Abstract
The EFSA Panel on Plant Health performed a pest categorisation of Phlyctinus callosus (Coleoptera: Curculionidae) for the EU territory. This species is not included in EU Commission Implementing Regulation 2019/2072. P. callosus is a polyphagous pest native to South Africa which has spread to Australia and New Zealand, Reunion and St Helena. Immature development takes place in the soil where larvae feed on the roots of a variety of plants including grasses, root vegetables and herbaceous plants; adults are noted as significant pests of apples, nectarines and grapes, feeding on foliage and the surface of fruit causing scarring. Soft fruits such as strawberries and blueberries can also be damaged by adult feeding. P. callosus has been intercepted in Europe on apples and peaches from South Africa. Table grapes could also provide a pathway for entry to the EU. Rooted plants for planting could also provide a potential pathway. Hosts are grown widely across the EU in areas with climates comparable to those in parts of South Africa, New Zealand and Australia where the pest is established suggesting that conditions in the EU are suitable for the establishment of P. callosus. If introduced into the EU, natural spread would be limited because adults cannot fly and must disperse by walking. However, the movement of host plants for planting within the EU could spread juvenile stages much faster and adults could spread with fruits. The prohibition of soil or growing media from third countries should prevent the entry of P. callosus larvae and pupae. Other phytosanitary measures are available to inhibit the entry of P. callosus. P. callosus satisfies the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest.
Keywords: banded fruit weevil, garden weevil, pest risk, plant health, plant pest, quarantine
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
The new Plant Health Regulation (EU) 2016/2031, on the protective measures against pests of plants, is applying from 14 December 2019. Conditions are laid down in this legislation in order for pests to qualify for listing as Union quarantine pests, protected zone quarantine pests or Union regulated non‐quarantine pests. The lists of the EU regulated pests together with the associated import or internal movement requirements of commodities are included in Commission Implementing Regulation (EU) 2019/2072. Additionally, as stipulated in the Commission Implementing Regulation 2018/2019, certain commodities are provisionally prohibited to enter in the EU (high‐risk plants, HRP). EFSA is performing the risk assessment of the dossiers submitted by exporting to the EU countries of the HRP commodities, as stipulated in Commission Implementing Regulation 2018/2018. Furthermore, EFSA has evaluated a number of requests from exporting to the EU countries for derogations from specific EU import requirements.
In line with the principles of the new plant health law, the European Commission with the Member States are discussing monthly the reports of the interceptions and the outbreaks of pests notified by the Member States. Notifications of an imminent danger from pests that may fulfil the conditions for inclusion in the list of the Union quarantine pest are included. Furthermore, EFSA has been performing horizon scanning of media and literature.
As a follow‐up of the above‐mentioned activities (reporting of interceptions and outbreaks, HRP, derogation requests and horizon scanning), a number of pests of concern have been identified. EFSA is requested to provide scientific opinions for these pests, in view of their potential inclusion by the risk manager in the lists of Commission Implementing Regulation (EU) 2019/2072 and the inclusion of specific import requirements for relevant host commodities, when deemed necessary by the risk manager.
1.1.2. Terms of reference
EFSA is requested, pursuant to Article 29(1) of Regulation (EC) No 178/2002, to provide scientific opinions in the field of plant health.
EFSA is requested to deliver 53 pest categorisations for the pests listed in Annexes 1A, 1B, 1D and 1E (for more details, see mandate M‐2021‐00027 on the Open.EFSA portal). Additionally, EFSA is requested to perform pest categorisations for the pests so far not regulated in the EU, identified as pests potentially associated with a commodity in the commodity risk assessments of the HRP dossiers (Annex 1C; for more details, see mandate M‐2021‐00027 on the Open.EFSA portal). Such pest categorisations are needed in the case where there are not available risk assessments for the EU.
When the pests of Annex 1A are qualifying as potential Union quarantine pests, EFSA should proceed to phase 2 risk assessment. The opinions should address entry pathways, spread, establishment, impact and include a risk reduction options analysis.
Additionally, EFSA is requested to develop further the quantitative methodology currently followed for risk assessment, in order to have the possibility to deliver an express risk assessment methodology. Such methodological development should take into account the EFSA Plant Health Panel Guidance on quantitative pest risk assessment and the experience obtained during its implementation for the Union candidate priority pests and for the likelihood of pest freedom at entry for the commodity risk assessment of high risk plants.
1.2. Interpretation of the Terms of Reference
Phlyctinus callosus is one of a number of pests listed in Annex 1 to the Terms of Reference (ToR) (Section 1.1.2.1) to be subject to pest categorisation to determine whether it fulfils the criteria of a regulated pest for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores, and so inform European Commission decision‐making as to its appropriateness for potential inclusion in the lists of pests of Commission Implementing Regulation (EU) 2019/2072. If a pest fulfils the criteria to be potentially listed as a union quarantine pest, specific import requirements for relevant host commodities will be identified; for pests already present in the EU additional risk reduction options to slow spread and facilitate eradication will be identified.
1.3. Additional information
P. callosus was added to the EPPO Alert List in 2020 (EPPO, 2020) having been judged by the EU project DROPSA (Fera, 2018) as a pest of some fruits and which may present a threat to the EPPO region (Suffert et al., 2018).
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on P. callosus was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the pest as search term. Papers relevant for the pest categorisation were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online), the CABI databases and scientific literature databases as referred above in Section 2.1.1.
Data about the import of commodity types that could potentially provide a pathway for the pest to enter the EU and about the area of hosts grown in the EU were obtained from EUROSTAT (Statistical Office of the European Communities).
The Europhyt and TRACES databases were consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission as a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. TRACES is the European Commission's multilingual online platform for sanitary and phytosanitary certification required for the importation of animals, animal products, food and feed of non‐animal origin and plants into the European Union, and the intra‐EU trade and EU exports of animals and certain animal products. Up until May 2020, the Europhyt database managed notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the Member States and the phytosanitary measures taken to eradicate or avoid their spread. The recording of interceptions switched from Europhyt Interceptions to TRACES in May 2020.
2.2. Methodologies
The Panel performed the pest categorisation for P. callosus, following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018), the EFSA guidance on the use of the weight of evidence approach in scientific assessments (EFSA Scientific Committee, 2017) and the International Standards for Phytosanitary Measures No. 11 (FAO, 2013) and No. 21 (FAO, 2004).
The criteria to be considered when categorising a pest as a Union quarantine pest (QP) are given in Regulation (EU) 2016/2031 Article 3 and Annex 1 to this Regulation. Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. In judging whether a criterion is met the Panel uses its best professional judgement (EFSA Scientific Committee, 2017) by integrating a range of evidence from a variety of sources (as presented above in Section 2.1) to reach an informed conclusion as to whether or not a criterion is satisfied.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest (article 3) |
|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) | Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future. |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? |
| Available measures (Specific import requirements) (Section 3.6) | Are there measures available to prevent the entry into the EU such that the likelihood of introduction becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met. |
The Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, deemed to be a risk management decision, the Panel will present a summary of the observed impacts in the areas where the pest occurs, and make a judgement about potential likely impacts in the EU. Whilst the Panel may quote impacts reported from areas where the pest occurs in monetary terms, the Panel will seek to express potential EU impacts in terms of yield and quality losses and not in monetary terms, in agreement with the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Article 3 (d) of Regulation (EU) 2016/2031 refers to unacceptable social impact as a criterion for quarantine pest status. Assessing social impact is outside the remit of the Panel.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
1.
Is the identity of the pest established, or has it been shown to produce consistent symptoms and/or to be transmissible?
Yes. The identity of the species is established and Phlyctinus callosus (Schoenherr, 1826) is the accepted name.
Phlyctinus callosus is an insect within the Order Coleoptera and Family Curculionidae (Figure 1). In South Africa, it has the common name banded fruit weevil; in New Zealand and Australia, it is referred to as the garden weevil.
Figure 1.

Phlyctinus callosus adult (Source: Pest and Diseases Image Library, Bugwood.org)
Following detailed morphological studies and using molecular diagnostic tools, Haran et al. (2020) described P. callosus as a species complex of six species (Phlyctinus callosus (Schoenherr), P. grootbosensis Haran sp. nov., P. xerophilus Haran sp. nov., P. planithorax Haran sp. nov., P. littoralis Haran sp. nov. and P. aloevorus Haran sp. nov.). Each of the five new species described by Haran et al. (2020) is reported from limited areas within South Africa with four found only in natural or semi‐natural habitats, i.e. not regarded as pests of commercial crops. The species described by Haran et al. (2020) as P. callosus sensu stricto is recognised as having spread from South Africa hence literature published from studies in Australia and New Zealand, before the species complex was recognised and elaborated, remains valid for the purposes of this pest categorisation. However, Haran et al. (2020) note that it is difficult to morphologically distinguish between female specimens of P. callosus and the newly described species P. xerophilus and that more taxonomic research is needed.
The EPPO code1 for Phlyctinus callosus is PHLYCA (EPPO, online).
3.1.2. Biology of the pest
P. callosus usually has one generation per year with peak adult emergence in the spring. However, in summer irrigated fruit orchards, a second smaller generation of adults can emerge over an extended period during the late summer and autumn (Barnes, 1989a; Dlamini et al., 2009a). Table 2 summarises key features of the biology of each life stage.
Table 2.
Important features of the life‐history strategy of Phlyctinus callosus
| Life stage | Phenology and relation to host | Other relevant information |
|---|---|---|
| Egg | Females lay their eggs on grasses and other plant matter at, or just below the soil surface, or in loose organic litter (May, 1966; Barnes and Pringle, 1989). Most eggs are laid close to weeds or grasses in orchards (Barnes, 1987). Eggs are usually laid in batches of ˜ 20 (Dlamini et al., 2019a,b) although batches can consist of up to 70 eggs. Batches are laid at intervals over a 7‐day period and eggs hatch after 7–14 days depending on temperature (May, 1966). | Based on data in Walker (1980), the threshold for egg development is estimated to be 6.0°C with ˜ 125 degree days required for egg development. Eggs can tolerate 12 weeks at 5°C (Walker, 1980). |
| Larva | Newly hatched larvae burrow underground to feed on the roots of grasses, ornamental bulbs, corms and root vegetables (May, 1966). Larvae develop feeding on roots, most larvae are found within 15 cm of the soil surface, (Barnes, 1989b). In South Africa, larvae can be found every month of the year with peak numbers occurring during the winter and the lowest numbers in the summer (Barnes, 1989a). | The larvae over‐winter in the soil and progress through a variable number of instars. There are usually between 6 and 8 larval instars but there can be from 4 to 11 (Walker, 1980; Barnes, 1989b). |
| Pupa | Larvae form pupal chambers in the soil; pupation occurs within 5 cm of soil surface (Barnes, 1989b) | The pupal stage lasts 1–3 weeks (Dlamini et al., 2009a). |
| Adult | Adults emerge from the soil and move to woody fruit trees and vines. Adults cannot fly so need to walk and climb up trunks and stems where they feed on aerial parts of fruit trees (leaves and fruit surfaces) (Pryke and Samways, 2007). Adults feed and mate during the night and spend the day in the leaf litter on the ground in the vicinity of the host plant; in South African vineyards during warm weather adults shelter under bark of vine, in cool weather adults shelter in grape bunches (Pryke and Samways, 2007). | Sexual reproduction is reported in South Africa where both males and females are found (Barnes, 1989a). However, in New Zealand, May (1966) reports P. callosus as being parthenogenetic (i.e. growth and development of embryos occur without fertilisation). A thorough review of life‐history strategies of the Entiminae (the subfamily in which P. callosus sits) by Marvaldi et al. (2014) does not suggest P. callosus as being one of the rare beetle species capable of parthenogenesis. |
3.1.3. Host range/species affected
P. callosus is a polyphagous pest. Larvae feed on a range of monocotyledonous and dicotyledonous plants, including grasses, herbs and woody plants. Adults feed on the aerial parts of a more limited range of crop plants. Apple, peach, nectarine and grapevine are considered major hosts in EPPO global database. Bredenhand et al. (2010) reported P. callosus as a pest of blueberries after commercialisation of the crop began in South Africa in the early 1990s. Appendix B provides an extensive list of hosts and food plants.
3.1.4. Intraspecific diversity
Haran et al. (2020) described P. callosus as a species complex of six species.
3.1.5. Detection and identification of the pest
1.
Are detection and identification methods available for the pest?
Yes, P. callosus can be found during visual inspection of infested consignments; adults can be found on the surface of fruit hosts; larvae can be found in the soil of plants for planting.
Morphological keys and molecular methods are available for identification.
If P. callosus is suspected, conventional sampling methods for soil‐dwelling insects, such as taking soil samples, or using suction samplers to collect adults feeding on low vegetation, can be used (Southwood, 1978; MacLeod et al., 1994). Soil samples should be taken from around the roots of hosts to examine for larvae if infestation is suspected in the field. Sticky barriers wrapped around the trunks of fruit trees can be used to detect and monitor adults climbing up the trunks (Pryke and Samways, 2007). Adults do not spread evenly within orchards but aggregate on fruit trees, perhaps due to aggregation pheromones in frass (Barnes and Capatos, 1989). Weevils have a habit of dropping off the host plant if disturbed; hence, adult P. callosus can be collected from trees by firmly jarring branches and collecting them on a white or plastic sheet surrounding the tree trunk (Southwood, 1978; Barnes, 1989a).
Summary descriptions of life stages are provided below:
Eggs are oblong, ~ 0.9 mm long and creamy white turning black at each end as they age (May, 1966).
Larvae are creamy white, legless and up to 6 mm long, with long setae on the body. The head capsule is orange, jaws are black (Walker, 1978).
Pupae are 7–8 mm long and have stout, hooked bristles (Butcher, 1984).
Adults are ~ 7 mm long, dull greyish‐brown with a light V‐shaped band at the rear of the abdomen; beyond the V‐shaped band the elytra are ‘lumpy’. Each lump has numerous setae (Annecke and Moran, 1982).
A factsheet on P. callosus produced by the Department for Primary Industries and Regional Development of Western Australia (DPIRD, 2020) provides photographs of eggs, larvae, pupae and adults. Haran et al. (2020) provide a morphological key to species of Phlyctinus. Molecular methods for species identification are available.
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
P. callosus is indigenous to South Africa (Barnes, 1989b). It has spread in the southern hemisphere, being first reported in New Zealand in 1893, from where it spreads to Tasmania and other southern Australian states e.g. reported in South Australia in 1930 (Kuschel, 1972). P. callosus also occurs on St Helena and Reunion (Figure 2). Appendix A provides national and subnational records of occurrence (EPPO, online).
Figure 2.
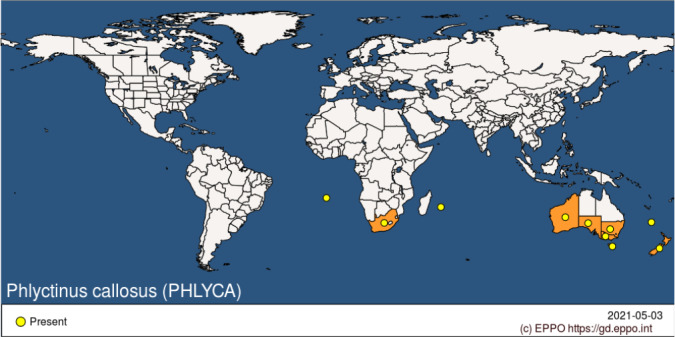
Global distribution of Phlyctinus callosus (Source: EPPO Global Database accessed on 3 May 2021)
3.2.2. Pest distribution in the EU
1.
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, P. callosus is not known to be present in the EU.
3.3. Regulatory status
3.3.1. Commission Implementing Regulation 2019/2072
P. callosus is not listed in Annex II of Commission Implementing Regulation (EU) 2019/2072, an implementing act of Regulation (EU) 2016/2031.
3.3.2. Hosts of Phlyctinus callosus that are prohibited from entering the Union from third countries
As specified in Annex VI of 2019/2072, some plants, which are also P. callosus host plants, or are affected by P. callosus (see Appendix B) are prohibited from entering the EU as plants for planting. Information on which plants are prohibited are shown in Table 3.
Table 3.
List of plants, plant products and other objects that are Phlyctinus callosus hosts whose introduction into the Union from certain third countries is prohibited (Source: Commission Implementing Regulation (EU) 2019/2072, Annex VI)
| List of plants, plant products and other objects whose introduction into the Union from certain third countries is prohibited | |||
|---|---|---|---|
| Description | CN Code | Third country, group of third countries or specific area of third country | |
| 8. | Plants for planting of […], Malus Mill., Prunus L., Pyrus L. and Rosa L., other than dormant plants free from leaves, flowers and fruits | See 2019/2072 Annex VI for details | Third countries other than: specified European third countries (see 2019/2072 Annex VI for details) |
| 9. | Plants for planting of […], Malus Mill., Prunus L. and Pyrus L. and their hybrids, and Fragaria L., other than seeds | “ | Third countries, other than: […]Australia, […], New Zealand […]. |
| 10. | Plants of Vitis L., other than fruits | “ | Third countries other than Switzerland |
| 11. | Plants of Citrus L.[…] other than fruits and seeds | “ | All third countries |
| 14. | Plants for planting of the family Poaceae, other than plants of ornamental perennial grasses of the subfamilies […], other than seeds | “ | Third countries other than: specified European third countries (see Annex VI for details) |
| 15. | Tubers of Solanum tuberosum L., seed potatoes | “ | Third countries other than Switzerland |
| 17. | Tubers of species of Solanum L., and their hybrids […] | “ | Third countries other than specified North African and Asian countries |
| 19. | Soil as such […] | “ | Third countries other than Switzerland |
| 20. | Growing medium as such […] | “ | Third countries other than Switzerland |
3.4. Entry, establishment and spread in the EU
3.4.1. Entry
1.
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes, eggs, larvae and pupae could be transported in soil and growing media accompanying plants for planting; soil contaminating root crops could be infested with immature stages (eggs, larvae or pupae) and the commodity itself could be infested by eggs and larvae feeding on root surfaces. Adults could be carried with fruits that they feed on.
Comment on plants for planting as a pathway.
Rooted plants for planting with growing media provide a potential pathway for eggs, larvae and pupae.
P. callosus has been found in table grapes intended for export from South Africa to USA and Israel (Myburgh and Kriegler, 1967; Pryke and Samways, 2007). In conducting a pest risk analysis on P. callosus for Israel, Opatowski (2001) reports that P. callosus is often found on (unspecified) commodities for export and is often the reason for USDA preclearance disqualifications after packing. In other words, P. callosus is often found with commodities intended for export from South Africa to USA, but the infested commodities are prevented from being exported due to the presence of P. callosus.
Potential pathways for entry of P. callosus into the EU are shown in Table 4.
Table 4.
Potential pathways for Phylyctinus callosus into the EU 27
| Pathways | Life stage | Relevant mitigations [e.g. prohibitions (Annex VI) or special requirements (Annex VII) within Implementing Regulation 2019/2072] |
|---|---|---|
| Plants for planting with roots | Eggs, larvae and pupae in soil, larvae feeding on roots, adults on leaves | Section 3.3.2 summarises plants for planting that are prohibited by Annex VI of 2019/2072. Annex I of EU 2018/2019 lists high‐risk plants whose introduction is prohibited pending risk assessment. The growing medium attached to or associated with plants, intended to sustain the vitality of the plants, are regulated in Article VII of Regulation 2019/2072 (point 1.) Plants for planting from third countries require a phytosanitary certificate and may be inspected on arrival No special requirements in Annex VII relate to P. callosus. |
| Soil | Eggs, larvae, pupae in soil | Soil from third countries is prohibited (Annex VI, 19. and 20.) |
| Fruit of hosts and affected plants (e.g. apples, blueberries, grapes, nectarines, peaches) | Adults amongst fruit | Annex XI, A indicates that a phytosanitary certificate is required for the import of fruits; imports may be inspected on arrival |
| Root and tuber vegetables | Larvae feeding on roots and tubers | Annex XI, A indicates that a phytosanitary certificate is required for the import of root and tubercle vegetables; imports may be inspected on arrival |
High uncertainty about adults being hitchhikers on fruit packaging.
Evidence from USA, Israel, UK and Ireland indicates fresh fruit as a pathway for adult P. callosus. In addition, Suffert et al. (2018a) listed P. callosus as one of a number of pests likely to be associated with apple fruit, table grapes and blueberry fruit imported into the EU. P. callosus was intercepted in the UK on apple fruits (Malus) from South Africa in 2014 and on peach fruit (Prunus) from South Africa in 2015 (Defra unpublished data). Within the EU, P. callosus has been intercepted on apple fruit for consumption from South Africa during a phytosanitary inspection at the Irish border in May 2020 (Bourke, 2020). Taking such evidence into account, the main pathway for entry into the EU is considered to be fresh fruits.
EU imports from South Africa, Australia and New Zealand of fruits that adult P. callosus feed on, as well as of root vegetables that larvae feed on are provided in Table 5.
Table 5.
Annual EU 27 imports of various hosts and plants affected by Phlyctinus callosus from South Africa, Australia and New Zealand, 2016–2020 (hundreds of kg) (Eurostat – Accessed 6 June 2021)
| Commodity | HS code | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Table grapes | 0806 1010 | 1,244,196 | 1,388,339 | 1,418,506 | 1,395,776 | 1,397,303 |
| Apples | 0808 10 | 1,050,839 | 1,011,732 | 1,310,696 | 994,440 | 1,092,098 |
| Pears | 0808 30 | 868,323 | 761,041 | 659,173 | 591,694 | 584,718 |
| Plums | 0809 4005 | 259,282 | 283,935 | 258,265 | 197,075 | 219,410 |
| Peaches, incl. nectarines | 0809 30 | 39,887 | 33,561 | 33,768 | 39,813 | 45,929 |
| Blueberries1 | 0810 4000 | 8,945 | 16,482 | 24,059 | 49,025 | 76,401 |
| Carrots and turnips | 0706 1000 | 16,351 | 12,438 | 12,294 | 12,932 | 12,044 |
| Strawberries | 0810 1000 | 20 | 64 | 176 | 25 | 125 |
| Potatoes (excl. seed) | 0701 90 | 2 | 2 | 0 | 236 | 0 |
| Asparagus | 0709 2000 | 80 | 20 | 1 | 0 | 136 |
Vaccinium macrocarpum and V. corymbosum.
In Europe, a single adult P. callosus was found on a potted azalea plant at a public market in Wolverhampton, England, in March 2004 (Smith, 2004).
Notifications of interceptions of harmful organisms began to be compiled in Europhyt in May 1994 and in TRACES in May 2020. As of 5 May 2021, there were no records of interceptions or outbreaks of P. callosus in the Europhyt and TRACES databases.
3.4.2. Establishment
1.
Is the pest able to become established in the EU territory?
Yes, biotic factors (host availability) and abiotic factors (climate suitability) suggest that areas of the EU would be suitable for establishment.
Climatic mapping is the principal method for identifying areas that could provide suitable conditions for the establishment of a pest taking key abiotic factors into account (Baker, 2002). Availability of hosts is considered in Section 3.4.2.1. Climatic factors are considered in Section 3.4.2.2.
3.4.2.1. EU distribution of main host plants
As noted above, and in Appendix B, P. callosus is polyphagous. Cultivated plants on which adults and/or larvae feed such as grapes, apples, peaches and nectarines are grown in central and southern EU. Grasses, weeds and roots vegetables that larvae feed on are grown throughout the EU. Many food plants are also grown in home gardens (de Rougemont, 1989).
Table 8 shows the harvested area of key hosts and food plants cultivated in the EU 27 in recent years.
Table 8.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Key uncertainties |
|---|---|---|
| Identity of the pest (Section 3.1) | The identity of the species is established and Phlyctinus callosus (Schoenherr, 1826) is the accepted name. | More taxonomic work to differentiate the species complex would help, nevertheless, P. callosus sensu stricto would still satisfy the criteria for QP status |
| Absence/presence of the pest in the EU (Section 3.2) | P. callosus is not known to be present in the EU | |
| Regulatory status (Section 3.3) | P. callosus is not regulated by EU plant health legislation. | |
| Pest potential for entry, establishment and spread in the EU (Section 3.4) | Eggs, larvae and pupae could be transported in soil and growing media accompanying plants for planting; adults can be carried with fruits that they feed on; soil contaminating root crops could be infested with juvenile stages (larvae or pupae) and the commodity itself could be infested by larvae feeding on root surfaces. Biotic factors (host availability) and abiotic factors (climate suitability) suggest that areas of the EU would be suitable for establishment of P. callosus. The pest is a free living organism and could spread within the EU, facilitated by movement of hosts. | As with many pests that can enter on produce, there are uncertainties regarding the ability of the organism to transfer to locate another host, complete development, reproduce and initiate a new population. |
| Potential for consequences in the EU (Section 3.5) | Larvae and adults are harmful to a range of plants and economic impacts would be expected if P. callosus established in the EU. | The effect of existing pest management practices used against other pests in the EU, and their consequences on the potential impact of P. callosus is unknown. |
| Available measures (Section 3.6) | Some plants affected by P. callosus are already prohibited as plants for planting from third countries and fruits imported into the EU require a phytosanitary certificate of which a proportion of consignments are inspected. Additional options are available to reduce the likelihood of pest entry into the EU. | |
| Conclusion (Section 4) | P. callosus satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest. | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | ||
3.4.2.2. Climatic conditions affecting establishment
P. callosus survives well in regions with dry hot summers and wet winters (Annecke and Moran, 1982). Smit et al. (2018) report collecting P. callosus from Piketberg (climate type Csa) and Grabouw (Csb) in the Western Cape in South Africa. Each area has a Mediterranean type climate. In New Zealand, Prestidge and Willoughby (1989) report P. callosus from the Bay of Plenty, Manawatu, Taranaki and Waikato regions; all regions have a Cfb‐type climate. The Cfb climate is found widely in central and northern EU countries; the Cfb‐type climate is represented in ˜ 46% of EU 27 five arcmin grid cells (MacLeod and Korycinska, 2019). Figure 3 shows the occurrence of EU type climates which also occur in South Africa, Australia and New Zealand.
Figure 3.
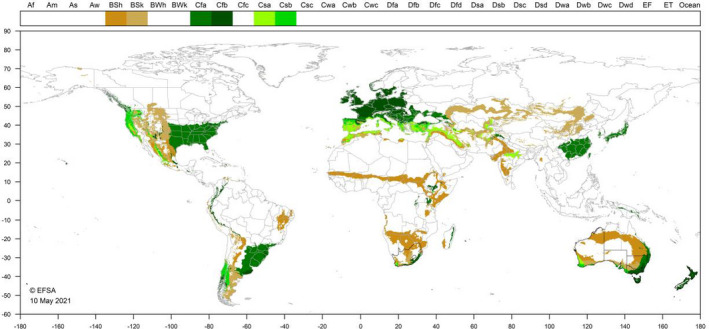
World distribution of six Köppen–Geiger climate types that occur in the EU and which occur in the countries where Phlyctinus callosus occurs. Black borders indicate countries or subnational areas where P. callosus occurs
Regarding establishment in protected cultivation, Miller (1979) reports P. callosus as a pest of horticultural crops in the field and in greenhouses in Tasmania and Lo et al. (1990) report P. callosus as a pest on grapevines in glasshouses in New Zealand. However, whether P. callosus is really established in greenhouses or whether it moves between outdoor hosts and glasshouse crops is unclear. For example, Lo et al. (1990) wrote ‘the weevils appear to be invading the glasshouse from outside, but may also be emerging inside’.
3.4.3. Spread
1.
Describe how the pest would be able to spread within the EU territory following establishment?
P. callosus is a free ‐living organism and could spread within the EU. Immature stages of P. callosus occur in the soil. Adults cannot fly so disperse by walking. If introduced into the EU, natural spread would occur locally and slowly.
Human‐mediated distribution of infested fruits could spread adults.
Comment on plants for planting as a mechanism of spread
Stages could be carried with plants for planting.
3.5. Impacts
1.
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes; Adults can cause direct damage to apples and nectarines via fruit scarring; they also impact other pome and stone fruit as well as blueberries and strawberries amongst other crops. Larvae can cause harm to root vegetables such as carrots and potatoes. Ornamentals in glasshouses could be damaged by larval root feeding and adults feeding on foliage. Given that larvae and adults are harmful to a range of plants, economic impacts would be expected if P. callosus established in the EU. Larvae are injurious to grasses and weeds in orchards and vineyards.
Adult P. callosus cause damage to deciduous fruit trees and fruit by feeding on leaves, fruit stalks and directly on fruit (Johnson and Neven, 2011; Dlamini et al., 2019a, 2019b). P. callosus has been regarded as a pest in South Africa since 1896 and Barnes (1989a) considered it as the most difficult pest to control in apple and nectarine orchards reporting annual losses worth more than Rands 5 million.2 Adults chew away the skin and eat the underlying flesh causing shallow craters in the fruit (Barnes, 1989a) less often damage is caused to pear, plums and peach fruit. The greatest damage to apples occurs to young fruit, up to 6 weeks after fruit set. The thickening of the apple wax cuticle and deterioration in the mandibles of adults reduces adult feeding on older apples (Barnes, 1987). In a 4‐year study, Barnes and Giliomee (1992) measured yield losses of between 1% and 66% from individual apple trees in orchards where P. callosus were not managed. Mean crop loss between seasons ranged from 5% to 29%. Witt et al. (1995) cite a report that estimated 40% of all damage to apples caused by pests and diseases in the Elgin area of the Western Cape during the 1970s could be attributed to P. callosus and that in 1981 average fruit damage in the three main apple growing areas in the Western Cape was estimated to be 1.25% of the harvest which amounted to an annual loss of at least US$ 100,000; losses in 1987 were estimated to be in the region of US$ 550,000.
Young fruit trees can be defoliated by large populations of adults (Barnes, 1989a). Following the commercialisation of blueberries in South Africa in the early 1990s, P. callosus has become a serious pest (Bredenhand et al., 2010). Although adults do not always cause yield or quality losses in table grapes in South Africa, P. callosus is highly relevant as one of the most serious pests due to the consequences of lost exports (Annecke and Moran, 1982; de Villiers and Pringle, 2008). Exports to Japan from New Zealand are also impacted by P. callosus (Lo et al., 1990).
In an article providing advice for the control of insect pests of potato in Western Australia, Learmonth and Matthiessen (1990) recognise that the main soil insect pests are Graphognathus (=Naupactus) leucoloma and Heteronychus arator; they also list P. callosus along with three other weevil species as pests that do cause damage to potato tubers, but over much smaller areas. Adult feeding also damages strawberry leaves and fruit, walnuts, plums and potato foliage. Adults also feed on leaves of ornamental succulents such as Cyclamen in Victoria (Australia) (Walker, 1980). In Tasmania, P. callosus is principally a pest of carrots and parsnips with larvae chewing holes in the root surface, ornamental flowers and shrubs are also damaged when adults feed on foliage at night (Miller, 1979).
3.6. Available measures and/or potential specific import requirements and limits of mitigation measures
1.
Are there measures available to prevent the entry into the EU such that the risk becomes mitigated?
Yes, some hosts are already prohibited as plants for planting from third countries (see 3.3.2). Fruits imported into the EU require a phytosanitary certificate and a proportion of consignments are inspected. Additional options are available to reduce the likelihood of pest entry into the EU.
Regarding plants for planting, host plants for planting could be grown under physical protection (e.g. secure greenhouses) to prevent them from becoming infested. However, once outdoors they could be exposed to P. callosus before export.
3.6.1. Identification of potential additional measures
Phytosanitary measures (prohibitions) are currently applied to some host plants for planting (see Section 3.3.2).
Potential control measures on hosts that are imported are listed in Table 5.
Table 7.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry in relation to currently unregulated hosts and pathways
| Special requirements summary (with hyperlink (in blue) to information sheet if available) | Potential control measure summary |
|---|---|
| Pest freedom | Source imports from pest‐free area. Used to mitigate likelihood of infestation by specified pest at origin, hence to mitigate entry. |
| Growing plants in isolation | Could be considered because P. callosus does not spread quickly; measures could be applied in vicinity of growing site |
| Chemical treatments on crops including reproductive material | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments |
| Soil treatment | Eggs, larvae and pupae develop in the soil. Used to mitigate likelihood of infestation of soil at origin |
| Inspections | Pre‐export inspections detect infested consignments and could be used to mitigate likelihood of infestation of consignments to EU |
| Chemical treatments on consignments or during processing | Used to mitigate likelihood of infestation of pests susceptible to chemical treatments |
| Physical treatments on consignments or during processing | Removal of soil from roots of plants for planting would lower likelihood of larvae being carried |
| Heat and cold treatments | Cold treatment with or without controlled atmosphere can achieve 100% mortality of larvae in stone fruit (Smit et al., 2018), but some fruit varieties are sensitive to chill injury, limiting the measure to tolerant fruit. |
| Controlled atmosphere | Controlled atmosphere combined with heat treatment could provide control post‐harvest (Johnson and Neven, 2011) |
| Cleaning and disinfection of facilities, tools and machinery | Used to mitigate likelihood of entry for spread of soil‐borne pests, could reduce likelihood of entry of eggs or larvae with soil. |
| Limits on soil | Used to mitigate likelihood of entry or spread via pests in soil |
| Phytosanitary certificate and plant passport | Used to attest which of the above requirements have been applied |
3.6.1.1. Biological or technical factors limiting the effectiveness of measures to prevent the entry of the pest
Larvae in the soil can be difficult to detect and treat
Limited range of pesticides available to treat fresh fruit and vegetables for consumption
Life stages emerge over extended periods so targeting measures at sites of production is a challenge; measures targeting specific life stages are needed multiple times.
3.7. Uncertainty
If entering on produce, there are uncertainties over the pests’ ability to transfer to a suitable host following arrival in the EU. The likelihood of transfer will generally be much lower for pests that arrive on produce than for pests that arrive on living plants (van der Gaag et al., 2019). Uncertainties affecting establishment, which are common to other pests that enter, also include the ability to find a mate and other Allee effects (effects causing reduced survival of new colonies with a small number of individuals (Tobin et al., 2011)) as well as the impact of natural enemies. There is uncertainty over P. callosus parthenogenesis. Marvaldi et al. (2014) did not recognise P. callosus as being parthenogenetic and males and females occur in South Africa. However, May (1966) in New Zealand states P. callosus as being parthenogenetic without any details.
4. Conclusions
P. callosus satisfies all the criteria that are within the remit of EFSA to assess for it to be regarded as a potential Union quarantine pest (Table 6).
Table 6.
EU 27 area of crop production of hosts and plants affected by Phlyctinus callosus (cultivation/harvested/production, thousand ha). Other hosts are also cultivated in the EU. Appendix B provides an extensive list of hosts and plants affected. Source: Eurostat
| Crop | Code | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|
| Grapes | W1000 | 3,136.0 | 3,134.9 | 3,137.2 | 3,160.7 | 3,162.5 |
| Apples | F1100 | 506.5 | 505.6 | 507.2 | 491.4 | 473.7 |
| Peaches & nectarines | F1210_1220 | 224.9 | 221.6 | 215.0 | 206.8 | 197.1 |
| Potatoes | R1000 | 1,550.5 | 1,601.2 | 1,562.9 | 1,607.4 | 1,672.2 |
| Citrus fruits | T0000 | 519.0 | 502.8 | 509.0 | 512.5 | 487.1 |
| Onions | V4210 | 169.9 | 170.7 | 171.8 | 176.6 | 176.3 |
| Plums | F1250 | 152.8 | 153.9 | 153.4 | 154.5 | 154.1 |
| Pears | F1120 | 115.8 | 114.8 | 114.8 | 111.8 | 108.8 |
| Carrots | V4100 | 106.4 | 106.7 | 107.4 | 108.5 | 107.3 |
| Strawberries | S0000 | 103.8 | 103.8 | 107.0 | 100.9 | 99.1 |
| Asparagus | V2600 | 56.4 | 59.1 | 60.2 | 58.9 | 59.7 |
| Blueberries | F3300 | 13.3 | 16.9 | 19.4 | 20.6 | : |
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 2018).
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 2018).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2018).
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2018).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2018).
- Greenhouse
A walk‐in, static, closed place of crop production with a usually translucent outer shell, which allows controlled exchange of material and energy with the surroundings and prevents release of plant protection products (PPPs) into the environment.
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2018).
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2018).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2018).
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2018).
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO 2018).
Appendix A – Distribution of Phlyctinus callosus
1.
Distribution records based on EPPO Global Database (EPPO, online).
| Region | Country | Subnational (e.g. State) | Status |
|---|---|---|---|
| North America | No records, presumed absent | ||
| Central America | No records, presumed absent | ||
| Caribbean | No records, presumed absent | ||
| South America | No records, presumed absent | ||
| Europe | Ireland | Intercepted only, presumed absent | |
| United Kingdom | Intercepted only, presumed absent | ||
| Africa | Reunion | Present, no details | |
| Saint Helena | Present, no details | ||
| South Africa | Present, no details | ||
| Asia | No records, presumed absent | ||
| Oceania | Australia | Present, restricted distribution | |
| New South Wales | Present, no details | ||
| Norfolk Island | Present, no details | ||
| South Australia | Present, no details | ||
| Tasmania | Present, no details | ||
| Victoria | Present, no details | ||
| Western Australia | Present, no details | ||
| New Zealand | Present, no details |
Appendix B – Phlyctinus callosus host plants/plants affected*
1.
*For a plant species to be regarded as a true host, the pest must be able to develop and reproduce feeding exclusively on the plant species, i.e. the plant species provides sufficient nutrition for larvae and adults to develop and reproduce successfully. It is common that literature on polyphagous plant pests list plant species upon which the pest feeds and regard them as hosts, without providing substantiating evidence that development and reproduction is possible. In a list of plants that Haran et al. (2020) provide as being verified host species of P. callosus no genera from the Rosaceae or Vitaceae are included. However, apples, peaches and nectarines (Rosaceae) and grapevine Vitis (Vitaceae) are commonly reported as hosts in literature. The table below lists true hosts as well as plants on which feeding has occurred (i.e. plants affected).
Source: EPPO Global Database (2021) unless indicated
| Host status | Plant family | Host name | Common name | Reference |
|---|---|---|---|---|
| Cultivated hosts | Aizoaceae | Lampranthus sp. | – | |
| Amaranthaceae | Beta vulgaris | Beet | Bourke (2020) | |
| Amaryllidaceae | Allium cepa | Onion | ||
| Tulbaghia | Society garlic | |||
| Apiaceae | Daucus carota subsp. sativus | Carrot | ||
| Pastinaca sativa | Parsnip | Bourke (2020) | ||
| Asparagaceae | Asparagus officinalis | Asparagus | Prestidge and Willoughby (1989) | |
| Asteraceae | Chrysanthemum | Bourke (2020) | ||
| Gazania sp. | – | |||
| Crassulaceae | Cotyledon orbiculata | Round‐leafed navel‐wort | ||
| Ericaceae | Vaccinium corymbosum | Northern highbush blueberry | ||
| Geraniaceae | Pelargonium sp. | Geranium | ||
| Juglandaceae | Juglans regia | Walnut | Bourke (2020) | |
| Plumbaginaceae | Plumbago auriculata | Cape leadwort | ||
| Poaceae | Lolium perenne | Perennial ryegrass | Bourke (2020) | |
| Polygonaceae | Rheum rhabarbarum | Rhubarb | Bourke (2020) | |
| Rosaceae | Fragaria | Strawberry | Bourke (2020) | |
| Malus domestica | Apple * | |||
| Prunus domestica | Plum | Bourke (2020) | ||
| Prunus persica | Peach * | |||
| Prunus persica var. nucipersica | Nectarine * | |||
| Pyrus communis | European pear | Bourke (2020) | ||
| Rosa | Rose | Bourke (2020) | ||
| Rutaceae | Citrus | Bourke (2020) | ||
| Solanaceae | Capsicum | Bourke (2020) | ||
| Solanum tuberosum | Potato | Learmonth and Matthiessen (1990) | ||
| Vitaceae | Vitis vinifera | Grape vine * | ||
| Wild/Weed hosts | Asteraceae | Osteospermum moniliferum | Bush‐tickberry | |
| Taraxacum officinale | Dandelion | Bourke (2020) | ||
| Plantaginaceae | Plantago lanceolata | Ribwort plantain | ||
| Polygonaceae | Rumex acetosa | Dock | Bourke (2020) | |
| Artificial/experimental hosts | None reported |
Plant frequently recorded in literature impacted by P. callosus.
Appendix C – EU 27 imports of fresh or chilled fruit and vegetable hosts and plants affected by Phlyctinus callosus from South Africa, New Zealand and Australia, 2016–2020. (Hundreds of kg)
1.
(Source Eurostat accessed 6 June 2021)
| Source | Commodity | HS Code | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|
| South Africa | Potatoes (excl. seed) | 0701 90 | 2 | 0 | – | 236 | – |
| Carrots and turnips | 0706 1000 | 6,757 | 3,760 | 3,608 | 4,005 | 3,235 | |
| Asparagus | 0709 2000 | 70 | 20 | 1 | 0 | 136 | |
| Table grapes | 0806 1010 | 1,244,196 | 1,388,339 | 1,418,506 | 1,395,776 | 1,397,303 | |
| Apples | 0808 10 | 298,163 | 252,069 | 334,616 | 258,077 | 329,088 | |
| Pears | 0808 30 | 865,863 | 759,193 | 655,429 | 590,939 | 583,341 | |
| Peaches, incl. nectarines | 0809 30 | 39,581 | 33,270 | 33,445 | 39,570 | 45,719 | |
| Plums | 0809 4005 | 259,282 | 283,935 | 258,257 | 197,060 | 219,213 | |
| Strawberries | 0810 1000 | 20 | 64 | 176 | 25 | 125 | |
| Blueberries1 | 0810 4000 | 8,914 | 16,482 | 24,059 | 49,025 | 76,401 | |
| New Zealand | Potatoes (excl. seed) | 0701 90 | – | 2 | – | – | – |
| Carrots and turnips | 0706 1000 | 244 | 244 | 511 | 756 | – | |
| Asparagus | 0709 2000 | – | 0 | – | – | – | |
| Table grapes | 0806 1010 | – | 0 | – | – | – | |
| Apples | 0808 10 | 751,628 | 754,737 | 966,921 | 728,052 | 759,371 | |
| Pears | 0808 30 | 2,460 | 1,847 | 2,520 | 755 | 1,377 | |
| Peaches, incl. nectarines | 0809 30 | 22 | 11 | – | – | – | |
| Plums | 0809 4005 | – | 0 | – | – | – | |
| Strawberries | 0810 1000 | – | 0 | – | – | – | |
| Blueberries1 | 0810 4000 | – | 0 | – | – | – | |
| Australia | Potatoes (excl. seed) | 0701 90 | – | 0 | – | – | – |
| Carrots and turnips | 0706 1000 | 9,350 | 8,434 | 8,176 | 8,171 | 8,809 | |
| Asparagus | 0709 2000 | 10 | 0 | – | – | – | |
| Table grapes | 0806 1010 | – | 1 | – | – | – | |
| Apples | 0808 10 | 1,049 | 4,926 | 9,159 | 8,311 | 3,639 | |
| Pears | 0808 30 | – | 0 | 1,225 | – | – | |
| Peaches, incl. nectarines | 0809 30 | 285 | 280 | 323 | 243 | 209 | |
| Plums | 0809 4005 | – | 0 | 8 | 15 | 197 | |
| Strawberries | 0810 1000 | – | 0 | – | – | – | |
| Blueberries1 | 0810 4000 | 31 | 0 | – | – | – |
Vaccinium macrocarpum and V. corymbosum.
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard C, Di Serio F, Gonthier P, Jaques Miret JA, Justesen AF, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Gregoire J‐C, Malumphy C, Campese C, Czwienczek E, Kertesz V, Maiorano A and MacLeod A, 2021. Scientific Opinion on the pest categorisation of Phlyctinus callosus . EFSA Journal 2021;19(8):6800, 22 pp. 10.2903/j.efsa.2021.6800
Requestor: European Commission
Question number: EFSA‐Q‐2021‐00244
Panel members: Claude Bragard, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © Pest and Diseases Image Library, Bugwood.org, Figure 2: © EPPO.
Adopted: 8 July 2021
Notes
An EPPO code, formerly known as a Bayer code, is a unique identifier linked to the name of a plant or plant pest important in agriculture and plant protection. Codes are based on genus and species names. However, if a scientific name is changed the EPPO code remains the same. This provides a harmonized system to facilitate the management of plant and pest names in computerized databases, as well as data exchange between IT systems (Griessinger and Roy, 2015; EPPO, 2019).
Based on an exchange rate of 0.3 in 1989, 5 million Rand equates to approximately € 1.5 million.
References
- Annecke DP and Moran VC, 1982. Insects and mites of cultivated plants in South Africa. Butterworths, Durban.
- Baker RHA, 2002. Predicting the limits to the potential distribution of alien crop pests. In: Hallman GJ and Schwalbe CP (eds.). Invasive Arthropods in Agriculture: problems and solutions. Science Publishers Inc, Enfield, USA. pp. 207–241. [Google Scholar]
- Barnes BN, 1987. Biology of Phlyctinus callosus in fruit orchards. Curculio 23, June 15th 1987.
- Barnes BN, 1989a. Different life and seasonal cycles of banded fruit weevil, Phlyctinus callosus (Coleoptera: Curculionidae), in apple orchards in the south‐western Cape. Phytophylactica, 21, 147–158. [Google Scholar]
- Barnes BN, 1989b. Embryonic and immature stages of Phlyctinus callosus Boh.(Coleoptera: Curculionidae): aspects of biology and behaviour with respect to control in deciduous fruit orchards. Journal of the Entomological Society of Southern Africa, 152, 165–778. [Google Scholar]
- Barnes BN and Capatos D, 1989. Evidence for an aggregation pheromone in adult frass of banded fruit weevil, Phlyctinus callosus (Schoenherr)(Col., Curculionidae). Journal of Applied Entomology, 108, 512–518. [Google Scholar]
- Barnes BN and Giliomee JH, 1992. Fruit‐feeding behaviour of banded fruit weevil, Phlyctinus callosus (Schönherr)(Col., Curculionidae), in apple orchards. Journal of Applied Entomology, 113, 407–415. [Google Scholar]
- Barnes BN and Pringle KL, 1989. Oviposition by the banded fruit weevil, Phlyctinus callosus (Schoenherr) (Coleoptera: Curculionidae), in deciduous fruit orchards in South Africa. Bulletin of Entomological Research, 79, 31–40. [Google Scholar]
- Bourke A, 2020. Rapid Pest Risk Analysis (PRA) for Phlyctinus callosus. Ireland Department of Agriculture Food and the Marine. 53 pp. Available online https://pra.eppo.int/getfile/9c71c4b6-fabc-4e68-a9ae-e4a27f863fd9 [Accessed: June 6]
- Bredenhand E, Hoorn A, van May F, Ferreira T and Johnson S, 2010. Evaluation of techniques for monitoring banded fruit weevil, Phlyctinus callosus (Schoenherr) (Coleoptera:Curculionidae), infestation in blueberry orchards. African Entomology, 18, 205–209. 10.4001/003.018.0118 [DOI] [Google Scholar]
- Butcher MR, 1984. Vegetable crop pests. In: Scott RR (ed.). New Zealand Pest and Beneficial Insects. Lincoln University College of Agriculture, Canterbury. pp. 93–118. [Google Scholar]
- Dlamini BE, Addison P and Malan AP, 2019a. A review of the biology and control of Phlyctinus callosus (Schönherr) (Coleoptera: Curculionidae), with special reference to biological control using entomopathogenic nematodes and fungi. African Entomology, 27, 279–288. [Google Scholar]
- Dlamini BE, Malan AP and Addison P, 2019b. Control of the banded fruit weevil Phlyctinus callosus (Coleoptera: Curculionidae) using entomopathogenic nematodes. Austral Entomology, 58, 687–695. [Google Scholar]
- DPIRD , 2020. Garden weevil in vineyards. Department for Primary Industries and Regional Development, Western Australia. Last updated 3rd July 2020. Available online: https://www.agric.wa.gov.au/print/node/2841 [Accessed: 12 June 2021]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Schlatter JR, Silano V, Solecki R, Turck D, Benfenati E, Chaudhry QM, Craig P, Frampton G, Greiner M, Hart A, Hogstrand C, Lambre C, Luttik R, Makowski D, Siani A, Wahlstroem H, Aguilera J, Dorne J‐L, Fernandez Dumont A, Hempen M, Valtueña Martinez S, Martino L, Smeraldi C, Terron A, Georgiadis N and Younes M, 2017. Scientific Opinion on the guidance on the use of the weight of evidence approach in scientific assessments. EFSA Journal 2017;15(8):4971, 69 pp. 10.2903/j.efsa.2017.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO , 2019. EPPO codes. Available online: https://www.eppo.int/RESOURCES/eppo_databases/eppo_codes
- EPPO , 2020. Addition to the EPPO Alert List: Phlyctinus callosus (Coleoptera: Curculionidae), banded fruit weevil. EPPO Reporting Service, 8, 165.
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 3 May 2021]
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2018. International Standards for Phytosanitary Measures. ISPM 5 Glossary of phytosanitary terms. Revised version adopted CPM 13, April 2018. FAO, Rome. Available online: https://www.ippc.int/en/publications/621/
- Fera , 2018. Final Report Summary ‐ DROPSA (Strategies to develop effective, innovative and practical approaches to protect major European fruit crops from pests and pathogens). Available online: https://cordis.europa.eu/project/id/613678/reporting [Accessed: 5 June 2021]
- van der Gaag DJ, Holt J, Leach AW and Loomans AJ, 2019. Model of the probability of pest transfer to a site suitable for establishment following their arrival on imported fruit, cut‐flower or vegetable produce. Crop Protection, 117, 135–146. [Google Scholar]
- Griessinger D and Roy A‐S, 2015. EPPO codes: a brief description. Available online: https://www.eppo.int/media/uploaded_images/RESOURCES/eppo_databases/A4_EPPO_Codes_2018.pdf
- Haran JM, Hansen S, Benoit I and Addison P, 2020. Description of five new species in the genus Phlyctinus Schoenherr (Coleoptera, Curculionidae): a first step in deciphering the P.callosus complex. European Journal of Taxonomy, 669, 1–29. 10.5852/ejt.2020.669 [DOI] [Google Scholar]
- Johnson SA and Neven LG, 2011. Heated‐controlled atmosphere postharvest treatments for Macchiademus diplopterus (Hemiptera: Lygaeidae) and Phlyctinus callosus (Coleoptera: Curculionidae). Journal of Economic Entomology, 104, 398–404. [DOI] [PubMed] [Google Scholar]
- Kuschel G, 1972. The foreign Curculionoidea established in New Zealand (Insecta: Coleoptera). New Zealand Journal of Science, 15, 273–289. [Google Scholar]
- Learmonth S and Matthiessen J, 1990. Integrated control of soil insect pests of potatoes. Journal of Agriculture, Western Australia, 31, 155–158. [Google Scholar]
- Lo PL, Blank RH and Parker RE, 1990. Insecticides for adult garden weevil and problems with control on glasshouse grapes. New Zealand Weed and Pest Control Society Inc., Palmerston North. pp. 100–103. [Google Scholar]
- MacLeod A and Korycinska A, 2019. Detailing Koppen‐Geiger climate zones at a country and regional level: are source for pest risk analysis. EPPO Bulletin, 49, 73–82. [Google Scholar]
- MacLeod A, Wratten SD and Harwood RW, 1994. The efficiency of a new lightweight suction sampler for sampling aphids and their predators in arable land. Annals of Applied Biology, 124, 11–17. [Google Scholar]
- Marvaldi AE, Lanteri AA, del Rio Guadalupe M and Oberprieler RG, 2014. 3.7.5 Entiminae Schoenherr, 1823. pp. 503–522. In: Leschen RAB and Beutel RG (eds.). Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles. Volume 3: Morphology and Systematics (Phytophaga). Walter de Gruyter, Berlin/Boston. [Google Scholar]
- May BM, 1966. Identification of the immature forms of some common soil‐inhabiting weevils, with notes on their biology. New Zealand Journal of Agricultural Research, 9, 286–316. [Google Scholar]
- Miller LA, 1979. Weevil pests of horticultural crops. Journal of Agriculture, Tasmania, 50, 52–53. [Google Scholar]
- Myburgh AC and Kriegler PJ, 1967. Experiments on sterilization of the Snout‐Beetles, Phlyctinus callosus Boh. and Eremnus setulosus Boh., on export grapes in cold‐storage. Journal of the Entomological Society of Southern Africa, 29, 96–101. [Google Scholar]
- Opatowski D, 2001. EPPO panel on pest risk analysis. OEPP/EPPO Bulletin, 31, 371–374. [Google Scholar]
- Prestidge RA and Willoughby B, 1989. Garden weevil life cycle and insecticides for its control in asparagus. In Proceedings of the Forty Second New Zealand Weed and Pest Control Conference, Taranki Country Lodge, New Plymouth, 8‐10 August, 1989. (pp. 238–242). New Zealand Weed and Pest Control Society Inc.
- Pryke JS and Samways MJ, 2007. Current control of phytosanitary insect pests in table grape vineyards of the Hex River Valley, South Africa. African Entomology, 15, 25–36. [Google Scholar]
- de Rougemont GM, 1989. A Field Guide to the Crops of Britain and Europe. Collins Sons and Co. Ltd, London. [Google Scholar]
- Smith RM, 2004. Pest Risk Analysis for Phlyctinus callosus. Central Science Laboratory, Sand Hutton, York: YO41 1LZ UK. [Available on request from Defra]. [Google Scholar]
- Smit R, Jooste MM and Johnson SA, 2018. CATTS technology: phytosanitary control and market expansion of chill sensitive Japanese plums for South Africa. Acta Horticulturae. 1194, Proceedings of VIII International Postharvest Symposium: Enhancing Supply Chain and Consumer Benefits – Ethical and Technological Issues, 201–208.
- Southwood TRE, 1978. Ecological methods with particular reference to the study of insect populations, 2nd Edition. Chapman & Hall, London. 524 pp. [Google Scholar]
- Suffert M, Wilstermann A, Petter F, Schrader G and Grousset F, 2018. Identification of new pests likely to be introduced into Europe with the fruit trade. EPPO Bulletin, 48, 144–154. [Google Scholar]
- Tobin PC, Berec L and Liebhold AM, 2011. Exploiting Allee effects for managing biological invasions. Ecology Letters, 14, 615–624. [DOI] [PubMed] [Google Scholar]
- de Villiers M and Pringle KL, 2008. Developing a generic sampling system for monitoring the key arthropod pests of table grapes, Vitis vinifera L. International Journal of Pest Management, 54, 207–217. [Google Scholar]
- Walker PL, 1978. A study of the biology, pest status and control of garden weevil, Phlyctinus callosus Boheman, and the development of techniques for laboratory studies. 73 pp. Research Project Report, Plant Research Institute, Burnley; Victorian Department of Agriculture.
- Walker PL, 1980. Laboratory rearing of the Garden Weevil, Phlyctinus callosus Boheman (Coleoptera: Curculionidae), and the effect of temperature on its growth and survival. Australian Journal of Zoology, 29, 25–32. [Google Scholar]
- Witt AR, Little RM and Crowe TM, 1995. The effectiveness of helmeted guineafowl Numida meleagris (Linnaeus 1766) in controlling the banded fruit weevil Phlyctinus callosus (Schönherr 1826), and their impact on other invertebrates in apple orchards in the Western Cape Province, South Africa. Agriculture, Ecosystems and Environment, 55, 169–179. [Google Scholar]


