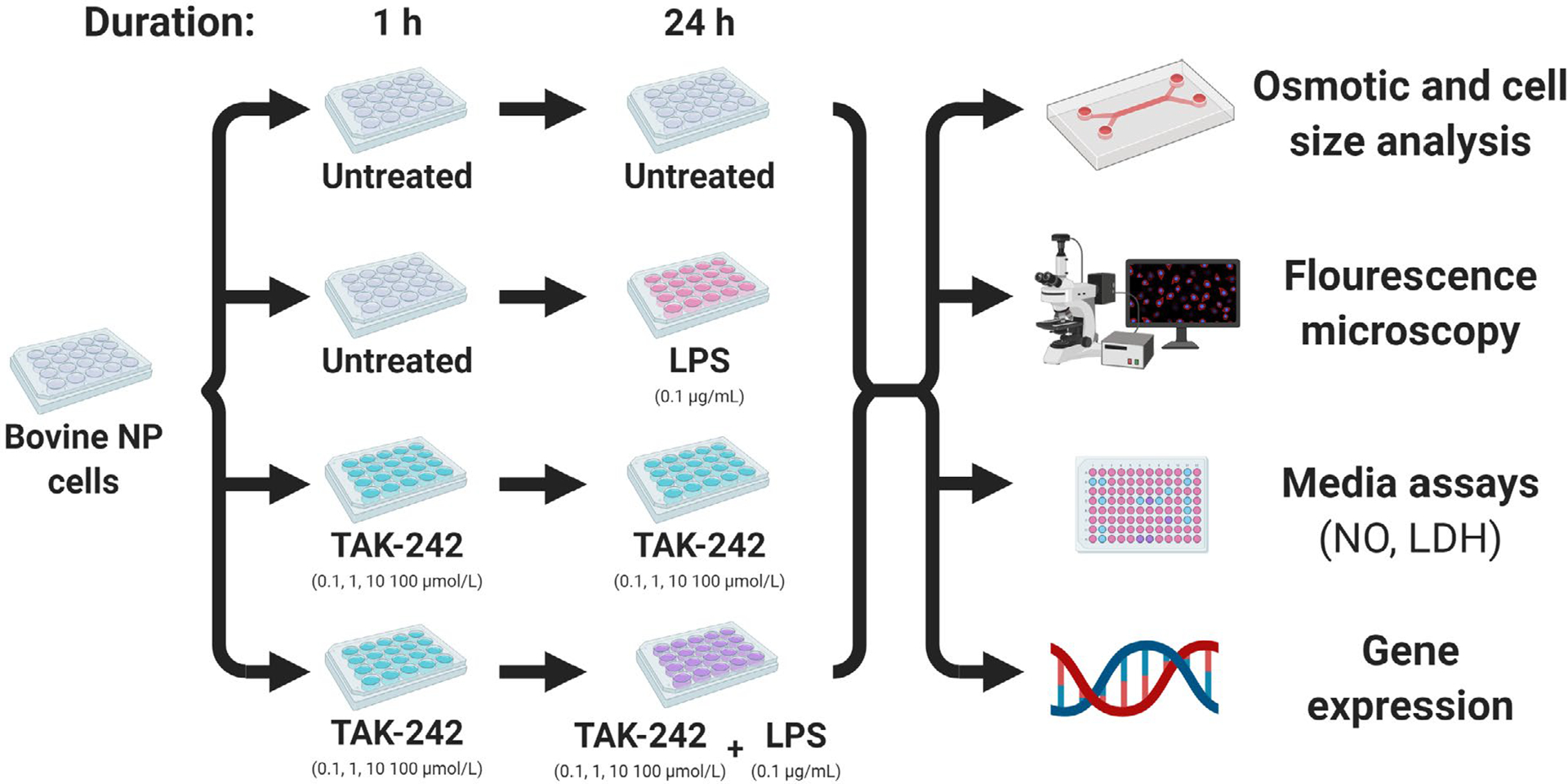
For part 2 studies, cells were tested under one of 4 treatment groups: (i) untreated, (ii) LPS, (iii) TAK-242, (iv) LPS + TAK-242. TAK-242 was introduced into the cell culture 1 h prior to 24 h co-treatment with LPS.
NP cells from each treatment group were trypsinised and seeded in a custom-made Y-shaped microfluidic channel (Albro et al., 2007; Chao et al., 2005; Maidhof et al., 2014). The PDMS channel (10 mm length, 300 μm width, 200 μm height) was sealed over a poly-D-lysine-treated glass slide. 40 μL of cell suspension were placed into the chamber. Then, the seeded cells were incubated at 37 °C for 30 min to allow attachment but retain a rounded morphology. Maintenance of cells in rounded morphology for this measurement was necessary to allow for accurate determination of cell volume by microscopy. Cells were equilibrated in a 333 mOsm/L NaCl solution for 5 min through the addition of 100 μL of 333 mOsm solution to each of the two upstream wells of the osmotic chamber. Loading was performed at room temperature to observe passive osmotic properties of the cells as cells do not exhibit active volume recovery when loaded in NaCl under these conditions and experimental duration (Albro et al., 2009). After equilibration, a single hyper-osmotic loading step was applied (333 mOsm to 466 mOsm) followed by a hypo-osmotic loading step (466 mOsm to 333 mOsm) using NaCl solutions. These concentrations were selected based on typical osmolarities found within the disc in vivo (Urban and McMullin, 1988) and were used in prior studies (Hernandez et al., 2020; Maidhof et al., 2014). During osmotic loading, cells were imaged using an inverted confocal microscope using DIC filters. Images (0.2 μm/pixel) were acquired at a frequency of 0.5 Hz. A MatLab routine was used to segment individual cells and calculate a volume response over time for each cell (Maidhof et al., 2014). The rounded morphology of imaged cells was used to compute cell volume from cross-sectional measurements. To track changes in basal cell radius due to treatments, NP cells post treatment were plated in the microfluidic chamber, incubated for 30 min and equilibrated at 333 mOsm NaCl. Due to the smaller effect size of treatment on radii measurements, a larger number of observations were made (n = 44–91 cells per group) when compared to the volume-response tests (n = 10–13 cells per group).
Analysis of volume response
The cell volume response was analysed with the Kedem-Kachalsky model using a mixture theory framework (Albro et al., 2007; Albro et al., 2009; Ateshian et al., 2006; Maidhof et al., 2014). NaCl at room temperature is modelled as a non-permeating solute (Albro et al., 2009; Bush and Hall, 2001), allowing the differential equation describing cell volume V(t) to be simplified to
where A is the volume-dependent cell surface area, a is the cell radius, Lp is hydraulic permeability, R is the universal gas constant, θ is the absolute temperature, Ci and Ce are the intracellular and extracellular osmolarities. Ce is defined by the experimentally applied osmotic solution, while Ci is governed by
where ni is the number of moles of solute within the cell. Since NaCl is modelled as a non-permeating solute, ni can be described by
where Φi is the volume fraction of osmotically active water in the cytoplasm that changes as water enters or leaves the cell according to the equation
The subscript r denotes the reference state of V and Φi (at the beginning of each loading step). Once the cell has reached equilibrium after an osmotic load is applied and
the reference intracellular water content (Φir) can be determined using the equation
The measured volume response of each cell and osmotic step was curve-fit to this system of equations using a custom MatLab protocol to calculate values for Φir and Lp.
Immunofluorescence
At end of treatment, some cells were trypsinised and plated on poly-L-lysine-coated glass coverslips for 25 min to maintain a rounded morphology. Cells were immediately fixed with 4 % paraformaldehyde in PBS for 10 min at room temperature, permeabilised with 0.1 % Triton-X100 in PBS for 10 min and blocked in 1 % BSA in PBS + 0.1 % Tween-20 for 1 h at room temperature. Primary antibodies anti-β-tubulin (1 : 1,000, Sigma, T8328) or anti-vimentin (1 : 1,000, Abcam, ab8069) were incubated in blocking buffer overnight at 4 °C. After three 5 min washes with PBS + 0.1 % Tween-20, samples were incubated with secondary antibody anti-mouse conjugated to Alexa 488 (1 : 500, A11029, Molecular Probes) and rhodamine-phalloidin (1 : 500, R415, Molecular Probes) in blocking buffer for 1 h at room temperature. Coverslips were again washed with PBS + 0.1 % Tween-20 for 5 min, mounted using Prolong Diamond (Thermo-Fisher Sci) and visualised in a Olympus XL70 laser scanning confocal microscope using a 40× objective.
Gene expression
To further characterise the effects of TAK-242 in mitigating the response of LPS, the expression of pro-inflammatory, matrix degradation and ECM-related genes was examined at the end of the 24 h treatment period. Total RNA was extracted from treated NP cells using the QIAGEN RNeasy kit following manufacturer’s instructions. Concentration and purity were measured by NanoDrop, with 260/280 ratios between 1.8 and 2.0 for all samples. 200 ng of total RNA were converted to cDNA using the iScript cDNA Synthesis kit (BioRad). Primers listed in Table 2 were designed using the Integrated DNA Technologies PrimerQuest Tool (Integrated DNA Technologies). Quantitative PCR was performed using QuantiStudio 6 Flex system (Applied Biosystems, ThermoFisher Scientific) and iTaq Universal SYBR Green kit (BioRad) with the following amplification protocol: 95 °C for 30 s followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Analysis of gene expression was performed by QuantiStudio Real-Time PCR Software v1.3 software (Applied Biosystems) using the 2− (ΔΔCt) method to calculate fold change relative to the untreated group.
Table 2.
Primers for gene expression.
| Gene | Forward | Reverse |
|---|---|---|
| IL-1β | GTTAGAGTGCCATCCCTTCTGTC | CCATTGCCTTCTCCGCTATT |
| IL-6 | CCAGAACGAGTATGAGGGAAATC | TTGTGGCTGGAGTGGTTATTAG |
| TNFα | GCCAACTCCCTCTGTTTATGT | GCCAACTCCCTCTGTTTATGT |
| TLR4 | CATGGGCTTAGAGCAACTAGAA | GCGGAGGTTTCTGAGTGATAG |
| HMGB1 | ACACTGCTGCGGATGATAAG | GGTTTCCCTTTAGCTCGGTATG |
| MIF | TGGCGCGCACAGATTC | GCGTCTCCACACCGTTTATT |
| ADAMTS4 | AGACACAAGCAGGGAGAAAC | ACCTTCAGAGGAGTTGGAGA |
| ADAMTS5 | GACCAGATTCACTGCCTACTT | CGTTGATGTCGATGATGGTTTC |
| MMP3 | GTTGGTTTCTTCAGCACCTTTC | CAGCATCTCTGGGTAAATCCTT |
| MMP13 | GAGCACTCATGTTTCCCATCTA | GAGTGGCAGTGGTGAATCTT |
| ACAN | GAGTGGCAGTGGTGAATCTT | CCACAGATCCTAAGCCTTCTTC |
| GAPDH (housekeeping) | GATGCTGGTGCTGAGTATGT | GCAGAAGGTGCAGAGATGAT |
Statistical analysis
Results are presented as mean ± standard deviation, unless otherwise noted.
Part 1: NO release, LDH release and IL-6 expression outcomes were analysed by one-way ANOVA, with TAK-242 or PmB dose as an independent variable. Pairwise comparison was made by Fisher LSD post-hoc test, with p < 0.05 considered statistically significant (Statistica, TIBCO Software, Palo Alto, CA, USA).
Part 2: osmotic analysis, cell size and gene expression data were analysed by two-way ANOVA for effect of LPS and TAK-242 as independent variables. Pairwise comparisons were made using the Fisher LSD post-hoc test, with p < 0.05 considered statistically significant (Statistica). Differences in hydraulic permeability and osmotically active water between hyper- and hypo-osmotic steps were tested for within each treatment group using unpaired t-test, with p < 0.05 considered statistically significant (Statistica).
Results
Part 1: evaluating dose-response of TAK-242 in LPS-stimulated NP cells
No significant changes in cytotoxicity, as demonstrated by LDH levels, were observed in the LPS- or TAK-242-treated groups versus untreated control (p > 0.72) (Fig. 2a). Moreover, LDH levels in all groups were significantly lower than levels measured in the cytotoxic kill control group (p < 0.0001) (Fig. 2a). These results indicated that LPS with or without TAK-242 did not induce a significant loss of cell viability. Stimulation of cells with LPS significantly increased NO release (p < 0.05, Fig. 2b). Treatment of NP cells with the TLR4 inhibitor TAK-242 at 0.1, 1, 10 and 100 μmol/L inhibited LPS-induced NO release in a dose-dependent manner (p < 0.05, Fig. 2b). An IC50 of 0.0467 μmol/L TAK-242 was determined from a linear regression of normalised NO inhibition data (R2 = 0.80). TAK-242 dosages above the IC50 (0.1, 1, 10 and 100 μmol/L) all significantly reduced NO levels by 52.3 %, 74.3 %, 81.1 % and 101.5 %, respectively (Fig. 2b). Cells stimulated with LPS had significantly higher IL-6 expression when compared to untreated cells (454 fold, p < 0.001). 0.1, 1 and 10 μmol/L TAK-242 had significantly lower IL-6 expression versus LPS-only group (48.2 %, 55.0 % and 71.6 % reduction vs. LPS, respectively, p < 0.05, Fig. 2c). To confirm the specificity of LPS responses, NO release from cells co-treated with LPS and PmB was measured. Cells co-treatment with PmB + LPS at all tested doses had significantly lower NO release (1 μg/mL: 0.515 ± 0.082 μmol/L; 25 μg/mL: 0.332 ± 0.05 μmol/L; 50 μg/mL: 0.497 ± 0.082 μmol/L) compared to LPS-treated cells (4.212 ± 0.429 μmol/L, p < 0.05 for each dose group comparison). No significant difference in NO levels were observed between untreated cells (0.259 ± 0.077 μmol/L) and cells treated with LPS + 25 μg/mL PmB (p = 0.56) or LPS + 50 μg/mL PmB (p = 0.056). Cells treated with 50 μg/mL PmB alone also had comparable NO levels (0.204 ± 0.077 μmol/L) to untreated cells (p = 0.86, Fig. 2d), confirming that a potentially low (background) level of endotoxin was not a major factor in this culture model/system.
Fig. 2. TLR4 inhibition mitigated LPS-induced inflammatory response.
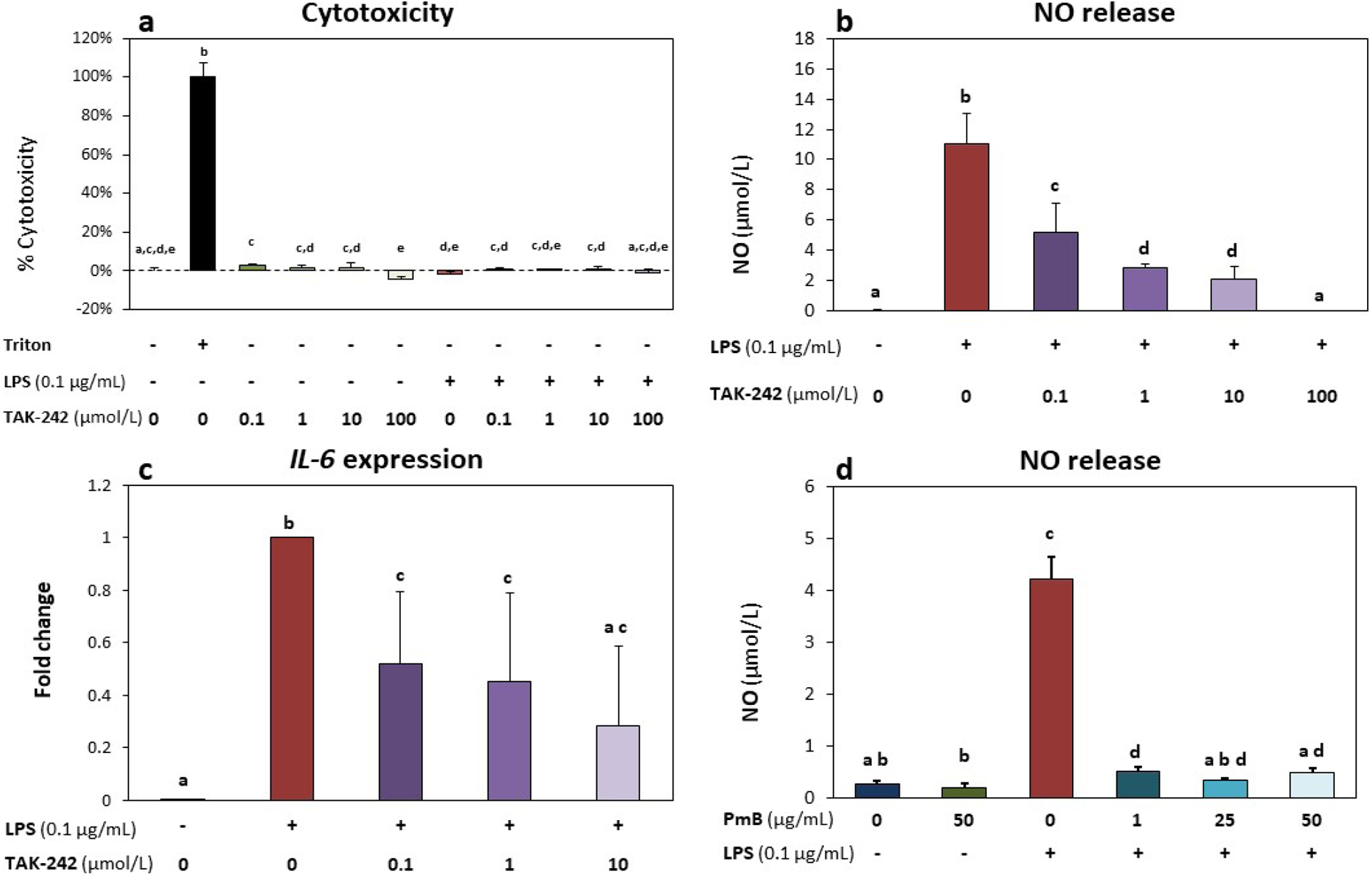
(a) Cytotoxicity (%) based on LDH levels did not increase with either LPS (0.1 μg/mL) or TAK-242 treatment, indicating no loss in cell viability in any of the experimental treatment group. Cells treated with 10× Triton X-100 for 10 min at the end of culture were used as a cytotoxic (kill/positive) control. (b) TAK-242 inhibited LPS-induced increases in NO release in a dose-dependent manner. (c) Cells treated with LPS showed significantly up-regulated expression of IL-6; TAK-242 co-treatment significantly decreased IL-6 expression when compared to LPS-only group. Gene expression data shown as fold change versus LPS-only group. (d) NO release increased in cells treated with LPS. PMB inhibited LPS-induced increase in NO release. All groups were supplemented with 0.4 % DMF, equivalent to vehicle levels in the highest dose of TAK-242. All graphs show mean ± standard deviation (n = 3 biological replicates per group); groups with different letters indicate significant difference (p < 0.05) between groups.
Part 2: osmotic properties and equilibrium cell radius
NP cell radius at isotonic osmolarity (333 mOsm/L NaCl) was found to increase with LPS (0.1 μg/mL) treatment. Significant increases in cell radius were found in cells treated with LPS (8.56 ± 1.52 μmol/L, p < 0.005) compared to untreated control (7.74 ± 1.59 μmol/L) (Fig. 3b). However, the radius of cells co-treated with TAK-242 + LPS (7.61 ± 1.41 μmol/L) was significantly lower than that of LPS-treated cells (p < 0.01) and showed no significant difference from that of untreated cells (p = 0.6).
Fig. 3. TLR4 inhibition mitigated LPS-induced changes in NP cell biophysical properties.
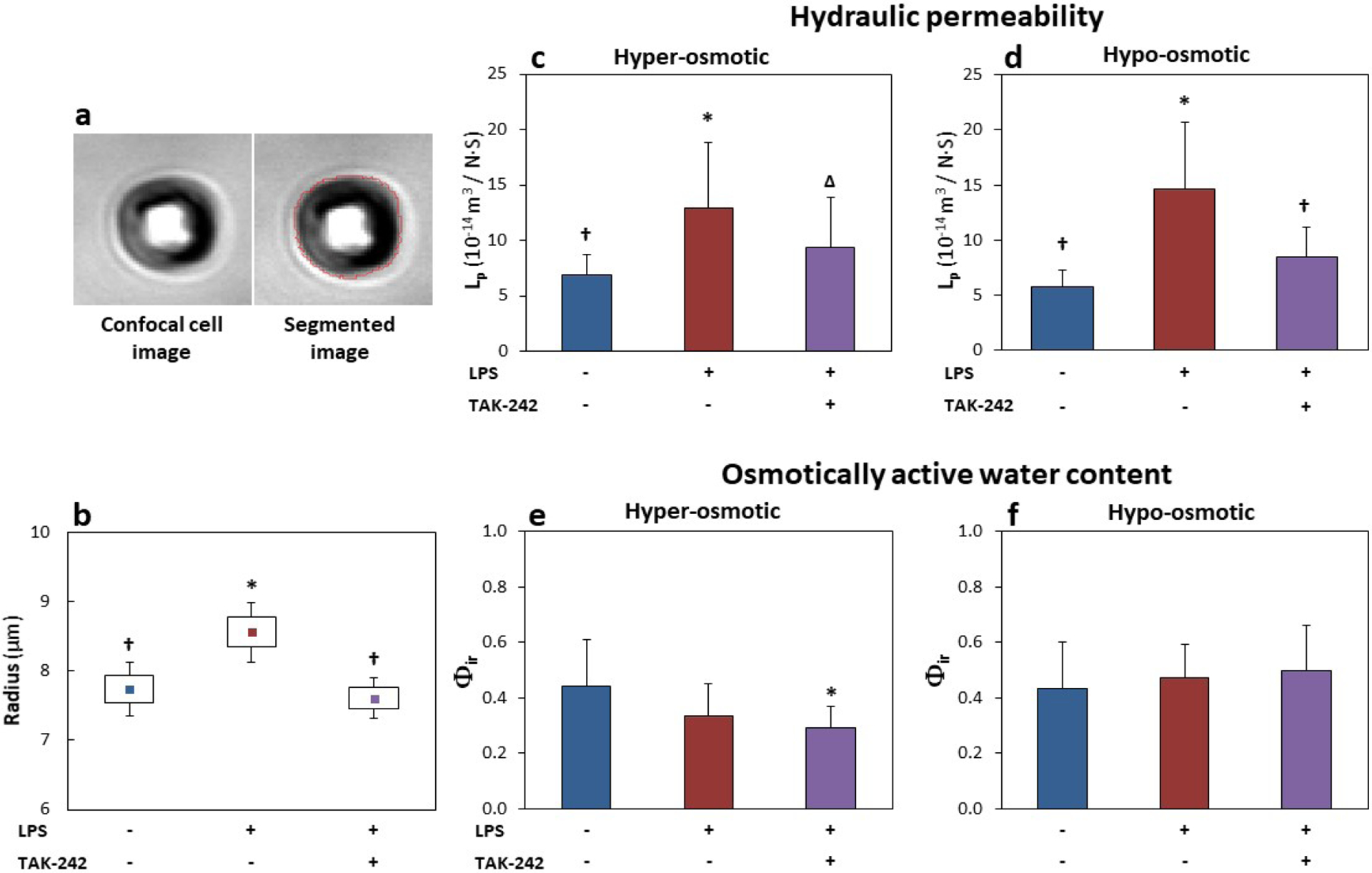
(a) Representative DIC cell image used in cell radius and osmotic analysis and the resulting cell area attained by image segmentation (red line). (b) Cell radius measured at 333 mOsm/L increased with LPS treatment (0.1 μg/mL) and returned to untreated levels with addition of 1 μmol/L TAK-242. (c,d) Lp was significantly increased with LPS stimulation and returned to untreated levels with TAK-242 treatment for both (b) hyper- and (c) hypo-osmotic steps. (e,f) Φir was only significantly affected in the LPS + TAK-242 co-treatment group for the hyper-osmotic step. (d) No other significant differences in Φir were noted. All groups were supplemented with 0.4 % DMF. (b) Data show mean (▪), standard error (boxes) and 95 % confidence intervals (n = 44–91 cells per group). (c-f) Data show mean ± standard deviation (n = 8–13 cells per group). * p < 0.05 vs. untreated; † p < 0.05 vs. LPS alone; Δ p = 0.11 vs. LPS alone.
Lp significantly increased with LPS stimulation in both hypo- and hyper-osmotic loading steps. Treatment with 1 μmol/L of TAK-242 significantly mitigated LPS-induced increase in Lp for the hypo-osmotic step. Co-treatment of cells with LPS + TAK-242 significantly decreased hypo-osmotic Lp compared to cells receiving LPS-only stimulation (Fig. 3c,d, Table 3). A trend for lower hyper osmotic Lp was observed in LPS + TAK-242 vs. LPS-only groups (p = 0.11), though this effect did not reach statistical significance. No significant differences in Lp were observed between untreated cells and TAK-242-treated groups. No significant differences in Lp were found between the hyper- and hypo-osmotic steps.
Table 3.
LP determined at each osmotic step.
| Lp (10−14 m3/N·S) | ||
|---|---|---|
| Treatment | Hyper-osmotic | Hypo-osmotic |
| Untreated | 6.85 ±1.91 | 5.75 ± 1.54 |
| LPS (0.1 μg/mL) | 12.86 ± 5.93* | 14.66 ± 6.08* |
| LPS + TAK-242 (1 μmol/L) | 9.32 ± 4.56Δ | 8.43 ± 2.72† |
p < 0.05 vs. untreated.
p < 0.05 vs. LPS.
p = 0.11 vs. LPS.
No significant changes in Φir were found due to inflammatory stimulation with LPS (Fig. 3e,f; Table 4). Cells co-treated with LPS + TAK-242 had a small but significant reduction in Φir in the hyper-osmotic step only.
Table 4.
Φir determined at each osmotic step (mean ± standard deviation).
| Φir | ||
|---|---|---|
| Treatment | Hyper-osmotic | Hypo-osmotic |
| Untreated | 0.441 ± 0.17 | 0.434 ± 0.17 |
| LPS (0.1 μg/mL) | 0.336 ± 0.12 | 0.472 ± 0.12 |
| LPS + TAK-242 (1 μmol/L) | 0.291 ± 0.08* | 0.501 ± 0.16 |
p < 0.05 vs. untreated.
Part 2: cytoskeleton
Cytoskeletal changes were investigated in rounded cells from all groups. While no apparent changes to microtubules (Fig. 4a) and vimentin intermediate filaments (Fig. 4b) were observed, more evident changes were observed for actin microfilaments. In untreated NP cells, actin was distributed in a punctuate pattern, with no presence of stress fibres in the perinuclear area and few small cytoplasmic processes (Fig. 4c). NP cells treated with LPS exhibited increased cytoplasmic processes rich in F-actin, with no increase in actin fibres within the perinuclear area. The changes in F-actin due to LPS treatment were blocked by the addition of TAK-242. Cells pre-treated with TAK-242 showed no presence of projections and instead appear to have a more homogeneous distribution of F-actin at the cell cortex similar to that in untreated cells.
Fig. 4. TLR4 inhibition mitigated LPS-induced cytoskeletal changes.
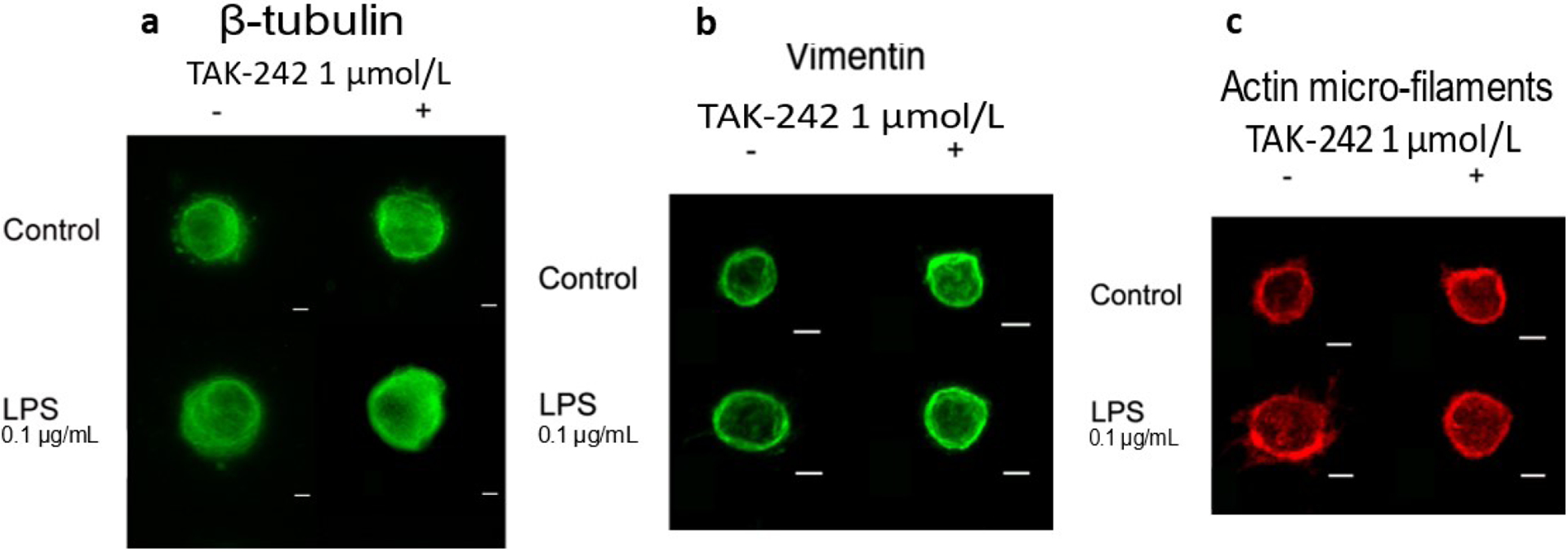
(a) No changes in β-tubulin were observed with LPS or TAK-242 treatment. (b) No changes in intermediate vimentin filaments were observed with LPS or TAK-242 treatment. (c) Changes in F-actin (rhodamine-phalloidin) induced by LPS treatment were mitigated by TAK-242. Cells treated with LPS showed presence of small cell projections and increase in cortical actin fibres, which were prevented in cells co-treated with TAK-242. All groups were supplemented with 0.4 % DMF. Scale bars: 10 μmol/L; images are Z-projections.
Part 2: gene expression
0.1 μg/mL LPS treatment was found to significantly increase IL-6 expression, in agreement with gene expression from part 1 study (Fig. 2c). Co-treatment with LPS + TAK-242 significantly reduced IL-6 expression by 40 % compared to LPS alone (Fig. 5). Both HMGB1 and MIF expression was significantly reduced in LPS + TAK-242 co-treated cells versus untreated cells (p < 0.05 and p < 0.001, respectively). MIF expression also decreased with TAK-242 treatment alone versus untreated cells (p < 0.01). MMP3 and ADMATS5 significantly increased with LPS stimulation (p < 0.01 and p < 0.05, respectively); however, LPS + TAK-242 co-treatment did not significantly alter MMP3 or ADAMTS5 expression versus LPS alone (Fig. 5). ACAN expression was not affected by LPS stimulation but significantly increased with TAK-242 treatment (TAK-242 vs. untreated and LPS + TAK-242 vs. untreated, p < 0.01). ADAMTS4 was significantly increased in LPS + TAK-242-treated cells versus untreated cells (p < 0.01) but no differences from untreated cells were observed in cells treated with LPS alone or TAK-242 alone. Lastly, no significant differences in IL-1β, TLR4, TNFα or MMP13 were observed between any treatment group (Fig. 5).
Fig. 5. Gene expression of NP cells from part 2 study groups.
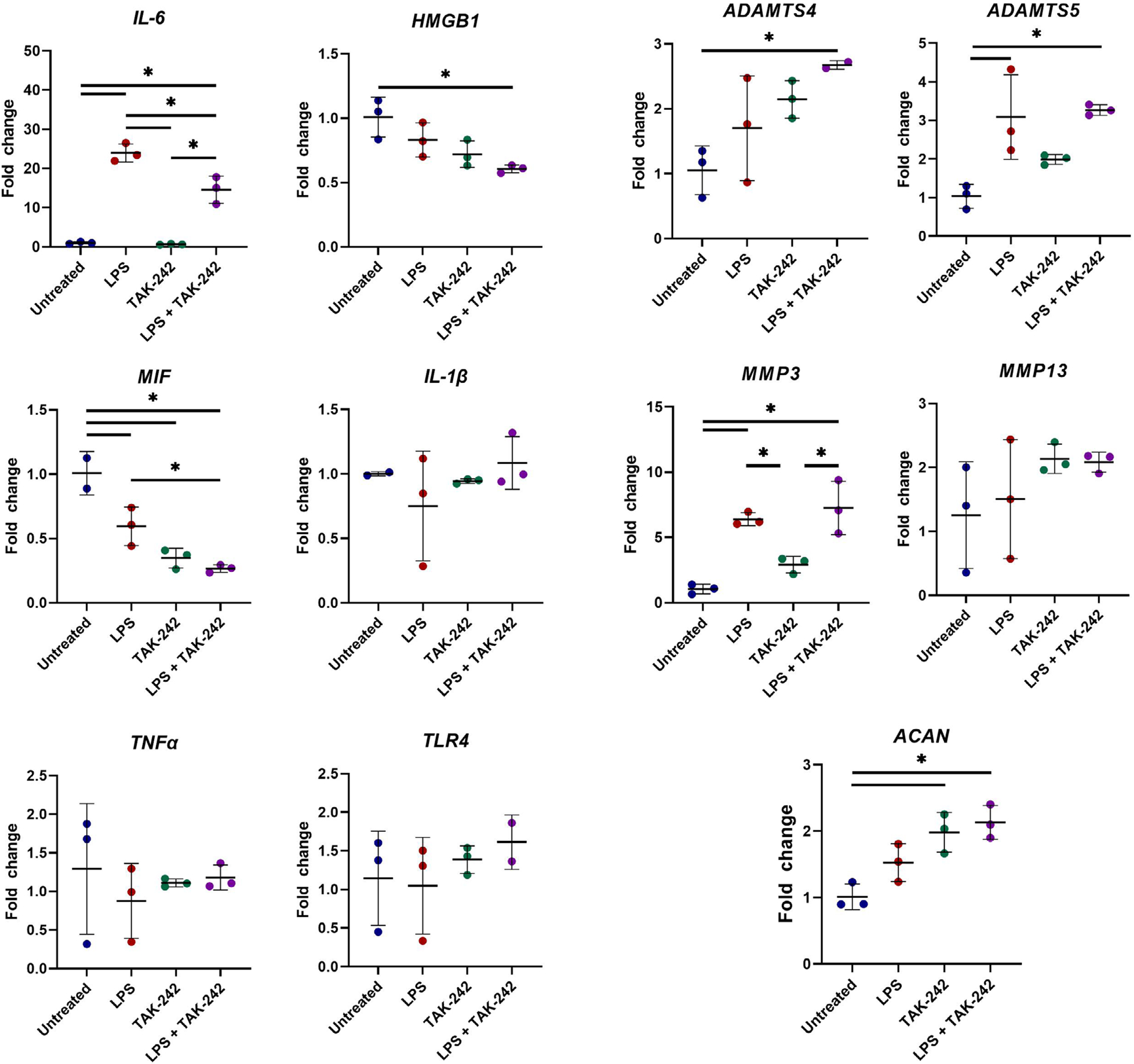
All graphs showed gene expression fold change versus untreated. (Left) Gene expression of pro-inflammatory cytokines IL-6, HMGB1, MIF, IL-1β, TNF-α, and TLR4. (Right) Gene expression of matrix-degrading enzymes MMP3, MMP13, ADATMS4, ADAMTS5 and ECM component ACAN. 0.1 μg/mL LPS significantly increased IL-6, MMP3 and ADAMTS5 expression. Addition of 1 μmol/L TAK-242 to LPS significantly reduced IL-6 but not MMP3 or ADAMTS5 expression as compared to LPS alone. TAK-242 significantly increased ACAN expression as compared to untreated. No significant changes in IL-1β, TLR4, TNFα or MMP13 were observed. All groups were supplemented with 0.4 % DMF. All graphs show mean ± standard deviation, with dots representing individual samples (n = 3 biological replicates per group). * p < 0.05 between indicated groups.
Discussion
The goal of the study was to investigate the role of TLR4 in mediating cell morphological, cytoskeletal and biomechanical changes in NP cells. The findings showed that stimulation of NP cells with LPS significantly increased hydraulic permeability and cell size and promoted formation of F-actin-rich cellular extensions. Blocking TLR4 signalling by TAK-242 mitigated LPS-induced changes in cell size and hydraulic permeability and protected against changes in actin cytoskeleton. Since TLR4 expression was unchanged with stimulation or treatment, the changes observed with TAK-242 treatment were a consequence of altered TLR4 signalling rather than changes in expression level of the TLR4 receptor. NP cell hydraulic permeability correlates with cell size (Maidhof et al., 2014), a relationship indicative of the cell’s ability to regulate cellular function in response to osmotic loading. Osmotic signals are important in the highly hydrated IVD, where reversible hydration changes that occur as part of the diurnal cycle of the spine or irreversible changes in hydration that occur with degeneration and GAG loss can alter NP cell gene expression (Wuertz et al., 2007). The finding that TLR4 played a role in mediating changes in cell hydraulic permeability and actin cytoskeleton suggests that inflammatory stimulation has consequences on both IVD cell biology and cell biomechanics that are preventable by blocking intracellular TLR4 signalling. Indeed, TAK-242 significantly mitigated increases in both hydraulic permeability and cell size, further confirming their relationship in NP cells.
Actomyosin regulates cell volume changes in dividing cells and in response to osmotic pressure (Stewart et al., 2011). Inflammatory treated cells had an increased cell size that was accompanied by a disrupted cortical actin cytoskeleton and extension of small cytoplasmic processes. Inhibiting cortical actomyosin contractility promotes the formation of cell processes rich in actin and increases cell hydraulic permeability (Hernandez et al., 2020). This confirms that an intact actin cytoskeleton is required to produce the forces that maintain cell shape. Interestingly, aquaporin-1 expression does not increase in LPS- or TNFα-stimulated NP cells (Maidhof et al., 2014). Conversely, LPS stimulation decreases aquaporin-1 protein expression. Decreased aquaporin protein expression may be a compensatory mechanism in response to increased hydraulic permeability.
In the present study, cytoskeletal evaluation was performed using the same cellular preparation (rounded NP cells) and state as used for the osmotic (permeability) measurement. The aim was to understand structure-function relationships between cytoskeletal changes and biomechanical properties in inflammatory conditions. Actin appeared to be the main cytoskeletal component affected by LPS stimulation, where no apparent changes in β-tubulin or vimentin were observed. This was mostly in agreement with a previous work showing no changes in β-tubulin, minor changes in vimentin and major changes in actin with TNFα inflammatory stimulation of bovine NP cells in a 3D environment (Hernandez et al., 2020). These findings are not surprising as tubulin plays a major role in organelle distribution and cell division, while functions related to mechano-response are mostly performed by actin and vimentin intermediate filaments (Blain, 2009). Of these two, actin is more intimately connected to myosin and myosin contraction while vimentin acts as a more passive shape and load stabilizer. Hernandez et al. (2020) found dysregulation in the transcriptome of myosin II and Rho GTPase family members following inflammatory stimulation that ultimately decreased actomyosin’s contractility and subsequently altered cell biophysical properties. Given the role of actomyosin contractility in affecting cell biophysical properties, actin was the main cytoskeletal element relatively affected compared to tubulin or vimentin. While previous results indicated that vimentin is mildly affected by inflammatory stimulation (Hernandez et al., 2020), in the context of LPS stimulation, no changes were observed.
Increasing actomyosin contractility is sufficient to prevent hydraulic permeability and actin cytoskeletal changes in TNFα-stimulated NP cells (Hernandez et al., 2020). In the present study, both the increase in cell volume and actin alteration due to LPS stimulation could be blocked by TAK-242 treatment. This finding pointed to a relationship between TLR4 signalling and actin regulation in NP cells. Changes in NP cell morphology and cytoskeleton due to LPS treatment might results in lower compressive stiffness in NP cells, based on similar changes in hydraulic permeability, morphology and cytoskeleton as seen with TNFα stimulation (Hernandez et al., 2020). Importantly, however, the effects of inflammation on cell biomechanics and cell cytoskeleton vary based on cell type and cell morphology. In monocyte and macrophages, studies indicate that TLR4 activation induces rapid and concentration-dependent increase in stiffness and increased F-actin staining (Doherty et al., 1994; Pi et al., 2014), while other studies find these cell types become considerably less stiff after stimulation (Leporatti et al., 2006).
TAK-242 (resatorvid) is a selective intracellular inhibitor of TLR4 that does not inhibit an interaction between the ligand (LPS) and its receptor. TAK-242 binds to the intracellular TIR domain of TLR4 and blocks interaction with the adaptor proteins TRIP and TRAP (Ii et al., 2006; Kawamoto et al., 2008; Matsunaga et al., 2011). TAK-242 inhibits production of inflammatory mediators including NO, IL-6 and TNFα in macrophages (Ii et al., 2006; Matsunaga et al., 2011; Sha et al., 2007) and myotube cell lines (Hussey et al., 2013; Ono et al., 2020). TAK-242 treatment mitigates LPS-induced acute lung injury in mice (Seki et al., 2010) as well as septic kidney injury in sheep (Fenhammar et al., 2011). TAK-242 also inhibits expression of IL-6 and matrix-degrading enzymes including MMP-1, following stimulation with the DAMP HMGB1 in human NP cells (Shah et al., 2019). Only a small number of clinical trials has evaluated TAK-242 in humans. Systemic TAK-242 administration was found to have a positive effect on traumatic brain injury recovery given systemic administration for 5 consecutive days after injury (Zhang et al., 2014). However, another study investigating the use of TAK-242 failed to show TAK-242 efficacy for the treatment of sepsis (Rice et al., 2010). LPS is recognised by TLR4 in a complex with MD2 and CD14 (Hoshino et al., 1999; Nomura et al., 2000; Poltorak et al., 1998; Schumann et al., 1990; Shimazu et al., 1999; Wright et al., 1990). The complex formed by TLR4-MD2-CD14 signals through a conserved pathway that results in the phosphorylation of NF-κB and initiates transcription of several pro-inflammatory cytokines such as IL-6 and TNFα (Goldring et al., 1998; Kastenbauer and Ziegler-Heitbrock, 1999; Ziegler-Heitbrock et al., 1994). These cytokines are responsible for the initiation of innate immune responses.
To characterise inflammatory response and signalling in the presence of LPS and TAK-242, gene expression of inflammatory factors (IL-6, IL-1β, TNFα, HMGB1, MIF), matrix degrading enzymes (MMP3, MMP13, ADMATS4, ADAMTS5) and ECM components (ACAN) was evaluated. IL-6, MMP-3 and ADAMTS5 expression significantly increased with LPS stimulation. However, only IL-6 expression decreased with co-treatment with LPS + TAK-242 as compared to LPS only. Unlike the study by Rajan et al. (2013), no significant changes in TNFα, IL-1β, HMGB1, ADAMTS4 or MMP13 with LPS treatment were observed. This may be a consequence of the DMF solvent used in all groups in the present study that can exert an anti-inflammatory and anti-oxidant activity (Zhao and Wen, 2018). Decreased expression of MIF was seen with TAK-242 treatment when compared with untreated cells and in LPS + TAK-242 co-treated cells compared with LPS-treated cells, indicating that TLR4 inhibition decreased MIF expression. ACAN expression was also unaffected by LPS but increased by TAK-242 treatment, indicating inhibition of TLR4 may have a positive effect on ECM production. Results suggested IL-6 was the predominant pro-inflammatory factor affected in this setting. While TAK-242 had a protective effect against the pro-inflammatory mediators IL-6 and NO, it did not mitigate the LPS-induced upregulation of matrix-degrading enzymes in this setting. One limitation was that the 24 h timepoint studied may not be aligned with the temporal profile of certain pro-inflammatory cytokines or MMPs/ADAMTSs following LPS stimulation. Another limitation was that this data represented the gene expression of matrix degrading enzyme in NP cells cultured in a 2D environment absent of a native or de novo ECM, potentially confounding the interpretation on MMPs/ADAMTS gene expression, which also did not fully represent changes in enzyme level or activity.
A growing body of evidence suggests that TLR4 is involved in the pathogenesis of IVD (Gomez et al., 2015; Klawitter et al., 2014; Quero et al., 2013). DAMPS such as HMGB1 or fibronectin fragments have degenerative effects in disc cells, including increased production of pro-inflammatory cytokine and matrix-degrading enzymes, mediated by signalling through TLRs, specifically TLR2 and TLR4 (Fang and Jiang, 2016; Krock et al., 2017; Shah et al., 2019). Furthermore, HMGB1 signalling has been shown to increase TLR2 and TLR4 expression (Fang and Jiang, 2016), suggesting a possible potentiation of signalling through TLRs. Lactoferrin can mitigate inflammatory disc signalling through TLR4 and, to a lesser extent, TLR2 (Kim et al., 2013). Naturally derived substances such as curcumin and triptolide prevent inflammation-induced catabolic and anabolic changes through TLR inhibition (Klawitter et al., 2012a; 2012b). Inhibition of MyD88, an adaptor of TLR2 and 4 signal transduction, also mitigates LPS-and IL-1β-induced upregulation of catabolic and inflammatory factors in the IVD (Ellman et al., 2012). While these studies identify TLR4 to be a critical regulator of joint pathology, cell mechanobiology contributions to these degenerative changes are unknown. The present study confirmed that LPS-induced pro-inflammatory signalling altered cell mechanobiology and the actin cytoskeleton. Moreover, pharmacological interventions that mitigate pro-inflammatory signalling, and specifically inhibition of TLR4 signalling, can protect cell mechanobiological function and potentially improve the long-term homeostasis of cells in IVD tissues. TLR4 inhibition has also been shown to protect against inflammatory stimulation in human NP cells (Shah et al., 2019), further enhancing the relevance of TLR4 inhibition via TAK-242 as a pharmacological intervention for human IVD degeneration. Interestingly, some studies have shown that stimulation of disc cells with ECM proteins, such as hyaluronic acid fragments and decorin, promotes pro-inflammatory signalling mediated by TLRs (Quero et al., 2013; Zwambag et al., 2020). However, whether TLR4 activation by ECM proteins induces cell biophysical changes is unknown. The preservation of cell biophysical properties has also been previously shown to protect against tissue-level degeneration and GAG loss (Hernandez et al., 2020). The preservation of cell biophysical properties by TAK-242 demonstrates its translational potential for mitigating DD. Indeed, a study by Krock et al. (2018) supports this premise, where SPARC-null mice (a DD and low-back pain model) treated with systemic TAK-242 exhibited decreases in cytokine release and less pain-like behaviour.
Potential limitations of the study include the use of cell plating for expansion, which may alter NP cell phenotype (Horner et al., 2002) as well as inflammatory response (Demoor-Fossard et al., 1999). However, for osmotic and cytoskeletal analysis, NP cells were observed in a rounded morphology that more closely mimicked the 3D morphology of NP cells in situ. The rounded re-attached state was used in cytoskeletal evaluation to match the morphological, structure-function state that the cells were in during osmotic (permeability) measurement. The aim was to understand how cytoskeletal changes related to the biomechanical properties measured. Importantly cytoskeletal morphology was compared across all treatment groups in the same conditions, following the same trypsinisation process, and differences between groups represented differences due to treatment regardless of the effects of trypsinisation or reattachment. Changes in cell actin cytoskeletal morphology in trypsinised and rounded NP cells following treatment with both LPS and the inflammatory cytokine TNFα have been previously observed and reported on (Maidhof et al., 2014), indicating that this method yields reproducible effects. Additionally, Hernandez et al. (2020) reported on the effect of inflammatory TNFα stimulation on cell actin cytoskeleton morphology in both trypsinised/rounded conditions as well as in adhered and spread 2D conditions, where similar disruptions to actin in both conditions were observed. Those findings are comparable to the ones presented in the present study. While detachment and re-attachment of NP cells and disruption of integrins following trypsinisation may affect the cell cytoskeleton, conclusions were based on treatment groups compared to one another where they all received the same cell processing. Therefore, the conclusions reached were sound and supported by the results of the study. Another limitation of the study was that osmotic properties of NP cells were observed in the absence of local pericellular and extracellular matrices, which may affect the ability of cells to swell, contributing to cell volume regulation (Cao et al., 2011). Lastly, the effect of LPS on pro-inflammatory signalling was complicated by the effects of the DMF solvent, which can exert anti-inflammatory and anti-oxidant effects (Zhao and Wen, 2018).
Conclusions
This study demonstrated that the TLR4 inhibitor TAK-242 could prevent LPS-induced changes to cellular biophysical properties of NP cells and block pro-inflammatory signalling of IL-6 and NO. Maintenance of cell biomechanical properties in the face of inflammatory insult mitigates tissue level DD (Hernandez et al., 2020). Therefore, inhibition of the inflammatory cascade via TLR4 signalling that results in altered cell biophysical properties is a promising strategy for mitigating DD.
Discussion with Reviewers
Reviewer: With regards to the TAK-242 concentration and exposure duration used in the study to achieve at least 50 % inhibition of TLR-4 activation by LPS and based on current evidence of the application of TAK-242 in humans, could TLR-4 inhibition with TAK-242 be a feasible treatment strategy for degenerative disc disease in the future?
Authors: To date, only a small number of clinical trials have evaluated TAK-242 effects in humans. Systemic TAK-242 administration was found to be tolerated in patients and efficacious for certain applications (Rice et al., 2010; Zang et al., 2014). Systemic administration of TAK-242 has also been found to decrease pain behaviour in a mouse model of DD (Krock et al., 2018). However, the standing paradigm for DD treatment strategies, given the avascular nature of the IVD, is that drug delivery will require targeted approaches or localised intradiscal injections. Several targeted and extended-release drug delivery strategies, such as injectable hydrogel of microparticle drug carries, are being investigated for application to the IVD (Tryfonidou et al., 2020, additional reference) and could allow for extended delivery of TAK-242. A recent study has shown that a nanostructure delivery system can promote solute retention within the IVD, which could allow for extended drug delivery for the treatment of DD (Wagner et al., 2020, additional reference).
Acknowledgements
The authors thank the lab of Dr Yousef AlAbed for assistance with endotoxin assay and PmB. This study was funded in part by NIH R01AR069668. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations
- ACAN
aggrecan
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- CD14
cluster of differentiation 14
- DAMPs
damage-associated matrix proteins
- DMEM
Dulbecco′s modified Eagle′s medium
- DMF
dimethylformamide
- DD
disc degeneration
- DIC
differential interference contrast
- ECM
extracellular matrix
- FBS
foetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HMGB1
high mobility group box protein 1
- IC50
half maximal inhibitory concentration
- IL
interleukin
- IVD
intervertebral disc
- LDH
lactate dehydrogenase
- Lp
hydraulic permeability
- LPS
lipopolysaccharide
- MD2
myeloid differentiation factor 2
- MIF
macrophage migration inhibitory factor
- MMPs
matrix metalloproteases
- MYD88
innate immune signal transduction adaptor
- NF-κB
nuclear factor kappa B
- NO
nitric oxide
- NP
nucleus pulposus
- PDMS
polydimethylsiloxane
- PGE2
prostaglandin E2
- PmB
polymyxin B
- SPARC
secreted protein acidic and rich in cysteine
- TIR
toll/IL-1 receptor
- TLR
toll-like receptor
- TNFα
tumour necrosis factor alpha
- TRAP
thrombospondin-related anonymous protein
- TRIP
TNF-receptor-associated factor interacting protein
- Φ
osmotically active water content
- Φir
reference intracellular water content
References
- Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS (2002) mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976) 27: 911–917. [DOI] [PubMed] [Google Scholar]
- Albro MB, Chahine NO, Caligaris M, Wei VI, Likhitpanichkul M, Ng KW, Hung CT, Ateshian GA (2007) Osmotic loading of spherical gels: a biomimetic study of hindered transport in the cell protoplasm. J Biomech Eng 129: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Petersen LE, Li R, Hung CT, Ateshian GA (2009) Influence of the partitioning of osmolytes by the cytoplasm on the passive response of cells to osmotic loading. Biophys J 97: 2886–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M (1996) The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98: 996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ateshian GA, Likhitpanichkul M, Hung CT (2006) A mixture theory analysis for passive transport in osmotic loading of cells. J Biomech 39: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmeier BE, Nerlich AG, Weiler C, Paesold G, Jochum M, Boos N (2007) Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann N Y Acad Sci 1096: 44–54. [DOI] [PubMed] [Google Scholar]
- Blain EJ (2009) Involvement of the cytoskeletal elements in articular cartilage homeostasis and pathology. Int J Exp Pathol 90: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush PG, Hall AC (2001) The osmotic sensitivity of isolated and in situ bovine articular chondrocytes. J Orthop Res 19: 768–778. [DOI] [PubMed] [Google Scholar]
- Cao L, Guilak F, Setton LA (2011) Three-dimensional finite element modeling of pericellular matrix and cell mechanics in the nucleus pulposus of the intervertebral disk based on in situ morphology. Biomech Model Mechanobiol 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PG, Tang Z, Angelini E, West AC, Costa KD, Hung CT (2005) Dynamic osmotic loading of chondrocytes using a novel microfluidic device. J Biomech 38: 1273–1281. [DOI] [PubMed] [Google Scholar]
- Chen J, Yan W, Setton LA (2004) Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol 22: 573–583. [DOI] [PubMed] [Google Scholar]
- Cheung KM, Karppinen J, Chan D, Ho DW, Song YQ, Sham P, Cheah KS, Leong JC, Luk KD (2009) Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 34: 934–940. [DOI] [PubMed] [Google Scholar]
- Demoor-Fossard M, Boittin M, Redini F, Pujol JP (1999) Differential effects of interleukin-1 and transforming growth factor beta on the synthesis of small proteoglycans by rabbit articular chondrocytes cultured in alginate beads as compared to monolayers. Mol Cell Biochem 199: 69–80. [DOI] [PubMed] [Google Scholar]
- Diseases GBD, Injuries C (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396: 1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty DE, Downey GP, Schwab B 3rd, Elson E, Worthen GS (1994) Lipolysaccharide-induced monocyte retention in the lung. Role of monocyte stiffness, actin assembly, and CD18-dependent adherence. J Immunol 153: 241–255. [PubMed] [Google Scholar]
- Ellman MB, Kim JS, An HS, Chen D, Kc R, An J, Dittakavi T, van Wijnen AJ, Cs-Szabo G, Li X, Xiao G, An S, Kim SG, Im HJ (2012) Toll-like receptor adaptor signaling molecule MyD88 on intervertebral disk homeostasis: in vitro, ex vivo studies. Gene 505: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Jiang D (2016) IL-1β/HMGB1 signalling promotes the inflammatory cytokines release via TLR signalling in human intervertebral disc cells. Biosci Rep 36: e00379. DOI: 10.1042/BSR20160118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenhammar J, Rundgren M, Forestier J, Kalman S, Eriksson S, Frithiof R (2011) Toll-like receptor 4 inhibitor TAK-242 attenuates acute kidney injury in endotoxemic sheep. Anesthesiology 114: 1130–1137. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Duncan NA, Lotz JC (1997) Radial tensile properties of the lumbar annulus fibrosus are site and degeneration dependent. J Orthop Res 15: 814–819. [DOI] [PubMed] [Google Scholar]
- Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, Haughton VM (2000) The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 25: 3036–3044. [DOI] [PubMed] [Google Scholar]
- Goldring CE, Reveneau S, Pinard D, Jeannin JF (1998) Hyporesponsiveness to lipopolysaccharide alters the composition of NF-kappaB binding to the regulatory regions of inducible nitric oxide synthase gene. Eur J Immunol 28: 2960–2970. [DOI] [PubMed] [Google Scholar]
- Gomez R, Villalvilla A, Largo R, Gualillo O, Herrero-Beaumont G (2015) TLR4 signalling in osteoarthritis – finding targets for candidate DMOADs. Nat Rev Rheumatol 11: 159–170. [DOI] [PubMed] [Google Scholar]
- Gu WY, Mao XG, Foster RJ, Weidenbaum M, Mow VC, Rawlins BA (1999a) The anisotropic hydraulic permeability of human lumbar anulus fibrosus. Influence of age, degeneration, direction, and water content. Spine (Phila Pa 1976) 24: 2449–2455. [DOI] [PubMed] [Google Scholar]
- Gu WY, Mao XG, Rawlins BA, Iatridis JC, Foster RJ, Sun DN, Weidenbaum M, Mow VC (1999b) Streaming potential of human lumbar anulus fibrosus is anisotropic and affected by disc degeneration. J Biomech 32: 1177–1182. [DOI] [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR (1999) Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn 215: 179–189. [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Jacobsen TD, Chahine NO (2020) Actomyosin contractility confers mechanoprotection against TNFα-induced disruption of the intervertebral disc. Sci Adv 6: eaba2368. DOI: 10.1126/sciadv.aba2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner HA, Roberts S, Bielby RC, Menage J, Evans H, Urban JP (2002) Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine (Phila Pa 1976) 27: 1018–1028. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S (1999) Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the LPS gene product. J Immunol 162: 3749–3752. [PubMed] [Google Scholar]
- Hurwitz EL, Randhawa K, Yu H, Cote P, Haldeman S (2018) The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J 27: 796–801. [DOI] [PubMed] [Google Scholar]
- Hussey SE, Liang H, Costford SR, Klip A, DeFronzo RA, Sanchez-Avila A, Ely B, Musi N (2013) TAK-242, a small-molecule inhibitor of Toll-like receptor 4 signalling, unveils similarities and differences in lipopolysaccharide- and lipid-induced inflammation and insulin resistance in muscle cells. Biosci Rep 33: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS (2013) Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J 13: 243–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y (2006) A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol 69: 1288–1295. [DOI] [PubMed] [Google Scholar]
- Johannessen W, Elliott DM (2005) Effects of degeneration on the biphasic material properties of human nucleus pulposus in confined compression. Spine (Phila Pa 1976) 30: E724–729. [DOI] [PubMed] [Google Scholar]
- Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF 3rd, Evans CH (1996) Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 21: 271–277. [DOI] [PubMed] [Google Scholar]
- Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH (1997) Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine (Phila Pa 1976) 22: 1065–1073. [DOI] [PubMed] [Google Scholar]
- Kastenbauer S, Ziegler-Heitbrock HW (1999) NF-kappaB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression. Infect Immun 67: 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H (2008) TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol 584: 40–48. [DOI] [PubMed] [Google Scholar]
- Keshari KR, Lotz JC, Link TM, Hu S, Majumdar S, Kurhanewicz J (2008) Lactic acid and proteoglycans as metabolic markers for discogenic back pain. Spine (Phila Pa 1976) 33: 312–317. [DOI] [PubMed] [Google Scholar]
- Kim JS, Ellman MB, Yan D, An HS, Kc R, Li X, Chen D, Xiao G, Cs-Szabo G, Hoskin DW, Buechter DD, Van Wijnen AJ, Im HJ (2013) Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. J Cell Physiol 228: 1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawitter M, Hakozaki M, Kobayashi H, Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T, Meier U, Sekiguchi M, Konno SI, Boos N, Ferguson SJ, Wuertz K (2014) Expression and regulation of toll-like receptors (TLRs) in human intervertebral disc cells. Eur Spine J 23: 1878–1891. [DOI] [PubMed] [Google Scholar]
- Klawitter M, Quero L, Klasen J, Gloess AN, Klopprogge B, Hausmann O, Boos N, Wuertz K (2012a) Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J Inflamm (Lond) 9: 29. DOI: 10.1186/1476-9255-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawitter M, Quero L, Klasen J, Liebscher T, Nerlich A, Boos N, Wuertz K (2012b) Triptolide exhibits anti-inflammatory, anti-catabolic as well as anabolic effects and suppresses TLR expression and MAPK activity in IL-1β treated human intervertebral disc cells. Eur Spine J 21 Suppl 6: S850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krock E, Millecamps M, Currie JB, Stone LS, Haglund L (2018) Low back pain and disc degeneration are decreased following chronic toll-like receptor 4 inhibition in a mouse model. Osteoarthritis Cartilage 26: 1236–1246. [DOI] [PubMed] [Google Scholar]
- Krock E, Rosenzweig DH, Currie JB, Bisson DG, Ouellet JA, Haglund L (2017) Toll-like receptor activation induces degeneration of human intervertebral discs. Sci Rep 7: 17184. DOI: 10.1038/s41598-017-17472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A, Moon A, Purmessur D, Skovrlj B, Laudier DM, Winkelstein BA, Cho SK, Hecht AC, Iatridis JC (2016) Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J 16: 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA (2005) The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther 7: R732–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Freemont AJ, Hoyland JA (2006) A preliminary in vitro study into the use of IL-1Ra gene therapy for the inhibition of intervertebral disc degeneration. Int J Exp Pathol 87: 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maitre CL, Hoyland JA, Freemont AJ (2007) Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther 9: R77. DOI: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporatti S, Gerth A, Kohler G, Kohlstrunk B, Hauschildt S, Donath E (2006) Elasticity and adhesion of resting and lipopolysaccharide-stimulated macrophages. FEBS Lett 580: 450–454. [DOI] [PubMed] [Google Scholar]
- Li S, Duance VC, Blain EJ (2008) Zonal variations in cytoskeletal element organization, mRNA and protein expression in the intervertebral disc. J Anat 213: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Li M, Fang D, Fang J, Su SB (2011) The essential roles of Toll-like receptor signaling pathways in sterile inflammatory diseases. Int Immunopharmacol 11: 1422–1432. [DOI] [PubMed] [Google Scholar]
- Liu GZ, Ishihara H, Osada R, Kimura T, Tsuji H (2001) Nitric oxide mediates the change of proteoglycan synthesis in the human lumbar intervertebral disc in response to hydrostatic pressure. Spine (Phila Pa 1976) 26: 134–141. [DOI] [PubMed] [Google Scholar]
- Lurie JD, Tosteson TD, Tosteson AN, Zhao W, Morgan TS, Abdu WA, Herkowitz H, Weinstein JN (2014) Surgical versus nonoperative treatment for lumbar disc herniation: eight-year results for the spine patient outcomes research trial. Spine (Phila Pa 1976) 39: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO (2014) Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One 9: e99621. DOI: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga N, Tsuchimori N, Matsumoto T, Ii M (2011) TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol 79: 34–41. [DOI] [PubMed] [Google Scholar]
- Mavrogonatou E, Kletsas D (2012) Differential response of nucleus pulposus intervertebral disc cells to high salt, sorbitol, and urea. J Cell Physiol 227: 1179–1187. [DOI] [PubMed] [Google Scholar]
- Millward-Sadler SJ, Costello PW, Freemont AJ, Hoyland JA (2009) Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther 11: R65. DOI: 10.1186/ar2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidlinger-Wilke C, Mietsch A, Rinkler C, Wilke HJ, Ignatius A, Urban J (2012) Interactions of environmental conditions and mechanical loads have influence on matrix turnover by nucleus pulposus cells. J Orthop Res 30: 112–121. [DOI] [PubMed] [Google Scholar]
- Neidlinger-Wilke C, Wurtz K, Urban JP, Borm W, Arand M, Ignatius A, Wilke HJ, Claes LE (2006) Regulation of gene expression in intervertebral disc cells by low and high hydrostatic pressure. Eur Spine J 15 Suppl 3: S372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S (2000) Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol 164: 3476–3479. [DOI] [PubMed] [Google Scholar]
- O’Connell GD, Vresilovic EJ, Elliott DM (2011) Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J Orthop Res 29: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Maejima Y, Saito M, Sakamoto K, Horita S, Shimomura K, Inoue S, Kotani J (2020) TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice. Sci Rep 10: 694. DOI: 10.1038/s41598-020-57714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RH, Grimmer BJ, Adams ME (1987) Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res 5: 198–205. [DOI] [PubMed] [Google Scholar]
- Pi J, Li T, Liu J, Su X, Wang R, Yang F, Bai H, Jin H, Cai J (2014) Detection of lipopolysaccharide induced inflammatory responses in RAW264.7 macrophages using atomic force microscope. Micron 65: 1–9. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282: 2085–2088. [DOI] [PubMed] [Google Scholar]
- Quero L, Klawitter M, Schmaus A, Rothley M, Sleeman J, Tiaden AN, Klasen J, Boos N, Hottiger MO, Wuertz K, Richards PJ (2013) Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signalling pathways. Arthritis Res Ther 15: R94. DOI: 10.1186/ar4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan N, Bloom O, Maidhof R, Stetson N, Sherry B, Levine M, Chahine NO (2013) Toll-like receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine (Phila Pa 1976) 38: 1343–1351. [DOI] [PubMed] [Google Scholar]
- Rice TW, Wheeler AP, Bernard GR, Vincent JL, Angus DC, Aikawa N, Demeyer I, Sainati S, Amlot N, Cao C, Ii M, Matsuda H, Mouri K, Cohen J (2010) A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med 38: 1685–1694. [DOI] [PubMed] [Google Scholar]
- Risbud MV, Shapiro IM (2014) Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 10: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley PJ, Alini M, Antoniou J (2002) The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochem Soc Trans 30: 869–874. [DOI] [PubMed] [Google Scholar]
- Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ (1990) Structure and function of lipopolysaccharide binding protein. Science 249: 1429–1431. [DOI] [PubMed] [Google Scholar]
- Seguin CA, Pilliar RM, Roughley PJ, Kandel RA (2005) Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 30: 1940–1948. [DOI] [PubMed] [Google Scholar]
- Seki H, Tasaka S, Fukunaga K, Shiraishi Y, Moriyama K, Miyamoto K, Nakano Y, Matsunaga N, Takashima K, Matsumoto T, Ii M, Ishizaka A, Takeda J (2010) Effect of Toll-like receptor 4 inhibitor on LPS-induced lung injury. Inflamm Res 59: 837–845. [DOI] [PubMed] [Google Scholar]
- Sha T, Sunamoto M, Kitazaki T, Sato J, Ii M, Iizawa Y (2007) Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur J Pharmacol 571: 231–239. [DOI] [PubMed] [Google Scholar]
- Shah BS, Burt KG, Jacobsen T, Fernandes TD, Alipui DO, Weber KT, Levine M, Chavan SS, Yang H, Tracey KJ, Chahine NO (2019) High mobility group box-1 induces pro-inflammatory signaling in human nucleus pulposus cells via toll-like receptor 4-dependent pathway. J Orthop Res 37: 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ (2010) Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum 62: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med 189: 1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivan S, Neidlinger-Wilke C, Wurtz K, Maroudas A, Urban JP (2006) Diurnal fluid expression and activity of intervertebral disc cells. Biorheology 43: 283–291. [PubMed] [Google Scholar]
- Stewart MP, Helenius J, Toyoda Y, Ramanathan SP, Muller DJ, Hyman AA (2011) Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature 469: 226–230. [DOI] [PubMed] [Google Scholar]
- Tracey KJ (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest 117: 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JP (2002) The role of the physicochemical environment in determining disc cell behaviour. Biochem Soc Trans 30: 858–864. [DOI] [PubMed] [Google Scholar]
- Urban JP, McMullin JF (1988) Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine (Phila Pa 1976) 13: 179–187. [DOI] [PubMed] [Google Scholar]
- Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS (2007) Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 356: 2257–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Lurie JD, Tosteson TD, Skinner JS, Hanscom B, Tosteson AN, Herkowitz H, Fischgrund J, Cammisa FP, Albert T, Deyo RA (2006) Surgical vs. nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT) observational cohort. Jama 296: 2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, Herkowitz H, Hilibrand A, Albert T, Fischgrund J (2008) Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976) 33: 2789–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, Birkmeyer N, Herkowitz H, Longley M, Lenke L, Emery S, Hu SS (2009) Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am 91: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson A, Blood E, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H (2010) Surgical versus nonoperative treatment for lumbar spinal stenosis four-year results of the Spine Patient Outcomes Research Trial. Spine (Phila Pa 1976) 35: 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433. [DOI] [PubMed] [Google Scholar]
- Wuertz K, Urban JP, Klasen J, Ignatius A, Wilke HJ, Claes L, Neidlinger-Wilke C (2007) Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res 25: 1513–1522. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li H, Li T, Zhou M, Hao S, Yan H, Yu Z, Li W, Li K, Hang C (2014) TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: implication in the treatment of human brain injury. Neurochem Int 75: 11–18. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chee A, Shi P, Adams SL, Markova DZ, Anderson DG, Smith HE, Deng Y, Plastaras CT, An HS (2016) Intervertebral disc cells produce interleukins found in patients with back pain. Am J Phys Med Rehabil 95: 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Wen LB (2018) DMF attenuates cisplatin-induced kidney injury via activating Nrf2 signaling pathway and inhibiting NF-kB signaling pathway. Eur Rev Med Pharmacol Sci 22: 8924–8931. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Wedel A, Schraut W, Strobel M, Wendelgass P, Sternsdorf T, Bauerle PA, Haas JG, Riethmuller G (1994) Tolerance to lipopolysaccharide involves mobilization of nuclear factor kappa B with predominance of p50 homodimers. J Biol Chem 269: 17001–17004. [PubMed] [Google Scholar]
- Zwambag DP, Molladavoodi S, Guerreiro MJ, DeWitte-Orr SJ, Gregory DE (2020) Immuno-stimulatory capacity of decorin in the rat tail intervertebral disc and the mechanical consequence of resultant inflammation. Eur Spine J 29: 1641–1648. [DOI] [PubMed] [Google Scholar]
Additional References
- Tryfonidou MA, de Vries G, Hennink WE, Creemers LB (2020) “Old Drugs, New Tricks” - Local controlled drug release systems for treatment of degenerative joint disease. Adv Drug Deliv Rev 160: 170–185. [DOI] [PubMed] [Google Scholar]
- Wagner EK, Vedadghavami A, Jacobsen TD, Goel SA, Chahine NO, Bajpayee AG (2020) Avidin grafted dextran nanostructure enables a month—long intradiscal retention. Sci Rep 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


